Abstract
Context: Pineal melatonin production is mediated by afferent signaling pathways that navigate through the cervicothoracic spinal cord. Melatonin profiles in individuals with complete cervical spinal cord injury (SCI) have not been systematically reviewed despite this proposed pathway.
Objectives: The primary objective was to understand melatonin profiles in individuals with complete cervical SCI, as compared to healthy controls and those with thoracolumbar and incomplete cervical SCI. Secondary objectives were to understand the impact of injury chronicity and melatonin supplementation on melatonin values in adults with complete cervical SCI.
Methods: This review (PROSPERO ID: CRD42017073767) searched several databases and gray literature sources from January 1978 to August 2017. Studies were eligible if they evaluated melatonin levels (blood, saliva or urinary metabolite measurements) in adults with complete cervical SCI. 390 studies were screened and 12 studies met final selection criteria. Given the heterogeneity in study designs, a narrative analysis was performed.
Results: There is evidence that adults with complete cervical SCI have absent diurnal melatonin rhythms as compared to healthy controls and individuals with thoracolumbar SCI below T3. There is limited evidence comparing levels in individuals with incomplete tetraplegia. There is insufficient evidence describing profiles immediately (<2 weeks) after cervical SCI. Based on a limited number of studies, melatonin supplementation does not appear to improve sleep outcomes in adults with long-standing complete cervical SCI.
Conclusions: Future research should explore melatonin levels acutely after cervical SCI and the impact of supplementation on non-sleep outcomes.
Keywords: Melatonin, Spinal cord injuries, Circadian rhythm, Tetraplegia, Quadriplegia
Introduction
Melatonin is a lipophilic hormone produced by the pineal gland.1 The timing of the pineal gland production of melatonin is controlled by the hypothalamic suprachiasmatic nucleus.1–2 The suprachiasmatic nucleus activates a signaling cascade which navigates through the intermediolateral column of the spinal cord and culminates in the superior cervical ganglion, where post-ganglion neurons stimulate the secretion of melatonin through a norepinephrine-induced effect on the pineal gland.1–2 During light exposure, pineal melatonin production is believed to be inhibited through GABA-mediated signals originating from the hypothalamus. As a result, plasma melatonin levels generally increase in the mid-to-late evening at the onset of darkness, following a square wave pattern with the highest concentrations occurring between 12 and 4 am.1–3 The fundamental role of dynamic melatonin profiles is to transmit day-length information. Although this function may assist with the synchronization of circadian processes such as sleep-wake cycles, it does not appear absolutely necessary for circadian physiology, as individuals with absent melatonin profiles can have normal sleep architecture.4–7 Melatonin may also play a role in pain modulation, cardiovascular function, neuroprotection and antioxidation.8–29
There are numerous factors that can influence melatonin secretion. Light exposure administered at night is a potent inhibitor of melatonin production.4,30 Beta-blockers, by suppressing post-ganglionic neurotransmission, are also responsible for reduced melatonin profiles.31 Other elements that may modify secretion include posture,4 opioids,4,32 caffeine,4 alcohol,4 benzodiazepines,33 smoking,4,34 transmeridian travel,5 acute illness,32,35 recent surgery,32,35 and traumatic brain injury.36,37 Additionally, complete tetraplegia has been suggested to be associated with reduced night-time melatonin levels and abolition of the diurnal variation of melatonin, due to anatomical disruption of the signaling pathway from hypothalamus to pineal gland via the cervical spine.38–41 However, there are no systematic reviews appraising the quality of this evidence. Furthermore, there are no systematic reviews evaluating melatonin levels according to level, chronicity and completeness of spinal cord injury. As well, there are no reviews to date that characterize the impact of melatonin supplementation on melatonin values and clinical outcomes in adults with complete cervical SCI and deficient melatonin production.
Given the potential clinical importance of normal melatonin rhythmicity in individuals with SCI, the primary objective of this systematic review was to compare melatonin profiles in adults with complete cervical SCI to those seen in healthy controls and individuals with incomplete cervical SCI and thoracolumbar SCI. The secondary objectives were to understand the chronicity of melatonin dysregulation in individuals with complete cervical SCI and to determine the clinical impact of melatonin supplementation on melatonin profiles in these individuals. Given the potential neuroprotective and cardioprotective benefits of melatonin, we were interested in understanding the impact of supplementation on both sleep and non-sleep-related outcomes. These objectives were created to identify the patient population most likely to benefit from melatonin supplementation, while also highlighting gaps in knowledge that may need to be addressed before considering future melatonin supplementation trials in the acute, sub-acute and chronic phases of spinal cord injury.
Methods
Study design
The protocol for the systematic review is registered at PROSPERO under the ID CRD42017073767 and was conducted in adherence to the PRISMA guidelines. The PRISMA checklist is found in the Supplementary Information. The primary objective of the review was to characterize melatonin profiles in adults with complete cervical SCI, in comparison to healthy controls, and to individuals with thoracolumbar SCI and incomplete cervical SCI. The secondary objective was to understand the effect of injury chronicity and melatonin supplementation on melatonin profiles in individuals with complete cervical SCI. The summary statistics included the binary presence or absence of circadian melatonin rhythms, as well as the absolute melatonin values during day and night-time. Pediatric data, which were defined as data from individuals younger than 16 years of age, were excluded given the confounding impact of age on melatonin profiles.2 If a study included both pediatric and adult data, attempts were made to exclude pediatric data if possible38; if not these studies were excluded. Complete cervical SCI was defined as an American Spinal Injury Association Impairment Scale (AIS) of A or as a Frankel Grade classification of A.
Search strategy
The search strategy covered the publication period from 1978 onwards; corresponding with the first study by Kneisley et al. describing melatonin levels in individuals with cervical SCI.39 No language restrictions were imposed. A combination of keywords and synonyms for “melatonin” and “cervical spinal cord injury” was designed with the consultation of a professional medical search strategist to guide the formal search strategy. The following databases were searched: PubMed, EMBASE, CENTRAL, Scopus, and ProQuest. The search strategy was first conducted on PubMed and then modeled to other search engines. Databases were searched from January 1978 to August 2017. The search strategy for PubMed is documented below:
(“melatonin”[MeSH Terms] OR “melatonin”[All Fields] OR circadin[tiab] OR melatonin [tiab] OR melovine[tiab] OR 5 methoxy n acetyltryptamine[tiab] OR 6 hydroxymelatonin [tiab]) AND (“spinal cord”[MeSH Terms] OR “spinal cord”[All Fields] OR sci[tiab] OR tetraplegi*[tiab] OR paraplegi*[tiab] OR quadraplegi*[tiab] OR quadripar*[tiab] OR paraparesis[tiab] OR tetraparesis[tiab].)
Additional gray-literature records that were searched for abstracts included conference proceedings from the Canadian Association of Physical Medicine and Rehabilitation, the American Academy of Physical Medicine and Rehabilitation, the American Thoracic Society, and the American Academy of Sleep Medicine. These conference proceedings were screened given their relevance to the subject matter.
Eligibility criteria
Studies were considered eligible for review provided that melatonin measurements were reported for individuals with complete cervical SCI, either in numerical or graphical form unless reported as below assay detection limits. For the purpose of this review, “melatonin measurements” included samples obtained from blood and saliva, as well as urinary 6-sulfatoxymelatonin (aMT6) levels. The urinary excretion of aMT6 was viewed as an appropriate surrogate for systemic melatonin values, as it closely resembles the circadian rhythm of serum melatonin concentrations.2 There is no standardized protocol for capturing the circadian rhythm of melatonin, as blood, saliva and urinary sampling each have their own benefits and weaknesses with respect to invasiveness, sleep disruption, sampling frequency and assay reliability.1,42–43 In order to maximize the number of studies to review, eligible studies included any non-review study that reported novel data, including but not limited to randomized control trials (RCTs), non-randomized interventional studies, observational studies, case studies, abstracts and conference proceedings. There was no requirement for having a non-spinal cord control group in this review. Studies were also deemed eligible if they provided melatonin measurements after melatonin supplementation. Eligible supplementation trials included those that yielded both supra-physiological and physiological levels of melatonin.
Articles captured by the search strategy were imported into Covidence, a screening and data extraction tool (https://www.covidence.org/). The titles and abstracts of these articles were screened by two reviewers using the outlined eligibility criteria. If disagreement existed, a final decision was made by a third reviewer. The full text of successfully screened articles was reviewed by two reviewers to determine final eligibility. Disagreement about final eligibility was overseen by a third reviewer if necessary. Studies presenting duplicate data were excluded.
Data extraction and analysis
Data extraction from eligible studies was completed by two investigators using a standardized form. The following data were collected: language of publication, country of study, total number of SCI participants, characteristics of SCI participants (age, biological sex, level of injury, AIS (ASIA Impairment Scale) score, chronicity of injury, medication and substance use), total number and characteristics of controls if applicable, inclusion and exclusion criteria, presence of melatonin supplementation (and if so, protocol for dose, route and duration), specified light conditions and sleep schedules, location of research (inpatient vs. outpatient), season of measurements, method and protocol of melatonin measurement, type of melatonin detection assay, detection limit of melatonin assay, and study outcome results. A narrative analysis of the primary and secondary outcomes was performed due to the heterogeneity in study designs.
Risk of bias within studies
Risk of bias for non-supplementation and pre-supplementation data was assessed by evaluating criteria derived from the Joanna Briggs Institute (JBI) Checklist for Analytical Cross-Sectional Studies (Supplementary Information).44 The supplementation trials were additionally assessed using the Cochrane Risk of Bias Tool for Randomized Controlled Trials (Supplementary Information) to evaluate the component of supplementation inherent to their design.45 Risk of bias was not formally assessed in eligible abstracts and conference proceedings due to the lack of detail surrounding their study design. For each study, risk of bias was calculated into high, fair and low-quality designations using Agency for Healthcare Research and Quality (AHRQ) standards (Supplementary Information).46
Results
General study characteristics
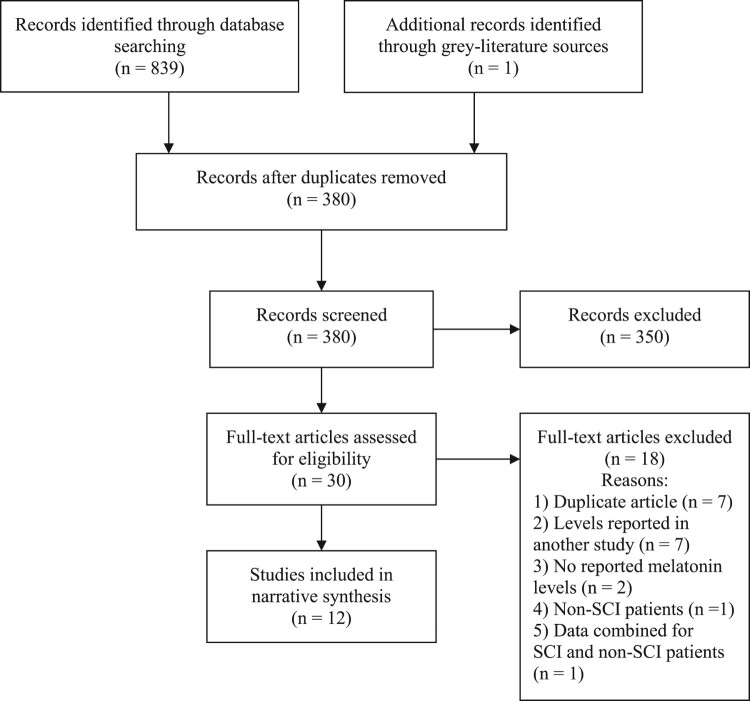
In total, 350 articles were screened and 12 studies were included for final data extraction (Fig. 1).38–41,47,48,49–54 The 12 selected studies included 130 individuals with SCI (Table 1). The majority of individuals with SCI were male (Table 1). Most studies recruited younger aged cohorts. Studies were conducted across several geographical regions, including India,52 Norway,51,53 China,38 Australia,47,50 South Africa,40 the Netherlands,49 and the United States of America.39,41,48,54 Most studies involved a comparison group with age and sex-matched healthy controls. There was a variety of melatonin sampling mediums and assay techniques used across the studies (Table 2). There was inter-study variability in the frequency and timing of melatonin sampling (Table 2).
Figure 1.
Search selection process. Full-text articles were excluded if there were duplicate articles, melatonin levels previously reported in another article (i.e. no new melatonin data), no reported melatonin levels, melatonin levels reported only in non-SCI individuals, or melatonin levels combined for non-SCI and SCI individuals that could not be sub-analyzed according to SCI individuals only.
Table 1. Baseline characteristics of spinal cord injury (SCI) individuals recruited in melatonin studies.
| Study | Number of SCI individuals | Age (years) | Sex | Level and AIS | Chronicity of SCI |
|---|---|---|---|---|---|
| Kneisley et al.39 | 6 C 1 L |
25.8 (x̅) 19 |
All male All male |
C5-C7, no AIS* L1, no AIS |
3 years (x̅) 5 weeks |
| Li et al.38 | 8 C 9 T-L |
30.1 (x̅) 34.1 (x̅) |
7M:1F 7M:2F |
C3-C7, AIS A-C* T9-L2, AIS A-C |
1.5 months (Md) 12 days (Md) |
| Papendorp et al.40 | 11 C 5 C-T |
25 (x̅) 31 (x̅) |
7M:4F 4M:3F |
C5-C7, complete C5-T4, incomplete |
20 days (x̅) 21 days (x̅) |
| Zeitzer et al.41 | 3 C 2 T |
32.7 (x̅) 31.5 (x̅) |
All male All male |
C4-C7, AIS A T4-T5, AIS A |
12 years (x̅) 5.5 years (x̅) |
| Verheggen et al.49 | 6 C 9 T |
43 (x̅) 49 (x̅) |
All male All male |
C4-C7, AIS A T4-T12, AIS A |
23 years (x̅) 22 years (x̅) |
| Spong et al.50 | 5 C | 38.4 (x̅) | 4M:1F | C4-C6, AIS A | 17.1 years (Md) |
| Spong et al.47 | 8 C | 49.5 (x̅) | 5M:3F | C4-C7, AIS A | 16 years (Md) |
| Zeitzer et al.48 | 8 C 2 C** |
55 (Md) NR |
7 M:1F NR |
C4-C8, AIS A-C NR, AIS C |
16.5 years (Md) NR |
| Kostovski et al.51 | 6 C | 45 (Md) | All male | C5-C8, AIS A | 16 years (Md) |
| Fatima et al.52 | 22 C | 35.7 (x̅) | All male | Cervical, AIS A | 2 weeks (x̅) |
| Kostovski et al.53 | 6 C | NR | NR | NR, AIS A | NR |
| Vaughan et al.54 | 9C 4T |
NR NR |
NR NR |
NR, C + I NR, C + I |
NR NR |
Studies are reported with the first name of the primary author. The number of SCI individuals per study is divided according to the level of injury (C, cervical; T, thoracic; L, lumbar; C-T, cervicothoracic; T-L, thoracolumbar). Patient age and chronicity of injury are reported in years as either mean (x̅) or median (Md) values. Completeness of injury is reported according to the ASIA Impairment Scale (AIS), or as I (incomplete) or C (complete) if not further specified in the study. If no data was reported, it is denoted as NR (not reported).
*Before the current ASIA Impairment Scale. A score was calculated if possible based on patient description.
**Zeitzer et al.48 described two cervical AIS C individuals who were excluded given significant overnight urinary aMT6 levels.
Table 2. Melatonin measurement protocols among included studies.
| Study | Sample Medium |
Assay Type |
Assay Limit | Assay CV | Melatonin sampling protocol |
|---|---|---|---|---|---|
| Kneisley et al.39 | Urine | RIA | NR | NR | 08:00–12:00, 12:00–20:00, 20:00–24:00, 24:00–04:00, 04:00–08:00 |
| Li et al.38 | Blood | RIA | NR | 6.2/16.5 | 02:00, 04:00, 10:00 and 14:00 |
| Papendorp et al.40 | Blood | RIA | NR | NR | 07:00, 14:00, 01:00 and 7:00 |
| Zeitzer et al.41 | Blood | RIA | 5 pg/ml | 8/13 | 1–3 samples/h, over 44-h total |
| Verheggen et al.49 | Saliva | ELISA | NR | NR | 19:00, 20:00, 20:30, 21:00, 21:30, 22:00, 22:30, 23:00 |
| Spong et al.50 | Saliva | NR | NR | NR | BT and UA |
| Spong et al.47 | Urine Blood |
RIA RIA |
2 ng/ml 2 pg/ml |
<15/<15 <15/<15 |
2-h BBT and UA OR urine hourly between 18:00 to 07:00 1-h BBT |
| Zeitzer et al.48 | Urine | NR | NR | NR | Overnight urine collection, times NR |
| Kostovski et al.51 | Blood | ELISA | 1 pg/ml | NR | 07:00, 12:00, 16:00, 18:00, 20:00, 22:00, 24:00, 02:00, 04:00, 07:00 |
| Fatima et al.52 | Blood | ELISA | NR | NR | 06:00, 12:00, 18:00 and 00:00 |
| Kostovski et al.53 | Blood | NR | NR | NR | Plasma samples collected 4 to 10 times during 24-h period, times NR |
| Vaughan et al.54 | Saliva | NR | NR | NR | 2-h BBT, 1-h BBT, BT, 1-h ABT |
Studies are reported with the first name of the primary author. Melatonin was assayed using either radioimmunoassay (RIA) or enzyme-linked immunosorbent assay (ELISA). The assay coefficient of variation (CV) is reported as the intra-assay reliability (%) / inter-assay reliability (%). NR, not reported; BT, bed-time; ABT, after bed-time; BBT, before bed-time; UA, upon awakening.
Risk of bias
The results of the risk of bias assessment are presented in Table 3. With respect to non- or pre-supplementation data, only one study, by Zeitzer et al., was of high quality, utilizing a constant routine protocol with strict inclusion criteria that minimized the risk of masking influences on melatonin synthesis and secretion.41 Although most studies reported such variables as age, sex, level, completeness and chronicity of injury, there was less consistency with respect to reporting substance use, medication use, acute co-morbidities, other concomitant neurological injury, and phase-shift influences like recent travel or shift work (Table 4). There was inconsistent adoption of strict light conditions among studies (Tables 3 and 4). There were often insufficient melatonin measurements to capture circadian rhythm (Table 3), with several plasma-based studies having only four measurements during a 24-h period (Table 2),38,40,52 and several saliva-derived studies using a limited window of sampling times around bed-time (Table 2).49,50,54 Only two studies employed appropriate outcome tools to analyze circadian rhythm. Zeitzer et al. determined the phase of melatonin for each individual as the midpoint between the times at which melatonin concentrations increased and decreased above the 24-h average.41 Alternatively, Verheggen et al. calculated individual dim-light melatonin onset as the marker of circadian rhythm.49 Other studies that explored circadian rhythm compared day and night-time melatonin concentrations among group-derived averages, using a limited number of sampling times. The best-conducted supplementation trials were by Zeitzer et al. and Kostvoski et al.48,51
Table 3. Risk of bias within included studies.
| Risk of bias tool | Risk of bias criteria |
K. 1978 |
L. 1989 |
V.P. 1995 |
Z. 2000 |
V. 2012 |
S. 2013 |
S. 2014 |
Z. 2014 |
K. 2015 |
F. 2016 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| JBI checklist for analytical studies | Clear inclusion criteria | + | + | + | + | + | + | + | + | + | |
| Study description | + | + | + | + | + | + | + | + | + | ||
| Consistent light conditions | – | – | + | + | + | – | – | – | – | ||
| Consistent melatonin measurement protocol | + | – | + | + | + | + | – | + | + | ||
| Clear description of confounders | – | – | – | + | – | – | – | – | + | ||
| Adjustment for confounding variables | – | – | – | + | – | – | – | – | + | ||
| Sufficient melatonin measurements | – | – | – | + | – | – | – | + | – | ||
| Outcome analysis | – | – | – | + | – | – | – | – | – | ||
| AHRQ Score | L | L | L | H | L | L | L | L | L | ||
| Cochrane Risk of Bias Tool | Random sequence generation | – | ? | ? | + | ||||||
| Allocation concealment | – | ? | ? | + | |||||||
| Selective reporting | + | + | + | + | |||||||
| Other bias | + | + | + | + | |||||||
| Blinding of participants/assessors | – | + | + | + | |||||||
| Blinding of outcome assessment | – | + | + | + | |||||||
| Incomplete outcome data | – | ? | + | + | |||||||
| AHRQ Score | L | L | F | H | |||||||
Studies are denoted using the main initial of the primary author and the date of publication. The risk of bias tools is given on the far left with the accompanying criteria. Boxes are shaded according to the following scale: meets full criterion (+), does not meet (–), not applicable (grayed out), or unsure (?). Conference proceedings and abstracts (Kostovski et al.53, Vaughan et al.54) were not analyzed for risk of bias given the lack of detail surrounding their study design. The study design by Zeitzer et al.48 was excluded (grayed out) from the JBI checklist given limited information regarding melatonin measurement and analysis, as the absence of nocturnal melatonin was used only as an inclusion criterion for study enrollment. For each checklist, studies were given an AHRQ score of L (low quality), F (fair quality) or H (high quality).
Table 4. Description of confounding variables within included studies.
| Study | Light conditions during sleep | Beta-blockers | GABA medications | Substance use | Brain injury | Phase-shift influences |
|---|---|---|---|---|---|---|
| Kneisley et al.39 | Lights off 23:00–24:00. Natural sunlight 06:00–07:30. | None | None | NR | None | NR |
| Li et al.38 | Samples collected under dim red light. Unclear otherwise. | None | None | No restriction | NR | NR |
| Papendorp et al.40 | Lights off 21:30. Samples collected with shielded flash-light. |
NR | NR | NR | NR | NR |
| Zeitzer et al.41 | Constant routine protocol | None | 66% BDZ & baclofen | None | None | Regular SWC |
| Verheggen et al.49 | Indoors under dim-light conditions after 18:00 | NR | NR | No alcohol, chocolate, coffee and tea 24 h before study | NR | NR |
| No restriction | ||||||
| Spong et al.50 | No restriction | NR | 66% BDZ & 100% baclofen | No restriction | None | No recent TT |
| Spong et al.47 | No restriction | None | 50% BDZ & baclofen | No restriction | NR | No recent TT |
| Zeitzer et al.48 | NR | NR | NR | NR | NR | No recent TT |
| Kostovski etal.51 | No restriction | NR | NR | No alcohol, max 2 cups of coffee | NR | NR |
| Fatima et al.52 | No restriction | None | None | None | None | No SW Regular SWC No TT |
| Kostovski et al.53 | NR | NR | NR | NR | NR | NR |
| Vaughan et al.54 | Dim light conditions, time initiated not specified | NR | NR | NR | NR | NR |
GABA medications included BDZ (benzodiazepines) and baclofen. Substance use included caffeine, nicotine and alcohol consumption. Phase-shift influences included recent shift work (SW), transmeridian travel (TT) and prior sleep-wake cycles (SWC) before study enrollment. NR, not reported.
Melatonin levels: Complete cervical SCI vs. healthy controls
There were eight studies that directly compared melatonin values between individuals with complete cervical SCI and healthy controls prior to any melatonin supplementation (Table 5). There was evidence that adults with complete cervical SCI had absent circadian rhythms when compared to healthy controls, supported by one high-quality study (Zeitzer et al.)41 and five low-quality studies.38–40,49,51 One of the five low-quality studies by Li et al. offered indirect evidence only, as they compared healthy controls to a heterogeneous group composed of individuals with both complete and incomplete cervical SCI.38 Only Fatima et al. reported the presence of circadian rhythm in participants with complete tetraplegia, but their results were limited by the lack of consistent light protocol, insufficient melatonin measurements to adequately capture melatonin rhythmicity and an outcome analysis that determined circadian rhythm based on mean melatonin values across combined group data rather than individually.52 There was evidence that individuals with complete cervical SCI had reduced night-time melatonin values when compared to healthy controls, supported by one high-quality study (Zeitzer et al.)41 and five low-quality studies.38,40,49,50,52 Only Kneisley et al. found no difference between nocturnal values in these two groups.39
Table 5. Melatonin values in individuals with complete cervical SCI in comparison to healthy controls.
| Study | Circadian rhythm | Day-time pattern | Day-time values |
Night-time pattern | Night-time values |
|---|---|---|---|---|---|
| Kneisley et al.39 | Absent P > 0.2 |
↑ (08:00–24:00) P < 0.01 |
SCI: (5.9 ± 2.7 ng)/4 h C: (0.46 ± 0.52ng)/4 h |
→ (24:00–08:00) P > 0.2 |
SCI: (4.6 ± 2.2 ng)/4 h C: (3.3 ± 2.3 ng)/4 h |
| Li et al.38* | Absent P > 0.05 |
→ (14:00) P > 0.05 |
SCI: 11.6 ± 1.9 pg/ml C: 16.3 ± 1.9 pg/ml** |
↓ (02:00) P < 0.05 |
SCI: 11.6 ± 3.3 pg/ml C: 67.4 ± 15.6 pg/ml** |
| Papendorp et al.40 | Absent P > 0.01 |
→ (14:00) P > 0.001 |
SCI: 30.7 ± 18.5 pg/ml C: 22 ± 5 pg/ml** |
↓ (01:00) P < 0.001 ↓ (07:00) P < 0.001 |
SCI: 33.2 ± 10.9 pg/ml C: 106 ± 10 pg/ml SCI: 27.6 ± 13.2 pg/ml C: 55 ± 5 pg/ml** |
| Zeitzer et al.41 | Absent*** | ↓*** | SCI: *** C: NR |
↓*** | SCI: *** C: 70 pg/ml (peak value) |
| Verheggen et al.49 | Absent P > 0.05 |
NA | NA | ↓ (22:30, 23:00) P < 0.05 → (19:00–22:00) P > 0.05 |
SCI: 2.41 ± 1.25 pg/ml C: 10.62 ± 4.59 pg/ml SCI: 5.25 ± 3.72 pg/ml C: 2.59 ± 1.04 pg/ml |
| Spong et al.50 | NA | NA | NA | ↓**** | NA |
| Kostovski et al.51 | Absent**** | NA | NA | NA | NA |
| Fatima et al.52 | Present**** | → (12:00) P > 0.01 → (18:00) P > 0.01 |
SCI: 16.9 pg/ml C: 14.4 pg/ml** SCI: 19.3 pg/ml C: 23.7 pg/ml** |
↓ (24:00) P < 0.01 ↑ (06:00) P < 0.01 |
SCI: 49.3 ± 19.5 pg/ml C: 65.6 ± 10.6 pg/ml SCI: 25.1 ± 6.7 pg/ml C: 13.7 ± 6.9 pg/ml |
Day and night-time melatonin patterns are in comparison to healthy controls (↑ = increased as compared to healthy controls, → = no difference, ↓ = decreased in relation to healthy controls). The exact measurement values (SCI = complete cervical SCI and C = control) are given with the standard deviation. The time points for the day and night-time melatonin values are given in parentheses. NA, not assessed; NR, not reported.
*Cervical individuals included a heterogenous group with both complete and incomplete injuries.
**Melatonin values and/or standard deviation were not reported numerically, and were derived from inspection of figures if possible.
***Melatonin levels were not detectable in complete cervical SCI, precluding statistical analysis.
****Determination was made with no accompanying statistical analysis.
Melatonin levels: Complete cervical SCI vs. thoracolumbar SCI
There is evidence to suggest that individuals with complete cervical SCI had reduced nocturnal melatonin levels and absent diurnal rhythms as compared to individuals with thoracolumbar SCI below T3 (Table 6), based on one high-quality study (Zeitzer et al.)41 and one low-quality study (Verheggen et al.).41,49 These were the only two studies that specifically compared these groups. There were four other studies that provided melatonin profiles for individuals with complete cervical SCI and thoracolumbar SCI, which are included in Table 6. Three of these four studies did not exclusively compare melatonin profiles between these groups. Li et al. compared a group of individuals with complete and incomplete cervical SCI with a sample of adults with thoracolumbar injuries ranging from T9 to L2.38 Vaughan et al. explored melatonin levels between a heterogeneous group of cervical SCI (both complete and incomplete) and thoracic SCI,54 whereas Van Papendorp et al. compared melatonin profiles between a group of individuals with complete tetraplegia and a comparison group with incomplete tetraplegia or undefined paraplegia.40 Although the study by Kneisley et al. compared melatonin values between individuals with cervical SCI and thoracolumbar SCI, there was only one thoracolumbar patient in the study, precluding statistical analysis.39 However, the results of these four studies generally did not conflict with the findings from Zeitzer et al. and Verheggen et al.41,49
Table 6. Melatonin values in individuals with complete cervical SCI (C) in comparison to adults with thoracolumbar (TL) SCI.
| Study | Circadian rhythm | Day-time pattern | Day-time values | Night-time pattern |
Night-time values |
|---|---|---|---|---|---|
| Kneisley et al.39 | C: Absent TL: Present |
NA | NA | NA | NA |
| Li et al.38* | C: Absent TL: Present |
→ (10:00) P > 0.05** → (14:00) P > 0.05** |
C: 15.8 ± 2.8 pg/ml TL: 13.5 ± 2.1 pg/ml C: 11.6 ± 1.9 pg/ml TL: 11.6 ± 2.3 pg/ml |
↓ (02:00) P < 0.05** ↓ (04:00) P < 0.05** |
C: 11.6 ± 3.3 pg/ml TL: 50.2 ± 10.5 pg/ml C: 12.6 ± 2.3 pg/ml TL: 46.5 ± 9.3 pg/ml |
| Papendorp et al.40 | C: Absent TL: Present*** |
NA | NA | NA | NA |
| Zeitzer et al.41 | C: Absent TL: Present |
↓ **** | **** | ↓ **** | C: **** TL: > 34.9 pg/ml (peak value) |
| Verheggen et al.49 | C: Absent TL: Present |
NA | NA | ↓ (22:30, 23:00) P < 0.05 → (19:00–22:00) P > 0.05 |
C: 2.41 ± 1.25 pg/ml T: 13.10 ± 7.39 pg/ml C: 5.25 ± 3.72 pg/ml T: 4.28 ± 3.28 pg/ml |
| Vaughan et al.54* | C: NA TL: NA |
NA | NA | → (2-h BBT to 1-h ABT) P > 0.05 |
C: 6.7 ± 7.4 pg/ml T: 6.3 ± 3.1 pg/ml |
The direction of the arrow indicates the difference seen in individuals with complete cervical SCI (↑ = increased as compared to adults with thoracolumbar injury, → = no difference, ↓ = decreased). The exact measurement values are given with the standard deviations. The time points for the day and night-time melatonin values are given in parentheses. NA, not assessed; BBT, before bed-time; ABT, after bed-time.
*The cervical group included individuals with both complete and incomplete injuries.
**Melatonin values and/or standard deviation were not reported numerically and were derived from inspection of figures if possible.
***Individuals with complete cervical SCI were compared with an incomplete group that included adults with either incomplete cervical SCI or thoracic SCI.
****Melatonin values were not detectable in participants with complete cervical SCI, precluding statistical analysis.
Melatonin levels: Cervical complete vs. incomplete injuries
There is limited evidence comparing melatonin profiles between individuals with cervical complete and incomplete injuries. Four studies offered indirect information only regarding the impact of completeness on melatonin profiles. Li et al. analyzed a heterogeneous group of cervical SCI individuals with both complete and incomplete injuries.38 These individuals, when grouped together, had reduced plasma melatonin levels and absent circadian melatonin rhythms, as compared to adults with thoracolumbar SCI and healthy controls.38 This outcome was not sub-analyzed according to completeness of cervical cord injury. Vaughan et al. reported that the completeness of the injury, rather than the level of the injury, was associated with increased night-time salivary levels of melatonin in adults with cervical and thoracic SCI.54 Van Papendorp et al. evaluated plasma melatonin values between a group of individuals with complete tetraplegia and a comparison group that included participants with incomplete tetraplegia or paraplegia.40 The comparison group, unlike the group with complete tetraplegia, demonstrated the presence of a circadian rhythm.40 This conclusion was not sub-analyzed to see whether it held true with participants with incomplete tetraplegia only.40 In Zeitzer et al., adults with cervical SCI were enrolled in a melatonin supplementation trial provided they had no significant overnight urinary excretion of aMT6.48 Of the three individuals with cervical AIS C spinal cord injury, one met criteria for inclusion.48 In addition to their high risk of bias (Table 3), these studies were not well designed to characterize melatonin levels according to completeness of injury, in part because they lacked appropriate sub-group analyses.
Melatonin levels in complete cervical SCI according to chronicity
The study by Fatima et al. represented the earliest documentation of melatonin levels in individuals with complete cervical SCI after spinal cord injury.52 They evaluated melatonin levels 2 weeks after injury. Overall, these individuals maintained a circadian rhythm, with reduced overnight plasma melatonin levels as compared to healthy controls. An absent circadian rhythm in individuals with complete cervical SCI has been documented as early as a mean of 20 days post-injury in the study by Van Papendorp et al.40 The pattern of low night-time melatonin values and absent circadian rhythms in cervical AIS A individuals has been demonstrated as far out as a mean of 23 years post-injury.49 Most studies examined melatonin values at least 10 years after the initial injury.41,47–51 There were no studies that compared melatonin levels longitudinally over time to evaluate the permanence of melatonin profiles.
Melatonin levels and clinical outcomes in individuals with complete cervical SCI after supplementation
There were four studies that examined melatonin levels pre- and post-melatonin supplementation in individuals with complete cervical SCI, with an additional study by Zeitzer et al. that evaluated the impact of Ramelteon, a melatonin receptor agonist, on sleep outcomes in adults with complete cervical SCI (Table 7).47,48,50,51,53 These studies generally involved melatonin and melatonin agonist administration long after the initial injury, with four of the five studies conducted at least 16 years after the presenting lesion. There was a variety of different melatonin supplementation protocols (Table 7). Melatonin values generally increased with melatonin supplementation (Table 7). There was limited information exploring the impact of melatonin on circadian rhythm, as several of the studies only had one to two measurements per 24-h period.47,50 Although there was no statistical analysis, the study by Kostovski et al. demonstrated that supplementation yielded circadian rhythms that mimicked those of normal controls, albeit with higher peak melatonin concentrations (2000–2500 pg/ml versus less than 200 pg/ml in healthy controls).51 There were no studies that compared the clinical effect of supraphysiologic versus physiologic doses of melatonin. Most studies revealed no impact of melatonin administration on subjective and objective parameters of sleep (Table 7). With respect to non-sleep outcomes, there was no major impact on mood, quality of life and hemostasis factors (Table 7). Kostovski et al. demonstrated that melatonin administration in complete cervical SCI may help align the expression of known clock genes to the timed expression seen in normal controls.53 These results, however, are limited by a lack of knowledge regarding the study design, as it is currently published as an abstract only.
Table 7. Melatonin supplementation trials: protocols and clinical outcomes in individuals with complete cervical SCI.
| Study | Protocol | Subjective sleep outcomes | Objective sleep outcomes | Other outcomes |
Post-supplementation melatonin values |
|---|---|---|---|---|---|
| Spong et al.50 | 3 mg qhs for 2 weeks | → (KSS,BNSQ) |
→ (PSG) |
NA | ↑ (undetectable vs. >50 pg/ml before bed-time) |
| Spong et al.47 | Cross-over trial 3 weeks of 3 mg qhs and placebo | → (sleep diary) |
↑ (only NREM1/2 on PSG) |
→ (HADS, POMS, AQoL) |
↑ (4.0 pg/ml vs. 10128.8 pg/ml 1 h before bed-time) |
| Zeitzer et al.48* | Cross-over trial 3 weeks of 8 mg of ramelteon qhs and placebo | → (sleep diary) |
→ (WA) |
→ (SF-36 subscale scores) |
NA |
| Kostvoski et al.51 | Cross-over trial of 4 days of 2 mg qhs and placebo | NA | NA | → (hemostatic factors)** |
↑ (peak concentrations overnight approached 2000–2500 pg/ml) |
| Kostvoski et al.53 | 2 mg qhs or placebo | NA | NA | Normalized 24-h expression profile of clock genes*** | ↑ (10– 500-time increase from baseline overnight) |
The protocol reports the dose of melatonin unless otherwise stated. Outcomes are reported using an arrow, with the direction of the arrow giving the difference with respect to placebo (in cross-over trials) or baseline (Spong et al.50) measurements (↑ = increased, → = no difference, ↓ = decreased). The outcome measurements are given in parentheses. KSS, Karolinska Sleepiness Scale; BNSQ, Basic Nordic Sleep Questionnaire; PSG, polysomnography; WA, wrist actigraphy; HADS, Hospital Anxiety and Depression Scale; POMS, Profile of Mood States; AQoL, Assessment of Quality of Life; SF-36, Short-Form Health Survey; NA, not assessed; NREM1, non-rapid eye movement sleep stage 1; NREM2; non-rapid eye movement sleep stage 2.
*Zeitzer et al.48 included individuals with incomplete cervical SCI with absent melatonin profiles.
**The hemostatic factors included prothrombin fragment 1+2, vWF, D-dimer, tissue factor pathway inhibitor, plasminogen activator inhibitory type 1.
***The clock genes included PER1, PER2, BMAL1, REV-ERB.
Discussion
To our knowledge, this is the first systematic review evaluating melatonin levels in individuals with complete cervical SCI. This review established that there is evidence that complete cervical SCI is associated with reduced night-time melatonin values and absent diurnal variation in melatonin profiles as compared to healthy controls. Although the included studies were limited by individual bias, these results were relatively consistent across several study designs, with adults from different ethnic backgrounds and exposed to different light conditions, melatonin assay kits, measurement mediums, sampling frequency, and circadian rhythm calculations.
The evidence owes much to the high-quality study conducted by Zeitzer et al.41 The strength of their study relates to the utilization of a constant routine protocol. Individuals were subjected to 46 h of wakefulness in a constant posture, with stable dim light conditions and room temperatures, with meals and fluids provided on an hourly basis to ensure that participants received their typical caloric intake over a 24-h period. The basis of the constant routine is to mask periodic changes in behavior – such as sleep, posture, food intake, temperature, and physical activity level – that may interfere with circadian rhythms.55,56 Besides the use of a constant routine, Zeitzer et al. created strict exclusion criteria that eliminated confounding variables such as recent substance use, phase-shift influences and other neurological injury.41 The administration of a constant routine may explain why Zeitzer et al. demonstrated undetectable melatonin levels in individuals with complete cervical SCI, unlike the other studies which reported reduced, but present, night-time levels.41 By controlling environmental inputs, the constant routine may have eliminated factors that triggered non-cervical spinal cord mediated melatonin production. The issue with the constant protocol is that it is time-intensive, demands sleep deprivation from its participants and requires an environment suitable for controlling temperature and light conditions, which is difficult to apply in standard hospital and home settings.55,56
With the study by Zeitzer et al. in mind, there were several common methodological biases that impaired the quality of the other individual studies. In particular, there was a lack of controlled light conditions, with only three studies enforcing strict overnight light settings.41,42,49 This is perhaps the most important confounding variable to control, as even ambient room light can suppress melatonin rhythmicity in healthy adults.4,30 There were also insufficient melatonin measurements among studies to properly determine circadian rhythm. For the ease of home monitoring, Benloucif et al. report that salivary melatonin can be measured every 30 minutes under dim light just before and throughout the anticipated rise in melatonin.43 Using this technique, melatonin can be sampled over a shortened period to determine the presence of diurnal variation. Unfortunately, it can be difficult to predict the expected rise in melatonin with this sampling protocol, as some individuals may have phase shifts in their rhythms. This may have been an issue in the study by Verheggen et al., as they measured melatonin from 19:00 to 23:00.49 Although they reported the absence of circadian rhythm in individuals with complete cervical SCI, these subjects may have had delayed melatonin production that was not captured within these limited time measurements. Conversely, the frequency of melatonin sampling over a 24-h period was a larger problem in plasma-based studies. Several articles had only one to two plasma melatonin measurements during the expected rise of melatonin, making it difficult to capture individual circadian rhythm.38,40,52 Unfortunately, frequent melatonin sampling over a 24-h period is challenging, as all sampling mediums are limited by cost, sleep disruption and invasiveness.43
Another common methodological flaw was the inappropriate calculation of circadian rhythm. With the exception of Zeitzer et al. and Verheggen et al., all other studies combined melatonin values from individuals and compared these averaged values between day and night-time sampling periods to determine the presence of circadian rhythm.41,49 There is great individual variability in the absolute concentration of melatonin values and in the timing of the expected melatonin rise in healthy individuals.43 The individual variability in melatonin profiles raises the question whether combining individual melatonin values into grouped averages is valid when applied to SCI cohorts, particularly with limited sampling periods. This was evident when reviewing the individual plots of urinary aMT6 excretion in Kneisley et al.39 On inspection of the individual plots, there appeared to be diurnal variation to the individual melatonin profiles, with some individuals demonstrating normal melatonin rhythms and others exhibiting phase shifts with higher values during the day. The conclusion of their grouped data was that adults with complete cervical SCI had abolished diurnal variation. By combining individual profiles, they may have masked the individual variability in melatonin rhythms. Unfortunately, unlike Kneisley et al.,39 most studies did not report individual melatonin profiles, making it difficult to assess the validity of analyzing group-based data. As well, there is no standard statistical measurement that best captures melatonin phase changes. For instance, dim-light melatonin onset (DLMO) is used as a circadian phase marker and represents an amenable tool for determining whether rhythmic melatonin production is present in SCI individuals.55 The setback is that DLMO can be calculated using several statistical methods, none of which is superior to the other.55 Regardless of the analytical model that is used, we believe that melatonin circadian rhythm should be based on individual rather than group-based measurements.
Although individuals with complete cervical SCI generally have absent circadian rhythms of melatonin, there is limited evidence exploring the changes in melatonin profiles that occur acutely after spinal cord injury. The earliest study documenting abnormal melatonin levels was by Fatima et al., which examined levels 2 weeks after injury.52 Individuals with acute illness, regardless of neurological compromise, may have depressed melatonin levels shortly after presentation, secondary to factors such as sedation, recent surgery, acute stress, disrupted sleep-wake cycles and hospital light settings.36 Since these factors may be common to all SCI individuals, it is conceivable that all patients, regardless of level and completeness of injury, may have depressed levels of melatonin shortly after presentation. These changes may only persist chronically in the setting of complete cervical SCI. The difficulty with measuring melatonin in the acute period is the ability to control for confounding variables that may influence melatonin secretion. Individuals with acute cervical SCI have care needs (e.g. sedation, ventilation, inotropic support, decompressive surgery) and environmental constraints (e.g. ICU settings) that would make enforcement of a constant routine protocol impossible. Despite these limitations, future research should explore the changes in melatonin levels in the first two weeks after injury and the longitudinal changes in individuals over time, in order to guide the timing of melatonin supplementation trials. To minimize confounding variables, studies in the acute period should have frequent melatonin measurements over a 24-h period, with artificial light conditions minimized overnight, with clear exclusion criteria that eliminate such factors as concomitant brain injury that may influence pineal melatonin synthesis and secretion.
There was also evidence that adults with complete cervical SCI have absent diurnal variation in melatonin profiles when compared to individuals with thoracolumbar SCI at T4 and below. No studies involved individuals with thoracic injuries from T1 to T3. The innervation of the pineal gland is believed to involve pre-ganglionic fibers originating from the intermediolateral cell column (IML) of the high thoracic spinal cord (T1–T4), which synapse on post-ganglionic fibers originating from the superior cervical ganglion.57 Although low thoracic and lumbar injuries may potentially spare neural connections between the supraventricular nucleus and more rostral nuclei of the IML, high thoracic injuries could potentially disrupt this pathway, affecting the pineal gland production of melatonin. Future research should include melatonin profiles in individuals with high thoracic cord injuries since they may behave similarly to those with complete tetraplegia.
There was limited information exploring the impact of melatonin supplementation on circadian rhythm and overnight melatonin levels in individuals with complete cervical SCI. Kostovski et al. demonstrated that supplementation induced overnight levels that mimicked melatonin rhythms seen in healthy controls, albeit with higher peak concentrations.51 In the studies by Spong et al. and Spong et al., there appeared to be individual variability in patient response to melatonin.47,50 Both studies were limited by a lack of melatonin measurements per patient, with only one to two measurements per 24-h period. Future supplementation studies should include enough measurement samples per patient to capture the rise and fall in melatonin levels to allow full evaluation of the individual pharmacokinetics of melatonin administration. By documenting the circadian rhythm before and after supplementation, researchers should be able to correlate clinical response with pharmacokinetics, to understand the individual factors, melatonin dose and timing of melatonin administration that are best associated with clinical outcomes.
Most studies revealed no clinical benefits from melatonin or Ramelteon administration in adults with complete cervical SCI. Of the three studies that evaluated sleep outcomes, only Spong et al. showed a change in sleep architecture, but this was only with respect to the duration of NREM1/NREM2 stages of sleep.47,48,50 Deeper and more restorative stages of sleep were unaffected. As a result, there is no evidence at this time to support melatonin administration for improving sleep outcomes in patients with complete cervical SCI. Preliminary data from individuals with pinealectomy have shown that standard measures of sleep polysomnography are unaffected by reduced or absent melatonin profiles, suggesting that melatonin secretion is not necessary for sleep.58 This review appears to corroborate that finding. However, melatonin supplementation is effective in advancing sleep-wake cycles in patients with delayed sleep phase disorder.59 As a result, melatonin, rather than being essential to circadian function, may be important for optimal function, particularly in situations where circadian rhythms are not entrained to day-length cycles. Although the studies in this review revealed no benefit on sleep-related outcomes, they were conducted several years after the initial injury. It is possible that after years of chronic pineal gland denervation, these individuals may have adapted their sleep cycles to reduced or absent night-time melatonin levels, potentially through changes in the output of the suprachiasmatic nucleus. As it has not been explored, supplementation trials should explore the impact on sleep in acute injuries. Ideally, these trials should rely on polysomnography rather than actigraphy or sleep-based questionnaires for outcome assessments. The reliance on questionnaires and wrist actigraphy likely affected the quality of evidence in this review. Questionnaires are prone to bias and wrist actigraphy may not be a valid tool for certain individuals with cervical SCI, as motor levels at C4 or above may have insufficient distal motor strength to facilitate accurate wrist actigraphy.60
At this time, there appears to be no benefits from melatonin supplementation on mood and quality of life subscales. Kostovski et al. found that exogenous melatonin can normalize the expression of several genes, including PER1 and PER2, two genes expressed in the suprachiasmatic nucleus that are involved in circadian rhythm.53,61 Melatonin also normalized the expression of BMAL1 and REV-ERB, two genes implicated in hypertension, diabetes, lipogenesis and adipogenesis.61,62 Abnormal melatonin production in complete cervical SCI, as shown by Kostvoski et al., may be associated with phase shifts in the circadian expression of genes important to cardiovascular health.53 Theoretically, this may predispose to adverse cardiovascular outcomes, which are more prevalent in the SCI population.20,23 Oral administration of melatonin in individuals with metabolic syndrome is associated with reduced inflammatory markers, and improved blood pressure and lipid profiles.20–22 Given the potential cardiovascular benefits of melatonin in non-SCI cohorts, future supplementation studies in adults with SCI should explore whether melatonin administration improves cardiac outcomes and whether this is related to normalized expression of genes implicated in cardiovascular circadian rhythms.
Relevant clinical parameters that have not been explored in SCI supplementation trials include pain and neurological outcomes. As summarized in the systematic review by Yang et al., melatonin supplementation in animal models of SCI is associated with improved biochemical and neurological recovery, enhanced antioxidant function, enhanced blood-brain barrier integrity, attenuated apoptosis, diminished edema and reduced ischemic damage.12 Although these neuroprotective effects seem promising, the results are limited by the fact that there are no human SCI studies that have explored the impact of melatonin on neurological recovery. As the mainstay of acute SCI treatment is minimization of secondary neurological injury, melatonin supplementation may represent an exciting avenue for minimizing the biochemical cascade responsible for secondary injury. This is worth considering given the innocuous nature of melatonin in clinical trials. Melatonin, through both spinal and supraspinal-mediated pathways, may also dampen peripheral and central sensitization to reduce the development of chronic pain.18 This effect does not appear to be related to its structural similarity to serotonin, as melatonin does not bind to 5-HT receptors.63 With the advent of new neuropathic pain medications that target melatonin receptors, it would be worthwhile to consider testing these agents in SCI individuals characterized by deficient melatonin profiles, especially given the increased prevalence of pain within the SCI population.28,29
Limitations
The difference in study design and measurement outcomes across the included articles made it difficult to incorporate a quantitative analysis into the results. The review is therefore limited to a narrative analysis. As well, conference proceeding and abstracts were eligible for inclusion, with two included for the purpose of this review.53,54 Although the data included in the abstracts was less robust than fully published articles – given the lack of details surrounding their study design – these abstracts were included to inform the scientific community about all relevant research in the domain of melatonin levels. Researchers of these abstracts were not contacted to solicit details surrounding the study designs.
Conclusion
There is evidence to suggest that complete cervical spinal cord injury is associated with reduced night-time melatonin values and absent circadian melatonin rhythms, as compared to healthy, age-matched controls. There is also evidence that these same individuals have absent rhythms when compared to adults with thoracolumbar SCI at T4 and below. There is limited evidence comparing levels with individuals with incomplete cervical SCI. There is no evidence describing melatonin levels acutely or longitudinally over multiple time points after complete cervical SCI. There is no apparent benefit from melatonin supplementation in improving sleep outcomes in individuals with complete cervical SCI of chronic duration. Melatonin supplementation trials in individuals with complete cervical SCI have only explored the clinical effect on sleep, with no trials documenting the impact on neurological recovery, pain scores and cardiovascular health. Given the proposed benefits of melatonin, future research should focus on these areas, while also characterizing melatonin levels acutely after injury, in order to optimize the timing of melatonin supplementation, as most trials have occurred several years after the initial injury, when the window of clinical benefits may be missed.
Supplementary Material
Acknowledgements
We would like to acknowledge Robin Parker, a librarian from the School of Information Management at Dalhousie University, for her help in creating the search strategy.
Disclaimer statements
Contributors None.
Funding None.
Declaration of interest None.
Conflicts of interest The authors report no conflict of interest.
References
- 1.Middleton B. Measurement of melatonin and 6-sulphatoxymelatonin. Methods Mol Biol 2006;324:235–54. [DOI] [PubMed] [Google Scholar]
- 2.Brzezinski A. Melatonin in humans. N Engl J Med 1997;336:186–95. [DOI] [PubMed] [Google Scholar]
- 3.Brown EN, Choe Y, Shanahan TL, Czeisler CA.. A mathematical model of diurnal variations in human plasma melatonin levels. Am J Physiol 1997;272(3 Pt 1):E506–16. [DOI] [PubMed] [Google Scholar]
- 4.Arendt J. Melatonin: characteristics, concerns, and prospects. J Biol Rhythms 2005;20(4):291–303. [DOI] [PubMed] [Google Scholar]
- 5.Claustrat B, Brun J, Chazot G.. The basic physiology and pathophysiology of melatonin. Sleep Med Rev 2005;9(1):11–24. [DOI] [PubMed] [Google Scholar]
- 6.Arendt J. Melatonin and human rhythms. Chronobiol Int 2006;23(1–2):21–37. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer M, Korf HW, Wicht H.. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen Comp Endocrinol 2018;258:215–21. [DOI] [PubMed] [Google Scholar]
- 8.Kaptanoglu E, Tuncel M, Palaoglu S, Konan A, Demirpençe E, Kilinç K.. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J Neurosurg 2000;93(1 Suppl):77–84. [DOI] [PubMed] [Google Scholar]
- 9.Cayli SR, Kocak A, Yilmaz U, Tekiner A, Erbil M, Ozturk Cet al. Effect of combined treatment with melatonin and methylprednisolone on neurological recovery after experimental spinal cord injury. Eur Spine J 2004;13(8):724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gül S, Celik SE, Kalayci M, Taşyürekli M, Cokar N, Bilge T.. Dose-dependent neuroprotective effects of melatonin on experimental spinal cord injury in rats. Surg Neurol 2005;64(4):355–61. [DOI] [PubMed] [Google Scholar]
- 11.Erten SF, Kocak A, Ozdemir I, Aydemir S, Colak A, Reeder BS.. Protective effect of melatonin on experimental spinal cord ischemia. Spinal Cord 2003;41(10):533–8. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Yao M, Lan Y, Mo W, Sun YL, Wang Jet al. Melatonin for spinal cord injury in animal models: a systematic review and network meta-analysis. J Neurotrauma 2016;33(3):290–300. [DOI] [PubMed] [Google Scholar]
- 13.Alluri H, Wilson RL, Anasooya Shaji C, Wiggins-Dohlvik K, Patel S, Liu Yet al. Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. PLoS One 2016;11(5):e0154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Z, Zhou Z, Gao S, Guo Y, Gao K, Wang Het al. Melatonin inhibits neural cell apoptosis and promotes locomotor recovery via activation of the Wnt/β-Catenin signaling pathway after spinal cord injury. Neurochem Res 2017;42(8):2336–43. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Chen X, Qiao S, Liu X, Liu C, Zhu Det al. Melatonin lowers edema after spinal cord injury. Neural Regen Res 2014;9(24):2205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aydemir S, Dogan D, Kocak A, Dilsiz N.. The effect of melatonin on spinal cord after ischemia in rats. Spinal Cord 2016;54(5):360–3. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I.. Analgesic effects of melatonin: a review of current evidence from experimental and clinical studies. J Pineal Res 2011;51(3):270–7. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan V, Ho KY, Castroviejo DA, Zakaria R, Brzezinski A, Lauterbach EC.. Melatonin and pain: therapeutic applications. In: Venkataramanujam S, Brzezinski A, Oter S, Shillcut SD, (eds.), Melatonin and Melatonergic Drugs in Clinical Practice. New York: Springer Press; 2014. p. 221–34. [Google Scholar]
- 19.Gonçalves AL, Martini Ferreira A, Ribeiro RT, Zukerman E, Cipolla-Neto J, Peres MF.. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry 2016;87(10):1127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nduhirabandi F, Lochner A.. Melatonin and the metabolic syndrome. In: Venkataramanujam S, Brzezinski A, Oter S, Shillcut SD (eds.), Melatonin and Melatonergic Drugs in Clinical Practice. New York: Springer Press. 2014. p. 71–95. [Google Scholar]
- 21.Mesri Alamdari N, Mahdavi R, Roshanravan N, Lotfi Yaghin N, Ostadrahimi AR, Faramarzi E.. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm Metab Res 2015;47(7):504–8. [DOI] [PubMed] [Google Scholar]
- 22.Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M.. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res 2011;50(3):261–6. [DOI] [PubMed] [Google Scholar]
- 23.Myers J, Lee M, Kiratli J.. Cardiovascular disease in spinal cord injury. Am J Phys Med Rehabil 2007;86(2):142–52. [DOI] [PubMed] [Google Scholar]
- 24.Citera G, Arias MA, Maldonado-Cocco JA, Lazaro MA, Rosemfett MG, Brusco LIet al. The effect of melatonin in patients with fibromyalgia: a pilot study. Clin Rheumatol 2000;19:9–13. [DOI] [PubMed] [Google Scholar]
- 25.Hussain SA, Al-Khalifa H, Jasim NA, Gorial FI.. Adjuvant use of melatonin for treatment of fibromyalgia. J Pineal Res 2011;50:267–71. [DOI] [PubMed] [Google Scholar]
- 26.Brun J, Claustrat B, Saddier P, Chazot G.. Nocturnal melatonin excretion is decreased in patients with migraine without aura attacks associated with menses. Cephalalgia 1995;15:136–9. [DOI] [PubMed] [Google Scholar]
- 27.Masruha MR, de Souza Viera DS, Minett TS, Cipolla-Neto J, Zuckerman E, Vilanova LCet al. Low urinary 6-sulphatoxy-melatonin concentrations in acute migraine. J Headache Pain 2008;9:221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabeeva GR, Sergeev AV, Gromova SA.. Possibilities of preventive treatment of migraine with the MT1- and MT2 agonist and 5-HT2с receptor antagonist agomelatin (valdoxan). Zh Nevrol Psikhiatr Im S S Korsakova 2011;111(9):32–6. [PubMed] [Google Scholar]
- 29.Modirian E, Pirouzi P, Soroush M, Karbalaei-Esmaeili S, Shojaei H, Zamani H.. Chronic pain after spinal cord injury: results of a long-term study. Pain Med 2010;11(7):1037–43. [DOI] [PubMed] [Google Scholar]
- 30.Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J.. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int 2015;32(9):1294–310. [DOI] [PubMed] [Google Scholar]
- 31.Stoschitsky K, Sakotnik A, Lercher P, Zweiker R, Maier R, Liebmann Pet al. Influence of beta-blockers on melatonin release. Eur J Clin Pharmacol 1999;55:111–5. [DOI] [PubMed] [Google Scholar]
- 32.Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl Wet al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med 2002;30(3):536–40. [DOI] [PubMed] [Google Scholar]
- 33.Monteleone P, Forziati D, Orazzo C, Maj M.. Preliminary observations on the suppression of nocturnal plasma melatonin levels by short-term administration of diazepam in humans. J Pineal Res 1989;6:253–8. [DOI] [PubMed] [Google Scholar]
- 34.Ursing C, von Bahr C, Brismar K, Rojdmark S.. Influence of cigarette smoking on melatonin levels in man. Eur J Clin Pharmacol 2005;61:197–201. [DOI] [PubMed] [Google Scholar]
- 35.Karkela J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M.. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand 2002;46:30–6. [DOI] [PubMed] [Google Scholar]
- 36.Seifman MA, Gomes K, Nguyen PN, Bailey M, Roseneld JV, Cooper DJet al. Measurement of serum melatonin in intensive care unit patients: changes in traumatic brain injury, trauma and medical conditions. Front Neurol 2014;5:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grima NA, Ponsford JL, St. Hilaire MA, Mansfield D, Rajaratnam SM.. Circadian melatonin rhythm following traumatic brain injury. Neurorehabil Neural Repair 2016;30(10):972–7. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Jiang DH, Wang ML, Jiao DR, Pang SF.. Rhythms of serum melatonin in patients with spinal lesions at the cervical, thoracic or lumbar region. Clin Endocrinol (Oxf) 1989;30(1):47–56. [DOI] [PubMed] [Google Scholar]
- 39.Kneisley LW, Moskowitz MA, Lynch HG.. Cervical spinal cord lesions disrupt the rhythm in human melatonin excretion. J Neural Transm Suppl 1978;13:311–23. [PubMed] [Google Scholar]
- 40.Van Papendorp DH, Theron JJ, Viljoen M, Kruger MC, Steyn ME.. Plasma melatonin levels in patients with spinal cord lesions. Med Sci Res 1995;23(3):189–90. [Google Scholar]
- 41.Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA.. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. J Clin Endocrinol Metab 2000;85(6):2189–96. [DOI] [PubMed] [Google Scholar]
- 42.Alves de Almeida E, Di Mascio P, Harumi T, Spence DW, Moscovitch A, Hardeland Ret al. Measurement of melatonin in body fluids: standards, protocols and procedures. Childs Nerv Syst 2011;27:879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benloucif S, Burgess HJ, Klerman EB, Lewy AF, Middleton B, Murphy PJet al. Measuring melatonin in humans. J Clin Sleep Med 2008;4(1):66–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Joanna Briggs Institute . JBI critical appraisal – checklist for analytical cross-sectional studies. 2017. Available from http://joannabriggs.org/assets/docs/critical-appraisal-tools/JBI_Critical_Appraisal-Checklist_for_Analytical_Cross_Sectional_Studies2017.pdf.
- 45.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman ADet al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, Santaguida PLet al. Individual studies in systematic reviews of health care interventions. In: Agency for healthcare research and quality, methods guide for comparative effectiveness reviews. March 2012. AHRQ Publication No. 12-EHC047-EF. Available from www.effectivehealthcare.ahrq.gov/. [PubMed]
- 47.Spong J, Kennedy GA, Tseng J, Brown DJ, Armstrong S, Berlowitz DJ.. Sleep disruption in tetraplegia: a randomised, double-blind, placebo-controlled crossover trial of 3 mg melatonin. Spinal Cord 2014;52(8):629–34. [DOI] [PubMed] [Google Scholar]
- 48.Zeitzer JM, Ku B, Ota D, Kiratli BJ.. Randomized controlled trial of pharmacological replacement of melatonin for sleep disruption in individuals with tetraplegia. J Spinal Cord Med 2014;37(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verheggen R, Jones H, Nyakayiru J, Thompson A, Groothuis JT, Atkinson Get al. Complete absence of evening melatonin increase in tetraplegics. FASEB J 2012;26:3059–64. [DOI] [PubMed] [Google Scholar]
- 50.Spong J, Kennedy GA, Brown DJ, Armstrong SM, Berlowitz DJ.. Melatonin supplementation in patients with complete tetraplegia and poor sleep. Sleep Disord 2013;2013:Article ID 128197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kostovski E, Dahm AEA, Mowinckel MC, Stranda A, Skretting G, Osterud Bet al. Circadian rhythms of hemostatic factors in tetraplegia: a double-blind, randomized, placebo-controlled cross-over study of melatonin. Spinal Cord 2015;53:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fatima G, Sharma VP, Verma NS.. Circadian variations in melatonin and cortisol in patients with cervical spinal cord injury. Spinal Cord 2016;54:364–7. [DOI] [PubMed] [Google Scholar]
- 53.Kostovski E, Frigato E, Dahm A, Skretting GA, Mowinkel MC, Sandset PMet al. Melatonin modified peripheral blood cell oscillators in humans. FASEB J 2017;31(1 Suppl):614.3. [Google Scholar]
- 54.Vaughan SE, Badr MS, Kruppe E, Abbas HK, Mukkavilli V, Sankari A.. Endogenous night time melatonin is preserved in patients with chronic spinal cord injury. Am J Respir Crit Care Med 2017;195:A4536. [Google Scholar]
- 55.Hofstra WA, de Weerd AW.. How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav 2008;13(3):438–44. [DOI] [PubMed] [Google Scholar]
- 56.Duffy JF, Dijk DJ.. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms 2002;17(1):4–13. [DOI] [PubMed] [Google Scholar]
- 57.Moore RY. Neural control of the pineal gland. Behav Brain Res 1996;73(1–2):125–30. [DOI] [PubMed] [Google Scholar]
- 58.Slawik H, Stoffel M, Riedl L, Veselý Z, Behr M, Lehmberg J, et al. Prospective study on salivary evening melatonin and sleep before and after pinealectomy in humans. J Biol Rhythms 2016;31(1):82–93. [DOI] [PubMed] [Google Scholar]
- 59.Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC.. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep 2005;28(10):1271–8. [DOI] [PubMed] [Google Scholar]
- 60.Spivak E, Oksenberg A, Catz A.. The feasibility of sleep assessment by actigraph in patients with tetraplegia. Spinal Cord 2007;45(12):765–70. [DOI] [PubMed] [Google Scholar]
- 61.Angelousi A, Kassi E, Nasiri-Ansari N, Weickert MO, Randeva H, Kaltsas G.. Clock genes alterations and endocrine disorders. Eur J Clin Invest 2018;48: e12927. [DOI] [PubMed] [Google Scholar]
- 62.Kojetin DJ, Burris TP.. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov 2014;13(3):197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamal M, Gbahou F, Guillaume JL, Daulat AM, Benleulmi-Chaachoua A, Luka M, et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J Biol Chem 2015;290(18):11537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.