Patients with visual snow syndrome suffer from subjective visual and non-visual symptoms, such as tinnitus and concentration problems. Schankin et al. reveal structural and functional changes in extrastriate visual and extra-visual cortex in those affected, emphasizing that the disorder extends beyond the visual system
Keywords: visual snow, non-visual symptoms, migraine, FDG PET, voxel-based morphometry
Abstract
Patients with visual snow syndrome suffer from a continuous pan-field visual disturbance, additional visual symptoms, tinnitus, and non-perceptional symptoms. The pathophysiology of visual symptoms might involve dysfunctional visual cortex. So far, the extra-visual system has not been investigated. We aimed at identifying structural and functional correlates for visual and non-visual symptoms in visual snow syndrome. Patients were compared to age- and sex-matched controls using 18F-2-fluoro-2-deoxy-d-glucose PET (n = 20 per group) and voxel-based morphometry (n = 17 per group). Guided by the PET results, region of interest analysis was done in voxel-based morphometry to identify structural-functional correspondence. Grey matter volume was assessed globally. Patients had corresponding hypermetabolism and cortical volume increase in the extrastriate visual cortex at the junction of the right lingual and fusiform gyrus. There was hypometabolism in the right superior temporal gyrus and the left inferior parietal lobule. Patients had grey matter volume increases in the temporal and limbic lobes and decrease in the superior temporal gyrus. The corresponding structural and functional alterations emphasize the relevance of the visual association cortex for visual snow syndrome. The broad structural and functional footprint, however, confirms the clinical impression that the disorder extends beyond the visual system.
Introduction
Patients with visual snow syndrome (VSS) suffer from a continuous visual disturbance consisting of visual snow and additional visual symptoms: palinopsia, enhanced entoptic phenomena, such as floaters and blue field entoptic phenomenon, photophobia and impaired night vision (Schankin et al., 2014a). The pathophysiology of the condition is poorly understood. The high co-morbidity with migraine (Schankin et al., 2014b), a disorder of sensory processing (Goadsby et al., 2017), suggests some pathophysiological overlap. This is supported by recent imaging and electrophysiological data suggesting a dysfunction of visual processing at the level of the visual association cortex (Schankin et al., 2014b; Eren et al., 2018; Luna et al., 2018). Treatment remains frustrating. Further, many patients with VSS also suffer from non-visual complaints, such as tinnitus, concentration problems, irritability, and lethargy (Schankin et al., 2014a). This suggests alterations in several extra-visual cortical areas, such as the auditory or the limbic system.
The objective of this study was to improve our understanding of VSS by exploring brain areas that differ in structure, or function, or both, from controls. Specifically, we sought to determine areas with corresponding structural and functional alterations. In addition, we assessed brain metabolism and structure in an exploratory, voxel-wise manner.
These data have been presented in preliminary form [17th Congress of the International Headache Society, Valencia 14–17 May 2015 (Schankin et al., 2015)]
Materials and methods
This prospective case-control study was carried out at a tertiary headache centre after approval by the local ethics committee (11-07431) and the radiation safety committee (58605-RU-04-URH). All subjects gave written informed consent. Patients were recruited via advertisements in social media with the support of a self-help group on visual snow (Eye on Vision Foundation; http://eyeonvision.org). Data on a subgroup of patients presented here (n = 17) have been published in a previous report investigating solely hypermetabolism (Schankin et al., 2014b).
Participants
Eligibility was assessed during telephone interviews. Inclusion criteria were black and white visual snow, i.e. dynamic, continuous, tiny dots in the entire visual field lasting longer than 3 months, with at least three additional visual symptoms (Schankin et al., 2014a). Controls were matched for age and sex. Exclusion criteria for controls were: presence of visual snow or any associated visual symptoms, a history of migraine attacks more often than once every 2 months, or of migraine aura (Headache Classification Committee of the International Headache Society, 2018). Exclusion criteria for both groups were ophthalmological pathologies other than refraction anomalies, any lifetime history of intake of hallucinogenic drugs or recent (<6 months) history of intake of recreational drugs, age older than 50 years, contraindications for PET or MRI, and pregnancy. Each subject underwent a semi-structured interview focusing on visual symptoms, migraine history including typical aura and general past medical history. Standard questionnaires were used to assess potential depression (PHQ-8) and anxiety (GAD-7) with clinically relevant depression or anxiety being defined by a score of >9 (Lowe et al., 2008; Kroenke et al., 2009).
Each patient had visual evoked potentials (standard pattern reversal stimuli, monocular response, both eyes) and 18-channel EEG (standard international 10-20 electrode placement).
Imaging
On the scanning day, each subject had a fasting period of at least 6 h. High-resolution T1-weighted anatomical MRI [axial T1, 3D IRSPGR (inversion recovery spoiled gradient echo), repetition time 7.252, echo time 1.6, inversion time 400, 180 images acquired, slice thickness 1 mm] was performed on a General Electric Signa HDxT 3.0 T scanner (GE Healthcare). In three patients with visual snow, a slice thickness of 1.5 mm was applied instead because of a technical problem on the scanning day. Afterwards, the subjects were injected with 370 MBq of 18F-2-fluoro-2-deoxy-d-glucose (FDG) as an intravenous bolus and were instructed to rest in a darkened room for 45 min with eyes closed. PET scans were acquired using a GE Discovery VCT PET/CT scanner (GE Healthcare) in 3D mode with septa retracted. A low-mA CT scan prior to the PET was used for attenuation correction. Scatter correction was also applied based on the CT-based attenuation map. During scanning, all subjects had their eyes closed and were instructed not to fall asleep. The scan duration was 15 min. One static frame was acquired. We visually observed the patient head motion during the scan. In our data, no significant blurring in reconstructed images was noted that might be caused by significant head motion. Motion correction was not applied. PET images were reconstructed using the manufacturer-provided 3D iterative reconstruction into 47 image planes (separation 3.27 mm) and into a 128 × 128 image matrix (pixel size: 2.1 × 2.1 mm2). These images were used as input for the Statistical Parametric Mapping (SPM) analysis. The image pixel values in activity concentration (Bq/ml) were used, instead of converting them to standardized uptake values (SUVs).
Analysis
The structural MRI data were first co-registered to the PET data using SPM8 (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm) followed by automated segmentation as well as stereotactic normalization to MNI (Montreal Neurological Institute) space.
The parameters obtained from this step were applied to normalize stereotactically the PET images. The final resliced voxel size of the PET images was 2 × 2 × 2 mm3. The PET images were smoothed with a Gaussian kernel (full-width at half-maximum of 12 mm). The VSS patient group was compared to controls using a two-sample t-test with masking of non-brain tissue (whole brain explicit mask generated with WFU PickAtlas, Advanced Neuroscience Imaging Research Laboratory, Department of Radiology of Wake Forest University School of Medicine, http://fmri.wfubmc.edu/) using proportional scaling. Statistical significance was assumed for areas with P < 0.001, uncorrected for multiple comparisons.
For voxel-based morphometry (VBM), only patients who had MRI with slice thickness of 1 mm were analysed. The VBM toolbox for SPM8 (VBM8, version 435, http://dbm.neuro.uni-jena.de/vbm/) was used to normalize semi-automatically (modulated normalization) and to segment the MRI data into grey matter, white matter and CSF using standard parameters. Grey matter images were smoothed with a kernel with 8mm full-width at half-maximum. The smoothed grey matter images were compared between VSS patients and controls using standard parameters.
For our primary research question, we assessed structural (VBM)-functional (PET) correspondence of abnormalities in VSS patients. The statistical peak coordinates from the PET clusters were used to guide region of interest analysis in VBM. For this purpose, spherical regions of interest with a radius of 10 mm (Carreiras et al., 2009) were generated around the PET peak coordinate clusters as explicit mask using the MarsBaR region of interest toolbox for SPM (Brett et al., 2002). The VSS patient group was compared to controls using a two-sample t-test with correction for multiple comparisons [familywise error (FWE) rate of P < 0.05 on cluster level).
For the secondary research question, an exploratory whole brain analysis was performed (two-sample t-test, P < 0.001, uncorrected for multiple comparisons).
SPSS (v20, IBM Corp., Armonk, New York, USA) was used for analysis of non-imaging data. Standard descriptive statistics were applied. Where appropriate, data are presented as mean ± standard deviation (SD). Nominal data were compared using chi-square test, continuous data using t-test. Significance was defined by P < 0.05.
Data availability
Anonymized data will be shared by reasonable request from any qualified investigator.
Results
Participant demographics
Of 20 patients with black and white visual snow and at least three additional visual symptoms (11 female, 18 right-handed, mean age ± SD = 31 ± 7 years), nine had had visual snow for as long as they could remember. Mean age of onset in the remaining was 24 ± 8 years. Sixteen (80%) had a history of migraine. Five of those had migraine with typical aura (Headache Classification Committee of the International Headache Society, 2018), and one had migraine aura without headache (Table 1). Sixteen (80%) patients suffered from concentration problems, 11 (55%) from irritability, 10 (50%) from tinnitus, and eight (40%) from lethargy. Past medical history was otherwise unremarkable. According to the questionnaires, one patient had depression and anxiety, one anxiety only, and one depression only. Reports of an ophthalmological exam were available in all patients (all had normal fundoscopy, automated visual fields abnormal in one with generalized reduced sensitivity, best corrected visual acuity normal in all except for 20/25 bilateral in one and 20/20-2 left in another). Visual evoked potentials (P100 latency and N75-P100 amplitude) and EEG were normal in all patients.
Table 1.
Demographics, visual symptoms and migraine history in the study population with visual snow syndrome
| Patient | Age | Age at onset | Sex | Visual symptoms | Migraine | Aura | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | Palinopsia | Entoptic phenomena | Nyctalopia | Photo- phobia | ||||||||||
| Trailing | After images | Floaters | Photopsia | BFEP | Self-light | |||||||||
| VS1 | 35 | 32 | M | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| VS2 | 39 | a | F | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| VS3 | 24 | a | F | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| VS4 | 37 | 20 | F | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| VS5 | 29 | 24 | F | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| VS6 | 23 | 17 | F | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| VS7 | 40 | 39 | F | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| VS8 | 29 | 28 | M | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| VS9 | 36 | 34 | F | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| VS10 | 32 | 24 | M | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 |
| VS11 | 35 | a | F | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| VS12 | 24 | a | F | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| VS13 | 25 | a | M | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| VS14 | 35 | a | M | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| VS15 | 47 | a | F | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| VS16 | 22 | 13 | M | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| VS17 | 21 | 20 | M | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| VS18 | 27 | a | F | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| VS19 | 21 | a | M | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| VS20 | 33 | 16 | M | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
Patients recall having visual snow as for long as they can remember.
0 = absent; 1 = present; Aura = history of typical migraine aura; BW = black and white visual snow; BFEP = blue field entoptic phenomenon; migraine = history of migraine with or without aura (depending on the presence of aura); nyctalopia = impaired night vision; photopsia = random flashes of light, mainly in the periphery and without mechanical stimulation of the retina; self-light of the eye = patients perceive coloured clouds or waves when briefly closing their eyes.
The 20 controls had the same age and sex distribution [11 female, 18 right-handed, 30 ± 7 years, t(38) = 0.25, P = 0.81 for age], but differed significantly from patients in respect of history of aura [exclusion criterion, χ2(1) = 7.06, P = 0.02] and history of migraine [no control subject had migraine, χ2(1) = 26.67, P < 0.001]. One control subject had anxiety, another one had depression [χ2(1) = 0.36, P = 1.00].
Imaging
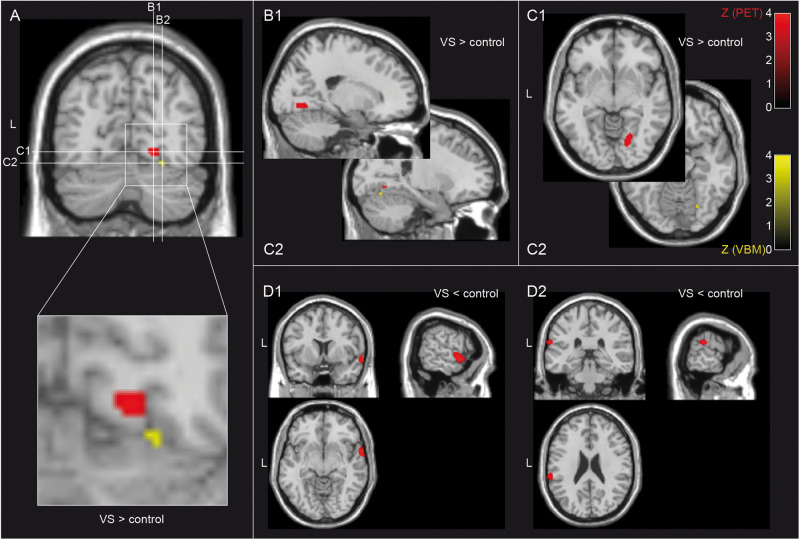
In patients with VSS, FDG PET demonstrated hypermetabolism in the right lingual gyrus in Brodmann area (BA) 19. For VBM, three patients had to be excluded due to slice thickness of 1.5 mm in MRI. Guided by the metabolic data, VBM identified increased grey matter volume in the adjacent lingual gyrus-fusiform gyrus junction (maximum of VBM at MNI: x = 26, y = −69, z = −14, Z = 3.46, P = 0.03 FWE-corrected on cluster-level, kE = 12). In FDG PET, there was hypometabolism in the right superior temporal gyrus (BA 22) and the left inferior parietal lobule (BA 40), without corresponding changes in grey matter volume (Fig. 1 and Table 2).
Figure 1.
Differences in brain metabolism between patients with VSS and controls. When compared to controls, patients with VSS demonstrated hypermetabolism in FDG PET (red) and a corresponding increase of grey matter volume as determined by VBM (yellow) at the junction of the lingual and fusiform gyrus on the right, both in Brodmann area (BA) 19 (A–C). VSS patients showed additional areas of hypometabolism in the right superior temporal gyrus (BA 22, D1) and left inferior parietal lobule (BA 40, D2) without corresponding alterations in grey matter volume. PET statistical parametric maps at a threshold of P < 0.001 uncorrected for multiple comparisons are overlaid on a normalized standard T1-weighted MRI. The VBM map was obtained using a 10-mm sphere as an explicit mask around the maximum from significant PET clusters and thus represents only a part of the grey matter alterations shown in Fig. 2 (threshold P < 0.05, family wise error rate on cluster level). VS = visual snow.
Table 2.
Coordinates of metabolic and structural differences between patients with visual snow syndrome and controls
| MNI | kE | Z-score | P-value | Side | Lobe | Gyrus | BA | |||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| PET | ||||||||||
| VS > C | 20 | −68 | −7 | 49 | 3.4 | 0 | Right | Occipital | Lingual | 19 |
| VS < C | 66 | 4 | −9 | 309 | 3.68 | 0 | Right | Temporal | Superior temporal | 22 |
| −64 | −32 | 21 | 58 | 3.57 | 0 | Left | Parietal | Inferior parietal lobule | 40 | |
| VBM | ||||||||||
| VS > C | 26 | −69 | −15 | 29 | 3.52 | 0 | Right | Temporal | Fusiform | 19 |
| 54 | −27 | −15 | 159 | 4.11 | 0 | Right | Temporal | Middle temporal | 21 | |
| 20 | −24 | −23 | 21 | 3.28 | 0.001 | Right | Limbic | Parahippocampal | 35 | |
| −50 | −15 | −14 | 71 | 3.41 | 0 | Left | Temporal | Superior temporal | 22 | |
| 15 | 35 | 7 | 57 | 3.28 | 0.001 | Right | Limbic | Anterior cingulate | 32 | |
| VS < C | −54 | 5 | 1 | 30 | 3.32 | 0 | Left | Temporal | Superior temporal | 22 |
Metabolic (FDG PET, n = 20 per group) and structural (VBM, n = 17 per group) changes in patients with visual snow syndrome in comparison to age and sex matched controls. Guided by the metabolic data, small volume correction using a sphere with 10-mm radius around the peak of metabolic activity (VS > C in PET, MNI 20, −68, −7) identified corresponding increased grey matter volume (VS > C in VBM, FWE corrected P = 0.03 on cluster level, peak at MNI 26, −69, −14, data not shown in table).
BA = Brodmann area; kE = number of contiguous voxels with Z-score > 3.3 (P < 0.001); MNI = standard space as defined by the Montreal Neurological Institute; VS < C = visual snow group is metabolically less active (FDG PET) or has less grey matter volume (VBM) than control group; VS > C = visual snow group is metabolically more active (FDG PET) or has larger grey matter volume (VBM) than control group.
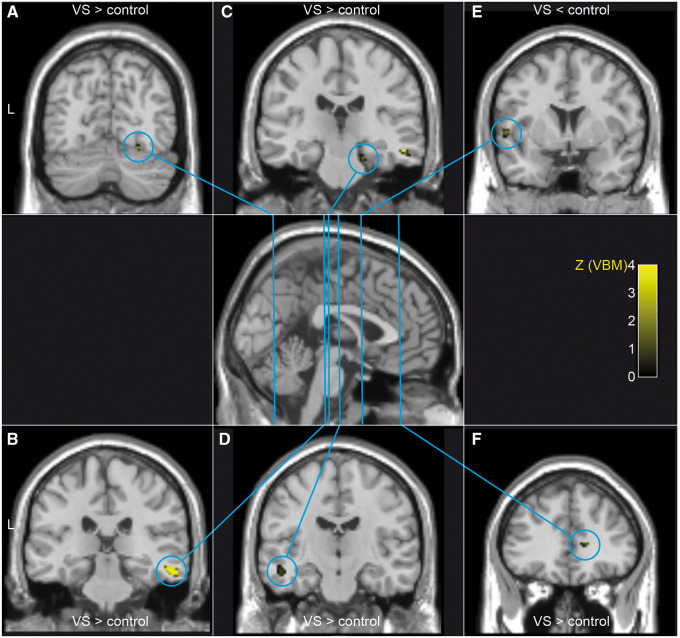
Using a whole-brain analysis, VBM identified additional grey matter volume increases in VSS that did not co-localize with PET changes in the right middle temporal gyrus, the right parahippocampal gyrus, the left superior temporal gyrus as well as the right anterior cingulate cortex. Grey matter decreases were found in the left superior temporal gyrus (Fig. 2 and Table 2).
Figure 2.
Grey matter volume differences between patients with VSS and controls. Using VBM, we observed grey matter volume increases (visual snow > control) in the right fusiform gyrus (A), the right middle temporal gyrus (B), the right parahippocampal gyrus (C), the left superior temporal gyrus (D) as well as the right anterior cingulate cortex (F) in patients with visual snow syndrome. Grey matter decreases were found in the left superior temporal gyrus (E). VBM statistical parametric maps at a threshold of P < 0.001 uncorrected for multiple comparisons are overlaid on a normalized standard T1-weighted MRI. VS = visual snow.
Discussion
From a clinical perspective, VSS presents with debilitating visual as well as non-visual symptoms. Using two different brain imaging modalities, FDG PET and VBM, this study assessed brain metabolism and grey matter volume changes as surrogate parameters for brain function and structure in patients with VSS.
The corresponding hypermetabolism and cortical volume increase in the extrastriate visual cortex at the junction of the right lingual and fusiform gyrus is consistent with a previous smaller group-size functional study (Schankin et al., 2014b) and electrophysiological evidence (Eren et al., 2018). It confirms at a structural level the importance of this region for VSS. Previous studies have shown involvement of the lingual, or fusiform gyrus, or both, in colour perception (Rizzo et al., 1992), face (Kanwisher et al., 1997), and shape recognition (Corbetta et al., 1991) supporting that this region is relevant for complex visual processing.
Palinopsia, i.e. the inability to suppress the just-seen (Critchley, 1951) is a defining associated symptom in patients with VSS (Schankin et al., 2014a), and was present in 17 patients of this study. Palinopsia can be found in occipito-parietal lesions (Gersztenkorn and Lee, 2015). The hypometabolism in the inferior parietal lobule found in our study might therefore represent a biological correlate of patients’ palinopsia. This is supported by a case report of a patient who had palinopsia with a haematoma in the angular gyrus of the inferior parietal lobule (Hayashi et al., 2002). Palinacousis, i.e. the prolonged perception of the just-heard, has been associated with the inferior parietal lobule (Mohamed et al., 2012) suggesting that this region might be important even for the suppression of the just-perceived in general, independent of the sensory modality.
Further, metabolic and grey matter volume alterations were found in the temporal lobe, the limbic system and the parietal lobe. These areas are not typically associated with visual processing. Hypothetically, this functional and structural footprint might reflect the clinical observation of VSS symptoms extending beyond the visual system (Schankin et al., 2014a). Tinnitus, for example, is a relevant non-visual symptom in VSS (Schankin et al., 2014a) and was present in 50% of our patients, potentially reflecting the acoustic equivalent of visual snow. Recent evidence suggests that the inferior parietal lobule (Lv et al., 2016) and the superior temporal gyrus (Liu et al., 2018) might both be involved in the development of tinnitus. In our patients, the hypometabolism of both regions and the altered grey matter volume in the superior temporal gyrus might therefore represent a pathophysiological correlate of tinnitus.
In clinical practice, patients further report non-perceptional symptoms, such as problems concentrating, irritability, and lethargy (Schankin et al., 2014a). This is sometimes mistaken as an underlying or causative psychiatric or psychosomatic condition. However, these symptoms might suggest involvement of limbic or temporal structures in VSS. The hypometabolism of the superior temporal gyrus found in our study is in agreement with its role for irritability (Besteher et al., 2017) and for thought suppression of pain (Chehadi et al., 2018). The latter study further demonstrated involvement of the middle temporal gyrus in cognitive pain control, an area that also exhibited grey matter volume increase in VSS. These areas might be correlates of the inability to suppress or control the continuous visual perception in VSS.
A limitation of our study is that there was an asymmetric distribution of co-morbid migraine and typical migraine aura in both groups. Two previous studies have investigated migraine and migraine aura using FDG PET, and neither demonstrated hypermetabolism in the visual association cortex (Aurora et al., 2007; Kim et al., 2010). With respect to grey matter volume, paediatric patients with migraine with aura demonstrated increased grey matter volume in the fusiform gyrus contralateral to our patients with VSS (i.e. left) (Rocca et al., 2014). In patients with chronic migraine, Coppola et al. (2017) found grey matter volume reductions in the left middle temporal gyrus and the visual association cortex, areas of grey matter volume increase in VSS. Similarly, Riederer et al. (2012) demonstrated grey matter volume decrease in medication overuse headache in the anterior cingulate cortex (Riederer et al., 2012), an area showing grey matter volume increase in VSS. One study found grey matter increases in migraineurs in the middle temporal gyrus, but no other overlap with our results (Palm-Meinders et al., 2017). The majority of studies in migraine did not demonstrate grey matter volume increases in patients (Schmidt-Wilcke et al., 2008; Valfre et al., 2008; Neeb et al., 2017). In patients with migraine with aura, Hougaard et al. (2015) found differences in cortical thickness in the inferior frontal lobe that were not altered in patients with VSS. The same group assessed response to visual stimulation in patients with strictly side-locked migraine aura. Compared to the contralateral side, there was an increased response in the inferior parietal lobule on the aura side (Hougaard et al., 2014). In VSS, we demonstrated hypometabolism in the same area. A bias from the higher frequency of co-morbid migraine aura in the VSS group, however, is unlikely in the view of only six patients having migraine aura that was side-locked in none. In summary, the metabolic and structural profile found in our study for VSS does not substantially overlap with the profile of migraine. It is therefore unlikely that our findings are a consequence of co-morbid migraine or aura rather than VSS.
Another limitation is that the power of the study was too low for subgroup analyses looking specifically for the areas responsible for individual additional symptoms, such as palinopsia or tinnitus.
The structural and functional footprint of VSS suggests dysfunction of several brain areas involved in visual, auditory, emotional, and cognitive processing. The co-localizing structural and functional alteration in the occipital lobe supports the idea that the disturbance causing visual symptoms might be located in the visual association cortex and not in the primary visual cortex. The alterations in the other areas shown in this study are first imaging evidence that VSS is indeed a complex clinical syndrome that affects more than just the visual system. It is in line with the various complaints stated by patients in daily clinical practice and suggests that multimodal and multidisciplinary treatment might be useful, in addition to a potential pharmacological treatment of the visual symptoms. Clinical profiling of visual, auditory, emotional, and cognitive functions might be important for better understanding the interactions between these domains. Correlating symptoms with neurobiology secure the identification of visual snow as an entity and offer directions for potential therapeutic developments for this currently very challenging to manage disorder.
Acknowledgements
We thank all patients who took part in the study. The study was supported by the self-help group for visual snow (Eye On Vision Foundation) by communicating the study to patients.
Funding
The work has been funded by the Eye on Vision Foundation (www.eyeonvision.org) and Visual Snow Initiative (www.visualsnowinitiative.org). C.J.S. was supported by the Deutsche Forschungsgemeinschaft (DFG; SCHA 1676/1-1), Deutsche Migräne- und Kopfschmerzgesellschaft (www.dmkg.de), and Baasch-Medicus Foundation.
Competing interests
C.J.S. reports grants from Deutsch Forschungsgemeinschaft DFG, grants from Baasch Medicus Foundation, during the conduct of the study; personal fees from Novartis, personal fees from Eli Lilly, personal fees and other from Teva Pharmaceuticals, personal fees from Allergan, personal fees from Almirall, personal fees and other from Amgen, outside the submitted work. F.H.M. reports personal fees and non-financial support from Novartis, personal fees and non-financial support from TEVA, personal fees and non-financial support from Allergan, outside the submitted work. D.E.C. is currently a full-time employee of Amgen (with stock ownership), though not at the time that this research was performed. Outside the submitted work, she has received research funding from Teva, Alder, Cefaly Technology, and Capnia; personal fees from Amgen, Teva, Eli Lilly, Allergan, and Pernix for serving on advisory boards; and personal fees from Gammacore, Medscape, and Peerview. M.E. reports speakers fees from Allergan, Novartis, an honorarium from Journal Watch and is on an advisory board with Novartis. T.S’s current and previous institutions have received compensation for author serving on scientific advisory boards/consultation and speaking from Actelion, ATI, Biogen, Desitin, Electrocore, Merck Serono, Novartis, Sanofi Genzyme, and Teva; research support from Novartis, Swiss MS Society, and Swiss National Science Foundation. P.J.G. reports, over the last 36 months, grants and personal fees from Amgen and Eli-Lilly and Company, and personal fees from Alder Biopharmaceuticals, Allergan, Autonomic Technologies Inc., Biohaven Pharmaceuticals Inc., Electrocore LLC, eNeura, Impel Neuropharma, MundiPharma, Novartis, Teva Pharmaceuticals, Trigemina Inc., WL Gore, and personal fees from MedicoLegal work, Massachusetts Medical Society, Up-to-Date, Oxford University Press, and Wolters Kluwer; and a patent magnetic stimulation device for headache assigned to eNeura without fee.
Glossary
- VBM =
voxel-based morphometry
- VSS =
visual snow syndrome
References
- Aurora SK, Barrodale PM, Tipton RL, Khodavirdi A.. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache 2007; 47: 996–1003; discussion 4–7. [DOI] [PubMed] [Google Scholar]
- Besteher B, Squarcina L, Spalthoff R, Bellani M, Gaser C, Brambilla P, et al. Brain structural correlates of irritability: findings in a large healthy cohort. Hum Brain Mapp 2017; 38: 6230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract]. 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan; 2002.
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, et al. An anatomical signature for literacy. Nature 2009; 461: 983–6. [DOI] [PubMed] [Google Scholar]
- Chehadi O, Rusu AC, Konietzny K, Schulz E, Koster O, Schmidt-Wilcke T, et al. Brain structural alterations associated with dysfunctional cognitive control of pain in patients with low back pain. Eur J Pain 2018; 22: 745–55. [DOI] [PubMed] [Google Scholar]
- Coppola G, Petolicchio B, Di Renzo A, Tinelli E, Di Lorenzo C, Parisi V, et al. Cerebral gray matter volume in patients with chronic migraine: correlations with clinical features. J Headache Pain 2017; 18: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE.. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 1991; 11: 2383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley M. Types of visual perseveration: “paliopsia” and “illusory visual spread”. Brain 1951; 74: 267–99. [DOI] [PubMed] [Google Scholar]
- Eren O, Rauschel V, Ruscheweyh R, Straube A, Schankin CJ.. Evidence of dysfunction in the visual association cortex in visual snow syndrome. Ann Neurol 2018; 84: 946–9. [DOI] [PubMed] [Google Scholar]
- Gersztenkorn D, Lee AG.. Palinopsia revamped: a systematic review of the literature. Surv Ophthalmol 2015; 60: 1–35. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S.. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017; 97: 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R, Shimizu S, Watanabe R, Katsumata Y, Mimura M.. Palinopsia and perilesional hyperperfusion following subcortical hemorrhage. Acta Neurol Scand 2002; 105: 228–31. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edn. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- Hougaard A, Amin FM, Hoffmann MB, Larsson HB, Magon S, Sprenger T, et al. Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia 2015; 35: 3–9. [DOI] [PubMed] [Google Scholar]
- Hougaard A, Amin FM, Hoffmann MB, Rostrup E, Larsson HB, Asghar MS, et al. Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp 2014; 35: 2714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM.. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 1997; 17: 4302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim S, Suh SI, Koh SB, Park KW, Oh K.. Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia 2010; 30: 53–61. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH.. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009; 114: 163–73. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lv H, Zhao P, Liu Z, Chen W, Gong S, et al. Neuroanatomical alterations in patients with early stage of unilateral pulsatile tinnitus: a voxel-based morphometry study. Neural Plast 2018; 2018: 4756471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe B, Decker O, Muller S, Brahler E, Schellberg D, Herzog W, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care 2008; 46: 266–74. [DOI] [PubMed] [Google Scholar]
- Luna S, Lai D, Harris A.. Antagonistic relationship between VEP potentiation and gamma power in visual snow syndrome. Headache 2018; 58: 138–44. [DOI] [PubMed] [Google Scholar]
- Lv H, Zhao P, Liu Z, Li R, Zhang L, Wang P, et al. Abnormal resting-state functional connectivity study in unilateral pulsatile tinnitus patients with single etiology: a seed-based functional connectivity study. Eur J Radiol 2016; 85: 2023–9. [DOI] [PubMed] [Google Scholar]
- Mohamed W, Ahuja N, Shah A.. Palinacousis–evidence to suggest a post-ictal phenomenon. J Neurol Sci 2012; 317: 6–12. [DOI] [PubMed] [Google Scholar]
- Neeb L, Bastian K, Villringer K, Israel H, Reuter U, Fiebach JB.. Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache 2017; 57: 400–16. [DOI] [PubMed] [Google Scholar]
- Palm-Meinders IH, Arkink EB, Koppen H, Amlal S, Terwindt GM, Launer LJ, et al. Volumetric brain changes in migraineurs from the general population. Neurology 2017; 89: 2066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer F, Marti M, Luechinger R, Lanzenberger R, von Meyenburg J, Gantenbein AR, et al. Grey matter changes associated with medication-overuse headache: correlations with disease related disability and anxiety. World J Biol Psychiatry 2012; 13: 517–25. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Nawrot M, Blake R, Damasio A.. A human visual disorder resembling area V4 dysfunction in the monkey. Neurology 1992; 42: 1175–80. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Messina R, Colombo B, Falini A, Comi G, Filippi M.. Structural brain MRI abnormalities in pediatric patients with migraine. J Neurol 2014; 261: 350–7. [DOI] [PubMed] [Google Scholar]
- Schankin CJ, Maniyar FH, Digre KB, Goadsby PJ.. ‘Visual snow’ - a disorder distinct from persistent migraine aura. Brain 2014a; 137: 1419–28. [DOI] [PubMed] [Google Scholar]
- Schankin CJ, Maniyar FH, Sprenger T, Chou DE, Eller M, Goadsby PJ.. The relation between migraine, typical migraine aura and “visual snow”. Headache 2014b; 54: 957–66. [DOI] [PubMed] [Google Scholar]
- Schankin CJ, Maniyar FH, Sprenger T, Chou DE, Eller M, Goadsby PJ.. Corresponding increase of gray matter volume and brain metabolism in the visual association cortex in patients with visual snow syndrome (OR21). Cephalalgia 2015; 35: 14. [Google Scholar]
- Schmidt-Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A.. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia 2008; 28: 1–4. [DOI] [PubMed] [Google Scholar]
- Valfre W, Rainero I, Bergui M, Pinessi L.. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008; 48: 109–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by reasonable request from any qualified investigator.