Abstract
In continuity of our search for novel anticancer agents acting as procaspase activators, we have designed and synthesised two series of (E)-N′-benzylidene-carbohydrazides (4a–m) and (Z)-N'-(2-oxoindolin-3-ylidene)carbohydrazides (5a–g) incorporating 1-(4-chlorobenzyl)-1H-indole core. Bioevaluation showed that the compounds, especially compounds in series 4a–m, exhibited potent cytotoxicity against three human cancer cell lines (SW620, colon cancer; PC-3, prostate cancer; NCI-H23, lung cancer). Within series 4a–m, compounds with 2-OH substituent (4g–i) exhibited very strong cytotoxicity in three human cancer cell lines assayed with IC50 values in the range of 0.56–0.83 µM. In particular, two compounds 4d and 4f bearing 4-Cl and 4-NO2 substituents, respectively, were the most potent in term of cytotoxicity with IC50 values of 0.011–0.001 µM. In caspase activation assay, compounds 4b and 4f were found to activate caspase activity by 314.3 and 270.7% relative to PAC-1. This investigation has demonstrated the potential of these simple acetohydrazides, especially compounds 4b, 4d, and 4f, as anticancer agents.
Keywords: Acetohydrazides, isatin, cytotoxicity, caspase activation
1. Introduction
Despite significant advances in anticancer therapy in the past decades, cancer remains one of the deadliest diseases with high mortality until today1. Current chemotherapeutic agents are mostly non-selective and are very prone to resistance by cancer cells, rendering the treatment less effective. Thus, development of more effective anticancer agents continues to be an urgent need. This also poses a great challenge due to the complexity of the disease1.
Apoptosis, a normal programmed cell death, is a tightly controlled and complex process in mammalian cells2. Dysregulation of this process, in which cells are able to escape apoptosis, is one among many causes of cancer initiation and progression2. Restoration of apoptosis, has therefore, become one of the most frequently used approaches in anticancer therapeutics nowadays2,3.
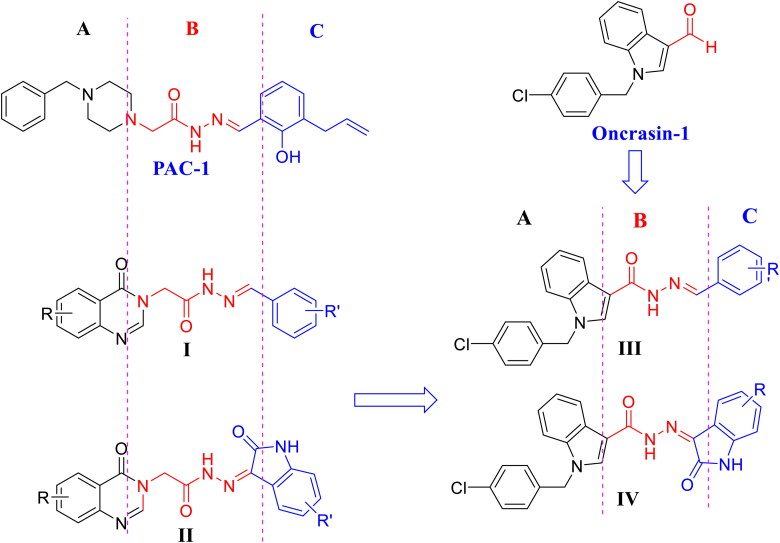
The mechanism of apoptosis is extremely complex and sophisticated4. This process can be initiated by many different pathways, e.g. intrinsic pathway, extrinsic pathway, perforin/granzyme pathway, among others4. Evidence has demonstrated that both extrinsic and intrinsic pathways end at the point of the execution phase, which is considered as the final pathway of apoptosis4. Activation of caspases plays a pivotal role in the initiation of this final pathway. Caspases is a family of cysteine-aspartic proteases with 14 members (caspases 1–14), in which caspase-3, caspase-6, and caspase-7 play a role as effector or “executioner” caspases5. Among these, caspase-3 is considered as the most important one of the executioner caspases. In cancer cells, caspase-3 exists as a low-activity zymogen, procaspase-35. Procaspase-3 has been shown to be overexpressed in many human cancers, such as lung cancer, melanoma, hepatoma, breast cancer, lymphoma, and neuroblastoma6–10. Experimental studies have shown that activation of procaspase-3 by appropriate small molecules resulted in restoration of apoptosis in cancer cells11. It is, therefore, targeting caspase has become an attractive approach for anticancer drug development in the last decades12. As a result, a number of small-molecule caspase activators have been reported13. Among these, PAC-1 (Figure 1), the first procaspase activating compound developed by Hergenrother’s group, demonstrated promising in vivo antitumor activity in human xenografted models and had been approved as an orphan drug for treatment of glioblastoma by the U.S. FDA in 201613. Based on the structural pharmacophore of PAC-1, we had designed and synthesised several series of 4-oxoquinazoline derivatives (compounds I, II) and evaluated for their cytotoxicity as well as their caspase activating activity14–17. It was found that many compounds in series I and II were potential as caspase activators and antitumor agents14,15. All compounds series I, II and PAC-1 share a common N-acylhydrazone functionality, which has been shown to be important for the formation of a strong complex with zinc ion in the caspase active binding site18. Continuing our research in this direction, we have designed two novel series III and IV incorporating a 1-(4-chlorobenzyl moiety (Figure 1). The 1-(4-chlorobenzyl) moiety is a core structure of oncrasin-1, a very potential anticancer agent19. Oncrasin-1 has been shown to strongly reduce the growth of various cancer cells and its mechanism of cytotoxicity has been demonstrated to interfering with cellular signalling proteins, such as c-jun N-terminal kinase, Fas, Fas-associated death domain (FADD), NF-kB, among others, and increasing caspase-8 activation and, thereby inducing apoptosis19–21. Thus, structures III and IV can be considered either as oncrasin-1 derivatives or hybrids of oncrasin-1 and other caspase activators (PAC-1, I, II) (Figure 1). We expected that these derivatives or hybrids would result in potent anticancer activity. This paper reports the results from synthesis, biological evaluation and docking studies of the designed compounds.
Figure 1.
Structure of PAC-1, oncrasin-1, acetohydrazides I, II and rational design of novel (E)-N'-arylidene-1-(4-chlorobenzyl)-1H-indol-3-carbohydrazides III and (Z)-1-(4-chlorobenzyl)-N'-(2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazides IV.
2. Materials and methods
2.1. Chemistry
Thin layer chromatography which was performed using Whatman® 250 µm Silica Gel GF Uniplates and visualised under UV light at 254 nm, was used to check the progress of reactions and preliminary evaluation of compounds’ homogeneity. Melting points were measured using a Gallenkamp Melting Point Apparatus (LabMerchant, London, UK) and are uncorrected. Purification of compounds was carried out using crystallisation methods and/or open silica gel column flash chromatography employing Merck silica gel 60 (240–400 mesh) as stationary phase. Nuclear magnetic resonance spectra (1H NMR) were recorded on a Bruker 500 MHz spectrometer with DMSO-d6 as solvent unless otherwise indicated. Tetramethylsilane was used as an internal standard. Chemical shifts are reported in parts per million (ppm), downfield from tetramethylsilane. Mass spectra with different ionisation modes including electron ionisation (EI), electrospray ionisation (ESI), were recorded using PE Biosystems API2000 (Perkin Elmer, Palo Alto, CA) and Mariner® (Azco Biotech, Inc., Oceanside, CA) mass spectrometers, respectively. The elemental (C, H, N) analyses were performed on a Perkin Elmer model 2400 elemental analyser. All reagents and solvents were purchased from Aldrich or Fluka Chemical Corp. (Milwaukee, WI) or Merck unless noted otherwise. Solvents were used directly as purchased unless otherwise indicated.
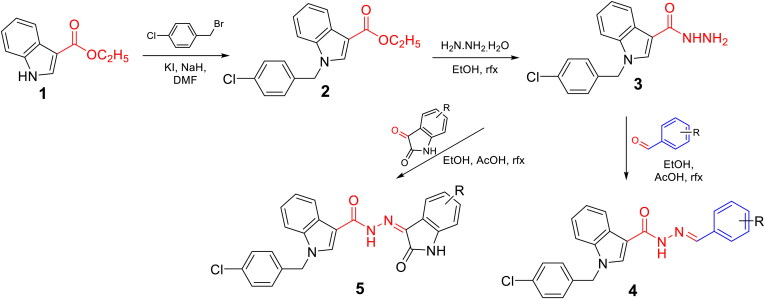
The synthesis of N'-substituted-carbohydrazides incorporating 1-(4-chlorobenzyl)-1H-indole core (4a–m and 5a–g) was carried out as illustrated in Scheme 1. Details are described below.
Scheme 1.
Synthesis of novel N'-substituted-1-(4-chlorobenzyl)-1H-indol-3-carbohydrazides (4a–m, 5a–g).
2.1.1. General procedures for the synthesis of compounds 4a–m and 5a–g
A solution of ethyl 1H-indole-3-carboxylate (1, 2 mmol) in DMF (5 ml) was treated with NaH (4 mmol). The mixture was stirred at room temperature for 1 h, then KI (49.8 mg, 0.3 mmol) was added. After stirring for further 15 min, 1-(bromomethyl)-4-chlorobenzene (2 mmol in 1 ml of DMF) was dropped slowly into the reaction mixture. The mixture was again stirred at room temperature for 24 h until the reaction completed. After completion of the reaction, the resulting mixture was cooled, poured into ice-cold water (20 ml). The precipitates were obtained, washed with water and dried to give the corresponding intermediate ester 2, which was used for the next step without further purification.
Compound 2 (1.5 mmol) was added to a solution of hydrazine monohydrate 80% in 1 ml ethanol. The reaction mixture was stirred at 80 °C until the reaction completed (3–4 h, checked by TLC). The resulting mixture was evaporated under reduced pressure to give the residue, which was cooled by addition of a water/ice mixture. The white solid was formed, filtered and dried to give compound 3 in quantitative yield.
The acetohydrazide 3 (1.0 mmol) was dissolved in ethanol (20 ml), two drops of glacial acetic acid, followed by benzaldehyde or isatin derivatives (1.0 mmol). The mixtures were refluxed until reaction completed. The precipitates formed were filtered and washed with ethanol (three times), the solid products were collected, dried under vacuum and recrystallised from ethanol or purified by column chromatography (MeOH:DCM) to obtain the target compounds 4a–m, 5a–g.
2.1.2. (E)-N′-Benzylidene-1-(4-chlorobenzyl)-1H-indole-3- carbohydrazide (4a)
Yellow solid; yield: 67%. mp: 176.7–177.5 °C. Rf=0.43 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3188 (NH); 3147, 3101, 3049 (CH aren); 2930, 2891 (CH, CH2); 1626 (C=N); 1544, 1525 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.43 (1H, s, CO-NH); 8.50–8.25 (3H, m, H-2, N=CH, H-4); 7.69–7.60 (2H, m, H-2″, H-6″); 7.58 (1H, d, J = 8.0 Hz, H-7); 7.47–7.41 (5H, m, H-3″, H-4″, H-5″, H-3′, H-5′); 7.34 (2H, d, J = 6.5 Hz, H-2′, H-6′); 7.25–7.19 (2H, m, H-5, H-6); 5.55 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 136.76; 135.15; 132.86; 130.02; 129.81; 129.30; 129.21; 127.24; 123.02; 122.04; 121.72; 11.16; 49.24. HR-MS (ESI) m/z 388.11826 [M + H]+. Anal. Calcd. for C23H18ClN3O (387.1138): C, 71.22; H, 4.68; N, 10.89. Found: C, 71.25; H, 4.60; N, 10.93.
2.1.3. (E)-1-(4-Chlorobenzyl)-N′-(2-chlorobenzylidene)-1H-indole-3- carbohydrazide (4b)
Yellow solid; yield: 61%. mp: 179.7–181.5 °C. Rf=0.52 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3190 (NH); 3103, 3045 (CH aren); 2920, 2852 (CH, CH2); 1703 (C=O); 1620 (C=N); 1543, 1521 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.70 (1H, s, CO-NH); 8.68 (1H, s, H-2); 8.37 (1H, s, N=CH); 8.25 (1H, m, H-4); 7.99–7.23 (11H, m, H-7, H-3″, H-4″, H-5″, H-6″, H-5′, H-6′, H-2′, H-3′, H-5, H-6); 5.56 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 136.77; 136.43; 133.29; 132.85; 132.41; 130.37; 129.73; 129.21; 128.06; 127.16; 123.11; 121.01; 121.82; 111.23; 49.29. HR-MS (ESI) m/z 422.07950 [M + H]+. Anal. Calcd. for C23H17Cl2N3O (421.0949): C, 65.41; H, 4.06; N, 9.95. Found: C, 65.44; H, 4.10; N, 9.90.
2.1.4. (E)-1-(4-Chlorobenzyl)-N′-(3-chlorobenzylidene)-1H-indole-3- carbohydrazide (4c)
Yellow solid; yield: 65%. mp: 179.7–181.5 °C. Rf=0.51 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3182 (NH); 3138, 3026 (CH aren); 2922, 2852 (CH, CH2); 1778 (C=O); 1618 (C=N); 1544, 1521, 1489 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.59 (1H, s, CO-NH); 8.49–8.25 (2H, m, H-2, N=CH); 8.23 (1H, d, J = 7.0 Hz, H-4); 7.76 (1H, s, H-2″); 7.65–7.61 (1H, m, H-6″); 7.58 (1H, d, J = 7.5 Hz, H-7); 7.50–7.44 (4H, m, H-5″, H-6″, H-3′, H-5′); 7.33 (2H, d, J = 6.5 Hz, H-2′, H-6′); 7.25–7.19 (2H, m, H-5, H-6); 5.55 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 137.44; 136.80; 134.13; 132.84; 131.18; 129.71; 129.65; 129.20; 126.60; 125.87; 123.09; 121.99; 121.79; 111.22; 49.26. HR-MS (ESI) m/z 422.07928 [M + H]+. Anal. Calcd. for C23H17Cl2N3O (421.0749): C, 65.41; H, 4.06; N, 9.95. Found: C, 65.49; H, 4.01; N, 9.97.
2.1.5. (E)-1-(4-Chlorobenzyl)-N′-(4-chlorobenzylidene)-1H-indole-3- carbohydrazide (4d)
Yellow solid; yield: 70%. mp: 179.7–181.5 °C. Rf=0.56 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3194 (NH); 3101, 3053 (CH aren); 2920, 2852 (CH, CH2); 1708 (C=O); 1625 (C=N); 1546, 1523, 1489 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.49 (1H, s, CO-NH); 8.34–8.24 (3H, m, H-2, N=CH, H-4); 7.58 (1H, d, J = 8.0 Hz, H-7); 7.51 (2H, d, J = 7.0 Hz, H-2″, H-6″); 7.45 (2H, d, J = 7.0 Hz, H-3″, H-5″); 7.44 (2H, d, J = 6.5 Hz, H-3′, H-5′); 7.33 (2H, d, J = 7.0 Hz, H-2′, H-6′); 7.25–7.19 (2H, m, H-5, H-6); 5.55 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 136.72; 134.42; 134.10; 132.86; 129.80; 129.37; 129.22; 128.85; 123.06; 122.05; 121.77; 111.21; 49.26. HR-MS (ESI) m/z 422.07980 [M + H]+. Anal. Calcd. for C23H17Cl2N3O (421.0749): C, 65.41; H, 4.06; N, 9.95. Found: C, 65.47; H, 4.16; N, 9.90.
2.1.6. (E)-1-(4-Chlorobenzyl)-N′-(2,6-dichlorobenzylidene)-1H- indole-3-carbohydrazide (4e)
Yellow solid; yield: 58%. mp: 179.7–181.5 °C. Rf=0.58 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3184 (NH); 3142, 3020 (CH aren); 2922, 2852 (CH, CH2); 1793 (C=O); 1616 (C=N); 1581, 1537, 1517 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.80 (1H, s, CO-NH), 8.48 (1H, s, H-2); 8.20 (1H, s, N=CH); 8.19 (1H, d, J = 8.0 Hz, H-4); 7.51 (1H, d, J = 8.0 Hz, H-7); 7.31 (2H, d, J = 7.5 Hz, H-3″, H-5″); 7.24–7.17 (5H, m, H-5, H-2′, H-3′, H-5′, H-6′); 6.90 (2H, t, J = 7.5 Hz, H-6, H-4″); 5.56 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 155.79; 147.50; 137.13; 136.65; 131.51; 129.34; 129.21; 127.51; 126.78; 122.89; 121.41; 119.43; 118.31; 116.15; 112.59; 33.87. HR-MS (ESI) m/z 456.04019 [M + H]+. Anal. Calcd. for C23H16Cl3N3O (455.0359): C, 60.48; H, 3.53; N, 9.20. Found: C, 60.40; H, 3.56; N, 9.21.
2.1.7. (E)-1-(4-Chlorobenzyl)-N′-(4-nitrobenzylidene)-1H-indole-3- carbohydrazide (4f)
Yellow solid; yield: 55%. mp: 179.7–181.5 °C. Rf=0.62 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3444 (NH); 3174, 3059 (CH aren); 2920, 2850 (CH, CH2); 1708 (C=O); 1637 (C=N); 1571, 1510 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.78 (1H, s, CO-NH); 8.39 (1H, s, N=CH); 8.31–8.24 (3H, m, H-4, H-3″, H-5″); 7.94 (2H, d, J = 6.5 Hz, H-2″, H-6″); 7.58 (1H, d, J = 8.5 Hz, H-7); 7.44 (2H, d, J = 8.0 Hz, H-3′, H-5′); 7.33 (2H, d, J = 8.0 Hz, H-2′, H-6′); 7.26–7.20 (2H, m, H-5, H-6); 5.57 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 147.95; 141.59; 136.70; 132.86; 129.77; 129.22; 128.09; 124.55; 123.18; 121.98; 121.91; 111.29; 49.32. HR-MS (ESI) m/z 433.10309 [M + H]+. Anal. Calcd. for C23H17ClN4O3 (432.0989): C, 63.82; H, 3.96; N, 12.94. Found: C, 63.83; H, 3.97; N, 12.92.
2.1.8. (E)-1-(4-Chlorobenzyl)-N′-(2-hydroxybenzylidene)-1H-indole- 3-carbohydrazide (4g)
Yellow solid; yield: 68%. mp: 179.7–181.5 °C. Rf=0.51 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3282 (NH); 3049 (CH aren); 2920, 2852 (CH, CH2); 1620 (C=N); 1521, 1487 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.50 (1H, s, CO-NH), 8.52 (1H, s, H-2), 8.22 (1H, s, N=CH), 8.20 (1H, d, J = 8.0 Hz, H-4), 7.61–7.43 (4H, m, H-3″, H-4″, H-5″, H-6″), 7.32–7.16 (5H, m, H-7, H-2′, H-3′, H-5′, H-6′), 6.95–6.92 (2H, m, H-5, H-6), 5.53 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 131.24; 129.50; 129.21; 129.14; 122.82; 121.84; 121.48; 121.34; 119.74; 116.81; 112.52. HR-MS (ESI) m/z 404.11316 [M + H]+. Anal. Calcd. for C23H18ClN3O2 (403.1088): C, 68.40; H, 4.49; N, 10.40. Found: C, 68.42; H, 4.47; N, 10.41.
2.1.9. (E)-N′-(4-Allyl-2-hydroxybenzylidene)-1-(4-chlorobenzyl)-1H- indole-3-carbohydrazide (4h)
Yellow solid; yield: 58%. mp: 179.7–181.5 °C. Rf=0.47 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3323 (NH); 3199 (OH); 3124, 3025 (CH aren); 2920 (CH, CH2); 1678 (C=O); 1620 (C=N); 1566, 1519 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.85 (1H, s, CO-NH); 8.59 (1H, s, H-2); 8.51 (1H, s, N=CH); 8.26 (1H, s, H-4); 7.57–7.55 (2H, m, H-7, H-6″); 7.45–7.39 (3H, m, H-6″, H-3′, H-5′); 7.29–7.18 (5H, m, H-3″, H-2′, H-6′, H-5, H-6); 5.51 (1H, s, CH2); 5.08–5.01 (3H, m, –CH2–CH=CH2); 3.41–3.35 (2H, m, –CH2–CH=CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 166.55; 136.65; 134.28; 132.82; 129.65; 129.17; 123.06; 122.04; 121.85; 116.91; 116.29; 116.18; 115.93; 111.19. HR-MS (ESI) m/z 444.14413 [M + H]+. Anal. Calcd. for C26H22ClN3O2 (443.1423): C, 70.35; H, 5.00; N, 9.47. Found: C, 70.33; H, 5.01; N, 9.49.
2.1.10. (E)-1-(4-Chlorobenzyl)-N′-(2-hydroxy-4- methoxybenzylidene)-1H-indole-3-carbohydrazide (4i)
Yellow solid; yield: 65%. mp: 179.7–181.5 °C. Rf=0.49 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3576 (NH); 3319 (OH); 3174, 3043 (CH aren); 2922 (CH, CH2); 1683 (C=O); 1624 (C=N); 1564, 1519 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.58 (1H, s, CO-NH); 8.44 (1H, s, H-2); 8.19 (1H, s, N=CH); 8.19 (1H, d, J = 7.0 Hz, H-4); 7.49 (1H, d, J = 8.0 Hz, H-7); 7.41 (1H, d, J = 7.5 Hz, H-5″); 7.22–7.15 (6H, m, H-2′, H-6′, H-3′, H-5′, H-5, H-6); 6.52 (1H, d, J = 9.0 Hz, H-5″); 6.51 (1H, s, H-3″); 5.53 (2H, s, CH2); 3.78 (3H, s, OCH3). 13C NMR (125 MHz, DMSO-d6, ppm): δ 162.10; 136.59; 126.84; 122.77; 121.46; 121.27; 108.76; 106.76; 101.67; 55.76. HR-MS (ESI) m/z 434.12378 [M + H]+. Anal. Calcd. for C24H20ClN3O3 (433.1193): C, 66.44; H, 4.65; N, 9.68. Found: C, 66.42; H, 4.65; N, 9.69.
2.1.11. (E)-1-(4-Chlorobenzyl)-N′-(3-hydroxy-4- methoxybenzylidene)-1H-indole-3-carbohydrazide (4j)
Yellow solid; yield: 59%. mp: 179.7–181.5 °C. Rf=0.48 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3523 (NH); 3219 (CH aren); 2924, 2852 (CH, CH2); 1708 (C=O); 1604 (C=N); 1575, 1510 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 8.33 (1H, s, H-2); 8.19 (1H, d, J = 9.0 Hz, H-4); 7.75–7.53 (1H, m, H-7); 7.40–7.38 (3H, m, H-3′, H-5′, H-2″); 7.30–7.28 (3H, m, H-5″, H-2′, H-6′); 7.23–7.21 (3H, m, H-4″, H-5, H-6); 5.52 (2H, s, CH2); 3.78 (3H, s, OCH3). 13C NMR (125 MHz, DMSO-d6, ppm): δ 164.48; 136.65; 136.62; 136.01; 132.78; 129.62; 129.13; 126.79; 122.19; 121.30; 111.68; 106.78; 59.62. HR-MS (ESI) m/z 434.12369 [M + H]+. Anal. Calcd. for C24H20ClN3O3 (433.1193): C, 66.44; H, 4.65; N, 9.68. Found: C, 66.45; H, 4.63; N, 9.66.
2.1.12. (E)-1-(4-Chlorobenzyl)-N′-(4-methoxybenzylidene)-1H- indole-3-carbohydrazide (4k)
Yellow solid; yield: 66%. mp: 179.7–181.5 °C. Rf=0.42 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3450 (NH); 3188, 3101, 3041 (CH aren); 2910, 2843 (CH, CH2); 1620 (C=N); 1552, 1510 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.47 (1H, s, CO-NH); 8.50–8.15 (3H, m, H-2, N=CH, H-4); 7.73–7.57 (3H, m, H-7, H-2″, H-6″); 7.45–7.39 (4H, m, H-2′, H-6′, H-3′, H-5′); 7.24–7.18 (2H, m, H-5, H-6); 7.01 (2H, d, J = 8.0 Hz, H-3″, H-5″); 5.54 (2H, s, CH2); 3.82 (3H, s, OCH3). 13C NMR (125 MHz, DMSO-d6, ppm): δ 160.92; 136.76; 132.84; 129.80; 129.21; 128.78; 127.74; 122.96; 122.04; 121.64; 114.80; 55.76; 49.23. HR-MS (ESI) m/z 418.12897 [M + H]+. Anal. Calcd. for C24H20ClN3O2 (417.1244): C, 68.98; H, 4.82; N, 10.06. Found: C, 68.97; H, 4.88; N, 10.03.
2.1.13. (E)-1-(4-Chlorobenzyl)-N′-(4-(dimethylamino)benzylidene)- 1H-indole-3-carbohydrazide (4l)
Yellow solid; yield: 72%. mp: 179.7–181.5 °C. Rf=0.56 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3210 (NH); 3091, 3062 (CH aren); 2906, 2802 (CH, CH2); 1708 (C=O); 1610 (C=N); 1556, 1523 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.19, 11.01 (0.6H, 0.4H, 2s, CO-NH); 8.50–7.96 (3H, m, H-2, N=CH, H-4); 7.56–7.32 (7H, m, H-7, H-2″, H-6″, H-2′, H-6′, H-3′, H-5′); 7.23–7.17 (2H, m, H-5, H-6); 6.75 (2H, d, J = 7.0 Hz, H-3″, H-5″); 5.53 (2H, s, CH2); 2.98 (6H, s, N(CH3)2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 151.67; 136.74; 132.85; 131.60; 129.73; 129.21; 128.51; 122.90; 122.53; 122.16; 121.57; 112.37; 49.20; 31.15. HR-MS (ESI) m/z 431.16071 [M + H]+. Anal. Calcd. for C25H23ClN4O (430.1560): C, 69.68; H, 5.38; N, 13.00. Found: C, 69.67; H, 5.36; N, 13.01.
2.1.14. (E)-1-(4-Chlorobenzyl)-N′-(3,4,5-trimethoxybenzylidene)-1H- indole-3-carbohydrazide (4m)
Yellow solid; yield: 60%. mp: 179.7–181.5 °C. Rf=0.50 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3180 (NH); 3028 (CH aren); 2922, 2850 (CH, CH2); 1784 (C=O); 1612 (C=N); 1550, 1521 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.56 (1H, s, CO-NH); 8.50–8.10 (3H, m, H-2, N=CH, H-4); 7.53 (1H, m, H-7); 7.40 (2H, d, J = 8.5 Hz, H-2″, H-6″); 7.26–7.18 (4H, m, H-2′, H-6′, H-5, H-6); 7.01 (2H, s, H-2″, H-6″); 5.56 (2H, s, CH2); 3.81 (9H, s, OCH3). 13C NMR (125 MHz, DMSO-d6, ppm): δ 153.65; 139.29; 136.92; 132.71; 130.76; 129.15; 123.02; 122.05; 121.70; 111.16; 104.47; 60.58; 56.29; 49.27. HR-MS (ESI) m/z 478.15005 [M + H]+. Anal. Calcd. for C26H24ClN3O4 (477.1455): C, 65.34; H, 5.06; CN, 8.79. Found: C, 65.38; H, 5.08; CN, 8.77.
2.1.15. (Z)-1-(4-Chlorobenzyl)-N′-(2-oxoindolin-3-ylidene)-1H- indole-3-carbohydrazide (5a)
Yellow solid; yield: 67%. mp: 176.7–177.5 °C. Rf=0.47 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3238 (NH); 3115, 3057 (CH aren); 2926, 2864 (CH, CH2); 1710 (C=O); 1660 (C=N); 1610, 1543, 1490 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 13.25, 11.21 (0.54H, 0.46H, 2s, CONH); 11.97, 11.94 (0.6H, 0.4H, 2s, NH-isatin); 8.59, 7.62 (0.43H, 0.57H, 2s, H-2); 8.25–8.11 (2H, m, H-4, H-4′); 7.63–7.61 (1H, d, J = 7.5 Hz, H-7); 7.36–7.28 (4H, m, H-3′, H-5′, H-6″, H-7″); 7.18–7.10 (3H, m, H-5, H-2′, H-6′); 7.10–6.94 (1H, m, H-6); 4.95, 4.91 (1.1H, 0.9H, 2s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 168.18; 162.11; 134.67; 133.17; 132.01; 131.76; 130.22; 129.58; 126.88; 124.19; 123.41; 122.47; 122.28; 121.36; 120.70; 116.13; 113.45; 111.19; 110.53; 108.47, 42.93. HR-MS (ESI) m/z 429.10867 [M + H]+. Anal. Calcd. for C24H17ClN4O2 (428.1040): C, 67.21; H, 4.00; N, 13.06. Found: C, 67.20; H, 4.05; N, 13.07.
2.1.16. (Z)-1-(4-Chlorobenzyl)-N′-(5-fluoro-2-oxoindolin-3- ylidene)-1H-indole-3-carbohydrazide (5b)
Yellow solid; yield: 61%. mp: 176.7–177.5 °C. Rf=0.53 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3272 (NH); 3113, 3057 (CH aren); 2976, 2927 (CH, CH2); 1697 (C=O); 1668 (C=N); 1645, 1581, 1523 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 13.72 (1H, s, CONH); 12.48 (s, NH-isatin, 1H); 8.77 (1H, s, H-2); 8.66 (1H, d, J = 7.0 Hz, H-4); 8.00 (1H, s, H-4″); 7.98 (1H, d, J = 7.5 Hz, H-7); 7.98–7.82 (4H, m, H-6″, H-7″, H-3′, H-5′); 7.70–7.64 (3H, m, H-5, H-2′, H-6′); 7.49–7.47 (1H, m, H-6); 5.46 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 162.03; 160.71; 158.81; 139.10; 137.18; 135.54; 134.10; 130.22; 129.59; 126.72; 123.72; 122.52; 122.10; 121.39; 118.02; 113.82; 112.31; 108.55; 108.19; 42.85. HR-MS (ESI) m/z 447.09891 [M + H]+. Anal. Calcd. for C24H16ClFN4O2 (446.0946): C, 64.51; H, 3.61; N, 12.54. Found: C, 64.50; H, 3.66; N, 12.56.
2.1.17. (Z)-N′-(5-chloro-2-oxoindolin-3-ylidene)-1-(4-chlorobenzyl)-1H-indole-3-carbohydrazide (5c)
Yellow solid; yield: 66%. mp: 176.7–177.5 °C. Rf=0.51 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3433 (NH); 3176, 3059 (CH aren); 2926, 2868 (CH, CH2); 1710 (C=O); 1658 (C=N); 1620, 1579, 1523 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 13.23 (1H, s, CONH); 12.06 (s, NH-isatin, 1H); 8.38 (1H, s, H-2); 8.24 (1H, d, J = 7.0 Hz, H-4); 7.73 (1H, s, H-4″); 7.57 (1H, d, J = 7.0 Hz, H-7); 7.45–7.51 (5H, m, H-5, H-6″, H-7″, H-3′, H-5′); 7.27–7.24 (2H, m, H-2′, H-6′); 7.09–7.08 (1H, m, H-6); 5.05 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 161.36; 141.12; 135.04; 132.78; 130.63; 129.80; 129.17; 128.05; 123.30; 122.09; 121.01; 120.48; 113.03; 112.31; 42.44. HR-MS (ESI) m/z 463.06921 [M + H]+. Anal. Calcd. for C24H16Cl2N4O2 (462.0650): C, 62.22; H, 3.48; N, 12.09. Found: C, 62.28; H, 3.45; N, 12.07.
2.1.18. (Z)-N′-(7-Chloro-2-oxoindolin-3-ylidene)-1-(4-chlorobenzyl)-1H-indole-3-carbohydrazide (5d)
Yellow solid; yield: 60%. mp: 176.7–177.5 °C. Rf=0.52 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3398 (NH); 3167, 3147, 3109 (CH aren); 2927, 2860 (CH, CH2); 1724 (C=O); 1658 (C=N); 1662, 1546, 1521 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 13.26, 11.49 (0.56H, 0.34H, 2s, CONH) ; 12.07 (1H, s, NH-isatin); 8.38 (1H, s, H-2); 8.23 (1H, d, J = 7.0 Hz, H-4); 7.75 (1H, d, J = 7.5 Hz, H-4″); 7.55 (1H, d, J = 7.0 Hz, H-7); 7.42–7.18 (8H, m, H-2′, H-6′, H-3′, H-5′, H-5, H-6, H-5″, H-6″); 5.35 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 162.05; 127.96; 136.70; 134.12; 132.99; 132.12; 129.06; 128.63; 125.40; 124.42; 123.69; 122.12. 121.93; 120.93; 119.66; 115.66; 113.07; 44.35. HR-MS (ESI) m/z 463.06927 [M + H]+. Anal. Calcd. for C24H16Cl2N4O2 (462.0650): C, 62.22; H, 3.48; N, 12.09. Found: C, 62.25; H, 3.42; N, 12.07.
2.1.19. (Z)-N′-(5-Bromo-2-oxoindolin-3-ylidene)-1-(4-chlorobenzyl)- 1H-indole-3-carbohydrazide (5e)
Yellow solid; yield: 57%. mp: 176.7–177.5 °C. Rf=0.50 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3296 (NH); 3161, 3057 (CH aren); 2929, 2873 (CH, CH2); 1707 (C=O); 1666 (C=N); 1612, 1587, 1525 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 13.20 (1H, s, CONH); 12.06 (1H, s, NH-isatin); 8.36 (1H, s, H-2); 8.24 (1H, d, J = 7.0 Hz, H-4); 7.80 (1H, s, H-4″); 7.56 (1H, d, J = 8.5 Hz, H-5″); 7.54 (1H, d, J = 8.0 Hz, H-7); 7.43–7.39 (4H, m, H-2′, H-3′, H-5′, H-6′); 7.27–7.22 (2H, m, H-5, H-6); 7.01 (1H, d, J = 8.5 Hz, H-6″); 5.02 (2H, s, CH2). 13C NMR (125 MHz, DMSO-d6, ppm): δ 161.18; 141.45; 136.73; 134.98; 133.41; 132.48; 129.77; 129.16; 126.35; 123.29; 122.41; 122.09; 121.00; 115.69; 113.01; 112.71; 42.41. HR-MS (ESI) m/z 507.01645 [M + H]+. Anal. Calcd. for C24H16BrClN4O2 (506.1045): C, 56.77; H, 3.18; N, 11.03. Found: C, 56.71; H, 3.19; N, 11.04.
2.1.20. (Z)-1-(4-Chlorobenzyl)-N′-(5-methyl-2-oxoindolin-3-ylidene)-1H-indole-3-carbohydrazide (5f)
Yellow solid; yield: 62%. mp: 176.7–177.5 °C. Rf=0.55 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3147 (NH); 3109, 3055 (CH aren); 2920, 2866 (CH, CH2); 1724 (C=O); 1683 (C=N); 1624, 1579, 1533 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 13.32 (1H, s, CONH); 12.07 (s, NH-isatin, 1H); 8.30 (1H, s, H-2); 8.25 (1H, d, J = 7.0 Hz, H-4); 7.57 (1H, d, J = 8.0 Hz, H-7); 7.49 (1H, s, H-4″); 7.42–7.39 (4H, m, H-2′, H-5′, H-3′, H-5′); 7.28–7.23 (2H, m, H-5, H-6); 7.16 (1H, d, J = 7.5 Hz, H-7″); 6.92 (1H, d, J = 7.5 Hz, H-6″); 5.05 (2H, s, CH2); 2.33 (3H, s, CH3). 13C NMR (125 MHz, DMSO-d6, ppm): δ 161.52; 140.26; 126.79; 135.33; 132.97; 132.70; 131.64; 129.75; 129.13; 123.22; 122.00; 121.26; 120.97; 120.24; 113.00; 110.54; 42.33; 21.01. HR-MS (ESI) m/z 443.12402 [M + H]+. Anal. Calcd. for C25H19ClN4O2 (442.1197): C, 67.80; H, 4.32; N, 12.65. Found: C, 67.85; H, 4.35; N, 12.61.
2.1.21. (Z)-1-(4-Chlorobenzyl)-N′-(5-methoxy-2-oxoindolin-3- ylidene)-1H-indole-3-carbohydrazide (5g)
Yellow solid; yield: 69%. mp: 176.7–177.5 °C. Rf=0.49 (DCM:MeOH = 14:1). IR (KBr, cm–1): 3221 (NH); 3109, 3015 (CH aren); 2931 (CH, CH2); 1714 (C=O); 1656 (C=N); 1519, 1487 (C=C). 1H NMR (500 MHz, DMSO-d6, ppm): δ 13.40 (1H, s, CONH); 12.07 (s, NH-isatin, 1H); 8.30 (1H, s, H-2); 8.23 (1H, d, J = 6.5 Hz, H-4); 7.60 (1H, s, H-4″); 7.56 (1H, d, J = 6.0 Hz, H-7); 7.44–7.48 (4H, m, H-2′, H-6′, H-3′, H-5′); 7.28–7.23 (3H, m, H-5, H-6, H-7″); 6.98 (1H, d, J = 9.0 Hz, H-6″); 5.02 (2H, s, CH2); 3.80 (3H, s, OCH3). 13C NMR (125 MHz, DMSO-d6, ppm): δ 161.56; 156.46; 136.82; 135.37; 132.69; 129.78; 129.16; 129.16; 123.25; 122.03; 121.16; 120.93; 117.07; 113.03; 111.67; 106.36; 56.19; 42.35. HR-MS (ESI) m/z 459.11905 [M + H]+. Anal. Calcd. for C25H19ClN4O3 (458.1146): C, 65.43; H, 4.17; N, 12.21. Found: C, 65.45; H, 4.13; N, 12.23.
2.2. Cytotoxicity assay
The cytotoxicity of the synthesised compounds was evaluated against three human cancer cell lines, including SW620 (colon cancer), PC3 (prostate cancer), and NCI-H23 (lung cancer). The cell lines were purchased from a Cancer Cell Bank at the Korea Research Institute of Bioscience and Biotechnology (KRIBB). The media, sera, and other reagents that were used for cell culture in this assay were obtained from GIBCO Co. Ltd. (Grand Island, NY). The cells were cultured in Dulbecco’s modified Eagle medium (DMEM) until confluence. The cells were then trypsinised and suspended at 3 × 104 cells/ml of cell culture medium. On day 0, each well of the 96-well plates was seeded with 180 µl of cell suspension. The plates were then incubated in a 5% CO2 incubator at 37 °C for 24 h. Compounds were initially dissolved in dimethyl sulphoxide (DMSO) and diluted to appropriate concentrations by culture medium. Then, 20 µl of each compounds’ samples, which were prepared as described above, were added to each well of the 96-well plates, which had been seeded with cell suspension and incubated for 24-h, at various concentrations. The plates were further incubated for 48 h. Cytotoxicity of the compounds was measured by the colorimetric method, as described previously22 with slight modifications23–26. The IC50 values were calculated using a probit method and were averages of three independent determinations (SD ≤ 10%)27.
2.3. Caspase-3 activation assay
Caspase activity was measured by using caspase 3 assay kit according to the manufacturer’s instructions (Abcam, Cambridge, MA). U937 5 × 105 cells/well seeding (2.5 × 105 cells/ml, 2 ml/well, six well) were plated in six-well culture plates and allowed to grow for 24 h. The cells were treated with compounds or PAC-1 (50 µM) for 24 h, and then harvested. The harvested cells were washed twice with ice-cold PBS and treated with lysis buffer included in the kit. Cell lysate (100 µg/50 µl) was mixed with 50 µl of 2× reaction buffer and 5 µl of DEVD-p-NA substrate as the instruction of caspase-3 assay kit (Abcam, Cambridge, MA, cat. no. ab39401). Fluorescence was measured after one-hour incubation.
2.4. Cell cycle analysis
U937 5 × 105 cells/well seeding (2.5 × 105 cells/ml, 2 ml/well, six well) were plated in six-well culture plates and allowed to grow for 24 h. The cells were treated with compounds (50 µM) for 24 h, and then harvested. The harvested cells were washed twice with ice-cold PBS, fixed in 75% ice-cold ethanol, and stained with propidium iodide (PI) in the presence of RNase at room temperature for 30 min. The stained cells were analysed for DNA content using a FACScalibur flow cytometer (BD Biosciences, San Jose, CA) and the data were processed using Cell Quest Pro software (BD Biosciences, San Jose, CA).
2.5. Apoptosis assay
The Annexin V-FITC/PI dual staining assay was used to determine the percentage of apoptotic cells. U937 5 × 105 cells/well seeding (2.5 × 105 cells/ml, 2 ml/well, six well) were plated in six-well culture plates and allowed to grow for 24 h. The cells were treated with compounds (50 µM) for 24 h, and then harvested. The harvested cells were washed twice with ice-cold PBS and incubated in the dark at room temperature in 100 ml of 1× binding buffer containing 1 µl Annexin V-FITC and 12.5 ml PI. After 15 min incubation, cells were analysed for percentage undergoing apoptosis using a FACScalibur flow cytometer (BD Biosciences, San Jose, CA). The data were processed using Cell Quest Pro software (BD Biosciences, San Jose, CA).
2.6. Docking studies
Molecular docking was carried out using MOE 2009.1028 to study the interactions of synthesised compounds in catalytic domain site of caspase-6 (PDB entry 4FXO)29. The structure of reference compound PAC-1 was obtained from Pubchem databases and those of synthesised compounds were built using the Builder module of MOE software. The docking procedures were mainly based on those reported previously14,25, that include removing co-crystal ligand, adding hydrogen atoms, parameter setting, minimising structure, and fixing charge. For estimating the negative binding free energy profile of the complex (kcal/mol), the London scoring function was first used with force field (MMFF94x) refinement, and each binding pose was then minimised and rescored with the GBVI/WSA S scoring function implemented in MOE software25. It is emphasised that for docking caspases, water molecules must be conserved as they are an integral part of allosteric mechanisms29. For interaction visualisation, all the figures were built using the snapshots taken in BIOVIA Discovery Studio v.3.5 program.
3. Results and discussion
3.1. Chemistry
The target N′-substituted-1-(4-chlorobenzyl)-1H-indol-3-carbohydrazides (4a–m, 5a–g) were synthesised as illustrated in Scheme 1. Starting from ethyl indol-3-carboxylate (1), a nucleophilic reaction with 4-chlorobenzyl bromide was affected using NaH as a base and a catalytic amount of KI. The reaction was best occurred in DMF. An acyl nucleophilic reaction in the second step between ethyl 1-(4-chlorobenzyl)-1H-indol-3-carboxylate (2) with hydrazine was smooth under refluxing conditions in EtOH to give a hydrazide (3) in a quantitative yield. Finally, the hydrazide (3) was condensed with respective benzaldehydes or isatins to afford the final series 4a–m, 5a–g in good overall yields.
The structures of the synthesised compounds were determined straightforwardly based on analysis of spectroscopic data, including IR, MS, 1H and 13C NMR. In all 1H NMR spectra of compounds in series 4a–m and 5a–g, there always appeared a singlet peak around 5.55 ppm, which was distinguished for two methylene protons from 1-(4-chlorobenzyl)indole part. In the 13C NMR spectra of compounds in series 4a–m, a downfield peak around 162 ppm was attributable for a carbonyl carbon of the acetohydrazide functionality. In the 13C NMR spectra of compounds in series 5a–g, one additional more downfield carbon around 168 ppm was characteristic for the carbonyl carbon of the 2-oxoindoline system.
3.2. Bioactivity
The synthesised compounds (4a–m, 5a–g) were first evaluated for their cytotoxicity using an SRB method as described previously20 with slight modifications as previously reported23–26. Three human cancer cell lines, including SW620 (a colon cancer), PC3 (a prostate cancer), and NCI-H23 (a lung cancer), were used in the screening. The results, which were averages of the triplicate measurements, are summarised in Table 1. 5-Fluorouracil, a currently used anticancer drug, was employed as a positive control. Also, PAC-1 and oncrasin-1 were included in the evaluation for comparison purpose. As can be seen from Table 1, all compounds in series 4a–m and 5a–g were active against three human cancer cell lines tested. In series 4a–m, compound 4a (IC50, 1.67–2.09 µM) displayed slightly more potent cytotoxicity in comparison to PAC-1 (IC50, 4.23–5.11 µM) and oncrasin-1 (IC50, 3.11–3.32 µM). This compound was approximately 4- to 8-fold more potent than 5-FU (IC50, 8.84–13.61 µM) in term of cytotoxicity. Substitution of Cl at the meta position on the phenyl ring resulted in compound 4c with decreased cytotoxicity (IC50, 4.70–5.25 µM). However, ortho substitution of Cl substantially enhanced cytotoxicity (4b, IC50, 0.12–0.16 µM). Especially, compound 4d with Cl substituted at para position exhibited extremely potent cytotoxicity with IC50 of 0.001–0.002 µM in all three human cancer cell lines. Compound 4d was virtually the most potent one in the series. Surprisingly, addition of an ortho chloro substituent led to the loss of antiproliferative activity (4e, IC50>10 µM). Compound 4f with 4-NO2 substituent also displayed very potent cytotoxicity (IC50, 0.005–0.011 µM). Thus, it seemed that substitution of electron withdrawing groups, e.g. Cl or NO2 at para-position greatly enhanced cytotoxicity. In contrast, substitution of electron releasing groups, e.g. –OCH3 (compound 4k) or –N(CH3)2 (compound 4l) at para-position did not improve, even led to the loss of cytotoxicity. Compound 4m with multiple methoxy substituents was also inactive. Finally, it was very interesting to note that in series 4a–m, three compounds (4g–i) with 2-OH substituent all exhibited potent cytotoxicity in three human cancer cell lines assayed with IC50 values in the range of 0.56–0.83 µM. The cytotoxic potency of compounds 4g–i was approximately 6- to 10-fold higher than that of PAC-1. From the results obtained, it was clear that for compounds in series 4a–m, 2-Cl or 2-OH substituents were favourable for cytotoxicity. Compounds with 4-Cl and 4-NO2 substituents, and likely other electron withdrawing groups, were the most potent ones.
Table 1.
Cytotoxicity of the selected compounds against some human cancer cell lines. 
| Cpd code | R | MW | Cytotoxicity (IC50a, µM)/cell linesb |
||
|---|---|---|---|---|---|
| SW620 | PC3 | NCI-H23 | |||
| 4a | H | 387.87 | 2.09 ± 0.138 | 2.97 ± 0.017 | 1.67 ± 0.056 |
| 4b | 2-Cl | 422.31 | 0.16 ± 0.002 | 0.16 ± 0.006 | 0.12 ± 0.002 |
| 4c | 3-Cl | 422.31 | 5.25 ± 0.059 | 4.70 ± 0.145 | 4.81 ± 0.098 |
| 4d | 4-Cl | 422.31 | 0.002 ± 0.000 | 0.002 ± 0.000 | 0.001 ± 0.000 |
| 4e | 2,6-Cl2 | 456.75 | >10 | >10 | >10 |
| 4f | 4-NO2 | 432.86 | 0.011 ± 0.001 | 0.010 ± 0.000 | 0.005 ± 0.001 |
| 4g | 2-OH | 403.87 | 0.62 ± 0.003 | 0.58 ± 0.014 | 0.69 ± 0.017 |
| 4h | 2-OH-3-allyl | 443.93 | 0.58 ± 0.000 | 0.61 ± 0.003 | 0.56 ± 0.000 |
| 4i | 2-OH-4-OCH3 | 433.89 | 0.80 ± 0.042 | 0.83 ± 0.020 | 0.62 ± 0.029 |
| 4j | 3-OH-4-OCH3 | 433.89 | 5.00 ± 0.065 | 7.31 ± 0.423 | 6.60 ± 0.407 |
| 4k | 4-OCH3 | 417.89 | 2.53 ± 0.055 | 3.29 ± 0.023 | 2.45 ± 0.028 |
| 4l | 4-N(CH3)2 | 430.94 | >10 | >10 | >10 |
| 4m | 3,4,5-(OCH3)3 | 477.94 | >10 | >10 | >10 |
| 5a | H | 428.88 | 1.96 ± 0.055 | 1.54 ± 0.020 | 1.71 ± 0.044 |
| 5b | 5-F | 446.87 | 2.28 ± 0.084 | 1.58 ± 0.012 | 1.71 ± 0.040 |
| 5c | 5-Cl | 463.32 | 4.33 ± 0.223 | 4.46 ± 0.039 | 3.83 ± 0.247 |
| 5d | 7-Cl | 463.32 | 4.49 ± 0.203 | 3.74 ± 0.142 | 2.68 ± 0.225 |
| 5e | 5-Br | 507.77 | 1.70 ± 0.051 | 1.61 ± 0.022 | 1.17 ± 0.076 |
| 5f | 5-CH3 | 442.90 | 7.56 ± 0.200 | 4.71 ± 0.006 | 5.13 ± 0.382 |
| 5g | 5-OCH3 | 458.90 | 5.27 ± 0.192 | 3.79 ± 0.180 | 3.86 ± 0.076 |
| 5-FUc | 130.08 | 8.84 ± 1.92 | 13.61 ± 0.46 | 13.45 ± 3.92 | |
| PAC-1d | 392.49 | 4.57 ± 0.17 | 4.23 ± 0.45 | 5.11 ± 0.07 | |
| Oncrasin-1e | 269.06 | 3.17 ± 0.24 | 3.11 ± 0.28 | 3.32 ± 0.31 | |
aThe concentration (µM) of compounds that produces a 50% reduction in cell growth, the numbers represent the averaged results from triplicate experiments with deviation of less than 10%.
bCell lines: SW620, colon cancer; PC3, prostate cancer; NCI-H23, lung cancer.
c5-FU: 5-fluorouracil, a positive control.
dPAC-1: a positive control.
eOncrasin-1: a positive control.
Seven compounds in series 5a–g bearing 2-oxoindoline ring were less potent than compounds in series 4a–m. Only three compounds, including 5a, 5b, and 5e, were cytotoxically equipotent in comparison to compound 4a. Other compounds showed relatively higher IC50 values (up to 7.56 µM).
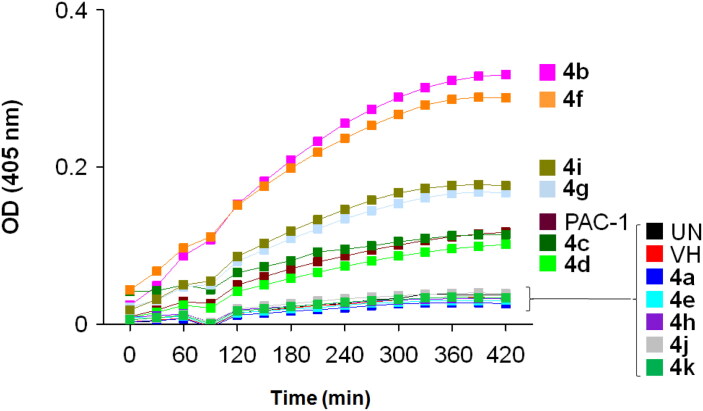
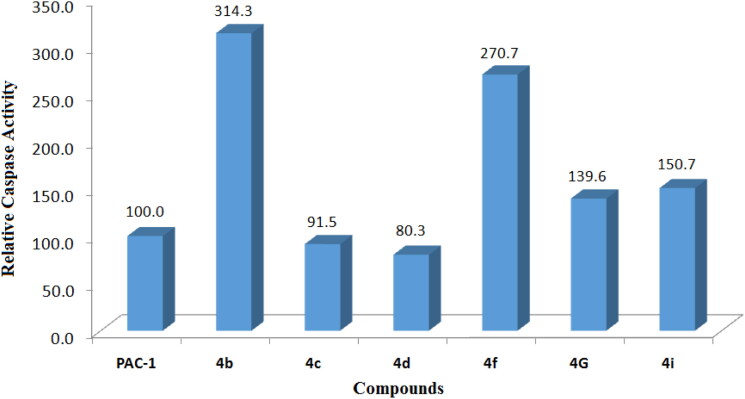
To get a preliminary insight into possible mechanisms of cytotoxicity of the synthesised compounds, compounds 4a–k were selected for further evaluation in caspase activation assay. U937 5 × 105 cells/well seeding (2.5 × 105 cells/ml, 2 ml/well, six well) were allowed to grow for 24 h, then treated with compounds or PAC-1 (50 µM) for 24 h, and harvested. The cells were lysed and caspase activity was measured using a caspase 3 assay kit according to the manufacturer’s instructions (Abcam, Cambridge, MA). The results shown in Figures 2 and 3 demonstrate that six compounds, including 4b–d, 4f, 4i, and 4g, clearly activated caspase activity. Overall, the caspase activation activity was found to be relatively correlated well with cytotoxicity in SRB assay. For example, compounds 4g and 4i, which were about 6- to 10-fold more potent than PAC-1 in term of cytotoxicity, showed relative activation of caspase activity by 139.6 and 150.7%, in comparison to that of PAC-1. Compound 4b, which exhibited approximately 40-fold more potent cytotoxicity than PAC-1, was found to activate caspase activity by 314.3% higher in relation to PAC-1. Also, compound 4f activated caspase activity by 270.7% relative to PAC-1. In SRB assay, this compound was found to be approximately 415- up to 1022-fold more cytotoxic than PAC-1. However, some exceptions in correlation between caspase activation activity and cytotoxicity were also noted. Compound 4h, which was similarly cytotoxic as compounds 4g and 4i, did not activate caspase activity. Compound 4d, which was virtually the most potent compound in terms of cytotoxicity, was found to activate the caspase activity by only 80.3% in relation to PAC-1. Since the assay performed in this study mainly measured caspase 3 activity, it is likely that these compounds might activate other caspase isoform. It is also possible that the compounds might act on other targets and additional investigation should be furthered to fully elucidate the cytotoxic mechanism of these acetohydrazides.
Figure 2.
Caspase-3 activation activity of compounds 4a–k. U937 5 × 105 cells/well seeding (2.5 × 105 cells/ml, 2 ml/well, six well) were treated with PAC-1 or compounds (50 µM) for 24 h. Cell lysate was used to detect caspase-3 activation by caspase-3 assay kit. UN: untreated; VH: vehicle (DMSO 0.05%).
Figure 3.
Relative caspase activation activity of some compounds in comparison to PAC-1. Compounds were tested at 50 µM.
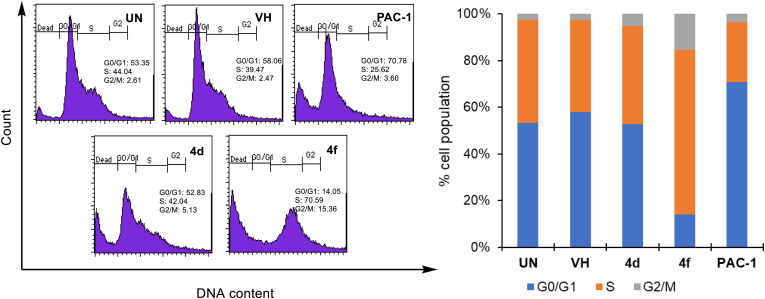
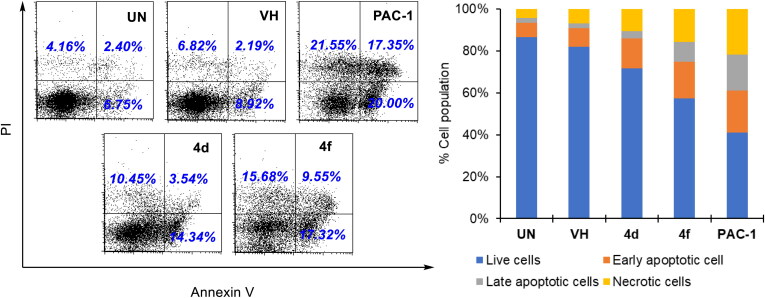
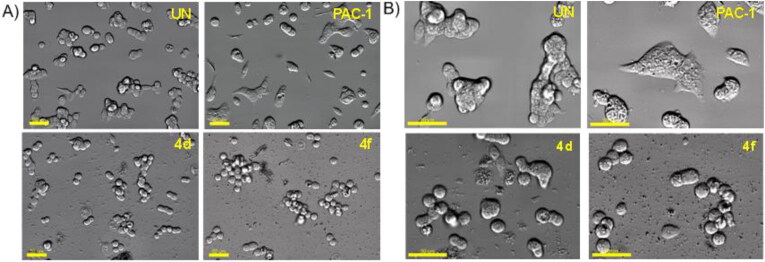
Next, we decided to select two compounds 4d and 4f to analyse for their effects on cell cycle and apoptosis. U937 cells were treated with compounds or PAC-1 for 24 h, then flow cytometry was used to analyse the DNA content after cell lysis. It could be seen that both compounds, especially compound 4f, caused substantial population of cells accumulated at S and G2/M phases (Figure 4). At this concentration, two compounds also caused a large population of cell death (19.29% for 4d and 35.36 for 4f). In Annexin V-FITC/PI dual staining assay, compounds 4d and 4f were found to substantially cause both early and late cellular apoptosis (Figure 5). These results demonstrate both compounds 4d and 4f were strong apoptotic inducers, similar to PAC-1. In line with their effects on the cell cycle and apoptosis, compounds 4d and 4f caused clear cellular morphological changes, as shown in Figure 6.
Figure 4.
Cell cycle analysis of some compounds. U937 5 × 105 cells/well seeding (2.5 × 105 cells/ml, 2 ml/well, six well) were treated with compounds (50 µM) for 24 h. The harvested cells were stained with propidium iodide (PI) in the presence of RNase and then were analysed for DNA content. UN: untreated; VH: vehicle (DMSO. 0.05%). Data were represented as histograms (left) and bar graphs (right).
Figure 5.
Apoptosis (Annexin V/PI) analysis of some compounds. U937 5 × 105 cells/well seeding (2.5 × 105 cells/ml, 2 ml/well, six well) were treated with compounds (50 µM) for 24 h. The harvested cells were incubated with Annexin V-FITC and PI. UN: untreated; VH: vehicle (DMSO. 0.05%). Area 1 = PI positive population, area 2: Annexin V-positive population. Data were represented as histograms (left) and bar graphs (right).
Figure 6.
Morphology changes of cells treated with representative compounds 4d and 4f. U937 5 × 105 cells/well seeding (2.5 × 105 cells/ml, 2 ml/well, six well) were incubated for 24 h, then compounds 4a, 4f or PAC-1 (50 μM) were added and incubated further for 24 h. The cells were then photographed using an Imaging Device: Zeiss, Celldiscoverer7 with ×40 lens, scale bar: 20 µm (A) and 50 µm (B). UN: untreated.
3.3. Molecular docking studies
As can be observed in Figure 3, 4b, 4f, 4g, and 4i were identified as potential procaspase-activating compounds, with activity up to 150–300 times higher than PAC-1. They also showed good cytotoxicity against three cancer cell lines (SW620, PC3, and NCI-H23), especially 4f with IC50 of 0.005–0.011 µM which is significantly higher than that of PAC-1. These compounds are therefore considered as potential hit compounds and needed to be explored for their structure–activity relationships that are useful for further hit-to-lead optimisation. To do so, these four derivatives were docked into the active site of procaspase enzymes taking into account the studies reported elsewhere.
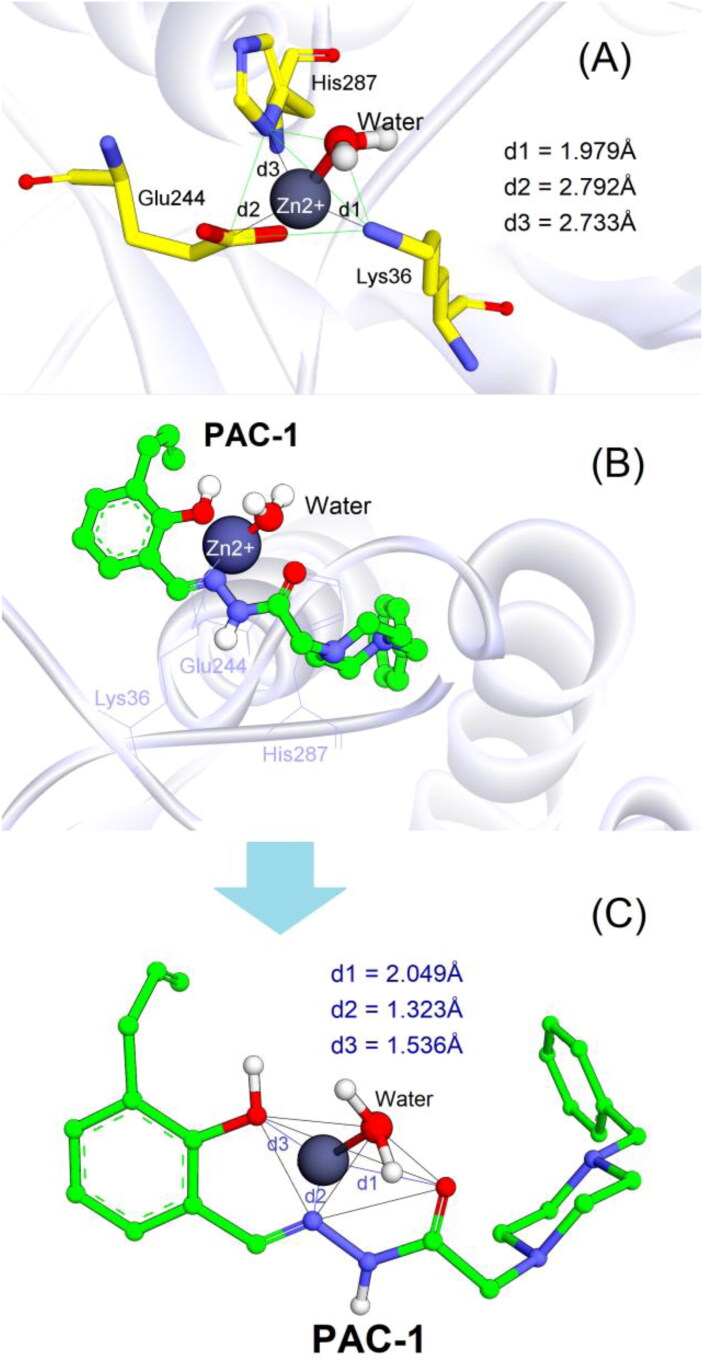
It is well known that caspases are cysteine-related proteases, which are originally synthesised as inactive dimers or zymogen procaspases and subsequently activated during apoptotic processes30. The procaspase activating derivatives, e.g. B-PAC-1, WF-208, and S-PAC-113,31,32, convert inactive executioner procaspases (caspase-3, -6, and -7) into their active cleaved forms by chelation of labile zinc ion which is a key physiological inhibitor of caspases. In the previous studies, a large number of protein data of caspases have been reviewed and the crystallographical structure of caspase-6 (a homologous structure of caspase-3) in complex with zinc ion reported by Delgado and Hardy (PDB ID: 4FXO) was identified as suitable protein model to investigate the ability of small molecules to chelate the intracellular zinc to activate caspases14,16,29. In the allosteric site of this crystal caspase structure29, zinc ion exerted its inhibitory role by establishing a tetrahedral geometry with Lys36, Glu244, His287, and water (Figure 7(A)).
Figure 7.
(A) Tetrahedral geometry of Lys36, Glu244, His287 and water molecule with zinc ion; (B) 3D structure of docked complex between PAC-1 and zinc ion in the allosteric binding site of caspase-6 (PDB ID: 4FXO); (C) tetrahedral geometry of the corresponding complex.
The PAC-1 compound was first docked into the allosteric site of zinc-bound caspase-6. As shown in Figure 7(B,C), the results clearly indicated that the metal chelating properties of ortho-hydroxy N-acylhydrazone is necessary for zinc binding and procaspase activation. By penetrating into the allosteric pocket of caspase-6, PAC-1 was able to form stable complex with zinc ion and one water molecule, showing similar coordination geometry formed between procaspase and zinc29. The binding free energy (S) estimated by GBVI/WSA force field-based scoring function was –5.03 kcal/mol, suggesting appropriate stability of PAC-1 in the complex.
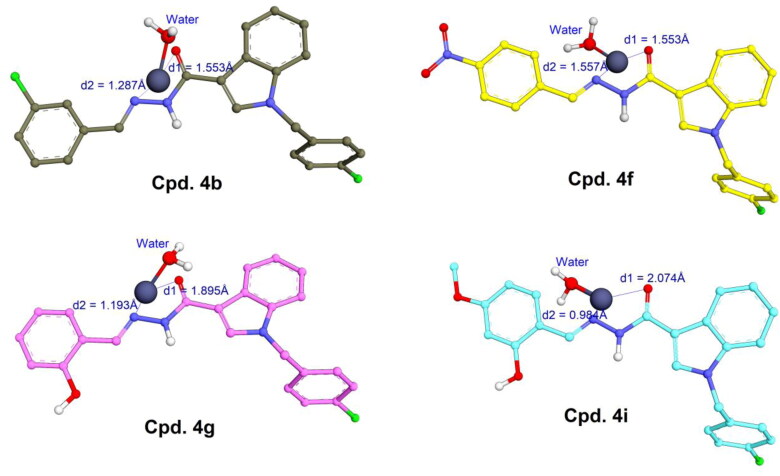
Four derivatives 4b, 4f, 4g, and 4i were subsequently docked with the zinc ion extracted from the allosteric binding site of procaspase enzyme. Compared to PAC-1, these compounds exhibited a slight difference in the zinc chelating mode (Figure 8). They were able to form bidentate chelations between =N– and =O groups of the N-acyl hydrazine moiety towards zinc ion. The similarly close distances (1–2 Å) between these atoms and zinc plays an important role in zinc-binding motif of the ligands. Despite the fact that compounds 4g and 4i owned ortho-hydroxy N-acyl hydrazine structures similar to PAC-1 the 2-hydroxyl groups could not participate in chelation with zinc like PAC-1. Interestingly, indole, N-acyl hydrazine, and phenyl moieties did not show the flexibility that PAC-1 did (see Figure 7(C)); instead, they formed a conjugated system and are almost aligned with zinc ion. Among four ligands, 4b and 4f exhibited quite low binding energies (–7.15 and –6.90 kcal/mol), meanwhile 4g and 4i showed S values of –5.15 and –5.36 kcal/mol, suggesting a higher affinity towards zinc compared to PAC-1.
Figure 8.
Metal complex and geometries between 4b, 4f, 4g, and 4i with zinc (grey spheres) extracted from locked caspase-6 (PDB ID: 4FXO).
4. Conclusions
In conclusion, we have designed and synthesised two series of (E)-N′-benzylidene-carbohydrazides (4a–m) and (Z)-N′-(2-oxoindolin-3-ylidene)carbohydrazides (5a–g) incorporating 1-(4-chlorobenzyl)-1H-indole core. The results from bioevaluation demonstrated that the compounds, especially compounds in series 4a–m, exhibited potent cytotoxicity against three human cancer cell lines (SW620, colon cancer; PC-3, prostate cancer; NCI-H23, lung cancer). Analysis of structure–activity relationships revealed that among series 4a–m, compounds with 2-OH substituent (4g–i) exhibited very strong cytotoxicity in three human cancer cell lines assayed with IC50 values in the range of 0.56–0.83 µM. Compound 4a with a 2-Cl substituent was even more potent with IC50 values of 0.12–0.16 µM. In particular, two compounds 4d and 4f, bearing 4-Cl and 4-NO2 substituents, respectively, were the most potent in terms of cytotoxicity with IC50 values of 0.011–0.001 µM. Compounds 4b and 4f strongly activated caspase activity by 314.3 and 270.7% relative to PAC-1. Annexin V-FITC/PI dual staining assay revealed two compounds 4d and 4f as strong apoptotic inducers, similar to PAC-1. In flow cytometry analysis, compound 4f was found to arrest U937 at S and G2/M phases. Subsequent docking simulations for 4b, 4f, 4g, and 4i also revealed that these compounds could form stable chelations with zinc ion that is a key physiological inhibitor of caspases. However, the geometries between the N-acylhydrazone moiety and zinc were significantly different from that of PAC-1. The conjugation effect between indole, N-acylhydrazone, and phenyl moieties played the central role for the penetration of the allosteric site of procaspase and zinc binding mode of the ligands. Thus, this investigation has demonstrated the potential of these simple acetohydrazides, especially compounds 4b, 4d, and 4f, as anticancer agents.
Supplementary Material
Funding Statement
We acknowledge the principal financial supports from the National Foundation for Science and Technology of Vietnam [NAFOSTED, Grant number 104.01-2018.01]. The work was also supported by a grant funded by the Korean Government [NRF, Grant number 2017R1A5A2015541] for indepth bioevaluation that was not supported by Nafosted Grant.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Khazaei S, Rezaeian S, Soheylizad M, et al. . Global incidence and mortality rates of stomach cancer and the human development index: an ecological study. Asian Pac J Cancer Prev 2016;17:1701–4. [DOI] [PubMed] [Google Scholar]
- 2.Storey S. Targeting apoptosis: selected anticancer strategies. Nat Rev Drug Discov 2008;7:971–2. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen-Hai N, Keykavous P.. Current targets for anticancer drug discovery. Curr Drug Targets 2003;4:159–79. [DOI] [PubMed] [Google Scholar]
- 4.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slee EA, Adrain C, Martin SJ.. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 2001;276:7320–6. [DOI] [PubMed] [Google Scholar]
- 6.Krepela E, Procházka J, Liu X, et al. . Increased expression of Apaf-1 and procaspase-3 and the functionality of intrinsic apoptosis apparatus in non-small cell lung carcinoma. Biol Chem 2004;385:153–68. [DOI] [PubMed] [Google Scholar]
- 7.Fink D, Schlagbauer-Wadl H, Selzer E, et al. . Elevated procaspase levels in human melanoma. Melanoma Res 2001;11:385–93. [DOI] [PubMed] [Google Scholar]
- 8.Persad R, Liu C, Wu T-T, et al. . Overexpression of caspase-3 in hepatocellular carcinomas. Mod Pathol 2004;17:861–7. [DOI] [PubMed] [Google Scholar]
- 9.O’Donovan N, Crown J, Stunell H, et al. . Caspase 3 in breast cancer. Clin Cancer Res 2003;9:738. [PubMed] [Google Scholar]
- 10.Izban KF, Wrone-Smith T, Hsi ED, et al. . Characterization of the interleukin-1beta-converting enzyme/Ced-3-family protease, caspase-3/CPP32, in Hodgkin’s disease: lack of caspase-3 expression in nodular lymphocyte predominance Hodgkin’s disease. Am J Pathol 1999;154:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putt KS, Chen GW, Pearson JM, et al. . Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat Chem Biol 2006;2:543–50. [DOI] [PubMed] [Google Scholar]
- 12.Patrick H, Murli M, Natasha K.. Targeting caspases in cancer therapeutics. Biol Chem 2013;394:831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard SR, Paul JH.. Derivatives of procaspase-activating compound 1 (PAC-1) and their anticancer activities. Curr Med Chem 2016;23:201–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huan LC, Phuong CV, Truc LC, et al. . (E)-N′-Arylidene-2-(4-oxoquinazolin-4(3H)-yl) acetohydrazides: synthesis and evaluation of antitumor cytotoxicity and caspase activation activity. J Enzyme Inhib Med Chem 2019;34:465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huan LC, Truc LC, Phuong CV, et al. . N′-[(E)-arylidene]-2-(2,3-dihydro-3-oxo-4H-1,4-benzoxazin-4-yl)-acetohydrazides: synthesis and evaluation of caspase activation activity and cytotoxicity. Chem Biodivers 2018;15:e1800322. [DOI] [PubMed] [Google Scholar]
- 16.Huan LC, Tran P-T, Phuong CV, et al. . Novel 3,4-dihydro-4-oxoquinazoline-based acetohydrazides: design, synthesis and evaluation of antitumor cytotoxicity and caspase activation activity. Bioorg Chem 2019;92:103202. [DOI] [PubMed] [Google Scholar]
- 17.Huan LC, Anh DT, Truong BX, et al. . New acetohydrazides incorporating 2-oxoindoline and 4-oxoquinazoline: synthesis and evaluation of cytotoxicity and caspase activation activity. Chem Biodivers 2020;17:e1900670. [DOI] [PubMed] [Google Scholar]
- 18.Hsu DC, Roth HS, West DC, et al. . Parallel synthesis and biological evaluation of 837 analogues of procaspase-activating compound 1 (PAC-1). ACS Comb Sci 2012;14:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo W, Wu S, Liu J, Fang B.. Identification of a small molecule with synthetic lethality for K-Ras and protein kinase C Iota. Cancer Res 2008;68:7403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W, Wu S, Wang L, et al. . Antitumor activity of a novel oncrasin analogue is mediated by JNK activation and STAT3 inhibition. PLoS One 2011;6:e28487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Wang L, Guo W, et al. . Analogues and derivatives of oncrasin-1, a novel inhibitor of the C-terminal domain of RNA polymerase II and their antitumor activities. J Med Chem 2011;54:2668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skehan P, Storeng R, Scudiero D, et al. . New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI 1990;82:1107–12. [DOI] [PubMed] [Google Scholar]
- 23.Nam NH, Huong TL, Dung DTM, et al. . Synthesis, bioevaluation and docking study of 5-substitutedphenyl-1,3,4-thiadiazole-based hydroxamic acids as histone deacetylase inhibitors and antitumor agents. J Enzyme Inhib Med Chem 2014;29:611–8. [DOI] [PubMed] [Google Scholar]
- 24.Nam NH, Pitts RL, Sun S, et al. . Design of tetrapeptide ligands as inhibitors of the Src SH2 domain. Bioorg Med Chem 2004;12:779–87. [DOI] [PubMed] [Google Scholar]
- 25.Nam NH, Hong DH, You YJ, et al. . Synthesis and cytotoxicity of 2,5-dihydroxychalcones and related compounds. Arch Pharm Res 2004;27:581–8. [DOI] [PubMed] [Google Scholar]
- 26.Min BS, Kim JH, Na MK, et al. . Furo-1,2-naphthoquinones from Crataegus pinnatifida with ICAM-1 expression inhibition activity. Planta Med 2004;70:1166–9. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Smythe AM, Stinson SF, et al. . Multidrug-resistant phenotype of disease-oriented panels of human tumor cell lines used for anticancer drug screening. Cancer Res 1992;52:3029–34. [PubMed] [Google Scholar]
- 28.Molecular Operating Environment (MOE). 2009.10. Montreal, Canada: Chemical Computing Group ULC; 2009. [Google Scholar]
- 29.Velázquez-Delgado EM, Hardy JA.. Zinc-mediated allosteric inhibition of caspase-6. J Biol Chem 2012;287:36000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson QP, Goode DR, West DC, et al. . PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J Mol Biol 2009;388:144–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel V, Balakrishnan K, Keating MJ, et al. . Expression of executioner procaspases and their activation by a procaspase-activating compound in chronic lymphocytic leukemia cells. Blood 2015;125:1126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, Liu Y, Wang L, et al. . Targeting procaspase-3 with WF-208, a novel PAC-1 derivative, causes selective cancer cell apoptosis. J Cell Mol Med 2015;19:1916–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.