Abstract
The frequency and gender distribution of thoracic aortic aneurysm as a cardiovascular manifestation of loss-of-function (LOF) X-linked FilaminA (FLNA) mutations are not known. Furthermore, there is very limited cardiovascular morbidity or mortality data in children and adults. We analyzed cardiac data on the largest series of 114 patients with LOF FLNA mutations, both children and adults, with periventricular nodular heterotopia (PVNH), including 48 study patients and 66 literature patients, median age of 22.0 years (88F, 26M, range: 0 – 71 years), with 75 FLNA mutations observed in 80 families. Most (64.9%) subjects had a cardiac anomaly or vascular abnormality (80.8% of males and 60.2% of females). Thoracic aortic aneurysms (TAA) were found in 18.4% (n= 21), and were associated with other structural cardiac malformations in 57.1% of patients, most commonly patent ductus arteriosus (PDA) and valvular abnormalities. TAA most frequently involved the aortic root and ascending aorta, and were associated with sinus of Valsalva aneurysms in almost half. Six TAA patients (28.5%) required surgery (median age 37 yrs, range 14 – 41). TAA was also the only recorded cause of premature, non-accidental mortality in adults (2M, 2F), with unanticipated aortic rupture found on autopsy in 2 adult patients (1F, 1M, median 38.5 yrs), at aortic dimensions smaller than current recommendations for surgery for other aortopathies. Data from this largest series of LOF FLNA mutation patients underscore the importance of serial follow-up to identify and manage these potentially devastating cardiovascular complications.
Keywords: FilaminA, periventricular nodular heterotopia, aortic aneurysm, thoracic, cardiac, anomalies, patent ductus arteriosus
INTRODUCTION
Although FLNA mutations have long been associated with an X-linked brain malformation and epilepsy (Fox et al., 1998), cardiovascular manifestations of FLNA mutations, including aortic aneurysm, have increasingly been recognized as being common and often life-threatening in this population (Oegema et al., 2013; Reinstein et al., 2013; Robertson et al., 2003; Sheen et al., 2005). FLNA loss-of-function mutations are usually male-lethal, and are associated most commonly in females with periventricular nodular heterotopia (PVNH), a disorder in which some neurons fail to migrate normally from where they are generated, in the ventricular region (Figure 1), to the cerebral cortex (Eksioglu et al., 1996; Fox et al., 1998; Kakita et al., 2002; Sheen et al., 2001). Frequently PVNH patients have normal intelligence and demonstrate few outward dysmorphic signs, despite the distinctive brain MRI pattern. Patients typically come to clinical attention when they develop seizures during late childhood or young adulthood, which usually triggers the brain MRI that results in the clinical diagnosis of PVNH (Chen & Walsh, 2015).
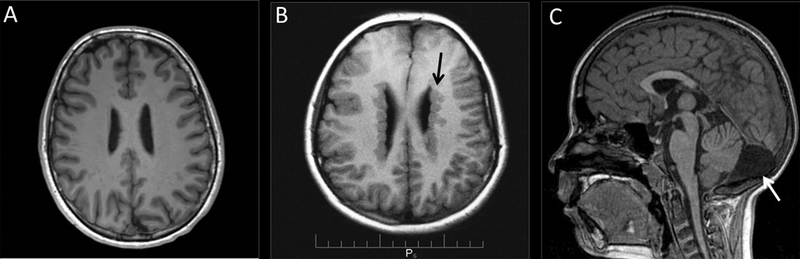
Figure 1: Representative axial T1-weighted brain Magnetic Resonance Imaging (MR) from normal and PVNH patients.
(A) Patient without PVNH. (B) PVNH patient with nodular heterotopias (arrow) along the lateral ventricles of the brain. (C) Mega cisterna magna (arrow) is seen on sagittal images in some PVNH patients.
FLNA encodes a homodimeric protein that crosslinks F-actin to transduce ligand-receptor binding into actin reorganization (Feng et al., 2006; Feng & Walsh, 2004; Sheen et al., 2002; Wang, Ash, & Singer, 1975). The FilaminA protein binds over 90 cellular proteins of varying function and is widely expressed throughout the body, a likely explanation for the many different organ systems including the brain, skin, heart and vasculature that can be adversely affected when the FLNA gene is mutated (Ehrlicher, Nakamura, Hartwig, Weitz, & Stossel, 2011; Nakamura, Stossel, & Hartwig, 2011; Razinia, Makela, Ylanne, & Calderwood, 2012; Retailleau et al., 2016; Zhou, Boren, & Akyurek, 2007). FLNA missense mutations also can produce a diverse spectrum of other rare developmental diseases such as frontometaphyseal dysplasia, intestinal pseudo-obstruction, Melnick-Needles syndrome, and otopalatodigital syndromes with involvement of the bones, muscles, lung, and gastrointestinal tract (Clark, Sawyer, Robertson, & Sutherland-Smith, 2009; Foley et al., 2010; Kapur et al., 2010; Masurel-Paulet et al., 2011; Reinstein et al., 2013; Robertson et al., 2006; Robertson et al., 2003; Sun et al., 2010).
Mouse studies suggest that FLNA is important during the embryonic development of the heart and vasculature. Male FLNA-null mice often died in utero from lethal hemorrhage and have abnormally developed blood vessels. FLNA knockout mice also showed anomalies of the left-ventricular outflow tract, atria, septum, and the great vessels of the heart (Feng et al., 2006; Hart et al., 2006). Blood vessels were found to be abnormally dilated, coarse, and with abnormal branching patterns. Importantly, vascular endothelial cells showed abnormal adherens junctions suggesting abnormalities in cell-to-cell contact (Feng et al., 2006). FLNA also regulates the structure and function of large arteries in adult mice (Retailleau et al., 2016).
Cardiovascular anomalies in FLNA deficient patients with PVNH have been described in case reports or small series (de Wit et al., 2011; de Wit et al., 2009; Hehr et al., 2006; Kapur et al., 2010; Sole et al., 2009; Srour et al., 2011), but these limited numbers have prevented systematic assessment of cardiovascular outcomes from aortic aneurysms and cardiac malformations. Recently, the cardiovascular consequences of FLNA mutations were studied in 11 patients (Reinstein et al., 2013) and X-linked PVNH found to be associated with a range of vascular and connective tissue anomalies. Serial long-term data on change in aortic aneurysm size in PVNH patients have only rarely been reported. Therefore, in this paper, we systematically analyze the aortic disease and associated structural cardiac malformations of FLNA patients, present serial data when available on aortic aneurysm growth, and present death data on a total of 114 mutation-positive patients—48 original study patients and 66 literature patients. Our study may help guide cardiac screening, frequency, and also follow-up in this population.
METHODS
Patients
Inclusion criteria for patients were 1) radiographically confirmed periventricular nodular heterotopia (PVNH), 2) a documented mutation of the FLNA gene, and 3) sufficient medical history, cardiac imaging, or records available to determine the presence or absence of a cardiac anomaly or cardiovascular issues in individuals.
Boston Research Patient Cohort
Patients were consecutively enrolled from an existing registry of patients with FLNA mutation with PVNH. Of >300 case reviews of PVNH patients, only patients with confirmed FLNA mutation were included. Informed consent was obtained according to Human Studies Protocols and the study was approved by the institutional review boards of the Beth Israel Deaconess Medical Center, Boston Children’s Hospital, and other participating institutions. In cases where cardiac information or outcomes was missing, the team re-contacted the subject’s physicians to review the patient’s cardiac history and available imaging; additionally, the study cardiologist (MHC) reviewed each subject’s existing medical information in the database for diagnoses of cardiovascular disease. Cardiac images in our database, when available (i.e. echocardiography, CT, cardiac MRI), were also reviewed. Outcomes on aortic deaths were directly derived from autopsy findings listed on death certificates. Our data collection produced a total of 48 individuals; of which only a minority had limited cardiac history included in previous publications.
Literature Review Cohort
We also performed a literature review of all FLNA mutation-positive PVNH patients published in the literature until 2016 with sufficient clinical information to ascertain cardiac history (see Supplement). A PubMed search was conducted using a combination of the following MESH terms: FilaminA, Filamin1, FLNA, periventricular nodular heterotopia, cardiovascular, congenital heart defects, thoracic aortic aneurysm, patent ductus arteriosus, mitral valve prolapse, and septal defects. Additionally, references relevant to this cohort cited in the literature but not found on the first series of PubMed searches, were also reviewed.
Literature review subjects met the same three criteria as original cohort patients. Excluded were those who tested negative for a FLNA mutation or did not have documented genetic testing or lacked cardiac data. Duplicate reports of the same patient in different articles were also eliminated. Also excluded were patients from studies that aggregated medical and cardiac data, wherein there was insufficient information to correlate an individual’s clinical phenotype to their genotype. All members of a family who were FLNA mutation carriers were included, not only the index case. This method generated over 36 articles, from which 66 patients met inclusion criteria (Supplemental Table 1). Thus 48 original patients with the 66 literature review subjects yielded the study cohort of 114 subjects.
Diagnoses and Definitions
Each subject was classified as having either 1) one or more cardiovascular abnormality or 2) no known cardiovascular issues. Cardiovascular abnormalities included both intracardiac lesions and cardiovascular lesions. An intracardiac lesion was defined as an anatomic defect involving the structures within the heart, i.e. atrial septal defect, ventricular septal defect, mitral valve prolapse, or other dysplastic valve. A cardiovascular lesion was defined as one that affects the vascular structures that are associated with the heart (i.e. aortic dilatation/aneurysm or pulmonary artery dilatation). With the literature cohort, we had to rely on definitions used by the authors. For our cohort, we reported the subjects’ clinical diagnoses (i.e. aortic aneurysm or aortic dilatation). Since the study cohort included both children and adults, aortic size and z-scores were used to allow comparison of aortic dilatation among individuals of differing sizes. Of note, aortic dilatation, by convention, is defined as a Z-score of ≥2 (i.e., ≥2 standard deviations above the predicted mean normal diameter (Chubb & Simpson, 2012; Lopez et al., 2010). In adults, aortic dimensions at different anatomic locations were presented as absolute values per convention. Nomenclature of anatomic segments of the aorta in our own cohort (i.e. aortic root, ascending aorta, aortic arch, descending aorta) were defined as per the American Society of Echocardiography (Goldstein et al., 2015).
Analysis
Microsoft Access was used as the database and analysis software. Median age of the cohort was calculated. Age data for the original cohort was based on the age of the subject at completion of the collection of cardiovascular data. Age data from the literature cohort was taken directly from the articles. Z scores were used in the reporting of serial aortic dimensions in growing children so that significance could be determined (Chubb & Simpson, 2012; Lopez et al., 2010).
RESULTS
Study Schema and Clinical Features (Figure 2)
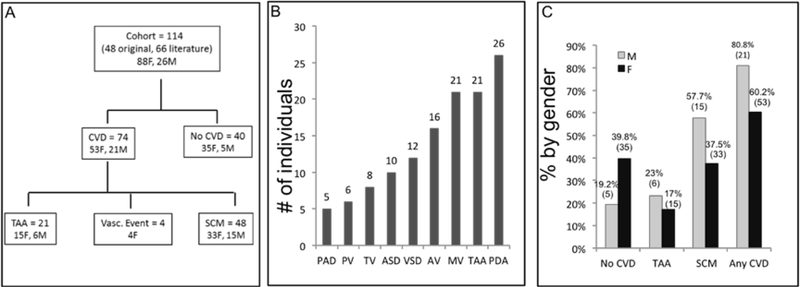
Figure 2:
(A) Study schema (n=114 individuals). 64.9% (n=74) of the cohort had either a vascular aneurysm or event (n=26) or a structural cardiac anomaly (n=48). Twenty-one had a thoracic aortic aneurysm (TAA), with 4/21 patients having both a TAA and a dilated pulmonary artery. One patient with a dilated pulmonary artery alone was not specifically depicted in schema. (B) Frequency of the vascular and structural cardiac abnormalities in individuals. TAA and PDAs were very common in subjects and frequently occurred in conjunction. (C) Gender distribution of cardiovascular malformations and aneurysms: There was no significant difference between males and females, by Fisher’s exact test. Absolute numbers are in parentheses. M = male, F = female. No CVD = No cardiovascular anomalies or abnormalities, TAA = Thoracic aortic aneurysm, SCM= Structural cardiovascular malformation, Vasc = Vascular event (i.e. stroke, myocardial infarction).
ASD = Atrial septal defect, AV= Aortic valve abnormality, MV= Mitral valve abnormality, PAD = Pulmonary artery dilatation, PDA = Patent ductus arteriosus, PV = Pulmonary valve abnormality, TV= Tricuspid valve abnormality, VSD = Ventricular septal defect.
A total of 114 subjects (76.4% female, 84F, 26M), with a median age of 22 years (range: 0 – 71 years), with 75 unique FLNA mutations from 80 families, comprised the cohort. All patients except four had cardiac imaging or autopsy performed. Given that FLNA is X-linked, the greater proportion of females than males was not unexpected. Seventy-four subjects (64.9%, 21 M, 53F) had at least one vascular and/or structural cardiac malformation, with the types of abnormalities categorized in Figure 2b. The remaining 40 individuals (35.6%, 35F, 5M) had no known cardiovascular issues by medical history. Prevalence of cardiovascular malformations and vascular abnormalities by gender is detailed in Figure 2c. Overall, 80.8% of males and 60.2% of females had at least one cardiac anomaly or vascular abnormality, but this difference was not statistically significant.
Thoracic Aorta Aneurysms (Table 1)
Table I:
Patients with Dilated Thoracic Aorta or Thoracic Aortic Aneurysm (n=21/114, M=6, F=15):
| Study ID | Sex (Age) | FLNA mutation | Aneurysm Location | Other significant cardiopulmonary or medical findings | Cardiovascular Outcome |
|---|---|---|---|---|---|
| Boston Cohort #9 | F (43y) | GT to GC at exon 4+2 | - Preop: Sinus of Valsalva aneurysm involving the non-coronary sinus (4.0×3.5 cm), eroding down toward the roof of the LA - Dilated thoracic aorta (5.1cm) from the sinuses to the mid-transverse aorta (between the left common carotid and the left subclavian artery) |
- Congenital bicuspid aortic valve with moderate aortic stenosis with mild AR prior to Ross - Seizures, prolactin-secreting adenoma |
1) S/p Dacron interposition graft in ascending aorta at age 36; S/p Ross procedure for aortic stenosis 2) Aortic arch began to dilate at age 40 years |
| Boston cohort #27 | F (16y) | c.4147 del G single base pair deletion exon 25, p.(Ala1383Leufs*10) | -Thoracic ascending aorta (unknown) | - Hyperflexibility and joint dislocations - No seizures |
n/a |
| Boston Cohort #29 | F (15y) | c.116 C>G exon 2 p.(Ala39Gly) | - Sinus of Valsalva aneurysm | - Hyperelasticity of the large vessels, hyperextensibility of skin, joint laxity and subluxations - Seizures, mild developmental delay |
n/a |
| Boston Cohort #36 | F (41y) | G>A intron 2 | - Sinuses of Valsalva aneurysm (non-coronary and right-coronary sinuses) - Mildly dilated thoracic ascending aorta |
- Dysplastic AV, perimembranous VSD, moderate AR, mild MR. Normal abdominal aorta - Hyperflexible with joint dislocations - Seizures |
1) Progressive increase in aortic size from age 29 to 41 years 2) Died age 41yrs of lethal aortic rupture, at an aortic root size of 4.2 cm |
| Boston Cohort #37 | F (17y) | C>T exon 48, p.(Gln2385*) | - Dilated aortic root (4.8cm) - Dilated thoracic ascending aorta (2.7cm) |
- PDA, PFO, perimembranous VSD, MVP. Pulmonary arterial dilation (3.7cm). Coronary fistula to proximal coronary artery - Developmental delay, no documented seizures |
1) s/p PDA repair neonatal period 2) s/p Bilateral lung transplant during infancy 3) s/p Aortic root and valve replacement at age 13 years 4) s/p mitral valve annuloplasty ring and mitral valve repair |
| Boston Cohort #39 | M (36y) | c.6915bp C>G exon 41, p.(Tyr2305*) | - Dilated thoracic ascending aorta | - AR - Seizures |
1) Died age 36 yrs from aortic rupture and cardiac tamponade |
| Reinstein 2013, F3 ++ Boston Cohort #85 |
F(11y) | c.2193C>A exon15, p.(Tyr731*) | - Sinus of Valsalva aneurysm involving the non-coronary cusp. - Mildly dilated thoracic ascending aorta - Mildly dilated descending aorta. |
- PDA, perimembranous VSD; dysplastic pulmonary, tricuspid and aortic valves. Moderate AR, PFO - Pulmonary hypertension with lung disease at birth that later stabilized. Dilatation of main pulmonary artery and its branches. Dilated head and neck branches. Hyperflexible. - No seizures |
1) PDA ligated at 10 weeks 2) Development of sinus of Valsalva aneurysm by age 6 2) Aortic root Z score normal at birth and increased to 5 by age 11 |
| Boston Cohort #107 | F(1Y) | Complete deletion | - Nl size aortic root - Dilated thoracic ascending aorta (z score=4.6) |
- Small PDA - Hyperflexible, no seizures |
No cardiac symptoms |
| Boston Cohort #114 | F(21) | c.62_64TCG exon 2,p.(Val21dup) | - Sinus of Valsalva aneurysm involving the right and non-coronary cusps - Mildly dilated thoracic ascending aorta |
- PDA, perimembranous VSD, MVP with mild mitral regurgitation - Asthma, normal intellect, no seizures |
1) PDA ligated at 10 months |
| Guerrini 2004, 3-II:1 | M (49y) | A>G at intron 11 | -Thoracic ascending aorta | - Dysplastic AV - Bifid epiglottis, seizures |
1) s/p aortic valve replacement and ascending aorta replacement at age 26 yrs |
| Sole* 2009, F8 | F(43y) | c.365 T>G exon 2 p.(Val122Gly) Mother of Sole F9. |
- Aortic aneurysm (no further detail) | - Joint hypermobility - Seizures |
1) s/p Aortic aneurysm surgery at age 38yrs |
| Sole* 2009, F9 | F(15y) | c.365T>G exon 2 p.(Val122Gly) | - Aortic aneurysm (no further detail) | - EDS with ankle dislocation and joint hyperlaxity - No Sz |
Treated with beta-blockers |
| Sole** 2009,F15 | F(35y) | c.356T>A exon 2 p.(Val122Gly) | - Aortic aneurysm (no further detail) | - EDS- with joint hyperlaxity, AR - Seizures |
Treated with beta-blockers |
| Fergelot** 2012, P1 | M(57y) | c.356T>A exon 2, p.(Val122Gly) Father of Sole 2009, F15 |
- Severely dilated aortic root by age 41 years - Dilated thoracic ascending aorta (6cm) |
- Severe AR, thrombocytopenia - Normal cognitive development, no seizures |
1) s/p Aortic root repair and also AV replacement at age 41 yrs 2) s/p Bentall at age 57 yrs for progressive dilatation of the ascending aorta and also stenosed aortic valve prosthesis 3) Died post- Bentall of severe bleeding complications at age 57 |
| de Wit 2011, P5 | F(42y) | c.6635delTCAG exon 41, p.(Ser2213*) | -Dilated ascending aorta | - Severe AR from dysplastic AV, VSD - No seizures |
1) s/p VSD repair and aortic valve replacement at age 24 yrs 2) s/p Bentall at age 36 yrs |
| Masurel-Paulet 2010, P1 | M(6y) | c.994delG exon 7 p.(Lys331*) | - Sinus of Valsalva aneurysm | - PDA. AR, enlarged RV with wall hypertrophy. Pan-pulmonary emphysema and pulmonary arterial hypertension - Developmental delay, poor feeding, no seizures |
s/p PDA ligation after 5 days of life |
| Reinstein 2013, F1 | F(38y) | c883-880 del8 exon 6, p.(Asn296Glu fs*38) | - Sinus of Valsalva aneurysm | - Diffuse ectasia of aortic branch vessels - No seizures |
s/p valve-sparing aortic root repair by age 38 years Root increased from 48mm to 57 cm (preop) |
| Reinstein 2013, F5 | F(23y) | c.7813delC exon 48, p.(Leu2605Trpfs*2) | - Dilated aortic root (4.5cm) - Dilated thoracic ascending aorta |
- AR, Pulmonary artery dilatation - Seizures |
n/a |
| Reinstein 2013, M2 | M(2mo) | c.5498-5504delCACCACinsAC exon 34, p.(Ala1833Aspfs*13) | - Dilated thoracic aorta - Dilated abdominal aorta - Elongated/tortuous supra-aortic vessels |
- PDA. Dysplastic AV, thickened mitral and tricuspid valves on autopsy, ASD, VSD - Pulmonary hypertension, with pulmonary artery dilatation progressive heart failure. - No seizures |
Died at age 2 months, secondary to sepsis following pulmonary hypertension and heart failure |
| Reinstein 2013, M3 | M(15y) | c.381G>C exon3, p.(Lys127Asn) | - Dilated aortic root (3.1cm, nl < 2.8cm) | - MVP, history of pneumothorax, slightly hyperextensible skin, joint hypermobility, and umbilical hernia. - No seizures |
n/a |
| LaPointe 2014 | F(38y) | c.2002C>T exon13, p.(Gln668Ter) | - Dilated aortic root - Dilated thoracic ascending aorta |
- Mild AR. PDA, bruising - Seizures |
PDA repaired at 18 months |
AR = aortic regurgitation. AVR = aortic valve replacement. AV= aortic valve. Bentall = Bentall cardiac surgical procedure to replace the aortic valve, aortic root, and ascending aorta. EDS = Ehlers Danlos Syndrome. LA = left atrium. MR = mitral regurgitation. PASP = pulmonary arterial pressures PDA = patent ductus arteriosus. PFO = patent foramen ovale. MVP = mitral valve prolapse. VSD = ventricular septal defect.
= relationship with another patient with *.
= relationship with another patient with **.
= Additional cardiac follow-up information ascertained since publication.
Twenty-one individuals (18.4%, 15F, 6M) had an aortic aneurysm or pathological aortic dilatation, making it the most common vascular abnormality in this cohort. Since definitions and usage of the terms TAA and aortic dilatation vary somewhat between institutions, and between pediatric and adult patients, we use the term TAA to refer to both. The FLNA mutations of these 21 patients with TAA are summarized in Figure 3. The median age of all subjects with TAA was 23 years (range 0 months - 57 years). Unexpectedly, a similar proportion of males and females had an aortic aneurysm (Figure 2c). Median age of males vs. females with a TAA was 26 yrs. vs. 23 yrs. respectively. Many of our patients with PVNH and TAA have symptoms and signs that are frequently also seen in Ehlers-Danlos syndrome, including joint hypermobility, skin hyperelasticity, and cutaneous abnormalities (Reinstein et al., 2013)(Sheen et al, 2005).
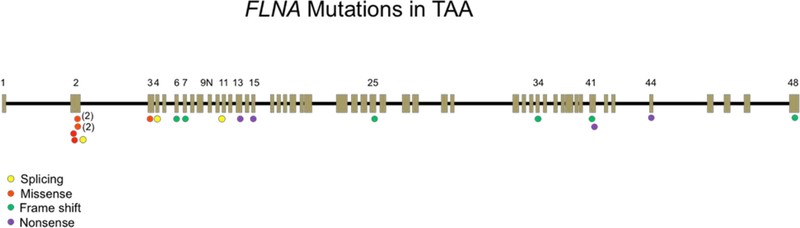
Figure 3: Genomic locations of exonic FLNA mutations associated with TAA.
A schematic representation of the FLNA gene. The FLNA gene is 25.9 kbp in size and encoded by 49 exons including a newly discovered “poison” exon 9N (Zhang et al, 2016), present in the contig: NT 025965. Exons are depicted by boxes, and introns are shown as black lines between the exons. Exons with relevant mutations are numbered. Types of FLNA mutations occurring in patients with TAA are localized on the FLNA map. The mutation in two families that each show two 2 individuals is indicated by one circle with total family members in parentheses. Five unique missense FLNA mutations (occurring in 7 individuals) associated with TAA localize most commonly to the actin-binding domains of FLNA gene (encoded by exons 2–5), whereas other loss-of-function mutations (nonsense, splicing, frameshift) are seen throughout the gene. One patient had a complete deletion of the FLNA gene.
Clinical Presentation
A brief history of five patients with PVNH with TAA is presented below, demonstrating the region of aortic involvement, progressive dilatation, and diverse outcomes (Table 1).
#114 was a 21 year-old college student, born at term, s/p PDA ligation at age 10 months, and with a history of mitral valve prolapse since infancy. She also has asthma responsive to inhalers. She developed seizures at age 16 years and was diagnosed with PVNH with a FLNA mutation. Echocardiogram at age 16 showed a new sinus of Valsalva aneurysm involving her right coronary cusp (RCC) and noncoronary cusp (NCC) and a perimembranous VSD (See Figure 4 A–D). Her aortic root measured 4.2 cm, and has been without significant progression in the last five years.
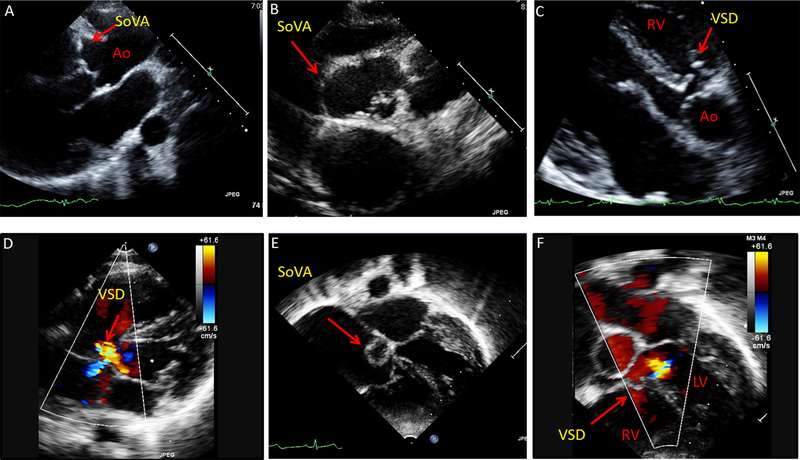
Figure 4: Sinus of Valsalva aneurysms (SoVA) with perimembranous ventricular septal defect in 2 young females.
(A-B) Echocardiography images from 21 year-old female with SoVA involving the right and non-coronary cusps of the aorta (parasternal long-axis and short-axis views). (C-D) Patient also has a perimembranous ventricular septal defect (VSD) with shunt flow, seen on parasternal and apical views. (E-F) Echocardiography images from an 11 year-old female depicting sinus of Valsalva aneurysm and a perimembranous VSD (apical 5-chamber view). Ao = aorta, LV = left ventricle, RV= right ventricle.
#85 was a 11 year-old female with PVNH with additional cardiac follow-up and serial echocardiography data analysis obtained for this study. Initially described as a 6 year-old, (Reinstein et al., 2013), she had a PDA s/p surgically ligation in infancy, a small sinus of Valvalva aneurysm, history of pulmonary hypertension and lung disease initially dependent on sildenafil, and has now improved with time. She was noted to have mild ascending aortic dilatation at birth, with a normal aortic root size. Serial analysis for this study showed progressive increase in her aortic root size, with continued eccentric enlargement of a sinus of Valsalva aneurysm involving her non-coronary cusp (See Figure 4 E–F). A perimembranous VSD is also visualized. Over the last decade, her aortic root z scores have transitioned from normal at birth to abnormal [z = 0.6 (birth) → 5.0 (11 years old)] (Figure 5A).
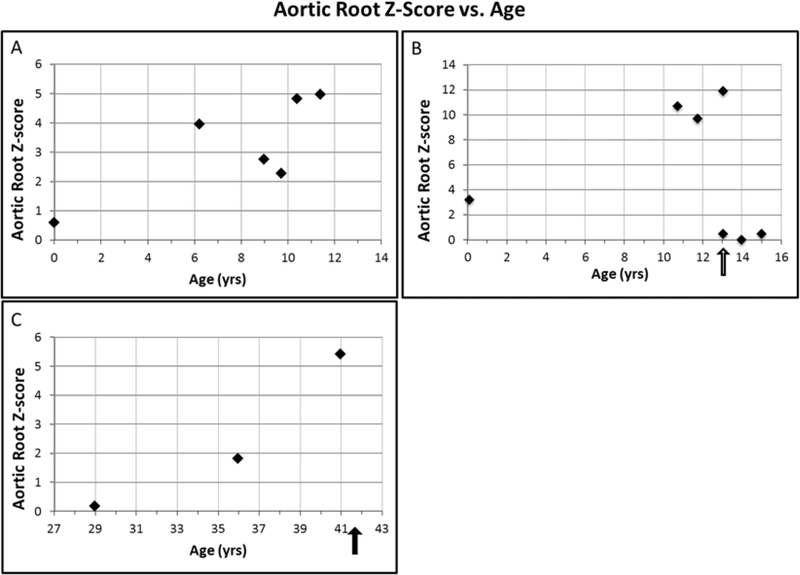
Figure 5: Aortic root growth vs. age in 3 patients, pediatric and adult.
Aortic size is displayed as Z-scores in all individuals to allow for comparison. (A) Female child (#85) has a normal Z score at birth, but over the next 10 years, her aortic root z-scores have increased to ~5. (B) #37 Female child (#37) was born with a dilated aorta that increased to a Z score of 11.9, before her aneurysm surgery. Root size was normalized by surgery. Black arrow indicates timing of aortic aneurysm surgery. (C) Middle-age adult female (#36) who had normal aortic root Z scores on initial imaging, with a rapid increase in aortic root size between age 35 and 41 years of age. Aortic root size was 4.2 cm (Z score = 5.4) at time of death. Black arrow denotes timing of patient demise.
#37 was a 17 year-old female diagnosed with PDA at 3 months, s/p surgical closure, and with a dilated aorta since birth (Figure 5B, 6). She underwent a double lung transplant in infancy for bronchoalveolar dysplasia. At age 5 years, in the setting of seizure-like activity, we diagnosed her with PVNH and a FLNA mutation. She was empirically treated with Losartan at age 10 years by her cardiologist, but her ascending aorta continued to dilate. At age 13 years, she underwent ascending aortic aneurysm repair surgery at an aortic root size of 4.2 cm (Z score = 11.9) (Figure 5B).
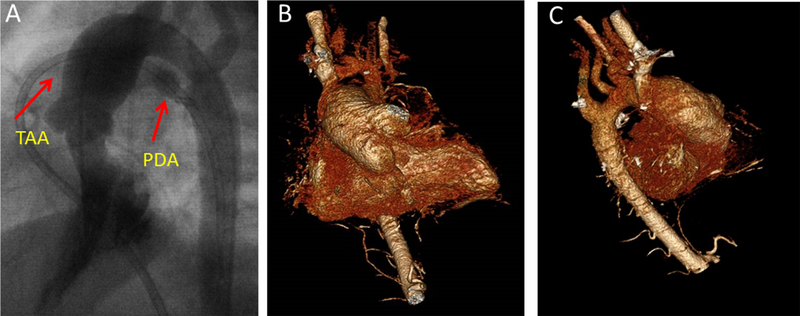
Figure 6: Female with progressive thoracic aortic dilatation requiring surgery.
(A) Dilated aorta and PDA seen on cardiac catheterization images during infancy. (B-C) 3D CT images of the patient’s aorta, obtained a decade later, demonstrate persistent dilatation of the aortic root and ascending aorta without involvement of the distal arch or descending aorta.
#39 was a 36 year-old male that died from a sudden aortic rupture. He was diagnosed with partial complex epilepsy which brought him to medical care at age 32 years. He was noted to have increasing voltages on his EKG for borderline left ventricular hypertrophy and also left atrial enlargement over the ensuing next 5 years. Last physical exam by neurology noted a pulsatile lesion in his neck area. He was lost to subsequent follow-up. His death certificate documented fatal aortic rupture with cardiac tamponade. No further records or details are available.
#36 was a 41 year-old female first found to have mild aortic root and ascending aortic dilatation at 29 years, after presenting with a seizure post-partum and being diagnosed with PVNH. She was otherwise well. Initial echocardiogram demonstrated eccentric sinus of Valsalva aneurysms involving the NCC and RCC, and an aortic root size of 2.7 cm. She remained asymptomatic, and serial echocardiograms over the next decade showed progressive aortic root dilatation from 2.7 cm (age 29 years) to 3.2 cm (36 years) to 4.2 cm (41 years) (Figure 5). At 41 years, several months after her last echocardiogram, she died suddenly. Autopsy identified the cause of death as sudden aortic rupture. Importantly, although an aortic root size of 4.2 cm is above normal limits, her aortic dimensions were smaller than current recommendations for aortic surgery in aortopathies.
Thoracic Location (Table 1)
The location of the aortic aneurysm was known in 10 patients, and not specified in 2 literature subjects. In all 19 patients, the aortic aneurysm involved the aortic root and/or thoracic ascending aorta, as typically seen in other genetic causes of TAA in the young (Isselbacher, Lino Cardenas, & Lindsay, 2016). The descending aorta was very infrequently involved, with only one patient having diffuse dilatation of his vasculature system including the entire thoracic aorta, pulmonary arteries, and also head and neck vessels. Another single patient had involvement of the abdominal aorta in addition to the thoracic aorta, and none had an abdominal aneurysm alone. For patients with aortic root aneurysms, there was frequent eccentric involvement of the sinuses of Valsalva (n= 9, 2M, 7F), with preferential involvement of the right and non-coronary cusps rather than the left coronary cusp. Of these 9 patients with sinus aneurysms, 4 also had dilatation of the thoracic ascending aorta.
Change in Size of Aortic Aneurysm
There was progressive dilatation of the TAA in individuals followed serially, leading to aortic aneurysm surgery in some patients, and deaths noted in some unoperated patients (Table 1). Serial aortic measurements were available in 5 individuals with TAA. Two young female subjects, with normal aortic root size at birth, developed sinus of Valsalva aneurysms during childhood (#85, #114 Figure 4). Longitudinal data of aortic root size over 10 years is presented in 3 patients (Figure 5).
Surgical Repair (Table 1)
Seven of 21 patients (33.3%, 2M, 5F) with TAA underwent aortic aneurysm repair, with one dying of a post-surgical bleed. The age of repair, known in 5 patients, was a median of 37 years (range 14 – 41 years). Two patients (1M, 1F) had progressive aortic dilatation after TAA surgery, with one needing a reoperation 16 years later. The size of the aorta at the time of surgical repair was not available in most subjects, except for three. One child underwent ascending aorta repair at 4.8 cm (z score=11.9); an adult female repaired with an ascending aorta of 5.1 cm, and a third patient, originally reported by Reinstein, was operated on when TAA increased to 5.7 cm (Reinstein et al., 2013)(Table 1).
Structural Cardiac Malformations (SCM)
The types and frequency of FLNA-associated SCM for the entire cohort are displayed in Figure 2b. Specifically, over half the patients with TAA (57.1%, 12/21) also had an associated SCM. Patent ductus arteriosus (PDA, n=7) and ventricular septal defects (VSD, n=6) were most common in patients with TAA. In 3 patients where the type of VSD was known, it was perimembranous. Not surprisingly, aortic valve abnormalities (bicuspid/dysplastic AV) were common, occurring in 28.6% (6/21) of TAA patients. Mitral valve abnormalities were noted in 5/21 TAA patients, with elongated leaflets resulting in prolapse or thickened, dysplastic valves.
Pulmonary Artery and other Involved Blood Vessels
Pulmonary artery dilatation was also common, occurring in 5 patients. Most patients (4/5) with pulmonary artery dilatation also had a TAA. Aneurysms of other vessels present in our study included the subclavian artery (1), the abdominal aorta (1), the middle cerebral artery (1), and the internal carotid artery (1).
Vascular Events and Death
In addition to the 21 patients with thoracic aortic aneurysms described above, four additional subjects (all female and without TAA) had a vascular event (myocardial ischemia, stroke/TIA, subarachnoid hemorrhage) at relatively young ages (median 40 yrs, range 19–71 yrs). One had a myocardial infarction at age 39. Another had a stroke at age 19 due to a thrombus without known source of embolus, but did have protein C deficiency. Another had a first stroke at age 41 and recurrent TIA/stroke thereafter at age 50 without source or clotting disorder found. Finally, one had a fatal subarachnoid hemorrhage at age 71 secondary to a rupture of an internal carotid artery aneurysm. One additional patient had dilatation of the pulmonary artery root without a TAA (Reinstein et al., 2013). Therefore, a total of twenty-six subjects (22.8%, 6M, 19F) of the entire cohort had a vascular aneurysm or dilatation, or suffered a vascular event. Vascular aneurysms were found in both children and adults, as well as in males and females.
Pediatric and Adult Deaths (Table 2)
Table 2.
Cardiovascular and Pulmonary Deaths in Children and Adults (n=8):
| Pediatric Deaths (n=4): | ||||
| Study ID | Sex (Age at Death) | FLNA Mutation | Cause of Death | Cardiovascular Findings |
| Boston Cohort #105 | F (7months) | 39 Kb deletion encompassing all of FLNA | Respiratory failure secondary to emphysema | TV and MV dysplasia, PFO, pulmonary hypertension, RV hypertrophy, normal size aorta on post-mortem. |
| DeWit 2011, P1, Reinstein 2013, M2 | M (2months) | c.5504delCACCACinsAC | Heart failure, pulmonary hypertension | TAA, dysplastic aortic valve, PDA,VSD, ASD, general enlargement of vessels |
| Gerard-Blanluet 2006, T2 | M (8months) | c.7922C>T p.(Pro2642leu) | Respiratory complications from bronchopulmonary dysplasia | PDA |
| Kapur 2010, P5 | M (1.5month) | c.7021C>T p.(Gln2341X) | Cerebral and spinal cord infarcts. Multiple congenital abnormalities with left diaphragmatic hernia, s/p surgical repair. | ASD |
| Adult Deaths (n=4) | ||||
| Study ID | Sex (Age at Death) | FLNA Mutation | Cause of Death | Cardiovascular Findings |
| Boston Cohort #39 | M (36yrs) | c.6915C>G p.(Glu2305Ter) | Aortic rupture (cardiac tamponade following rupture) | TAA |
| Boston Cohort #36 | F (41yrs) | IVS2DS G-A +1 (splicing mutation) | Aortic rupture | TAA, VSD |
| Fergelot 2011, P1 | M (57yrs) | c.356T>A p.(Ile119Asp) | Bleeding s/p 2nd aorta surgery | TAA requiring 2 surgeries (2nd lethal) |
| DeWit 2009, F1 | F (71yrs) | c.3045-3049del5 exon 21 | Subarachnoid hemorrhage secondary to internal carotid aneurysm. | s/pAVR @56y. Intracranial vascular disease. Heart failure requiring cardiac transplantation. |
ASD = Atrial Septal Defect. AR = Aortic Insufficiency. AS = Aortic Stenosis. CAD = Coronary Artery Disease. PDA = Patent Ductus Arteriosus, HF= Heart Failure, LV = Left Ventricle, RV = Right Ventricle, TAA = Thoracic Aortic Aneurysm, VSD = Ventricular Septal Defect.
Eight patients (7.3%, 3F, 5M) suffered nontraumatic death at the time of their respective study, with a median age of death of 18 years old (range: 0–71 years). A ninth patient died at age 48 years in a motorcycle accident and was not included in the analysis below. Half of all non-traumatic deaths (50.0%, 3M, 1F) occurred in infants < 1 year of age, with the remainder occurring during adulthood (2M, 2F, median age 41 years, range 36–57 years). The cause of death in infancy was predominantly secondary to pulmonary disease with or without heart failure, or the sequelae of multiple congenital defects. In contrast, the most common cause of death in adults (3/4) was autopsy-confirmed aortic rupture, or post-operative bleeding s/p aortic root repair for aortic aneurysm.
DISCUSSION
Here we provide the largest systematic analysis to-date of the frequency of aortic aneurysms and cardiovascular outcomes in both pediatric and adult patients with PVNH and FLNA loss-of-function mutations. Although ascertainment of cardiovascular abnormalities in the majority was performed by reviewing existing genetic records and medical records in this relatively young population, we find a surprisingly high 18.4% of PVNH patients had aneurysms of the aortic root and/or the thoracic ascending aorta, and almost 42.1% (48/114) had SCM. Furthermore, we found that a) the location of the aortic aneurysms was almost entirely thoracic, commonly involving the aortic root and ascending aorta, and frequently associated with sinus of Valsalva aneurysms, b) there was progressive dilatation of the TAA in both children and adults, frequently resulting in the need for surgery during early adulthood, and c) TAA rupture was a cause of premature death in middle-age adults with PVNH. Rupture of the aorta and the frequency of deaths from TAA have not been reported previously in FLNA patients. Our data underscores the importance of long-term cardiovascular screening of all FLNA mutation positive PVNH patients for this silent, life-threatening disease.
Eighty percent of male FLNA mutation carriers in our cohort show a SCM, vascular abnormality, or lung disease, and are at risk for death during childhood (Table 1). Of course, most males with FLNA mutations die before birth, and those that survive childhood often have mutations that preserve some residual FLNA function. Nevertheless, occasional males in our cohort survived into adulthood, although 2 of them subsequently died from TAA (Table 2). We presume that the postnatal survival of these males reflects mutations that leave some residual FLNA function intact, and the nature of the mutations in males associated with postnatal survival (missense, splicing, truncating mutations late in the protein sequence in the case of older males) are generally consistent with this notion (Guerrini et al., 2004; Hehr et al., 2006; Oegema et al., 2013; Parrini, Mei, Wright, Dorn, & Guerrini, 2004; Sheen et al., 2005; Zhang et al., 2016).
In mice, FLNA has essential functions both in the vascular endothelium, neural crest, and arterial muscle layers, all of which might contribute in principal to the pathophysiology of the TAA in humans. Germline FLNA mutant mice show widespread abnormalities of vascular patterning and growth, as well as abnormal endothelial adherens junctions between vascular endothelial cells, which results in decreased integrity of the vascular walls and widespread hemorrhage prenatally in males (Feng et al., 2006). Moreover, FLNA has essential roles in cardiac and valvular development that are at least in part reproduced by conditional removal from neural crest cells (Feng et al., 2006; Sauls et al., 2012) so that essential roles in neural crest might also lead to TAA (Sherif, 2014; Weinreich, Yu, & Trost, 2015). The preferential involvement of the RCC and the NCC, compared to the LCC, may be explained by their embryologic derivation from neural crest cells (Sherif, 2014). Sinus of Valsalva aneurysms are thought to result from a lack of continuity between the aortic media and the aortic annulus (Feldman & Roman, 2006). Interestingly, this region of the aorta is subject to rupture and not dissection. The association of perimembranous VSD with sinus of Valsalva aneurysms is consistent with the observation that this portion of the septum serves as the base which forms the right and noncoronary sinuses (Taguchi, Sasaki, Matsuura, & Uemura, 1969). Our FLNA mouse model also developed a VSD, also likely reflecting a known embryologic derivative of the distal left ventricular outflow tract from neural crest cells (Feng et al., 2006).
Although FLNA binds a host of other intracellular molecules, the distribution of missense mutations associated with TAA might give some tentative insight into cellular/molecular mechanisms. FLNA is thought to anchor the actin cytoskeleton to transmembrane integrin molecules (Karimi & Milewicz, 2016). Interestingly in our current analysis we find that 5 unique missense mutations in 7 patients with TAA reside in the first five exons of the coding region (Figure 3). Since this segment of the gene encodes the actin-binding domain, these mutations suggest that TAA might involve at some level a dysregulation of the polymerization of actin filaments, but this suggestion would need further substantiation in animal models.
It is not known in patients when the aortic aneurysms first present, since cardiac imaging is not always performed in this population and serial follow-up is even rarer, and TAA is thus often not diagnosed until symptomatic. Of note, our data demonstrates that aortic dilatation can be present at birth, as evident in several of our patients. In other patients with available serial data, we observe progressive dilatation of the aorta over 5–10 years. Our preliminary findings suggest that FLNA mutations may behave somewhat differently than genetic forms of Ehlers-Danlos Syndrome, which tend to show greater stability of aortic size over time (Reinstein et al., 2013), and underscores that serial follow-up and imaging of the thoracic aorta are critical in FLNA patients.
Our findings are consistent with data that TAA are often genetic in etiology and occur generally in a younger age group than abdominal aortic aneurysms, which tend to be atherosclerotic in nature (Isselbacher et al., 2016; Lindsay & Dietz, 2014). Currently, almost 20 genes have been previously identified as causing TAA: ACTA2, COL3A1, FBN1, TGFB 2–3, TGFBR1–2, SMAD3, MFAP5, MYH11, MYLK, PRKG1, AAT1, AAT2, and MAT2A (Milewicz et al., 2008; Milewicz & Regalado, 2016). They have been generally categorized into genes affecting extracellular matrix proteins (i.e. COL3A1, FBN1), TGF-Beta signaling (TGFB 2–3, TGFBR1–2, SMAD2–4), or cytoskeletal/smooth muscle contraction apparatus proteins (e.g., MYH11, MYLK, PRKG1) and others. There are several similarities between our PVNH FLNA cohort and patients with other cytoskeletal/smooth muscle contraction apparatus gene mutations. MYH11 encodes a regulator of affects smooth muscle myosin and the actin-binding site (Isselbacher et al., 2016) and patients with MYH11 mutations also develop TAA and PDA (Zhu et al., 2006). ACTA2 mutations are also associated with both TAA and PDA (Regalado et al., 2015). Our data further support the hypothesis that FLNA, MYH11, and ACTA2 share defects in mechanical strength of the vascular walls or the ability of the arterial walls to contract, potentially in part through a common pathway (Karimi & Milewicz, 2016; Retailleau et al., 2016).
For FLNA, ACTA2, TGFBR2, and FBN1 individuals with thoracic aneurysms, there were a high percentage of patients who suffered a vascular event. However, unlike Marfan, Loeys-Dietz syndrome, and ACTA2 patients, our cohort often suffered sudden aortic rupture rather than acute or chronic aortic dissection. None of the death certificates reviewed on our patients indicated that they had an aortic dissection, although further work needs to be done in this area, and there is a great need to obtain pathological material to characterize the histopathology of the FLNA TAA. Sinus of Valsalva aneurysms typically rupture and tend not to dissect. Given the frequency of sinus of Valsalva aneurysms in this study and the abnormal adherens junctions in FLNA mutant mice, it may not be unexpected that sudden rupture occurs, and often at aortic root diameters much smaller than in standard thoracic aortic dissections. Like Loeys-Dietz and Marfan disease, FLNA patients mainly have dilatation of the aortic root and the thoracic ascending aorta, and but also show dilatation of the pulmonary arteries, and also mitral valve dysplasia or prolapse (Lindsay & Dietz, 2014). Like Marfan and TGFBR2 patients, a high percentage of FLNA patients require aortic surgical repair. In contrast, ACTA2 patients have frequent involvement of their entire arch, whereas most of our cohort only had thoracic ascending aorta dilatation, with very rare involvement of the descending aorta or abdominal aorta, by history or by type of surgical repair required (Regalado et al., 2015).
Our results suggest that a comprehensive baseline cardiac evaluation should be undertaken at the time of PVNH diagnosis to identify any existing SCM, as well as to document baseline dimensions of the thoracic aorta. Imaging should include at least an echocardiogram for identification of any structural anomaly including septal defects, valvular abnormalities, and PDA (Chen & Walsh, 2015). At the same time, measurements of the aortic root, and the visualized portion of the ascending and descending thoracic aorta should also be performed (Lai, Mertens, Geva, & Cohen 2009). For any patient with difficult acoustic windows, a CT or a MR should be performed to examine the vasculature of the transverse aorta and the great vessels (Lai et al., 2009). Data on vascular tortuosity also can also be obtained via MRI or CT, although its usefulness to the FLNA population is unknown (Reinstein, Morris, Rimoin, Robertson, & Lacro, 2014). We recommend that serial cardiac imaging and cardiac follow-up be performed for all FLNA positive patients given the documented progressive nature of TAA associated with this disease, even in patients who have normal sized aortas. Childhood and times of rapid growth may be when even closer follow-up is warranted. Also women who are contemplating pregnancy or those undergo other surgical procedures should be followed more closely.
As with other aortopathies, the frequency of imaging in patients with an aortic aneurysm or dilated aortic dimensions is based on size and rate of progression. Furthermore, there are currently no specific recommendations of aortic size to consider aortic root or ascending aorta surgery in FLNA patients, and much more data is needed. Our cohort may more resemble patients with Loeys-Dietz syndrome, where surgical repair is considered when the patient’s aortic size approaches 4.0–4.5 cm (the range at which our FLNA patient ruptured), and obviously more urgently if the aneurysm is growing rapidly. There is very little known about specific medical therapy that might modulate the course of aortic enlargement due to FLNA mutation, and specifically no published data about potential efficacy of atenolol or losartan, which have shown some efficacy in Marfan syndrome (Franken et al., 2015; Lacro et al., 2014; Sandor et al., 2015). Increased awareness of the natural history of FLNA mutations may allow identification of multi-center cohorts needed to perform such clinical studies. Given the catastrophic consequences of aortic rupture and the importance of early diagnosis of silent aortic aneurysms, DNA testing or MRI analysis of any first degree of affected individuals should be performed, and cardiac imaging is recommended.
Since early studies were mainly conducted to look for neurologic disease, the association of PVNH with aortic aneurysms and cardiac anomalies was not always systematically gathered. Since detailed cardiac history and serial cardiac screening was not always performed, even our reported frequency of TAA may well be an underestimation of the true frequency of SCM and TAA in PVNH patients. The majority of findings was derived from medical history, post-mortem examination, and not by comprehensive prospectively conducted serial cardiovascular screening, which would be the gold standard. Even in our cohort where we attempted to contact each patient, and recommended cardiac screening, some did not receive it. Despite these issues, the high frequency of TAA and the unexpected premature death of patients make greater awareness and management of FLNA and aortic aneurysms imperative.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to all the PVNH families who have contributed to our studies over the last 2 decades, and for all the physicians who have cared for these patients from many different disciplines, including geneticists, neurologists, cardiologists, internists, pediatricians and subspecialists. We appreciate Dr. Terry Robertson’s help in updating the information from his patient with TAA.
SOURCE OF FUNDING
This work was supported by the Manton Center for Orphan Disease Research, the American Heart Association (Association-Wide AHA Scientist Development Award #17SDG33410147 to Sangita Choudhury, PhD) and by the NINDS (RO1 NS35129). B.S.C. is supported by the NINDS (RO1 NS073601). C.A.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
DISCLOSURES
None
REFERENCES
- Chen MH, & Walsh CA (2015). FLNA-Related Periventricular Nodular Heterotopia. In Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, & Stephens K (Eds.), GeneReviews(R) Seattle (WA). [Google Scholar]
- Chubb H, & Simpson JM (2012). The use of Z-scores in paediatric cardiology. Ann Pediatr Cardiol, 5(2), 179–184. doi: 10.4103/0974-2069.99622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AR, Sawyer GM, Robertson SP, & Sutherland-Smith AJ (2009). Skeletal dysplasias due to filamin A mutations result from a gain-of-function mechanism distinct from allelic neurological disorders. Hum Mol Genet, 18(24), 4791–4800. doi: 10.1093/hmg/ddp442 [DOI] [PubMed] [Google Scholar]
- de Wit MC, de Coo IF, Lequin MH, Halley DJ, Roos-Hesselink JW, & Mancini GM (2011). Combined cardiological and neurological abnormalities due to filamin A gene mutation. Clin Res Cardiol, 100(1), 45–50. doi: 10.1007/s00392-010-0206-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit MC, Kros JM, Halley DJ, de Coo IF, Verdijk R, Jacobs BC, & Mancini GM (2009). Filamin A mutation, a common cause for periventricular heterotopia, aneurysms and cardiac defects. J Neurol Neurosurg Psychiatry, 80(4), 426–428. doi: 10.1136/jnnp.2008.149419 [DOI] [PubMed] [Google Scholar]
- Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, & Stossel TP (2011). Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature, 478(7368), 260–263. doi: 10.1038/nature10430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksioglu YZ, Scheffer IE, Cardenas P, Knoll J, DiMario F, Ramsby G, Berg M, Kamuro K, Berkovic SF, Duyk GM, Parisi J, Huttenlocher PR, & Walsh CA (1996). Periventricular heterotopia: an X-linked dominant epilepsy locus causing aberrant cerebral cortical development. Neuron, 16(1), 77–87. [DOI] [PubMed] [Google Scholar]
- Feldman DN, & Roman MJ (2006). Aneurysms of the sinuses of Valsalva. Cardiology, 106(2), 73–81. doi: 10.1159/000092635 [DOI] [PubMed] [Google Scholar]
- Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, & Walsh CA (2006). Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A, 103(52), 19836–19841. doi: 10.1073/pnas.0609628104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, & Walsh CA (2004). The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol, 6(11), 1034–1038. doi: 10.1038/ncb1104-1034 [DOI] [PubMed] [Google Scholar]
- Foley C, Roberts K, Tchrakian N, Morgan T, Fryer A, Robertson SP, & Tubridy N (2010). Expansion of the Spectrum of FLNA Mutations Associated with Melnick-Needles Syndrome. Mol Syndromol, 1(3), 121–126. doi: 10.1159/000320184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng Y, Graham DA, Scheffer IE, Dobyns WB, Hirsch BA, Radtke RA, Berkovic SF, Huttenlocher PR, & Walsh CA (1998). Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron, 21(6), 1315–1325. [DOI] [PubMed] [Google Scholar]
- Franken R, den Hartog A, Radonic T, Micha D, Maugeri A, van Dijk FS, Meijers-Heijboer HE, Timmermans J, Scholte AJ, van den Berg MP, Groenink M, Mulder BJ, Zwinderman AH, de Waard V, & Pals G (2015). Beneficial Outcome of Losartan Therapy Depends on Type of FBN1 Mutation in Marfan Syndrome. Circ Cardiovasc Genet. doi: 10.1161/CIRCGENETICS.114.000950 [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, Bolen MA, Connolly HM, Cuellar-Calabria H, Czerny M, Devereux RB, Erbel RA, Fattori R, Isselbacher EM, Lindsay JM, McCulloch M, Michelena HI, Nienaber CA, Oh JK, Pepi M, Taylor AJ, Weinsaft JW, Zamorano JL, Dietz H, Eagle K, Elefteriades J, Jondeau G, Rousseau H, & Schepens M (2015). Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr, 28(2), 119–182. doi: 10.1016/j.echo.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Guerrini R, Mei D, Sisodiya S, Sicca F, Harding B, Takahashi Y, Dorn T, Yoshida A, Campistol J, Kramer G, Moro F, Dobyns WB, & Parrini E (2004). Germline and mosaic mutations of FLN1 in men with periventricular heterotopia. Neurology, 63(1), 51–56. [DOI] [PubMed] [Google Scholar]
- Hart AW, Morgan JE, Schneider J, West K, McKie L, Bhattacharya S, Jackson IJ, & Cross SH (2006). Cardiac malformations and midline skeletal defects in mice lacking filamin A. Hum Mol Genet, 15(16), 2457–2467. doi: 10.1093/hmg/ddl168 [DOI] [PubMed] [Google Scholar]
- Hehr U, Hehr A, Uyanik G, Phelan E, Winkler J, & Reardon W (2006). A filamin A splice mutation resulting in a syndrome of facial dysmorphism, periventricular nodular heterotopia, and severe constipation reminiscent of cerebro-fronto-facial syndrome. J Med Genet, 43(6), 541–544. doi: 10.1136/jmg.2005.038505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isselbacher EM, Lino Cardenas CL, & Lindsay ME (2016). Hereditary Influence in Thoracic Aortic Aneurysm and Dissection. Circulation, 133(24), 2516–2528. doi: 10.1161/CIRCULATIONAHA.116.009762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakita A, Hayashi S, Moro F, Guerrini R, Ozawa T, Ono K, Kameyama S, Walsh CA, & Takahashi H (2002). Bilateral periventricular nodular heterotopia due to filamin 1 gene mutation: widespread glomeruloid microvascular anomaly and dysplastic cytoarchitecture in the cerebral cortex. Acta Neuropathol, 104(6), 649–657. doi: 10.1007/s00401-002-0594-9 [DOI] [PubMed] [Google Scholar]
- Kapur RP, Robertson SP, Hannibal MC, Finn LS, Morgan T, van Kogelenberg M, & Loren DJ (2010). Diffuse abnormal layering of small intestinal smooth muscle is present in patients with FLNA mutations and x-linked intestinal pseudo-obstruction. Am J Surg Pathol, 34(10), 1528–1543. doi: 10.1097/PAS.0b013e3181f0ae47 [DOI] [PubMed] [Google Scholar]
- Karimi A, & Milewicz DM (2016). Structure of the Elastin-Contractile Units in the Thoracic Aorta and How Genes That Cause Thoracic Aortic Aneurysms and Dissections Disrupt This Structure. Can J Cardiol, 32(1), 26–34. doi: 10.1016/j.cjca.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, Pearson GD, Selamet Tierney ES, Levine JC, Atz AM, Benson DW, Braverman AC, Chen S, De Backer J, Gelb BD, Grossfeld PD, Klein GL, Lai WW, Liou A, Loeys BL, Markham LW, Olson AK, Paridon SM, Pemberton VL, Pierpont ME, Pyeritz RE, Radojewski E, Roman MJ, Sharkey AM, Stylianou MP, Wechsler SB, Young LT, Mahony L, & Pediatric Heart Network, I. (2014). Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med, 371(22), 2061–2071. doi: 10.1056/NEJMoa1404731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WW, Mertens LL, Geva T, & Cohen MS (Eds.). (2009). Echocardiography in pediatric and congenital heart disease: from fetus to adult (1 ed.): Wiley-Blackwell. [Google Scholar]
- Lindsay ME, & Dietz HC (2014). The genetic basis of aortic aneurysm. Cold Spring Harb Perspect Med, 4(9), a015909. doi: 10.1101/cshperspect.a015909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, & Geva T (2010). Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr, 23(5), 465–495; quiz 576–467. doi: 10.1016/j.echo.2010.03.019 [DOI] [PubMed] [Google Scholar]
- Masurel-Paulet A, Haan E, Thompson EM, Goizet C, Thauvin-Robinet C, Tai A, Kennedy D, Smith G, Khong TY, Sole G, Guerineau E, Coupry I, Huet F, Robertson S, & Faivre L (2011). Lung disease associated with periventricular nodular heterotopia and an FLNA mutation. Eur J Med Genet, 54(1), 25–28. doi: 10.1016/j.ejmg.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, & Pannu H (2008). Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet, 9, 283–302. doi: 10.1146/annurev.genom.8.080706.092303 [DOI] [PubMed] [Google Scholar]
- Milewicz DM, & Regalado E (2016). Heritable Thoracic Aortic Disease Overview. In Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, & Stephens K (Eds.), GeneReviews(R) Seattle (WA). [Google Scholar]
- Nakamura F, Stossel TP, & Hartwig JH (2011). The filamins: organizers of cell structure and function. Cell Adh Migr, 5(2), 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema R, Hulst JM, Theuns-Valks SD, van Unen LM, Schot R, Mancini GM, Schipper ME, de Wit MC, Sibbles BJ, de Coo IF, Nanninga V, Hofstra RM, Halley DJ, & Brooks AS (2013). Novel no-stop FLNA mutation causes multi-organ involvement in males. Am J Med Genet A, 161(9), 2376–2384. doi: 10.1002/ajmg.a.36109 [DOI] [PubMed] [Google Scholar]
- Parrini E, Mei D, Wright M, Dorn T, & Guerrini R (2004). Mosaic mutations of the FLN1 gene cause a mild phenotype in patients with periventricular heterotopia. Neurogenetics, 5(3), 191–196. doi: 10.1007/s10048-004-0187-y [DOI] [PubMed] [Google Scholar]
- Razinia Z, Makela T, Ylanne J, & Calderwood DA (2012). Filamins in mechanosensing and signaling. Annu Rev Biophys, 41, 227–246. doi: 10.1146/annurev-biophys-050511-102252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado ES, Guo DC, Prakash S, Bensend TA, Flynn K, Estrera A, Safi H, Liang D, Hyland J, Child A, Arno G, Boileau C, Jondeau G, Braverman A, Moran R, Morisaki T, Morisaki H, Montalcino Aortic C, Pyeritz R, Coselli J, LeMaire S, & Milewicz DM (2015). Aortic Disease Presentation and Outcome Associated With ACTA2 Mutations. Circ Cardiovasc Genet, 8(3), 457–464. doi: 10.1161/CIRCGENETICS.114.000943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinstein E, Frentz S, Morgan T, Garcia-Minaur S, Leventer RJ, McGillivray G, Pariani M, van der Steen A, Pope M, Holder-Espinasse M, Scott R, Thompson EM, Robertson T, Coppin B, Siegel R, Bret Zurita M, Rodriguez JI, Morales C, Rodrigues Y, Arcas J, Saggar A, Horton M, Zackai E, Graham JM, Rimoin DL, & Robertson SP (2013). Vascular and connective tissue anomalies associated with X-linked periventricular heterotopia due to mutations in Filamin A. Eur J Hum Genet, 21(5), 494–502. doi: 10.1038/ejhg.2012.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinstein E, Morris SA, Rimoin DL, Robertson SP, & Lacro RV (2014). Arterial tortuosity in patients with Filamin A- associated vascular aneurysms. Am J Med Genet A, 164A(11), 2961–2963. doi: 10.1002/ajmg.a.36717 [DOI] [PubMed] [Google Scholar]
- Retailleau K, Arhatte M, Demolombe S, Jodar M, Baudrie V, Offermanns S, Feng Y, Patel A, Honore E, & Duprat F (2016). Smooth muscle filamin A is a major determinant of conduit artery structure and function at the adult stage. Pflugers Arch, 468(7), 1151–1160. doi: 10.1007/s00424-016-1813-x [DOI] [PubMed] [Google Scholar]
- Robertson SP, Jenkins ZA, Morgan T, Ades L, Aftimos S, Boute O, Fiskerstrand T, Garcia-Minaur S, Grix A, Green A, Der Kaloustian V, Lewkonia R, McInnes B, van Haelst MM, Mancini G, Illes T, Mortier G, Newbury-Ecob R, Nicholson L, Scott CI, Ochman K, Brozek I, Shears DJ, Superti-Furga A, Suri M, Whiteford M, Wilkie AO, & Krakow D (2006). Frontometaphyseal dysplasia: mutations in FLNA and phenotypic diversity. Am J Med Genet A, 140(16), 1726–1736. [DOI] [PubMed] [Google Scholar]
- Robertson SP, Twigg SR, Sutherland-Smith AJ, Biancalana V, Gorlin RJ, Horn D, Kenwrick SJ, Kim CA, Morava E, Newbury-Ecob R, Orstavik KH, Quarrell OW, Schwartz CE, Shears DJ, Suri M, Kendrick-Jones J, & Wilkie AO (2003). Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat Genet, 33(4), 487–491. doi: 10.1038/ng1119 [DOI] [PubMed] [Google Scholar]
- Sandor GG, Alghamdi MH, Raffin LA, Potts MT, Williams LD, Potts JE, Kiess M, & van Breemen C (2015). A randomized, double blind pilot study to assess the effects of losartan vs. atenolol on the biophysical properties of the aorta in patients with Marfan and Loeys-Dietz syndromes. Int J Cardiol, 179, 470–475. doi: 10.1016/j.ijcard.2014.11.082 [DOI] [PubMed] [Google Scholar]
- Sauls K, de Vlaming A, Harris BS, Williams K, Wessels A, Levine RA, Slaugenhaupt SA, Goodwin RL, Pavone LM, Merot J, Schott JJ, Le Tourneau T, Dix T, Jesinkey S, Feng Y, Walsh C, Zhou B, Baldwin S, Markwald RR, & Norris RA (2012). Developmental basis for filamin-A-associated myxomatous mitral valve disease. Cardiovasc Res, 96(1), 109–119. doi: 10.1093/cvr/cvs238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen VL, Dixon PH, Fox JW, Hong SE, Kinton L, Sisodiya SM, Duncan JS, Dubeau F, Scheffer IE, Schachter SC, Wilner A, Henchy R, Crino P, Kamuro K, DiMario F, Berg M, Kuzniecky R, Cole AJ, Bromfield E, Biber M, Schomer D, Wheless J, Silver K, Mochida GH, Berkovic SF, Andermann F, Andermann E, Dobyns WB, Wood NW, & Walsh CA (2001). Mutations in the X-linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum Mol Genet, 10(17), 1775–1783. [DOI] [PubMed] [Google Scholar]
- Sheen VL, Feng Y, Graham D, Takafuta T, Shapiro SS, & Walsh CA (2002). Filamin A and Filamin B are co-expressed within neurons during periods of neuronal migration and can physically interact. Hum Mol Genet, 11(23), 2845–2854. [DOI] [PubMed] [Google Scholar]
- Sheen VL, Jansen A, Chen MH, Parrini E, Morgan T, Ravenscroft R, Ganesh V, Underwood T, Wiley J, Leventer R, Vaid RR, Ruiz DE, Hutchins GM, Menasha J, Willner J, Geng Y, Gripp KW, Nicholson L, Berry-Kravis E, Bodell A, Apse K, Hill RS, Dubeau F, Andermann F, Barkovich J, Andermann E, Shugart YY, Thomas P, Viri M, Veggiotti P, Robertson S, Guerrini R, & Walsh CA (2005). Filamin A mutations cause periventricular heterotopia with Ehlers-Danlos syndrome. Neurology, 64(2), 254–262. doi: 10.1212/01.wnl.0000149512.79621.df [DOI] [PubMed] [Google Scholar]
- Sherif HM (2014). Heterogeneity in the Segmental Development of the Aortic Tree: Impact on Management of Genetically Triggered Aortic Aneurysms. Aorta (Stamford), 2(5), 186–195. doi: 10.12945/j.aorta.2014.14-032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole G, Coupry I, Rooryck C, Guerineau E, Martins F, Deves S, Hubert C, Souakri N, Boute O, Marchal C, Faivre L, Landre E, Debruxelles S, Dieux-Coeslier A, Boulay C, Chassagnon S, Michel V, Routon MC, Toutain A, Philip N, Lacombe D, Villard L, Arveiler B, & Goizet C (2009). Bilateral periventricular nodular heterotopia in France: frequency of mutations in FLNA, phenotypic heterogeneity and spectrum of mutations. J Neurol Neurosurg Psychiatry, 80(12), 1394–1398. doi: 10.1136/jnnp.2008.162263 [DOI] [PubMed] [Google Scholar]
- Srour M, Rioux MF, Varga C, Lortie A, Major P, Robitaille Y, Decarie JC, Michaud J, & Carmant L (2011). The clinical spectrum of nodular heterotopias in children: report of 31 patients. Epilepsia, 52(4), 728–737. doi: 10.1111/j.1528-1167.2010.02975.x [DOI] [PubMed] [Google Scholar]
- Sun Y, Almomani R, Aten E, Celli J, van der Heijden J, Venselaar H, Robertson SP, Baroncini A, Franco B, Basel-Vanagaite L, Horii E, Drut R, Ariyurek Y, den Dunnen JT, & Breuning MH (2010). Terminal osseous dysplasia is caused by a single recurrent mutation in the FLNA gene. Am J Hum Genet, 87(1), 146–153. doi:S0002–9297(10)00311–3 [pii] 10.1016/j.ajhg.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Sasaki N, Matsuura Y, & Uemura R (1969). Surgical correction of aneurysm of the sinus of Valsalva. A report of forty-five consecutive patients including eight with total replacement of the aortic valve. Am J Cardiol, 23(2), 180–191. [DOI] [PubMed] [Google Scholar]
- Wang K, Ash JF, & Singer SJ (1975). Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A, 72(11), 4483–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich M, Yu PJ, & Trost B (2015). Sinus of valsalva aneurysms: review of the literature and an update on management. Clin Cardiol, 38(3), 185–189. doi: 10.1002/clc.22359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen MH, Wu X, Kodani A, Fan J, Doan R, Ozawa M, Ma J, Yoshida N, Reiter JF, Black DL, Kharchenko PV, Sharp PA, & Walsh CA (2016). Cell-Type-Specific Alternative Splicing Governs Cell Fate in the Developing Cerebral Cortex. Cell, 166(5), 1147–1162 e1115. doi: 10.1016/j.cell.2016.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Boren J, & Akyurek LM (2007). Filamins in cardiovascular development. Trends Cardiovasc Med, 17(7), 222–229. doi: 10.1016/j.tcm.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, & Jeunemaitre X (2006). Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet, 38(3), 343–349. doi: 10.1038/ng1721 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.