Co12P7, synthesized at high pressure/temperature conditions, crystallizes isotypically with ordered Cr12P7 in space-group type P
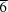 .
.
Keywords: crystal structure, synchrotron, high-pressure synthesis, cobalt phosphide, M12P7 phase
Abstract
The structural properties of cobalt phosphides were investigated at high pressures and temperatures to better understand the behavior of metal-rich phosphides in Earth and planetary interiors. Using single-crystal X-ray diffraction synchrotron data and a laser-heated diamond anvil cell, we discovered a new high pressure–temperature (HP–HT) cobalt phosphide, Co12P7, dodecacobalt heptaphosphide, synthesized at 27 GPa and 1740 K, and at 48 GPa and 1790 K. Co12P7 adopts a structure initially proposed for Cr12P7 (space-group type P
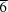 , Z =1), consisting of chains of edge-sharing CoP5 square pyramids and chains of corner-sharing CoP4 tetrahedra. This arrangement leaves space for trigonal–prismatic channels running parallel to the c axis. Coupled disordering of metal and phosphorus atoms has been observed in this structure for related M
12P7 (M = Cr, V) compounds, but all Co and P sites are ordered in Co12P7. All atomic sites in this crystal structure are situated on special positions. Upon decompression to ambient conditions, peak broadening and loss of reflections at high angles was observed, suggesting phase instability.
, Z =1), consisting of chains of edge-sharing CoP5 square pyramids and chains of corner-sharing CoP4 tetrahedra. This arrangement leaves space for trigonal–prismatic channels running parallel to the c axis. Coupled disordering of metal and phosphorus atoms has been observed in this structure for related M
12P7 (M = Cr, V) compounds, but all Co and P sites are ordered in Co12P7. All atomic sites in this crystal structure are situated on special positions. Upon decompression to ambient conditions, peak broadening and loss of reflections at high angles was observed, suggesting phase instability.
Chemical context
Cobalt phosphides have previously been examined in the context of binary phase relations and thermodynamics (Okamoto & Massalski, 1990 ▸; Schlesinger, 2002 ▸) and have gained attention for their unique conductive properties (Prins & Bussell, 2012 ▸; Popczun et al., 2014 ▸; Pan et al., 2016 ▸; Pramanik et al., 2017 ▸), magnetic properties (Fujii et al., 1988 ▸; Jeitschko et al., 1978 ▸; Jeitschko & Jaberg, 1980 ▸; Reehuis & Jeitschko, 1989 ▸), and ability to store lanthanide cations (Jeitschko et al., 1978 ▸). Cobalt phosphides also serve as structural analogs to iron-rich phosphides and sulfides in planetary core-forming alloys. Previous studies of CoP and Co2P indicate that their phase relations tend to precede in pressure the stability of isostructural Fe-phosphides and Fe-sulfides (Rundqvist, 1960 ▸; Ellner & Mittemeijer, 2001 ▸; Dera et al., 2008 ▸; Tateno et al., 2019 ▸; Rundqvist, 1962 ▸; Ono & Kikegawa, 2006 ▸; Ono et al. 2008 ▸). Hence, understanding the behavior of cobalt phosphides at high pressures provides insight into the ultra-high pressure behavior of iron sulfides and phosphides.
There are few structures reported in the literature for transition-metal phosphides with the composition M
12P7. Baurecht et al. (1971 ▸) first examined Cr12P7 and determined that it adopts a hexagonal lattice with space group P
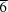 , Z = 1. The structure consists of columns of alternating tetrahedral and pyramidal polyhedra and columns of stacked triangular–prismatic polyhedra extending along the c-axis direction. Chromium atoms occupy half of all possible tetrahedral and pyramidal sites while the triangular–prismatic sites are empty (Baurecht et al., 1971 ▸). The polyhedra in the unit cell can be described as Cr9
PCr3
T[] 2
PrP7 (P = pyramidal, T = tetrahedral, Pr = trigonal–prismatic, [] = empty site) (Maaref et al., 1981 ▸). Coupled disordering of two half-atoms of the corresponding metal with two half-atoms of phosphorus within the tetrahedral and pyramidal sites has been observed in this structure for compounds Th7S12, V12P7, and Cr12P7, increasing the symmetry to the P63/m space group (Zachariasen, 1949 ▸; Olofsson & Ganglberger 1970 ▸; Chun & Carpenter, 1979 ▸).
, Z = 1. The structure consists of columns of alternating tetrahedral and pyramidal polyhedra and columns of stacked triangular–prismatic polyhedra extending along the c-axis direction. Chromium atoms occupy half of all possible tetrahedral and pyramidal sites while the triangular–prismatic sites are empty (Baurecht et al., 1971 ▸). The polyhedra in the unit cell can be described as Cr9
PCr3
T[] 2
PrP7 (P = pyramidal, T = tetrahedral, Pr = trigonal–prismatic, [] = empty site) (Maaref et al., 1981 ▸). Coupled disordering of two half-atoms of the corresponding metal with two half-atoms of phosphorus within the tetrahedral and pyramidal sites has been observed in this structure for compounds Th7S12, V12P7, and Cr12P7, increasing the symmetry to the P63/m space group (Zachariasen, 1949 ▸; Olofsson & Ganglberger 1970 ▸; Chun & Carpenter, 1979 ▸).
At ambient conditions the M
12P7 composition is not observed in the binary systems with M = Co, Ni, Fe. Dhahri (1996 ▸) concluded that Co12P7, Ni12P7 and Fe12P7 do not occur in the Cr12P7 structure type at ambient conditions because, unlike Cr and V, the elements Co, Ni and Fe do not preferentially occupy pyramidal sites. In support of this conclusion, the Zn2Fe12P7 structure type (P
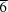 , Z = 1) with many structural similarities to the Cr12P7 structure type, has been observed in Ln
2
M
12P7 (Ln = rare-earth element; M = Co, Ni, Fe) compounds where the pyramidal-to-tetrahedral site ratio is 1:3 (Jeitschko et al., 1978 ▸; Jeitschko & Jaberg, 1980 ▸; Reehuis & Jeitschko, 1989 ▸). Ordering is present in the Co-, Fe-, Ni-rich Zn2Fe12P7 isomorphs (Jeitschko et al., 1984 ▸). No other structure types for the composition M
12P7 (M = Co, Ni, Fe) have been reported so far.
, Z = 1) with many structural similarities to the Cr12P7 structure type, has been observed in Ln
2
M
12P7 (Ln = rare-earth element; M = Co, Ni, Fe) compounds where the pyramidal-to-tetrahedral site ratio is 1:3 (Jeitschko et al., 1978 ▸; Jeitschko & Jaberg, 1980 ▸; Reehuis & Jeitschko, 1989 ▸). Ordering is present in the Co-, Fe-, Ni-rich Zn2Fe12P7 isomorphs (Jeitschko et al., 1984 ▸). No other structure types for the composition M
12P7 (M = Co, Ni, Fe) have been reported so far.
The effect of pressure and temperature on stabilizing Co in both the tetrahedral and pyramidal sites and ordering of Co and P in the Cr12P7-type structure has not been examined previously. In the current study, we report the synthesis of a Co12P7 phase at 27 GPa and 1750 K, and at 48 GPa and 1790 K; both phases are isostructural and crystallize in space group P
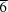 . Structure refinements revealed that Co and P sites are ordered in the high P–T structure and Co atoms occupy tetrahedral and pyramidal coordinations. Using single-crystal diffraction techniques, we report refined atomic coordinate sites of Co12P7 at 48 GPa and 15 GPa.
. Structure refinements revealed that Co and P sites are ordered in the high P–T structure and Co atoms occupy tetrahedral and pyramidal coordinations. Using single-crystal diffraction techniques, we report refined atomic coordinate sites of Co12P7 at 48 GPa and 15 GPa.
Structural commentary
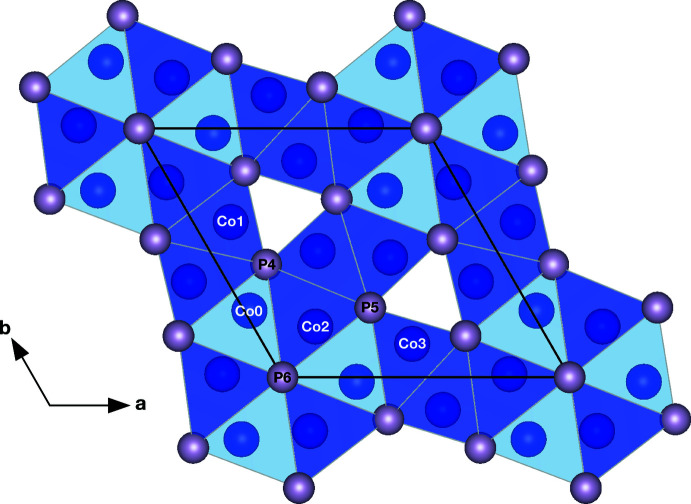
Refinement of the structure confirms that Co12P7 assumes the ordered Cr12P7 structure type (Baurecht et al., 1971 ▸; Chun & Carpenter, 1979 ▸). Two of the Co sites (Co0, Co1) occupy Wyckoff position 3 j (point group symmetry m..), the other two Co sites (Co2, Co3) Wyckoff position 3 k (m..), one P site (P5) Wyckoff position 3 j, one P site (P4) Wyckoff position 3 k, and one P site (P6) Wyckoff position 1 a (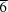 ..). The Co sites occupy tetrahedral (cyan) and pyramidal (violet) sites as imaged in Fig. 1 ▸. Chains of edge-sharing CoP5 square pyramids and chains of corner-sharing CoP4 tetrahedra build up the framework with trigonal–prismatic channels running parallel to the c axis.
..). The Co sites occupy tetrahedral (cyan) and pyramidal (violet) sites as imaged in Fig. 1 ▸. Chains of edge-sharing CoP5 square pyramids and chains of corner-sharing CoP4 tetrahedra build up the framework with trigonal–prismatic channels running parallel to the c axis.
Figure 1.
Crystal structure of Co12P7 based on the 48 GPa data set with atoms of the asymmetric unit labeled. CoP4 tetrahedra are shaded in cyan and CoP5 square pyramids are shaded in violet.
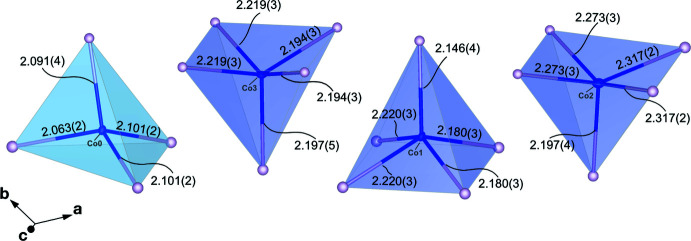
Ranges of interatomic Co—P distances and polyhedral volumes are provided in Table 1 ▸ and Fig. 2 ▸ with CoP4 tetrahedra represented by a cyan polyhedron and CoP5 pyramids represented by violet polyhedra. Co0 atoms occupy a distorted tetrahedral site with one P atom at a short distance, two at intermediate distances and one at a long distance (Table 1 ▸, Fig. 2 ▸). Co1 and Co2 atoms occupy square pyramids with two intermediate and two long interatomic distances at the base. Co3 atoms occupy a less distorted square pyramid with two elongated and two truncated bonds at the base (Fig. 2 ▸). Interatomic distances at 48 GPa range from 2.063 (2)–2.102 (2) Å in the tetrahedral polyhedra, 2.147 (4)–2.220 (4) Å for Co1—P polyhedra, 2.197 (4)–2.317 (2) Å for Co2—P polyhedra and 2.194 (3)–2.219 (3) Å for Co3—P polyhedra (Table 1 ▸). These interatomic distances are comparable to those observed in Co2P and CoP (Rundqvist 1960 ▸, 1962 ▸).
Table 1. Selected structural parameters for Co12P7 at 48 GPa.
| Group | Maximal bond length (Å) | minimal bond length (Å) | Polyhedron volume (Å3) | Distortion index |
|---|---|---|---|---|
| CoP4 (Co0—P4, —P5, —P6) | 2.102 (2) | 2.063 (2) | 4.5433 | 0.00656 |
| CoP5 (Co1—P4, —P5) | 2.220 (4) | 2.147 (4) | 8.1257 | 0.01085 |
| CoP5 (Co2—P4, —P5, —P6) | 2.317 (2) | 2.197 (4) | 9.0766 | 0.01432 |
| CoP5 (Co3—P4, —P5) | 2.219 (3) | 2.194 (3) | 8.3239 | 0.00514 |
Figure 2.
Co—P polyhedra as observed in the Co12P7 structure (48 GPa data set) showing varying degrees of volume and distortion, quantified in Table 1 ▸. CoP4 tetrahedra are shaded in cyan and CoP5 square pyramids are shaded in violet. Displacement ellipsoids are drawn at the 50% probability level.
A grain of Co12P7 was decompressed to ambient conditions where 44 total reflections were identified in reciprocal space and indexed to a unit cell of a = 8.47 (1) Å, c = 3.37 (1) Å. These unit-cell parameters are in agreement with the pressure–volume trend observed, but peak broadening and loss of reflections at high angles may reflect the onset of phase instability on decompression.
Synthesis and crystallization
The synthesis of Co12P7 was performed at high pressures and temperatures in a laser-heated diamond anvil cell (LHDAC). Two samples were loaded for this study in which Co12P7 was synthesized at 26.9 (8) GPa and 1740 (110) K and 48.2 (5) GPa and 1790 (200) K, respectively. Pressure was generated in BX-90-type (70° angular opening) diamond anvil cells (DACs) with 300 µm culet, Boehler–Almax type diamonds and seats. Co–P samples and a ruby sphere for pressure calibration were loaded into a sample chamber drilled from a rhenium gasket. The chamber was subsequently filled with compressed neon gas (Rivers et al., 2008 ▸). Pressure was determined using the ruby fluorescence scale and the Ne equation of state (Mao & Bell, 1976 ▸; Fei et al., 2007 ▸).
Samples were heated from both sides with 100W Yb-doped fiber lasers at beamline 13-ID-D (GeoSoilEnviroCARS) of the Advanced Photon Source (APS), Argonne National Laboratory. Heating cycles typically lasted ∼15 minutes at target temperatures prior to quench. The lasers were shaped with ∼15 µm flat tops and temperature was measured spectroradiometrically from a 6 µm central region of the laser heated spot using a gray body approximation (Heinz & Jeanloz, 1987 ▸). Axial temperature gradients through the sample were accounted for by applying a 3% correction on temperature measurements (Campbell et al., 2007 ▸, 2009 ▸).
Upon quench from high temperatures, high-pressure samples consisted of agglomerates of Co12P7 and Pnma Co2P (Rundqvist, 1960 ▸) crystals of variable grain sizes up to ∼5 µm in diameter. Grains of target phases were identified in reciprocal space and sorted out from the scattering contribution of other grains, neon and diamond. Diffraction data were processed using Dioptas (Prescher & Prakapenka, 2015 ▸) and CrysAlis Pro (Rigaku OD, 2018 ▸). Decompression data were collected for both samples in two experimental stations; here we report two selected refinements of the Co12P7 structure at 48.2 (5) GPa and 15.4 (2) GPa.
Refinement
Crystal data, data collection and structure refinement details at 48 GPa and 15 GPa are summarized in Table 2 ▸.
Table 2. Experimental details.
| 48 GPa | 15 GPa | |
|---|---|---|
| Crystal data | ||
| Chemical formula | Co12P7 | Co12P7 |
| M r | 923.95 | 923.95 |
| Crystal system, space group | Hexagonal, P
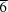
|
Hexagonal, P
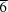
|
| Temperature (K) | 293 | 293 |
| a, c (Å) | 7.9700 (14), 3.2034 (4) | 8.253 (5), 3.2902 (18) |
| V (Å3) | 176.22 (7) | 194.1 (3) |
| Z | 1 | 1 |
| Radiation type | Synchrotron, λ = 0.29521 Å | Synchrotron, λ = 0.3344 Å |
| μ (mm−1) | 2.47 | 3.17 |
| Crystal size (mm) | 0.01 × 0.01 × 0.01 | 0.01 × 0.01 × 0.01 |
| Data collection | ||
| Diffractometer | 13IDD @ APS | 13BMD @ APS |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2018 ▸) | Multi-scan (CrysAlis PRO; Rigaku OD, 2018 ▸) |
| T min, T max | 0.789, 1.000 | 0.546, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 336, 292, 279 | 592, 321, 253 |
| R int | 0.006 | 0.055 |
| (sin θ/λ)max (Å−1) | 0.874 | 0.762 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.037, 0.096, 1.12 | 0.053, 0.105, 1.11 |
| No. of reflections | 292 | 321 |
| No. of parameters | 32 | 32 |
| Δρmax, Δρmin (e Å−3) | 2.35, −1.81 | 1.70, −1.74 |
| Absolute structure | Flack x determined using 75 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) | Flack x determined using 78 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | 0.42 (6) | 0.4 (2) |
Monochromatic X-ray diffraction measurements took place at beamlines 13-ID-D (2 µm x 3 µm beam, λ = 0.2952 Å) and 13-BM-D (5 µm × 8 µm beam, λ = 0.3344 Å) at APS (Table 2 ▸). Diffraction measurements were collected at synthesis pressures and upon decompression. At target pressure steps, 10 x 10 µm still image maps were collected in 2 µm steps around the heated region. At selected map locations exhibiting the largest crystallites, rotation images were collected spanning ±30° at a rate of 1s per 0.5° step.
Grains of Co12P7 identified in reciprocal space were indexed to a primitive hexagonal lattice. Analysis of systematic absences indicated space group P
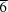 with Z = 1. Two grains from distinct loadings and measured at different beamlines were selected for structural refinements as they showed the largest number of observed reflections and good statistical parameters (Table 2 ▸). Structure factors measured in microdiffraction in the LHDAC show some well-known limitations, such as limited resolution and redundancy, reflections overlapped by parasitic scattering, diamond diffraction (Loveday et al., 1990 ▸) and, more notably, variable volume of illuminated crystal during rotation. As could be expected, we identified eight and five outlier reflections in the refinements for the 48 GPa and 15 GPa data sets, respectively, and omitted them in the final calculations. Based on the ratio ‘observed reflections/refined parameters’ and statistical tests (Hamilton, 1965 ▸), we concluded that the P sites should be refined with isotropic displacement parameters (U
iso) whereas the Co sites could be refined with anisotropic displacement parameters. After convergence, site occupancies of Co atoms and P atoms were released in alternate runs. Within uncertainty (< 1.2% for Co and < 1.3% for P), all sites are fully occupied.
with Z = 1. Two grains from distinct loadings and measured at different beamlines were selected for structural refinements as they showed the largest number of observed reflections and good statistical parameters (Table 2 ▸). Structure factors measured in microdiffraction in the LHDAC show some well-known limitations, such as limited resolution and redundancy, reflections overlapped by parasitic scattering, diamond diffraction (Loveday et al., 1990 ▸) and, more notably, variable volume of illuminated crystal during rotation. As could be expected, we identified eight and five outlier reflections in the refinements for the 48 GPa and 15 GPa data sets, respectively, and omitted them in the final calculations. Based on the ratio ‘observed reflections/refined parameters’ and statistical tests (Hamilton, 1965 ▸), we concluded that the P sites should be refined with isotropic displacement parameters (U
iso) whereas the Co sites could be refined with anisotropic displacement parameters. After convergence, site occupancies of Co atoms and P atoms were released in alternate runs. Within uncertainty (< 1.2% for Co and < 1.3% for P), all sites are fully occupied.
Supplementary Material
Crystal structure: contains datablock(s) Co12P7_at_48GPa, Co12P7_at_15GPa. DOI: 10.1107/S2056989020012657/wm5583sup1.cif
Structure factors: contains datablock(s) Co12P7_at_48GPa. DOI: 10.1107/S2056989020012657/wm5583Co12P7_at_48GPasup2.hkl
Structure factors: contains datablock(s) Co12P7_at_15GPa. DOI: 10.1107/S2056989020012657/wm5583Co12P7_at_15GPasup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Dodecacobalt heptaphosphide (Co12P7_at_48GPa). Crystal data
| Co12P7 | Dx = 8.706 Mg m−3 |
| Mr = 923.95 | Synchrotron radiation, λ = 0.29521 Å |
| Hexagonal, P6 | Cell parameters from 292 reflections |
| a = 7.9700 (14) Å | θ = 2.3–14.9° |
| c = 3.2034 (4) Å | µ = 2.47 mm−1 |
| V = 176.22 (7) Å3 | T = 293 K |
| Z = 1 | Irregular, black |
| F(000) = 429 | 0.01 × 0.01 × 0.01 mm |
Dodecacobalt heptaphosphide (Co12P7_at_48GPa). Data collection
| 13IDD @ APS diffractometer | 279 reflections with I > 2σ(I) |
| Radiation source: synchrotron | Rint = 0.006 |
| ω scans | θmax = 15.0°, θmin = 2.1° |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2018) | h = −6→8 |
| Tmin = 0.789, Tmax = 1.000 | k = −10→9 |
| 336 measured reflections | l = −5→5 |
| 292 independent reflections |
Dodecacobalt heptaphosphide (Co12P7_at_48GPa). Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.0802P)2] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.037 | (Δ/σ)max < 0.001 |
| wR(F2) = 0.096 | Δρmax = 2.35 e Å−3 |
| S = 1.12 | Δρmin = −1.81 e Å−3 |
| 292 reflections | Absolute structure: Flack x determined using 75 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 32 parameters | Absolute structure parameter: 0.42 (6) |
Dodecacobalt heptaphosphide (Co12P7_at_48GPa). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Dodecacobalt heptaphosphide (Co12P7_at_48GPa). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Co0 | 0.0185 (3) | 0.2676 (3) | 0.0000 | 0.0047 (4) | |
| Co1 | 0.1313 (3) | 0.6239 (3) | 0.0000 | 0.0047 (4) | |
| Co2 | 0.2161 (3) | 0.2037 (4) | 0.5000 | 0.0071 (4) | |
| Co3 | 0.5185 (3) | 0.1341 (3) | 0.5000 | 0.0051 (4) | |
| P4 | 0.1693 (5) | 0.4529 (5) | 0.5000 | 0.0062 (6)* | |
| P5 | 0.4454 (5) | 0.2795 (6) | 0.0000 | 0.0050 (6)* | |
| P6 | 0.0000 | 0.0000 | 0.0000 | 0.0069 (9)* |
Dodecacobalt heptaphosphide (Co12P7_at_48GPa). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Co0 | 0.0044 (8) | 0.0030 (8) | 0.0062 (5) | 0.0015 (7) | 0.000 | 0.000 |
| Co1 | 0.0038 (8) | 0.0024 (7) | 0.0071 (8) | 0.0009 (6) | 0.000 | 0.000 |
| Co2 | 0.0081 (8) | 0.0069 (9) | 0.0079 (6) | 0.0049 (7) | 0.000 | 0.000 |
| Co3 | 0.0038 (8) | 0.0028 (8) | 0.0069 (7) | 0.0005 (6) | 0.000 | 0.000 |
Dodecacobalt heptaphosphide (Co12P7_at_48GPa). Geometric parameters (Å, º)
| Co0—P6 | 2.0629 (18) | Co2—Co3 | 2.730 (2) |
| Co0—P5i | 2.091 (4) | Co3—P5xi | 2.194 (3) |
| Co0—P4 | 2.102 (2) | Co3—P5xii | 2.194 (3) |
| Co0—P4ii | 2.102 (2) | Co3—P4viii | 2.197 (5) |
| Co0—Co3iii | 2.458 (2) | Co3—P5vii | 2.218 (3) |
| Co0—Co3i | 2.458 (2) | Co3—P5 | 2.219 (3) |
| Co0—Co2ii | 2.4710 (19) | Co3—Co0viii | 2.458 (2) |
| Co0—Co2 | 2.4710 (19) | Co3—Co0ix | 2.458 (2) |
| Co0—Co2iii | 2.497 (2) | Co3—Co3xii | 2.474 (3) |
| Co0—Co2i | 2.497 (2) | Co3—Co3xiii | 2.475 (3) |
| Co0—Co1 | 2.514 (3) | Co3—Co1viii | 2.578 (2) |
| Co0—Co1iv | 2.515 (2) | Co3—Co1ix | 2.578 (2) |
| Co1—P5v | 2.147 (4) | P4—Co0vii | 2.102 (2) |
| Co1—P4v | 2.180 (3) | P4—Co1x | 2.180 (3) |
| Co1—P4vi | 2.180 (3) | P4—Co1iv | 2.180 (3) |
| Co1—P4ii | 2.220 (3) | P4—Co3i | 2.197 (5) |
| Co1—P4 | 2.220 (3) | P4—Co1vii | 2.220 (3) |
| Co1—Co0v | 2.515 (2) | P4—P4iv | 2.674 (6) |
| Co1—Co1v | 2.546 (3) | P4—P4v | 2.674 (6) |
| Co1—Co1iv | 2.546 (3) | P5—Co0viii | 2.091 (4) |
| Co1—Co3iii | 2.578 (2) | P5—Co1iv | 2.147 (4) |
| Co1—Co3i | 2.578 (2) | P5—Co3xiii | 2.194 (3) |
| Co1—Co2v | 2.639 (2) | P5—Co3xiv | 2.194 (3) |
| Co2—P4 | 2.197 (4) | P5—Co3ii | 2.218 (3) |
| Co2—P5 | 2.273 (3) | P5—Co2ii | 2.273 (3) |
| Co2—P5vii | 2.273 (3) | P6—Co0i | 2.0629 (18) |
| Co2—P6 | 2.3174 (17) | P6—Co0viii | 2.0629 (18) |
| Co2—P6vii | 2.3174 (17) | P6—Co2xv | 2.3174 (17) |
| Co2—Co0vii | 2.4710 (19) | P6—Co2viii | 2.3174 (17) |
| Co2—Co0viii | 2.497 (2) | P6—Co2ii | 2.3174 (17) |
| Co2—Co0ix | 2.497 (2) | P6—Co2iii | 2.3175 (17) |
| Co2—Co1x | 2.639 (2) | P6—Co2i | 2.3175 (17) |
| Co2—Co1iv | 2.639 (2) | ||
| P6—Co0—P5i | 96.84 (13) | P4—Co2—Co3 | 138.57 (14) |
| P6—Co0—P4 | 116.54 (11) | P5—Co2—Co3 | 51.66 (8) |
| P5i—Co0—P4 | 114.33 (11) | P5vii—Co2—Co3 | 51.66 (8) |
| P6—Co0—P4ii | 116.54 (11) | P6—Co2—Co3 | 106.26 (8) |
| P5i—Co0—P4ii | 114.33 (11) | P6vii—Co2—Co3 | 106.26 (8) |
| P4—Co0—P4ii | 99.31 (14) | Co0vii—Co2—Co3 | 139.47 (4) |
| P6—Co0—Co3iii | 126.74 (6) | Co0—Co2—Co3 | 139.47 (4) |
| P5i—Co0—Co3iii | 57.69 (9) | Co0viii—Co2—Co3 | 55.89 (7) |
| P4—Co0—Co3iii | 116.61 (12) | Co0ix—Co2—Co3 | 55.89 (7) |
| P4ii—Co0—Co3iii | 56.96 (11) | Co1x—Co2—Co3 | 96.63 (8) |
| P6—Co0—Co3i | 126.74 (6) | Co1iv—Co2—Co3 | 96.63 (8) |
| P5i—Co0—Co3i | 57.69 (9) | P5xi—Co3—P5xii | 93.78 (15) |
| P4—Co0—Co3i | 56.96 (11) | P5xi—Co3—P4viii | 107.43 (14) |
| P4ii—Co0—Co3i | 116.61 (12) | P5xii—Co3—P4viii | 107.43 (14) |
| Co3iii—Co0—Co3i | 81.31 (9) | P5xi—Co3—P5vii | 77.38 (14) |
| P6—Co0—Co2ii | 60.69 (6) | P5xii—Co3—P5vii | 146.69 (16) |
| P5i—Co0—Co2ii | 129.60 (8) | P4viii—Co3—P5vii | 105.86 (14) |
| P4—Co0—Co2ii | 116.07 (13) | P5xi—Co3—P5 | 146.69 (16) |
| P4ii—Co0—Co2ii | 56.73 (11) | P5xii—Co3—P5 | 77.38 (14) |
| Co3iii—Co0—Co2ii | 98.20 (5) | P4viii—Co3—P5 | 105.86 (14) |
| Co3i—Co0—Co2ii | 170.85 (9) | P5vii—Co3—P5 | 92.44 (15) |
| P6—Co0—Co2 | 60.69 (6) | P5xi—Co3—Co0viii | 160.35 (15) |
| P5i—Co0—Co2 | 129.60 (8) | P5xii—Co3—Co0viii | 89.46 (9) |
| P4—Co0—Co2 | 56.73 (11) | P4viii—Co3—Co0viii | 53.31 (7) |
| P4ii—Co0—Co2 | 116.07 (13) | P5vii—Co3—Co0viii | 109.66 (12) |
| Co3iii—Co0—Co2 | 170.85 (9) | P5—Co3—Co0viii | 52.82 (11) |
| Co3i—Co0—Co2 | 98.20 (5) | P5xi—Co3—Co0ix | 89.46 (9) |
| Co2ii—Co0—Co2 | 80.81 (8) | P5xii—Co3—Co0ix | 160.35 (15) |
| P6—Co0—Co2iii | 60.19 (6) | P4viii—Co3—Co0ix | 53.31 (7) |
| P5i—Co0—Co2iii | 58.60 (11) | P5vii—Co3—Co0ix | 52.82 (11) |
| P4—Co0—Co2iii | 169.97 (10) | P5—Co3—Co0ix | 109.66 (12) |
| P4ii—Co0—Co2iii | 90.40 (8) | Co0viii—Co3—Co0ix | 81.32 (9) |
| Co3iii—Co0—Co2iii | 66.85 (7) | P5xi—Co3—Co3xii | 56.36 (10) |
| Co3i—Co0—Co2iii | 116.27 (10) | P5xii—Co3—Co3xii | 56.36 (10) |
| Co2ii—Co0—Co2iii | 71.44 (10) | P4viii—Co3—Co3xii | 151.84 (16) |
| Co2—Co0—Co2iii | 120.88 (10) | P5vii—Co3—Co3xii | 93.38 (11) |
| P6—Co0—Co2i | 60.19 (6) | P5—Co3—Co3xii | 93.38 (11) |
| P5i—Co0—Co2i | 58.60 (11) | Co0viii—Co3—Co3xii | 138.36 (5) |
| P4—Co0—Co2i | 90.40 (8) | Co0ix—Co3—Co3xii | 138.36 (5) |
| P4ii—Co0—Co2i | 169.97 (10) | P5xi—Co3—Co3xiii | 93.99 (11) |
| Co3iii—Co0—Co2i | 116.27 (10) | P5xii—Co3—Co3xiii | 93.99 (11) |
| Co3i—Co0—Co2i | 66.85 (7) | P4viii—Co3—Co3xiii | 148.16 (16) |
| Co2ii—Co0—Co2i | 120.88 (10) | P5vii—Co3—Co3xiii | 55.42 (11) |
| Co2—Co0—Co2i | 71.44 (10) | P5—Co3—Co3xiii | 55.42 (11) |
| Co2iii—Co0—Co2i | 79.79 (9) | Co0viii—Co3—Co3xiii | 105.12 (10) |
| P6—Co0—Co1 | 165.52 (9) | Co0ix—Co3—Co3xiii | 105.12 (10) |
| P5i—Co0—Co1 | 97.64 (13) | Co3xii—Co3—Co3xiii | 60.0 |
| P4—Co0—Co1 | 56.65 (9) | P5xi—Co3—Co1viii | 107.55 (12) |
| P4ii—Co0—Co1 | 56.65 (9) | P5xii—Co3—Co1viii | 52.72 (10) |
| Co3iii—Co0—Co1 | 62.44 (6) | P4viii—Co3—Co1viii | 54.71 (8) |
| Co3i—Co0—Co1 | 62.44 (6) | P5vii—Co3—Co1viii | 160.55 (15) |
| Co2ii—Co0—Co1 | 109.16 (7) | P5—Co3—Co1viii | 92.62 (8) |
| Co2—Co0—Co1 | 109.16 (7) | Co0viii—Co3—Co1viii | 59.83 (7) |
| Co2iii—Co0—Co1 | 128.86 (7) | Co0ix—Co3—Co1viii | 107.89 (10) |
| Co2i—Co0—Co1 | 128.86 (7) | Co3xii—Co3—Co1viii | 105.04 (11) |
| P6—Co0—Co1iv | 104.70 (9) | Co3xiii—Co3—Co1viii | 140.36 (4) |
| P5i—Co0—Co1iv | 158.46 (14) | P5xi—Co3—Co1ix | 52.72 (10) |
| P4—Co0—Co1iv | 55.49 (9) | P5xii—Co3—Co1ix | 107.55 (12) |
| P4ii—Co0—Co1iv | 55.49 (9) | P4viii—Co3—Co1ix | 54.71 (8) |
| Co3iii—Co0—Co1iv | 107.42 (7) | P5vii—Co3—Co1ix | 92.62 (8) |
| Co3i—Co0—Co1iv | 107.42 (7) | P5—Co3—Co1ix | 160.55 (15) |
| Co2ii—Co0—Co1iv | 63.90 (7) | Co0viii—Co3—Co1ix | 107.89 (10) |
| Co2—Co0—Co1iv | 63.90 (7) | Co0ix—Co3—Co1ix | 59.83 (7) |
| Co2iii—Co0—Co1iv | 133.74 (7) | Co3xii—Co3—Co1ix | 105.04 (11) |
| Co2i—Co0—Co1iv | 133.74 (7) | Co3xiii—Co3—Co1ix | 140.36 (4) |
| Co1—Co0—Co1iv | 60.82 (8) | Co1viii—Co3—Co1ix | 76.82 (8) |
| P5v—Co1—P4v | 108.68 (12) | P5xi—Co3—Co2 | 130.38 (8) |
| P5v—Co1—P4vi | 108.68 (12) | P5xii—Co3—Co2 | 130.38 (8) |
| P4v—Co1—P4vi | 94.55 (18) | P4viii—Co3—Co2 | 82.57 (13) |
| P5v—Co1—P4ii | 108.29 (12) | P5vii—Co3—Co2 | 53.49 (10) |
| P4v—Co1—P4ii | 143.01 (13) | P5—Co3—Co2 | 53.49 (10) |
| P4vi—Co1—P4ii | 74.85 (14) | Co0viii—Co3—Co2 | 57.26 (7) |
| P5v—Co1—P4 | 108.29 (12) | Co0ix—Co3—Co2 | 57.26 (7) |
| P4v—Co1—P4 | 74.85 (14) | Co3xii—Co3—Co2 | 125.59 (11) |
| P4vi—Co1—P4 | 143.01 (13) | Co3xiii—Co3—Co2 | 65.59 (11) |
| P4ii—Co1—P4 | 92.36 (16) | Co1viii—Co3—Co2 | 116.75 (8) |
| P5v—Co1—Co0 | 89.08 (13) | Co1ix—Co3—Co2 | 116.75 (8) |
| P4v—Co1—Co0 | 127.10 (9) | Co0—P4—Co0vii | 99.31 (14) |
| P4vi—Co1—Co0 | 127.10 (9) | Co0—P4—Co1x | 144.1 (2) |
| P4ii—Co1—Co0 | 52.26 (8) | Co0vii—P4—Co1x | 71.92 (7) |
| P4—Co1—Co0 | 52.26 (8) | Co0—P4—Co1iv | 71.92 (7) |
| P5v—Co1—Co0v | 91.74 (12) | Co0vii—P4—Co1iv | 144.1 (2) |
| P4v—Co1—Co0v | 52.58 (9) | Co1x—P4—Co1iv | 94.55 (17) |
| P4vi—Co1—Co0v | 52.58 (9) | Co0—P4—Co2 | 70.15 (11) |
| P4ii—Co1—Co0v | 127.40 (9) | Co0vii—P4—Co2 | 70.15 (11) |
| P4—Co1—Co0v | 127.40 (9) | Co1x—P4—Co2 | 74.16 (12) |
| Co0—Co1—Co0v | 179.18 (8) | Co1iv—P4—Co2 | 74.16 (12) |
| P5v—Co1—Co1v | 151.31 (16) | Co0—P4—Co3i | 69.73 (11) |
| P4v—Co1—Co1v | 55.38 (11) | Co0vii—P4—Co3i | 69.73 (11) |
| P4vi—Co1—Co1v | 55.38 (11) | Co1x—P4—Co3i | 132.68 (9) |
| P4ii—Co1—Co1v | 91.21 (10) | Co1iv—P4—Co3i | 132.68 (9) |
| P4—Co1—Co1v | 91.21 (10) | Co2—P4—Co3i | 116.01 (16) |
| Co0—Co1—Co1v | 119.61 (8) | Co0—P4—Co1 | 71.09 (7) |
| Co0v—Co1—Co1v | 59.56 (10) | Co0vii—P4—Co1 | 140.9 (2) |
| P5v—Co1—Co1iv | 148.69 (16) | Co1x—P4—Co1 | 136.84 (16) |
| P4v—Co1—Co1iv | 92.13 (10) | Co1iv—P4—Co1 | 70.69 (9) |
| P4vi—Co1—Co1iv | 92.13 (10) | Co2—P4—Co1 | 133.80 (8) |
| P4ii—Co1—Co1iv | 53.93 (10) | Co3i—P4—Co1 | 71.42 (12) |
| P4—Co1—Co1iv | 53.93 (10) | Co0—P4—Co1vii | 140.9 (2) |
| Co0—Co1—Co1iv | 59.61 (8) | Co0vii—P4—Co1vii | 71.09 (7) |
| Co0v—Co1—Co1iv | 119.56 (10) | Co1x—P4—Co1vii | 70.69 (9) |
| Co1v—Co1—Co1iv | 60.0 | Co1iv—P4—Co1vii | 136.84 (17) |
| P5v—Co1—Co3iii | 54.42 (8) | Co2—P4—Co1vii | 133.80 (8) |
| P4v—Co1—Co3iii | 163.07 (13) | Co3i—P4—Co1vii | 71.42 (12) |
| P4vi—Co1—Co3iii | 92.51 (9) | Co1—P4—Co1vii | 92.36 (16) |
| P4ii—Co1—Co3iii | 53.87 (11) | Co0—P4—P4iv | 125.14 (14) |
| P4—Co1—Co3iii | 107.88 (12) | Co0vii—P4—P4iv | 125.14 (14) |
| Co0—Co1—Co3iii | 57.72 (8) | Co1x—P4—P4iv | 53.25 (12) |
| Co0v—Co1—Co3iii | 122.84 (8) | Co1iv—P4—P4iv | 53.25 (12) |
| Co1v—Co1—Co3iii | 139.67 (4) | Co2—P4—P4iv | 94.4 (2) |
| Co1iv—Co1—Co3iii | 102.97 (11) | Co3i—P4—P4iv | 149.6 (2) |
| P5v—Co1—Co3i | 54.42 (8) | Co1—P4—P4iv | 87.91 (10) |
| P4v—Co1—Co3i | 92.51 (9) | Co1vii—P4—P4iv | 87.91 (10) |
| P4vi—Co1—Co3i | 163.07 (13) | Co0—P4—P4v | 122.98 (14) |
| P4ii—Co1—Co3i | 107.88 (12) | Co0vii—P4—P4v | 122.98 (14) |
| P4—Co1—Co3i | 53.87 (11) | Co1x—P4—P4v | 88.73 (10) |
| Co0—Co1—Co3i | 57.72 (8) | Co1iv—P4—P4v | 88.73 (10) |
| Co0v—Co1—Co3i | 122.84 (8) | Co2—P4—P4v | 154.4 (2) |
| Co1v—Co1—Co3i | 139.67 (4) | Co3i—P4—P4v | 89.6 (2) |
| Co1iv—Co1—Co3i | 102.97 (11) | Co1—P4—P4v | 51.90 (11) |
| Co3iii—Co1—Co3i | 76.82 (8) | Co1vii—P4—P4v | 51.90 (11) |
| P5v—Co1—Co2v | 55.58 (8) | P4iv—P4—P4v | 60.0 |
| P4v—Co1—Co2v | 53.20 (10) | Co0viii—P5—Co1iv | 126.72 (19) |
| P4vi—Co1—Co2v | 107.02 (11) | Co0viii—P5—Co3xiii | 132.10 (9) |
| P4ii—Co1—Co2v | 163.76 (12) | Co1iv—P5—Co3xiii | 72.86 (12) |
| P4—Co1—Co2v | 94.83 (9) | Co0viii—P5—Co3xiv | 132.10 (9) |
| Co0—Co1—Co2v | 123.33 (7) | Co1iv—P5—Co3xiv | 72.86 (12) |
| Co0v—Co1—Co2v | 57.23 (6) | Co3xiii—P5—Co3xiv | 93.78 (15) |
| Co1v—Co1—Co2v | 103.16 (10) | Co0viii—P5—Co3ii | 69.48 (13) |
| Co1iv—Co1—Co2v | 140.65 (5) | Co1iv—P5—Co3ii | 133.45 (8) |
| Co3iii—Co1—Co2v | 109.96 (9) | Co3xiii—P5—Co3ii | 133.08 (18) |
| Co3i—Co1—Co2v | 65.62 (7) | Co3xiv—P5—Co3ii | 68.22 (10) |
| P4—Co2—P5 | 103.71 (12) | Co0viii—P5—Co3 | 69.48 (13) |
| P4—Co2—P5vii | 103.71 (12) | Co1iv—P5—Co3 | 133.45 (8) |
| P5—Co2—P5vii | 89.59 (15) | Co3xiii—P5—Co3 | 68.22 (10) |
| P4—Co2—P6 | 103.35 (9) | Co3xiv—P5—Co3 | 133.08 (18) |
| P5—Co2—P6 | 85.20 (8) | Co3ii—P5—Co3 | 92.44 (15) |
| P5vii—Co2—P6 | 152.92 (13) | Co0viii—P5—Co2 | 69.66 (13) |
| P4—Co2—P6vii | 103.35 (9) | Co1iv—P5—Co2 | 73.26 (11) |
| P5—Co2—P6vii | 152.92 (13) | Co3xiii—P5—Co2 | 78.50 (8) |
| P5vii—Co2—P6vii | 85.20 (8) | Co3xiv—P5—Co2 | 146.03 (19) |
| P6—Co2—P6vii | 87.44 (8) | Co3ii—P5—Co2 | 139.1 (2) |
| P4—Co2—Co0vii | 53.12 (8) | Co3—P5—Co2 | 74.85 (8) |
| P5—Co2—Co0vii | 155.85 (14) | Co0viii—P5—Co2ii | 69.66 (13) |
| P5vii—Co2—Co0vii | 89.95 (7) | Co1iv—P5—Co2ii | 73.25 (11) |
| P6—Co2—Co0vii | 105.40 (8) | Co3xiii—P5—Co2ii | 146.03 (19) |
| P6vii—Co2—Co0vii | 50.91 (5) | Co3xiv—P5—Co2ii | 78.50 (8) |
| P4—Co2—Co0 | 53.12 (8) | Co3ii—P5—Co2ii | 74.85 (8) |
| P5—Co2—Co0 | 89.95 (7) | Co3—P5—Co2ii | 139.1 (2) |
| P5vii—Co2—Co0 | 155.85 (14) | Co2—P5—Co2ii | 89.59 (15) |
| P6—Co2—Co0 | 50.91 (5) | Co0i—P6—Co0 | 120.0 |
| P6vii—Co2—Co0 | 105.40 (8) | Co0i—P6—Co0viii | 120.0 |
| Co0vii—Co2—Co0 | 80.81 (8) | Co0—P6—Co0viii | 120.0 |
| P4—Co2—Co0viii | 140.08 (4) | Co0i—P6—Co2xv | 69.24 (6) |
| P5—Co2—Co0viii | 51.74 (11) | Co0—P6—Co2xv | 136.27 (4) |
| P5vii—Co2—Co0viii | 106.53 (11) | Co0viii—P6—Co2xv | 68.40 (6) |
| P6—Co2—Co0viii | 50.57 (5) | Co0i—P6—Co2viii | 69.24 (6) |
| P6vii—Co2—Co0viii | 104.56 (10) | Co0—P6—Co2viii | 136.27 (4) |
| Co0vii—Co2—Co0viii | 149.98 (11) | Co0viii—P6—Co2viii | 68.40 (6) |
| Co0—Co2—Co0viii | 91.97 (7) | Co2xv—P6—Co2viii | 87.45 (8) |
| P4—Co2—Co0ix | 140.08 (4) | Co0i—P6—Co2 | 136.27 (4) |
| P5—Co2—Co0ix | 106.54 (11) | Co0—P6—Co2 | 68.40 (6) |
| P5vii—Co2—Co0ix | 51.74 (11) | Co0viii—P6—Co2 | 69.24 (6) |
| P6—Co2—Co0ix | 104.56 (10) | Co2xv—P6—Co2 | 137.63 (3) |
| P6vii—Co2—Co0ix | 50.57 (5) | Co2viii—P6—Co2 | 77.49 (6) |
| Co0vii—Co2—Co0ix | 91.97 (7) | Co0i—P6—Co2ii | 136.27 (4) |
| Co0—Co2—Co0ix | 149.98 (11) | Co0—P6—Co2ii | 68.40 (6) |
| Co0viii—Co2—Co0ix | 79.79 (9) | Co0viii—P6—Co2ii | 69.24 (6) |
| P4—Co2—Co1x | 52.64 (9) | Co2xv—P6—Co2ii | 77.49 (6) |
| P5—Co2—Co1x | 103.19 (12) | Co2viii—P6—Co2ii | 137.64 (3) |
| P5vii—Co2—Co1x | 51.16 (10) | Co2—P6—Co2ii | 87.44 (8) |
| P6—Co2—Co1x | 155.65 (9) | Co0i—P6—Co2iii | 68.40 (6) |
| P6vii—Co2—Co1x | 94.13 (4) | Co0—P6—Co2iii | 69.24 (6) |
| Co0vii—Co2—Co1x | 58.87 (6) | Co0viii—P6—Co2iii | 136.27 (4) |
| Co0—Co2—Co1x | 105.65 (9) | Co2xv—P6—Co2iii | 77.49 (6) |
| Co0viii—Co2—Co1x | 149.97 (10) | Co2viii—P6—Co2iii | 137.63 (3) |
| Co0ix—Co2—Co1x | 95.00 (6) | Co2—P6—Co2iii | 137.63 (3) |
| P4—Co2—Co1iv | 52.64 (9) | Co2ii—P6—Co2iii | 77.49 (6) |
| P5—Co2—Co1iv | 51.16 (10) | Co0i—P6—Co2i | 68.40 (6) |
| P5vii—Co2—Co1iv | 103.19 (13) | Co0—P6—Co2i | 69.24 (6) |
| P6—Co2—Co1iv | 94.13 (4) | Co0viii—P6—Co2i | 136.27 (4) |
| P6vii—Co2—Co1iv | 155.65 (9) | Co2xv—P6—Co2i | 137.63 (3) |
| Co0vii—Co2—Co1iv | 105.65 (9) | Co2viii—P6—Co2i | 77.49 (6) |
| Co0—Co2—Co1iv | 58.87 (6) | Co2—P6—Co2i | 77.49 (6) |
| Co0viii—Co2—Co1iv | 95.00 (6) | Co2ii—P6—Co2i | 137.63 (3) |
| Co0ix—Co2—Co1iv | 149.97 (10) | Co2iii—P6—Co2i | 87.44 (8) |
| Co1x—Co2—Co1iv | 74.74 (7) |
Symmetry codes: (i) −y, x−y, z; (ii) x, y, z−1; (iii) −y, x−y, z−1; (iv) −y+1, x−y+1, z; (v) −x+y, −x+1, z; (vi) −x+y, −x+1, z−1; (vii) x, y, z+1; (viii) −x+y, −x, z; (ix) −x+y, −x, z+1; (x) −y+1, x−y+1, z+1; (xi) −y+1, x−y, z+1; (xii) −y+1, x−y, z; (xiii) −x+y+1, −x+1, z; (xiv) −x+y+1, −x+1, z−1; (xv) −x+y, −x, z−1.
(Co12P7_at_15GPa). Crystal data
| Co12P7 | Dx = 7.905 Mg m−3 |
| Mr = 923.95 | Synchrotron radiation, λ = 0.3344 Å |
| Hexagonal, P6 | Cell parameters from 249 reflections |
| a = 8.253 (5) Å | θ = 2.9–14.7° |
| c = 3.2902 (18) Å | µ = 3.17 mm−1 |
| V = 194.1 (3) Å3 | T = 293 K |
| Z = 1 | Irregular, black |
| F(000) = 429 | 0.01 × 0.01 × 0.01 mm |
(Co12P7_at_15GPa). Data collection
| 13BMD @ APS diffractometer | 253 reflections with I > 2σ(I) |
| Radiation source: synchrotron | Rint = 0.055 |
| /w scan | θmax = 14.8°, θmin = 3.2° |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2018) | h = −11→12 |
| Tmin = 0.546, Tmax = 1.000 | k = −9→8 |
| 592 measured reflections | l = −4→4 |
| 321 independent reflections |
(Co12P7_at_15GPa). Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.0219P)2 + 2.8589P] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.053 | (Δ/σ)max < 0.001 |
| wR(F2) = 0.105 | Δρmax = 1.70 e Å−3 |
| S = 1.11 | Δρmin = −1.74 e Å−3 |
| 321 reflections | Absolute structure: Flack x determined using 78 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 32 parameters | Absolute structure parameter: 0.4 (2) |
(Co12P7_at_15GPa). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(Co12P7_at_15GPa). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Co0 | 0.0153 (6) | 0.2651 (6) | 0.0000 | 0.0114 (8) | |
| Co1 | 0.1320 (7) | 0.6234 (7) | 0.0000 | 0.0123 (9) | |
| Co2 | 0.2135 (6) | 0.2038 (8) | 0.5000 | 0.0151 (9) | |
| Co3 | 0.5195 (7) | 0.1363 (7) | 0.5000 | 0.0107 (9) | |
| P4 | 0.1656 (11) | 0.4503 (11) | 0.5000 | 0.0102 (15)* | |
| P5 | 0.4425 (12) | 0.2809 (13) | 0.0000 | 0.0086 (15)* | |
| P6 | 0.0000 | 0.0000 | 0.0000 | 0.012 (3)* |
(Co12P7_at_15GPa). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Co0 | 0.014 (2) | 0.016 (2) | 0.0072 (18) | 0.009 (2) | 0.000 | 0.000 |
| Co1 | 0.019 (2) | 0.016 (2) | 0.003 (2) | 0.0101 (18) | 0.000 | 0.000 |
| Co2 | 0.024 (2) | 0.020 (3) | 0.005 (2) | 0.014 (2) | 0.000 | 0.000 |
| Co3 | 0.011 (2) | 0.012 (2) | 0.008 (2) | 0.0052 (16) | 0.000 | 0.000 |
(Co12P7_at_15GPa). Geometric parameters (Å, º)
| Co0—P6 | 2.128 (5) | Co2—Co1iv | 2.731 (5) |
| Co0—P5i | 2.148 (10) | Co2—Co3 | 2.846 (7) |
| Co0—P4 | 2.165 (6) | Co3—P5xi | 2.263 (7) |
| Co0—P4ii | 2.165 (6) | Co3—P5xii | 2.263 (7) |
| Co0—Co3iii | 2.538 (5) | Co3—P4viii | 2.266 (10) |
| Co0—Co3i | 2.538 (5) | Co3—P5vii | 2.301 (8) |
| Co0—Co2ii | 2.543 (6) | Co3—P5 | 2.301 (8) |
| Co0—Co2 | 2.543 (6) | Co3—Co3xiii | 2.536 (9) |
| Co0—Co2iii | 2.571 (6) | Co3—Co3xii | 2.536 (9) |
| Co0—Co2i | 2.571 (6) | Co3—Co0viii | 2.538 (5) |
| Co0—Co1 | 2.612 (7) | Co3—Co0ix | 2.538 (5) |
| Co0—Co1iv | 2.634 (7) | Co3—Co1viii | 2.674 (5) |
| Co1—P5v | 2.202 (10) | Co3—Co1ix | 2.674 (5) |
| Co1—P4v | 2.266 (7) | P4—Co0vii | 2.165 (6) |
| Co1—P4vi | 2.266 (7) | P4—Co1x | 2.266 (7) |
| Co1—P4ii | 2.284 (7) | P4—Co1iv | 2.266 (7) |
| Co1—P4 | 2.285 (7) | P4—Co3i | 2.266 (10) |
| Co1—Co1v | 2.624 (9) | P4—Co1vii | 2.285 (7) |
| Co1—Co1iv | 2.624 (9) | P5—Co0viii | 2.148 (10) |
| Co1—Co0v | 2.634 (7) | P5—Co1iv | 2.202 (10) |
| Co1—Co3iii | 2.674 (5) | P5—Co3xiii | 2.263 (7) |
| Co1—Co3i | 2.674 (5) | P5—Co3xiv | 2.263 (7) |
| Co1—Co2v | 2.731 (5) | P5—Co3ii | 2.301 (8) |
| Co2—P4 | 2.258 (9) | P5—Co2ii | 2.341 (7) |
| Co2—P5vii | 2.341 (7) | P6—Co0i | 2.128 (5) |
| Co2—P5 | 2.341 (7) | P6—Co0viii | 2.128 (5) |
| Co2—P6 | 2.383 (4) | P6—Co2xv | 2.383 (4) |
| Co2—P6vii | 2.383 (4) | P6—Co2iii | 2.383 (4) |
| Co2—Co0vii | 2.543 (6) | P6—Co2i | 2.383 (4) |
| Co2—Co0viii | 2.571 (6) | P6—Co2viii | 2.383 (4) |
| Co2—Co0ix | 2.571 (6) | P6—Co2ii | 2.383 (4) |
| Co2—Co1x | 2.731 (5) | ||
| P6—Co0—P5i | 96.9 (3) | P5vii—Co2—Co1iv | 102.3 (3) |
| P6—Co0—P4 | 116.4 (2) | P5—Co2—Co1iv | 50.7 (2) |
| P5i—Co0—P4 | 114.7 (3) | P6—Co2—Co1iv | 94.84 (11) |
| P6—Co0—P4ii | 116.4 (2) | P6vii—Co2—Co1iv | 156.4 (2) |
| P5i—Co0—P4ii | 114.7 (3) | Co0vii—Co2—Co1iv | 106.0 (2) |
| P4—Co0—P4ii | 98.9 (4) | Co0—Co2—Co1iv | 59.81 (15) |
| P6—Co0—Co3iii | 127.43 (16) | Co0viii—Co2—Co1iv | 94.89 (12) |
| P5i—Co0—Co3iii | 58.1 (2) | Co0ix—Co2—Co1iv | 148.9 (2) |
| P4—Co0—Co3iii | 116.1 (3) | Co1x—Co2—Co1iv | 74.08 (17) |
| P4ii—Co0—Co3iii | 56.9 (2) | P4—Co2—Co3 | 138.5 (3) |
| P6—Co0—Co3i | 127.43 (16) | P5vii—Co2—Co3 | 51.6 (2) |
| P5i—Co0—Co3i | 58.1 (2) | P5—Co2—Co3 | 51.6 (2) |
| P4—Co0—Co3i | 56.9 (2) | P6—Co2—Co3 | 106.1 (2) |
| P4ii—Co0—Co3i | 116.1 (3) | P6vii—Co2—Co3 | 106.1 (2) |
| Co3iii—Co0—Co3i | 80.8 (2) | Co0vii—Co2—Co3 | 139.57 (11) |
| P6—Co0—Co2ii | 60.57 (16) | Co0—Co2—Co3 | 139.58 (11) |
| P5i—Co0—Co2ii | 129.6 (2) | Co0viii—Co2—Co3 | 55.61 (16) |
| P4—Co0—Co2ii | 115.7 (3) | Co0ix—Co2—Co3 | 55.61 (16) |
| P4ii—Co0—Co2ii | 56.6 (2) | Co1x—Co2—Co3 | 95.9 (2) |
| Co3iii—Co0—Co2ii | 98.44 (12) | Co1iv—Co2—Co3 | 95.9 (2) |
| Co3i—Co0—Co2ii | 170.2 (3) | P5xi—Co3—P5xii | 93.3 (4) |
| P6—Co0—Co2 | 60.57 (16) | P5xi—Co3—P4viii | 106.5 (3) |
| P5i—Co0—Co2 | 129.6 (2) | P5xii—Co3—P4viii | 106.5 (3) |
| P4—Co0—Co2 | 56.6 (2) | P5xi—Co3—P5vii | 79.0 (4) |
| P4ii—Co0—Co2 | 115.7 (3) | P5xii—Co3—P5vii | 148.1 (4) |
| Co3iii—Co0—Co2 | 170.2 (3) | P4viii—Co3—P5vii | 105.3 (3) |
| Co3i—Co0—Co2 | 98.44 (12) | P5xi—Co3—P5 | 148.1 (4) |
| Co2ii—Co0—Co2 | 80.6 (2) | P5xii—Co3—P5 | 79.0 (4) |
| P6—Co0—Co2iii | 60.07 (15) | P4viii—Co3—P5 | 105.3 (3) |
| P5i—Co0—Co2iii | 58.7 (2) | P5vii—Co3—P5 | 91.3 (4) |
| P4—Co0—Co2iii | 170.1 (3) | P5xi—Co3—Co3xiii | 95.1 (3) |
| P4ii—Co0—Co2iii | 90.7 (2) | P5xii—Co3—Co3xiii | 95.1 (3) |
| Co3iii—Co0—Co2iii | 67.70 (14) | P4viii—Co3—Co3xiii | 148.1 (4) |
| Co3i—Co0—Co2iii | 116.8 (2) | P5vii—Co3—Co3xiii | 55.5 (3) |
| Co2ii—Co0—Co2iii | 71.4 (2) | P5—Co3—Co3xiii | 55.5 (3) |
| Co2—Co0—Co2iii | 120.6 (2) | P5xi—Co3—Co3xii | 57.0 (3) |
| P6—Co0—Co2i | 60.07 (15) | P5xii—Co3—Co3xii | 57.0 (3) |
| P5i—Co0—Co2i | 58.7 (2) | P4viii—Co3—Co3xii | 151.9 (4) |
| P4—Co0—Co2i | 90.7 (2) | P5vii—Co3—Co3xii | 94.1 (3) |
| P4ii—Co0—Co2i | 170.1 (3) | P5—Co3—Co3xii | 94.1 (3) |
| Co3iii—Co0—Co2i | 116.8 (2) | Co3xiii—Co3—Co3xii | 60.0 |
| Co3i—Co0—Co2i | 67.70 (14) | P5xi—Co3—Co0viii | 159.3 (3) |
| Co2ii—Co0—Co2i | 120.6 (2) | P5xii—Co3—Co0viii | 89.59 (18) |
| Co2—Co0—Co2i | 71.4 (2) | P4viii—Co3—Co0viii | 53.20 (19) |
| Co2iii—Co0—Co2i | 79.6 (2) | P5vii—Co3—Co0viii | 108.5 (3) |
| P6—Co0—Co1 | 164.3 (3) | P5—Co3—Co0viii | 52.4 (2) |
| P5i—Co0—Co1 | 98.8 (3) | Co3xiii—Co3—Co0viii | 105.1 (2) |
| P4—Co0—Co1 | 56.2 (2) | Co3xii—Co3—Co0viii | 138.58 (11) |
| P4ii—Co0—Co1 | 56.2 (2) | P5xi—Co3—Co0ix | 89.59 (18) |
| Co3iii—Co0—Co1 | 62.53 (14) | P5xii—Co3—Co0ix | 159.3 (3) |
| Co3i—Co0—Co1 | 62.53 (14) | P4viii—Co3—Co0ix | 53.20 (19) |
| Co2ii—Co0—Co1 | 108.4 (2) | P5vii—Co3—Co0ix | 52.4 (2) |
| Co2—Co0—Co1 | 108.4 (2) | P5—Co3—Co0ix | 108.5 (3) |
| Co2iii—Co0—Co1 | 129.67 (15) | Co3xiii—Co3—Co0ix | 105.1 (2) |
| Co2i—Co0—Co1 | 129.67 (15) | Co3xii—Co3—Co0ix | 138.58 (11) |
| P6—Co0—Co1iv | 104.29 (19) | Co0viii—Co3—Co0ix | 80.8 (2) |
| P5i—Co0—Co1iv | 158.8 (3) | P5xi—Co3—Co1viii | 106.4 (3) |
| P4—Co0—Co1iv | 55.3 (2) | P5xii—Co3—Co1viii | 52.2 (2) |
| P4ii—Co0—Co1iv | 55.3 (2) | P4viii—Co3—Co1viii | 54.34 (19) |
| Co3iii—Co0—Co1iv | 107.12 (19) | P5vii—Co3—Co1viii | 159.6 (3) |
| Co3i—Co0—Co1iv | 107.12 (19) | P5—Co3—Co1viii | 93.3 (2) |
| Co2ii—Co0—Co1iv | 63.65 (16) | Co3xiii—Co3—Co1viii | 140.83 (9) |
| Co2—Co0—Co1iv | 63.65 (16) | Co3xii—Co3—Co1viii | 105.3 (2) |
| Co2iii—Co0—Co1iv | 133.59 (16) | Co0viii—Co3—Co1viii | 60.09 (15) |
| Co2i—Co0—Co1iv | 133.59 (16) | Co0ix—Co3—Co1viii | 107.4 (2) |
| Co1—Co0—Co1iv | 60.0 (2) | P5xi—Co3—Co1ix | 52.2 (2) |
| P5v—Co1—P4v | 108.1 (2) | P5xii—Co3—Co1ix | 106.4 (3) |
| P5v—Co1—P4vi | 108.1 (2) | P4viii—Co3—Co1ix | 54.34 (19) |
| P4v—Co1—P4vi | 93.1 (4) | P5vii—Co3—Co1ix | 93.3 (2) |
| P5v—Co1—P4ii | 108.0 (3) | P5—Co3—Co1ix | 159.6 (3) |
| P4v—Co1—P4ii | 144.0 (3) | Co3xiii—Co3—Co1ix | 140.83 (9) |
| P4vi—Co1—P4ii | 76.3 (3) | Co3xii—Co3—Co1ix | 105.3 (2) |
| P5v—Co1—P4 | 108.0 (3) | Co0viii—Co3—Co1ix | 107.4 (2) |
| P4v—Co1—P4 | 76.3 (3) | Co0ix—Co3—Co1ix | 60.09 (15) |
| P4vi—Co1—P4 | 144.0 (3) | Co1viii—Co3—Co1ix | 75.95 (16) |
| P4ii—Co1—P4 | 92.1 (4) | P5xi—Co3—Co2 | 131.18 (19) |
| P5v—Co1—Co0 | 89.0 (3) | P5xii—Co3—Co2 | 131.18 (19) |
| P4v—Co1—Co0 | 128.2 (2) | P4viii—Co3—Co2 | 82.0 (3) |
| P4vi—Co1—Co0 | 128.2 (2) | P5vii—Co3—Co2 | 52.8 (2) |
| P4ii—Co1—Co0 | 51.9 (2) | P5—Co3—Co2 | 52.8 (2) |
| P4—Co1—Co0 | 51.9 (2) | Co3xiii—Co3—Co2 | 66.1 (3) |
| P5v—Co1—Co1v | 150.6 (4) | Co3xii—Co3—Co2 | 126.1 (3) |
| P4v—Co1—Co1v | 55.1 (2) | Co0viii—Co3—Co2 | 56.70 (15) |
| P4vi—Co1—Co1v | 55.1 (2) | Co0ix—Co3—Co2 | 56.70 (15) |
| P4ii—Co1—Co1v | 92.1 (2) | Co1viii—Co3—Co2 | 116.37 (18) |
| P4—Co1—Co1v | 92.1 (2) | Co1ix—Co3—Co2 | 116.37 (18) |
| Co0—Co1—Co1v | 120.4 (2) | Co0—P4—Co0vii | 98.9 (4) |
| P5v—Co1—Co1iv | 149.4 (4) | Co0—P4—Co2 | 70.2 (2) |
| P4v—Co1—Co1iv | 92.6 (2) | Co0vii—P4—Co2 | 70.2 (2) |
| P4vi—Co1—Co1iv | 92.6 (2) | Co0—P4—Co1x | 144.1 (4) |
| P4ii—Co1—Co1iv | 54.4 (2) | Co0vii—P4—Co1x | 72.93 (17) |
| P4—Co1—Co1iv | 54.4 (2) | Co2—P4—Co1x | 74.3 (3) |
| Co0—Co1—Co1iv | 60.4 (2) | Co0—P4—Co1iv | 72.93 (17) |
| Co1v—Co1—Co1iv | 60.0 | Co0vii—P4—Co1iv | 144.1 (4) |
| P5v—Co1—Co0v | 91.0 (3) | Co2—P4—Co1iv | 74.3 (3) |
| P4v—Co1—Co0v | 51.8 (2) | Co1x—P4—Co1iv | 93.1 (4) |
| P4vi—Co1—Co0v | 51.8 (2) | Co0—P4—Co3i | 69.9 (3) |
| P4ii—Co1—Co0v | 128.0 (2) | Co0vii—P4—Co3i | 69.9 (3) |
| P4—Co1—Co0v | 128.0 (2) | Co2—P4—Co3i | 116.5 (4) |
| Co0—Co1—Co0v | 180.0 (2) | Co1x—P4—Co3i | 133.35 (18) |
| Co1v—Co1—Co0v | 59.6 (2) | Co1iv—P4—Co3i | 133.35 (18) |
| Co1iv—Co1—Co0v | 119.6 (2) | Co0—P4—Co1 | 71.84 (17) |
| P5v—Co1—Co3iii | 54.26 (19) | Co0vii—P4—Co1 | 141.5 (4) |
| P4v—Co1—Co3iii | 162.3 (3) | Co2—P4—Co1 | 133.94 (18) |
| P4vi—Co1—Co3iii | 93.40 (17) | Co1x—P4—Co1 | 135.3 (4) |
| P4ii—Co1—Co3iii | 53.7 (2) | Co1iv—P4—Co1 | 70.4 (2) |
| P4—Co1—Co3iii | 107.1 (3) | Co3i—P4—Co1 | 72.0 (3) |
| Co0—Co1—Co3iii | 57.38 (16) | Co0—P4—Co1vii | 141.5 (4) |
| Co1v—Co1—Co3iii | 140.25 (9) | Co0vii—P4—Co1vii | 71.84 (17) |
| Co1iv—Co1—Co3iii | 103.5 (2) | Co2—P4—Co1vii | 133.94 (18) |
| Co0v—Co1—Co3iii | 122.64 (18) | Co1x—P4—Co1vii | 70.4 (2) |
| P5v—Co1—Co3i | 54.26 (19) | Co1iv—P4—Co1vii | 135.3 (4) |
| P4v—Co1—Co3i | 93.40 (17) | Co3i—P4—Co1vii | 72.0 (3) |
| P4vi—Co1—Co3i | 162.3 (3) | Co1—P4—Co1vii | 92.1 (4) |
| P4ii—Co1—Co3i | 107.1 (3) | Co0viii—P5—Co1iv | 127.8 (5) |
| P4—Co1—Co3i | 53.7 (2) | Co0viii—P5—Co3xiii | 131.9 (2) |
| Co0—Co1—Co3i | 57.38 (15) | Co1iv—P5—Co3xiii | 73.6 (3) |
| Co1v—Co1—Co3i | 140.25 (9) | Co0viii—P5—Co3xiv | 131.9 (2) |
| Co1iv—Co1—Co3i | 103.5 (2) | Co1iv—P5—Co3xiv | 73.6 (3) |
| Co0v—Co1—Co3i | 122.64 (18) | Co3xiii—P5—Co3xiv | 93.3 (4) |
| Co3iii—Co1—Co3i | 75.95 (16) | Co0viii—P5—Co3 | 69.5 (3) |
| P5v—Co1—Co2v | 55.41 (19) | Co1iv—P5—Co3 | 133.9 (2) |
| P4v—Co1—Co2v | 52.7 (2) | Co3xiii—P5—Co3 | 67.5 (2) |
| P4vi—Co1—Co2v | 105.6 (3) | Co3xiv—P5—Co3 | 131.1 (4) |
| P4ii—Co1—Co2v | 163.3 (3) | Co0viii—P5—Co3ii | 69.5 (3) |
| P4—Co1—Co2v | 95.17 (19) | Co1iv—P5—Co3ii | 133.9 (2) |
| Co0—Co1—Co2v | 123.5 (2) | Co3xiii—P5—Co3ii | 131.1 (4) |
| Co1v—Co1—Co2v | 102.6 (2) | Co3xiv—P5—Co3ii | 67.5 (2) |
| Co1iv—Co1—Co2v | 140.73 (11) | Co3—P5—Co3ii | 91.3 (4) |
| Co0v—Co1—Co2v | 56.54 (14) | Co0viii—P5—Co2 | 69.7 (3) |
| Co3iii—Co1—Co2v | 109.6 (2) | Co1iv—P5—Co2 | 73.8 (2) |
| Co3i—Co1—Co2v | 66.10 (15) | Co3xiii—P5—Co2 | 79.62 (16) |
| P4—Co2—P5vii | 103.7 (3) | Co3xiv—P5—Co2 | 147.3 (4) |
| P4—Co2—P5 | 103.7 (3) | Co3—P5—Co2 | 75.6 (2) |
| P5vii—Co2—P5 | 89.3 (3) | Co3ii—P5—Co2 | 139.2 (4) |
| P4—Co2—P6 | 103.6 (2) | Co0viii—P5—Co2ii | 69.7 (3) |
| P5vii—Co2—P6 | 152.7 (3) | Co1iv—P5—Co2ii | 73.8 (2) |
| P5—Co2—P6 | 85.31 (18) | Co3xiii—P5—Co2ii | 147.3 (4) |
| P4—Co2—P6vii | 103.6 (2) | Co3xiv—P5—Co2ii | 79.62 (16) |
| P5vii—Co2—P6vii | 85.30 (18) | Co3—P5—Co2ii | 139.2 (4) |
| P5—Co2—P6vii | 152.7 (3) | Co3ii—P5—Co2ii | 75.6 (2) |
| P6—Co2—P6vii | 87.33 (17) | Co2—P5—Co2ii | 89.3 (3) |
| P4—Co2—Co0vii | 53.20 (19) | Co0i—P6—Co0viii | 120.0 |
| P5vii—Co2—Co0vii | 90.21 (18) | Co0i—P6—Co0 | 120.0 |
| P5—Co2—Co0vii | 155.9 (3) | Co0viii—P6—Co0 | 120.0 |
| P6—Co2—Co0vii | 105.4 (2) | Co0i—P6—Co2xv | 69.23 (17) |
| P6vii—Co2—Co0vii | 51.06 (12) | Co0viii—P6—Co2xv | 68.36 (17) |
| P4—Co2—Co0 | 53.20 (19) | Co0—P6—Co2xv | 136.33 (9) |
| P5vii—Co2—Co0 | 155.9 (3) | Co0i—P6—Co2iii | 68.36 (17) |
| P5—Co2—Co0 | 90.21 (18) | Co0viii—P6—Co2iii | 136.33 (9) |
| P6—Co2—Co0 | 51.06 (12) | Co0—P6—Co2iii | 69.23 (17) |
| P6vii—Co2—Co0 | 105.4 (2) | Co2xv—P6—Co2iii | 77.58 (13) |
| Co0vii—Co2—Co0 | 80.6 (2) | Co0i—P6—Co2i | 68.36 (17) |
| P4—Co2—Co0viii | 140.20 (12) | Co0viii—P6—Co2i | 136.33 (9) |
| P5vii—Co2—Co0viii | 106.2 (3) | Co0—P6—Co2i | 69.23 (17) |
| P5—Co2—Co0viii | 51.6 (2) | Co2xv—P6—Co2i | 137.59 (6) |
| P6—Co2—Co0viii | 50.70 (12) | Co2iii—P6—Co2i | 87.34 (17) |
| P6vii—Co2—Co0viii | 104.5 (2) | Co0i—P6—Co2viii | 69.23 (17) |
| Co0vii—Co2—Co0viii | 150.1 (2) | Co0viii—P6—Co2viii | 68.36 (17) |
| Co0—Co2—Co0viii | 92.22 (16) | Co0—P6—Co2viii | 136.33 (9) |
| P4—Co2—Co0ix | 140.20 (12) | Co2xv—P6—Co2viii | 87.34 (17) |
| P5vii—Co2—Co0ix | 51.6 (2) | Co2iii—P6—Co2viii | 137.59 (6) |
| P5—Co2—Co0ix | 106.2 (3) | Co2i—P6—Co2viii | 77.58 (13) |
| P6—Co2—Co0ix | 104.5 (2) | Co0i—P6—Co2ii | 136.33 (9) |
| P6vii—Co2—Co0ix | 50.70 (12) | Co0viii—P6—Co2ii | 69.23 (17) |
| Co0vii—Co2—Co0ix | 92.22 (16) | Co0—P6—Co2ii | 68.37 (17) |
| Co0—Co2—Co0ix | 150.1 (2) | Co2xv—P6—Co2ii | 77.58 (13) |
| Co0viii—Co2—Co0ix | 79.6 (2) | Co2iii—P6—Co2ii | 77.58 (13) |
| P4—Co2—Co1x | 52.99 (19) | Co2i—P6—Co2ii | 137.59 (6) |
| P5vii—Co2—Co1x | 50.7 (2) | Co2viii—P6—Co2ii | 137.59 (6) |
| P5—Co2—Co1x | 102.3 (3) | Co0i—P6—Co2 | 136.33 (9) |
| P6—Co2—Co1x | 156.4 (2) | Co0viii—P6—Co2 | 69.23 (17) |
| P6vii—Co2—Co1x | 94.84 (11) | Co0—P6—Co2 | 68.37 (17) |
| Co0vii—Co2—Co1x | 59.81 (15) | Co2xv—P6—Co2 | 137.59 (6) |
| Co0—Co2—Co1x | 106.0 (2) | Co2iii—P6—Co2 | 137.59 (6) |
| Co0viii—Co2—Co1x | 148.9 (2) | Co2i—P6—Co2 | 77.58 (13) |
| Co0ix—Co2—Co1x | 94.89 (12) | Co2viii—P6—Co2 | 77.58 (13) |
| P4—Co2—Co1iv | 52.99 (19) | Co2ii—P6—Co2 | 87.33 (17) |
Symmetry codes: (i) −y, x−y, z; (ii) x, y, z−1; (iii) −y, x−y, z−1; (iv) −y+1, x−y+1, z; (v) −x+y, −x+1, z; (vi) −x+y, −x+1, z−1; (vii) x, y, z+1; (viii) −x+y, −x, z; (ix) −x+y, −x, z+1; (x) −y+1, x−y+1, z+1; (xi) −y+1, x−y, z+1; (xii) −y+1, x−y, z; (xiii) −x+y+1, −x+1, z; (xiv) −x+y+1, −x+1, z−1; (xv) −x+y, −x, z−1.
Funding Statement
This work was funded by National Science Foundation grant EAR – 1651017 to A. Campbell.
References
- Baurecht, H. E., Boller, H. & Nowotny, H. (1971). Monats. Chem. 102, 373–384.
- Campbell, A. J., Danielson, L., Righter, K., Seagle, C. T., Wang, Y. & Prakapenka, V. B. (2009). Earth Planet. Sci. Lett. 286, 556–564.
- Campbell, A. J., Seagle, C. T., Heinz, D. L., Shen, G. & Prakapenka, V. B. (2007). Phys. Earth Planet. Inter. 162, 119–128.
- Chun, H. K. & Carpenter, G. B. (1979). Acta Cryst. B35, 30–33.
- Dera, P., Lavina, B., Borkowski, L. A., Prakapenka, V. B., Sutton, S. R., Rivers, M. L., Downs, R. T., Boctor, N. Z. & Prewitt, C. T. (2008). Geophys. Res. Lett. 35, l10301.
- Dhahri, E. (1996). J. Phys. Condens. Matter, 8, 4351–4360.
- Ellner, M. & Mittemeijer, E. J. (2001). Z. Anorg. Allg. Chem. 627, 2257–2260.
- Fei, Y., Ricolleau, A., Frank, M., Mibe, K., Shen, G. & Prakapenka, V. (2007). PNAS, 104, 9182–9186. [DOI] [PMC free article] [PubMed]
- Fujii, S., Ishida, S. & Asano, S. (1988). J. Phys. F: Met. Phys. 18, 971–980.
- Hamilton, W. C. (1965). Acta Cryst. 18, 502–510.
- Heinz, D. L. & Jeanloz, R. (1987). High Press. Res. Miner. Phys. 2, 113–127.
- Jeitschko, W., Braun, D. J., Ashcraft, R. H. & Marchand, R. (1978). J. Solid State Chem. 25, 309–313.
- Jeitschko, W. & Jaberg, B. (1980). Z. Anorg. Allg. Chem. 467, 95–104.
- Jeitschko, W., Meisen, U. & Scholz, U. D. (1984). J. Solid State Chem. 55, 331–336.
- Loveday, J. S., McMahon, M. I. & Nelmes, R. J. (1990). J. Appl. Cryst. 23, 392–396.
- Maaref, S., Madar, R., Chaudouet, P., Senateur, J. P. & Fruchart, R. (1981). J. Solid State Chem. 40, 131–135.
- Mao, H. K. & Bell, P. M. (1976). Science, 191, 851–852. [DOI] [PubMed]
- Momma, K. & Izumi, F. (2011). J. Appl. Cryst. 44, 1272–1276.
- Okamoto, H. & Massalski, T. B. (1990). Editors. Binary Alloy Phase Diagrams. OH, USA: ASM International.
- Olofsson, O. & Ganglberger, E. (1970). Acta Chem. Scand. 24, 2389–2396.
- Ono, S. & Kikegawa, T. (2006). Am. Mineral. 91, 1941–1944.
- Ono, S., Oganov, A. R., Brodholt, J. P., Vočadlo, L., Wood, I. G., Lyakhov, A., Glass, C. W., Côté, A. S. & Price, G. D. (2008). Earth Planet. Sci. Lett. 272, 481–487.
- Pan, Y., Lin, Y., Chen, Y., Liu, Y. & Liu, C. (2016). J. Mater. Chem. A, 4, 4745–4754.
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Popczun, E. J., Read, C. G., Roske, C. W., Lewis, N. S. & Schaak, R. E. (2014). Angew. Chem. 126, 5531–5534. [DOI] [PubMed]
- Pramanik, M., Tominaka, S., Wang, Z. L., Takei, T. & Yamauchi, Y. (2017). Angew. Chem. 129, 13693–13697. [DOI] [PubMed]
- Prescher, C. & Prakapenka, V. B. (2015). High. Press. Res. 35, 223–230.
- Prins, R. & Bussell, M. E. (2012). Catal. Lett. 142, 1413–1436.
- Reehuis, M. & Jeitschko, W. (1989). J. Phys. Chem. Solids, 50, 563–569.
- Rigaku OD (2018). CrysAlis PRO. Rigaku Oxford Diffraction Ltd, Yarnton, England.
- Rivers, M., Prakapenka, V. B., Kubo, A., Pullins, C., Holl, C. M. & Jacobsen, S. D. (2008). High Pressure Res. 28, 273–292.
- Rundqvist, S. (1960). Acta Chem. Scand. 14, 1961–1979.
- Rundqvist, S. (1962). Acta Chem. Scand. 16, 1–19.
- Schlesinger, M. E. (2002). Chem. Rev. 102, 4267–4302. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Tateno, S., Ozawa, H., Hirose, K., Suzuki, T., I–Kawaguchi, S. & Hirao, N. (2019). Geophys. Res. Lett. 46, 11944–11949.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Zachariasen, W. H. (1949). Acta Cryst. 2, 288–291.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) Co12P7_at_48GPa, Co12P7_at_15GPa. DOI: 10.1107/S2056989020012657/wm5583sup1.cif
Structure factors: contains datablock(s) Co12P7_at_48GPa. DOI: 10.1107/S2056989020012657/wm5583Co12P7_at_48GPasup2.hkl
Structure factors: contains datablock(s) Co12P7_at_15GPa. DOI: 10.1107/S2056989020012657/wm5583Co12P7_at_15GPasup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report