Abstract
Infections, contaminations, and biofouling resulted from micro- and/or macro- organisms remained a prominent threat to the public health, food industry, and aqua/marine-related applications. Considering environmental and drug resistance concerns as well as insufficient efficacy on biofilms associated with conventional disinfecting reagents, developing an antimicrobial surface potentially improved the antimicrobial performance by directly working on the microbes surrounding the surface area. Here we provide an engineering perspective on the logic of choosing materials and strategies for designing antimicrobial surfaces, as well as an application perspective on their potential impacts. In particular, we analyze and discuss requirements and expectations for specific applications and provide insights on potential misconnection between the antimicrobial solution and its targeted applications. Given the high translational barrier for antimicrobial surfaces, future research would benefit from a comprehensive understanding of working mechanisms for potential materials/strategies, and challenges/requirements for a targeted application.
Keywords: Antimicrobial, biocide, interface, coating, microbe-resistance
Graphical Abstract
Introduction
The adhesion of microbes on surfaces has caused prominent health, environmental, and societal issues. Significant effort and investment have been made to deal with the contamination/fouling of micro- or macro- organisms on indwelling medical devices1, food processing industry2, ship hulls and marine devices3, etc. Take the threat to public health, for example, an annual of 1.7 million people suffered from healthcare-associated infections (HCAI) in the USA, and about 90, 000 died due to the infection each year.4
Conventional ways to disinfect (kill or inhibit) microorganisms involve antimicrobial reagents, including antibiotics, fungicides, antiviral drugs, as well as a wide selection of non-pharmaceutical chemicals. Extensive use of these reagents caused the concern of environmental pollution and potential microbial drug resistance.5 Furthermore, once microbes were attached and concentrated on a surface, a notorious biofilm would be developed. Conventional antimicrobial agents such as antibiotics were known for treating planktonic microbe infection but became insufficient to inhibit or eliminate a biofilm.6 Part of the reasons have been attributed to the biofilm matrix which provides a protective diffusion barrier for antimicrobial reagents.7–8 The applied reagents, typically in solution form, also have limitations in achieving high and durable local concentrations on the surface, preventing an effective treatment of biofilm.9–10
For most of the application scenarios (e.g., implanted medical devices, food packaging, vessels submerged into fresh or saltwater, etc.), the infection, biofouling, and/or biofilm formation typically happened on the substrate surface interfacing with the microbe environment. Compared to conventional bulk disinfection methods, engineering an antimicrobial surface in these cases potentially made more sense by directly treating the incoming and/or colonized microbes surrounding the surface area, resulting in an improved antimicrobial efficacy.8, 10
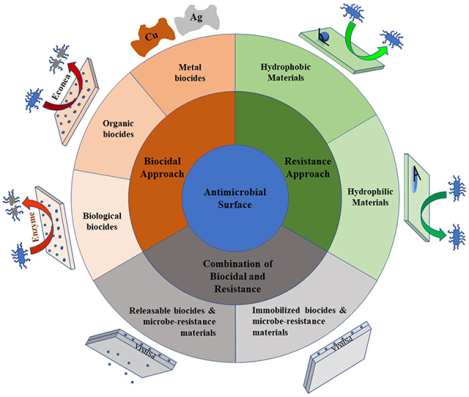
There are multiple strategies to construct an antimicrobial surface, including the approach integrating biocides onto the surface (microbicidal surface)11–12, integrating materials showing microbe resistance properties (microbe resistance surface)13–14, and potential combination of the two approach15–16 (Figure 1). Prior reviews have focused on antimicrobial materials, their functioning mechanisms, and test results.17–20 As a deviation from previous reviews, we provide an engineering perspective on the logic of choosing materials and strategies for designing antimicrobial surfaces, as well as an application perspective on their potential impacts (e.g., environmental, longevity/durability, capability to extend use). Majority discussions involve examples, either from the research field or industry, within the past five years. When possible, we provide insight on potential misconnection between a particular antimicrobial surface and its potential targeted applications, and desired properties/performance from the application standpoint. These perspectives are crucial given the high translational barrier in this field.
Figure 1.
Design strategies for antimicrobial surface.
Surface Microbicidal Approach
The most commonly used antimicrobial approach is by incorporating materials/reagents with biocidal properties onto a surface and performing antimicrobial functions through releasing biocide21–23 or contact killing24–26. An immediate convenience of this strategy is that a wide selection of biocides is available; many of them might have been approved by regulatory agencies and are ready to be formulated for specific applications.
For a typical biocides releasing process, biocide was released from the coating layer into the solid/liquid interface and the liquid phase and formed a toxic atmosphere with biocide concentration decreasing from the interference to the environment.7 Locally, the surface biocide concentration can easily exceed the minimal inhibitory concentration (MIC) and the biocide can effectively kill the approaching microbes and prevent their contamination on the surface.10 A major concern with this approach is that the biocide released pollute the environment, such as water, to some extent.27–28 This may or may not be an issue depending on the type of the biocides and the local regulatory standards. Additionally, once the biocide is consumed, the surface loses the antimicrobial property.29 The functioning duration of an antimicrobial surface is one of the key application-specific factors. This may require re-prepare the surface for biocide reloading, such as a paint removal and re-painting process.
For a contact killing process, biocide was typically covalently or firmly immobilized on the top surface and killed the microbes attempting to adsorb on the surface. Compared with the biocide releasing process, contact killing has limited biocide leakage to the environment and only functions when the microorganisms contact to the surface.29 Common bactericidal mechanism is through physical damage, such as by destroying the bacterial membranes.9, 30 The benefits of this approach are that no or limited toxic compound being released, which is environmentally friendly.31 Besides, compared to the biocide releasing strategy, contact killing, in theory, can function for a relatively longer time, assuming that the surface can be reactivated after the killed microbes are removed.10 Nevertheless, surface absorption by contaminators such as bacterial fragments and/or inorganic or organic pollutants can disable the antimicrobial capability of these surface-immobilized biocides; This typically resulted in fouling gradually.32–34
Different from the traditional classification based on antimicrobial mechanisms29–30, below we classified some commonly used biocidal reagents based on their material types, including metal derivatives21, 35, 36, organic biocides11–12, 22, 37, and biological biocides34, 38 (this is for the convenience of biocide selections for a particular application scenario), and discussed their characters/functional mechanisms, reagent-stability, and pros and cons in terms of multiple aspects closely relating to their applications.
Metal Biocides
Metal-based biocides are among the most popular for a broad range of applications, including industrial, agricultural, marine, residential, and medical-related, and can be either deposited39–40 or adsorbed41–42 on a substrate. Copper oxidants43–45, for example, are the main active ingredients in an antifouling marine coating,46 which is nowadays classified as self-polished coating (SPC)47. The copper-based biocide was mixed with a base paint and deposited/applied on the substrate through a curing process. The paint includes polymer matrix providing mechanical support for the coating and the biocide contained is responsible for the antimicrobial or antifouling functions. The antimicrobial SPC, in general, has good durability despite the top surface layer continuously being polished/removed. The duration for copper-based biocide to function is determined by the amount of biocide trapped, the type of coating matrix, self-polishing rate, and the coating thickness (e.g., several layers of paint). In practice, the commercial copper-based SPC can function from months to years, which was subjected to variations caused by the longevity of coated vessels in water and the surrounding water conditions (temperature, salinity, etc.). Nevertheless, once the biocide is consumed, there is no way for reloading, and the remaining polymer coating has to be removed, followed by re-prepare the substrate for a new paint.
Before the copper oxidants were adopted for marine antifouling coating, tin-based biocide, such as organic tin complexes (Tributyltin, TBT), was the gold standard performing superior antifouling functions that can hardly be achieved even by any current antifouling paints, including those copper based48–49. Nevertheless, tin-based biocide was banned by the International Maritime Organization for biocidal surface applications in 2003 because it was very toxic to the shellfish and the toxicity can be transferred and enriched to other marine organisms even to human beings.48, 50–51 The copper biocide has a safer profile than tin biocide, but toxic concern for marine environment remains, with regulation coming into place for certain vessel coating applications in California and Washington State of the US.52
Comparatively, the silver-based biocide has an efficient antimicrobial property meanwhile with a relatively safer profile in living bodies.35,36 A typical silver-based coating can be obtained through surface reduction process particularly for a small scale surface53. For large scale surfaces, they can be coated with silver nanoparticles (Ag NPs) based coatings54–55 which is a more cost-effective option. Nanoparticle coatings had an increased specific surface area and further improved the antibacterial efficiency of silver54. It should be noted that silver-based antimicrobial performance has been widely documented, but limited results available for long-term performance.35,15 Theoretically, the Ag NPs were trapped by the 3D structure of a coating matrix (such as polymer/gel-based)56 and oxidized to Ag+ very slowly57. But without a chemical linkage58, the retention of the Ag NPs within the coating matrix could be weak, limiting their antimicrobial performance for a long term. Generally, the silver-based antimicrobial materials, under certain lab-test conditions, can function for several days59–60, and with proper encapsulation for months.61–62 Caution should be made that real application condition is still needed to evaluate the time frame of the release of biocides and its long-term performance.
With limited types of metal biocides available, current research in this area focused more on improving efficacy, such as through nano-scale preparation, and on controllable release, such as by tuning the adsorption to/retention by the substrate and/or through encapsulation. On the list of metal biocides, Zn and Ag are under more intensive study due to the lower toxicity compared to traditional Cu and Sn.
Organic Biocides
Organic biocides are small molecules or macromolecules exhibit antimicrobial properties. Different from the biological biocides, the organic biocides are mostly designed artificially and produced by the chemical industry in large scales. There are a lot more choices of organic biocides compared with metal biocides, including small biocidal molecules such as antibiotics37,63 and triclosan11, econea64–65 and high molecular weight polymers such as chitosans66–67, N-halamine polymers68, sulfonium salts69. The organic biocides could be encapsulated by polymers37, 70, or directly immobilized to surfaces11–12.
In a typical encapsulation strategy, organic biocides could be directly mixed with the coating matrix materials before curing71, or nano/micrometer-sized capsules could be formed with the biocide encapsulated before formulated into the coating matrix or immobilized onto the substrate.71–72 The biocide release could be a purely diffusion dependent process which could be further determined by hydrophobicity/hydrophilicity and molecular weight of the biocide and potential interaction with the polymer matrix. Or the release could be controlled through potential degradation of the matrix polymers/capsules73–74 (4,5-Dichloro-2-n-octyl-4-isothiazoline-3-one in polysaccharide reported to several days75; 3-iodo-2propynyl butyl carbamate in polymethylmethacrylate (PMMA) reported to last for 15 years74). A potential benefit of organic biocides is their release to the environment could be considered “safe” given that they are degraded into non-toxic products. Econea is one of them which strongly resists barnacles meanwhile is quickly degraded in sea water65, and has been extensively used in many of current marine antifouling paint formulations. Nevertheless, as a general issue to any biocide releasing approach, organic biocides released can be hard to be recharged, resulting in a loss of surface antimicrobial functions over time. It should be noted that under certain conditions, the loss of antimicrobial/antifouling functionalities might happen suddenly without warning, a potential combinatory effect of biocide release and degradation, which might cause unexpected troubles.
When organic biocides are immobilized onto the surface, they perform contact killing functions.11–12, 76 The immobilization could be achieved by grafting polymer chains onto a substrate followed by anchoring the organic biocidal molecules to the terminal functional groups of the polymer through chemical reactions.12, 77 A covalent linkage between the biocide and the polymer base is preferred to strengthen the immobilization. This leads to no/minimum release of biocide to the environment31, and a working antimicrobial surface for a relatively long period78. Potential degradation mechanism for the organic biocides should be considered when designing the contact killing surface, which might limit the longevity of the antimicrobial performance. Potential contamination/absorption of bacterial cells or fragments on the biocide surface should also be considered since they compromised the immediate contact between the biocide and live microbes, deteriorating the bactericidal functions over time.15, 19, 79–80
Cationic polymers, such as the chitosan66–67 and poly quaternary ammonium salt81–82 are well known antimicrobial materials working though the contact killing mechanism.9, 30 The polymer chain appeared to penetrate the cell wall of the bacteria, leading to a cytoplasmic leakage and killing the cells.83–84 Length and density of the cationic polymer chains are considered critical parameters of modulating the penetration and thus the biocidal capability. For example, it was found that cationic polymer of 2-(dimethylamino)ethyl methacrylate (pDMAEMA) exhibited the highest antibacterial efficacy with greater than 5×1015 charges/cm2.25 Besides, the longer chain length (> 20 nm) of the cationic polymers improved the efficiency of killing the E. coli up to 20 folds compared to the polymer layer as thin as 10 nm.25, 85 Cationic polymers would not induce drug resistance through known mechanisms, presumably there is no similar structure in nature.85 Nevertheless, the cationic polymers had the same problem of non-specific contamination/absorption as other immobilized biocides, gradually losing their antimicrobial performance due to the coverage of bacteria or microbe fragments.
Biological Biocides
Biological biocides are antimicrobial compounds obtained from living bodies and/or produced through biological engineering methods, mainly including enzymes34, 86–87, antimicrobial peptides38, 88–89, bacteriophages90–92, etc. Consider the molecular size and relatively high cost compared to metal and organic biocides, biological biocides were majorly immobilized on surfaces and functioned through contact killing.
Biocidal enzymes and peptides can be covalently anchored to the terminal of a polymer brush, which was grafted on the substrate through various surface reactions.93–94 Durable immobilization could be obtained, and so is the antimicrobial activity; a covalently linked antimicrobial peptide on surfaces was able to maintain sufficient biocidal capability for up to 6 months as published.95 The durability of the immobilization can be further improved by enhancing the linkage between substrate and spacer polymers. Similar to the biocidal mechanism for certain organic biocides, antimicrobial enzymes, and peptides functioned by non-specifically destroying the cytomembrane, which led to cellular content leakage.34 The stability and performance of enzymes were restricted by environmental factors including temperature96, pH97, ultraviolet radiation98, etc. Lysozyme is known as more stable among traditional enzymes99, but still has limited applicability in a complex environment such as the less stable in warm marine area100. Again, simple modification of biological biocides cannot resist the adhesion of the protein and/or dead microbial debris, which compromised the biocidal efficiency over time.34, 101
Bacteriophages have been introduced as a biocide and immobilized onto surfaces in more recent years.90–92, 102 They were immobilized though physisorption103, electrostatic interactions104 and covalent bonds92, and functioned as a virus by infecting the microbes through surface contact. During the immobilization, the orientation of the bacteriophages has been considered; for example to ensure their tails pointing away from the surface to improve their capability of infecting the microorganisms,90,102 and this is because the bacteriophages inject their genetic material through the tails into the cytoplasm of the host to infect the microorganism105. The naturally occurred bacteriophages are environmentally friendly and can perform efficient biocidal functions for months.91 Nevertheless, they are non-rechargeable after the infectious genetic materials within the phages are consumed. Compared with other organic and biological biocides (e.g., cationic polymers and enzymes), bacteriophages infected and fought very specific microbes as they contacted the surface. It is expected that bacterial resistance of the phages would be developed and an equilibrium between the phage/host-microbe populations would be established.102
Surface Microbial Resistance Approach
In addition to the surface biocidal strategy, surface can be engineered to resist the microbial adhesion/adsorption/accumulation.29–30 Microbe adhesion is considered as the critical step of colonization, invasion and biofilm formation, so the prevention of adhesion can reduce the bacterial virulence and resist the contamination efficiently.106 Unlike biocidal materials, materials performing anti-adhesion or resisting function do not damage the bacteria or reduce their numbers, but resist their attachment by reducing the adhesive force,29, 107–108 or enable ease of removal of the loosely attached microbes such as through water shearing or physical turbulence.109 Because this is a passive strategy involving no biocide, the surface resistance approach is considered safe, environmentally friendly, and without drug resistance concerns.29, 110 Because of the non-leaching and non-specific resisting properties, this approach potentially provides a long period of microbial/fouling resistance.13, 111–112 Certain low-surface-energy (hydrophobic)113–114 and hydrophilic13, 115 materials have been used as microbial resistance surface and were discussed below.
Low-surface-energy (Hydrophobic) Materials
Theoretically, a low-surface-energy material coated surface would resist the adhesion and spreading of high-surface-energy biofoulers such as proteins, cells, and bacteria since the potential coverage of which would further increase the surface energy of the coated substrate- an unfavored process according to Gibbs law of free energy.116 Fluorocarbon and silicone-based materials are the most widely used low-surface energy materials. They are among the few materials showing surface free energy below 30 mN/m (e.g., polytetrafluoroethylene PTFE (Teflon™): 20 mN/m117; polydimethylsiloxane (PDMS): 19.8 mN/m118). In reality, the biofoulers did adhere to the low surface energy materials, but could be relatively easier to remove compared with regular material surfaces. For example, barnacle, one of the most notable marine fouler, has drastically reduced adhesion force on PDMS (~0.05 MPa119; 0.069 MPa120) as compared to a regular plastic substrate PMMA (0.5 MPa119). There is a number of ways to explain the rationale for the ease of removal or fouling release. For the PTFE surface, because of its hydrophobic nature, protein tends to approach to the surface through its hydrophobic region through hydrophobic-hydrophobic interactions.121 However, the unique lipophobic nature of PTFE122 vs. the lipophilic nature of the protein hydrophobic domain weakened such adhesion force123. For the PDMS surface, there is a significant mobility of the molecular chains accounting for a higher level of surface entropy. Potential protein absorption tends to restrict the chain movement, resulting in an unfavored status of decreased surface entropy. Fluorocarbon coat such as PTFE was typically applied through a spraying or electrostatic coating followed by thermal baking/curing124–126, a process suitable for small to medium-sized substrates (larger size is tough). Silicone-based materials have been formulated into a series of commercial paints such as those for marine fouling-release purposes and could be conveniently coated on substrates of any size through conventional painting or rolling methods. Compared to biocide based antifouling paints, silicone-based marine fouling-release paints show the advantage of environmental friendly and relative ease of cleaning, however, their market share was quite small due to a general lower antifouling performance (e.g., fouling-release coating may require a minimum of 15 knots sailing speed for at least 70% of time in water).127
As a common way to improve the low-surface-energy/hydrophobic properties, the surface morphologies or roughness could be further modified such as on PTFE and PDMS surfaces. Inspired by the natural superhydrophobic phenomenon of the lotus leaves128, nano-scale morphology has been produced on PTFE surfaces and was found to improve the fouling-release performance (e.g., more than 99% S. aureus was easily removed by flowing water).124 A porous surface based on a ZnO nano framework combined with a PDMS matrix has also been developed to achieve superhydrophobicity (contact angle increased to over 150°).129 The increased surface roughness and potential air entrapment by the 3-D morphology reduced the contact area between the microbe and the surface, further decreasing the adhesive force in-between. A common pitfall with complex surface morphology (e.g., micro/nano roughness feature) is their subjective to mechanical damage, impacting the durability and performance of use.113 Compared to PTFE, PDMS has a relatively higher resistance to surface abrasions or damage due to its elastic nature129. A desired mechanical property can also be obtained through a composite material strategy. Additional pitfall includes a gradual entrapment of fouling debris onto the rough surface, particularly the valley region, and a gradual loss of the entrapped air, leading to deteriorated fouling release performance. Furthermore, translating the complex surface morphology to real-world substrates could be challenging with potential scaling-up, inconvenience, and high cost issues.
When a porous low-surface-energy surface (e.g., wrinkled PTFE) was infused with low-surface-energy liquid (e.g., silicone oil), a surface called SLIPS (slippery porous lubricant-infused surfaces) was obtained showing superhydrophobicity with water contact angle >160°.130 High resistance to bacterial adhesion (Pseudoalteromonas spp.) up to 99% was achieved within 24 h incubation. Better marine antifouling performance (inhibiting algal attachment) was observed with SLIPS compared with un-infused control when tested in the waters of Sydney Harbor for over 7 weeks. Despite reasonable retention by the porous surface due to similar polarities, the silicone oil was subject to gradual leakage through diffusion and shear stress of the water, leading to the loss of antifouling performance over time.130 Silicone oil can be recharged into the surface, but their retention shall be further improved given that oil discharge could be an environmental concern and a demand for the longevity of a coating product, particularly in the marine antifouling market.
Hydrophilic Materials
Different from low-surface-energy (superhydrophobic) strategy, certain hydrophilic polymers resist the microbial adhesion because of their strong hydration capability.30 The tightly bound water layer around these materials shows strong repulsive hydration forces to resist protein adsorption, as well as cells/bacteria adhesion.29–30, 131 Note that these hydrophilic materials here would resist the fouling in the first place, different from low-surface-energy materials that do encourage fouling but allowing the ease of fouling release/removal. Typical hydrophilic/water-soluble polymers would have structural characteristics of non-ionic or zwitterionic, which enables hydrogen bonding with water or ionic solvation, respectively.131 Among them, nonionic polyethylene glycol (PEG) and zwitterionic polymers are the most well-known for surface coatings achieving superior microbial resistance.3, 112, 132
PEG is one of the most popular hydrophilic polymers for a broad range of applications; it has been FDA-approved to formulate drugs for both oral and injective administrations indicating an excellent safety profile.133–134 There is abundant oxygen within the -CH2CH2O- repeating unit of PEG to form a large number of hydrogen bonds with water molecules. This afforded a strongly hydrated surface once a PEG coating was formed, and was responsible for its high resistance of more than 99% of bacteria from nonspecific adhesion.14 The microbial-resistance property could be optimized with chain length and coating density132, and overall a flexible PEG chain and high surface coverage are preferred to achieve a maximized surface entropy and minimized coating defect. One main challenge unique to PEG coatings is their subjective to oxidative damage, prohibiting their long-term applicability.135 In addition, known anti-PEG antibodies have been/could be developed within certain group of people, which may or may not jeopardize their applications inside the body.136–137
Zwitterionic polymers are characterized as balanced positively and negatively charged groups within the same repeating units. Unlike nonionic polymers such as PEG typically able to dissolve in both water and organic solvents and showing both hydrophilic and hydrophobic (amphiphilic) properties, zwitterionic polymers may only be dissolvable in water (e.g., certain carboxybetaine polymers) and show a superhydrophilic property.131 Different from nonionic hydrophilic polymers forming hydration layer through hydrogen bonds, zwitterionic polymers provide strong hydration through ionic solvation resulted from plenty of negative and positive charges within the material.131 Since the net charge of zwitterionic polymer is nearly zero with opposite charges homogeneously distributed, the material itself would not preferably interact with charged proteins or microbes through electrostatic interactions. A number of zwitterionic polymer surfaces prepared from 2-methacryloyloxylethyl phosphorylcholine (MPC)138–139, sulfobetaine methacrylate (SBMA)115, 140, and carboxybetaine methacrylate (CBMA)13, 141 showed high antifouling performance in resisting proteins/cells/bacteria attachment. Certain report indicated that zwitterionic PCBMA coating can reduce the formation of P. aeruginosa biofilm by 95% and maintained such high performance for 10 days, which might function superior over other reported PEG coatings that can only delay biofilm formation for one day.110 A potential limitation unique to zwitterionic polymers is their solubility issue (only dissolvable in water for certain types of polymers), which might complicate potential reaction with hydrophobic materials such as when forming an amphiphilic diblock copolymer for potential coating applications.131, 142–143 Yet a zwitterionic polymer coatings can still be applied on many substrates through surface-initiated polymerization (graft-from method)144, and covalent linkage through terminal functional groups (graft-to method)145.
A notable drawback for any hydrophilic coatings is their low durability in an aqueous environment; they drastically tend to dissolve in water, together with insufficient coating immobilization, resulting in a gradual loss of the coating from the substrate.13, 141, 146 The material loss may further pose a safety concern when the coating was applied in a medical environment where they are contacting with circulating blood. For these reasons, it was rarely found any antifouling hydrophilic coatings in the market such as in medical device fields and marine coating fields. From the perspective of resisting microbe adhesion and biofilm formation, as long as the hydrophilic coating shows high antifouling performance and maintains their coating integrity on a substrate, theoretically, a biofilm should never form. Nevertheless, majority hydrophilic coatings reported to date showed a delay of biofilm formation for days but failed to resist the biofilm for a long term, which could be partly explained by the durability challenge.141 Recently, super glue has been used to immobilize zwitterionic gel network onto a variety of common substrates and the resulting zwitterionic hydrogel coating showed unusually high durability under aqueous, shearing, and mechanical impacting conditions, and remained the high antifouling performance for 3 months.13 Figure 2 shows the performance of this coating in resisting different microbes from adhesion and biofilm formation (nearly “zero” attachment), showing high potential to fully address the biofilm issue.
Figure 2.
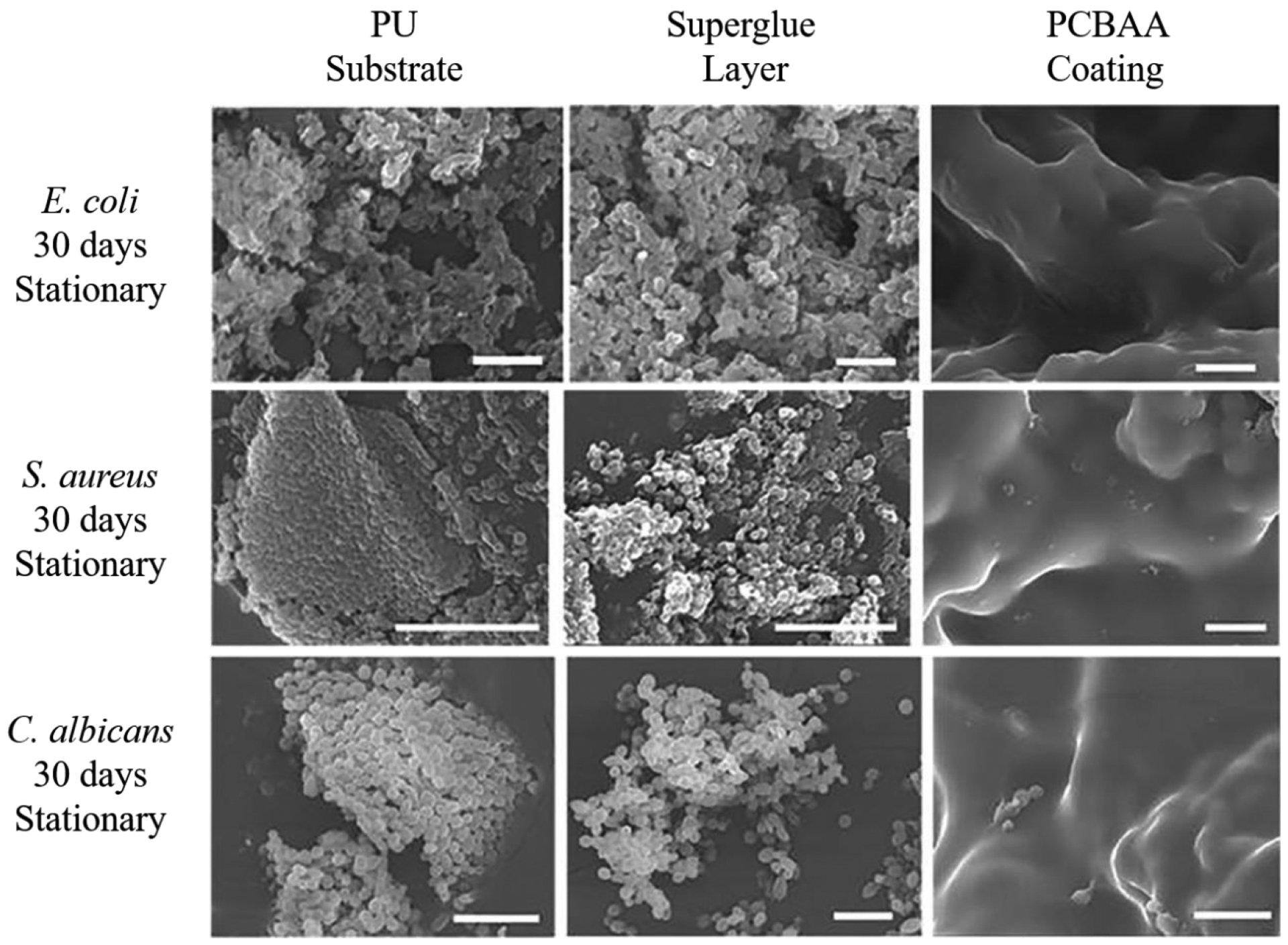
Surface microbial resistance of superdurable PCBAA (poly-3-((3-acrylamidopropyl) dimethylammonio)propanoate)) coating for long term performance.13 The SEM images exhibited the microbial adhesion on the PU (polyurethane), superglue and PCBAA coating surface respectively after 30 days of stationary with different types of microbes. Reproduced with permission from ref 13. Copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Combination of Microbicidal and Resistance Approach
As discussed above, surface microbicidal approach and surface microbe resistance approach involve different technical, environmental, and functioning features, which further guide their applications in suitable scenarios. One can think of the potential combination of the two strategies to achieve maximum antimicrobial performance meanwhile balancing the pros and cons of each of the strategies.30, 33, 80. Here we discuss the possibility of combining these two approaches without compromising the performance for each, note that a bold combination may lead to unexpected results. Despite the potential improvement in antimicrobial efficacy, the combination approach will most likely increase the complexity of coating development and implementation, which requires additional justification.
Releasable Biocides Plus Microbe-resistance Materials
When a releasable biocidal material was combined with microbe-resistance material (either fouling resistance or fouling release type), there is a high chance that the released biocide plays the role of first defense by killing majority planktonic microbes (since the biocide can leave the surface) and the microbe-resistant material serves the second defense by preventing the attachment of dead microbes, a small portion of live microbes escaped from the biocide-killing, or other contaminators, or by allowing the ease of removal of the fouling attached. When the releasing rate for the biocide is slow, which is preferred to improve the longevity of microbicidal approach (e.g., as seen in commercial copper-based SPC), a fouling-resistant material would function as the first defense by resisting majority planktonic microbes from absorption and the releasable biocide serves the second defense and kills the microbes ever adhere to the surface. For a fouling-release material, it may function simultaneously with the releasable biocide while the microbes are attached on the surface.
Since releasable biocides are typically small molecules and ultimately would leave the surface, their interference with microbe-resistance materials is minimum, assuming no other materials competing the presence on surfaces with microbe-resistance materials. Thus, this combination perhaps represents the best coordination between the two strategies without sacrificing much of each of the biocidal/resistance functions. This combination also shows a potential advantage to continue performing the antimicrobial function, by resisting and/or enabling easy cleaning, after the releasing biocides were consumed up. Figure 3 shows one example of this combination147 among many others148, where Ag NPs were deposited within a zwitterionic PSBAA or PSBMA polymer coating. This combined approach showed a resistance of over 95% of E. coli from attaching to the surface, meanwhile killed up to 98% bacteria that attached.
Figure 3.
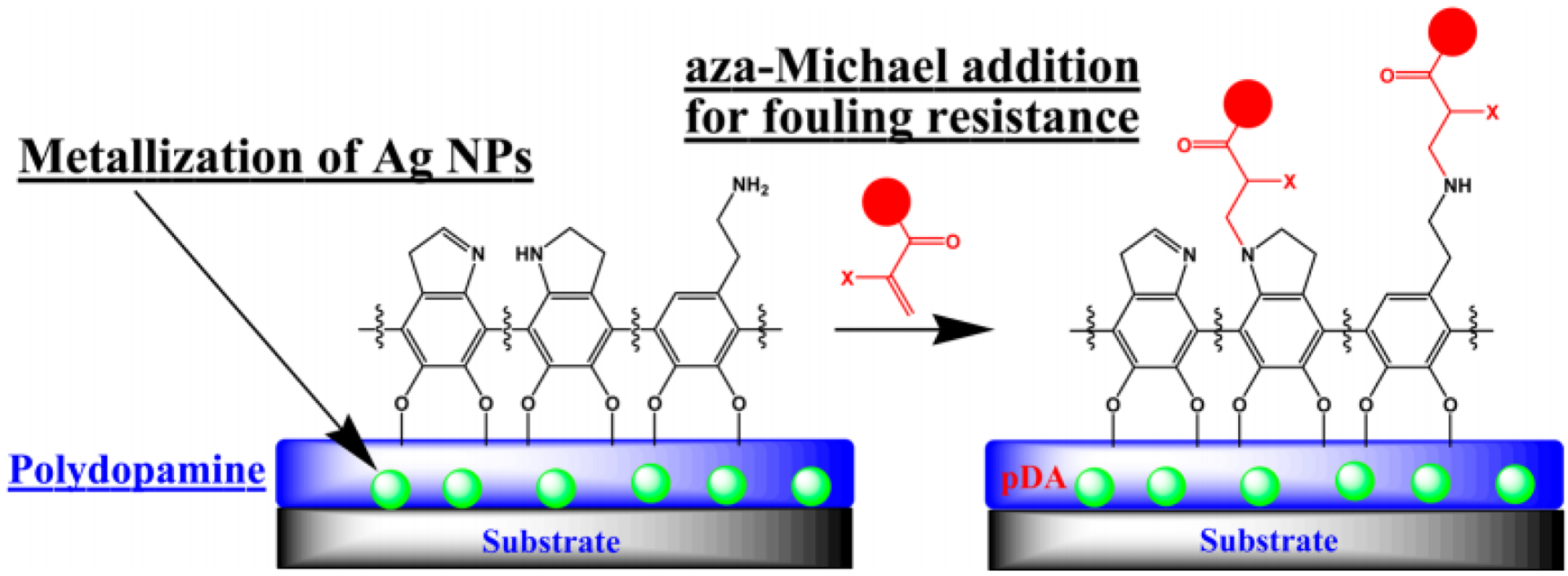
Releasable biocides within microbe-resistance surface for coordinated antimicrobial functions.147 The Ag NPs (green balls) were deposited in the pDA layer (blue) in situ as the releasable biocide before the pDA surface was further functionalized and linked covalently to antifouling materials such as the SBAA, SBMA or PEGMA (red) to provide the microbe-resistance functions. Reproduced with permission from ref 147. Copyright 2016, American Chemical Society.
The releasable biocides could be recharged under certain conditions, e.g., Ag NPs can be repeatedly loaded into a microbe-resistant hydrogel to sustain the antimicrobial functions148. These types of rechargeable surfaces might find applications for short-term usage per recharging cycle, since an effective recharging requires high diffusion property of the coating matrix, which in turn results in a faster release of the biocide out of the matrix. For long-term usage, the biocide was much stronger retained by the coating matrix; recharging the biocide would be difficult and the coating would need to be re-applied to sustain the microbicidal functions.
Immobilized Biocides Plus Microbe-resistance Materials
When an immobilized biocidal material was combined with microbe-resistance material (either fouling resistance or fouling release type) to form a surface coating, they would compete on their interaction with an approaching or an attached microbe. Depending on physical and chemical characters for these two materials (chain length, size, hydrophilicity/hydrophobicity), their spatial-temporal presence on the surface might be different, and this may or may not cause a compromise of each of the biocidal/resistance functions.89, 149–150 For example, biocidal materials tend to interact with microbes while fouling resistance materials tend to repel. The presence of both on surface leads to the respective compromise of their functions. However, when biocidal materials and fouling release materials are co-presented on the surface, the compromise could be minimum since both tend to interact with microbes to some extent. Additionally, when the two types of materials are not on the same surface levels, such as the example shown in Figure 4, where zwitterionic PSBMA (fouling-resistance material) located at the outer surface and N-halamine (immobilized biocide) presented closer to the substrate149, the compromise could also be minimum.
Figure 4.
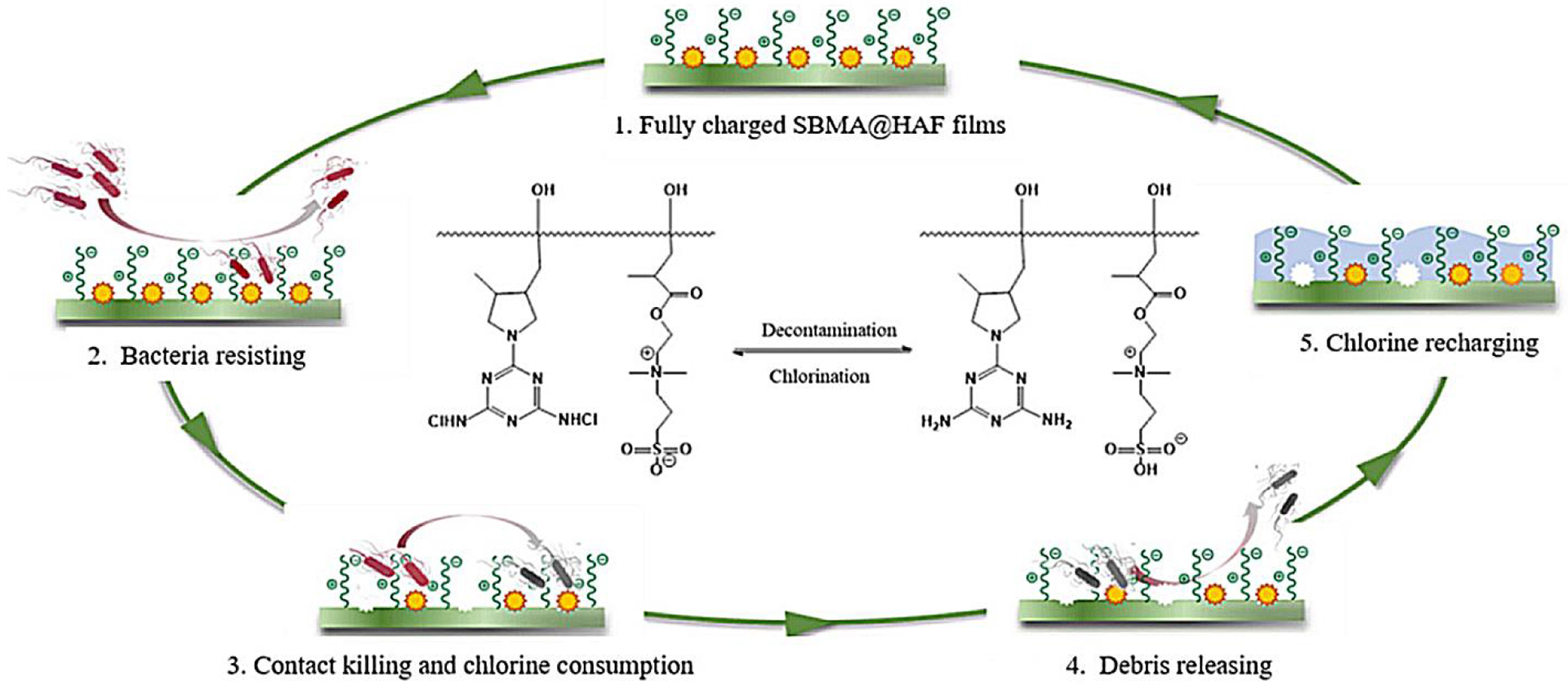
The immobilized biocidal N-halamine film integrated with the microbe-resistance PSBMA brush for coordinated antimicrobial application.149 The HAF (immobilized rechargeable biocide) film was prepared with the modification of BPTCD (photoinitiator) before the zwitterionic polymers (green line, PSBMA, microbe-resistance coating) were grafted on the surface. The chlorine (yellow balls) on the HAF would be consumed during the contact biocidal process while the PSBMA resisted the adhesion of the bacteria/debris. The biocidal chlorine could be recharged by immersing the surface in a household bleach solution. Reproduced with permission from ref 149. Copyright 2019, American Chemical Society.
Special design of the combination approach could adjust the temporal presence of the two materials through external stimuli, allowing only majorly one type of material to appear and function at a time (to resolve potential compromise). For example, an antimicrobial surface has been engineered using immobilized cationic polymers to perform biocidal functions on attached microbes. After special treatment to hydrolyze part of the polymers, zwitterionic carboxybetaine was generated on the surface allowing release and/or easy removal of the prior contact-killed microbes.151 Additionally, novel structured polymer surface would allow unlimited switch between cationic polymers and zwitterionic polymers through reversible ester bonds forming/breaking triggered by acidic/basic conditions, performing either microbicidal or resistance function as demanded.16 It should be noted that the external manipulation to control the spatial-temporal presence of the two materials might be a hassle or might be demanded in a particular application scenario, which requires further validation.
Conclusion and Outlook
Overall, both microbicidal surface and microbe resistance surface could effectively address the antimicrobial requirement for a variety of applications, and some of them have already been implemented as commercial products. Current research in each of these areas and their potential combination further advanced the knowledge and technology to address the ever-increasing needs for antimicrobials. Despite enormous research publications on antimicrobial surfaces, the translational barrier was high and few of them succeeded in serving the real-world applications. Common reasons include a significantly high cost to register and approve a novel biocide with safety/environmental agencies, manufacturing barrier and difficulty to apply the coating on a real substrate, not being able to meet durability/mechanical/longevity requirement, e.g., medical devices and marine-related applications requires both coating stability and high performance for months or even years, and additional issues that might be out of the radar of researchers, such as coating removal/repair, spec to meet for targeted applications, and cost issues. It is believed that future research on antimicrobial surfaces would benefit from a comprehensive understanding of fundamentals, e.g., working mechanisms for potential materials/strategies, as well as the challenges/requirements from an application perspective.
Acknowledgments
This work was supported by the National Science Foundation (1843790 and 1809229) and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DP2DK111910 and R01DK123293).
References
- (1).Donlan RM Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis 2001, 33 (8), 1387–1392, DOI: 10.1086/322972 [DOI] [PubMed] [Google Scholar]
- (2).Srey S; Jahid IK; Ha S-D Biofilm Formation in Food Industries: A Food Safety Concern. Food Control 2013, 31 (2), 572–585, DOI: 10.1016/j.foodcont.2012.12.001 [DOI] [Google Scholar]
- (3).Banerjee I; Pangule RC; Kane RS Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater 2011, 23 (6), 690–718, DOI: 10.1002/adma.201001215 [DOI] [PubMed] [Google Scholar]
- (4).Haque M; Sartelli M; McKimm J; Abu Bakar M Health Care-Associated Infections — an Overview. Infect. Drug. Resist 2018, 11, 2321–2333, DOI: 10.2147/IDR.S177247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Boda SK; Basu B Engineered Biomaterial and Biophysical Stimulation as Combinatorial Strategies to Address Prosthetic Infection by Pathogenic Bacteria. J. Biomed. Mater. Res. B. Appl. Biomater 2017, 105 (7), 2174–2190, DOI: 10.1002/jbm.b.33740 [DOI] [PubMed] [Google Scholar]
- (6).Costerton JW; Lewandowski Z; Caldwell DE; Korber DR; Lappin-Scott HM Mirobial Biofilms. Annual. Rev. Microbial 1995, 49, 711–745, DOI: 10.1146/annurev.mi.49.100195.003431 [DOI] [PubMed] [Google Scholar]
- (7).Gladis F; Eggert A; Karsten U; Schumann R Prevention of Biofilm Growth on Man-Made Surfaces: Evaluation of Antialgal Activity of Two Biocides and Photocatalytic Nanoparticles. Biofouling 2010, 26 (1), 89–101, DOI: 10.1080/08927010903278184 [DOI] [PubMed] [Google Scholar]
- (8).Cloutier M; Mantovani D; Rosei F Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol 2015, 33 (11), 637–652, DOI: 10.1016/j.tibtech.2015.09.002 [DOI] [PubMed] [Google Scholar]
- (9).Chen A; Peng H; Blakey I; Whittaker AK Biocidal Polymers: A Mechanistic Overview. Polymer Reviews 2016, 57 (2), 276–310, DOI: 10.1080/15583724.2016.1223131 [DOI] [Google Scholar]
- (10).Siedenbiedel F; Tiller JC Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4 (1), 46–71, DOI: 10.3390/polym4010046 [DOI] [Google Scholar]
- (11).Wu HX; Tan L; Tang ZW; Yang MY; Xiao JY; Liu CJ; Zhuo RX Highly Efficient Antibacterial Surface Grafted with a Triclosan-Decorated Poly(N-Hydroxyethylacrylamide) Brush. ACS Appl. Mater. Interfaces 2015, 7 (12), 7008–7015, DOI: 10.1021/acsami.5b01210 [DOI] [PubMed] [Google Scholar]
- (12).Nattharika A; Sabine H; Marek WU The Effectiveness of Antibiotic Activity of Penicillin Attached to Expanded Poly(Tetrafluoroethylene) (ePTFE) Surfaces: A Quantitative Assessment. Biomacromolecules 2007, 8, 3525–3530, DOI: 10.1021/bm700803e [DOI] [PubMed] [Google Scholar]
- (13).Wang W; Lu Y; Zhu H; Cao Z Superdurable Coating Fabricated from a Double-Sided Tape with Long Term “Zero” Bacterial Adhesion. Adv. Mater 2017, 29 (34), 1606506, DOI: 10.1002/adma.201606506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Chapman RG; Ostuni E; Liang MN; Meluleni G; Kim E; Yan L; Pier G; Warren HS; Whitesides GM Polymeric Thin Films That Resist the Adsorption of Proteins and the Adhesion of Bacteria. Langmuir 2001, 17, 1225–1233, DOI: 10.1021/la001222d [DOI] [Google Scholar]
- (15).Yang H; Li G; Stansbury JW; Zhu X; Wang X; Nie J Smart Antibacterial Surface Made by Photopolymerization. ACS Appl. Mater. Interfaces 2016, 8 (41), 28047–28054, DOI: 10.1021/acsami.6b09343 [DOI] [PubMed] [Google Scholar]
- (16).Cao Z; Mi L; Mendiola J; Ella-Menye JR; Zhang L; Xue H; Jiang S Reversibly Switching the Function of a Surface between Attacking and Defending against Bacteria. Angew. Chem. Int. Ed. Engl 2012, 51 (11), 2602–2605, DOI: 10.1002/anie.201106466 [DOI] [PubMed] [Google Scholar]
- (17).Lemire JA; Harrison JJ; Turner RJ Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol 2013, 11 (6), 371–384, DOI: 10.1038/nrmicro3028 [DOI] [PubMed] [Google Scholar]
- (18).El-Refaie K; D. WS; Roy B The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384, DOI: 10.1021/bm061150q [DOI] [PubMed] [Google Scholar]
- (19).Wei T; Tang Z; Yu Q; Chen H Smart Antibacterial Surfaces with Switchable Bacteria-Killing and Bacteria-Releasing Capabilities. ACS Appl. Mater. Interfaces 2017, 9 (43), 37511–37523, DOI: 10.1021/acsami.7b13565 [DOI] [PubMed] [Google Scholar]
- (20).Li X; Wu B; Chen H; Nan K; Jin Y; Sun L; Wang B Recent Developments in Smart Antibacterial Surfaces to Inhibit Biofilm Formation and Bacterial Infections. Journal of Materials Chemistry B 2018, 6 (26), 4274–4292, DOI: 10.1039/c8tb01245h [DOI] [PubMed] [Google Scholar]
- (21).Shtykova L; Fant C; Handa P; Larsson A; Berntsson K; Blanck H; Simonsson R; Nydén M; Ingelsten Härelind H Adsorption of Antifouling Booster Biocides on Metal Oxide Nanoparticles: Effect of Different Metal Oxides and Solvents. Prog. Org. Coatings 2009, 64 (1), 20–26, DOI: 10.1016/j.porgcoat.2008.07.005 [DOI] [Google Scholar]
- (22).Sørensen G; Nielsen AL; Pedersen MM; Poulsen S; Nissen H; Poulsen M; Nygaard SD Controlled Release of Biocide from Silica Microparticles in Wood Paint. Prog. Org. Coatings 2010, 68 (4), 299–306, DOI: 10.1016/j.porgcoat.2010.03.009 [DOI] [Google Scholar]
- (23).Hu R; Li G; Jiang Y; Zhang Y; Zou JJ; Wang L; Zhang X Silver-Zwitterion Organic-Inorganic Nanocomposite with Antimicrobial and Antiadhesive Capabilities. Langmuir 2013, 29 (11), 3773–3779, DOI: 10.1021/la304708b [DOI] [PubMed] [Google Scholar]
- (24).Pinar Kurt LW, Dennis E Ohman, and Kenneth J. Wynne. Highly Effective Contact Antimicrobial Surfaces via Polymer Surface Modifiers. Langmuir 2007, 23, 4719–4723, DOI: 10.1021/la063718m [DOI] [PubMed] [Google Scholar]
- (25).Murata H; Koepsel RR; Matyjaszewski K; Russell AJ Permanent, Non-Leaching Antibacterial Surface — 2: How High Density Cationic Surfaces Kill Bacterial Cells. Biomaterials 2007, 28 (32), 4870–4879, DOI: 10.1016/j.biomaterials.2007.06.012 [DOI] [PubMed] [Google Scholar]
- (26).Tiller Joerg C; Liao C-J; Lewis K; Klibanov AM Designing Surfaces That Kill Bacteria on Contact. Proc. Natl. Acad. Sci 2001, 98, 5981–5985, DOI: 10.1073/pnas.111143098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hoch M Organotin Compounds in the Environment — an Overview. Applied Geochemistry 2001, 16, 719–743, DOI: 10.1016/S0883-2927(00)00067-6 [DOI] [Google Scholar]
- (28).Turner A Marine Pollution from Antifouling Paint Particles. Mar. Pollut. Bull 2010, 60 (2), 159–171, DOI: 10.1016/j.marpolbul.2009.12.004 [DOI] [PubMed] [Google Scholar]
- (29).Adlhart C; Verran J; Azevedo NF; Olmez H; Keinanen-Toivola MM; Gouveia I; Melo LF; Crijns F Surface Modifications for Antimicrobial Effects in the Healthcare Setting: A Critical Overview. J. Hosp. Infect 2018, 99 (3), 239–249, DOI: 10.1016/j.jhin.2018.01.018 [DOI] [PubMed] [Google Scholar]
- (30).Lu Y; Yue Z; Wang W; Cao Z Strategies on Designing Multifunctional Surfaces to Prevent Biofilm Formation. Front. Chem. Sci. Eng 2015, 9 (3), 324–335, DOI: 10.1007/s11705-015-1529-z [DOI] [Google Scholar]
- (31).Kaur R; Liu S Antibacterial Surface Design — Contact Kill. Prog. Surf. Sci 2016, 91 (3), 136–153, DOI: 10.1016/j.progsurf.2016.09.001 [DOI] [Google Scholar]
- (32).Yu Q; Cho J; Shivapooja P; Ista LK; Lopez GP Nanopatterned Smart Polymer Surfaces for Controlled Attachment, Killing, and Release of Bacteria. ACS Appl. Mater. Interfaces 2013, 5 (19), 9295–9304, DOI: 10.1021/am4022279 [DOI] [PubMed] [Google Scholar]
- (33).Wei T; Zhan W; Cao L; Hu C; Qu Y; Yu Q; Chen H Multifunctional and Regenerable Antibacterial Surfaces Fabricated by a Universal Strategy. ACS Appl. Mater. Interfaces 2016, 8 (44), 30048–30057, DOI: 10.1021/acsami.6b11187 [DOI] [PubMed] [Google Scholar]
- (34).Yu Q; Ista LK; Lopez GP Nanopatterned Antimicrobial Enzymatic Surfaces Combining Biocidal and Fouling Release Properties. Nanoscale 2014, 6 (9), 4750–4757, DOI: 10.1039/c3nr06497b [DOI] [PubMed] [Google Scholar]
- (35).Suwatthanarak T; Than-ardna B; Danwanichakul D; Danwanichakul P Synthesis of Silver Nanoparticles in Skim Natural Rubber Latex at Room Temperature. Mater 2016, 168, 31–35, DOI: 10.1016/j.matlet.2016.01.026 [DOI] [Google Scholar]
- (36).Sharma VK; Yngard RA; Lin Y Silver Nanoparticles: Green Synthesis and Their Antimicrobial Activities. Adv. Colloid Interface Sci 2009, 145 (1–2), 83–96, DOI: 10.1016/j.cis.2008.09.002 [DOI] [PubMed] [Google Scholar]
- (37).Dilek S; Ihsan G; Donald WL; Vasıf H Antibiotic Release from Biodegradable PHBV Microparticles. J. Control Release 1999, 59, 207–217, DOI: 10.1016/s0168-3659(98)00195-3 [DOI] [PubMed] [Google Scholar]
- (38).Costa F; Carvalho IF; Montelaro RC; Gomes P; Martins MC Covalent Immobilization of Antimicrobial Peptides (AMPs) onto Biomaterial Surfaces. Acta Biomater 2011, 7 (4), 1431–1440, DOI: 10.1016/j.actbio.2010.11.005 [DOI] [PubMed] [Google Scholar]
- (39).Jiang H; Manolache S; Wong ACL; Denes FS Plasma-Enhanced Deposition of Silver Nanoparticles onto Polymer and Metal Surfaces for the Generation of Antimicrobial Characteristics. J. Appl. Polym 2004, 93 (3), 1411–1422, DOI: 10.1002/app.20561 [DOI] [Google Scholar]
- (40).Paladini F; Pollini M; Sannino A; Ambrosio L Metal-Based Antibacterial Substrates for Biomedical Applications. Biomacromolecules 2015, 16 (7), 1873–1885, DOI: 10.1021/acs.biomac.5b00773 [DOI] [PubMed] [Google Scholar]
- (41).Li JX; Wang J; Shen LR; Xu ZJ; Li P; Wan GJ; Huang N The Influence of Polyethylene Terephthalate Surfaces Modified by Silver Ion Implantation on Bacterial Adhesion Behavior. Surf. Coat. Technol 2007, 201 (19–20), 8155–8159, DOI: 10.1016/j.surfcoat.2006.02.069 [DOI] [Google Scholar]
- (42).Qin Y; Zhu C; Chen J; Chen Y; Zhang C The Absorption and Release of Silver and Zinc Ions by Chitosan Fibers. J. Appl. Polym. Sci 2006, 101 (1), 766–771, DOI: 10.1002/app.23985 [DOI] [Google Scholar]
- (43).Monk AB; Kanmukhla V; Trinder K; Borkow G Potent Bactericidal Efficacy of Copper Oxide Impregnated Non-Porous Solid Surfaces. BMC Microbiol 2014, 14, 57, DOI: 10.1186/1471-2180-14-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Gabbay J; Borkow G; Mishal J; Magen E; Zatcoff R; Shemer-Avni Y Copper Oxide Impregnated Textiles with Potent Biocidal Activities. J. Ind. Text 2006, 35 (4), 323–335, DOI: 10.1177/1528083706060785 [DOI] [Google Scholar]
- (45).Crisp D; Austin A The Action of Copper in Antifouling Paints. Ann. Appl. Biol 1960, 48 (4), 787–799, DOI: 10.1111/j.1744-7348.1960.tb03580.x [DOI] [Google Scholar]
- (46).Parks R; Donnier-Marechal M; Frickers PE; Turner A; Readman JW Antifouling Biocides in Discarded Marine Paint Particles. Mar. Pollut. Bull 2010, 60 (8), 1226–1230, DOI: 10.1016/j.marpolbul.2010.03.022 [DOI] [PubMed] [Google Scholar]
- (47).Callow ME; Callow JA Marine Biofouling: A Sticky Problem. Biologist 2002, 49 (1), 1–5, [PubMed] [Google Scholar]
- (48).Valkirs AO; Seligman PF; Haslbeck E; Caso JS Measurement of Copper Release Rates from Antifouling Paint under Laboratory and in Situ Conditions: Implications for Loading Estimation to Marine Water Bodies. Mar. Pollut. Bull 2003, 46 (6), 763–779, DOI: 10.1016/s0025-326x(03)00044-4 [DOI] [PubMed] [Google Scholar]
- (49).Yebra DM; Kiil S; Dam-Johansen K Antifouling Technology — Past, Present and Future Steps Towards Efficient and Environmentally Friendly Antifouling Coatings. Prog. Org. Coat 2004, 50 (2), 75–104, DOI: 10.1016/j.porgcoat.2003.06.001 [DOI] [Google Scholar]
- (50).Vouloulis N; Scrimshaw MD; Lester JN Occurrence of Four Biocides Utilized in Antifouling Paints, as Alternatives to Organotin Compounds, in Waters and Sediments of a Commercial Estuary in the UK. Mar. Pollut. Bull 2000, 40, 938–946, DOI: 10.1016/S0025-326X(00)00034-5 [DOI] [Google Scholar]
- (51).Christen K Imo Will Ban the Use of a Popular Biocide. Environ. Sci. Technol 1999, 33 (1), 11A, DOI: 10.1021/es992609+ [DOI] [PubMed] [Google Scholar]
- (52).Lotz A Marine Coatings: Making Sense of Us, State, and Local Mandates of Copper-Based Antifouling Regulations. JCT COATINGSTECH 2016, 13 (9), 50–54, [Google Scholar]
- (53).Roe D; Karandikar B; Bonn-Savage N; Gibbins B; Roullet J-B Antimicrobial Surface Functionalization of Plastic Catheters by Silver Nanoparticles. J. Antimicrob. Chemother 2008, 61 (4), 869–876, DOI: 10.1093/jac/dkn034 [DOI] [PubMed] [Google Scholar]
- (54).Duran N; Duran M; de Jesus MB; Seabra AB; Favaro WJ; Nakazato G Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomedicine: NBM 2016, 12 (3), 789–799, DOI: 10.1016/j.nano.2015.11.016 [DOI] [PubMed] [Google Scholar]
- (55).Liu L; Yang J; Xie J; Luo Z; Jiang J; Yang YY; Liu S The Potent Antimicrobial Properties of Cell Penetrating Peptide-Conjugated Silver Nanoparticles with Excellent Selectivity for Gram-Positive Bacteria over Erythrocytes. Nanoscale 2013, 5 (9), 3834–3840, DOI: 10.1039/c3nr34254a [DOI] [PubMed] [Google Scholar]
- (56).James C; Johnson AL; Jenkins AT Antimicrobial Surface Grafted Thermally Responsive Pnipam-Co-Ala Nano-Gels. Chem. Commun 2011, 47 (48), 12777–12779, DOI: 10.1039/c1cc15372b [DOI] [PubMed] [Google Scholar]
- (57).Le Ouay B; Stellacci F Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10 (3), 339–354, DOI: 10.1016/j.nantod.2015.04.002 [DOI] [Google Scholar]
- (58).Lv Y; Liu H; Wang Z; Hao L; Liu J; Wang Y; Du G; Liu D; Zhan J; Wang J Antibiotic Glass Slide Coated with Silver Nanoparticles and Its Antimicrobial Capabilities. Polym. Adv. Technol 2008, 19 (11), 1455–1460, DOI: 10.1002/pat.1138 [DOI] [Google Scholar]
- (59).Ertem E; Gutt B; Zuber F; Allegri S; Le Ouay B; Mefti S; Formentin K; Stellacci F; Ren Q Core-Shell Silver Nanoparticles in Endodontic Disinfection Solutions Enable Long-Term Antimicrobial Effect on Oral Biofilms. ACS Appl. Mater. Interfaces 2017, 9 (40), 34762–34772, DOI: 10.1021/acsami.7b13929 [DOI] [PubMed] [Google Scholar]
- (60).Wu J; Yu C; Li Q Novel Regenerable Antimicrobial Nanocomposite Membranes: Effect of Silver Loading and Valence State. J. Membrane. Sci 2017, 531, 68–76, DOI: 10.1016/j.memsci.2017.02.047 [DOI] [Google Scholar]
- (61).Damm C; Münstedt H; Rösch A Long-Term Antimicrobial Polyamide 6/Silver-Nanocomposites. J. Mater. Sci 2007, 42 (15), 6067–6073, DOI: 10.1007/s10853-006-1158-5 [DOI] [Google Scholar]
- (62).Benhacine F; Hadj-Hamou A. s. ; Habi A Development of Long-Term Antimicrobial Poly (E-Caprolactone)/Silver Exchanged Montmorillonite Nanocomposite Films with Silver Ion Release Property for Active Packaging Use. Polym. Bull 2015, 73 (5), 1207–1227, DOI: 10.1007/s00289-015-1543-9 [DOI] [Google Scholar]
- (63).Qi R; Guo R; Zheng F; Liu H; Yu J; Shi X Controlled Release and Antibacterial Activity of Antibiotic-Loaded Electrospun Halloysite/Poly(Lactic-Co-Glycolic Acid) Composite Nanofibers. Colloids Surf. B Biointerfaces 2013, 110, 148–155, DOI: 10.1016/j.colsurfb.2013.04.036 [DOI] [PubMed] [Google Scholar]
- (64).Silva ER; Ferreira O; Rijo P; Bordado JC; Calhorda MJ Antifouling Eco-Filters for Water Bio-Econtamination. Proceedings 2017, 2 (5), 181, DOI: 10.3390/ecws-2-04950 [DOI] [Google Scholar]
- (65).Downs R; Dean J; Downer A; Perry J Determination of the Biocide Econea® in Artificial Seawater by Solid Phase Extraction and High Performance Liquid Chromatography Mass Spectrometry. Separations 2017, 4 (4), 34, DOI: 10.3390/separations4040034 [DOI] [Google Scholar]
- (66).Kong M; Chen XG; Xing K; Park HJ Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol 2010, 144 (1), 51–63, DOI: 10.1016/j.ijfoodmicro.2010.09.012 [DOI] [PubMed] [Google Scholar]
- (67).Helander IM; Nurmiaho-Lassila E-L; Ahvenainen R; Rhoades J; Roller S Chitosan Disrupts the Barrier Properties of the Outer Membrane of Gram-Negative Bacteria. Int. J. Food Microbiol 2001, 71, 235–244, DOI: 10.1016/s0168-1605(01)00609-2 [DOI] [PubMed] [Google Scholar]
- (68).Hui F; Debiemme-Chouvy C Antimicrobial N-Halamine Polymers and Coatings: A Review of Their Synthesis, Characterization, and Applications. Biomacromolecules 2013, 14 (3), 585–601, DOI: 10.1021/bm301980q [DOI] [PubMed] [Google Scholar]
- (69).Akihiko K; Tomiki I; Takeshi E Antibacterial Activity of Polymeric Sulfonium Salts. J. Polym. Sci. Part A: Polym. Chem 1993, 31, 2873–2876, DOI: 10.1002/pola.1993.080311126 [DOI] [Google Scholar]
- (70).Faÿ F; Linossier I; Langlois V; Vallee-Rehel K Biodegradable Poly(Ester-Anhydride) for New Antifouling Coating. Biomacromolecules 2007, 8, 1751–1758, DOI: 10.1021/bm061013t [DOI] [PubMed] [Google Scholar]
- (71).Nordstierna L; Abdalla AA; Masuda M; Skarnemark G; Nydén M Molecular Release from Painted Surfaces: Free and Encapsulated Biocides. Prog. Org. Coat 2010, 69 (1), 45–48, DOI: 10.1016/j.porgcoat.2010.05.002 [DOI] [Google Scholar]
- (72).Kamtsikakis A; Kavetsou E; Chronaki K; Kiosidou E; Pavlatou E; Karana A; Papaspyrides C; Detsi A; Karantonis A; Vouyiouka S Encapsulation of Antifouling Organic Biocides in Poly(Lactic Acid) Nanoparticles. Bioengineering 2017, 4 (4), 81, DOI: 10.3390/bioengineering4040081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Cai H; Wang P; Zhang D Ph-Responsive Linkages-Enabled Layer-by-Layer Assembled Antibacterial and Antiadhesive Multilayer Films with Polyelectrolyte Nanocapsules as Biocide Delivery Vehicles. J. Drug Deliv. Sci. Technol 2019, 54, 101251, DOI: 10.1016/j.jddst.2019.101251 [DOI] [Google Scholar]
- (74).Jämsä S; Mahlberg R; Holopainen U; Ropponen J; Savolainen A; Ritschkoff AC Slow Release of a Biocidal Agent from Polymeric Microcapsules for Preventing Biodeterioration. Prog. Org. Coat 2013, 76 (1), 269–276, DOI: 10.1016/j.porgcoat.2012.09.018 [DOI] [Google Scholar]
- (75).Borodina TN; Grigoriev DO; Carillo MA; Hartmann J; Moehwald H; Shchukin DG Preparation of Multifunctional Polysaccharide Microcontainers for Lipophilic Bioactive Agents. ACS Appl. Mater. Interfaces 2014, 6 (9), 6570–8, DOI: 10.1021/am406039r [DOI] [PubMed] [Google Scholar]
- (76).Nattharika A; Matthew MS; Marek UW Tunable Antimicrobial Polypropylene Surfaces: Simultaneous Attachment of Penicillin (Gram +) and Gentamicin (Gram −). Biomacromolecules 2009, 10, 623–629, DOI: 10.1021/bm8013473 [DOI] [PubMed] [Google Scholar]
- (77).Aumsuwan N; Heinhorst S; Urban WM Antibacterial Surfaces on Expanded Polytetrafluoroethylene; Penicillin Attachment. Biomacromolecules 2007, 8, 713–718, DOI: 10.1021/bm061050k [DOI] [PubMed] [Google Scholar]
- (78).Ferreira L; Zumbuehl A Non-Leaching Surfaces Capable of Killing Microorganisms on Contact. J. Mater. Chem 2009, 19 (42), 7796–7806, DOI: 10.1039/b905668h [DOI] [Google Scholar]
- (79).Gao Q; Li P; Zhao H; Chen Y; Jiang L; Ma PX Methacrylate-Ended Polypeptides and Polypeptoids for Antimicrobial and Antifouling Coatings. Polym. Chem 2017, 8 (41), 6386–6397, DOI: 10.1039/c7py01495c [DOI] [Google Scholar]
- (80).Yu Q; Wu Z; Chen H Dual-Function Antibacterial Surfaces for Biomedical Applications. Acta Biomater 2015, 16, 1–13, DOI: 10.1016/j.actbio.2015.01.018 [DOI] [PubMed] [Google Scholar]
- (81).Chen CZ; Beck-Tan NC; Dhurjati P; Van Dyk TK; LaRossa RA; Cooper SL Quaternary Ammonium Functionalized Poly(Propylene Imine) Dendrimers as Effective Antimicrobials: Structure-Activity Studies. Biomacromolecules 2000, 1, 473–480, DOI: 10.1021/bm0055495 [DOI] [PubMed] [Google Scholar]
- (82).Ravikumar T; Murata H; Koepsel RR; Russell JA Surface-Active Antifungal Polyquaternary Amine. Biomacromolecules 2006, 7, 2762–2769, DOI: 10.1021/bm060476w [DOI] [PubMed] [Google Scholar]
- (83).Ikeda T; Yamaguchi H; Tazuke S New Polymeric Biocides: Synthesis and Antibacterial Activities of Polycations with Pendant Biguanide Groups. Antimicrob. Agents Chemother 1984, 26, 139–144, DOI: 10.1128/aac.26.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Xue Y; Xiao H; Zhang Y Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci 2015, 16 (2), 3626–3655, DOI: 10.3390/ijms16023626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Lewis K; Klibanov AM Surpassing Nature: Rational Design of Sterile-Surface Materials. Trends Biotechnol 2005, 23 (7), 343–348, DOI: 10.1016/j.tibtech.2005.05.004 [DOI] [PubMed] [Google Scholar]
- (86).Liu K; Su Z; Miao S; Ma G; Zhang S UV-Curable Enzymatic Antibacterial Waterborne Polyurethane Coating. Biochem. Eng. J 2016, 113, 107–113, DOI: 10.1016/j.bej.2016.06.004 [DOI] [Google Scholar]
- (87).Muszanska AK; Busscher HJ; Herrmann A; van der Mei HC; Norde W Pluronic-Lysozyme Conjugates as Anti-Adhesive and Antibacterial Bifunctional Polymers for Surface Coating. Biomaterials 2011, 32 (26), 6333–6341, DOI: 10.1016/j.biomaterials.2011.05.016 [DOI] [PubMed] [Google Scholar]
- (88).Appendini P; Hotchkiss JH Surface Modification of Poly(Styrene) by the Attachment of an Antimicrobial Peptide. J. Appl. Polym. Sci 2001, 81, 609–616, DOI: 10.1002/app.1476 [DOI] [Google Scholar]
- (89).Caro A; Humblot V; Méthivier C; Minier M; Salmain M. l.; Pradier C-M Grafting of Lysozyme and/or Poly(Ethylene Glycol) to Prevent Biofilm Growth on Stainless Steel Surfaces. J. Phys. Chem. B 2009, 113, 2101–2109, DOI: 10.1021/jp805284s [DOI] [PubMed] [Google Scholar]
- (90).Melo LD; Veiga P; Cerca N; Kropinski AM; Almeida C; Azeredo J; Sillankorva S Development of a Phage Cocktail to Control Proteus Mirabilis Catheter-Associated Urinary Tract Infections. Front. Microbiol 2016, 7, 1024, DOI: 10.3389/fmicb.2016.01024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Pearson HA; Sahukhal GS; Elasri MO; Urban MW Phage-Bacterium War on Polymeric Surfaces: Can Surface-Anchored Bacteriophages Eliminate Microbial Infections? Biomacromolecules 2013, 14 (5), 1257–1261, DOI: 10.1021/bm400290u [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Wang C; Sauvageau D; Elias A Immobilization of Active Bacteriophages on Polyhydroxyalkanoate Surfaces. ACS Appl. Mater. Interfaces 2016, 8 (2), 1128–1138, DOI: 10.1021/acsami.5b08664 [DOI] [PubMed] [Google Scholar]
- (93).Hilpert K; Elliott M; Jenssen H; Kindrachuk J; Fjell CD; Korner J; Winkler DF; Weaver LL; Henklein P; Ulrich AS; Chiang SH; Farmer SW; Pante N; Volkmer R; Hancock RE Screening and Characterization of Surface-Tethered Cationic Peptides for Antimicrobial Activity. Chem. Biol 2009, 16 (1), 58–69, DOI: 10.1016/j.chembiol.2008.11.006 [DOI] [PubMed] [Google Scholar]
- (94).Fillat A; Gallardo O; Vidal T; Pastor FIJ; Díaz P; Roncero MB Enzymatic Grafting of Natural Phenols to Flax Fibres: Development of Antimicrobial Properties. Carbohydr. Polym 2012, 87 (1), 146–152, DOI: 10.1016/j.carbpol.2011.07.030 [DOI] [PubMed] [Google Scholar]
- (95).Humblot V; Yala JF; Thebault P; Boukerma K; Hequet A; Berjeaud JM; Pradier CM The Antibacterial Activity of Magainin I Immobilized onto Mixed Thiols Self-Assembled Monolayers. Biomaterials 2009, 30 (21), 3503–3512, DOI: 10.1016/j.biomaterials.2009.03.025 [DOI] [PubMed] [Google Scholar]
- (96).Czeslik C; Winter R Effect of Temperature on the Conformation of Lysozyme Adsorbed to Silica Particles. Phys. Chem. Chem. Phys 2001, 3 (2), 235–239, DOI: 10.1039/b005900p [DOI] [Google Scholar]
- (97).Lu JR; Su TJ; Thirtle PN; Thomas RK; Rennie AR; Cubitt R The Denaturation of Lysozyme Layers Adsorbed at the Hydrophobic Solid/Liquid Surface Studied by Neutron Reflection. J. Colloid. Interface. Sci 1998, 206, 212–223, DOI: 10.1006/jcis.1998.5680 [DOI] [PubMed] [Google Scholar]
- (98).Durchschlag H; Hefferle T; Zipper P Comparative Investigations of the Effects of X- and UV-Irradiation on Lysozyme in the Absence or Presence of Additives. Radiat. Phys. Chem 2003, 67, 479–486, DOI: 10.1016/s0969-806x(03)00089-6 [DOI] [Google Scholar]
- (99).Matthews BW Studies on Protein Stability with T4 Lysozyme In Advances in Protein Chemistry; Elsevier: 1995; pp 249–278 [DOI] [PubMed] [Google Scholar]
- (100).Kristensen JB; Meyer RL; Laursen BS; Shipovskov S; Besenbacher F; Poulsen CH Antifouling Enzymes and the Biochemistry of Marine Settlement. Biotechnol. Adv 2008, 26 (5), 471–481, DOI: 10.1016/j.biotechadv.2008.05.005 [DOI] [PubMed] [Google Scholar]
- (101).Zhi Z; Su Y; Xi Y; Tian L; Xu M; Wang Q; Pandidan S; Padidan S; Li P; Huang W Dual-Functional Polyethylene Glycol-B-Polyhexanide Surface Coating with in Vitro and in Vivo Antimicrobial and Antifouling Activities. ACS Appl. Mater. Interfaces 2017, 9 (12), 10383–10397, DOI: 10.1021/acsami.6b12979 [DOI] [PubMed] [Google Scholar]
- (102).Hosseinidoust Z; Van de Ven TG; Tufenkji N Bacterial Capture Efficiency and Antimicrobial Activity of Phage-Functionalized Model Surfaces. Langmuir 2011, 27 (9), 5472–5480, DOI: 10.1021/la200102z [DOI] [PubMed] [Google Scholar]
- (103).Fu L; Li S; Zhang K; Chen IH; Barbaree JM; Zhang A; Cheng Z Detection of Bacillus Anthracis Spores Using Phage-Immobilized Magnetostrictive Milli/Micro Cantilevers. IEEE Sens. J 2011, 11 (8), 1684–1691, DOI: 10.1109/jsen.2010.2095002 [DOI] [Google Scholar]
- (104).Cademartiri R; Anany H; Gross I; Bhayani R; Griffiths M; Brook MA Immobilization of Bacteriophages on Modified Silica Particles. Biomaterials 2010, 31, 1904–1910, DOI: 10.1016/j.biomaterials.2009.11.029 [DOI] [PubMed] [Google Scholar]
- (105).Ma W; Panecka M; Tufenkji N; Rahaman MS Bacteriophage-Based Strategies for Biofouling Control in Ultrafiltration: In Situ Biofouling Mitigation, Biocidal Additives and Biofilm Cleanser. J. Colloid Interface Sci 2018, 523, 254–265, DOI: 10.1016/j.jcis.2018.03.105 [DOI] [PubMed] [Google Scholar]
- (106).Klemm P; Vejborg RM; Hancock V Prevention of Bacterial Adhesion. Appl. Microbiol. Biotechnol 2010, 88, 451–459, DOI: 10.1007/s00253-010-2805-y [DOI] [PubMed] [Google Scholar]
- (107).Tang L; Gu W; Yi P; Bitter JL; Hong JY; Fairbrother DH; Chen KL Bacterial Anti-Adhesive Properties of Polysulfone Membranes Modified with Polyelectrolyte Multilayers. J. Membrane Sci 2013, 446, 201–211, DOI: 10.1016/j.memsci.2013.06.031 [DOI] [Google Scholar]
- (108).Tsibouklis J; Stone M; Thorpe AA; Graham P; Peters V; Heerlien R; Smith JR; Green KL; Nevell TG Preventing Bacterial Adhesion onto Surfaces: The Low-Surface-Energy Approach. Biomaterials 1999, 20 (13), 1229–1235, DOI: 10.1016/s0142-9612(99)00023-x [DOI] [PubMed] [Google Scholar]
- (109).Nejadnik MR; van der Mei HC; Norde W; Busscher HJ Bacterial Adhesion and Growth on a Polymer Brush-Coating. Biomaterials 2008, 29, 4117–4121, DOI: 10.1016/j.biomaterials.2008.07.014 [DOI] [PubMed] [Google Scholar]
- (110).Cheng G; Li G; Xue H; Chen S; Bryers JD; Jiang S Zwitterionic Carboxybetaine Polymer Surfaces and Their Resistance to Long-Term Biofilm Formation. Biomaterials 2009, 30, 5234–5240, DOI: 10.1016/j.biomaterials.2009.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Kelly CG; Younson JS Anti-Adhesive Strategies in the Prevention of Infectious Disease at Mucosal Surfaces. Expert. Opin. Investig. Drugs 2000, 9 (8), 1711–1721, DOI: 10.1517/13543784.9.8.1711 [DOI] [PubMed] [Google Scholar]
- (112).Krishnan S; Weinman CJ; Ober CK Advances in Polymers for Anti-Biofouling Surfaces. J. Mater. Chem 2008, 18 (29), 3405–3413, DOI: 10.1039/b801491d [DOI] [Google Scholar]
- (113).Zhang X; Wang L; Levänen E Superhydrophobic Surfaces for the Reduction of Bacterial Adhesion. RSC Adv 2013, 3 (30), 12003, DOI: 10.1039/c3ra40497h [DOI] [Google Scholar]
- (114).Stallard CP; McDonnell KA; Onayemi OD; O’Gara JP; Dowling DP Evaluation of Protein Adsorption on Atmospheric Plasma Deposited Coatings Exhibiting Superhydrophilic to Superhydrophobic Properties. Biointerphases 2012, 7 (1–4), 31, DOI: 10.1007/s13758-012-0031-0 [DOI] [PubMed] [Google Scholar]
- (115).Cheng G; Zhang Z; Chen S; Bryers JD; Jiang S Inhibition of Bacterial Adhesion and Biofilm Formation on Zwitterionic Surfaces. Biomaterials 2007, 28 (29), 4192–4199, DOI: 10.1016/j.biomaterials.2007.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Williams DL; O’Bryon TM Chapter 5 — Cleanliness Verification on Large Surfaces: Instilling Confidence in Contact Angle Techniques In Developments in Surface Contamination and Cleaning; Kohli R; Mittal KL, Eds.; William Andrew Publishing: Oxford, 2013; pp 163–181 [Google Scholar]
- (117).Kinloch AJ Adhesion & Adhesives - Science & Technology, Chapman & Hall: London, 1987; [Google Scholar]
- (118).Kuo ACM Polymer Data Handbook, Oxford University Press: 1999; [Google Scholar]
- (119).Berglin M; Gatenholm P The Nature of Bioadhesive Bonding between Barnacles and Fouling-Release Silicone Coatings. J. Adhesion Sci. Technol 1999, 13 (6), 713–727, DOI: 10.1163/156856199X00956 [DOI] [Google Scholar]
- (120).Wendt DE; Kowalke GL; Kim J; Singer IL Factors That Influence Elastomeric Coating Performance: The Effect of Coating Thickness on Basal Plate Morphology, Growth and Critical Removal Stress of the Barnacle Balanus Amphitrite. Biofouling 2006, 22 (1), 1–9, DOI: 10.1080/08927010500499563 [DOI] [PubMed] [Google Scholar]
- (121).Zardeneta G; Mukai H; Marker V; Milam SB Protein Interactions with Particulate Teflon: Implications for the Foreign Body Response. J. Oral Maxillofac. Surg 1996, 54 (7), 873–878, DOI: 10.1016/S0278-2391(96)90540-6 [DOI] [PubMed] [Google Scholar]
- (122).Chen G; Li J; Yang X; Wu Y Surface-Appropriate Lipophobicity — Application in Isobutene Oligomerization over Teflon-Modified Silica-Supported 12-Silicotungstic Acid. Appl. Catal., A 2006, 310, 16–23, DOI: 10.1016/j.apcata.2006.04.046 [DOI] [Google Scholar]
- (123).Alluri C; Ji HF; Sit PS Strong Resistance of (Tridecafluoro-1,1,2,2-Tetrahydrooctyl)Triethoxysilane (TTS) Nanofilm to Protein Adsorption. Biotechnol. Appl. Biochem 2013, 60 (5), 494–501, DOI: 10.1002/bab.1136 [DOI] [PubMed] [Google Scholar]
- (124).Hizal F; Rungraeng N; Lee J; Jun S; Busscher HJ; van der Mei HC; Choi CH Nanoengineered Superhydrophobic Surfaces of Aluminum with Extremely Low Bacterial Adhesivity. ACS Appl. Mater. Interfaces 2017, 9 (13), 12118–12129, DOI: 10.1021/acsami.7b01322 [DOI] [PubMed] [Google Scholar]
- (125).Zhang Y-Y; Ge Q; Yang L-L; Shi X-J; Li J-J; Yang D-Q; Sacher E Durable Superhydrophobic PTFE Films through the Introduction of Micro- and Nanostructured Pores. Appl. Surf. Sci 2015, 339, 151–157, DOI: 10.1016/j.apsusc.2015.02.143 [DOI] [Google Scholar]
- (126).Wang H; Zhao J; Zhu Y; Meng Y; Zhu Y The Fabrication, Nano/Micro-Structure, Heat- and Wear-Resistance of the Superhydrophobic PPS/PTFE Composite Coatings. J. Colloid Interface Sci 2013, 402, 253–258, DOI: 10.1016/j.jcis.2012.11.011 [DOI] [PubMed] [Google Scholar]
- (127).Harder T; Yee LH 5 - Bacterial Adhesion and Marine Fouling In Advances in Marine Antifouling Coatings and Technologies; Hellio C; Yebra D, Eds.; Woodhead Publishing: 2009; pp 113–131 [Google Scholar]
- (128).Barthlott W; Neinhuis C Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202, 1–8, DOI: 10.1007/s004250050096 [DOI] [Google Scholar]
- (129).Yamauchi Y; Tenjimbayashi M; Samitsu S; Naito M Durable and Flexible Superhydrophobic Materials: Abrasion/Scratching/Slicing/Droplet Impacting/Bending/Twisting-Tolerant Composite with Porcupinefish-Like Structure. ACS Appl. Mater. Interfaces 2019, 11 (35), 32381–32389, DOI: 10.1021/acsami.9b09524 [DOI] [PubMed] [Google Scholar]
- (130).Ware CS; Smith-Palmer T; Peppou-Chapman S; Scarratt LRJ; Humphries EM; Balzer D; Neto C Marine Antifouling Behavior of Lubricant-Infused Nanowrinkled Polymeric Surfaces. ACS Appl. Mater. Interfaces 2018, 10 (4), 4173–4182, DOI: 10.1021/acsami.7b14736 [DOI] [PubMed] [Google Scholar]
- (131).Cao Z; Jiang S Super-Hydrophilic Zwitterionic Poly(Carboxybetaine) and Amphiphilic Non-Ionic Poly(Ethylene Glycol) for Stealth Nanoparticles. Nano Today 2012, 7 (5), 404–413, DOI: 10.1016/j.nantod.2012.08.001 [DOI] [Google Scholar]
- (132).Saldarriaga Fernandez IC; van der Mei HC; Lochhead MJ; Grainger DW; Busscher HJ The Inhibition of the Adhesion of Clinically Isolated Bacterial Strains on Multi-Component Cross-Linked Poly(Ethylene Glycol)-Based Polymer Coatings. Biomaterials 2007, 28 (28), 4105–4112, DOI: 10.1016/j.biomaterials.2007.05.023 [DOI] [PubMed] [Google Scholar]
- (133).Alconcel SNS; Baas AS; Maynard HD FDA-Approved Poly(Ethylene Glycol)–Protein Conjugate Drugs. Polym. Chem 2011, 2 (7), 1442–1448, DOI: 10.1039/c1py00034a [DOI] [Google Scholar]
- (134).Gong C; Shi S; Dong P; Kan B; Gou M; Wang X; Li X; Luo F; Zhao X; Wei Y; Qian Z Synthesis and Characterization of PEG-PCL-PEG Thermosensitive Hydrogel. Int. J. Pharm 2009, 365 (1–2), 89–99, DOI: 10.1016/j.ijpharm.2008.08.027 [DOI] [PubMed] [Google Scholar]
- (135).Han S; Kim C; Kwon D Thermal/Oxidative Degradation and Stabilization of Polyethylene Glycol. Polymer 1997, 38 (2), 317–323, DOI: 10.1016/S0032–3861(97)88175-X [Google Scholar]
- (136).Garay R,P; Labaune J,P In Immunogenicity of Polyethylene Glycol (PEG), The Open Conference Proceedings Journal, 2011; pp 104–107 [Google Scholar]
- (137).Harris JM; Chess RB Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discov 2003, 2 (3), 214–221, DOI: 10.1038/nrd1033 [DOI] [PubMed] [Google Scholar]
- (138).Chen J-S; Ting Y-S; Tsou H-M; Liu T-Y Highly Hydrophilic and Antibiofouling Surface of Zwitterionic Polymer Immobilized on Polydimethylsiloxane by Initiator-Free Atmospheric Plasma-Induced Polymerization. Surf. Coat. Technol 2018, 344, 621–625, DOI: 10.1016/j.surfcoat.2018.03.078 [DOI] [Google Scholar]
- (139).Jin YJ; Kang S; Park P; Choi D; Kim DW; Jung D; Koh J; Jeon J; Lee M; Ham J; Seo JH; Jin HR; Lee Y Anti-Inflammatory and Antibacterial Effects of Covalently Attached Biomembrane-Mimic Polymer Grafts on Gore-Tex Implants. ACS Appl. Mater. Interfaces 2017, 9 (22), 19161–19175, DOI: 10.1021/acsami.7b02696 [DOI] [PubMed] [Google Scholar]
- (140).Tang Z-W; Ma C-Y; Wu H-X; Tan L; Xiao J-Y; Zhuo R-X; Liu C-J Antiadhesive Zwitterionic Poly-(Sulphobetaine Methacrylate) Brush Coating Functionalized with Triclosan for High-Efficiency Antibacterial Performance. Prog. Org. Coat 2016, 97, 277–287, DOI: 10.1016/j.porgcoat.2016.04.038 [DOI] [Google Scholar]
- (141).Wang W; Lu Y; Xie J; Zhu H; Cao Z A Zwitterionic Macro-Crosslinker for Durable Non-Fouling Coatings. Chem. Commun 2016, 52 (25), 4671–4674, DOI: 10.1039/c6cc00109b [DOI] [PubMed] [Google Scholar]
- (142).Wang H; Zhang C; Wang J; Feng X; He C Dual-Mode Antifouling Ability of Thiol–Ene Amphiphilic Conetworks: Minimally Adhesive Coatings via the Surface Zwitterionization. ACS Sustain. Chem. Eng 2016, 4 (7), 3803–3811, DOI: 10.1021/acssuschemeng.6b00525 [DOI] [Google Scholar]
- (143).Zhao X; He C Efficient Preparation of Super Antifouling PVDF Ultrafiltration Membrane with One Step Fabricated Zwitterionic Surface. ACS Appl. Mater. Interfaces 2015, 7 (32), 17947–17953, DOI: 10.1021/acsami.5b04648 [DOI] [PubMed] [Google Scholar]
- (144).Yu Y; Yuk H; Parada GA; Wu Y; Liu X; Nabzdyk CS; Youcef-Toumi K; Zang J; Zhao X Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv. Mater 2019, 31 (7), 1807101, DOI: 10.1002/adma.201807101 [DOI] [PubMed] [Google Scholar]
- (145).Inoue Y; Kamichatani W; Saito M; Kobayashi Y; Yamamoto A Extraction and Separation Properties of Hydrophilic Compounds Using Novel Water-Holding Adsorbents Bonded with a Zwitter-Ionic Polymer. Chromatographia 2011, 73 (9–10), 849–855, DOI: 10.1007/s10337-011-1965-y [DOI] [Google Scholar]
- (146).Wang G; Wang L; Lin W; Wang Z; Zhang J; Ji F; Ma G; Yuan Z; Chen S Development of Robust and Recoverable Ultralow-Fouling Coatings Based on Poly(Carboxybetaine) Ester Analogue. ACS Appl. Mater. Interfaces 2015, 7 (31), 16938–16945, DOI: 10.1021/acsami.5b05162 [DOI] [PubMed] [Google Scholar]
- (147).Liu CY; Huang CJ Functionalization of Polydopamine via the Aza-Michael Reaction for Antimicrobial Interfaces. Langmuir 2016, 32 (19), 5019–5028, DOI: 10.1021/acs.langmuir.6b00990 [DOI] [PubMed] [Google Scholar]
- (148).Zhang D; Fu Y; Huang L; Zhang Y; Ren B; Zhong M; Yang J; Zheng J Integration of Antifouling and Antibacterial Properties in Salt-Responsive Hydrogels with Surface Regeneration Capacity. J. Mater. Chem. B 2018, 6 (6), 950–960, DOI: 10.1039/c7tb03018e [DOI] [PubMed] [Google Scholar]
- (149).Ma Y; Li J; Si Y; Huang K; Nitin N; Sun G Rechargeable Antibacterial N-Halamine Films with Antifouling Function for Food Packaging Applications. ACS Appl. Mater. Interfaces 2019, 11 (19), 17814–17822, DOI: 10.1021/acsami.9b03464 [DOI] [PubMed] [Google Scholar]
- (150).Wang B; Ye Z; Tang Y; Han Y; Lin Q; Liu H; Chen H; Nan K Fabrication of Nonfouling, Bactericidal, and Bacteria Corpse Release Multifunctional Surface through Surface-Initiated Raft Polymerization. Int. J. Nanomedicine 2017, 12, 111–125, DOI: 10.2147/IJN.S107472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Cheng G; Xue H; Zhang Z; Chen S; Jiang S A Switchable Biocompatible Polymer Surface with Self-Sterilizing and Nonfouling Capabilities. Angew. Chem. Int. Ed. Engl 2008, 47 (46), 8831–8834, DOI: 10.1002/anie.200803570 [DOI] [PubMed] [Google Scholar]


