The crystal structure of aqua(citric acid)(hydrogen citrato)calcium monohydrate has been solved and refined using synchrotron X-ray powder diffraction data, and optimized using density functional techniques. A DFT-optimized structure is also reported for calcium hydrogen citrate trihydrate.
Keywords: powder diffraction, citrate, calcium, Rietveld refinement, density functional theory
Abstract
The crystal structure of ‘aquabis(dihydrogen citrato)calcium hydrate’, better formulated as aqua(citric acid)(hydrogen citrato)calcium monohydrate, (I), has been solved and refined using synchrotron X-ray powder diffraction data, and optimized using density functional techniques. The CaO8 coordination polyhedra are isolated, but occur in layers parallel to the ab plane. Both the Rietveld-refined and DFT-optimized structures indicate that one citrate is doubly ionized and that the other is citric acid. All of the active hydrogen atoms participate in strong (11–16 kcal mol−1) hydrogen bonds. Hydrogen atoms were added to the existing calcium hydrogen citrate trihydrate structure [Sheldrick (1974 ▸). Acta Cryst. B30, 2056–2057; CSD refcode: CAHCIT], (II), and a DFT calculation was carried out to assess the hydrogen bonding and compare it to this optimized structure.
Chemical context
A systematic study of the crystal structures of Group 1 (alkali metal) citrate salts has been reported in Rammohan & Kaduk (2018 ▸). This paper is part of an extension of the study to Group 2 (alkaline earth) citrates. Calcium citrate (as the tetrahydrate) is a common dietary supplement. Previously reported calcium citrate structures include calcium hydrogen citrate trihydrate (Sheldrick, 1974 ▸; CSD refcode: CAHCIT) and calcium citrate tetrahydrate (Herdtweck et al., 2011 ▸; ISEQIH). The ISEQIH structure was derived from a hydrothermally synthesized pseudomerohedrally twinned crystal at 123 K, and in this study a Rietveld refinement using room-temperature powder data was also reported.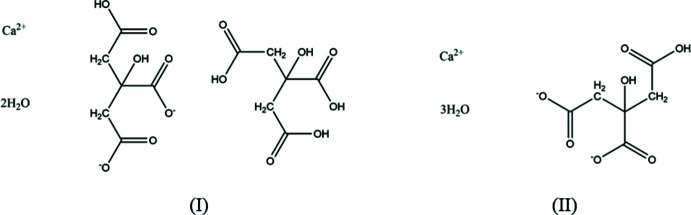
The crystal structures of anhydrous calcium citrate, a new (and commercially relevant, for it is the phase that occurs in dietary supplements) polymorph of calcium citrate tetrahydrate, and calcium citrate hexahydrate have been reported recently (Kaduk, 2018 ▸).
Structural commentary
The crystal structure of ‘aquabis(dihydrogen citrato)calcium hydrate’, (I), has been solved and refined using synchrotron X-ray powder diffraction data, and optimized using density functional techniques (Fig. 1 ▸). The root-mean-square Cartesian displacement of the non-hydrogen citrate atoms in the Rietveld refined and DFT-optimized structures is 0.213 Å (Fig. 2 ▸) The absolute difference in the position of the Ca2+ cation in the unit cell is 0.318 Å. The good agreement between the structures is evidence that the experimental structure is correct (van de Streek & Neumann, 2014 ▸). The rest of the discussion will emphasize the DFT-optimized structure. Almost all of the citrate bond lengths, bond angles, and torsion angles fall within the normal ranges indicated by a Mercury Mogul geometry check (Macrae et al., 2020 ▸). Only the C4—C3—C6 angle of 102.6° [average = 110.4 (19), Z-score = 4. 2] is flagged as unusual. One citrate occurs in the trans,trans-conformation, and the other occurs in the gauche,trans-conformation. Both conformations are equivalent in energy (Rammohan & Kaduk, 2018 ▸). Both central carboxylate groups and the hydroxyl groups exhibit slight twists (O10—C6—C3—O13 = −18.1 and O29—C26—C23—O33 = −4.1°) from the normal planar arrangement.
Figure 1.
The asymmetric unit of aqua(citric acid)(hydrogen citrato)calcium monohydrate with the atom numbering and 50% probability spheroids.
Figure 2.
Comparison of the refined and optimized structures of aqua(citric acid)(hydrogen citrato)calcium monohydrate. The refined structure is in red, and the DFT-optimized structure is in blue.
Both the refined and optimized structures indicate that one citrate group (C1–C6) is doubly ionized (in the normal fashion central/terminal), with the C1—O8—O7—H40 moiety being an intact carboxylic acid group. The other ‘citrate’ (C21–C26) is in fact citric acid. The compound may therefore be better characterized as aqua(citric acid)(hydrogen citrato)calcium monohydrate. The C—O bond lengths in both the refined (restrained) and optimized structures are consistent with this formulation. Removing the restraints on the C—O bond lengths did not change the refined values significantly. Given the preparation (in a probable excess of citric acid), the crystallization of a mixed salt/co-crystal is not unreasonable. It is probably wise to be cautious about locating hydrogen atoms using X-ray powder (even synchrotron) data, even when the structure is confirmed by a DFT calculation. As noted below, some of the hydrogen bonds are very strong, and perhaps have double minima. A more sophisticated quantum calculation may be required to understand the details of the hydrogen bonding in this compound.
The Ca2+ cation is eight-coordinate, with seven shorter and one long bond (Table 1 ▸), resulting in a distorted bicapped octahedral coordination polyhedron; the ligands are two terminal carboxylate groups, one central carboxylate group, one terminal CO2H, one central CO2H, two hydroxyl groups, and one water molecule. The Ca bond-valence sum amounts to 2.10 valence units (v.u.). Both citrate and citric acid chelate to the Ca2+ cation through the hydroxyl groups and the central carboxyl groups (O13/O10 and O33/O29), respectively, forming a five-membered ring. The citrate anion also exhibits a monodentate mode to two other Ca2+ cations (through O11 and O12) whereas the citric acid molecule shows a monodentate coordination mode only through O27.
Table 1. Selected bond lengths (Å) for (I) .
| Ca19—O12i | 2.332 | Ca19—O29 | 2.446 |
| Ca19—O33 | 2.400 | Ca19—O39 | 2.466 |
| Ca19—O11ii | 2.417 | Ca19—O27iii | 2.564 |
| Ca19—O10 | 2.421 | Ca19—O13 | 2.818 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
The Bravais–Friedel–Donnay–Harker (Bravais, 1866 ▸; Friedel, 1907 ▸; Donnay & Harker, 1937 ▸) method suggests that we might expect blocky morphology for aqua(citric acid)(hydrogen citrato)calcium monohydrate. A 2nd order spherical harmonic model was included in the refinement. The texture index was only 1.003, indicating that preferred orientation was not significant in this rotated capillary specimen.
In the known crystal structure of CAHCIT, (II), the citrate anion is also in the gauche,trans-conformation, and chelates to the Ca2+ cation through the hydroxyl group and a terminal carboxylate group as well as through the ionized central and terminal carboxylate groups. The root-mean-square Cartesian displacement between the single crystal and the DFT-optimized structures is only 0.0399 Å, confirming the excellent quality of the single-crystal study (Sheldrick, 1974 ▸). The Ca bond-valence sum is 2.07 v.u. With a limited number of calcium citrate structures, it is hard to make grand generalizations, but several more such compounds have been synthesized and await structural characterization.
Supramolecular features
The CaO8 coordination polyhedra in aqua(citric acid)(hydrogen citrato)calcium monohydrate are isolated (Fig. 3 ▸), but occur in layers parallel to the ab plane. Numerical values of the hydrogen bonds are summarized in Table 2 ▸. The free water molecule O20 acts as a hydrogen-bond donor to the hydroxyl group O13 and the carbonyl group O8. The coordinating water molecule O39 acts as a donor to the carbonyl group O32 and the free water molecule O20. The carboxylic acid group O7—H40 in the hydrogen citrate anion acts as a donor to the coordinating water molecule O39. The carboxylic acid function O28—H46 acts as a donor to the ionized central carboxylate O9. The carboxylic acids O31—H43 and O30—H44 act as donors to the free water molecule O20. The hydroxyl group O13—H16 forms an intramolecular hydrogen bond to the ionized central carboxylate O11 atom, while the hydroxyl group O33—H36 forms an intermolecular hydrogen bond to the ionized central carboxylate O10 atom.
Figure 3.
Crystal structure of aqua(citric acid)(hydrogen citrato)calcium monohydrate, viewed down the a axis.
Table 2. Hydrogen-bond geometry (Å, °, electrons, kcal mol−1) for (I).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A | Mulliken overlap | H-bond energy |
|---|---|---|---|---|---|---|
| O28i—H46⋯O9 | 1.022 | 1.542 | 2.545 | 165.8 | 0.087 | 16.1 |
| O20—H47⋯O13ii | 0.999 | 1.726 | 2.706 | 166.0 | 0.078 | 15.3 |
| O7—H40⋯O39iii | 1.001 | 1.706 | 2.677 | 162.4 | 0.076 | 15.1 |
| O33—H36⋯O10iv | 1.002 | 1.683 | 2.672 | 168.4 | 0.070 | 14.5 |
| O30—H44⋯O20 | 0.999 | 1.687 | 2.685 | 178.1 | 0.066 | 14.0 |
| O13—H16⋯O11 | 0.995 | 1.852 | 2.739 | 146.8 | 0.061 | 13.5 |
| O20—H45⋯O8v | 0.991 | 1.798 | 2.754 | 161.0 | 0.059 | 13.3 |
| O39—H41⋯O20ii | 0.978 | 1.815 | 2.765 | 163.2 | 0.057 | 13.0 |
| O39—H42⋯O32vi | 0.986 | 1.816 | 2.739 | 154.3 | 0.049 | 12.1 |
| O31—H43⋯O20vii | 0.984 | 1.816 | 2.851 | 159.6 | 0.042 | 11.2 |
Symmetry codes: (i) 1 − x, 1 − y, 1 − z; (ii) 1 − x, −y, 1 − z; (iii) 1 − x, −y, −z; (iv) 2 − x, 1 − y, 1 − z; (v) x, y, 1 + z; (vi) 2 − x, −y, 1 − z; (vii) 1 + x, y, z.
In CAHCIT, the Ca2+ cation is seven-coordinated in the form of a distorted side-capped trigonal prism. The polyhedra share corners to form chains along the [010] direction. In Ca3(C6H5O7)2 and its hydrates (Kaduk, 2018 ▸), the coordination numbers are larger, and the Ca/O coordination spheres share edges to form layers, which condense to a three-dimensional framework in anhydrous calcium citrate. Since the hydrogen atoms were not located in the CAHCIT structure, approximate positions were deduced, and a DFT calculation was carried out to assess the hydrogen bonding (Table 3 ▸). The carboxylic acid function O13—H23 acts as a donor to the ionized carboxylate O8 atom. The hydroxyl group O9—H20 forms an intermolecular hydrogen bond to the carbonyl O12 atom. The water molecules act as donors to both ionized carboxylate groups and other water molecules. The crystal structure of calcium hydrogen citrate trihydrate is shown in Fig. 4 ▸.
Table 3. Hydrogen-bond geometry (Å, °, electrons, kcal mol−1) for (II).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A | Mulliken overlap | H-bond energy |
|---|---|---|---|---|---|---|
| O15i—H29⋯O8ii | 0.974 | 1.940 | 2.891 | 164.6 | 0.035 | 10.2 |
| O15iii—H28⋯O10iv | 0.979 | 1.831 | 2.806 | 173.5 | 0.046 | 11.7 |
| O16i—H27⋯O7vi | 0.971 | 1.970 | 2.926 | 167.6 | 0.037 | 10.5 |
| O16i—H26⋯O7i | 0.981 | 1.838 | 2.817 | 176.3 | 0.055 | 12.8 |
| O14v—H25⋯O15i | 0.992 | 1.719 | 2.702 | 170.9 | 0.081 | 15.6 |
| O14v—H24⋯O8v | 0.975 | 1.782 | 2.742 | 167.1 | 0.047 | 11.8 |
| O13v—H23⋯O8v | 1.018 | 1.560 | 2.571 | 171.4 | 0.076 | 15.1 |
| O9v—H20⋯O12i | 0.988 | 1.764 | 2.749 | 174.9 | 0.061 | 13.5 |
Symmetry codes: (i) x,  − y,
− y,  + z; (ii) 1 − x, −y, 1 − z; (iii) 1 − x,
+ z; (ii) 1 − x, −y, 1 − z; (iii) 1 − x,  + y,
+ y,  − z; (iv) x, 1 + y, z; (v) 1 − x, 1 − y, 1 − z.
− z; (iv) x, 1 + y, z; (v) 1 − x, 1 − y, 1 − z.
Figure 4.
Crystal structure of [Ca(C6H6O7(H2O)3], viewed down the b axis.
Database survey
Details of the comprehensive literature search for citrate structures are presented in Rammohan & Kaduk (2018 ▸). A search of the Cambridge Structural Database (Groom et al., 2016 ▸, version 2020.2.0) using a citrate fragment and Ca, C, H, and O only yielded two hits, viz. calcium hydrogen citrate trihydrate (Sheldrick, 1974 ▸; CAHCIT) and calcium citrate tetrahydrate (Herdtweck et al., 2011 ▸; ISEQIH). A search of the Powder Diffraction File (Gates-Rector & Blanton, 2019 ▸) for C, H, Ca, and O only with ‘citrat’ in the compound name yielded entry 00-028-2003 for the mineral earlandite (calcium citrate tetrahydrate, isolated from an unconsolidated ocean floor sediment from the Weddell Sea near Antarctica), 00-069-1272, 1273, and 1274 for the three compounds from Kaduk (2018 ▸), 01-084-5956 calculated from ISEQIH, and 02-060-8946 calculated from CAHCIT.
Synthesis and crystallization
This solid was obtained from the scale [94.5 (1) wt% magnesian calcite Ca0.84Mg0.16CO3, 5.3 (4) wt% brucite Mg(OH)2, and 0.2 (1) wt% vaterite polymorph of CaCO3] in a Megahome water still. The still was cleaned by filling the tank with tap water (from Lake Michigan; 47 ppm Ca and 11 ppm Mg), adding several tablespoons of citric acid monohydrate, and boiling for ∼2 h. The pale-yellow solution was decanted into a plastic pail, and allowed to evaporate at ambient conditions. This solid was recovered after 90 days, with isolation of intermediate phases. The wet solid was washed with ethanol to remove the yellow syrup from the white solid. The slurry was filtered and dried in an oven at 338 K. The solid was first ground in a mortar and pestle, then in a Spex 8000 mixer/mill.
Refinement
The laboratory pattern (measured on a Bruker D2 Phaser using Cu radiation) was indexed on a primitive triclinic unit cell using DICVOL06 (Louër & Boultif, 2007 ▸). The indexing was carried out on a pattern from a specimen blended with NIST SRM 640b Si internal standard; a = 8.37261 (3), b = 10.90306 (4), c = 11.06287 (4) Å, α = 105.2026 (4), β = 100.6846 (4), γ = 110.7096 (3)°, V = 867.2026 (4) Å3, and Z = 2. This cell was used to solve the structure with data collected from beamline 11-BM (Lee et al., 2008 ▸; Wang et al., 2008 ▸) at the Advanced Photon Source, Argonne National Laboratory using direct methods in EXPO2009 (Altomare et al., 2009 ▸).
Crystal data, data collection and structure refinement details are summarized in Table 4 ▸. The structure was refined by the Rietveld method using GSAS-II (Toby & Von Dreele, 2013 ▸). Initial positions for the active hydrogen atoms were derived by an analysis of potential hydrogen-bonding patterns. All non-H bond lengths and angles in the citrate anion/citric acid molecule were subjected to restraints, based on a Mercury Mogul geometry check (Sykes et al., 2011 ▸; Bruno et al., 2004 ▸) of the molecule; the Ca—O bond lengths were not restrained. The Mogul average and standard deviation for each quantity were used as the restraint parameters. The hydrogen atoms were included in calculated positions, which were recalculated during the refinement using Materials Studio (Dassault Systèmes, 2018 ▸). U iso values were grouped by chemical similarity, and the U iso value in the two anions were constrained to be the same. The U iso value of each H atom was constrained to be 1.3× that of the heavy atom to which is is attached. A Rietveld plot is given in Fig. 5 ▸. The largest errors in the difference plot reflect the presence of an unidentified impurity and misfits of the peak profiles. The peaks of the impurity phase can be indexed on a primitive monoclinic unit cell with a = 15.3648, b = 7.2713, c = 19.3755 Å, β = 109.116°, and V = 2045.30 Å3, but no structure solution has as yet been obtained.
Table 4. Experimental details.
| (I) | |
|---|---|
| Crystal data | |
| Chemical formula | [Ca(C6H6O7)(C6H8O7)(H2O)]·H2O |
| M r | 458.34 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 295 |
| a, b, c (Å) | 8.37267 (11), 10.9032 (3), 11.0629 (3) |
| α, β, γ (°) | 105.2029 (6), 100.6847 (4), 110.7096 (3) |
| V (Å3) | 867.24 (1) |
| Z | 2 |
| Radiation type | Synchrotron, λ = 0.41307 Å |
| Specimen shape, size (mm) | Cylinder, 3 × 1.5 |
| Data collection | |
| Diffractometer | 11-BM, APS |
| Specimen mounting | Kapton capillary |
| Data collection mode | Transmission |
| Scan method | Step |
| 2θ values (°) | 2θmin = 0.500, 2θmax = 49.991, 2θstep = 0.001 |
| Refinement | |
| R factors and goodness of fit | R p = 0.116, R wp = 0.154, R exp = 0.066, R(F 2) = 0.11717, χ2 = 5.532 |
| No. of parameters | 115 |
| No. of restraints | 68 |
| H-atom treatment | Only H-atom displacement parameters refined |
| (Δ/σ)max | 0.163 |
Figure 5.
Rietveld plot for aqua(citric acid)(hydrogen citrato)calcium monohydrate. The blue crosses represent the observed data points, and the green line is the calculated pattern. The cyan curve is the normalized error plot. The vertical scale has been multiplied by a factor of 10× for 2θ > 10.0°, and by a factor of 40× for 2θ > 17.0°. The row of blue tick marks indicates the calculated reflection positions; the red line is the background curve.
Density functional geometry optimizations (fixed experimental unit cell) for the two structures were carried out using CRYSTAL09 (Dovesi et al., 2005 ▸). The basis sets for the H, C and O atoms were those of Gatti et al. (1994 ▸), and the basis set for Ca was that of Catti et al. (1991 ▸). The calculations used 8 k-points and the B3LYP functional, and each took around seven days on a 2.4 GHz PC.
Supplementary Material
Crystal structure: contains datablock(s) I, I_DFT, CAHCIT_DFT. DOI: 10.1107/S2056989020012864/wm5577sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020012864/wm5577Isup2.hkl
Rietveld powder data: contains datablock(s) I. DOI: 10.1107/S2056989020012864/wm5577Isup3.rtv
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02–06CH11357. I thank Matthew Suchomel and Lynn Ribaud for assistance with the data collection.
supplementary crystallographic information
Aqua(citric acid)(hydrogen citrato)calcium monohydrate (I). Crystal data
| [Ca(C6H6O7)(C6H8O7)(H2O)]·H2O | V = 867.24 (1) Å3 |
| Mr = 458.34 | Z = 2 |
| Triclinic, P1 | Dx = 1.755 Mg m−3 |
| Hall symbol: -P 1 | Synchrotron radiation, λ = 0.41307 Å |
| a = 8.37267 (11) Å | T = 295 K |
| b = 10.9032 (3) Å | Particle morphology: white powder |
| c = 11.0629 (3) Å | white |
| α = 105.2029 (6)° | cylinder, 3 × 1.5 mm |
| β = 100.6847 (4)° | Specimen preparation: Prepared at 300 K |
| γ = 110.7096 (3)° |
Aqua(citric acid)(hydrogen citrato)calcium monohydrate (I). Data collection
| 11-BM, APS diffractometer | Scan method: step |
| Specimen mounting: Kapton capillary | 2θmin = 0.500°, 2θmax = 49.991°, 2θstep = 0.001° |
| Data collection mode: transmission |
Aqua(citric acid)(hydrogen citrato)calcium monohydrate (I). Refinement
| Least-squares matrix: full | 115 parameters |
| Rp = 0.116 | 68 restraints |
| Rwp = 0.154 | Only H-atom displacement parameters refined |
| Rexp = 0.066 | Weighting scheme based on measured s.u.'s |
| R(F2) = 0.11717 | (Δ/σ)max = 0.163 |
| 49492 data points | Background function: Background function: "chebyschev-1" function with 6 terms: 86.9(17), 10.6(26), -9.1(12), -14.2(8), 19.3(10), -23.7(7), Background peak parameters: pos, int, sig, gam: 5.20(3), 5.0(4)e5, 3.10(18)e4, 0.100, |
| Profile function: Finger-Cox-Jephcoat function parameters U, V, W, X, Y, SH/L: peak variance(Gauss) = Utan(Th)2+Vtan(Th)+W: peak HW(Lorentz) = X/cos(Th)+Ytan(Th); SH/L = S/L+H/L U, V, W in (centideg)2, X & Y in centideg 1.163, -0.126, 0.063, 0.000, 0.000, 0.002, Crystallite size in microns with "isotropic" model: parameters: Size, G/L mix 1.41(5), 1.000, Microstrain, "isotropic" model (106 * delta Q/Q) parameters: Mustrain, G/L mix 1026(17), 1.000, | Preferred orientation correction: Simple spherical harmonic correction Order = 2 Coefficients: 0:0:C(2,-2) = 0.041(5); 0:0:C(2,-1) = 0.002(5); 0:0:C(2,0) = -0.053(5); 0:0:C(2,1) = -0.047(5); 0:0:C(2,2) = 0.076(5) |
Aqua(citric acid)(hydrogen citrato)calcium monohydrate (I). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.3002 (7) | 0.0929 (11) | 0.1064 (5) | 0.0317 (7)* | |
| C2 | 0.2629 (8) | 0.1243 (8) | 0.2378 (6) | 0.0194 (16)* | |
| C3 | 0.4299 (6) | 0.1958 (4) | 0.3587 (4) | 0.0194* | |
| C4 | 0.3777 (8) | 0.2398 (5) | 0.4829 (6) | 0.0194* | |
| C5 | 0.2425 (8) | 0.1186 (7) | 0.5031 (8) | 0.0317* | |
| C6 | 0.5717 (6) | 0.3282 (6) | 0.3500 (8) | 0.0317* | |
| O7 | 0.1573 (8) | 0.0272 (7) | 0.0020 (6) | 0.0317* | |
| O8 | 0.4552 (7) | 0.1313 (7) | 0.0985 (6) | 0.0317* | |
| O9 | 0.5212 (8) | 0.4142 (6) | 0.3257 (7) | 0.0317* | |
| O10 | 0.7340 (7) | 0.3519 (6) | 0.3923 (7) | 0.0317* | |
| O11 | 0.2937 (8) | 0.0305 (7) | 0.5283 (7) | 0.0317* | |
| O12 | 0.0867 (8) | 0.1091 (6) | 0.4861 (7) | 0.0317* | |
| O13 | 0.5123 (7) | 0.1008 (6) | 0.3667 (6) | 0.0317* | |
| H14 | 0.19243 | 0.179707 | 0.214454 | 0.0252* | |
| H15 | 0.16994 | 0.02156 | 0.24318 | 0.0252* | |
| H16 | 0.46014 | 0.04456 | 0.40231 | 0.0412* | |
| H17 | 0.50220 | 0.289194 | 0.57285 | 0.0252* | |
| H18 | 0.31942 | 0.31872 | 0.47649 | 0.0252* | |
| Ca19 | 0.8557 (3) | 0.2040 (3) | 0.4459 (3) | 0.0265 (10)* | |
| O20 | 0.5714 (9) | 0.1614 (7) | 0.8826 (7) | 0.034 (3)* | |
| C21 | 0.9040 (8) | 0.6012 (10) | 0.7264 (9) | 0.0317* | |
| C22 | 1.0577 (9) | 0.5859 (7) | 0.8073 (6) | 0.0194* | |
| C23 | 1.0641 (5) | 0.4425 (5) | 0.7610 (4) | 0.0194* | |
| C24 | 1.2319 (8) | 0.4523 (7) | 0.8537 (6) | 0.0194* | |
| C25 | 1.2572 (8) | 0.3207 (6) | 0.8292 (11) | 0.0317* | |
| C26 | 0.8915 (7) | 0.3260 (7) | 0.7618 (5) | 0.0317* | |
| O27 | 0.9197 (8) | 0.6604 (7) | 0.6442 (7) | 0.0317* | |
| O28 | 0.7536 (8) | 0.5408 (7) | 0.7496 (7) | 0.0317* | |
| O29 | 0.7793 (8) | 0.2414 (7) | 0.6568 (5) | 0.0317* | |
| O30 | 0.8722 (8) | 0.3353 (7) | 0.8750 (6) | 0.0317* | |
| O31 | 1.4295 (8) | 0.3460 (6) | 0.8536 (7) | 0.0317* | |
| O32 | 1.1330 (8) | 0.2043 (6) | 0.7770 (7) | 0.0317* | |
| O33 | 1.0718 (7) | 0.4071 (6) | 0.6278 (5) | 0.0317* | |
| H34 | 1.19050 | 0.66534 | 0.810791 | 0.0252* | |
| H35 | 1.05265 | 0.61039 | 0.91286 | 0.0252* | |
| H36 | 1.12701 | 0.48610 | 0.62146 | 0.0412* | |
| H37 | 1.35653 | 0.53313 | 0.854557 | 0.0252* | |
| H38 | 1.22758 | 0.48943 | 0.95894 | 0.0252* | |
| O39 | 0.8031 (10) | 0.0427 (8) | 0.2335 (7) | 0.051 (3)* | |
| H40 | 0.18003 | −0.01867 | −0.07989 | 0.0412* | |
| H41 | 0.68916 | −0.03767 | 0.23211 | 0.0663* | |
| H42 | 0.85248 | −0.02514 | 0.24834 | 0.0663* | |
| H43 | 1.43719 | 0.26928 | 0.82734 | 0.0412* | |
| H44 | 0.74472 | 0.27820 | 0.86966 | 0.0412* | |
| H45 | 0.55021 | 0.11781 | 0.94379 | 0.0441* | |
| H46 | 0.65968 | 0.57637 | 0.77733 | 0.0412* | |
| H47 | 0.55075 | 0.08274 | 0.80181 | 0.0441* |
Aqua(citric acid)(hydrogen citrato)calcium monohydrate (I). Geometric parameters (Å, º)
| C1—C2 | 1.519 (6) | Ca19—O27iv | 2.494 (6) |
| C1—O7 | 1.314 (6) | Ca19—O29 | 2.501 (6) |
| C1—O8 | 1.243 (6) | Ca19—O33 | 2.388 (6) |
| C2—C1 | 1.519 (6) | Ca19—O39 | 2.389 (7) |
| C2—C3 | 1.530 (4) | C21—C22 | 1.512 (6) |
| C3—C2 | 1.530 (4) | C21—O27 | 1.246 (6) |
| C3—C4 | 1.527 (4) | C21—O28 | 1.306 (5) |
| C3—C6 | 1.551 (3) | C22—C21 | 1.512 (6) |
| C3—O13 | 1.445 (3) | C22—C23 | 1.536 (4) |
| C4—C3 | 1.527 (4) | C23—C22 | 1.536 (4) |
| C4—C5 | 1.507 (4) | C23—C24 | 1.529 (4) |
| C5—C4 | 1.507 (4) | C23—C26 | 1.554 (3) |
| C5—O11 | 1.253 (5) | C23—O33 | 1.442 (3) |
| C5—O12 | 1.246 (5) | C24—C23 | 1.529 (4) |
| C6—C3 | 1.551 (3) | C24—C25 | 1.486 (6) |
| C6—O9 | 1.225 (4) | C25—C24 | 1.486 (6) |
| C6—O10 | 1.259 (4) | C25—O31 | 1.328 (6) |
| O7—C1 | 1.314 (6) | C25—O32 | 1.219 (6) |
| O8—C1 | 1.243 (6) | C26—C23 | 1.554 (3) |
| O9—C6 | 1.225 (4) | C26—O29 | 1.226 (5) |
| O10—C6 | 1.259 (4) | C26—O30 | 1.275 (6) |
| O10—Ca19 | 2.330 (6) | O27—Ca19iv | 2.494 (6) |
| O11—C5 | 1.253 (5) | O27—C21 | 1.246 (6) |
| O11—Ca19i | 2.537 (6) | O28—C21 | 1.306 (5) |
| O12—C5 | 1.246 (5) | O29—Ca19 | 2.501 (6) |
| O12—Ca19ii | 2.519 (6) | O29—C26 | 1.226 (5) |
| O13—C3 | 1.445 (3) | O30—C26 | 1.275 (6) |
| O13—Ca19 | 2.562 (6) | O31—C25 | 1.328 (6) |
| Ca19—O10 | 2.330 (6) | O32—C25 | 1.219 (6) |
| Ca19—O11i | 2.537 (6) | O33—Ca19 | 2.388 (6) |
| Ca19—O12iii | 2.519 (6) | O33—C23 | 1.442 (3) |
| Ca19—O13 | 2.562 (6) | O39—Ca19 | 2.389 (7) |
| C2—C1—O7 | 115.3 (4) | O33—Ca19—O39 | 145.5 (2) |
| C2—C1—O8 | 122.2 (4) | C22—C21—O27 | 123.3 (5) |
| O7—C1—O8 | 122.5 (4) | C22—C21—O28 | 112.1 (5) |
| C1—C2—C3 | 114.8 (4) | O27—C21—O28 | 124.6 (4) |
| C2—C3—C4 | 109.7 (3) | C21—C22—C23 | 116.7 (6) |
| C2—C3—C6 | 111.3 (3) | C22—C23—C24 | 107.8 (3) |
| C4—C3—C6 | 108.6 (3) | C22—C23—C26 | 110.2 (4) |
| C2—C3—O13 | 109.8 (2) | C24—C23—C26 | 111.1 (3) |
| C4—C3—O13 | 110.13 (19) | C22—C23—O33 | 110.3 (2) |
| C6—C3—O13 | 107.4 (3) | C24—C23—O33 | 110.1 (2) |
| C3—C4—C5 | 113.3 (3) | C26—C23—O33 | 107.4 (3) |
| C4—C5—O11 | 116.9 (5) | C23—C24—C25 | 116.0 (5) |
| C4—C5—O12 | 117.8 (4) | C24—C25—O31 | 111.7 (4) |
| O11—C5—O12 | 125.1 (4) | C24—C25—O32 | 123.0 (5) |
| C3—C6—O9 | 117.5 (3) | O31—C25—O32 | 124.6 (4) |
| C3—C6—O10 | 117.1 (2) | C23—C26—O29 | 119.2 (3) |
| O9—C6—O10 | 124.0 (4) | C23—C26—O30 | 115.3 (3) |
| C6—O10—Ca19 | 129.0 (3) | O29—C26—O30 | 125.10 (19) |
| O10—Ca19—O33 | 86.2 (2) | Ca19—O33—C23 | 125.0 (3) |
| O10—Ca19—O39 | 101.9 (3) |
Symmetry codes: (i) −x+1, −y, −z+1; (ii) x−1, y, z; (iii) x+1, y, z; (iv) −x+2, −y+1, −z+1.
(I_DFT). Crystal data
| C12H18CaO16 | c = 11.0629 Å |
| Mr = 458.34 | α = 105.2026° |
| Triclinic, P1 | β = 100.6846° |
| Hall symbol: -P 1 | γ = 110.7096° |
| a = 8.3726 Å | V = 867.24 Å3 |
| b = 10.9031 Å | Z = 2 |
(I_DFT). Data collection
| h = → | l = → |
| k = → |
(I_DFT). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.31016 | 0.07285 | 0.08749 | 0.02690* | |
| C2 | 0.26956 | 0.10747 | 0.21719 | 0.01140* | |
| C3 | 0.43277 | 0.17813 | 0.34179 | 0.01140* | |
| C4 | 0.38652 | 0.23372 | 0.46820 | 0.01140* | |
| C5 | 0.25177 | 0.12171 | 0.50135 | 0.02690* | |
| C6 | 0.57901 | 0.31142 | 0.34106 | 0.02690* | |
| O7 | 0.16469 | −0.01796 | −0.01141 | 0.02690* | |
| O8 | 0.46026 | 0.12456 | 0.07496 | 0.02690* | |
| O9 | 0.53090 | 0.39795 | 0.30853 | 0.02690* | |
| O10 | 0.74165 | 0.33221 | 0.38382 | 0.02690* | |
| O11 | 0.28897 | 0.01869 | 0.50683 | 0.02690* | |
| O12 | 0.11984 | 0.13979 | 0.52504 | 0.02690* | |
| O13 | 0.50583 | 0.07864 | 0.35311 | 0.02690* | |
| H14 | 0.19948 | 0.17489 | 0.21364 | 0.01480* | |
| H15 | 0.17267 | 0.01068 | 0.22038 | 0.01480* | |
| H16 | 0.44873 | 0.03225 | 0.41011 | 0.03500* | |
| H17 | 0.51107 | 0.28420 | 0.55055 | 0.01480* | |
| H18 | 0.33695 | 0.31178 | 0.46016 | 0.01480* | |
| Ca19 | 0.88141 | 0.19783 | 0.46615 | 0.01810* | |
| O20 | 0.54811 | 0.14774 | 0.85153 | 0.03720* | |
| C21 | 0.89574 | 0.61406 | 0.72810 | 0.02690* | |
| C22 | 1.05088 | 0.60366 | 0.81326 | 0.01140* | |
| C23 | 1.05835 | 0.45929 | 0.76591 | 0.01140* | |
| C24 | 1.22583 | 0.46859 | 0.86206 | 0.01140* | |
| C25 | 1.24934 | 0.33401 | 0.83148 | 0.02690* | |
| C26 | 0.88489 | 0.34266 | 0.76154 | 0.02690* | |
| O27 | 0.91283 | 0.67660 | 0.65013 | 0.02690* | |
| O28 | 0.74438 | 0.55486 | 0.75249 | 0.02690* | |
| O29 | 0.77908 | 0.24851 | 0.65899 | 0.02690* | |
| O30 | 0.85938 | 0.35541 | 0.87736 | 0.02690* | |
| O31 | 1.41952 | 0.35627 | 0.84442 | 0.02690* | |
| O32 | 1.12639 | 0.21838 | 0.80356 | 0.02690* | |
| O33 | 1.06496 | 0.42342 | 0.63541 | 0.02690* | |
| H34 | 1.17517 | 0.68282 | 0.81372 | 0.01480* | |
| H35 | 1.03899 | 0.62482 | 0.91229 | 0.01480* | |
| H36 | 1.14234 | 0.50804 | 0.61969 | 0.03500* | |
| H37 | 1.34467 | 0.55122 | 0.85978 | 0.01480* | |
| H38 | 1.21569 | 0.49440 | 0.96204 | 0.01480* | |
| O39 | 0.81696 | 0.02002 | 0.25093 | 0.04720* | |
| H40 | 0.18537 | −0.02945 | −0.09914 | 0.04120* | |
| H41 | 0.68916 | −0.03767 | 0.23211 | 0.06630* | |
| H42 | 0.86902 | −0.04787 | 0.25331 | 0.06630* | |
| H43 | 1.43590 | 0.26928 | 0.83176 | 0.04120* | |
| H44 | 0.74472 | 0.27820 | 0.86966 | 0.04120* | |
| H45 | 0.53070 | 0.12674 | 0.93125 | 0.04410* | |
| H46 | 0.35770 | 0.42024 | 0.28145 | 0.04120* | |
| H47 | 0.54619 | 0.06538 | 0.78398 | 0.04410* |
(I_DFT). Bond lengths (Å)
| C1—C2 | 1.518 | Ca19—O13 | 2.818 |
| C1—O7 | 1.321 | O20—H45 | 0.991 |
| C1—O8 | 1.233 | O20—H47 | 0.999 |
| C2—C3 | 1.537 | C21—C22 | 1.515 |
| C2—H14 | 1.094 | C21—O27 | 1.231 |
| C2—H15 | 1.094 | C21—O28 | 1.317 |
| C3—C4 | 1.548 | C22—C23 | 1.551 |
| C3—C6 | 1.537 | C22—H34 | 1.090 |
| C3—O13 | 1.442 | C22—H35 | 1.090 |
| C4—C5 | 1.521 | C23—C24 | 1.548 |
| C4—H17 | 1.095 | C23—C26 | 1.538 |
| C4—H18 | 1.086 | C23—O33 | 1.410 |
| C5—O11 | 1.280 | C24—C25 | 1.508 |
| C5—O12 | 1.250 | C24—H37 | 1.091 |
| C6—O9 | 1.255 | C24—H38 | 1.095 |
| C6—O10 | 1.271 | C25—O31 | 1.331 |
| O7—H40 | 1.001 | C25—O32 | 1.227 |
| O11—Ca19i | 2.417 | C26—O29 | 1.224 |
| O12—Ca19ii | 2.332 | C26—O30 | 1.317 |
| O13—H16 | 0.995 | O27—Ca19iv | 2.564 |
| Ca19—O12iii | 2.332 | O28—H46v | 1.022 |
| Ca19—O33 | 2.400 | O30—H44 | 0.999 |
| Ca19—O11i | 2.417 | O31—H43 | 0.984 |
| Ca19—O10 | 2.421 | O33—H36 | 1.002 |
| Ca19—O29 | 2.446 | O39—H41 | 0.978 |
| Ca19—O39 | 2.466 | O39—H42 | 0.986 |
| Ca19—O27iv | 2.564 | H46—O28v | 1.022 |
Symmetry codes: (i) −x+1, −y, −z+1; (ii) x−1, y, z; (iii) x+1, y, z; (iv) −x+2, −y+1, −z+1; (v) −x+1, −y+1, −z+1.
(I_DFT). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O28v—H46···O9 | 1.022 | 1.542 | 2.545 | 165.8 |
| O20—H47···O13i | 0.999 | 1.726 | 2.706 | 166.0 |
| O7—H40···O39vi | 1.001 | 1.706 | 2.677 | 162.4 |
| O33—H36···O10iv | 1.002 | 1.683 | 2.672 | 168.4 |
| O30—H44···O20 | 0.999 | 1.687 | 2.685 | 178.1 |
| O13—H16···O11 | 0.995 | 1.852 | 2.739 | 146.8 |
| O20—H45···O8vii | 0.991 | 1.798 | 2.754 | 161.0 |
| O39—H41···O20i | 0.978 | 1.815 | 2.765 | 163.2 |
| O39—H42···O32viii | 0.986 | 1.816 | 2.739 | 154.3 |
| O31—H43···O20iii | 0.984 | 1.816 | 2.851 | 159.6 |
Symmetry codes: (i) −x+1, −y, −z+1; (iii) x+1, y, z; (iv) −x+2, −y+1, −z+1; (v) −x+1, −y+1, −z+1; (vi) −x+1, −y, −z; (vii) x, y, z+1; (viii) −x+2, −y, −z+1.
Calcium hydrogen citrate trihydrate (CAHCIT_DFT). Crystal data
| [Ca(C6H6O7)(H2O)3] | c = 23.8176 Å |
| Mr = 284.2 | β = 116.7700° |
| Monoclinic, P21/c | V = 1045.35 Å3 |
| a = 8.7955 Å | Z = 4 |
| b = 5.5891 Å |
Calcium hydrogen citrate trihydrate (CAHCIT_DFT). Data collection
| h = → | l = → |
| k = → |
Calcium hydrogen citrate trihydrate (CAHCIT_DFT). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.76524 | 0.05348 | 0.37245 | 0.01640* | |
| C2 | 0.95140 | 0.08798 | 0.41838 | 0.01460* | |
| C3 | 0.03847 | 0.27647 | 0.39546 | 0.01420* | |
| C4 | 0.04728 | 0.18511 | 0.33591 | 0.01370* | |
| C5 | 0.22520 | 0.32338 | 0.44588 | 0.01720* | |
| C6 | 0.23352 | 0.46010 | 0.50207 | 0.01570* | |
| O7 | 0.27344 | 0.46657 | 0.18178 | 0.02140* | |
| O8 | 0.65243 | 0.11625 | 0.38870 | 0.02060* | |
| O9 | 0.94176 | 0.49387 | 0.37832 | 0.01870* | |
| O10 | 0.09279 | −0.02877 | 0.33519 | 0.01850* | |
| O11 | 0.01008 | 0.32908 | 0.29062 | 0.01980* | |
| O12 | 0.17785 | 0.12176 | 0.03732 | 0.02810* | |
| O13 | 0.69827 | 0.32564 | 0.49124 | 0.02930* | |
| O14 | 0.36036 | 0.36647 | 0.31711 | 0.02640* | |
| O15 | 0.58350 | 0.25887 | 0.10989 | 0.03520* | |
| O16 | 0.62980 | 0.41541 | 0.24756 | 0.02480* | |
| Ca17 | 0.14260 | 0.19608 | 0.22556 | 0.01080* | |
| H18 | 0.03696 | 0.85761 | 0.53607 | 0.02070* | |
| H19 | −0.01870 | 0.08077 | 0.57649 | 0.02070* | |
| H20 | 0.10289 | 0.46976 | 0.59127 | 0.02070* | |
| H21 | 0.71042 | 0.84819 | 0.53720 | 0.02070* | |
| H22 | 0.70600 | 0.57636 | 0.57478 | 0.02070* | |
| H23 | 0.30870 | 0.75353 | 0.54836 | 0.03810* | |
| H24 | 0.53176 | 0.71932 | 0.66252 | 0.03330* | |
| H25 | 0.62196 | 0.47982 | 0.65997 | 0.03330* | |
| H26 | 0.50523 | 0.07373 | 0.72512 | 0.03330* | |
| H27 | 0.65711 | 0.24461 | 0.76515 | 0.03330* | |
| H28 | 0.30452 | 0.83594 | 0.37404 | 0.04570* | |
| H29 | 0.51280 | 0.12685 | 0.61841 | 0.04570* |
Calcium hydrogen citrate trihydrate (CAHCIT_DFT). Bond lengths (Å)
| C1—C2 | 1.517 | O13—C6iii | 1.317 |
| C1—O7i | 1.274 | O13—H23iii | 1.018 |
| C1—O8 | 1.266 | O14—H24iii | 0.975 |
| C2—C3ii | 1.540 | O14—H25iii | 0.992 |
| C2—H18iii | 1.086 | O15—H28i | 0.981 |
| C2—H19iv | 1.091 | O15—H29viii | 0.974 |
| C3—C2v | 1.540 | O16—H26viii | 0.981 |
| C3—C4 | 1.542 | O16—H27viii | 0.971 |
| C3—C5 | 1.559 | H18—C2iii | 1.086 |
| C3—O9v | 1.433 | H19—C2iv | 1.091 |
| C4—O10 | 1.263 | H20—O9iii | 0.988 |
| C4—O11 | 1.265 | H21—C5iii | 1.094 |
| C5—C6 | 1.514 | H22—C5iii | 1.091 |
| C5—H21iii | 1.094 | H23—O13iii | 1.018 |
| C5—H22iii | 1.091 | H24—O14iii | 0.975 |
| C6—O12vi | 1.235 | H25—O14iii | 0.992 |
| C6—O13iii | 1.317 | H26—O16vi | 0.981 |
| O7—C1vii | 1.274 | H27—O16vi | 0.971 |
| O9—C3ii | 1.433 | H28—O15vii | 0.981 |
| O9—H20iii | 0.988 | H29—O15vi | 0.974 |
| O12—C6viii | 1.235 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) x+1, y, z; (iii) −x+1, −y+1, −z+1; (iv) −x+1, −y, −z+1; (v) x−1, y, z; (vi) x, −y+1/2, z+1/2; (vii) −x+1, y+1/2, −z+1/2; (viii) x, −y+1/2, z−1/2.
Calcium hydrogen citrate trihydrate (CAHCIT_DFT). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O15vi—H29···O8iv | 0.974 | 1.940 | 2.891 | 164.6 |
| O15vii—H28···O10ix | 0.979 | 1.831 | 2.806 | 173.5 |
| O16vi—H27···O7iii | 0.971 | 1.970 | 2.926 | 167.6 |
| O16vi—H26···O7vi | 0.981 | 1.838 | 2.817 | 176.3 |
| O14iii—H25···O15vi | 0.992 | 1.719 | 2.702 | 170.9 |
| O14iii—H24···O8iii | 0.975 | 1.782 | 2.742 | 167.1 |
| O13iii—H23···O8iii | 1.018 | 1.560 | 2.571 | 171.4 |
| O9iii—H20···O12vi | 0.988 | 1.764 | 2.749 | 174.9 |
Symmetry codes: (iii) −x+1, −y+1, −z+1; (iv) −x+1, −y, −z+1; (vi) x, −y+1/2, z+1/2; (vii) −x+1, y+1/2, −z+1/2; (ix) x, y+1, z.
References
- Altomare, A., Camalli, M., Cuocci, C., Giacovazzo, C., Moliterni, A. & Rizzi, R. (2009). J. Appl. Cryst. 42, 1197–1202.
- Brandenburg, K. (2016). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bravais, A. (1866). Études Cristallographiques. Paris: Gauthier Villars.
- Bruno, I. J., Cole, J. C., Kessler, M., Luo, J., Motherwell, W. D. S., Purkis, L. H., Smith, B. R., Taylor, R., Cooper, R. I., Harris, S. E. & Orpen, A. G. (2004). J. Chem. Inf. Comput. Sci. 44, 2133–2144. [DOI] [PubMed]
- Catti, M., Pavese, A. & Saunders, V. R. (1991). J. Phys. Condens. Matter, 3, 4151–4164.
- Dassault Systèmes (2018). Materials Studio. BIOVIA, San Diego, USA.
- Donnay, J. D. H. & Harker, D. (1937). Am. Mineral. 22, 446–467.
- Dovesi, R., Orlando, R., Civalleri, B., Roetti, C., Saunders, V. R. & Zicovich-Wilson, C. M. (2005). Z. Kristallogr. 220, 571–573.
- Friedel, G. (1907). Bull. Soc. Fr. Mineral. 30, 326–455.
- Gates-Rector, S. & Blanton, T. N. (2019). Powder Diffr. 34, 352–360.
- Gatti, C., Saunders, V. R. & Roetti, C. (1994). J. Chem. Phys. 101, 10686–10696.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Herdtweck, E., Kornprobst, T., Sieber, R., Straver, L. & Plank, J. (2011). Z. Anorg. Allg. Chem. 637, 655–659.
- Kaduk, J. A. (2018). Powder Diffr. 33, 98–107.
- Lee, P. L., Shu, D., Ramanathan, M., Preissner, C., Wang, J., Beno, M. A., Von Dreele, R. B., Ribaud, L., Kurtz, C., Antao, S. M., Jiao, X. & Toby, B. H. (2008). J. Synchrotron Rad. 15, 427–432. [DOI] [PubMed]
- Louër, D. & Boultif, A. (2007). Z. Kristallogr. Suppl. 2007, 191–196.
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Rammohan, A. & Kaduk, J. A. (2018). Acta Cryst. B74, 239–252. [DOI] [PubMed]
- Sheldrick, B. (1974). Acta Cryst. B30, 2056–2057.
- Streek, J. van de & Neumann, M. A. (2014). Acta Cryst. B70, 1020–1032. [DOI] [PMC free article] [PubMed]
- Sykes, R. A., McCabe, P., Allen, F. H., Battle, G. M., Bruno, I. J. & Wood, P. A. (2011). J. Appl. Cryst. 44, 882–886. [DOI] [PMC free article] [PubMed]
- Toby, B. H. & Von Dreele, R. B. (2013). J. Appl. Cryst. 46, 544–549.
- Wang, J., Toby, B. H., Lee, P. L., Ribaud, L., Antao, S. M., Kurtz, C., Ramanathan, M., Von Dreele, R. B. & Beno, M. A. (2008). Rev. Sci. Instrum. 79, 085105. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, I_DFT, CAHCIT_DFT. DOI: 10.1107/S2056989020012864/wm5577sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020012864/wm5577Isup2.hkl
Rietveld powder data: contains datablock(s) I. DOI: 10.1107/S2056989020012864/wm5577Isup3.rtv
Additional supporting information: crystallographic information; 3D view; checkCIF report







