Abstract
Objective: To identify T-score values at the total hip (TH) and femoral neck (FN) that correspond to the cutoff value of <0.60 g/cm2 for heightened risk of fracture at the distal femur (DF) and proximal tibia (PT).
Design: Retrospective analysis of data in a research center’s database.
Setting: Community-based individuals with spinal cord injury (SCI).
Participants: 105 unique individuals with SCI.
Outcome Measurements: DXA derived areal BMD (aBMD) and T-score of the DF, PT, TH, and FN.
Results: The aBMD at the DF and PT regions were predictors of T-scores at the TH (R2 = 0.63, P < 0.001 and R2 = 0.65, P < 0.001) and FN (R2 = 0.55, P < 0.001 and R2 = 0.58, P < 0.001). Using the DF and PT aBMD of 0.60 g/cm2 as a value below which fractures were more likely to occur, the predicted T-score was −3.1 and −3.5 at the TH and −2.6 and −2.9 at the FN, respectively. However, when the predicted and observed T-score values disagree outside the 95% limit of agreement, the predicted T-score values are lower than the measured T-score values, overestimating the measured values between −2.0 and −4.0 SD.
Conclusion: The DF and PT cutoff value for aBMD of 0.60 g/cm2 was a moderate predictor of T-score values at the TH and FN, with considerable inaccuracies outside the clinically acceptable limits of agreement. As such, the direct measurement of knee aBMD in persons with SCI should be performed, whenever possible, prior to prescribing weight bearing upright activities, such as robotic exoskeletal-assisted walking.
Keywords: Spinal cord injury, Dual energy X-ray absorptiometry, Bone mineral density, T-score, Distal femur, Proximal tibia
Background
Persons with spinal cord injury (SCI) undergo immediate unloading of the skeleton and, as a result, have marked loss of bone below the level of lesion that predisposes them to a greatly increased risk of long-bone fractures of the lower extremities. This distribution of bone demineralization in individuals with SCI is considerably different than other models of bone loss because the skeleton above the level of lesion remains unaffected by the injury, while pronounced bone loss occurs in the regions below the level of lesion.1,2 The impact of skeletal unloading is further clarified when assessing bone loss in individuals with spinal cord lesions that are less neurologically motor-complete, specifically in those who can participate in weight-bearing activities and have partial preservation of voluntary lower extremity muscle contraction; bone demineralization in these individuals is generally not as extreme, and these more mobile, upright individuals are at considerably lower risk to have LE fractures.3,4 In persons with chronic SCI, the epiphysis and metaphysis of the distal femur (DF) and proximal tibia (PT) regions at the knee are the sites most vulnerable to fracture,5–7 with considerably lower fracture prevalence reported at the proximal hip region.8 Fragility fractures most often occur after a low velocity fall or minor trauma creating torsional and compressive forces at the DF and PT; these types of fracture have been demonstrated to occur during bending, surface-to-surface transfers, and routine physical therapy exercises.5–7 The use of dual x-ray absorptiometry (DXA) to assess changes in areal bone mineral density (aBMD) has become routine in both clinical practice and research studies due to its general availability, precise measurements with low levels of radiation exposure, relatively low cost compared to quantitative computed tomography (QCT) and magnetic resonance imaging, as well as the existence of reference data for DXA that has proven efficacy in predicting future risk of fracture.9 At the current time, CT and peripheral quantitative computed tomography (pQCT) have provided additional information concerning bone loss of the trabecular and cortical compartments of the femur and tibia,10,11 but DXA has been the primary method used to assess bone loss at the DF and PT in persons with SCI.12,13 Although several studies have demonstrated good reliability at quantifying aBMD at the knee region of interest (ROI) using non-specific software adapted from the lumbar spine and forearm,14,15 a more specific orthopedic knee software package with algorithms unique to the ratio of bone and soft tissue of the joint has been used exclusively for research since its availability,16 with a commercial version becoming available in 2014 (enCore V.16) as the Orthopaedic Knee software;17 Hologic DXA systems do not have knee-specific software commercially available.
With the advent of robotically powered exoskeletal-assisted ambulation for persons with SCI, as well as other advanced rehabilitation medicine interventions that position the individual in an upright posture and place increased forces on the lower extremities, the increased relevance of identifying those individuals who are at heightened risk of fracture is becoming ever more apparent. Because there is a paucity of evidence supporting the aBMD or T-score cut-off to exclude persons with SCI from rehabilitation interventions, several studies have incorporated DXA-derived cut-off criteria using a T-score range between −2.5 to −4.0 SD below the mean of a young-healthy able-bodied (AB) reference population at the TH or FN.18–23 In the clinical research environment where imaging is available to image the knee region, aBMD at the DF or PT in the range of 0.60–0.70 g/cm2 has been used to exclude persons with SCI from advanced upright rehabilitation strategies.18,23–25 The threshold aBMD value of 0.60 g/cm2 at the DF and PT was first described by Garland et al., below which aBMD value fractures were observed more likely to occur at these skeletal sites.26,27 Appreciating that these cut-off values have not been validated in studies that assessed incident fracture, it is important to note that recent studies have supported the use of an aBMD cut-off value at the knee of <0.60 g/cm2.18 Measurement of the TH and FN is routinely obtained, has excellent reliability, and standardized with well-established reference young normal (T-score) datasets to account for calibration differences between DXA manufacturers. As opposed to the use of T-score criteria at the TH and FN that are used to diagnose osteoporosis in other populations, the aBMD values at the DF and PT are the more appropriate regions to use to identify low aBMD and those with SCI who are at increased risk of fracture.6,8 However, aBMD measurements at the DF and PT are not routinely available to the clinician and are rarely obtained by clinical DXA technicians. If the aBMD at the DF and PT and T-score at the TH and FN have a proven strong association, then calculation of the optimum T-score cutoff values at the TH and FN that correspond to commonly used aBMD cutoff values at the DF and PT where fracture most commonly occurs could be identified and utilized. The purpose of this report was to characterize the T-score values at the TH and FN that best represent the DF or PT aBMD cutoff value (<0.60 g/cm2) commonly used to identify persons with SCI at the greatest risk of fracture.
Methods
A retrospective analysis of DXA measurements that were previously collected as part of separate prospective investigations in our clinical research units at the James J. Peters VA Medical Center (JJP VAMC) and the Kessler Institute for Rehabilitation (KIR) was performed. This retrospective analysis was approved by the institutional review boards at both the JJP VAMC and KIR. The prior investigations from which these DXA scans were obtained for this analysis had uniform inclusion/exclusion criteria and all participants provided written informed consent. At the time of informed consent during the original trial, participants were informed that their DXA data could be used in future cross-sectional exploratory analyses to explore bone health after SCI, and participants had the right to permit or restrict their data to be used in this manner. To ensure institutional regulatory compliance, additional IRB approval from the respective institutions was obtained to extract demographic and DXA data from de-identified and de-coded databases to perform the retrospective review and to analyze the data. Much of the relevant demographic information for skeletal parameters, such as physical activity, smoking, alcohol consumption, calcium intake, vitamin D levels, presence of spasticity, and fracture history, was not available for analysis. The prior studies, from which data were analyzed for this work, were conducted in accordance with the Declaration of Helsinki.
Participants
A retrospective data mining review was obtained on 105 unique individuals with SCI whose data were housed in our research center DXA database. In the primary studies, the inclusion criteria were male and female participants with traumatic SCI who were non-ambulatory (wheelchair reliant 100% of the time) and all participants were between the ages of 18 and 65 years. In those with SCI, the neurological level of injury was between cervical level-5 (C5) and lumbar level-2 (L2), and the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) grade was A, B, or C (28). Exclusion criteria for participants with SCI were the presence of severe spasticity and contracture that would interfere with acquiring an acceptable DXA scan of the hip and knee regions, decubitus pressure ulcer(s), current use of bisphosphonates or other medications known to affect bone metabolism, history of alcohol and drug abuse, chronic glucocorticoid use, hormone replacement therapy, current treatment for acute medical condition, bilateral knee and/or hip replacement, metal hardware in both lower extremities, history of heterotopic ossification at the hip and knee, history of cardiovascular disease, and a history of diseases known to affect bone metabolism (e.g. ankylosing spondylitis, rheumatoid arthritis, Paget’s disease, hyperparathyroidism, diabetes mellitus, and/or cancer).
DXA: knee and hip aBMD
The aBMD values and reference scores were obtained by DXA (GE Lunar iDXA, all software versions 16.0; enCORE, GE Medical Systems, Madison, WI). To minimize inter-rater variability, two International Society for Clinical Densitometry (ISCD) certified technicians with more than 15 years of experience performing DXA imaging in persons with SCI performed the acquisition and analysis of all DXA scans in the original studies. As part of quality assurance procedures in our unit, a spine phantom (i.e. aluminum spine L1-L4 encased in acrylic) was scanned >300 times over a 5-year period and the coefficient of variation was found to be <1% (CV = 0.54 ± .006). Appreciating there were no significant differences between the right and left sides for each ROI, the values for aBMD of the sides were averaged. For acquisition of the DF and PT aBMD, and their use in the subsequent computation of T-scores, DXA scans were acquired with the use of a Food and Drug Administration (FDA)-approved orthopedic knee software program with a custom ROI, as previously described.28 To obtain accurate assessment of aBMD at the DF and PT metaphysis, this method was employed to avoid overlap with the patella and fibula and has been shown to have a high reliability between multiple raters with intra-class correlation coefficient values of 0.98 for the DF and 0.89 for the PT.28 The FN and TH aBMD was obtained using the standard proximal femur software provided by the manufacturer and, as per convention, the young-normal T-score [Caucasian (non-race adjusted) female normative database] for the TH and FN were obtained using the combined manufacturer and third National Health and Nutrition Examination Survey (NHANES III/GE Lunar) reference database provided with the manufacturer’s software to diagnose osteoporosis (T-scores < −2.5 SD). The investigators appreciate that premenopausal woman and men under the age of 50 years are recommended to be compared to age-matched cohorts using Z-scores. However, because the investigators are attempting to identify persons with SCI who are at heightened risk of fracture, and persons with SCI represent a unique population sample, hip T-scores < 2.5 SD, values which have been defined to identify persons with osteoporosis, were applied in the analyses rather than Z-scores.
Statistical analysis
Results are expressed as group mean ± standard deviation (SD). Independent sample t-tests were performed to determine the difference in aBMD and T-score values between the hip and knee regions and the confounding effect of demographic variables on aBMD and T-score variables presented. To determine the relationship between the hip and knee regions, simple linear regression models were used to determine the associations between the DF and PT aBMD and T-scores at the TH and FN. The direction of this relationship was intentional to focus on the clinically relevant aBMD that has been identified at the DF and PT (0.60 g/cm2) and the well appreciated fact that T-score at the TH and FN is the commonly performed and clinically relevant measurement used by physicians to diagnose osteoporosis. The linear relationship was established by visual inspection of scatterplots with a superimposed regression line and normal distribution of residuals was established by visual inspection of probability plots. Homoscedasticity was evaluated by visual inspection of standardized residuals versus standardized predicted values plot and tested using the Breusch–Pagan test. To determine agreement between the predicted and measured T-score values at the TH and FN, a Bland–Altman analysis was completed with the proportion of the sample likely to be accurately predicted calculated. Multiple regression analysis was also used to determine the effect of other predictors (weight, BMI, age, level of lesion, motor completeness, and sex) of aBMD at the TH and FN. Applying the commonly used aBMD cutoff value at the DF or PT (<0.60 g/cm2) to dichotomize participants with low bone mass at heightened risk of fracture at the knee, a receiver operating characteristic (ROC) curve was used to identify the optimum T-score cutoff at the TH and FN (i.e. maximum sensitivity and specificity) using Youden Index [YI; intersecting point or “turn” along the curve that provides the best balance between sensitivity and specificity]. The AUC, sensitivity, and specificity were interpolated for the TH and FN T-score cutoff value from the coordinates of the curve of the YI and then compared to the sensitivity and specificity of the World Health Organization (WHO) criteria to diagnose osteoporosis (−2.5 SD from the reference mean). Additional sensitivity and specificity analysis was determined for the TH and FN cutoff values of <−3.5 SD (a value that has been used as an exclusion criterion for entry into exoskeletal-assisted walking (EAW) protocols), <−2.5 SD (WHO criterion to diagnose osteoporosis), and <−3.1 SD (obtained from ROC curve T-scoreYI and linear regression predicting T-score at the TH from the DF) using the aBMD cutoff value at the DF or PT (<0.60 g/cm2) as the reference standard and the number of false negative and false positive cases presented. Statistical analyses were completed using IBM SPSS Statistics version 22, IBM, Armonk, NY and graphs were generated by Prism GraphPad, version 6.0 for Windows, GraphPad Software, San Diego, CA. An a priori level of significance was set at p ≤ 0.05.
Results
Demographic and other descriptive characteristics for the study groups are provided (Table 1). A significantly higher aBMD was observed at the PT compared to the DF, TH, and FN regions (t = 12.4, t = 19.9, and t = 12.6, respectively, P < 0.001), as well as a significantly lower T-score at the TH compared to the FN (t = −5.3, P < 0.01). All continuous aBMD and T-score variables were tested for possible demographic confounders of bone loss. There were no significant differences in aBMD and reference scores when stratifying groups by weight, BMI, age, level of lesion, motor completeness, and sex.
Table 1. Demographic and densitometry characteristics of study participants.
| SCI (n = 105) |
Range | |
|---|---|---|
| Age (y) | 40.1 ± 11.9 | 20 to 65 |
| Height (cm) | 175.2 ± 9.2 | 152.4 to195.6 |
| Weight (kg) | 69.4 ± 21.2 | 25.7 to 141.4 |
| BMI (kg/m2) | 22.6 ± 6.5 | 8 to 43 |
| Sex (m/f) | 82/23 | – |
| TSI (y) | 9.3 ± 10.5 | 0.1 to 43 |
| Para/Tetra (n) | 68/37 | – |
| ISNCSCI (A/B/C) | 46/28/31 | – |
| Motor Complete (y/n) | 73/32 | – |
| aBMD (g/cm2) | ||
| Distal Femur | 0.852 ± 0.212a | 0.483 to 1.475 |
| Proximal Tibia | 1.019 ± 0.293 | 0.541 to 1.849 |
| Total Hip | 0.825 ± 0.207a | 0.379 to 1.429 |
| Femoral Neck | 0.860 ± 0.189a | 0.475 to 1.443 |
| T-score (SD) | ||
| Total Hip | −1.55 ± 1.67b | −5.00 to 3.30 |
| Femoral Neck | −1.37 ± 1.40 | −4.15 to 2.90 |
Continuous variables are expressed as group mean ± SD.
n, number of participants; y, years; cm, centimeters; kg, kilograms; m2= meters squared; BMI, body mass index; TSI, time since injury; para, paraplegia; tetra, tetraplegia; ISNCSCI, International Standards for Neurological Classification of Spinal Cord Injury; T-score, SD difference from young, healthy adults.
ap < 0.001 vs. proximal tibia aBMD.
bp < 0.01 vs. femoral neck T-score.
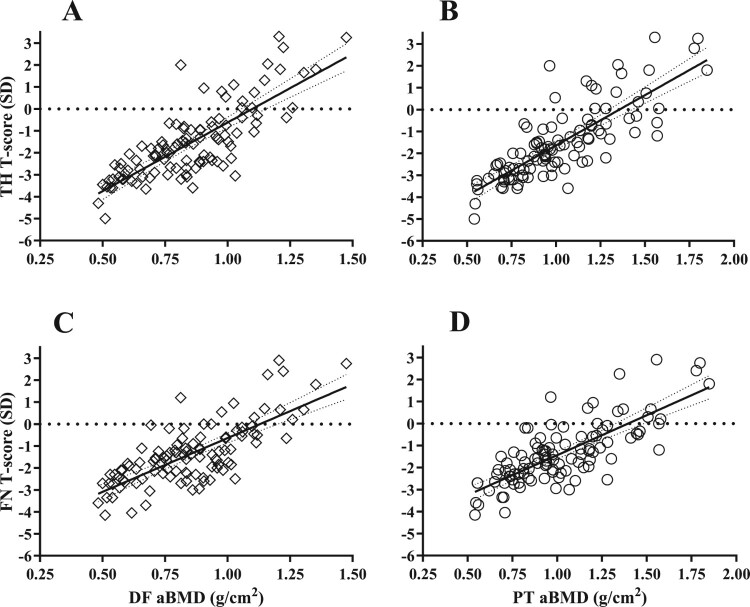
To determine the ability of the DF and PT to predict T-scores at the TH and FN, linear regression analysis was performed. The aBMD at the DF and PT regions were significant predictors of T-score at the TH (R2 = 0.63, P < 0.001 and R2 = 0.65, P < 0.001, respectively) and FN (R2 = 0.55, P < 0.001 and R2 = 0.58, P < 0.001, respectively). Additional analysis of the regression coefficients revealed that for a 0.1 g/cm2 change in aBMD at the DF and PT there was a 0.63 and 0.46 change in T-score at the TH, respectively, (Fig. 1 A and B) and a 0.49 and 0.36 change in T-score at the FN, respectively, (Fig. 1 C and D). Furthermore, using the DF and PT aBMD value of 0.60 g/cm2 to designate heightened risk of fracture, the predicted T-score was −3.1 and −3.5 at the TH, respectively, (Fig. 1 A and B) and −2.6 and −2.9 at the FN, respectively (Fig. 1 C and D). An additional multivariate regression model was performed to account for other factors that may contribute to predicting aBMD at the DF and PT, but no significant contributions were found for the additional covariates of age, BMI, level of lesion, motor completeness, and sex into the model (data not presented).
Figure 1.
Scatter plots of total hip (TH) T-score regressed against (A) distal femur (DF) [TH T-score (SD) = 6.257 X DF aBMD (g/cm2)−6.878, slope 95% CI: 5.320−7.194, R2 = 0.63, SE = 0.472, P < 0.0001] and (B) proximal tibia (PT) [TH T-score (SD) = 4.596 X PT aBMD (g/cm2) - 6.234, slope 95% CI: 3.936−5.256, R2 = 0.65, SE = 0.333, P < 0.0001]. A similar relationship was observed when regressing the femoral neck (FN) T-score against the (C) DF [FN T-score (SD) = 4.902 X DF aBMD (g/cm2) - 5.542, slope 95% CI: 4.042–5.762, R2 = 0.55, SE = 0.434, P < 0.0001] and (D) PT [FN T-score (SD) = 3.635 X PT aBMD (g/cm2) - 5.073, slope 95% CI: 3.032−4.237, R2 = 0.58, SE = 0.0.304, P < 0.0001]. Diamonds indicate DF and circles indicate PT. Solid line = line of identity. Thin dashed line = 95% CI. Thick dashed line = zero T-score.
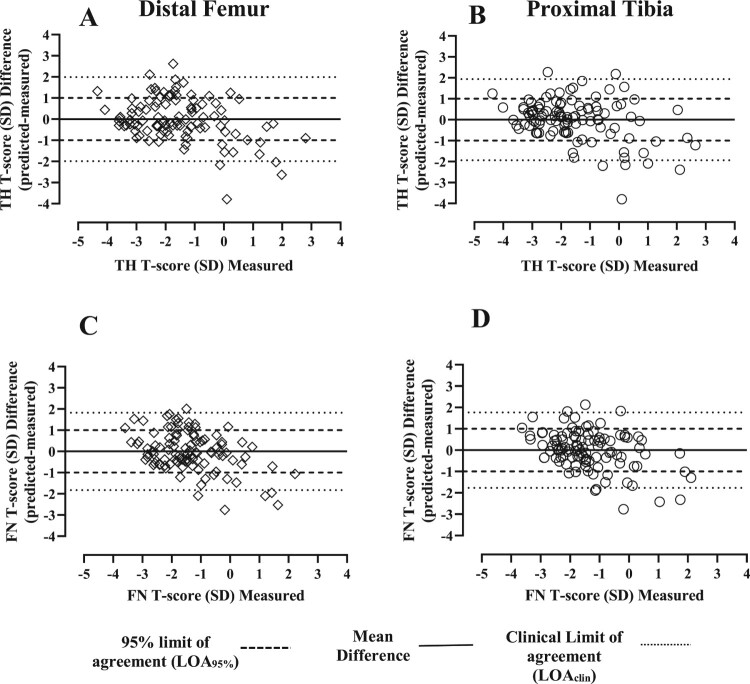
The Bland–Altman analysis was used to examine agreement between predicted and measured values based on the linear regression equations obtained. A small mean difference (bias) between the predicted and measured values was observed for all regions (<0.1 SD). Analysis of the 95% CI limit of agreement (LOA) using the DF and PT to predict T-scores ranged from −1.991 to 1.990 and −1.938 to 1.939 at the TH, respectively, (Fig. 2 A and B, light dashed lines) and −1.825 to 1.827 and −1.770 to 1.770 at the FN, respectively (Fig. 2 C and D, light dashed lines). For TH and FN regions, the regression estimates from the DF and PT predicted between 93 and 94% of the total sample of T-scores (Fig. 2 A-D). A second clinically relevant LOA (LOAclin) was set at the acceptable threshold (±1 SD) with the DF and PT accurately predicting 75% and 71% of TH T-score values, respectively, and 76% of FN T-score values, respectively (Fig. 2 A-D). Despite the overall good levels of agreement between the difference and measured values, when the predicted and observed T-score values are outside the 95% limit of agreement, the predicted T-score values are lower than the measured T-score values, overestimating the measured values between −2.0 and −4.0 SD, evidence that measures at the hip and knee region should be obtained separately in populations with a high prevalence of osteoporosis that are at increased risk for fracture.
Figure 2.
Bland-Altman agreement analysis: The differences between measured and predicted T-score values were plotted against measured T-score values at the TH and FN. Prediction of T-score at the total hip and femoral neck using the DF (A and B) and PT (C and D). Solid line = mean difference. Light dashed line = 95% limit of agreement (LOA95%, mean difference ± 2SD). Thick dashed line = clinical limit of agreement (LOAclin).
Using the aBMD cutoff value at the DF or PT (<0.60 g/cm2) to diagnosis participants with high fracture risk (HFR) and lower fracture risk (LFR), a receiver operating characteristic (ROC) curve was used to identify the sensitivity and specificity of the T-score at the point of the YI (T-scoreYI). The sensitivity and specificity along the curve was also identified for the WHO T-score cutoff (T-scoreWHO) of −2.5 used to diagnose osteoporosis at the hip. From a ROC curve, T-score of −3.1 (AUC = 0.95, SE = 0.021, 95% CI = 0.911−0.995, P < 0.001) was identified at the TH and −2.4 (AUC = 0.92, SE = 0.027, 95% CI = 0.868−0.974, P < 0.001) at the FN. Furthermore, interpolation of the TH T-scoreYI and T-scoreWHO along the curve revealed a sensitivity and specificity of 81% and 92% and 94 and 80%, respectively. At the FN, T-scoreYI and T-scoreWHO had a sensitivity and specificity of 69 and 86% and 69 and 90%, respectively (Table 2). By interpretation of the AUC in the ROC curve, the T-score cutoff value at the TH and FN can be used to diagnose osteoporosis 95 and 92% of the time, with the best balance between sensitivity and specificity demonstrated at the TH.
Table 2. Receiver operating characteristic curve to determine sensitivity and specificity for femoral neck and total hip cut-off values categorized by an aBMD cut-off value of <0.60 g/cm2 at the DF or PT.
| Sensitivity (%) | Specificity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | AUC | Std. Error | P-value | 95% CI Lower | 95% CI Upper | T-scoreWHO | T-scoreYI | T-scoreWHO | T-scoreYI |
| TH | 0.95 | 0.021 | <0.0001 | 0.911 | 0.995 | 94 | 81 | 80 | 92 |
| FN | 0.92 | 0.027 | <0.0001 | 0.868 | 0.974 | 69 | 69 | 90 | 86 |
DF, distal femur; PT, proximal tibia; TH, total hip; FN, femoral neck; AUC, area under the curve; ROC: receiver operating characteristic; CI: confidence interval; T-scoreWHO: World Health Organization T-score criteria of −2.5 SD below the young adult reference mean used to diagnose osteoporosis; T-scoreYI, Youden Index T-score cutoff of −3.1 SD at the TH and −2.4 SD at the FN.
Using the TH or FN T-score criterion of −3.5 SD, and using the cutoff criteria at the DF or PT (<0.60 g/cm2), both exclusion criteria that would prevent participation in EAW protocols, 89 (85%) of the participants reached or exceeded eligibility by either the TH/FN or DF/PT cutoff criteria, 5 (6%) did not meet eligibility by both the TH/FN or DF/PT cutoff criteria, 3(3%) did not meet the eligibility cutoff criteria at the DF or PT but reached or exceeded the cutoff criteria at the TH or FN, and 10 (10%) did not meet the eligibility cutoff criteria at the TH or FN but reached or exceeded the eligibility criteria at the DF or PT; thus using a T-score criterion at the hip of −3.5, the sensitivity and specificity was 33% and 97%, respectively (Table 3). Applying the TH or FN T-scoreWHO criterion (−2.5 SD) and the cutoff value at the DF or PT to this dataset, 68 (65%) participants reached or exceeded eligibility by either the TH/FN or DF/PT cutoff criteria, 17 (16%) did not meet eligibility by both the TH/FN or DF/PT cutoff criteria, 20 (19%) did not meet the eligibility criteria at the DF or PT but reached or exceeded the cutoff criteria at the TH or FN. There were no participants who reached the eligibility criteria at the DF or PT and did not meet the eligibility cutoff criteria at the TH or FN. Thus, using a T-score criterion at the hip of −2.5, the sensitivity and specificity was 100% and 77%, respectively (Table 3). Finally, applying the TH or FN T-score criterion of −3.1 SD (obtained from ROC curve T-scoreYI and linear regression analysis at the TH from the previous analysis), and using the same cutoff criteria at the DF or PT, 82 (78%) participants reached or exceeded eligibility by either the TH/FN or DF/PT cutoff criteria, 13 (12%) did not meet eligibility by both the TH/FN or DF/PT cutoff criteria, 6 (6%) did not meet the eligibility cutoff criteria at the DF or PT but reached or exceeded the cutoff criteria at the TH or FN, and 4 (4%) did not meet the eligibility cutoff criteria at the TH or FN but reached or exceeded the eligibility criteria at the DF or PT; thus using a T-score criterion at the hip of −3.1, the sensitivity and specificity was 76% and 93%, respectively (Table 3).
Table 3. Sensitivity and specificity for fracture risk at the DF and PT using different FN or TH T-score values in participants with SCI.
| DF/PT aBMD (≤ 0.60g/cm2) | ||||||
|---|---|---|---|---|---|---|
| FN/TH T-score cutoffs | HFR | LFR | Sensitivity | Specificity | ||
| <−3.5 SD | HFR | 5 | 3 | 33% | 97% | |
| LFR | 10 | 89 | ||||
| HFR | LFR | Sensitivity | Specificity | |||
| <−2.5 SD | HFR | 17 | 20 | 100% | 77% | |
| LFR | 0 | 68 | ||||
| HFR | LFR | Sensitivity | Specificity | |||
| <−3.1 SD | HFR | 13 | 6 | 76% | 93% | |
| LFR | 4 | 82 | ||||
FN and TH cutoff values: <−3.5 SD (a value that has been used as an exclusion criterion for entry into EAW protocols), <−2.5 SD (WHO criteria to diagnose osteoporosis), and <−3.1 SD (a value obtained from the ROC curve T-scoreYI and linear regression T-score predicted at the TH from the DF); DF/PT cutoff value used was <0.60 g/cm2.
HFR, high fracture risk; LFR, lower fracture risk; DF, distal femur; PT, proximal tibia; TH, total hip; FN, femoral neck; SD, standard deviation; ROC, receiver operating characteristic; T-scoreYI, Youden Index T-score cutoff of −3.1 SD.
Sensitivity = True-Positive/(True-Positive + False-Negative)
Specificity = True-Negative/(False-Positive + True-Negative)
Discussion
With the increasing availability of sophisticated rehabilitation modalities for upright posture and ambulation for use in persons with SCI, there is an increased importance to identify candidates who may be at increased risk of fracture if prescribed these activities. Recommendations are needed to identify appropriate candidates for these interventions because bone deteriorates dramatically below the level of the lesion and fragility fractures of the long bones of the lower extremities occur with increased frequency with longer duration of SCI. Several clinical investigations have been applying fracture threshold values from cross-sectional studies that identified the BMD in small samples of persons with chronic SCI who sustained fragility fractures. These cutoff values to identify the fracture threshold vary considerably, with fractures occurring, not infrequently, above and not below the reported mean aBMD (DF and PT) and T-scores (TH and FN) by non-evidence based cutoff values. The Department of Veterans Affairs Cooperative Studies Program (NCT02658656) has an ongoing nationwide clinical trial that has the following skeletal exclusion criteria: (1) lower extremity fracture within the past 2 years, (2) DF or PT aBMD <0.6 gm/cm2, and (3) TH and FN T-score <−3.5 SD. The exclusion criterion for BMD at the knee in this multicenter study was based largely on work by Garland et al. in which fractures of the lower extremities were observed to occur more frequently when the BMD at the knee was below a value of 0.6 gm/cm2.27,29 The exclusion criterion for the cutoff value for T-score at hip was determined using a weighted ranking method that enables a group to generate and prioritize a large number of issues giving all parties an equal voice from a consensus of experts (i.e. nominal group method) as to what the predicted hip T-score value would be when the cutoff value at the knee was <0.6 gm/cm2
This report developed regression equations to predict T-scores at the TH and FN regions from aBMD values at the DF and PT and provide statistics with diagnostic accuracy from this relationship. When using the cutoff value at the knee of <0.6 gm/cm2, as described by Garland et al., 27,29 as the independent predictor of risk of fracture, the question arises as to whether the T-score values at the hip are different than the WHO criteria to diagnose osteoporosis, defined as a T-score of < −2.5 SD below the mean. Because fractures of the leg have been reported to occur more frequently below a BMD value at the knee of 0.6 gm/cm2, this value for BMD at the DF and PT was found to be equivalent to T-scores at the TH of −3.1 and −3.5 and T-scores at the FN of −2.6 and −2.9, respectively. Despite the moderate strength of our prediction equations, which have been demonstrated to account for between 50 and 60% of the variance in TH and FN T-score values, our model still reveals considerable inaccuracies when comparing the proportion of predicted values outside the clinically acceptable limits of agreement (±1 SD). In addition to the linear modeling, utilizing the same aBMD cutoff value of < 0.60 g/cm2 at the DF or PT to differentiate participants with increased risk of fracture ROC regression was also performed. Extrapolating the intersecting point along the curve that provides the best balance between sensitivity and specificity, a T-score cutoff of −3.1 SD at the TH and −2.4 at the FN could diagnosis osteoporosis at the knee region 95 and 92% of the time. Utilizing different aBMD values at the DF and PT in a more recent study that documented aBMD and prevalent fracture, Lala et al.15 found considerably lower BMD values at the DF (0.454 g/cm2) and PT (0.371 g/cm2), demonstrating the variability in defining a fracture threshold among studies. Applying the lower mean aBMD values at the DF and PT epiphysis reported by Lala et al. to the equations generated in this report yielded T-score values at the TH of −4.0 and −4.5, respectively, and a T-scores at the FN of −3.3 and −3.7, respectively. Our work provides empirical data, and thus clarifies what BMD values at the DF and PT relate to T-score values at the TH and FN. Overall, these findings strongly support the need to diagnose osteoporosis and fracture risk by acquiring aBMD of the DF and PT in persons with SCI. Furthermore, while the cutoff values at the knee of <0.6 gm/cm2 are the best cutoff values for fracture available at this time, normative reference data at the DF and PT (T-scores) that control for differences in aBMD calculations between densitometer manufacturers would allow clinicians to more accurately diagnosis osteoporosis in persons with SCI.
Having predicted T-score values at the hip from aBMD at the knee, the reverse may then be performed: the ability to predict aBMD values at the knee from T-scores at the hip. The approach used in this study has clinical relevance because the ability to acquire aBMD at the knee is not currently available in most clinical settings. If healthcare professionals had the ability to directly measure aBMD at the knee and exclude patients with SCI who have a heightened risk of fracture from participating in upright ambulatory activities, then this exercise would be unnecessary. However, for clinicians who do not have the resources to capture aBMD of the DF and PT because of the unavailability of knee software, adequate technician knowledge and experience, and/or the absence of third party reimbursement, identification of a TH or FN T-score value that can be used as a surrogate marker to identify individuals with SCI at the greatest risk of fracture at the DF or PT has clinical utility. A lower sensitivity was observed at the FN, compared to the TH for both the T-scoreWHO and T-scoreYI criteria (See Table 2). Comparing the sensitivity and specificity between the T-scoreWHO (−2.5 SD), the T-score commonly used to exclude participants from EAW protocols (−3.5 SD), and the T-score value identified from the regression and diagnostic accuracy techniques (−3.1 SD), the best balance between sensitivity and specificity was demonstrated at a T-score cutoff of −3.1 SD (See Table 3). Using a T-score cutoff of −2.5 SD at the TH/FN, the number of SCI individuals diagnosed as having a high fracture risk at the DF/PT (true positive cases, n = 17), is at the expense of a considerable number of SCI individuals misdiagnosed as having a high fracture risk [HFR, FP cases (n = 20)], a cutoff value that could result in a considerable number of SCI participants denied entry into upright ambulatory EAW. Conversely, a diagnostic scenario of greater clinical impact can be observed using the T-score cutoff −3.5 SD at the TH/FN. Using this cutoff value, a considerable number of SCI participants with HFR at the TH/FN, would be misdiagnosed as having a lower fracture risk [LFR, FN cases (n = 10)] at the DF/PT, and would be allowed entry into upright ambulatory EAW. This evidence presented suggests that the use of a T-score of −3.1 SD for TH, a cutoff value predicted at the TH from regression and diagnostic accuracy analysis in the study cohort presented, would yield the greatest balance in maximizing entry into advanced upright rehabilitation therapy who have a LFR, while restricting the majority of participants who have a HFR, when direct measurement of aBMD at the DF and PT is not possible. However, each physician must weigh the potential risks and benefits of a more conservative or more liberal cutoff value for the TH T-score for the particular patient being offered the upright rehabilitation therapy.
In the current study, the protocol by Shields et al.28 was employed to avoid overlap with the fibula and patella but resulted in capturing aBMD of the DF and PT epiphysis and a portion of the metaphysis, which represents a combination trabecular and cortical compartments. This knee acquisition protocol28 used a cross sectional region that may have yielded a substantially different aBMD at the DF and PT compared to protocols employed by Lala et al.15 and McPherson et al.30 These investigators captured aBMD at the DF and PT epiphysis, metaphysis, and diaphysis, with the latter study validating the epiphysis region against volumetric BMD (vBMD) by quantitative computed tomography (QCT) scan. While DXA-derived measures of aBMD loss are a combination of trabecular loss and thinning of the endocortical envelope, QCT and pQCT-derived measures of vBMD can delineate between loss at the trabecular and/or cortical bone compartments. In a study by Eser et al.,31 trabecular vBMD by pQCT of the femur and tibia distal epiphyses was effective in identifying subjects with a history of fracture; subjects with a history of fracture had trabecular BMD values that were <114 mg/cm3 and <72 mg/cm3 for the DF and PT, respectively. The aforementioned studies by Garland et al. 27,29 and Eser et al.31 have provided guidance on prevalent fracture and cutoff values by DXA and pQCT in the SCI population but, to date, no prospective cohort studies in persons with SCI have been performed that have acquired BMD of the DF and PT at or in temporal proximity to the occurrence of the fracture.
There are several limitations of this investigation. Information describing the level of physical activity, smoking history, alcohol consumption, fracture history, spasticity, vitamin D concentrations, calcium intake, and medication history was not available to determine the potential effect of these previously reported determinants of skeletal integrity. By study design, the use of DXA methodology did not permit measurement of trabecular and cortical vBMD of the hip and knee regions. If the distribution of trabecular and cortical bone at the hip and knee regions had been acquired, such information may have permitted greater insight into relationships between the DF and PT and the FN and TH regions, with the prediction of trabecular and cortical compartments from one region possibly able to be predicted from the other region. The absence of standardized protocols to acquire aBMD at the distal femur and proximal tibia is a major source of the variability between values reported by investigators. In addition to the standardized protocols to capture and analyze aBMD at the DF and PT, programs should be standardized among the various manufacturers to permit the acquisition of these regions of interest to be compared across studies and between clinical imaging facilities. Because validated software programs have not been commercially offered by DXA manufacturers, the majority of clinical investigation in SCI to date have relied on the adaptation of software applications of the forearm and lumbar spine to measure aBMD of the knee region.15,30 However, the orthopedic knee software used in this study, as well as other clinical trials reported by our group,16 was approved in 2014 by the FDA to monitor aBMD. This knee software package is now commercially available and should be used when available at an imaging facility to acquire the knee in persons with SCI. In addition, acquiring an aBMD of young-normal persons that comprises an adequately powered reference dataset to calculate T-scores at the DF and PT is required to diagnose osteoporosis at the knee in persons with chronic SCI or in any other population sample. Finally, T-scores at the knee should be related in prospective cohort studies to the occurrence of subsequent fractures of the long-bones of the lower extremity, or, as a less acceptable alternative, DXA measurements should be obtained at the knee as soon as possible after a fracture of the leg occurs in persons with SCI.
Conclusion
The findings from this analysis advance our understanding of the relationships between the T-score values at the hip and aBMD values at the knee in persons with SCI. Despite the ability to predict T-scores at the TH and FN from aBMD values at the DF and PT using statistical constructs, there still exists a degree of uncertainty for a given individual to be able to predict knee aBMD values, especially. As such, the direct measurement of knee aBMD in persons with SCI should be performed, whenever possible, prior to prescribing vigorous upright activities, such as treadmill gait or robotic exoskeletal-assist ambulation, because of the heightened risk of precipitating fractures of long-bones of the lower extremity when additional stresses are placed upon regions of the skeleton that are vulnerable to structural failure.
Acknowledgments
The authors wish to thank the James J Peters VA Medical Center, Bronx, NY, the Department of Veterans Affairs Rehabilitation Research & Development Service, the Kessler Institute for Rehabilitation and the Kessler Foundation, West Orange, NJ, for their support.
Disclaimer statements
Contributors None.
Funding This material is from work supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service National Center for the Medical Consequences of Spinal Cord Injury (B9212-C), [DOD/ CDMRP Award: W81XWH-14-2-0170], New Jersey Commission on Spinal Cord Research [CSCR131RG013], Craig H. Neilsen Foundation [297267], the New York State Department of Health Spinal Cord Injury Research Board [DOH01-PART3-2019-00002], and the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) [90SI5026]; Congressionally Directed Medical Research Programs.
Conflicts of interest Authors have no conflict of interests to declare.
Ethics approval This study was approved by the James J. Peters VA Medical Center, Rutgers University, and Kessler Foundation Institutional Review Boards and all participants gave their informed consent to participate.
References
- 1.Battaglino RA, Lazzari AA, Garshick E, Morse LR.. Spinal cord injury-induced osteoporosis: pathogenesis and emerging therapies. Curr Osteoporos Rep. 2012;10:278–85. doi: 10.1007/s11914-012-0117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirnigliaro CM, LaFountaine MF, Dengel DR, Bosch TA, Emmons RR, Kirshblum SC, et al. . Visceral adiposity in persons with chronic spinal cord injury determined by dual energy X-ray absorptiometry. Obesity (Silver Spring. 2015;23:1811–7. doi: 10.1002/oby.21194 [DOI] [PubMed] [Google Scholar]
- 3.Biering-Sorensen F, Bohr HH, Schaadt OP.. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–5. doi: 10.1111/j.1365-2362.1990.tb01865.x [DOI] [PubMed] [Google Scholar]
- 4.Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF.. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–9. doi: 10.1016/S8756-3282(00)00326-4 [DOI] [PubMed] [Google Scholar]
- 5.Gifre L, Vidal J, Carrasco J, Portell E, Puig J, Monegal A, et al. . Incidence of skeletal fractures after traumatic spinal cord injury: a 10-year follow-up study. Clin Rehabil. 2014;28:361–9. doi: 10.1177/0269215513501905 [DOI] [PubMed] [Google Scholar]
- 6.Ingram RR, Suman RK, Freeman PA.. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia. 1989;27:133–9. [DOI] [PubMed] [Google Scholar]
- 7.Ragnarsson KT, Sell GH.. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil. 1981;62:418–23. [PubMed] [Google Scholar]
- 8.Frotzler A, Cheikh-Sarraf B, Pourtehrani M, Krebs J, Lippuner K.. Long-bone fractures in persons with spinal cord injury. Spinal Cord. 2015;53:701–4. doi: 10.1038/sc.2015.74 [DOI] [PubMed] [Google Scholar]
- 9.Cirnigliaro CM, Myslinski MJ, La Fountaine MF, Kirshblum SC, Forrest GF, Bauman WA.. Bone loss at the distal femur and proximal tibia in persons with spinal cord injury: imaging approaches, risk of fracture, and potential treatment options. Osteoporos Int. 2017;28:747–65. doi: 10.1007/s00198-016-3798-x [DOI] [PubMed] [Google Scholar]
- 10.de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E.. Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord. 2005;43:96–101. doi: 10.1038/sj.sc.3101685 [DOI] [PubMed] [Google Scholar]
- 11.Gibbs JC, Brown ZM, Wong AKO, Craven BC, Adachi JD, Giangregorio LM.. Measuring marrow density and area using peripheral quantitative computed tomography at the tibia: precision in young and older adults and individuals with spinal cord injury. J Clin Densitom. 2018;21:269–80. doi: 10.1016/j.jocd.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 12.Peppler WT, Kim WJ, Ethans K, Cowley KC.. Precision of dual-energy X-ray absorptiometry of the knee and heel: methodology and implications for research to reduce bone mineral loss after spinal cord injury. Spinal Cord. 2017;55:483–8. doi: 10.1038/sc.2016.170 [DOI] [PubMed] [Google Scholar]
- 13.Lobos S, Cooke A, Simonett G, Ho C, Boyd SK, Edwards WB.. Assessment of bone mineral density at the distal femur and the proximal tibia by dual-energy X-ray absorptiometry in individuals With Spinal Cord injury: Precision of protocol and Relation to injury duration. J Clin Densitom. 2018;21:338–46. doi: 10.1016/j.jocd.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 14.Bakkum AJ, Janssen TW, Rolf MP, Roos JC, Burcksen J, Knol DL, et al. . A reliable method for measuring proximal tibia and distal femur bone mineral density using dual-energy X-ray absorptiometry. Med Eng Phys. 2014;36:387–90. doi: 10.1016/j.medengphy.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 15.Lala D, Craven BC, Thabane L, Papaioannou A, Adachi JD, Popovic MR, et al. . Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int. 2014;25:177–85. doi: 10.1007/s00198-013-2419-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauman WA, Cirnigliaro CM, La Fountaine MF, Martinez L, Kirshblum SC, Spungen AM.. Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: an observational cohort study. J Bone Miner Metab. 2015;33:410–21. doi: 10.1007/s00774-014-0602-x [DOI] [PubMed] [Google Scholar]
- 17.Cirnigliaro CM, Myslinski MJ, Asselin P, Hobson JC, Specht A, La Fountaine MF, et al. . Progressive sublesional bone loss extends into the second decade after spinal cord injury. J Clin Densitom. 2019;22:185–94. doi: 10.1016/j.jocd.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 18.Asselin P, Knezevic S, Kornfeld S, Cirnigliaro C, Agranova-Breyter I, Bauman WA, et al. . Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J Rehabil Res Dev. 2015;52:147–58. doi: 10.1682/JRRD.2014.02.0060 [DOI] [PubMed] [Google Scholar]
- 19.Dolbow DR, Credeur DP.. Effects of resistance-guided high intensity interval functional electrical stimulation cycling on an individual with paraplegia: a case report. J Spinal Cord Med. 2018;41:248–52. doi: 10.1080/10790268.2017.1367358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorgey AS, Wade R, Sumrell R, Villadelgado L, Khalil RE, Lavis T.. Exoskeleton training may improve level of physical activity after spinal cord injury: a case series. Top Spinal Cord Inj Rehabil. 2017;23:245–55. doi: 10.1310/sci16-00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorman PH, Geigle PR, Chen K, York H, Scott W.. Reliability and relatedness of peak VO2 assessments during body weight supported treadmill training and arm cycle ergometry in individuals with chronic motor incomplete spinal cord injury. Spinal Cord. 2014;52:287–91. doi: 10.1038/sc.2014.6 [DOI] [PubMed] [Google Scholar]
- 22.Guimaraes JA, da Fonseca LO, de Sousa AC, Paredes ME, Brindeiro GA, Bó AP, et al. . FES bike race preparation to Cybathlon 2016 by EMA team: a short case report. Eur J Transl Myol. 2017;27:7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang A, Asselin P, Knezevic S, Kornfeld S, Spungen AM.. Assessment of in-hospital walking velocity and level of assistance in a powered exoskeleton in persons with spinal cord injury. Top Spinal Cord Inj Rehabil. 2015;21:100–9. doi: 10.1310/sci2102-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanujam A, Cirnigliaro CM, Garbarini E, Asselin P, Pilkar R, Forrest GF.. Neuromechanical adaptations during a robotic powered exoskeleton assisted walking session. J Spinal Cord Med. 2018;41:518–28. doi: 10.1080/10790268.2017.1314900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lester RM, Gorgey AS.. Feasibility of robotic exoskeleton ambulation in a C4 person with incomplete spinal cord injury: a case report. Spinal Cord Ser Cases. 2018;4:36. doi: 10.1038/s41394-018-0053-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garland DE, Maric Z, Adkins RH, Stewart CA.. Bone mineral denisty about the knee in spinal cord injured patients with pathologic fractures. Contemp Orthop. 1993;26:375–9. [Google Scholar]
- 27.Garland DE, Adkins RH, Kushwaha V, Stewart C.. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27:202–6. doi: 10.1080/10790268.2004.11753748 [DOI] [PubMed] [Google Scholar]
- 28.Shields RK, Schlechte J, Dudley-Javoroski S, Zwart BD, Clark SD, Grant SA, et al. . Bone mineral density after spinal cord injury: a reliable method for knee measurement. Arch Phys Med Rehabil. 2005;86:1969–73. doi: 10.1016/j.apmr.2005.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garland DEA H, Stewart CH.. Fracture threshold and risk for osteoporosis and pathologic fractures in individuals with spinal cord injury. Top Spinal Cord Inj. 2005;11:61–9. doi: 10.1310/G6TD-HPGC-XM3Q-7YJH [DOI] [Google Scholar]
- 30.McPherson JG, Edwards WB, Prasad A, Troy KL, Griffith JW, Schnitzer TJ.. Dual energy X-ray absorptiometry of the knee in spinal cord injury: methodology and correlation with quantitative computed tomography. Spinal Cord. 2014;52:821–5. doi: 10.1038/sc.2014.122 [DOI] [PubMed] [Google Scholar]
- 31.Eser P, Frotzler A, Zehnder Y, Denoth J.. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86:498–504. doi: 10.1016/j.apmr.2004.09.006 [DOI] [PubMed] [Google Scholar]