Abstract
Context: While it is well recognized that physical and physiological changes are more prominent in individuals with higher neurologic levels of spinal cord injury (SCI), the impact of level of lesion on cognition is less clear.
Design: Cross-sectional, 3-group.
Setting: Non-profit rehabilitation research foundation.
Participants: 59 individuals with SCI (30 with tetraplegia, 29 with paraplegia) and 30 age-matched healthy controls (HC).
Interventions: None.
Outcome Measures: Neuropsychological tests in the domains of attention, working memory, processing speed, executive control, and learning and memory.
Results: Results indicated significantly lower test performance in individuals with paraplegia on new learning and memory testing compared to HC. In contrast, compared to HC the group with tetraplegia, showed a significantly impaired performance on a processing speed task, and both the tetraplegia and the paraplegia groups were similarly impaired on a verbal fluency measure. SCI groups did not differ on any cognitive measure.
Conclusion: Individuals with SCI may display different patterns of cognitive performance based on their level of injury.
Keywords: Spinal cord injury, Cognitive deficits, Memory, Processing speed, Executive functioning
Introduction
Traumatic spinal cord injury (SCI) is prevalent.1 Research commonly focuses on physical consequences of SCI and the majority of rehabilitation efforts target motor function. However, cognitive consequences also ensue, with up to 60% of individuals with SCI showing cognitive deficits.2–6 The relative risk of cognitive impairment in persons with SCI is 13 times greater than in able-bodied individuals,7 with deficits documented in attention,2,3,8–10 concentration,3,8–10 new learning and memory (NLM),3,8,9,11 abstract reasoning,3 and processing speed (PS).12 These cognitive deficits occur in both younger and older individuals with SCI2–5 during both acute13 and chronic14 stages. In fact, cognitive deficits that present acutely post-injury appear to worsen over the initial 1–2 years post-injury.13
Cognitive deficits have a negative impact on rehabilitation outcomes following SCI, with less functional gains noted in persons with cognitive deficits.15 In addition, increased risk for re-hospitalization has been reported in persons with SCI with cognitive deficits.16 Not surprisingly, these cognitive deficits exert a significant impact on everyday life, with community and social re-integration,12,17 self-perception and quality of life (QOL) adversely impacted.12,17–19
Despite the evidence of cognitive deficits in persons with SCI and their adverse impact, the cause of these deficits remains undetermined. Concomitant traumatic brain injury (TBI)2–5,20,21 has often been implicated, in addition to secondary trauma (e.g. cerebral edema, hypoxia) and cardiovascular and cerebrovascular dysfunction.11,22,23 Other potential contributors include core body temperature dysregulation,24,25 medication management,26 sleep apnea,27 or treatment for neurogenic urinary tract dysfunction.28,29 Our group recently proposed accelerated brain aging post-injury as a possible explanation for observed cognitive decline.14,22,30
It is widely recognized that individuals with higher neurologic levels of SCI (i.e. T1 and above) have a greater physical disability as compared to lower levels of neurologic injury.31 However, the impact of lesion level on cognition is less clear. Several published studies show no relationship between cognitive impairment and level of SCI.3,7,32–36 However, the literature regarding SCI and cognition remains nascent, and is hampered by significant limitations. For example, several studies use cognitive subscales included within general disability scales32–37,38 to assess cognition, rather than conducting specialized neuropsychological examinations designed to examine cognition specifically. Differences by lesion level emerge with detailed neuropsychological assessment.39
The application of technology to better understand the relationship between cognition and level of lesion has shed light on the relationship between the two. Event related potentials (ERPs) have shown slower response times in those with tetraplegia compared to paraplegia; false-negative errors were also significantly more common in the tetraplegia group.40–43 Positron emission tomography studies have shown altered activation in sensorimotor and subcortical networks with higher injury levels.44,45 Significant differences have also been noted between individuals with tetraplegia and paraplegia in Grey Matter Volume (GMV), with higher-level injuries presenting with reduced levels of GMV.46 The impact of GMV on cognition has not yet been examined.
Much work thus remains to be done to understand the impact of level of injury on cognition. In an effort to identify patterns of cognitive deficits in persons with SCI of different lesion levels, we examined cognition in persons with traumatic SCI at the thoracic versus the cervical level, as compared to age-matched non-SCI healthy controls (HC) using a comprehensive neuropsychological battery. We hypothesized that neuropsychological performance in persons with SCI would differ by level of injury, and that, regardless of level of injury, performance would significantly differ from HC.
Methods
Participants
Recruitment ran from October 2013-March 2016. Fifty-nine individuals with SCI were enrolled, including 30 individuals with tetraplegia (injury level C3-T1) and 29 individuals with paraplegia (T2-T12). We additionally enrolled 30 non-SCI healthy controls (HC), age-matched to participants with SCI (30–40 years old).
All participants demonstrated intact visual acuity (minimum 20/60 in worst eye with prescription eyewear) and English proficiency. Participants with SCI had an American Spinal Injury Association Impairment Scale (AIS) grade A, B or C; all were non-ambulatory (wheelchair usage ≥40 h/week) and were at least 1-year post-injury (range: 1–31 years). Exclusion criteria included positive neurological history (e.g. stroke, multiple sclerosis), significant psychiatric history (e.g. post-traumatic stress disorder, schizophrenia, bipolar disorder), and illicit drug abuse within the past 6-months. Participants were excluded for a documented history of TBI, including TBI co-occurring with SCI, via medical record review and patient self-report. As the study recruitment site also operates as a TBI Model System, research assistants were trained to recognize diagnostic criteria for TBI based on TBIMS standards (i.e. combination of Glasgow Coma Scale, loss of consciousness, post-traumatic amnesia, and neuroimaging). Additionally, participants were excluded for evidence of metabolic syndrome, including diabetes. Other risk factors for cardiac disease were noted including obesity and high cholesterol (Table 1). Individuals with diagnosis of dementia were excluded, as well as individuals scoring ≤22 on a motor-free adaptation (i.e. revised 3-step command to “smile, look at the door, and wiggle your nose”, visuospatial discrimination task instead of constructional praxis) of the Mini-Mental Status Examination (MMSE).47
Table 1. Demographic information by group.
| Tetraplegia n = 30 |
Paraplegia n = 29 |
HC n = 30 |
ANOVA | Chi-square | |
|---|---|---|---|---|---|
| Age (years) | 36.57 (7.57) | 34.31 (6.38) | 35.73 (7.35) | 0.754 | – |
| Sex (% male) | 83.33% | 89.65% | 76.67% | 1.776 | |
| Ethnicity (%) | – | 3.779 | |||
| Caucasian | 53.33% | 41.38% | 50.00% | 0.895 | |
| African American | 30.00% | 44.83% | 26.67% | 2.455 | |
| Asian | 3.33% | 3.45% | 10.00% | 1.639 | |
| Hispanic | 13.33% | 10.34% | 13.33% | 0.161 | |
| Native American | 0.00% | 0.00% | 0.00% | – | |
| Other | 0.00% | 0.00% | 0.00% | – | |
| Education (years) | 13.87 (2.36) | 13.31 (2.06) | 15.5 (1.7) | 9.102*** | – |
| WASI-II Vocabulary SS | 10.22 (2.56) | 8.66 (3.10) | 11.79 (2.6) | 9.340*** | – |
| ASIA Classifications | |||||
| A | 46.67% | 55.17% | – | ||
| B | 33.33% | 20.69% | – | ||
| C | 20.00% | 24.14% | – | ||
| D | 0.00% | 0.00% | – | ||
| E | 0.00% | 0.00% | – | ||
| Cardiovascular Risk Comorbidities | |||||
| Obesity | 3 | 9 | 7 | – | 3.992 |
| Hypercholesterolemia | 0 | 1 | 0 | – | 2.092 |
| Hypertension | 0 | 4 | 4 | – | 4.473 |
| Medications | |||||
| Statins | 0 | 0 | 1 | – | 2.938 |
| Psychotropic/pain Rx | 8 | 9 | 0 | – | 8.457* |
| Baclofen | 18 | 10 | 0 | – | 19.857*** |
| Oxybutynin | 17 | 8 | 0 | – | 19.100*** |
| Chicago Multiscale Depression Inventory (Total T-score) | 53.05 | 47.40 | 47.35 | 1.041 | – |
| State Trait Anxiety Inventory – State (Standard Score) | 53.52 | 46.40 | 44.83 | 2.567 | – |
| State Trait Anxiety Inventory – Trait (Standard Score) | 54.09 | 47.20 | 47.75 | 1.515 | – |
*P < 0.05, **P < 0.01, ***P < .001.
Participants with SCI were enrolled prior to HCs to facilitate age-matching. Individuals with SCI were recruited from the Northern New Jersey SCI Model (NNJSCIS) System, and through posted flyers at support groups and treatment facilities. HC participants were recruited from the local community and flyers and were matched to SCI participants for sex, race, smoking status, socioeconomic status, IQ, and education (±1 year), to the best of our ability. Participants with SCI were screened for level and severity of injury based on prior evaluation using the International Standards for the Neurological Classification of SCI (ISNCSCI).48
There was no significant difference between the groups in age (Table 1). However, the groups were significantly different in education (F(2,86) = 9.10, P < .001) and pre-morbid Verbal IQ (VIQ) as estimated by the WASI-II Vocabulary subtest (F(2,85) = 9.34, P < .001). The tetraplegia and paraplegia groups both had significantly fewer years of education (t(58) = 3.08, P = 0.008; t(58) = 4.09, P < .001, respectively) as compared with HC. The paraplegia group also had a lower estimated pre-morbid verbal IQ (t(58) = 4.32, P < 0.001) compared to HC. Given that education and WASI-II VIQ were significantly correlated (P < 0.001), all subsequent analyses utilized only education as a co-variate, rather than both variables. Additionally, there were significant differences between the groups on prescribed medications (i.e. oxybutynin, baclofen, psychotropic, pain medications). However, due to the risk of multicollinearity between these variables and the grouping variable, as well as the diminished power if these factors were all included as covariates, these variables were not included in each model.
Neuropsychological Assessment was completed by all participants and was motor-free. (Normative scores were calculated based on reference listed with test name below.)
Digit Span, Wechsler Intelligence Scale–III (WAIS-III)49 assessed simple attention (forward) and WM (backward). In each segment, the examiner reads random number sequences aloud at the rate of one-per-second. WAIS III Digit Span has high internal consistency reliability (r = .90) and construct validity.49
Symbol Digit Modalities Test (SDMT)50 – oral version examined PS. Participants are asked to quickly orally substitute numbers for geometric symbols based on a provided key over 90 s. The SDMT has good alternate forms (r = .82, r = .84) and test-retest (r = .76) reliability. It is sensitive to cognitive deficits across populations.50,51
Letter-Number Sequencing, (WAIS-III LNS)49 quantified auditory WM. Numbers and letters are orally presented in a specified random order. The subjects reorganize the numbers in ascending order, followed by the letters in alphabetical order. High internal consistency reliability (r = .90) and construct validity has been demonstrated, as well as differential sensitivity to several neurocognitive disorders.49
The California Verbal Learning Test-II (CVLT-II)52 assesses verbal NLM. Sixteen words from 4 semantic categories are read aloud by the examiner over 5 trials; after each trial, the examinee freely recalls as many words as possible. A 20-minute delayed recall and recognition trial follow. High internal consistency (r = .94), split-half reliability (r = .83), test-retest reliability (r = .82) and construct validity have been shown.52
The Paced Auditory Serial Addition Test (PASAT)53 places demands on both PS and WM, by asking participants to add a series of aurally presented digits to the one immediately preceding it. Four trials are presented, each containing 50 digits; each of the 4 trials differs in speed of presentation. The PASAT has high internal consistency54 and spilt half reliability (r = .96),54 is sensitive to PS deficits,55 neurocognitive syndromes including concussion56,57 and diffuse cerebral damage.58
The Delis-Kaplan Executive Function System (D-KEFS)59
Verbal Fluency subtest requires the verbal generation of words in 60 s to both letters and categories. Prompts are provided by the examiner for letters (F, A, S), followed by semantic categories (e.g. animals).
The Stroop Color-Word Interference Test measures inhibitory control and cognitive flexibility through color naming and word reading tasks, followed by a color-word interference trial, which requires the examinee to inhibit the automatic response (reading) with the desired response (naming ink color). The speed component was removed from the total to allow for a pure measure of inhibitory control (Trial 3-Trial 1).
The Wechsler Abbreviated Scale of Intelligence- Second Edition (WASI-II), Vocabulary subtest60 assessed pre-morbid IQ. High intercorrelations between the WASI and WAIS-III IQ scales have consistently been documented (range: 0.66–0.92).60
The Chicago Multiscale Depression Inventory (CMDI)61 is a self-report depression measure that differentiates between aspects of depression (i.e. vegetative, affective, evaluative). It has high internal consistency (e.g. Cronbach’s Alpha = 0.84) and demonstrates convergent validity with the Beck Depression Inventory (r = 0.68).61 Higher T-scores indicate greater symptoms.
The State Trait Anxiety Inventory (STAI)62 is a standardized, well-established measure of current feelings of anxiety, including calmness, security, fear. Test-retest reliability is relatively high for trait anxiety and appropriately lower for state anxiety, given its natural fluctuations. The STAI-Trait Anxiety Scale shows high correlations with other measures of trait anxiety (IAPT Anxiety Scale r = .75;63 Taylor Manifest Anxiety Scale r = .8064). Higher T-scores indicate greater symptoms.
Procedures
Potential participants were initially screened via telephone and, if eligible, invited to complete study procedures. The neuropsychological test battery was administered over 2–2.5 h by a Bachelor’s level research assistant (RA) trained in testing procedures. Complete assessment also included cardiovascular/cerebrovascular evaluation (heart rate[HR], finger blood pressure[BP], cerebral blood flow velocity[CBFv], and brachial BP), collected while the subject was resting in the seated position and during neuropsychological testing. The cardiovascular/cerebrovascular evaluation was conducted by a Master’s level RA well-trained in the collection of cardiovascular/cerebrovascular data. The current paper focuses on patterns of neuropsychological test performance between groups; the cardiovascular/cerebrovascular data have been published elsewhere.65 Data were collected at Kessler Foundation (KF) in collaboration with investigators at the James J. Peters Veterans Affair Medical Center (JJPVAMC). Institutional Review Board approvals were obtained at both institutions and informed consent was obtained prior to initiating study procedures. To minimize bias, data collectors remained blind to study hypotheses.
Data analysis
Statistical analyses were performed using JASP (Version 0.9.2; JASP Team (2019). JASP (Version 0.9.2) [Computer software] (BibTeX); University of Amsterdam); continuous data are reported as mean ± standard deviation. Analysis of Covariance (ANCOVA) with group as the between subjects’ variable (HC, Tetraplegia, Paraplegia) was conducted on standardized scores from each test. Normatively corrected scores were utilized for all analyses to facilitate the identification of differences in performance between groups and allow interpretation of the extent of deficits. Education was a covariate in all analyses due to significant group differences. Note that, despite a similar significant group difference in WASI-II Vocabulary, only education was used as a covariate due to the strong correlation between both proxies of intelligence, as consistent with neuropsychological normative practice.66 Tukey LSD tests were calculated for pair-wise comparisons where appropriate. Significance was set at P < 0.05, and analyses were well-powered to detect medium effect sizes at the omnibus level (1 – β = 0.83 for effect size f = 0.31). There were no missing data points on any primary outcomes.
Results
Analyses were conducted on standardized scores wherever available to control for age-related variability in performance and provide a common metric across tests through which we could interpret performance. Results indicated significant differences between groups in specific cognitive domains, with distinct patterns noted between those with paraplegia and tetraplegia relative to HC.
New learning and memory (NLM)
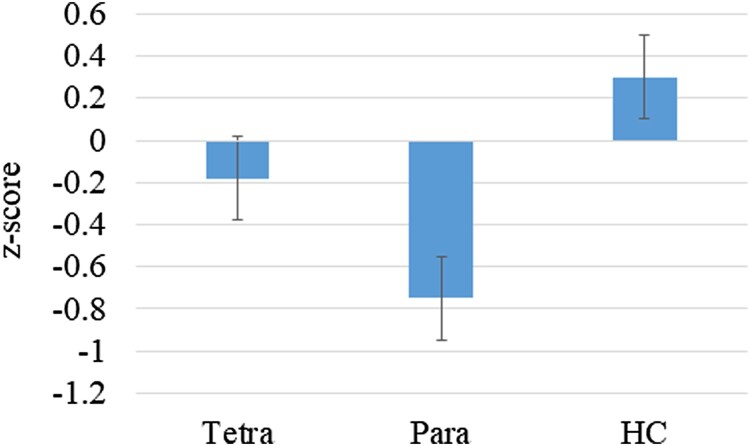
A significant difference between the groups was noted on CVLT-II short delay free recall (F(2,85) = 5.76, P = .004, η2 = .12; Fig. 1), with a trend on total words recalled on initial learning trials 1–5 (F(2,85) = 2.90, P = .06; η2 = .063). In both cases, the paraplegia group performed at lower levels than HC (Table 2); there were no significant differences between HC and the tetraplegia group. There were no significant differences between the groups on long delay free recall.
Figure 1.
Performance on the CVLT-II short delay free recall by group (z scores); P < .01.
Table 2. Neuropsychological test performance by group.
| Tetraplegia | Paraplegia | HC | F Value | |
|---|---|---|---|---|
| Performance Validity | ||||
| WAIS-III Reliable Digit Span Score | 9.77 (2.01) | 9.36 (2.02) | 9.67 (1.86) | 0.34, ns |
| Learning & Memory | ||||
| CVLT Total Recall across 5 trials (T) | 46.65 (14.10) | 45.24 (8.90) | 53.93 (11.98) | 2.90, P = 0.06 |
| CVLT SDFR (z) | –0.18 (1.02) | –0.76 (1.15) | 0.3 (1.01) | 5.76, P = 0.004 |
| CVLT LDFR (z) | –0.48 (1.3) | –0.62 (1.09) | 0.1 (1.11) | 2.06, ns |
| Working Memory / Processing Speed | ||||
| Digit Span ss | 9.30 (2.83) | 9.14 (2.72) | 9.9 (2.35) | 0.30, ns |
| Letter Number Sequencing ss | 9.68 (2.57) | 9.52 (3.20) | 9.67 (1.58) | 0.01, ns |
| SDMT z score | –1.52 (0.77) | –1.04 (1.19) | –0.59 (1.11) | 5.18, P = 0.008 |
| PASAT Total | –0.87 (1.02) | –1.14 (1.09) | –0.36 (0.85) | 1.99, ns |
| Executive Control | ||||
| Letter Fluency | 9.63 (2.61) | 8.83 (3.23) | 11.87 (3.12) | 3.85, P = 0.03 |
| Category Fluency | 9.17 (3.62) | 9.45 (3.43) | 11.67 (3.26) | 1.53, ns |
| Stroop–Color Naming | 8.60 (3.04) | 9.07 (2.32) | 9.17 (2.31) | 0.94, ns |
| Stroop-Word Reading | 9.13 (2.47) | 9.21 (3.11) | 10.43 (2.0) | 1.78, ns |
| Stroop Inhibition | 9.80 (3.21) | 9.43 (4.07) | 10.93 (1.86) | 0.96, ns |
*Peach shading denotes pairwise significant difference between shaded groups; significant differences are noted in SCI group (paraplegia, tetraplegia) vs HC; no significant differences were noted between the 2 SCI groups directly. F value is reported for omnibus statistical test.
Attention (ATTN), working memory (WM), processing speed (PS)
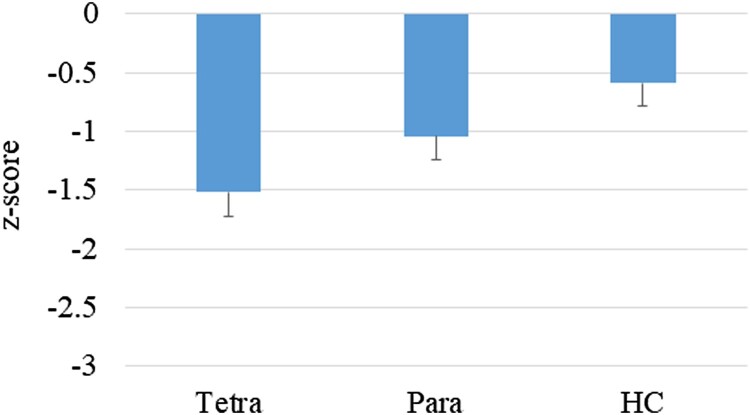
A significant difference was noted on the SDMT, a test of PS (F(2,85) = 5.18, P = .008; η2 = 0.11; Fig. 2). The group with tetraplegia performed at significantly lower levels than HC (P = .006, Cohen’s d = 0.95; Table 2). There was no significant difference between the HC and paraplegia groups.
Figure 2.
Performance on the SDMT by group (z-scores); P = .008.
There were no significant between-group differences on Digit Span, Letter-Number Sequencing or the PASAT.
Executive control
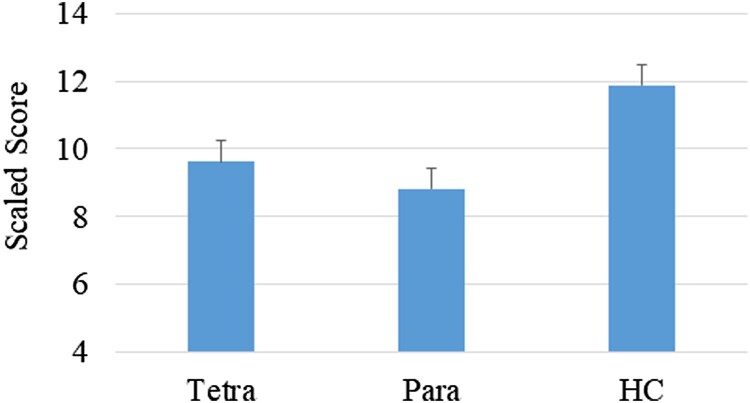
A significant difference was noted between the groups on letter fluency (F(2,85) = 3.86, P = .03, η2 = .08), namely between the HC group and both the paraplegia (P = 0.04,Cohen’s d = 0.73) and the tetraplegia (P = .04, Cohen’s d = 0.64) groups; no significant difference was noted between the 2 SCI groups (Fig. 3). There were no significant group differences on the DKEFS-Color Word Interference test.
Figure 3.
Performance on letter fluency by group (scaled scores); P = .03.
(Note that there were no cognitive domains in which the tetraplegia and paraplegia groups significantly differed in performance.)
Discussion
It is accepted that higher injury levels injury result in greater physical disability;31 however, few studies to date have examined the relationship between lesion level and cognitive performance in persons with SCI. Consistent with our hypotheses, results showed that neuropsychological performance indeed differs by level of injury, and performance overall significantly differed from HC. Significantly poorer NLM was noted in those with paraplegia and impaired PS in those with tetraplegia, as compared to HC. Both tetraplegia and paraplegia groups demonstrated impaired verbal fluency as compared with the HC.
Study findings are contradictory to much of the current literature indicating no relationship between cognitive impairment and level of SCI.3,7,32–36 When examining the methodological commonalities and distinctions between other studies and ours, one notable difference is the method for assessing cognition. Specifically, cognition can be assessed using general screening measures that evaluate global cognition versus using a detailed neuropsychological assessment, sampling all domains of cognition specifically. It is only through these detailed assessments that more subtle cognitive changes can be detected. Oftentimes, these two types of measurement result in vastly different results, as is the case in the SCI literature. That is, several of the studies that failed to show a relationship between cognitive profile and level of injury utilized cognitive subscales that are part of general disability scales, such as the Functional Independence Measure (FIM)32–37 and the Craig Handicap Assessment and Reporting Technique (CHART),38 rather than specialized neuropsychological testing designed to examine cognition specifically. In fact, many of these studies explicitly noted ceiling effects in SCI groups on measures such as the FIM.32,35–37 Hall and colleagues32 noted that the FIM cognition items are best seen as rudimentary screening tools for cognitive deficits.
Other studies have utilized domain-driven neuropsychological assessment to identify patterns of cognitive deficits in different levels of SCI. Importantly however, these existing studies did not approach analysis of the neuropsychological assessment exactly as in the current study. Specifically, Richards et al.5 focused on the change in cognition over time and only examined level of injury by time, noting no differences. The authors did not examine the impact of level of injury solely on patterns of cognitive performance. Additionally, Richard et al. focused on the delayed aspects of memory function, wherein we did not note differences by level of injury in the current study, and they did not examine PS, which has been frequently observed in the SCI literature as an impaired domain.67 Similarly, neither Wilmot and colleagues3 nor Tun and colleagues68 assessed PS or list learning, the tests on which those with paraplegia and tetraplegia differed in our sample. The current study is most comparable in approach to work conducted by Macciocchi and colleagues.69 While this group utilized a similar neuropsychological assessment, they did not report differences in cognitive profile by level of injury; however, the participants were within 1-year of injury (on average one month post-injury), which differed from our chronically injured cohort (on average 10 years post-injury). This difference in time post-SCI may have implications regarding the pattern and trajectory of cognitive deficits post-injury.
Due to differences in the anatomic level of injury to the autonomic nervous system, systemic and cerebral hemodynamic responses during testing may explain, in part, the differences noted in cognitive profiles between individuals with tetraplegia and paraplegia. Anatomic differences in the level of injury to the autonomic nervous system results in an inability to adequately regulate systemic blood pressure or cerebral blood flow during testing, which may account for differences in cognitive performance between individuals with paraplegia compared to tetraplegia. That is, we and others have noted associations between performance on tasks of memory and PS and changes in blood pressure and cerebral blood flow velocity in individuals with tetraplegia due to persistent and episodic hypotension.11,23,30 Whereas, individuals with paraplegia are not generally hypotensive, we previously reported increases in the cerebral vascular resistance index associated with poorer performance during a task of attention and cognitive flexibility, suggesting differential cardiovascular risk factors between those with upper level and lower level injuries.39,70 Additionally, improved cognitive performance has been noted in patients with Parkinson’s disease71 and in otherwise healthy but sedentary controls72 following endurance training designed to improve cardiovascular outcomes. Therefore, these cognitive deficits may be improved with relatively minimal physical activity in persons with SCI, regardless of level of injury. Because the etiology of cognitive deficits in persons with SCI remains elusive and likely multifactorial, understanding patterns of deficits by level of injury will provide insight regarding the underpinnings and will help guide clinical interventions.
Other approaches to quantify brain activity have shown corroborating findings by injury level. Lazzaro and colleagues43 examined abnormalities in ERPs by level of injury, and reported slowed response time in individuals with tetraplegia compared paraplegia; false-negative errors were also significantly more common in the tetraplegia group. Imaging studies have further shown distinctly different patterns of brain function between those with paraplegia and tetraplegia. Increased alteration in activation of sensorimotor and subcortical networks with higher levels of injury have been noted in positron emission tomography studies,44,45 while Karunakaran46 and colleagues showed a significant difference in grey matter volume (GMV) by injury level, with higher-level cord injuries presenting with reduced GMV levels.46 The impact of these alterations in brain structure and function on cognition is an important future direction.
We administered 3 tasks of executive functioning and individuals with SCI, regardless of level of injury, performed more poorly on the letter fluency test, but neither group demonstrated impaired function on category fluency or color-word inhibition relative to HC. Importantly, category fluency relies less on frontal-striatal circuitry (i.e. executive processes) and more on medial temporal circuitry,73,74 while the color-word inhibition task measures inhibition specifically, with less dependence on the speed component. Thus, this pattern of deficit in executive function suggests that persons with SCI, regardless of the level of injury, have difficulty on tasks requiring the contribution of multiple cognitive domains for successful completion (e.g. speed, working memory, executive control). This is a hypothesis that has yet to be addressed due to limitations inherent in the neuropsychological tests currently available. That is, there is only a limited pool of neuropsychological measures that allow for motor-free administration. Future work should develop motor-free neuropsychological measures to examine the assessment of each cognitive domain more comprehensively in the SCI population.
The current study is not without its limitations. First is the general lack of motor-free neuropsychological assessment measures, which substantially limits the measures available for administration to persons with SCI as well as the conclusions that can be drawn. Research should focus on the development of motor-free measures of multiple cognitive domains to enable complete neuropsychological assessment in persons with upper extremity motor limitations. We also limited our assessment to cognitive domains in which we anticipated the greatest likelihood of deficit (cf. visuospatial ability), and therefore did not evaluate the full gamut of neuropsychological functions. Furthermore, our decision to exclude individuals with comorbid or prior history of TBI may reduce generalization to the broader SCI population. Relatedly, we were unable to comprehensively assess the full range of comorbid conditions that may have impacted our findings (e.g. sleep apnea); future research should explore the relationship of these conditions as potential underlying mechanisms for cognitive deficits at different injury levels. In addition, the sample size in the current research study was smaller than ideal. A larger sample size would have increased power so that all potential confounding factors could have been included in each model (e.g. medications). Replication of this work with larger samples is warranted.
Conclusion
Despite these limitations, findings of the current study have important implications. It is essential that SCI be recognized as a neurological injury that can adversely impact cognition. Screening for such deficits and subsequent treatment, if appropriate, is necessary to maximize quality of life and facilitate full participation in society. Further research is necessary to identify the source of these deficits in an effort to effectively treat cognitive limitations and maximize functional independence.
Disclaimer statements
Contributors None.
Funding This work was supported by the New Jersey Commission on Spinal Cord Research [grant number CSCR13IRG018], the Rehabilitation Research and Development Service [grant number B9212-C & B2020-C] and the National Institute on Disability, Independent Living, and Rehabilitation Research [NIDILRR grant number 90SI5026].
Conflicts of interest None.
References
- 1.National Spinal Cord Injury Statistical Center . Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2017. doi: 10.1179/1079026813Z.000000000136 [DOI] [Google Scholar]
- 2.Davidoff G, Morris J, Roth E, Bleiberg J.. Cognitive dysfunction and mild closed head-injury in traumatic spinal-cord injury. Arch Phys Med Rehabil. 1985. doi:0003-9993(85)91232-8 [pii] [PubMed] [Google Scholar]
- 3.Wilmot CB, Cope DNN, Hall KM, Acker M.. Occult head injury: its incidence in spinal cord injury. Arch Phys Med Rehabil. 1985;66(4):227–31. doi: 10.1016/0003-9993(85)90148-0 doi: 10.1016/0003-9993(85)90148-0 [DOI] [PubMed] [Google Scholar]
- 4.Davidoff G, Thomas P, Johnson M, Berent S, Dijkers M, Doljanac R.. Closed head injury in acute traumatic spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil. 1988. doi: 10.1159/000103226 [DOI] [PubMed] [Google Scholar]
- 5.Richards J, Brown L, Hagglund K, Bua G, Reeder K.. Spinal cord injury and concomitant traumatic brain injury. Results of a longitudinal investigation. Am J Phys Med Rehabil. 1988;67(5):211–6. doi: 10.1097/00002060-198810000-00005 doi: 10.1097/00002060-198810000-00005 [DOI] [PubMed] [Google Scholar]
- 6.Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K.. Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc. 1997;3(5):464–72. doi: 10.1017/S1355617797004645 doi: 10.1017/S1355617797004645 [DOI] [PubMed] [Google Scholar]
- 7.Craig A, Guest R, Tran Y, Middleton J.. Cognitive impairment and Mood States after spinal cord injury. J Neurotrauma. 2017;34(6):1156–63. doi: 10.1089/neu.2016.4632 [DOI] [PubMed] [Google Scholar]
- 8.Davidoff G, Roth E, Thomas P, et al. Depression and neuropsychological test peformance in acute spinal cord injury patients: lack of correlation. Arch Clin Neuropsychol. 1990;5(1):77–88. doi: 10.1016/0887-6177(90)90009-E doi: [DOI] [PubMed] [Google Scholar]
- 9.Davidoff GN, Roth EJ, Haughton JS, Ardner MS.. Cognitive dysfunction in spinal cord injury patients: sensitivity of the functional independence measure subscales vs neuropsychologic assessment. Arch Phys Med Rehabil. 1990;71(5):326–9. [accessed 2019 September 12]. Available from https://www.ncbi.nlm.nih.gov/pubmed/2327886. [PubMed] [Google Scholar]
- 10.Roth E, Davidoff G, Thomas P, et al. A controlled study of neuropsychological deficits in acute spinal cord injury patients. Paraplegia. 1989;27(6):480–9. doi: 10.1038/sc.1989.75 [DOI] [PubMed] [Google Scholar]
- 11.Jegede AB, Rosado-Rivera D, Bauman WA, et al. Cognitive performance in hypotensive persons with spinal cord injury. Clin Auton Res. 2010;20(1):3–9. doi: 10.1007/s10286-009-0036-z doi: 10.1007/s10286-009-0036-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowler RN, O’Brien SA, Haaland KY, et al. Neuropsychological functioning following a spinal cord injury. Appl Neuropsychol. 1995. doi: 10.1080/09084282.1995.9645349 [DOI] [PubMed] [Google Scholar]
- 13.Molina B, Segura A, Serrano JP, et al. Cognitive performance of people with traumatic spinal cord injury: a cross-sectional study comparing people with subacute and chronic injuries. Spinal Cord. 2018;56(8):796–805. doi: 10.1038/s41393-018-0076-0 doi: 10.1038/s41393-018-0076-0 [DOI] [PubMed] [Google Scholar]
- 14.Chiaravalloti ND, Weber E, Wylie G, Dyson-Hudson T, Wecht JM.. Patterns of cognitive deficits in persons with spinal cord injury as compared with both age-matched and older individuals without spinal cord injury. J Spinal Cord Med. 2018. doi: 10.1080/10790268.2018.1543103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macciocchi SN, Bowman B, Coker J, Apple D, Leslie D.. Effect of co-morbid traumatic brain injury on functional outcome of persons with spinal cord injuries. Am J Phys Med Rehabil. 2004;83(1):22–6. doi: 10.1097/01.PHM.0000104661.86307.91 doi: 10.1097/01.PHM.0000104661.86307.91 [DOI] [PubMed] [Google Scholar]
- 16.Davidoff G, Schultz JS, Lieb T, et al. Rehospitalization after initial rehabilitation for acute spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil. 1990;71:121–24. [PubMed] [Google Scholar]
- 17.Davidoff GN, Roth EJ, Richards JS.. Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch Phys Med Rehabil. 1992;73(3):275–84. doi: 10.1140/epjc/s10052-010-1415-2 [DOI] [PubMed] [Google Scholar]
- 18.Weber E, Wecht JM, Katzelnick C, Dyson-Hudson T, Chiaravalloti N.. Learning and Memory Profile of Individuals with Spinal Cord Injury. Washington, DC: International Neuropsychological Society Annual Convention; 2018. [Google Scholar]
- 19.Murray RF, Asghari A, Egorov DD, et al. Impact of spinal cord injury on self-perceived pre- and postmorbid cognitive, emotional and physical functioning. Spinal Cord. 2007. doi: 10.1038/sj.sc.3102022 [DOI] [PubMed] [Google Scholar]
- 20.Davidoff G, Morris J, Roth E, Bleiberg J.. Closed head injury in spinal cord injured patients: retrospective study of loss of consciousness and post-traumatic amnesia. Arch Phys Med Rehabil. 1985;66(1):41–3. [accessed 2019 September 12]. Available from https://www.ncbi.nlm.nih.gov/pubmed/3966867. [PubMed] [Google Scholar]
- 21.Schueneman A, Morris J.. Neuropsychological deficits associated with spinal cord injury. Spinal Cord Inj Dig. 1982;4(35):64. [Google Scholar]
- 22.Katzelnick CG, Weir JP, Chiaravalloti ND, et al. Impact of blood pressure, lesion level, and physical activity on aortic augmentation index in persons with spinal cord injury. J Neurotrauma. 2017;34(24). doi: 10.1089/neu.2017.5065 doi: 10.1089/neu.2017.5065 [DOI] [PubMed] [Google Scholar]
- 23.Phillips AAA, Krassioukov AV V., Ainslie PNN, Cote ATT, Warburton DERER.. Increased central arterial stiffness explains baroreflex dysfunction in spinal cord injury. J Neurotrauma. 2014;31(12):1122–8. doi: 10.1089/neu.2013.3280 doi: 10.1089/neu.2013.3280 [DOI] [PubMed] [Google Scholar]
- 24.Handrakis JP, Liu SA, Rosado-Rivera D, et al. Effect of mild cold exposure on cognition in persons with tetraplegia. J Neurotrauma. 2015. doi: 10.1089/neu.2014.3719 [DOI] [PubMed] [Google Scholar]
- 25.Handrakis JP, Ni Guan Z, Nulty JW, et al. Effect of heat exposure on cognition in persons with tetraplegia. J Neurotrauma. 2017;34(24):3372–80. doi: 10.1089/neu.2016.4850 doi: 10.1089/neu.2016.4850 [DOI] [PubMed] [Google Scholar]
- 26.Krystal JH, Perry EB, Gueorguieva R, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005. doi: 10.1001/archpsyc.62.9.985 [DOI] [PubMed] [Google Scholar]
- 27.Schembri R, Spong J, Graco M, Berlowitz DJJ.. Neuropsychological function in patients with acute tetraplegia and sleep disordered breathing. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw037 doi: 10.1093/sleep/zsw037 [DOI] [PubMed] [Google Scholar]
- 28.Wyndaele JJ. The management of neurogenic lower urinary tract dysfunction after spinal cord injury. Nat Rev Urol. 2016;13(12):705–14. doi: 10.1038/nrurol.2016.206 doi: 10.1038/nrurol.2016.206 [DOI] [PubMed] [Google Scholar]
- 29.Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721–32. doi: 10.1001/jamaneurol.2016.0580 doi: 10.1001/jamaneurol.2016.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wecht J, Weir J, Katzelnick C, et al. Systemic and cerebral hemodynamic contribution to cognitive performance in spinal cord injury. J Neurotrauma. 2018;35:2957–64. [DOI] [PubMed] [Google Scholar]
- 31.Bedbrook G. The Care and Management of Spinal Cord Injuries. New York: Springer Verlag; 1981. [Google Scholar]
- 32.Hall KMM, Cohen MEE, Wright J, Call M, Werner P.. Characteristics of the functional independence measure in traumatic spinal cord injury. Arch Phys Med Rehabil. 1999;80(11):1471–6. doi: 10.1016/S0003-9993(99)90260-5 doi: 10.1016/S0003-9993(99)90260-5 [DOI] [PubMed] [Google Scholar]
- 33.Zonfrillo MR, Durbin DR, Winston FK, Zhang X, Stineman MG.. Residual cognitive disability after completion of inpatient rehabilitation among injured children. J Pediatr. 2014;164(1):130–5. [accessed 2019 September 12]. Available from https://www.sciencedirect.com/science/article/abs/pii/S0022347613011438. doi: 10.1016/j.jpeds.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Copes WS, Stark MM, Lawnick MM, et al. Linking data from national trauma and rehabilitation registries. J Trauma - Inj Infect Crit Care. 1996. doi: 10.1097/00005373-199603000-00018 [DOI] [PubMed] [Google Scholar]
- 35.Masedo AI, Hanley M, Jensen MP, Ehde D, Cardenas DD.. Reliability and validity of a self-report FIM (FIM-SR) in persons with amputation or spinal cord injury and chronic pain. Am J Phys Med Rehabil. 2005;84(3):167–76. doi: 10.1097/01.PHM.0000154898.25609.4A doi: 10.1097/01.PHM.0000154898.25609.4A [DOI] [PubMed] [Google Scholar]
- 36.Middleton JWW, Truman G, Geraghty TJJ.. Neurological level effect on the discharge functional status of spinal cord injured persons after rehabilitation. Arch Phys Med Rehabil. 1998;79(11):1428–32. doi: 10.1016/S0003-9993(98)90239-8 doi: 10.1016/S0003-9993(98)90239-8 [DOI] [PubMed] [Google Scholar]
- 37.Barbetta DCC, Cassemiro LCC, Assis MRR.. The experience of using the scale of functional independence measure in individuals undergoing spinal cord injury rehabilitation in Brazil. Spinal Cord. 2014;52(4):276–81. doi: 10.1038/sc.2013.179 doi: 10.1038/sc.2013.179 [DOI] [PubMed] [Google Scholar]
- 38.Samuelkamaleshkumar S, Radhika S, Cherian B, et al. Community reintegration in rehabilitated south Indian persons with spinal cord injury. Arch Phys Med Rehabil. 2010;91(7):1117–21. [accessed 2019 September 12]. Available from https://www.sciencedirect.com/science/article/pii/S0003999310002327. doi: 10.1016/j.apmr.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 39.Wecht JM, Rosado-Rivera D, Jegede A, et al. Systemic and cerebral hemodynamics during cognitive testing. Clin Auton Res. 2012;22(1):25–33. doi: 10.1007/s10286-011-0139-1 doi: 10.1007/s10286-011-0139-1 [DOI] [PubMed] [Google Scholar]
- 40.Ament PA, Cohen MJ, Schandler SL, Sowa M, Vulpe M.. Auditory P3 event related potentials (ERP) and brainstem auditory evoked responses (BAER) after spinal cord injury in humans. J Spinal Cord Med. 1995. doi: 10.1080/10790268.1995.11719395 [DOI] [PubMed] [Google Scholar]
- 41.Lacourse MGG, Cohen MJJ, Lawrence KEE, Romero DHH.. Cortical potentials during imagined movements in individuals with chronic spinal cord injuries. Behav Brain Res. 1999;104(1-2):73–88. doi: 10.1016/S0166-4328(99)00052-2 doi: 10.1016/S0166-4328(99)00052-2 [DOI] [PubMed] [Google Scholar]
- 42.Di Russo F, Bultrini A, Brunelli S, et al. Benefits of sports participation for executive function. J Neurotrauma. 2010. doi: 10.1089/neu.2010.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazzaro I, Tran Y, Wijesuriya N, Craig A.. Central correlates of impaired information processing in people with spinal cord injury. J Clin Neurophysiol. 2013;30(1):59–65. doi: 10.1097/WNP.0b013e31827edb0c doi: 10.1097/WNP.0b013e31827edb0c [DOI] [PubMed] [Google Scholar]
- 44.Bruehlmeier M, Dietz V, Leenders KLL, Roelcke U, Missimer J, Curt A.. How does the human brain deal with a spinal cord injury? Eur J Neurosci. 1998;10(12):3918–22. doi: 10.1046/j.1460-9568.1998.00454.x doi: 10.1046/j.1460-9568.1998.00454.x [DOI] [PubMed] [Google Scholar]
- 45.Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V.. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma. 2002. doi: 10.1089/089771502753460222 [DOI] [PubMed] [Google Scholar]
- 46.Karunakaran KD, He J, Zhao J, et al. Differences in cortical gray matter atrophy of paraplegia and tetraplegia after complete spinal cord injury. J Neurotrauma. 2019;36(12):2045–51. [DOI] [PubMed] [Google Scholar]
- 47.Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 48.Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wechsler D. Wechsler adult intelligence scale (WAIS-3R). Psychol Corp. 1997. [Google Scholar]
- 50.Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 51.Costa SL, Genova HM, Deluca J, Chiaravalloti ND.. Information processing speed in multiple sclerosis: past, present, and future. Mult Scler. 2017;23(6). doi: 10.1177/1352458516645869 doi: 10.1177/1352458516645869 [DOI] [PubMed] [Google Scholar]
- 52.Delis D, Kramer J, Kaplan E, Ober B.. California Verbal Learning Test® – Second Edition (CVLT®-II). San Antonio, TX: Pearson; 2000. [Google Scholar]
- 53.Brittain JL, La Marche JA, Reeder KP, Roth DL, Boll TJ.. Effects of age and IQ on paced auditory serial addition task (PASAT) performance. Clin Neuropsychol. 1991. doi: 10.1080/13854049108403300 [DOI] [Google Scholar]
- 54.Strauss EH, Sherman EMS, Spreen O.. A compendium of neuropsychological tests. Adm Norms Comment. 2006. doi:2168651.2168654. [Google Scholar]
- 55.Gronwall D, Wrightson P.. Memory and information processing capacity after closed head injury. J Neurol Neurosurg Psychiatry. 1981. doi: 10.1136/jnnp.44.10.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gronwall D, Wrightson P.. Delayed recovery of intellectual function after minor head injury. Lancet. 1974;304(7881):605–9. doi: 10.1016/S0140-6736(74)91939-4 doi: 10.1016/S0140-6736(74)91939-4 [DOI] [PubMed] [Google Scholar]
- 57.Gronwall D, Sampson H.. The Psychological Effects of Concussion. New Zealand: Oxford University Press; 1974. [Google Scholar]
- 58.Roman DD, Edwall GE, Buchanan RJ, Patton JH.. Extended norms for the paced auditory serial addition task. Clin Neuropsychol. 1991. doi: 10.1080/13854049108401840 [DOI] [Google Scholar]
- 59.Delis DC, Kaplan E, Kramer JH.. Delis-Kaplan Executive Function Scale. San Antonio: TX Psychol Corp. 2001. doi: 10.3109/02770903.2012.715704 [DOI] [Google Scholar]
- 60.Wechsler D. Wechsler Abbreviated Scale of Intelligence–Second Edition (WASI-II); 2011. doi:citeulike-article-id:6135820.
- 61.Nyenhuis DL, Luchetta T.. The development, standardization, and initial validation of the Chicago multiscale depression inventory. J Pers Assess. 1998;70(2):386–401. doi: 10.1207/s15327752jpa7002_14 doi: 10.1207/s15327752jpa7002_14 [DOI] [PubMed] [Google Scholar]
- 62.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Spielberger C, ed. Menlo Park: Mind Garden Publishers; 1983. [Google Scholar]
- 63.Cattell RB, Scheier IH.. Handbook for the IPAT Anxiety Scale. 2nd ed. Chicago, IL: Institute for Personality and Ability Testing; 1963. [Google Scholar]
- 64.Taylor JA. A personality scale of manifest anxiety. J Abnorm Psychol. 1953;48:285–90. [DOI] [PubMed] [Google Scholar]
- 65.Wecht JM, Weir JP, Katzelnick CG, Wylie G, Eraifej M, Nguyen N, et al. Systemic and cerebral hemodynamic contribution to cognitive performance in spinal cord injury. J Neurotrauma. 2018;35(24):2957–64. [DOI] [PubMed] [Google Scholar]
- 66.Heaton RK, Miller S, Taylor M, Grant I.. Revised comprehensive norms for an expanded halstead-reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults scoring programs. Psychol Assess Resour. 2004. doi: 10.1051/matecconf/201710303003 [DOI] [Google Scholar]
- 67.Sachdeva R, Gao F, Chan C, Krassioukov A.. Cognitive function after spinal cord injury: a systematic review. Neurology. 2018;91(13):611–21. doi: 10.1212/WNL.0000000000006244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tun CG, Tun PA, Wingfield A.. Cognitive function following long-term spinal cord injury. Rehabil Psychol. 1997;42(3):163–82. doi: 10.1037/0090-5550.42.3.163 doi: 10.1037/0090-5550.42.3.163 [DOI] [Google Scholar]
- 69.Macciocchi S, Seel RT, Warshowsky A, Thompson N, Barlow K.. Co-occurring traumatic brain injury and acute spinal cord injury rehabilitation outcomes. Arch Phys Med Rehabil. 2012;93(10):1788–94. [accessed 2019 September 12]. Available from https://www.sciencedirect.com/science/article/pii/S0003999312001037. doi: 10.1016/j.apmr.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 70.Wecht JM, Bauman WA.. Decentralized cardiovascular autonomic control and cognitive deficits in persons with spinal cord injury. J Spinal Cord Med. 2013;36(2):74–81. doi: 10.1179/2045772312y.0000000056 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reuter I, Mehnert S, Sammer G, Oechsner M, Engelhardt M.. Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson’s disease. J Aging Res. 2012;2012:1–15. doi: 10.1155/2012/235765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muscari A, Giannoni C, Pierpaoli L, et al. Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: a randomized controlled trial. Int J Geriatr Psychiatry. 2009;25(10):1055–64. doi: 10.1002/gps.2462 doi: 10.1002/gps.2462 [DOI] [PubMed] [Google Scholar]
- 73.Birn RMM, Kenworthy L, Case L, et al. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49(1):1099–107. [accessed 2019 September 12]. Available from https://www.sciencedirect.com/science/article/abs/pii/S105381190900809X. doi: 10.1016/j.neuroimage.2009.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin A, Wiggs CLL, Lalonde F, Mack C.. Word retrieval to letter and semantic cues: a double dissociation in normal subjects using interference tasks. Neuropsychologia. 1994;32(12):1487–94. doi: 10.1016/0028-3932(94)90120-1 doi: 10.1016/0028-3932(94)90120-1 [DOI] [PubMed] [Google Scholar]