Abstract
TWIK-related acid-sensitive K+ (TASK) homomeric channels, TASK1 and TASK3, are present in PC12 cells. The channels do not heteromerize due plausibly to a lack of p11 protein. Single-channel recording reveals that most of the rat carotid body (CB) glomus cells express heteromeric TASK1-TASK3 channels, but the presence of p11 in glomus cells has not yet been verified. TASK1, but not TASK3, binds to p11, which has a retention signal for the endoplasmic reticulum. We hypothesized that p11 could facilitate heteromeric TASK1-TASK3 formation in glomus cells. We investigated this hypothesis in isolated immunocytochemically identified rat CB glomus cells. The findings were that glomus cells expressed p11-like immunoreactive (IR) material, and TASK1- and TASK3-like IR material mainly coincided in the cytoplasm. The proximity ligation assay showed that TASK1 and TASK3 heteromerized. In separate experiments, supporting evidence for the major role of p11 for channel heteromerization was provided in PC12 cells stimulated by nerve growth factor. p11 production took place there via multiple signaling pathways comprising mitogen-activated protein kinase and phospholipase C, and heteromeric TASK1-TASK3 channels were formed. We conclude that p11 is expressed and TASK1 and TASK3 heteromerize in rat CB glomus cells.
Keywords: carotid body glomus cell, PC12 cell, p11, TASK1, TASK3
Introduction
TWIK-related acid-sensitive K+ (TASK) channels contribute to the resting membrane potential in a variety of cells, such as carotid body (CB) glomus cells,1 brain neurons,2,3 and endocrine cells.4–8 They belong to the two-pore domain K+ (K2P) channel family,9,10 which comprises 15 subunits in humans. Having two pore domains in each subunit, K2P channels exist as a homodimer or heterodimer of subunits. The TASK subfamily consists of TASK1, TASK3, and TASK5: TASK1 and TASK3 function as homomeric or heteromeric channels,11,12 whereas TASK5 is devoid of channel activity in exogenous expression systems.13 The molecular structure of TASK channels constitutively expressed in some cells7,12,14,15 or exogenously expressed in oocytes16 or cell lines3,11 has been investigated with biochemical or electrophysiological approaches. Single-channel analysis in mouse12 and rat CB glomus cells14 revealed that most of the TASK channels are heteromeric TASK1-TASK3 structures. As homomeric TASK1 or TASK3 channel activity has been recorded in glomus cells of TASK3 or TASK1 knockout mice,12 respectively, it has been surmised that heteromeric TASK1-TASK3 channels are more readily assembled and/or trafficked to the plasma membrane than either homodimer. In contradistinction, endogenous homomeric TASK1 and TASK3 channels are found in the plasma membrane of PC12 cells17 which originate from rat adrenal medullary chromaffin cells.18 Interestingly, when green fluorescent protein (GFP)-tagged TASK1 or TASK3 proteins are exogenously expressed in PC12 cells,17 they do not heteromerize with endogenous TASK subunits in the plasma membrane. We have recently proposed that a lack of heteromeric TASK1-TASK3 channels in PC12 cells is due to a lack of p11,7 a cytosolic protein which contains a retention signal in the C-terminus.19,20 As p11 is not expressed in PC12 cells,21 TASK1 protein translated at the rough endoplasmic reticulum (ER) may be promptly folded in a mature form without encountering TASK3 and trafficked to the plasma membrane through the Golgi complex. If this notion is true, the cells where heteromeric TASK1-TASK3 channels are formed will be expected to produce p11. The present immunocytochemical study addresses this issue in rat CB glomus cells where heteromeric TASK1-TASK3 channels have been functionally identified in the plasma membrane,14 but the presence of p11 has not yet been substantiated. In addition, to enhance the understanding of how p11 expression is regulated, we explored the signal transduction pathways for the nerve growth factor (NGF)-induced p11 production in PC12 cells,7,21 which have the same catecholamine synthesizing potency18 as the glomus cells do.22
Materials and Methods
Animals
Eleven male Wistar rats weighing 150 to 300 g were used. Rats were housed in standard cages with free access to food and water. Animals were kept under a light/dark cycle of 12/12 hr under the normoxic condition. All procedures for the care and treatment of animals were carried out according to the Japanese Act on the Welfare and Management of Animals and the Guidelines for the Proper Conduct of Animal Experiments issued by the Science Council of Japan. The experiments were approved by the Institutional Animal Care and Use Committee of the University of Occupational and Environmental Health in Kitakyushu (permit AE19-014). The procedures complied with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. All efforts were made to minimize potential animal distress.
Immunocytochemistry
The rats were anesthetized with a mixture of medetomidine, midazolam, and butorphanol, i.p., according to a published protocol.23 After tracheostomy, the trachea-esophagus bundle was cut through and retracted rostrally to get bilateral access to the carotid artery bifurcation area. The superior sympathetic cervical ganglions covering the CBs were removed. The CB was carefully sliced off from the artery wall on either side under an operating microscope and immediately immersed in ice-cold Ca2+-deficient saline solution, where 1.8 mM CaCl2 was omitted. The standard saline contained 137 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.5 mM MgCl2, 0.53 mM NaH2PO4, 5 mM d-glucose, and 5 mM Hepes with pH adjusted to 7.4 with 4 mM NaOH. The preparations were incubated in a 0.5% collagenase-containing Ca2+-free solution at 36C for 30 min. Then, one CB was placed in a 35-mm glass-bottom dish (P35GCOL-1.0-14-C; MatTek, Ashland, MA), and cells were dissociated using fine needles under a microscope. Dissociated CB cells, allowed to attach to the glass for 30 min, were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.2) for 1 hr and preincubated in PBS with 5% fetal bovine serum (FBS; cat. no. 172012; Sigma-Aldrich, Tokyo, Japan) and 0.3% Triton-X for 30 min. For indirect immunofluorescence studies, cells were treated overnight with a combination of mouse anti–tyrosine hydroxylase (TH) (cat. no. MAB318; Millipore, Temecula, CA) (RRID: AB_2201528) and goat anti-p11 antibodies (cat. no. AF2377; R&D Systems, Minneapolis, MN) (RRID: AB_2183469), rabbit anti-TASK1 (cat. no. sc-28635; Santa Cruz Biotechnology, Dallas, TX) (RRID: AB_661017) and goat anti-TASK3 antibodies (cat. no. sc-11317; Santa Cruz Biotechnology) (RRID: AB_2131233), or anti-TASK1 and anti-p11 antibodies. The specific immunoreactivities of anti-p11, anti-TASK1, and anti-TASK3 antibodies used have been immunocytochemically confirmed in our previous experiments where the proteins tagged with GFP or myc were exogenously expressed in PC12 cells,6,17 and the Kcnk3 or Kcnk9 gene was deleted in the mouse germ line.7 The selectivity of anti-TH antibody has been well documented.24 After incubation, cells were washed 3× with PBS and treated with a respective secondary antibody conjugated with Alexa 488, 546, or 633 (Molecular Probes; Eugene, OR). Fluorescence was observed under a laser confocal microscope (LSM5 Pascal; Carl Zeiss, Tokyo, Japan). The objective lens was an oil immersion with a magnification of 63× and a numerical aperture of 1.4. For Alexa 488, a 488-nm laser was used and 510 to 560 nm emission was observed (FITC-like fluorescence); for Alexa 546, a 543-nm laser was used and emission above 560 nm was observed (rhodamine-like fluorescence). For GFP, a 514-nm laser was used and 530 to 600 nm emission was observed. The cell periphery was defined as an area of 1 µm width at the cell boundary, which was determined in a differential interference contrast (DIC) image. The immunofluorescence of the whole cell and at the cell periphery was measured with ImageJ software (NIH; Bethesda, MD). The coincidence of immunoreactivities to two different antibodies was assessed with MetaMorph software (Universal Imaging; Downingtown, PA). The fluorescence intensity in the nucleus was set as a threshold for a specific immunoreaction.
Cell Culture and Transfection
PC12 cells (RRID: CVCL_0481) originating from male rat adrenal medullary cells18 and PC12nnr5 cells lacking TrkA (RRID: CVCL_128)25 were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco/Life Technologies, Tokyo, Japan) supplemented with 10% FBS, as previously decribed.17 The Lipofectamine 2000 reagent (Invitrogen/Life Technologies) was used to transfect the cells with expression vectors, according to the manufacturer’s instructions. Briefly, plasmid (1.6 µg) and lipofectamine 2000 (4.0 µl) were added to an individual Ependorf tube that was filled with 50 µl of DMEM, and then left for 5 min at a room temperature before mixing. After 20 min, the mixture was added to 2 ml of DMEM in a 35-mm glass-bottom dish where PC12 cells had been cultured. The transfected cells were further cultured for 24 hr. The cells were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature. After washing 3× with PBS, the cells were incubated in PBS with 0.1% Triton X-100 for 30 min and then with PBS containing 1% FBS for 1 hr at room temperature. The cells were treated with primary and secondary antibodies. Coverslips were mounted in 50% glycerol containing 1 mg/ml 4-diaminobenzene. To differentiate PC12 cells into neuron-like cells, the cells were treated with NGF (50 ng/ml) in the absence of FBS.
Plasmids
The following mammalian expression vectors were used: GFP-tagged TASK1 (GFP-TASK1) and GFP-tagged TASK3 (GFP-TASK3)3 where rat TASK1 and TASK3 cDNAs were each subcloned into pEGFP with the consequent ligation of GFP to the N-terminus of TASK; myc-DDK-tagged p11 (myc-p11) where mouse p11 was into pCMV6-Entry with the consequent ligation of myc-DDK to the C-terminus of p11; hemagglutinin (HA)-tagged TrkA26 where rat TrkA cDNA was into pCMX with the insertion of HA tag at the N-terminus of TrkA; HA-tagged TrkA Y794F and HA-tagged TrkA 499F26,27 where Y at amino acid position 794 or 499 was replaced with F using PCR mutagenesis; and Flag-tagged TrkA Y760F28 where Y at 760 in rat TrkA subcloned into pcDNA3 was replaced with F using PCR mutagenesis.
Proximal Ligation Assay (PLA)
To detect heteromeric TASK1-TASK3 channel formation in CB cells and PC12 cells, an in situ PLA,29 which visualizes subcellular localization and protein–protein interaction, was performed using the Duolink in situ PLA kit (Olink Bioscience, Uppsala, Sweden), according to the manufacturer’s instructions. The fixed cells were treated overnight with a combination of rabbit anti-TASK1 and goat anti-TASK3 antibodies or mouse anti-GFP (cat. no. sc-9996; Santa Cruz Biotechnology) (RRID: AB_627695) and rabbit anti-myc antibodies (cat. no. sc-789: Santa Cruz Biotechnology) (RRID: AB_631274). After treatment with primary antibodies, cells were incubated with secondary antibodies conjugated with oligonucleotides (PLA probe MINUS and PLA probe PLUS). The oligonucleotides of two PLA probes were hybridized by ligase if they were in close proximity (<40 nm). A rolling-circle amplification was carried out with polymerase and fluorescently labeled oligonucleotides, resulting in amplification of the fluorescent signal. The fluorescence emanating from PLA products was observed with the LSM 5 Pascal Microscope System.
Sources of Reagents
The plasmid for myc-DDK-tagged p11 (cat. no. MR226807) was purchased from OriGene Technologies (Rockville, MD), NGF (cat. no. N6009) was from Sigma-Aldrich, mouse anti-HA antibody (cat. no. sc-7392) (RRID: AB_1537399) was from Santa Cruz Biotechnology, and mouse anti-Flag antibody (cat. no. 200-301-383) (RRID: AB_627809) was from Rockland Immunochemicals (Limerick, PA).
Statistics
Data are expressed as mean ± SEM. The significance of differences between experimental and control results was assessed with unpaired Student’s t-test for data with normal distribution (Shapiro–Wilk test). Otherwise, Mann–Whitney rank-sum test or Kruskal–Wallis analysis of variance on ranks was used. A value of p<0.05 defined a statistically significant difference. Statistical analysis was performed with Sigma Plot v13.0 software (Systat Software; San Jose, CA).
Results
Expression of p11 in Tyrosine Hydroxylase–positive Cells
Carotid bodies are composed of excitable glomus or type I cells that detect hypoxia, and glia-like sustentacular or type II cells that surround clusters of type I cells. Type II cells are non-excitable and do not contain voltage-dependent channels.30 TH is a rate-limiting enzyme for catecholamine biosynthesis in both CB glomus and PC12 cells.
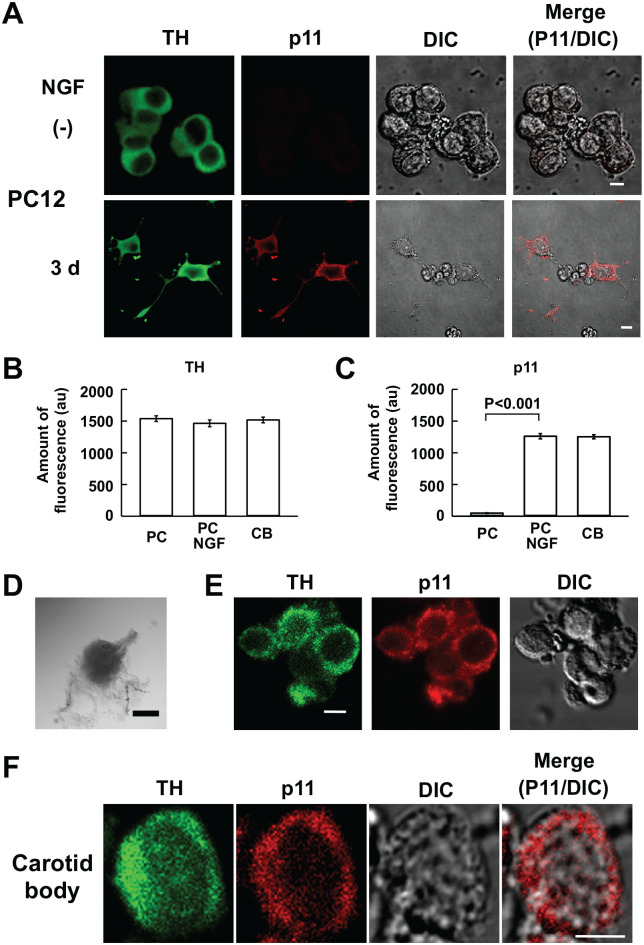
First, immunoreactivities to anti-HT and anti-p11 antibodies were examined in NGF-stimulated and non-stimulated PC12 cells. As is evident in Fig. 1A to C, TH-like IR material was detected in both stimulated and non-stimulated PC12 cells, whereas p11-like IR material predominantly occurred in the stimulated cells, consistent with references.7,21 These results warranted double immunostaining with these antibodies.
Figure 1.
Immunocytochemistry for TH and p11 in rat CB glomus cells and PC12 cells. (A) Confocal images of TH- and p11-like immunofluorescence in PC12 cells stimulated and non-stimulated (−) with NGF for 3 days (3 d). First and second columns represent TH- and p11-like immunofluorescence images, respectively; third, DIC images; fourth, merge of second and third images. Unless otherwise noted, green and red represent FITC- and rhodamine-like fluorescence (this and following figures), respectively. (B and C) Summaries of TH- and p11-like fluorescence present in stimulated (PC NGF) and non-stimulated (PC) PC12 cells and CB glomus cells. The amounts of fluorescence are expressed in arbitrary unit (au). Data are mean ± SEM (PC: n=20 from two culture dishes; PC NGF: n=20 from two culture dishes; CB: n=10 from three CBs); Student’s t-test. (D) Transmitted image of a CB treated with collagenase. The CB was obtained from a rat weighing 150 g. (E) Confocal images of TH- and p11-like immunofluorescence and DIC image in clustered CB cells. (F) Confocal images of TH- and p11-like immunofluorescence in a CB cell. Bars in A, E and F, D indicate 5 µm and 0.5 mm, respectively. Abbreviations: CB, carotid body; DIC, differential interference contrast; NGF, nerve growth factor; PC, pheochromocytoma; TH, tyrosine hydroxylase.
TH expression was immunocytochemically examined in isolated CB glomus cells to verify their identity.31 Mechanical dispersion of collagenase-treated CBs (Fig. 1D) yielded glomus cell clusters (Fig. 1E) and single cells (n=10; Fig. 1F). All of them showed both TH- and p11-like IR material (Fig. 1B and C). Thus, clustered cells comprised only glomus cells with no contribution by type II cells.
Expression of TASK1 and TASK3
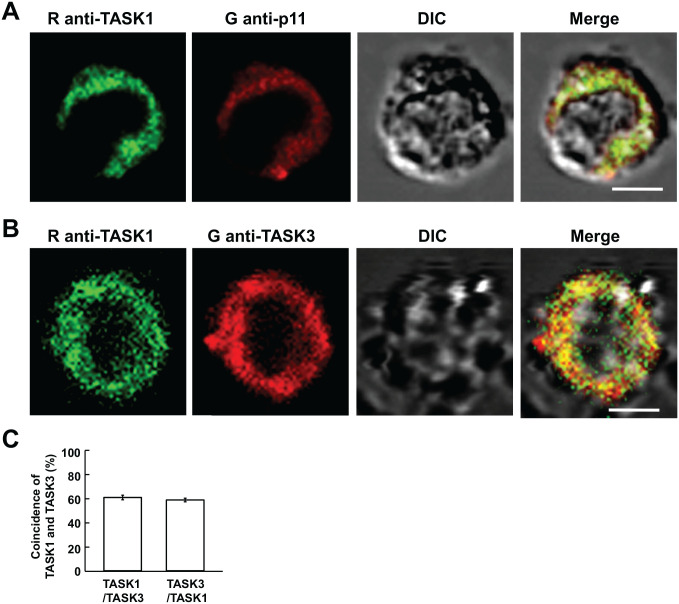
Because p11-immunopositive cells turned out to be glomus cells, isolated cells were subjected to double immunostaining for p11 and TASK1. All the seven p11-immunopositive cells showed TASK1-like IR material as exemplified in Fig. 2A. The expression of TASK1 and TASK3 was simultaneously explored with rabbit anti-TASK1 and goat anti-TASK3 antibodies. Most of TASK1- and TASK3-like IR material was present in the cytoplasm, and both immunoreactivities were mainly colocalized (Fig. 2B). The fraction of TASK1-like IR material colocalized with TASK3-like IR material was 60.4 ± 5.8%, whereas that of TASK3-like IR material with TASK1 was 58.1 ± 5.2% (Fig. 2C).
Figure 2.
Immunocytochemistry for TASK1 and TASK3 in CB cells. (A and B) Confocal images for TASK1- and p11-like immunofluorescence and TASK1- and TASK3-like immunofluorescence in CB glomus cells, respectively. First column represents immunostaining with rabbit anti-TASK1 antibody; second, immunostaining with goat anti-p11 or goat anti-TASK3 antibodies; third, DIC images; fourth, merge of immunofluorescence and DIC images. Yellow indicates coincidence of TASK1-like IR material with p11 (A) or TASK3-like IR material (B). (C) Summary of coincidence (%) of TASK3 with TASK1-like immunofluorescence and vice versa. Data are mean ± SEM (n=10 from three CBs); bars are 5 µm. Abbreviations: CB, carotid body; DIC, differential interference contrast; IR, immunoreactive; TASK, TWIK-related acid-sensitive K+.
Heteromeric Channel Formation
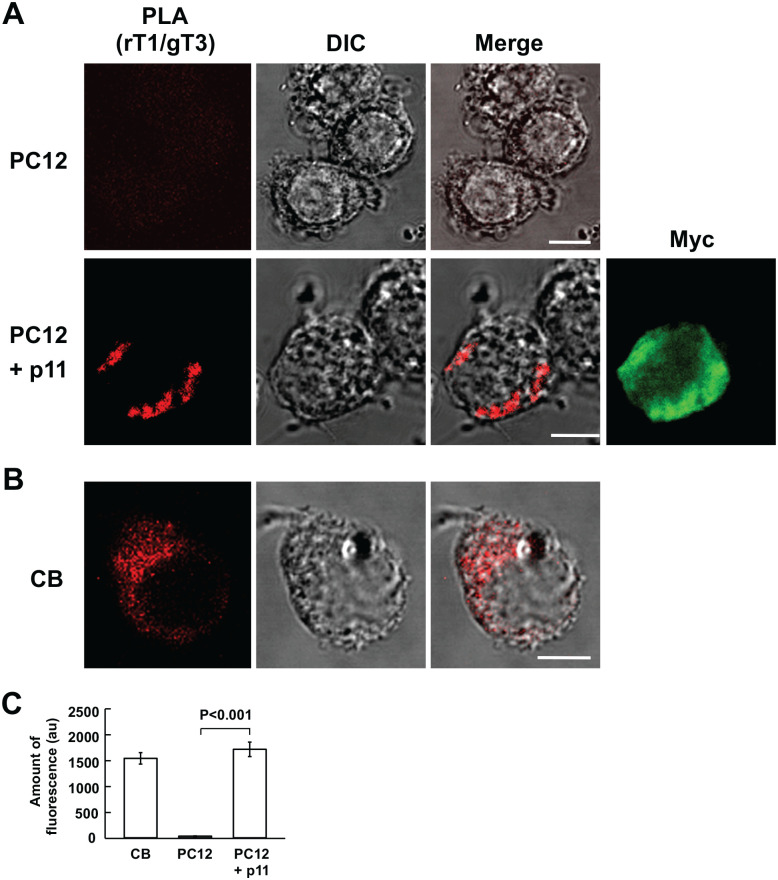
The coincidence of TASK1- with TASK3-like IR material strongly suggests that both form a heteromeric channel. This notion was further explored using PLA with rabbit anti-TASK1 and goat anti-TASK3 antibodies. The PLA products between TASK1 and TASK3 developed only in p11-expressing PC12 cells and were mainly present in the cytoplasm (Fig. 3A), although all the cells endogenously exhibited TASK1- and TASK3-like IR material (Fig. 4E), which is consistent with a previous report.7 Similarly, five CB cells exhibited the PLA reaction in the cytoplasm (Fig. 3B and C), indicating that TASK1 and TASK3 heteromerized there.
Figure 3.
Proximity ligation assay for heteromeric TASK1-TASK3 channel formation in PC12 cells and CB glomus cells. (A and B) Confocal images of PLA products in wild (PC12) and p11-expressing PC12 (PC12 + p11) cells and CB cells, respectively. First column represents fluorescence images of PLA products; second, DIC images; third, merge of fluorescence and DIC images; fourth, myc-like fluorescence image; bars are 5 µm. (C) Summary of fluorescence of PLA products in CB cells, and wild (PC12) and p11-expressing PC12 cells (PC12 + p11); data are mean ± SEM; Student’s t test. (CB: n=6 from two CBs; PC: n=6 from two culture dishes; PC12 + p11: n=6 from two culture dishes). PC12 cells were stimulated with NGF for 1 day (p11-expressing PC12 cells). Bars are 5 µm. Abbreviations: CB, carotid body; DIC, differential interference contrast; PC, pheochromocytoma; PLA, proximity ligation assay; NGF, nerve growth factor; TASK, TWIK-related acid-sensitive K+.
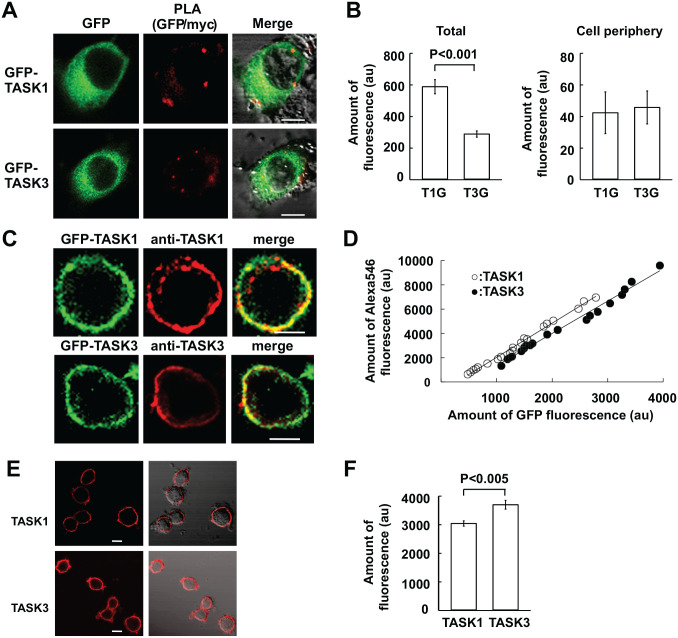
Figure 4.
PLA for myc-p11 binding to GFP-TASK channels. (A) Confocal images of GFP-TASK1 or GFP-TASK3 and PLA reaction between GFP and myc in PC12 cells exogenously expressing myc-p11 and GFP-TASK1 (T1G) or GFP-TASK3 (T3G). (B) Summaries of PLA products in the total area and at the cell periphery of PC12 cells. Data are mean ± SEM; Mann–Whitney rank-sum test (T1G: n=10 from three culture dishes; T3G: n=10 from two culture dishes). Bars are 5 µm. (C) Exogenous expression of GFP-TASK1 or GFP-TASK3 in PC12 cells. First and second columns represent confocal images of GFP and TASK1- or TASK3-like IR material, respectively; third represents merge of first and second images. GFP-TASK and TASK-like IR material were visible as GFP and rhodamine-like fluorescence, respectively. (D) TASK1- and TASK3-like IR material are plotted against those of GFP fluorescence (TASK1: n=20 from five culture dishes; TASK3: n=19 from four culture dishes). Linear regression lines are y = −7911 + 2.811x (correlation coefficient r = 0.996) and y = −13330 + 2.662x (r = 0.994) for TASK1 and TASK3, respectively. (E) Immunocytochemical staining for TASK1 and TASK3 endogenously expressed in PC12 cells. First and second columns represent confocal images and merge of fluorescence and DIC images. (F) TASK1- and TASK3-like IR material in PC12 cells. Data are mean ± SEM (n=20 from two culture dishes for each of TASK1 and TASK3). Bars are 5 µm. Abbreviations: DIC, differential interference contrast; GFP, green fluorescent protein; IR, immunoreactive; PC, pheochromocytoma; PLA, proximity ligation assay; TASK, TWIK-related acid-sensitive K+.
p11 Binding to Heteromeric TASK1-TASK3 Channels
The results outlined above indicate that the expression of p11 results in the formation of heteromeric TASK1-TASK3 channels. Because the binding site for p11, called i20, is in the C-terminus of TASK1, but not TASK3,20 the heteromeric TASK1-TASK3 channels are expected to complex with p11. This possibility was examined with PLA in PC12 cells where myc-p11 and either GFP-TASK1 or GFP-TASK3 were exogenously expressed. In this constellation, GFP-TASK and PLA products between GFP and myc were colocalized in the cytoplasm and at the cell periphery (Fig. 4A), irrespective of whether the TASK subtype was TASK1 or TASK3. This result suggests that GFP-TASK3 forms a heteromeric channel with endogenous TASK1 and p11 suppresses its ER exit by binding to the TASK1 subunit of the heteromeric channel.
Detailed inspection of the PLA reaction reveals that the amount of PLA products between GFP-TASK1 and myc-p11 was significantly more than that of PLA products between GFP-TASK3 and myc-p11 (Fig. 4B). This difference might be ascribed to a difference in the number of TASK dimer channels capable of binding to p11. Exogenous GFP-TASK1 will make a heteromeric channel with endogenous TASK3 and a homomeric channel with endogenous TASK1 or exogenous GFP-TASK1. On the contrary, exogenous GFP-TASK3 will make a heteromeric channel with endogenous TASK1 and a homomeric channel with endogenous TASK3 or exogenous GFP-TASK3 that is incapable of binding to p11. If endogenous TASK1 and TASK3 and exogenous GFP-TASK1 and GFP-TASK3 are expressed to a similar level, PLA products between GFP and myc in GFP-TASK1-expressing cells reflect the number of both heteromeric and homomeric channels, whereas those in GFP-TASK3-expressing cells reflect the number of heteromeric channels alone. Thus, it would be a reasonable assumption that the amount of PLA products in GFP-TASK1-expressing cells was larger than that in GFP-TASK3-expressing cells.
To verify the validity of our reasoning, the amounts of TASK1 and TASK3 endogenously expressed in PC12 cells were immunocytochemically measured. To this end, the immunoactivity of anti-TASK1 antibody was compared with that of anti-TASK3 antibody in PC12 cells exogenously expressing GFP-TASK1 or GFP-TASK3 (Fig. 4C). The amount of TASK1- or TASK3-like immunofluorescence was plotted against that of GFP fluorescence. As shown in Fig. 4D, the slope for TASK1-like immunofluorescence was akin to that of TASK3. This indicates that both antibodies had similar immunoreactivity. When endogenous TASK1 and TASK3 were stained with these antibodies (Fig. 4E), the amount of TASK1-like IR material was smaller than that of TASK3 (Fig. 4F), indicating that a larger amount of PLA products in GFP-TASK1-producing cells cannot be explained by a larger amount of endogenous TASK1. Thus, the difference in the PLA reaction between GFP-TASK1- and GFP-TASK3-producing cells may be accounted for by the notion that PLA products represent both heteromeric TASK1-TASK3 channels and homomeric TASK1 channels in the former.
Signal Transduction for p11 Production
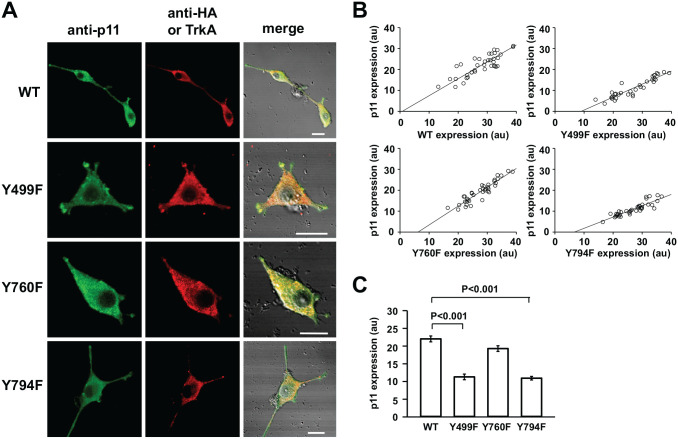
PC12nnr5 cells, where p75NTR, but not TrkA, was expressed,25 were used to explore the signaling mechanism for NGF-induced p11 production. The treatment with NGF of PC12nnr5 cells did not result in p11 production (not shown), indicating that TrkA is essential for p11 production. There are mainly three signaling pathways downstream of TrkA: mitogen-activated protein (MAP) kinase, phosphoinositide 3 (PI3)-kinase, and phospholipase C (PLC).32 Thus, TrkA mutants were used to elucidate which signaling pathways were involved in p11 production. In PC12nnr5 cells expressing either wild-type or mutant TrkA (Fig. 5A), the amount of p11-like IR fluorescence depended on the extent of TrkA expression. Thus, the amount of p11-like IR fluorescence was plotted against that of HA or Flag-like IR. The extent of p11 expression increased with that of exogenous TrkA, irrespective of its type (Fig. 5B). However, detailed inspection of the plots reveals that p11 expression differed among the TrkA mutants, which were expressed to similar extents. In TrkA Y499F-expressing cells where the MAP kinase pathway is mainly disrupted,27 NGF stimulation induced short growth-cone-like protrusions, but not long neurites, and the amount of p11 elicited by NGF was significantly less than that in wild-type TrkA-expressing cells (Fig. 5C). A similar decrease in p11 production occurred in PC12nnr5 cells expressing Y794F-TrkA lacking in PLC signaling28,33 (Fig. 5C). In this TrkA mutant–expressing cells, however, a few long neurites were extended in response to NGF (Fig. 5A). On the contrary, cells expressing Y760F-TrkA, where the PI3-kinase pathway is mainly disrupted,28,34 produced p11 to the same extent as the control cells, but did not extend neurites in response to NGF (Fig. 5A and C). Thus, this mutation in TrkA impairs neurite extension and has no effect on p11 production in response to NGF.
Figure 5.
Immunocytochemical analysis of signaling pathways involved in NGF-induced p11 production. (A) Confocal images of p11 and HA- or TrkA-like immunofluorescence in PC12nnr5 cells expressing wild-type (WT), Y499F-TrkA, Y760F-TrkA, or Y794F-TrkA. First and second columns represent p11- and HA- or Flag-like immunofluorescence images, respectively; third, merge of DIC and fluorescence images. HA-like fluorescence was observed in cells expressing WT, Y760F-TrkA, and Y794F-TrkA, whereas TrkA-like fluorescence was observed in cells expressing Y760F-TrkA. (B) p11-like fluorescence is plotted against those of HA- (WT, Y499F, and Y794F) or TrkA-like (Y760F) fluorescence observed in the same cells. Linear regression lines are as follows—WT: y = −0.634 + 0.810x, correlation coefficient r = 0.823; Y499F: y = −5.595 + 0.621x, r = 0.924; Y760F: y = −5.594 + 0.901x, r = 0.929; Y794F: y = −3.623 + 0.541x, r = 0.888. (C) Summary of p11-like IR material in PC12nnr5 cells expressing WT, Y499F-TrkA, Y760F-TrkA, or Y794F-TrkA. P11-like IR material was measured over the whole cell area including protrusions or neurites. Data are mean ± SEM; Kruskal–Wallis one-way analysis of variance on ranks (WT: n=38 from three culture dishes; Y499F: n=33 from two; Y760F: n=38 from three; Y794F: n=38 from 3). Bars are 10 µm. Abbreviations: DIC, differential interference contrast; HA, hemagglutinin; IR, immunoreactive; NGF, nerve growth factor; PC, pheochromocytoma.
Discussion
Heteromeric TASK Channels in CB Glomus and PC12 Cells
The main findings of this study were that (1) p11, TASK1, and TASK3 were expressed in rat CB glomus cells; (2) TASK1- and TASK3-like IR material mainly coincided in the cytoplasm; and (3) PLA products between TASK1 and TASK3 were predominantly located in the cytoplasm. These results suggest that TASK1 and TASK3 are present as a heteromeric channel mainly in the cytoplasm of glomus cells.
We have previously proposed that the cytoplasmic localization of heteromeric channels is due to retention of channels in the ER through binding to p117 which has a lysine-based retention/retrieval motif in the C-terminus.20 This notion is supported by the following evidence: (1) both TASK1 and TASK3, endogenously or exogenously expressed, are in the plasma membrane and do not heteromerize in PC12 cells in which p11 is not endogenously expressed7,21; (2) exogenous expression of p11 in PC12 cells results in the formation of heteromeric TASK1-TASK3 channels, which are mainly located in the cytoplasm (present results)7; and (3) in CB glomus cells endogenously expressing p11, TASK1 and TASK3 proteins are mainly present in the cytoplasm (present results). The presence of TASK3 in the cytoplasm may be explained by the notion that endogenously expressed p11 suppresses the ER exit of TASK1 through binding to its terminus and subsequently facilitates the heteromeric channel formation of TASK1 and TASK3. As a result, heteromeric TASK channels are retained in the cytoplasm. Consistent with this notion, in PC12 cells expressing GFP-TASK3 and myc-p11, GFP-TASK3 proteins are predominantly located in the cytoplasm and PLA reaction develops between GFP-TASK3 and myc-p11 probably through endogenous TASK1.
Signal Transduction for p11 Production
The content of brain-derived neurotrophic factor (BDNF), which is known to produce p11 via TrkB,35 decreases in humans with depression, and treatment with antidepressants results in BDNF restoration in the cerebrospinal fluid.36 In in vitro experiments, p11 enhances plasma membrane expression of the serotonin receptors 5HT1BR37 and 5HT4R.38 The biological plausibility that p11 plays an important role in the pathophysiology of depression arises. Thus, elucidation of signaling mechanisms for p11 production could enlighten the pathophysiology of depression and the development of new remedies.
Aside from brain neurons,29 p11 is expressed in various kinds of cells, and its production is facilitated by growth factors and biologically active substances, such as NGF7,21 and NO.39 p11, which functions as a homodimer or heteromer with annexin II,40 is involved in trafficking of membrane proteins,20 such as receptors37 and ion channels.41 Therefore, a change in p11 expression is assumed to induce a change in plasma membrane expression of receptors and ion channels, shaping cellular functions. Brain-derived neurotrophic factor is involved in neurite extension, synapse formation, plasticity in synaptic transmission, and cellular excitability.32 Several of these BDNF actions are mediated by p11.40 A pharmacological study shows that BDNF induces p11 formation in cultured neurons via the MAP kinase pathway.35 This study also shows that Y499A mutation in TrkA, which mainly disrupts MAP kinase signaling,27 resulted in a significant decrease in p11 expression in response to NGF. However, a similar extent of p11 diminution occurred in cells expressing the Y794A mutant lacking the PLC signaling.42,43 The result with the Y794F mutant might be due to a deficit in MAP kinase signaling. Protein kinase C has been suggested to activate MAP kinase signaling via Ras.33 This possibility, however, is not feasible because Y794A mutation did not affect neurite extension in response to NGF. The MAP kinase pathway is known to be primarily involved in neurite extension. Thus, the fact that Y794F mutation did not affect neurite extension indicates that MAP kinase signaling is not disturbed in cells expressing Y794F-TrkA. The present findings that p11 production was diminished in TrkA mutated at Y499 or Y794, but not Y760, suggest that both MAP kinase and PLC pathways were involved in NGF-induced p11 production. It should be noted that p11 is expressed in glomus cells, but not adrenal medullary cells, which are developmentally closely related to glomus cells.44 The molecular mechanisms elucidated for NGF-induced p11 expression in PC12 cells would pave the way to explore how p11 is expressed in glomus cells but not in adrenal medullary cells.
In conclusion, we elucidated the expression of p11 and heteromeric TASK1-TASK3 channels in rat CB glomus cells. In mouse hypoglossal motoneurons3 and cerebellar granule cells,2 where heteromeric TASK1-TASK3 channels are putatively formed, p11 expression is confirmed at the mRNA level.45 Those and the present findings suggest that p11 plays a major role in the generation of heteromeric TASK1-TASK3 channels. A further study, such as downregulation of p11 expression using a small interfering RNA approach, would be needed to elucidate whether p11 is essential for heteromeric channel formation in glomus cells or not.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HM, MP, RY, and MI contributed to the conception and design of the work; MP and MI made the preparation; HM acquired the data; HM and KH analyzed the data; and MI and MP wrote the manuscript. All authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was in part supported by grants JSPS KAKENHI (17K08555 to M. I. and 18K06865 to H. M.).
Contributor Information
Hidetada Matsuoka, Department of Cell and Systems Physiology, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
Mieczyslaw Pokorski, Department of Cell and Systems Physiology, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan; Institute of Sciences, University of Opole, Opole, Poland.
Keita Harada, Department of Cell and Systems Physiology, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
Reiji Yoshimura, Department of Psychiatry, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
Masumi Inoue, Department of Cell and Systems Physiology, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
Literature Cited
- 1. Yamamoto Y, Kummer W, Atoji Y, Suzuki Y. TASK-1, TASK-2, TASK-3 and TRAAK immunoreactivities in the rat carotid body. Brain Res. 2002;950:304–7. [DOI] [PubMed] [Google Scholar]
- 2. Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Czirjak G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002;16:621–9. [DOI] [PubMed] [Google Scholar]
- 5. Penton D, Bandulik S, Schweda F, Haubs S, Tauber P, Reichold M, Cong LD, El Wakil A, Budde T, Lesage F, Lalli E, Zennaro MC, Warth R, Barhanin J. Task3 potassium channel gene invalidation causes low renin and salt-sensitive arterial hypertension. Endocrinology. 2012;153:4740–8. [DOI] [PubMed] [Google Scholar]
- 6. Inoue M, Harada K, Matsuoka H, Sata T, Warashina A. Inhibition of TASK1-like channels by muscarinic receptor stimulation in rat adrenal medullary cells. J Neurochem. 2008;106:1804–14. doi: 10.1111/j.1471-4159. [DOI] [PubMed] [Google Scholar]
- 7. Inoue M, Matsuoka H, Lesage F, Harada K. Lack of p11 expression facilitates acidity-sensing function of TASK1 channels in mouse adrenal medullary cells. FASEB J. 2019;33:455–68. [DOI] [PubMed] [Google Scholar]
- 8. Matsuoka H, Inoue M. Molecular mechanism for muscarinic M1 receptor-mediated endocytosis of TWIK-related acid-sensitive K+ 1 channels in rat adrenal medullary cells. J Physiol. 2017;595:6851–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. [DOI] [PubMed] [Google Scholar]
- 10. Lesage F, Barhanin J. Molecular physiology of pH-sensitive background K(2P) channels. Physiology. 2011;26:424–37. [DOI] [PubMed] [Google Scholar]
- 11. Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner PJ, Buckler KJ. Oxygen and mitochondrial inhibitors modulate both monomeric and heteromeric TASK-1 and TASK-3 channels in mouse carotid body type-1 cells. J Physiol. 2013;591:5977–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim D, Gnatenco C. TASK-5, a new member of the tandem-pore K(+) channel family. Biochem Biophys Res Commun. 2001;284:923–30. [DOI] [PubMed] [Google Scholar]
- 14. Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inoue M, Harada K, Matsuoka H, Nakamura J, Warashina A. Mechanisms and roles of muscarinic activation in guinea-pig adrenal medullary cells. Am J Physiol Cell Physiol. 2012;303:C635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002;277:5426–32. [DOI] [PubMed] [Google Scholar]
- 17. Matsuoka H, Harada K, Nakamura J, Inoue M. Nerve growth factor-induced endocytosis of TWIK-related acid-sensitive K+ 1 channels in adrenal medullary cells and PC12 cells. Pflugers Arch. 2013;465:1051–64. [DOI] [PubMed] [Google Scholar]
- 18. Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pflugers Arch. 2008;455:575–82. [DOI] [PubMed] [Google Scholar]
- 20. Renigunta V, Yuan H, Zuzarte M, Rinne S, Koch A, Wischmeyer E, Schlichthorl G, Gao Y, Karschin A, Jacob R, Schwappach B, Daut J, Preisig-Muller R. The retention factor p11 confers an endoplasmic reticulum-localization signal to the potassium channel TASK-1. Traffic. 2006;7:168–81. [DOI] [PubMed] [Google Scholar]
- 21. Masiakowski P, Shooter EM. Nerve growth factor induces the genes for two proteins related to a family of calcium-binding proteins in PC12 cells. Proc Natl Acad Sci U S A. 1988;85:1277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–98. [DOI] [PubMed] [Google Scholar]
- 23. Kawai S, Takagi Y, Kaneko S, Kurosawa T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim. 2011;60:481–7. [DOI] [PubMed] [Google Scholar]
- 24. Gamble JA, Karunadasa DK, Pape J-R, Skynner MJ, Todman MG, Bicknell RJ, Allen JP, Herbison AE. Disruption of ephrin signaling associates with disordered axophilic migration of the gonadotropin-releasing hormone neurons. J Neurosci. 2005;25:3142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Green SH, Rydel RE, Connolly JL, Greene LA. PC12 cell mutants that possess low- but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J Cell Biol. 1986;102:830–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meakin SO, MacDonald JI. A novel juxtamembrane deletion in rat TrkA blocks differentiative but not mitogenic cell signaling in response to nerve growth factor. J Neurochem. 1998;71:1875–88. [DOI] [PubMed] [Google Scholar]
- 27. Meakin SO, MacDonald JI, Gryz EA, Kubu CJ, Verdi JM. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem. 1999;274:9861–70. [DOI] [PubMed] [Google Scholar]
- 28. Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Söderberg O, Gullberg M, Jarvius M, Ridderstrãle Leuchowius K-J, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson L-G, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. [DOI] [PubMed] [Google Scholar]
- 30. Lopez-Barneo J, Ortega-Saenz P, Pardal R, Pascual A, Piruat JI. Carotid body oxygen sensing. Eur Respir J. 2008;32:1386–98. [DOI] [PubMed] [Google Scholar]
- 31. Kato K, Fushuku S, Yamamoto Y. Age-related changes in immunoreactivity for dopamine β-hydroxylase in carotid body glomus cells in spontaneously hypertensive rats. Auton Neurosci. 2017;205:50–6. [DOI] [PubMed] [Google Scholar]
- 32. Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–42. [DOI] [PubMed] [Google Scholar]
- 33. Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. [DOI] [PubMed] [Google Scholar]
- 34. Obermeier A, Lammers R, Wiesmuller KH, Jung G, Schlessinger J, Ullrich A. Identification of Trk binding sites for SHC and phosphatidylinositol 3′-kinase and formation of a multimeric signaling complex. J Biol Chem. 1993;268:22963–6. [PubMed] [Google Scholar]
- 35. Warner-Schmidt JL, Chen EY, Zhang X, Marshall JJ, Morozov A, Svenningsson P, Greengard P. A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol Psychiatry. 2010;68:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312. [DOI] [PubMed] [Google Scholar]
- 37. Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. [DOI] [PubMed] [Google Scholar]
- 38. Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, Greengard P. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pawliczak R, Cowan MJ, Huang X, Nanavaty UB, Alsaaty S, Logun C, Shelhamer JH. p11 expression in human bronchial epithelial cells is increased by nitric oxide in a cGMP-dependent pathway involving protein kinase G activation. J Biol Chem. 2001;276:44613–21. [DOI] [PubMed] [Google Scholar]
- 40. Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. P11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci. 2013;14:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV, Wood JN. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature. 2002;417:653–6. [DOI] [PubMed] [Google Scholar]
- 42. Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–52. [DOI] [PubMed] [Google Scholar]
- 43. Zhang K, Duan L, Ong Q, Lin Z, Varman PM, Sung K, Cui B. Light-mediated kinetic control reveals the temporal effect of the Raf/MEK/ERK pathway in PC12 cell neurite outgrowth. PLoS ONE. 2014;9:e92917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Donoghue PCJ, Graham A, Kelsh RN. The origin and evolution of the neural crest. Bioessays. 2008;30:530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Milosevic A, Liebmann T, Knudsen M, Schintu N, Svenningsson P, Greengard P. Cell- and region-specific expression of depression-related protein p11 (S100a10) in the brain. J Comp Neurol. 2017;525:955–75. [DOI] [PMC free article] [PubMed] [Google Scholar]