ABSTRACT
Acinetobacter baumannii emerged as one of the most important pathogens that causes nosocomial infections due to its increased multidrug resistance. Identifying capsular epidemiology in A. baumannii can aid in the development of effective treatments and preventive measures against this emerging pathogen. Here we established a wzc-based method, and combined it with wzy-PCR to determine capsular types of A. baumannii causing nosocomial bacteraemia collected at two medical centres in Taiwan from 2015 to 2017. Among the 237 patients with A. baumannii bacteraemia, 98 (41.4%) isolates were resistant to carbapenems. Four prevalent capsular types (KL2, KL10, KL22, and KL52) accounted for 84.7% of carbapenem-resistant A. baumannii (CRAB) and 12.2% of non-CRAB. The rate of pneumonia, intensive care unit admission, APACHE II score, and Pitt bacteraemia score were higher in patients with KL2/10/22/52 infection than in those with non-KL2/10/22/52 infection. Patients with KL2/10/22/52 infection and patients with CRAB infection have a higher cumulative incidence of attributable and all-cause in-hospital 30-day mortality. On multivariate analysis, appropriate empirical antimicrobial therapy within 24 h was associated with a lower risk of 30-day attributable mortality in the KL2/10/22/52 isolates (odds ratio = 0.19, 95% CI: 0.06–0.66, p = 0.008) but not in non-KL2/10/22/52 isolates. Early recognition of carbapenem resistance-associated capsular types may help clinicians to promptly implement appropriate antimicrobial therapy for improving the outcomes in patients with CRAB bacteraemia.
KEYWORDS: Acinetobacter baumannii, capsular type, serotype, typing system, carbapenem resistance
Introduction
Acinetobacter baumannii has emerged as a predominant cause of nosocomial infections in the last decade. It poses characteristics of environmental persistence, resistance to dryness, and evasion of host immunity, which makes it a major threat in health-care facilities, particularly among immunocompromised patients [1]. Studies have documented that mortality associated with gram-negative bacteraemia was significantly increased in A. baumannii infections compared to other gram-negative bacilli [2,3]. It has emerged as one of the most troublesome gram-negative bacteria worldwide due to its high level of antibiotic resistance, with some exhibiting resistance to most clinically available antibiotics [4]. The spread of multidrug-resistant A. baumannii (MDRAB), especially carbapenem-resistant A. baumannii (CRAB) in intensive care units (ICU), has been growing globally [5]. More than 50% of A. baumannii isolates from US ICU during 2009–2012 were resistant to carbapenem and all other antibiotics except colistin or tigecycline [6]; the rate of CRAB has reached 70.2% in 2018, observed in Taiwan Nosocomial Infection Surveillance (TNIS) system supported by Centers for Disease Control (CDC), Taiwan [7]. Crude mortality for CRAB infections ranged from 16 to 76% [8]. A systemic review suggested that infection with CRAB may be associated with a two-fold increase in the risk of mortality compared to carbapenem susceptible A. baumannii [8]. Therefore, the World Health Organization declared CRAB as a top priority pathogen, which desperately needed the development of active antimicrobials [9].
Capsular polysaccharide (CPS) is a critical virulence factor for A. baumannii [10]. The layer of CPS makes A. baumannii more resistant to external stresses such as complement-mediated killing and certain antibiotics, and contributes to its ability to survive in the hospital environment for long periods [10,11,12]. Functional studies also revealed the importance of CPS during A. baumannii infection and growth in serum [10,13]. Capsular serotype-specific antibody against A. baumannii increased neutrophil opsonopagocytosis in vitro and increased bacterial clearance in vivo [14]. It is biologically plausible that capsule of A. baumannii is an adequate preventative and therapeutic target for the development of novel control strategies for A. baumannii infection. However, available capsular typing methods for A. baumannii rely on complete sequences of ∼20 kb cps region and thus is difficult for comprehensively serotyping multiple infecting strains.
A wzc genotyping method was reported in Klebsiella pneumoniae [15], which permits detection of capsular types (K-types) of K. pneumoniae, including the ones where cps sequences are unavailable; moreover, only one round of sequencing of PCR products is needed, and has been used for identifying K-types of K. pneumoniae in different studies [16,17,18]. Here we establish a wzc-based method for A. baumannii, and combined it with wzy-PCR to determine K-types of A. baumannii causing nosocomial bacteraemia. To our knowledge, this is the first study to screen K-types in large numbers of clinical isolate of A. baumannii without whole genome sequencing, and discovered the prevalent K-types in CRAB.
Materials and methods
Study population
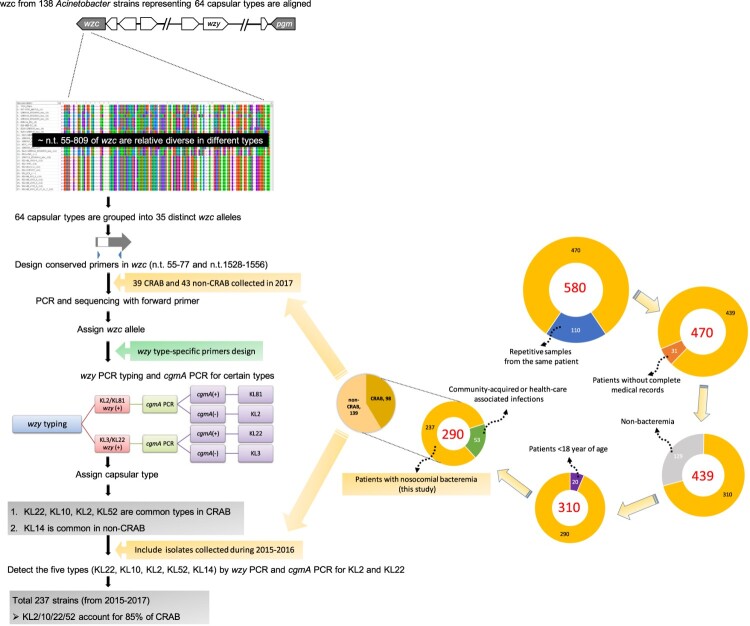
This study was conducted at Chang Gung Memorial Hospital (CGMH)-Lin Kou branch, a 3700-bed medical centre in northern Taiwan, and CGMH-Kaohsiung branch, a 2700-bed medical center in southern Taiwan, from January 2015 to December 2017. Nosocomial A. baumannii bacteraemia was defined by the presence of ≥1 positive blood culture results for patients with sign and symptoms of infection, which occurred >48 h after hospital admission. For patients with multiple episodes of bacteraemia, only the first episode was included. Patients<18 years of age or with incomplete medical records were excluded. This protocol was approved by the institutional review board of CGMH (Approval number: 201801433B0). The iterative process of sample collection was described as follows. A total of 580 samples were collected, repetitive samples from the same patient (n = 110) were excluded, patients without complete medical records (n = 31) were excluded, non-bacteraemia samples (n = 129) were excluded, patients <18 year of age (n = 20) were excluded, and community-acquired or health-care associated infection (n = 53) were excluded. Finally, a total of 237 samples from patients with nosocomial bacteraemia were analysed in this study (Figure 1 and supplementary file).
Figure 1.
. Flow diagram of capsular typing using wzc-based method followed by wzy-PCR genotyping and samples collection.
Microbiological studies
Identification of A. baumannii was performed using a matrix-assisted laser desorption-time of flight mass spectrometry (MALDI-TOF-MS) [19]. Susceptibility to all tested antibiotics except tigecycline [20] was determined according to the Clinical and Laboratory Standards Institute (CLSI) interpretive criteria for the disk diffusion method [21]. Minimum inhibitory concentration (MIC) of imipenem and meropenem was further determined using the broth dilution method [21], respectively. Carbapenem resistance was defined as MICs of >4 μg/ml for both imipenem/ meropenem.
K-typing
Owing to the successful K-typing of Klebsiella on the wzc gene [15], and because Wzc is believed to function as a tyrosine kinase, which interacts with the outer membrane protein Wza and phosphatase Wzb to form a trans-envelope capsule translocation complex, it is thus reasonable that Wzc domains might interact with type-specific capsular polysaccharides during the process of translocation. Therefore, we analysed the sequences of wzc genes from 138 Acinetobacter strains representing 64 K-types (see Supplementary Table 1; 5 non-baumannii strains described in a previous study analysing Acinetobacter cps [22] were also included because different Acinetobacter spp. may share the same capsule structure). Two nomenclature systems for capsule biosynthesis locus, KL(K) types [23], and PSgc types [22], have been used in different reports. KL type and corresponding PSgc types were given in Supplementary Table 1. Since KL has been widely adopted, we use KL nomenclature in the text unless KL type was unavailable.
The results showed that ∼n.t.55 to ∼n.t.809 of wzc genes were relatively diverse in different types but conserved in strains with the same type. The wzc sequences are provided in the supplementary file. Most of the strains with the same type showed ≧99% DNA identity with some exceptions. KL2 and Psgc63 showed 97% identity with their same K-type strains and four KL10 strains were clustered into two groups (TYTH_1 and NCGM_237) showed 98% DNA identity, and BAL_030 and XH857 showed 100% DNA identity, and the DNA identity between the two groups is 91% (Supplementary Table 2). On the other hand, most of the strains with different K-types showed <95% DNA identity with the exception of some K-types sharing relative high similarity with each other (Supplementary Table 3). We assigned the sequences given ≧95% DNA identity as an allele. A total of 64 K-types were grouped into 35 distinct alleles (Supplementary Table 3). We further designed conserved primers AB_wzcF1 and AB_wzcR5-plus located at n.t. 55–77 and n.t. 1528–1556, respectively. After wzc PCR was conducted, the expected PCR products were subjected to Sanger sequencing with AB_wzcF1. The sequences were compared to the 138 wzc sequences in our wzc panel (sequences are provided in the supplementary file). wzy PCR was further conducted to confirm the K-types with type-specific wzy primers (Supplementary Table 4), starting with the corresponding wzc type with highest DNA identify. If wzy PCR was negative for the most likely type (the type with highest identity), the second possible type would be tested. The iterative PCR process was continued to assign a wzy type for the strain. For certain K-types share the same wzy, further examination relies on the difference in other cps genes is needed to identify the K-types. For example, KL2 and KL81 share a wzy gene with three nucleotide differences and thus were further distinguished by cgmA PCR (cgmA was present in KL81 but not in KL2). KL3 and KL22 share a wzy with only one nucleotide difference, and thus were further distinguished by cgmA PCR (cgmA was present in KL22 but not in KL3).
Data collection and definitions
Medical records were reviewed to collect clinical information, including demographic characteristics; comorbid conditions, hospital stays; ICU stays; time of antimicrobial therapy; and the presence of a ventilator, central venous catheters, or a foley catheter at the time of bacteraemia onset. Hospital stays were defined from the date of bacteria sample collection to date of discharge. Comorbidity at diagnosis was classified using the Charlson comorbidity index. The severity of illness was assessed using Acute Physiology and Chronic Health Evaluation II (APACHE II) score [24] in ICU patients and the Pitt bacteraemia score [25] in all patients. Immunosuppressive therapy was defined as receipt of cytotoxic agents, or other immunosuppressive agents within 6 weeks, or corticosteroids at a dosage equivalent to or higher than 20 mg of prednisolone daily for 2 weeks or 30 mg of prednisolone daily for at least 1 week before bacteraemia onset. The primary infection source of bacteraemia was determined according to the definitions of the Centers for Disease Control and Prevention [26]. If no infectious focus was identified, the bacteraemia was counted as primary. Polymicrobial bacteraemia was defined as isolation of ≥1 microorganisms other than A. baumannii from blood during the same bacteraemic episode. Antimicrobial therapy was considered appropriate if the drugs used at therapeutic doses had in vitro activity against the strain isolated after the onset of bacteraemia [27,28](we use “appropriate empirical antimicrobial therapy” in this study). Antimicrobial therapy that did not meet this definition was considered inappropriate. Data on 14-day and 30-day mortality and all-cause in-hospital mortalities were recorded. Attributable mortality (bacteraemia-related death) was defined as death before resolution of symptoms and signs of bacteraemia and at least one blood culture positive for A. baumannii.
Statistical analysis
We examined whether the distribution of demographic characteristics underlying diseases, sources of infection, and clinical characteristics differed in terms of A. baumannii K-types. The analysis of variance (ANOVA) tests was performed for continuous variables and Fisher’s exact tests were performed for categorical variables. We used the Kaplan-Meier survival curves to display the cumulative probabilities of all-cause mortality and attributable mortality within 30 days, and used a log-rank test to compare the difference in survival functions between A. baumannii K-type and carbapenem resistance.
With adjustment for potential confounders, we used multivariate regression models to estimate the effects of A. baumannii K-type for four clinical outcomes; logistic regression models were performed for 30-day attributable mortality, 30-day all-cause mortality, and carbapenem resistance, and linear regression model was performed for Pitts score. We performed different models with varied adjustments to evaluate the influence of A. baumannii K-type after adjusting different covariates. To further explore the interactive effects between A. baumannii K-type, carbapenem resistance, and appropriate empirical antimicrobial therapy on 30-day attributable mortality, stratified analysis based on A. baumannii K-type or carbapenem resistance were carried out. All statistical analyses were conducted with the SAS 9.4. A p-value of < 0.05 was considered statistically significant.
Results
Antibiotic susceptibility
A total of 237 non-redundant A. baumannii strains causing nosocomial bacteraemia were collected from the Linkou CGMH (156 strains) and Kaohsiung CGMH (81 strains) from 2015 to 2017. Out of these strains, 41% (n = 98) were defined as carbapenem-resistant. The meropenem MIC50 and MIC90 of CRAB were both >32 μg/mL, and the imipenem MIC50 and MIC90 were 32 μg/mL and >32 μg/mL, respectively. All isolates were susceptible to colistin. The non-susceptibility rates were 41% to amikacin, 49% to ceftazidime, 48% to ciprofloxacin, 47% to cefepime, 49% to gentamicin, 44% to ampicillin-sulbactam, 49% to tazobactam, and 15% to tigecycline.
K-typing of A. baumannii
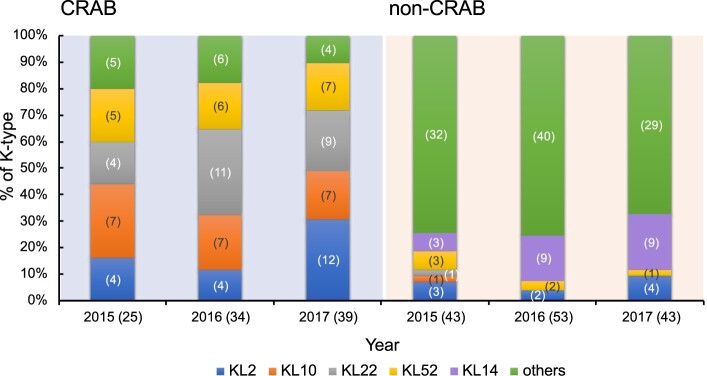
Wzc typing were conducted in 39 CRAB and 43 non-CRAB collected in 2017 (Figure 1). Sequences of 39 CRAB strains showed ≧97% DNA identity with 7 different wzc alleles and those of the 43 non-CRAB strains showed 87%-100% DNA identity with 16 wzc alleles (Supplementary Table 5). Based on wzc typing results, K-types of these strains were further determined by wzy-PCR with type-specific wzy primers. The distribution of KL types in 2017 is shown in (Figure 2). The four types KL2/10/22/52 account for 89.7% CRAB. For 43 non-CRAB, 28 strains were assigned to 12 different K-types; 15 strains were unknown (i.e. wzy-PCR was negative for possible wzc types and these strains were also confirmed to be non-KL2/10/22/52 type). In contrast to CRAB, type KL2/10/22/52 only account for 11.6% non-CRAB and the most prevalent type is KL14 (20.9%). We then tested strains from 2015to 2016 with wzy primers to determine the prevalence of the five types (KL2, KL10, KL14, KL22, and KL52) (Figure 2). In total, the four major KL2/10/22/52 types were predominant in CRAB, but not non-CRAB (85% vs. 12%) during the study period. Notably, the number of KL2 apparently increased in CRAB (from 16% in 2015 to 30.8% in 2017) but not in non-CRAB (7.0% in 2015 and 9.3% in 2017).
Figure 2.
The distribution of Acinetobacter baumannii capsular types with or without carbapenem resistance during 2015–2017. Number of isolates were shown in parentheses.
The distribution of demographic and clinical factors by A. baumannii capsular type
The demographic characteristics, underlying diseases, sources of infection, and clinical characteristics of patients with A. baumannii bacteraemia are shown in Table 1. There were no significant differences among patients with different K-types with respect to age, sex, and the Charlson scores. However, patients with the four major types of KL2/10/22/52 bacteraemia had more frequent chronic renal insufficiency and had less metastatic tumour than did patients with non-KL2/10/22/52 bacteraemia. The source of bacteraemia being pneumonia, ICU admission rate, ICU stays, APACHE II score, and Pitt bacteraemia score were significantly higher in the KL2/10/22/52 isolates. Besides, the KL2/10/22/52 isolates showed more carbapenem resistance. There were lower rates of appropriate empirical antimicrobial therapy within 24 h among patients with the KL2/10/22/52 isolates than there were among those with non- KL2/10/22/52 isolates.
Table 1.
Demographic characteristics, underlying diseases, sources of infection, clinical characteristics of patients with Acinetobacter baumannii Bacteraemia.
| KL type | p* for comparing KL 2, 10, 22, 52, and other | p* for comparing total number of KL2/10/22/52 and other | |||||
|---|---|---|---|---|---|---|---|
| 2 (n = 29) | 10 (n = 22) | 22 (n = 25) | 52 (n = 24) | Other (n = 137) | |||
| Male, number (%) | 19 (65.5) | 16 (72.7) | 13 (52.0) | 16 (66.7) | 77 (56.2) | 0.46 | 0.28 |
| Age (years), mean (SD) | 68.0 (12.6) | 58.5 (13.7) | 65.9 (13.1) | 60.3 (16.2) | 62.8 (13.9) | 0.09 | 0.68 |
| Charlson score, mean (SD) | 4.5 (2.8) | 5.2 (3.4) | 4.3 (2.2) | 4.0 (2.2) | 5.0 (2.5) | 0.31 | 0.15 |
| Underlying conditions, number (%) | |||||||
| DM with end organ disease | 1 (3.5) | 3 (13.6) | 5 (20.0) | 5 (20.8) | 10 (7.3) | 0.05 | 0.13 |
| Liver cirrhosis | 8 (27.6) | 4 (18.2) | 4 (16.0) | 3 (12.5) | 21 (15.3) | 0.57 | 0.49 |
| Hypertension | 12 (41.4) | 14 (63.6) | 10 (40.0) | 11 (45.8) | 55 (40.2) | 0.35 | 0.35 |
| Coronary artery disease | 1 (3.5) | 5 (22.7) | 2 (8.0) | 1 (4.2) | 13 (9.5) | 0.21 | 0.99 |
| Congestive heart failure | 0 (0.0) | 3 (13.6) | 2 (8.0) | 5 (20.8) | 14 (10.2) | 0.1 | 0.99 |
| Chronic renal insufficiency | 4 (13.8) | 11 (50.0) | 10 (40.0) | 11 (45.8) | 28 (20.4) | 0.001 | 0.01 |
| Chronic obstructive pulmonary disease | 6 (20.7) | 2 (9.1) | 5 (20.0) | 2 (8.3) | 11 (8.0) | 0.16 | 0.1 |
| Autoimmune disease | 2 (6.9) | 1 (4.6) | 2 (8.0) | 1 (4.2) | 2 (1.5) | 0.11 | 0.07 |
| Tumour with metastases | 3 (10.3) | 6 (27.3) | 2 (8.0) | 2 (8.3) | 49 (35.8) | 0.0006 | <0.0001 |
| Leukemia | 1 (3.5) | 1 (4.6) | 1 (4.0) | 1 (4.2) | 9 (6.6) | 0.99 | 0.57 |
| Lymphoma | 1 (3.5) | 1 (4.6) | 0 (0.0) | 1 (4.2) | 0 (0.0) | 0.06 | 0.07 |
| Solid malignancy | 11 (37.9) | 9 (40.9) | 8 (32.0) | 3 (12.5) | 61 (44.5) | 0.04 | 0.04 |
| Use of immunosuppressive agent, number (%) | 4 (13.8) | 9 (40.9) | 7 (28.0) | 5 (20.8) | 43 (31.4) | 0.2 | 0.31 |
| Source of bacteraemia, number (%) | 2 (6.9) | 1 (4.6) | 1 (4.0) | 2 (8.3) | 19 (13.9) | 0.55 | 0.06 |
| Primary bacteraemia | |||||||
| Pneumonia | 19 (65.5) | 13 (59.1) | 16 (64.0) | 12 (50.0) | 37 (27.0) | <0.0001 | <0.0001 |
| Ventilator-associated pneumonia | 16 (55.2) | 10 (45.5) | 14 (56.0) | 8 (33.3) | 7 (5.1) | <0.0001 | <0.0001 |
| Central venous catheter (CLABSI) | 13 (44.8) | 9 (40.9) | 3 (12.0) | 6 (25.0) | 50 (36.5) | 0.06 | 0.41 |
| Intra-abdominal infection | 1 (3.5) | 1 (4.6) | 1 (4.0) | 3 (12.5) | 19 (13.9) | 0.35 | 0.06 |
| Surgical site infection | 3 (10.3) | 2 (9.1) | 2 (8.0) | 1 (4.2) | 6 (4.4) | 0.5 | 0.27 |
| Urinary tract infection | 3 (10.3) | 2 (9.1) | 2 (8.0) | 2 (8.3) | 11 (8.0) | 0.99 | 0.82 |
| Foley’s catheter | 2 (6.9) | 2 (9.1) | 0 (0.0) | 1 (4.2) | 2 (1.5) | 0.09 | 0.14 |
| Polymicrobial bacteraemia, number (%) | 11 (37.9) | 6 (27.3) | 7 (28.0) | 10 (41.7) | 56 (40.9) | 0.62 | 0.34 |
| Carbapenem resistant, number (%) | 20 (69.0) | 21 (95.5) | 24 (96.0) | 18 (75.0) | 15 (11.0) | <0.0001 | <0.0001 |
| Appropriate empirical antimicrobial therapya within 24 h, number (%) | 9 (31.0) | 7 (31.8) | 3 (12.0) | 11 (45.8) | 83 (60.6) | <0.0001 | <0.0001 |
| ICU, number (%) | 13 (44.8) | 14 (63.6) | 12 (48.0) | 15 (62.5) | 30 (21.9) | <0.0001 | <0.0001 |
| ICU stay days, mean (SD) | 36.8 (21.4) | 42.8 (50.9) | 25.0 (17.6) | 24.8 (26.2) | 16.2 (18.4) | 0.04 | 0.01 |
| APACHE II score (if ICU = y), mean (SD) | 25.0 (5.5) | 25.0 (6.0) | 24.8 (9.1) | 24.9 (9.0) | 19.6 (8.8) | 0.08 | 0.004 |
| Pitt score, mean (SD) | 3.6 (3.6) | 4.9 (4.0) | 4.8 (3.9) | 4.5 (3.5) | 2.4 (3.2) | 0.0003 | <0.0001 |
| Hospital stay days, mean (SD) | 22.0 (22.0) | 27.3 (36.1) | 15.4 (28.3) | 25.5 (29.3) | 21.2 (25.5) | 0.58 | 0.74 |
| All cause in hospital mortality, number (%) | 15 (51.7) | 14 (63.6) | 22 (88.0) | 14 (58.3) | 45 (32.9) | <0.0001 | <0.0001 |
| Within 14 days | 10 (34.5) | 8 (36.4) | 15 (60.0) | 9 (37.5) | 31 (22.6) | 0.005 | 0.002 |
| Within 30 days | 13 (44.8) | 10 (45.5) | 21 (84.0) | 10 (41.7) | 38 (27.7) | <0.0001 | <0.0001 |
| Attributable mortality, number (%) | 9 (31.0) | 10 (45.5) | 19 (76.0) | 10 (41.7) | 33 (24.1) | <0.0001 | 0.0002 |
| Within 14 days | 8 (27.6) | 9 (40.9) | 14 (56.0) | 8 (33.3) | 30 (21.9) | 0.009 | 0.006 |
| Within 30 days | 9 (31.0) | 10 (45.5) | 18 (72.0) | 8 (33.3) | 33 (24.1) | 0.0001 | 0.0008 |
Note: DM: diabetes mellitus, ICU: intensive care unit, APACHE: acute physiology and chronic health evaluation, CLABSI, central line associated blood stream infection.
appropriate empirical antimicrobial therapy within 24 h was defined by in vitro susceptibility test.
*ANOVA test or Fisher exact test.
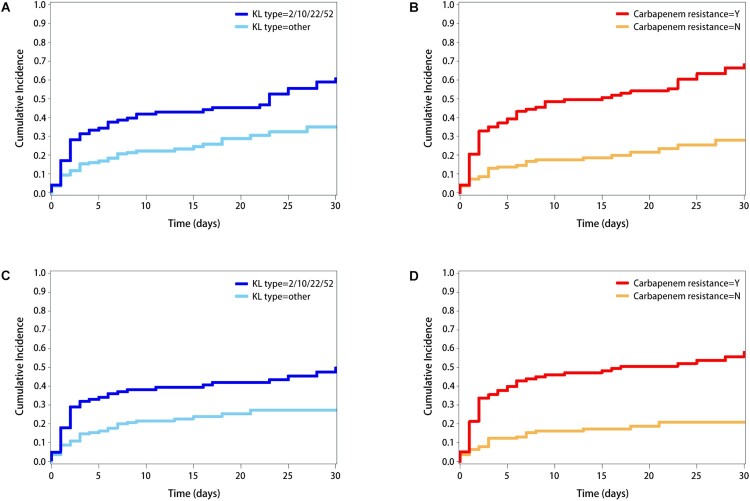
Both attributable and all-cause in-hospital mortality were higher for those with the KL2/10/22/52 isolates. Kaplen–Meier survival curves were shown in Figure 3. The patients infected with the major four types of KL2/10/22/52 and patients with carbapenem resistance had a higher cumulative incidence of attributable and all-cause in-hospital mortality in 30 days (all p < 0.001 for log-rank tests).
Figure 3.
Time to occurrence of all-cause mortality within 30 days in (A) Four major K-type KL2/10/22/52 v.s. other types (B) Carbapenem resistance, Yes v.s. No. Time to occurrence of attributable mortality within 30 days in (C) Four major K-type KL2/10/22/52 v.s. other types (D) Carbapenem resistance, Yes v.s. No.
Association between K-type and outcomes
With adjustment for sex, age, charlson score, and ICU, the patients infected with the KL2/10/22/52 isolates had a higher risk of 30-days attributable and all-cause mortality and a higher Pitt score (Table 2). However, the risk effect disappeared after further adjusting pneumonia, appropriate empirical antimicrobial therapy, and carbapenem resistance. The KL2/10/22/52 isolates were strongly associated with carbapenem resistance regardless of adjustments.
Table 2.
Association between capsular type and outcomes.
| 30-days Attributable Mortality | 30-days all-cause Mortality | Carbapenem resistance | Pitt score | |||||
|---|---|---|---|---|---|---|---|---|
| Model: adjustment | OR (95% CI)* | p | OR (95% CI)* | p | OR (95% CI)* | p | beta (95% CI)# | p |
| M0: Crude model | 2.58 (1.48–4.49) | 0.0008 | 3.06 (1.78–5.26) | <0.0001 | 39.71 (18.79–83.92) | <0.0001 | 2.01 (1.12, 2.90) | <0.0001 |
| M1: sex, age, Charlson score, ICU | 2.26 (1.25–4.09) | 0.007 | 2.60 (1.46–4.65) | 0.001 | 38.93 (16.90–89.70) | <0.0001 | 1.62 (0.68, 2.57) | 0.0008 |
| M2: M1, Pneumonia | 1.60 (0.84–3.04) | 0.15 | 1.88 (1.01–3.51) | 0.05 | 35.93 (14.90–86.68) | <0.0001 | 0.97 (0.03, 1.91) | 0.04 |
| M3: M2, Appropriate empirical antimicrobial therapy a within 24 h | 1.30 (0.66–2.56) | 0.45 | 1.80 (0.93–3.48) | 0.08 | 28.26 (11.54–69.21) | <0.0001 | 0.92 (−0.07, 1.92) | 0.07 |
| M4: M3, Carbapenem resistance | 0.47 (0.17–1.24) | 0.13 | 0.64 (0.25–1.63) | 0.35 | 0.26 (−0.97, 1.50) | 0.68 | ||
Note: ICU: intensive care unit.
appropriate empirical antimicrobial therapy within 24 h was defined by in vitro susceptibility test.
*estimated from logistic regression model; #estimated from linear regression model. Severity of illness was assessed using Pitt score [25].
In the stratified analysis by K-types (Table 3), appropriate empirical antimicrobial therapy was associated with a lower risk of 30-days attributable mortality in the KL2/10/22/52 isolates (OR = 0.19, 95% confidence interval 0.06–0.66, p = 0.008), whereas such association was lacking in other types. The difference in the effect of appropriate empirical antimicrobial therapy by K-type reached statistical significance (p for interaction between appropriate empirical antimicrobial therapy and K-type = 0.02). In both KL2/10/22/52 and non-KL2/10/22/52 types, carbapenem resistance was the strongest risk factor for 30-days attributable mortality. In the stratified analysis by carbapenem resistance (Table 4), K-type was not associated with 30-days attributable mortality. Appropriate empirical antimicrobial therapy was associated with a lower risk of 30-days attributable mortality in patients with carbapenem resistance (OR = 0.23, 95% confidence interval 0.07–0.77, p = 0.02), whereas such association lacked in patients without carbapenem resistance, though such difference did not reach statistical significance.
Table 3.
Association between carbapenem resistance, appropriate antibiotic treatment within 24 h, and 30-days attributable mortality, stratified by capsular types.
| Non-2/10/22/52 (n = 137) | 2/10/22/52 (n = 100) | P of interaction | |||
|---|---|---|---|---|---|
| OR (95% CI)* | p-value | OR (95% CI)* | p-value | ||
| Sex | 1.47 (0.54–4.01) | 0.45 | 0.75 (0.25–2.32) | 0.62 | 0.43 |
| Age | 1.03 (0.99–1.07) | 0.18 | 0.99 (0.95–1.04) | 0.79 | 0.58 |
| Charlson score | 1.10 (0.89–1.35) | 0.39 | 1.13 (0.90–1.42) | 0.30 | 0.92 |
| Pneumonia | 1.64 (0.58–4.61) | 0.35 | 3.89 (1.20–12.63) | 0.02 | 0.37 |
| Pitt score | 1.43 (1.21–1.69) | <0.0001 | 1.40 (1.15–1.70) | 0.0007 | 0.70 |
| Appropriate empirical antimicrobial therapy a within 24 h | 1.50 (0.51–4.39) | 0.46 | 0.19 (0.06–0.66) | 0.008 | 0.02 |
| Carbapenem resistance | 3.86 (0.88–16.93) | 0.07 | 7.90 (1.25–49.82) | 0.03 | 0.33 |
appropriate empirical antimicrobial therapy within 24 h was defined by in vitro susceptibility test.
*estimated from logistic regression model.
Table 4.
Association between capsular type, appropriate antibiotic treatment within 24 h, and 30-days attributable mortality, stratified by carbapenem resistance.
| Carbapenem resistance: N (n = 139) |
Carbapenem resistance: Y (n = 98) | P of interaction | |||
|---|---|---|---|---|---|
| OR (95% CI)* | p-value | OR (95% CI)* | p-value | ||
| KL2/10/22/52 v.s. non-KL2/10/22/52 | 0.24 (0.04–1.47) | 0.13 | 0.59 (0.13–2.64) | 0.49 | 0.33 |
| Sex | 1.81 (0.64–5.08) | 0.26 | 0.76 (0.26–2.21) | 0.61 | 0.29 |
| Age | 1.03 (0.99–1.08) | 0.12 | 0.99 (0.95–1.03) | 0.57 | 0.20 |
| Charlson score | 1.08 (0.88–1.32) | 0.46 | 1.09 (0.87–1.37) | 0.44 | 0.99 |
| Pneumonia | 2.26 (0.81–6.35) | 0.12 | 1.51 (0.50–4.52) | 0.46 | 0.95 |
| Pitt score | 1.31 (1.13–1.52) | 0.0004 | 1.56 (1.25–1.96) | 0.0001 | 0.17 |
| Appropriate empirical antimicrobial therapy a within 24 h | 1.05 (0.37–2.97) | 0.93 | 0.23 (0.07–0.77) | 0.02 | 0.22 |
appropriate empirical antimicrobial therapy within 24 h was defined by in vitro susceptibility test.
*estimated from logistic regression model.
Discussion
Biosynthesis of capsular polysaccharides in Acinetobacter is similar to Escherichia coli K30, as a model system of E. coli group 1 capsule assembly involved in wza/wzb/wzc export pathway [29,30]. The process is initiated by initial glycosyltransferase, which transfers a sugar precursor to inner membrane lipid carrier, and is followed by joining additional sugars catalysed by other specific glycosyltransferases [30]. The lipid-linked repeat units are translocated across the inner membrane into the periplasm by Wzx flippase and then polymerized into a chain by Wzy polymerase [30,31]. In general, genes required for capsule biosynthesis are clustered and variation in these loci leads to structural heterogeneity in K antigens. Previous reports described the difference of A. baumannii strains in capsule biosynthesis locus/polysaccharide gene cluster using two nomenclature systems, KL(K) types [23] and PSgc types [22], respectively. KL refers to K (capsule) locus, whereas PSgc refers to polysaccharide gene clusters. Kenyon et al. identified nine distinct capsule biosynthesis loci, designated KL1-KL9 [23]. Hu et al. analysed the polysaccharide gene clusters from 190 Acinetobacter genome sequences, and identified 25 pre-existing gene clusters [designated PSgc1-PSgc27 (PSgc7 and PSgc16 are identical to PSgc9 and PSgc23, respectively)] [22] and additional 52 new gene clusters. Both studies indicated the conservation of genetic organization, i.e. the gene clusters were between fkpA and lldP genes, with wza, wzb, and wzc export genes on the left, followed by a highly variable region (wzx, wzy, and glycosyltranferase genes), and several genes for synthesis of common sugar precursors. It was proposed that a cps locus with specific genetic contents represents a specific K-type. A recent study has provided a useful tool – Kaptive to compare the whole cps region with known K-types of A. baumannii when full sequences of cps locus is available [32]. It’s a reliable method if recombination occurs in cps region, however, amplification of the whole cps region or high cost of sequencing an entire genome is challenging for large numbers of clinical isolates. Therefore, for the rapid detection of K-type, an easier alternative strategy could be applied. One method based on wzy has been documented [22]. Hu et al. proposed that wzy, which is highly variable in sequence between distinct types, would be a candidate gene for a PCR-based molecular serotyping scheme. Despite the fact that wzy PCR is a reliable method, it’s difficult to conduct wzy typing for large numbers of clinical strains because a separate pair of primers is needed for each type (>106 K-types exist in A. baumannii). The wzc typing method reported here provides an alternative way to determine possible K-types for A. baumannii. According to our results, wzc sequencing can be used for prediction of possible K-types and then wzy PCR can be performed to confirm the type. It should be noted that even wzy PCR is not a gold standard for K-typing because it has been known that two distinct K-types may share the same wzy which may be due to recombination events in cps region. In these cases, further examination relies on the difference in other cps genes is needed (eg. cgmA PCR for KL2/KL81). However, if complete cps region or whole genome sequences were unavailable, wzc-wzy typing systems would be helpful for K-type analysis. To date, wzc typing has been used in two bacteria, K. pneumoniae and A. baumannii. Compared to wzc typing system in K. pneumoniae which needs four primer pairs to amplify wzc regions, only one primer pair for A. baumannii we designed in this current study can successfully amplify all 82 tested strains. However, wzc typing seems more discriminative in K. pneumoniae K-typing than in A. baumannii. Thus, combined with wzy genotyping would be strongly suggested in A. baumannii.
Previous studies conducted whole genome sequence analysis for MDRAB and KL1 and KL2 seem to be common K-types in global clone (GC) 1 and GC2, respectively [33,34]. A recent study analysed 134 ST1 of GC1 and 2016 ST2 of GC2 A. baumannii genome from NCBI and identified the most common K-types were KL1(31.3%) and KL4(18.7%) for ST1 and KL2(32.2%) and KL22(14.4%) for ST2 [32]. Despite that the analysis is not exclusively for MDRAB, KL1 and KL2 were predominant in these strains. In our current study, 4 major K-types were predominant in CRAB with a prevalence rate of KL2(20.4%), KL10(21.4%), KL22(24.5%), and KL52(18.4%) and account for a total of 85% for CRAB. Interestingly, no or rare studies report on KL10 and KL52. Therefore, we speculated that there could be new clones emerging in Taiwan. To clarify this notion, more strains from different hospitals should be included and the characteristics including K-types, virulence genes, and drug resistance genes should be further examined.
Among patients with KL2/10/22/52 infections, coherent with other studies, Pittsburgh bacteraemia scores and inappropriate empirical antimicrobial therapy were risk factors for mortality of A. baumannii bacteraemia [35]. Although KL2/10/22/52 were significantly more often associated with pneumonia, severe infection, carbapenem resistance, and high mortality, appropriate empirical antimicrobial therapy within 24 h was associated with higher reduction in mortality caused by KL2/10/22/52 infection. In this current study, we evaluate whether the patients received appropriate empirical antimicrobial therapy within 24 h by in vitro drug susceptibility test (please see the definition in the methods section). It should be noted that some drugs may show activity in vitro but still does not cause an effective cure in vivo, however, our results provided evidence on effectiveness which indicated that appropriate empirical antimicrobial therapy within 24 h decreases mortality for KL2/10/22/52 infection. Evidence to date has shown that early institution of appropriate antimicrobial therapy can improve the survival of patients with CRAB infection [35,36]. More than 50% of patients with CRAB infection received discordant antimicrobial therapy after illness, which led to death rates as high as 60–70% [35,36]. Therefore, recognition of KL2/10/22/52 types as soon as possible, especially in patients with high Pittsburgh bacteraemia scores and pneumonia, assist clinicians to promptly implement effective antimicrobial therapy for improving the outcomes in patients with KL2/10/22/52 bacteraemia before the result of in vitro susceptibility test. Generally, in vitro antimicrobial susceptibility test takes >12 h to determine the results. Thus, we suggested that for clinical application in patients with A. baumannii infection, wzy PCR for four common K-types (KL2, KL10, KL22, KL52) which is associated with carbapenem resistance can be performed (time spent <2 h). Even though an additional primer pair will be needed for distinguishing KL3/KL22 and KL2/KL81, it’s not necessary to perform the second PCR for them because KL3 and KL81 are rare compared to KL22 and KL2. If the strain is positive for common K-type, colistin could be considered for treatment. The procedure seems more tedious than in vitro antibiotics susceptibility test, however, it would be a time-saving method. Notably, despite that K-typing can be considered a rapid strategy for CRAB detection because of the strong association between K-type and carbapenem resistance, a combination of K-typing and in vitro antibiotics susceptibility test will be suggested (former for early detection, latter for confirmation). On the other hand, if researchers work on A. baumannii with no antibiotic assessment data, wzc typing could be conducted for initial K-type classification (time spent ∼1 day for PCR and sequencing), after that, wzy and other specific cps genes PCR will be further performed for K-type determination (time spent <2 h).
KL2/10/22/52 may frequently colonize in the respiratory tract and be exposed to antibiotic drugs, therefore may have been potentially submitted to resistance selective pressure, turning into pneumonia-causing CRAB strains. Unexpectedly, we found that appropriate empirical antimicrobial therapy within 24 h did not reduce mortality in patients with non- KL2/10/22/52 infections. The result may indicate that not all strains of A. baumannii are equally virulent, there is the propensity for mortality to be associated with the structure of a specific polysaccharide capsule or other capsule-related bacterial factors in certain types of A. baumannii.
Polysaccharide capsule, which shields A. baumannii from the host immune system, is critical to A. baumannii survival during interactions with host immunity. Nevertheless, not all capsule types appear to be similarly effective in shielding. Our study suggested that the disease pattern, antibiotic resistance, and outcome vary with capsule types. There may be variability in the uptake of exogenous DNA (natural competence) [37], transmission, and invasiveness of a given K-type in A. baumannii. K-type identification is important for understanding the virulence characterization and clinical manifestation of A. baumannii infection. In the future, an investigation into the K-type in A. baumannii serve as a target for medical treatment with phage and immune sera, rapid prediction of resistance, preventive measures such as vaccination, and novel strategies for intervention against this emerging pathogen.
Supplementary Material
Funding Statement
This work was supported by the Chang Gung Memorial Hospital, Taiwan under [Grant CMRPG3F1872 and CMRPG3F1592]; Ministry of Science and Technology, Taiwan under Grant [MOST 108-2320-B-039-059, MOST 109-2320-B-039-051]; China Medical University, Taiwan under [Grant CMU107-N-23 and CMU108-MF-62]; and China Medical University Hospital, Taiwan under [Grant DMR-108-136].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Gaynes R, Edwards JR.. National nosocomial infections surveillance system. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005 Sep 15;41(6):848–854. DOI: 10.1086/432803. PubMed PMID: 16107985. [DOI] [PubMed] [Google Scholar]
- 2.Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005 Nov;11(11):868–873. DOI: 10.1111/j.1469-0691.2005.01227.x. PubMed PMID: 16216100. [DOI] [PubMed] [Google Scholar]
- 3.Jerassy Z, Yinnon AM, Mazouz-Cohen S, et al. Prospective hospital-wide studies of 505 patients with nosocomial bacteraemia in 1997 and 2002. J Hosp Infect. 2006 Feb;62(2):230–236. DOI: 10.1016/j.jhin.2005.07.007. PubMed PMID: 16307825. [DOI] [PubMed] [Google Scholar]
- 4.Valencia R, Arroyo LA, Conde M, et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol. 2009 Mar;30(3):257–263. DOI: 10.1086/595977. PubMed PMID: 19199531. [DOI] [PubMed] [Google Scholar]
- 5.Mathlouthi N, El Salabi AA, Ben Jomaa-Jemili M, et al. Early detection of metallo-beta-lactamase NDM-1- and OXA-23 carbapenemase-producing Acinetobacter baumannii in Libyan hospitals. Int J Antimicrob Agents. 2016 Jul;48(1):46–50. DOI: 10.1016/j.ijantimicag.2016.03.007. PubMed PMID: 27216382. [DOI] [PubMed] [Google Scholar]
- 6.Shlaes DM, Sahm D, Opiela C, et al. The FDA reboot of antibiotic development. Antimicrob Agents Chemother. 2013 Oct;57(10):4605–4607. DOI: 10.1128/AAC.01277-13. PubMed PMID: 23896479; PubMed Central PMCID: PMCPMC3811409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control (Taiwan). Annual Report of Nosocomial Infections Surveillance System in Taiwan. Available from: https://www.cdc.gov.tw/english/info.aspx?treeid=00ED75D6C887BB27&nowtreeid=F0131176AA46D5DB&tid=1A8C498AF5F8AF5D
- 8.Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014 May;20(5):416–423. DOI: 10.1111/1469-0691.12363. PubMed PMID: 24131374. [DOI] [PubMed] [Google Scholar]
- 9.Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017 Feb 28;543(7643):15. DOI: 10.1038/nature.2017.21550nature.2017.21550 [pii]. PubMed PMID: 28252092; eng. [DOI] [PubMed] [Google Scholar]
- 10.Russo TA, Luke NR, Beanan JM, et al. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun. 2010 Sep;78(9):3993–4000. DOI: 10.1128/IAI.00366-10. PubMed PMID: 20643860; PubMed Central PMCID: PMCPMC2937447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisinger E, Isberg RR.. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015;11(2):e1004691. DOI: 10.1371/journal.ppat.1004691. PubMed PMID: 25679516; PubMed Central PMCID: PMCPMC4334535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tipton KA, Chin CY, Farokhyfar M, et al. Role of capsule in resistance to disinfectants, host antimicrobials, and desiccation in Acinetobacter baumannii. Antimicrob Agents Chemother. 2018 Dec;62(12). DOI: 10.1128/AAC.01188-18. PubMed PMID: 30297362; PubMed Central PMCID: PMCPMC6256795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang N, Ozer EA, Mandel MJ, et al. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. MBio. 2014 Jun 3;5(3):e01163–14. DOI: 10.1128/mBio.01163-14. PubMed PMID: 24895306; PubMed Central PMCID: PMCPMC4049102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo TA, Beanan JM, Olson R, et al. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect Immun. 2013 Mar;81(3):915–922. DOI: 10.1128/IAI.01184-12. PubMed PMID: 23297385; PubMed Central PMCID: PMCPMC3584894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan YJ, Lin TL, Chen YH, et al. Capsular types of Klebsiella pneumoniae revisited by wzc sequencing. PLoS One. 2013;8(12):e80670. DOI: 10.1371/journal.pone.0080670. PubMed PMID: 24349011; PubMed Central PMCID: PMCPMC3857182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan YJ, Lin TL, Lin YT, et al. Identification of capsular types in carbapenem-resistant Klebsiella pneumoniae strains by wzc sequencing and implications for capsule depolymerase treatment. Antimicrob Agents Chemother. 2015 Feb;59(2):1038–1047. DOI: 10.1128/AAC.03560-14. PubMed PMID: 25451047; PubMed Central PMCID: PMCPMC4335867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meletis G, Chatzopoulou F, Chatzidimitriou D, et al. Whole genome sequencing of NDM-1-producing ST11 Klebsiella pneumoniae isolated in a private laboratory in Greece. Microb Drug Resist. 2019 Jan/Feb;25(1):80–86. DOI: 10.1089/mdr.2017.0411. PubMed PMID: 29698126. [DOI] [PubMed] [Google Scholar]
- 18.Bellich B, Ravenscroft N, Rizzo R, et al. Structure of the capsular polysaccharide of the KPC-2-producing Klebsiella pneumoniae strain KK207-2 and assignment of the glycosyltransferases functions. Int J Biol Macromol. 2019 Jun 1;130:536–544. DOI: 10.1016/j.ijbiomac.2019.02.128. PubMed PMID: 30802520. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh SY, Tseng CL, Lee YS, et al. Highly efficient classification and identification of human pathogenic bacteria by MALDI-TOF MS. Mol Cell Proteomics. 2008 Feb;7(2):448–456. DOI: 10.1074/mcp.M700339-MCP200. PubMed PMID: 18045801. [DOI] [PubMed] [Google Scholar]
- 20.European Committee on Antimicrobial Susceptibility Testing (EUCAST) . Breakpoint tables for interpretation of MICs and Zone diameters. Available from: http://wwweucastorg/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_80_Breakpoint_Tablesxls. 2018
- 21.CLSI . Performance standards for antimicrobial susceptibility testing. 28th ed. CLSI supplement M100. Wayne (PA: ): Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 22.Hu D, Liu B, Dijkshoorn L, et al. Diversity in the major polysaccharide antigen of Acinetobacter baumannii assessed by DNA sequencing, and development of a molecular serotyping scheme. PLoS One. 2013;8(7):e70329. DOI: 10.1371/journal.pone.0070329. PubMed PMID: 23922982; PubMed Central PMCID: PMCPMC3726653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenyon JJ, Hall RM.. Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii genomes. PLoS One. 2013;8(4):e62160. DOI: 10.1371/journal.pone.0062160. PubMed PMID: 23614028; PubMed Central PMCID: PMCPMC3628348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818–829. PubMed PMID: 3928249. [PubMed] [Google Scholar]
- 25.Chow JW, Yu VL.. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999 Jan;11(1):7–12. DOI: 10.1016/s0924-8579(98)00060-0. PubMed PMID: 10075272. [DOI] [PubMed] [Google Scholar]
- 26.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988 Jun;16(3):128–140. DOI:0196-6553(88)90053-3 [pii]. PubMed PMID: 2841893; eng. [DOI] [PubMed] [Google Scholar]
- 27.Chuang YC, Sheng WH, Li SY, et al. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with acinetobacter bacteremia. Clin Infect Dis. 2011 Feb 1;52(3):352–360. DOI: 10.1093/cid/ciq154. PubMed PMID: 21193494. [DOI] [PubMed] [Google Scholar]
- 28.Lee YT, Kuo SC, Yang SP, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis. 2012 Jul;55(2):209–215. DOI: 10.1093/cid/cis385. PubMed PMID: 22495546. [DOI] [PubMed] [Google Scholar]
- 29.Cuthbertson L, Kos V, Whitfield C.. ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol Rev. 2010 Sep;74(3):341–362. DOI: 10.1128/MMBR.00009-10. PubMed PMID: 20805402; PubMed Central PMCID: PMCPMC2937517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. DOI: 10.1146/annurev.biochem.75.103004.142545. PubMed PMID: 16756484. [DOI] [PubMed] [Google Scholar]
- 31.Whitfield C, Roberts IS.. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol Microbiol. 1999 Mar;31(5):1307–1319. PubMed PMID: 10200953. [DOI] [PubMed] [Google Scholar]
- 32.Wyres KL, Cahill SM, Holt KE, et al. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb Genom. 2020 Mar;6(3):e000339. DOI: 10.1099/mgen.0.000339. PubMed PMID: 32118530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holt K, Kenyon JJ, Hamidian M, et al. Five decades of genome evolution in the globally distributed, extensively antibiotic-resistant Acinetobacter baumannii global clone 1. Microb Genom. 2016 Feb;2(2):e000052. DOI: 10.1099/mgen.0.000052. PubMed PMID: 28348844; PubMed Central PMCID: PMCPMC5320584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz MB, Pham Thanh D, Tran Do Hoan N, et al. Repeated local emergence of carbapenem-resistant Acinetobacter baumannii in a single hospital ward. Microb Genom. 2016 Mar;2(3):e000050. DOI: 10.1099/mgen.0.000050. PubMed PMID: 28348846; PubMed Central PMCID: PMCPMC5320574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon KT, Oh WS, Song JH, et al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother. 2007 Mar;59(3):525–530. DOI: 10.1093/jac/dkl499. PubMed PMID: 17213265. [DOI] [PubMed] [Google Scholar]
- 36.Lee HY, Chen CL, Wu SR, et al. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med. 2014 May;42(5):1081–1088. DOI: 10.1097/CCM.0000000000000125. PubMed PMID: 24394630. [DOI] [PubMed] [Google Scholar]
- 37.Traglia GM, Quinn B, Schramm ST, et al. Serum Albumin and Ca2+ are natural competence inducers in the human pathogen Acinetobacter baumannii. Antimicrob Agents Chemother. 2016 Aug;60(8):4920–4929. DOI: 10.1128/AAC.00529-16. PubMed PMID: 27270286; PubMed Central PMCID: PMCPMC4958237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.