Abstract
Context/objective: Information on the safety and feasibility of lower extremity powered exoskeletons for persons with acute/sub-acute spinal cord injury (SCI) is limited. Understanding the safety and feasibility of employing powered exoskeletons in acute/sub-acute (<6 months post injury) at a SCI acute inpatient rehabilitation (SCI-AIR) facility could guide clinical practice and provide a basis for larger clinical trials on efficacy and effectiveness.
Design: Single group observational study.
Setting: SCI-AIR.
Participants: Participants (n = 12; age: 28–71 years; 58% AIS D; 58% male) with neurological levels of injuries ranging from C2 to L3.
Interventions: Up to 90 min of exoskeleton-assisted locomotor training was provided up to three times per week during SCI-AIR.
Outcome measures: Safety of device use during inpatient locomotor training was quantified as the number of adverse events (AE) per device exposure hour. Feasibility of device use was defined in terms of protocol compliance, intensity, and proficiency.
Results: Concerning safety, symptomatic hypotension was the most common AE reported at 111-events/exoskeleton-hours. Protocol compliance had a mean (SD) of 54% (30%). For intensity, 77% of participants incorporated variable assistance into at least 1 walking session; 70% of participants' sessions were completed with a higher RPE than the physical therapist. In proficiency, 58% achieved at least minimal assistance when walking with the device.
Conclusion: Exoskeleton training in SCI-AIR can be safe and feasible for newly injured individuals with SCI who have clinically defined ambulatory goals. Nonetheless, sufficient controls to minimize risks for AEs, such as hypotensive events, are required.
Keywords: Spinal cord injuries, Physical therapy modalities, Walking, Robotics, Physical medicine and rehabilitation
Introduction
Interventions utilizing assisted walking are employed to facilitate recovery of ambulation and reduce secondary complications following spinal cord injury (SCI). The use of either manual/robot-assisted body-weight supported treadmill training (BWSTT) or conventional over-ground walking have demonstrated effectiveness at improving walking ability for persons with incomplete SCI or other similar neurological deficits (e.g. stroke).1–4 These interventions have been shown to increase muscle mass,5,6 improve cardiovascular functioning,7 decrease muscular tone or spasticity,8 decrease pain,9 help preserve bone density,6,10 and bowel function.11
Recently, powered exoskeletons have been introduced as an alternative to BWSTT for assisted locomotor training.12–16 The U.S. Food and Drug Administration (FDA) 17 has currently approved three class II medical devices in the United States (Eksoa, Indegob, and ReWalkc).12–14 Benefits of these devices have mainly been studied in small, single group design studies in outpatients with chronic SCI.18–26 Interventions with exoskeleton-assisted walking (EAW) have: (1) demonstrated that persons with SCI can become proficient with the device (learnability),21–23,25,26 (2) confirmed ambulation patterns similar to able-bodied individuals,20 (3) established improvements in quality of life,25 (4) illustrated that EAW may be equivalent to moderate intensity exercise,18,19 and (5) resulted in minor adverse events (AEs) – most commonly as skin integrity issues.21,22,24,25 Although Platz et al. performed their study in an inpatient rehabilitation setting, all subjects were individuals with chronic SCI (mean injury duration, 11.4 years).25 Consequently, the literature still lacks evidence on the safety and feasibility of EAW for recently injured SCI (acute/sub-acute; <6 months post injury) in a SCI acute inpatient rehabilitation (SCI-AIR) facility.18–26
The primary aim of this study was to examine the safety of EAW in a SCI-AIR facility. The study’s secondary aim was to examine the feasibility of incorporating exoskeleton walking within daily therapy sessions.
Methods
Study Design and Setting
This was a single group observational study to investigate the safety and feasibility of powered EAW in a SCI-AIR facility. All study procedures were conducted in a SCI-AIR facility within Mount Sinai Hospital in New York, NY. The study was approved by the Clinical Institutional Review Board at the Icahn School of Medicine at Mount Sinai (IRB# 15-2253).
Participants
Eligible participants were adults admitted to SCI-AIR facility following acute/sub-acute SCI who had ambulation goals (Table 1). Candidates for ambulation goals included individuals with American Spinal Injury Association (ASIA) Impairment Scale (AIS) C or D and individuals with A or B who had any preserved lower extremity motor strength (e.g. lumbar and sacral level injuries with LEMS < 3) (Table 1). All participants provided signed informed consent and underwent medical screening by a physiatrist who was board certified in SCI medicine and an evaluation for compatibility and fit with the device by a physical therapist (PT). Enrolled subjects who passed these assessments were scheduled for walking sessions.
Table 1. Inclusion/exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Admission into the spinal cord injury rehabilitation facility | 1. Poor upright tolerance for less than 30 min (i.e. orthostatic hypotension) |
| 2. 18 years or older | 2. Uncontrolled cardiovascular conditions |
| 3. Physician-confirmed diagnosis of SCI with lower extremity weakness/paralysis | 3. Colostomy that could impact proper device fitting |
| 4. Clinically indicated ambulation goals | 4. Pregnant |
| 5. Sufficient strength and function in the upper extremities to manage walking aids | 5. Fracture risk – based on clinician assessment |
| 6. Able to fit within the exoskeleton device | 6. Skin integrity issues in areas that would contact the device or get worse with device usage |
| 7. Anything that would prevent safe standing and walking in the device |
Device
The powered lower extremity exoskeleton used in this study was the Ekso GT.a This device has motors at both the hip and knee joints which move the lower extremities. Detailed descriptions of this device and its workings have been reported elsewhere.14,18 The optional SmartAssist12 (SA) feature allowed the PT to alter the walking program during sessions to accommodate for changes in the amount of motor assistance needed. There are two modes within SA, adaptive and fixed. Adaptive mode dynamically adjusts to the user’s needs and compensates with hip and knee motors as the user takes steps. Fixed mode allows the PT to set a particular threshold (i.e. percentage of exoskeleton motor assistance versus participant assistance throughout the walking motion) to augment the user’s efforts. This mode can be more challenging to the user depending on the threshold set by the PT. If the user does not provide enough force to propel the limb, the device will hesitate briefly before completing the step, and indicate to the PT that the user is not meeting the threshold. Subsequently, the PT can either encourage the user to “push harder” or modify the setting to fit the user’s needs. In either SA mode, the device has a set time limit after which the device will complete the step regardless whether the threshold is met (i.e. time limit or assistance level).
Exoskeleton Walking Sessions
A detailed description of screening, fit, and training for persons with SCI has been reported elsewhere.27 The walking intervention was provided from enrollment to discharge from the SCI-AIR facility with the primary objective of incorporating EAW sessions as part of the participant’s total therapy of 15 hours per week. Sessions were scheduled for 90 min at three times per week. Prior to the first walking session, participants were measured for device fit and evaluated for contraindications, which are outlined in the inclusion/exclusion criteria (Table 2). Participants were progressed from static standing balance with use of a walker, to standing weight shifting activities, assisted stepping, pivot turns, assisted walking for short distances, and finally to reduced assistance with SA. The PT would begin applying SA in ‘adaptive’ mode and then progress toward ‘fixed’ mode as the participant shows improvements in their ability to match the programmed walking parameters with less motor assistance. Participants progressed towards a goal of tolerating more than 30 min of up-time, the total time study participants spent standing or walking in the device, allowing for short rests as needed. The session schedule was adapted to fit participant availability and readiness; missed sessions were not rescheduled but the reasons were noted.
Table 2. Participant characteristics.
| Participant | Age* | Sex | BMI (kg/m2) | AIS | NLI | Days between admission and 1st session | No. of sessions |
|---|---|---|---|---|---|---|---|
| 1 | 45–54 | Male | 29.3 | D | C4 | 33 | 2 |
| 2 | 55–64 | Male | 26.9 | C | T9 | 37 | 4 |
| 3 | 25–34 | Female | 25.6 | B | L2 | 17 | 4 |
| 4 | 65–74 | Male | 21.9 | C | C3 | 32 | 1 |
| 5 | 45–54 | Male | 24.2 | D | T9 | 5 | 4 |
| 6 | 25–34 | Male | 20.9 | C | T4 | 9 | 5 |
| 7 | 35–44 | Male | 31.1 | D | C5 | 28 | 6 |
| 8 | 45–54 | Female | 33.6 | D | C4 | 23 | 4 |
| 9 | 35–44 | Male | 27.4 | A | L3 | 9 | 5 |
| 10 | 55–64 | Female | 30.7 | D | C4 | 15 | 4 |
| 11 | 65–74 | Female | 21.5 | D | C2 | 35 | 4 |
| 12 | 65–74 | Female | 30.6 | D | T4 | 15 | 1 |
| Median (min, max) | 51.5 (28,71) | – | 27.2 (20.9, 33.6) | – | – | 20 (5, 37) | 4 (1,6) |
| Mean | 50.9 | – | 27.0 | – | – | 21.5 | 3.7 |
| (SD) | (15.6) | (4.2) | (11.3) | (1.6) |
BMI = body mass index; m = meters; kg = kilograms; AIS = American Spinal Injury Association (ASIA) Impairment Scale; NLI = neurological level of injury.
* Age is at time of enrollment reported in 10-year age classes due to small sample size.
6-Minute Walking Test
The 6-Minute Walk Test (6MWT) measures the total distance traveled in a 6-minute period and has been reported to be a reliable measure of walking endurance for persons with SCI.28 The 6MWT is often used with EAW as a metric for progress in a wide variety of lower extremity exoskeleton-related studies.18,19,24,27,29 The 6MWT was administered at least once a week, if the participants were able to easily make consecutive steps and progress toward longer periods of ambulation.
Outcomes
The primary and secondary outcomes were, respectively, safety and feasibility of incorporating exoskeleton walking into SCI-AIR.
Safety was defined using the FDA’s list of known adverse events (AEs) for EAW, and for unforeseen AEs.17 The potential for AEs is predicated on both the type of, and exposure to, a given risk factor. Thus, event rates were calculated as the count for each AE divided by person-hours spent in the exoskeleton (i.e. EAW-hours), which accounts for varied exposure time among participants.
Feasibility was expressed in terms of compliance, intensity, and proficiency. Compliance was defined as the number of EAW sessions completed as a percentage of scheduled EAW sessions which were incorporated into the standard amount of daily therapy. This was calculated by dividing the number of EAW sessions completed by the number of expected EAW sessions (assuming 3 EAW session per week for the duration of the inpatient rehabilitation stay). Intensity was defined using 4 distinct domains: (1) the distance traveled walking with the device during a 6MWT,30 (2) the perceived exertion ratings from both the participants and PTs using the Borg Rating of Perceived Exertion (RPE) Scale at mid-session,25,26,31 (3) the lowest percentage of SA achieved for all EAW sessions, and (4) the proportion of EAW sessions which used SA at any point. Gait speed was also determined using the longest distance traveled in the device during the 6MWT – which has been demonstrated to a valid prognostic and contain predictive value in overall health.32 Proficiency was defined by the proportion of participants in the study that achieved the lowest assistance level during EAW, as rated by the PTs using a rating scale adapted for EAW from the FIM Instrument (sit-to-stand, walking, stand-to-sit).17 Other outcomes included donning and doffing time.
Statistical methods
All data were collected and entered into a REDCap database,33,d with analyses performed using SPSS version 20e and Microsoft Excel version 14.0f software. Descriptive statistics were reported for participant demographics. Distributions were examined for normality, results for all continuous variables are presented as mean (SD), and the median (min, max) are also reported for variables with non-normal distributions. Compliance and intensity outcomes were reported as proportions and rates as previously defined. To generate radar graphs of the intensity outcomes, only those who completed the protocol, regardless of session number were included in this analysis; those who were withdrawn were not analyzed. All 4 domains for intensity were scaled between 0 and 1 within their respective categories purely for visual comparison purposes; 0 was the lowest value among the cohort and 1 being the highest value.
Results
Participants
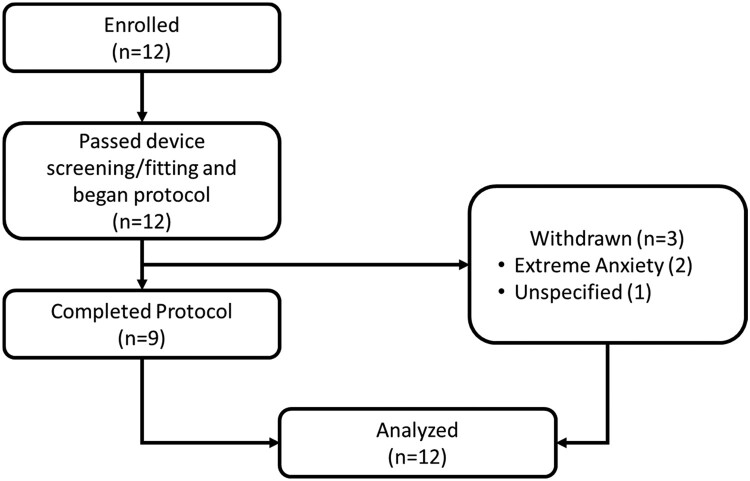
Of the 12 participants enrolled in the study, none screen-failed and 3 were withdrawn, two due to anxiety (both preexisting with diagnosis) and one for an unspecified reason (Fig. 1). The sample had more males (58%) and most participants were motor incomplete except for two individuals with lower level injuries and complete injuries (Table 2). There were an equal number of cervical and thoracic-lumbar level lesions. The mean (SD) number of sessions for the sample was 3.7 (1.6). The mean (SD) for number of days between inpatient admission and first EAW session was 21.5 (11.3).
Figure 1.
Study flow diagram. Of the 12 participants enrolled and passed device screening, 9 completed the protocol and 3 withdrew, primarily due to extreme anxiety (preexisting). All participants were analyzed.
Safety outcomes
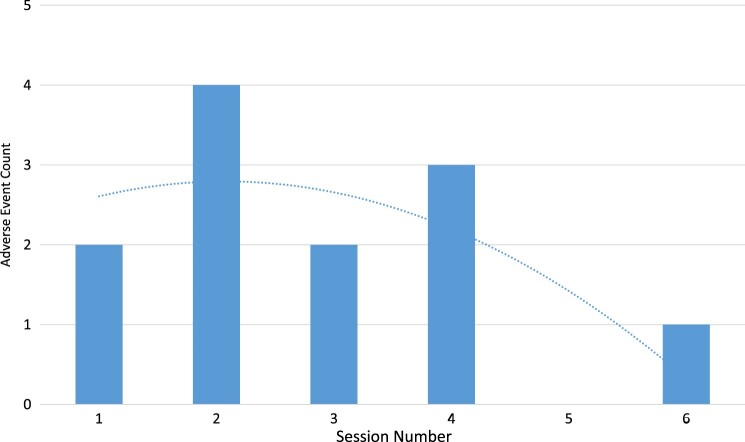
There were no serious AEs observed, and event rates for the minor AEs were low overall. The most frequently recorded individual AE was systolic hypotension (< 90 mmHg), observed at a rate of 111 events/1000 EAW-hours. Skin integrity issues and mechanical/electrical issues were both observed at 111 events/1000 EAW-hours. The least common minor AE recorded was use error at 37 events/1000 EAW-hours (Table 3). The number of There seemed to be a pattern with the increased number of AEs between Sessions 1 through 2, yet this tapered off after session 4; overall trend was a negative and parabolic (Fig. 2).
Table 3. Adverse events.
| Adverse event (AE) type | Count | Event rate per 1000 h.* |
|---|---|---|
| Fall | 0 | 0 |
| Instability (no fall) | 0 | 0 |
| Skin integrity issues | 3 | 111 |
| Bruising | 0 | 0 |
| Skin abrasion | 2 | 74 |
| Pressure injuries | 0 | 0 |
| Soft tissue injury | 0 | 0 |
| Tissue/skin reaction from device-skin contact | 1 | 37 |
| Soft tissue injury | 0 | 0 |
| Blood pressure issues | 5 | 185 |
| Systolic hypertension (140 ≥ mmHg) | 2 | 74 |
| Systolic hypotension (<90 mmHg) | 3 | 111 |
| Mechanical/electrical issues | 3 | 111 |
| Premature battery failure | 1 | 37 |
| Electrical shock | 0 | 0 |
| Burns | 0 | 0 |
| Device malfunction resulting in unanticipated operation | 1 | 37 |
| Device breakdown | 1 | 37 |
| Use error | 1 | 37 |
| Participant error | 0 | 0 |
| Researcher error | 1 | 37 |
| Companion error | 0 | 0 |
| Additional pain from exoskeleton-assisted walking | 0 | 0 |
| Other | 0 | 0 |
*Event rates are standardized per 1000 h of exposure to standing, walking, and sitting in the powered exoskeleton, for an average of 38.05 min exposure per session for all 43 sessions completed by 12 participants (27.27 h).
Figure 2.
Adverse event count by session. This illustrates the adverse event count by EAW Session along with a trendline which demonstrates that as the Session number increases there AEs are less frequent overall.
Feasibility outcomes
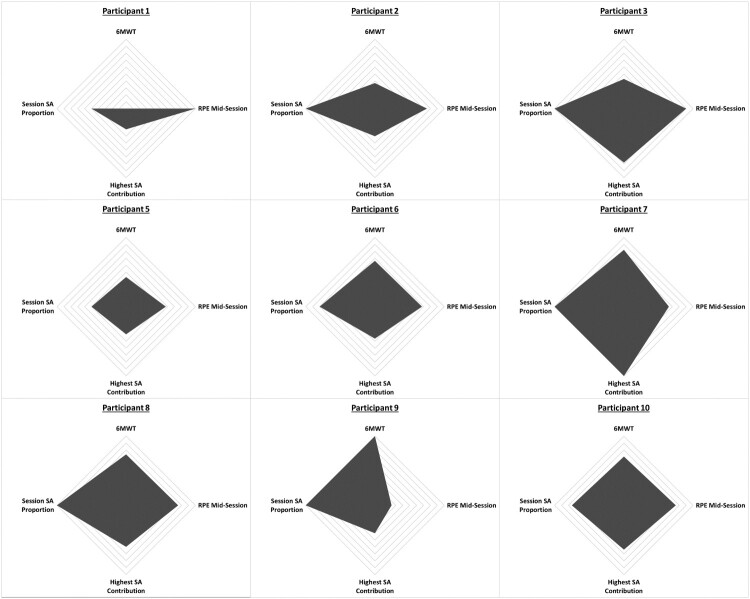
Participants with cervical SCI saw the largest gap in time to the first session from the dates of injury and from inpatient admission. Mean (SD) compliance was 54% (30%); median (min, max) compliance was 61% (6%, 100%) (Table 4). For the total of 43 sessions across all participants, the mean (SD) up time and walk time was 38.0 (11.4) and 22.5 (9.6) min, respectively. Most of the participants were able to increase EAW intensity, with 77% of participants using SA in their walking sessions, where median (min, max) proportion for each participant’s usage of SA and lowest percentage of assistance given by the device for each participant was 78% (0, 100%) and 77% (50%, 85%), respectively. The median (min, max) distance on the 6MWT was of 45 (25, 68) meters. Overall, intensity domains did not demonstrate any patterns for increasing session number, but did illustrate participant variability in response to the device (Fig. 3). Gait speed had a mean (SD) of 0.12 (0.04). Regarding proficiency, seven participants (58%), achieved at least minimal assistance for walking with the device (Table 5), and six of those seven (85%) achieved this by Session 2. Overall, average RPE ratings for both the participants and PTs increased from pre-session measure. By the end of the session 70% of the participants rated RPE higher than the PT (average participant vs. PT RPE; 14.8 vs. 14.1); mean (SD) PT RPE across participants for pre-, mid-, and post-session were 9.2 (2.3), 13.5 (2.0), and 14.1 (2.4), respectively. Donning and doffing time (in minutes) had a mean (SD) of 7.3 (1.9) and 4.4 (1.2), respectively.
Table 4. Feasibility: compliance and intensity.
| Participant | Compliance* | 6MWT Longest distance, m | 6MWT Longest distance gait speed, m/s† | Lowest %SA achieved‡ | Sessions using SA§ |
|---|---|---|---|---|---|
| 1 | 67% | – | – | 85% | 50% |
| 2 | 67% | 25 | 0.07 | 80% | 100% |
| 3 | 33% | 29 | 0.08 | 61% | 100% |
| 4 | 6% | – | – | – | 0% |
| 5 | 67% | 29 | 0.08 | 80% | 50% |
| 6 | 56% | 45 | 0.13 | 77% | 80% |
| 7 | 100% | 56 | 0.16 | 50% | 100% |
| 8 | 44% | 50 | 0.14 | 70% | 100% |
| 9 | 83% | 68 | 0.19 | 80% | 100% |
| 10 | 67% | 48 | 0.13 | 68% | 75% |
| 11 | 44% | 27 | 0.08 | – | 0% |
| 12 | 17% | – | – | – | 0% |
| Median | 61% | 45 | 0.13 | 77% | 78% |
| (min, max) | (6%, 100%) | (25, 68) | (0.07, 0.19) | (50%, 85%) | (0%, 100%) |
| Mean | 54% | 42 | 0.12 | 72% | 63% |
| (SD) | (30%) | (15.1) | (0.04) | (11%) | (42%) |
6MWT, 6-Minute Walk Test; m, meter; s, second; SA, SmartAssist.
* Calculated as:
†Calculated as:
‡The lowest percentage of SmartAssist (SA) achieved, where SA is a function of total motor contribution toward walking. The device used was capable of unilateral control, however this was not differentiated, and the percentage reported is solely the lowest level of assistance required.
§Calculated as:
Figure 3.
Radar chart of intensity domains. This figure demonstrates the 4 domains of Intensity outlined in this study: (1) 6-Minute Walk Test, (2) the RPE at mid-session, (3) Highest amount of SA-motor assistance, and (4) Proportion of sessions which utilized SA. A larger area inside the polygon corresponds to a greater performance for Intensity. All domains were scaled to between 0 and 1 which correspond to the minimum and maximum scores in each respective domain across participants. Those who completed the protocol, regardless of session number were included in this analysis; those who were withdrawn were not analyzed.
Table 5. Proficiency of exoskeleton-assisted walking skills.
| Walking level of assistance – minimal assistance, n* | Session | Cumulative percent† | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Walking | 2 | 4 | 1 | 0 | 0 | 0 | 58 |
*No. of participants who achieved the skill at the specified level by the session.
†Cumulative percent of participants who achieved the skill at the specified level of the total (n = 12) who began the intervention.
Discussion
Presently, our findings align with previous studies which reported on safety and feasibility following SCI-AIR.34–37
Overall, use of EAW in SCI-AIR under the study protocol appears to be safe. Blood pressure changes, namely orthostatic hypotension, were the most frequent AE. Skin issues related to the interface between the user and the device followed, then, mechanical/electrical issues, and finally, use error. There was an interesting relationship between AE and session number (Fig. 2). In the first session, participants primarily focused on standing and weight shifting in the device, taking fewer steps versus later sessions. The second session had participants focus on higher step counts (increasing walking proficiency) – this increased movement may have increased the AE risk. Later sessions (e.g. session 4) may have had lower AE counts because the participants gained proficiency with the device – decreasing the AE risk.
Hypotensive episodes were observed in approximately 50% of the participants by at least mid-session after participants were standing or walking for an extended period. However, no clear patterns were evident in the hypotensive AEs. Orthostatic hypotension, which accounted for 80% of all blood pressure AEs, is a common issue in acute SCI recovery with a variety of physiological mechanisms.38–40 A recent systematic review of the literature demonstrated that orthostatic hypotension was a seemingly rare event (18% of the sample; 4/22 subjects) where only one study reported orthostatic hypotension as an AE,41 however this study used a different lower extremity exoskeleton and in a stroke population.36 Consequently, the risk of orthostatic hypotension may have been under-represented in previous studies.36 Regardless, hypotension should be managed before initiating EAW, and blood pressure should be monitored throughout sessions – a perspective that seems to be shared by others.34,35
This study demonstrates that the use of EAW for locomotor training in SCI-AIR may be feasible if logistical and clinical needs are met. Utilizing these devices as a tool for locomotor training may be helpful, particularly if these devices are to be used as an adjunct therapy for functional recovery (i.e. improve walking independence).42 However, the short duration of SCI-AIR limits the number of EAW sessions. A short SCI-AIR stay is important to consider given that, according to the most recent SCI Model System’s Report, the median length of stay for SCI-AIR has been reduced from 42 days in 2000 to 31 days in 2004.43 Consequently, shortened SCI-AIR duration can potentially prevent the user from engaging in a therapy which provides a high volume of steps (following the principles of motor learning).31,44 Another important point to consider is that this device, at maximum, only allowed participants to walk at 0.19 m/s and would be marginally considered household walkers.32 It is unclear how use of these devices may improve functional ambulation post-discharge assuming individuals have access.
Employing these devices would involve clinician buy-in, requiring exoskeleton-certified PT’s to feel comfortable incorporating EAW into their practice. In many ways EAW provides gait training analogous to BWSTT and manual over-ground training, however an important difference with EAW is that BWSTT may allow for gait training in individuals who might not otherwise receive it (e.g. marginal upper extremity strength). This is particularly important; however, this was not seen with the present cohort which could objectively fall into a category that possesses greater functional capacity (i.e. AIS C/D). On the contrary, EAW may provide advantages over BWSTT due to using a biomechanical reciprocating pattern which allows for hip extension and full loading of the lower limbs thus facilitating neural plasticity.31 This relationship takes for granted that the EAW user does not require assistance to walk in the device, as less mastery has less ground reaction force – translating to less forces acting on the lower limbs.20 Albeit, integrating this type of locomotor training to standard care is both costly (e.g. device cost and training costs) and time-consuming (e.g. device setup between users).37 Ultimately, time in EAW is time not spent in other proven effective therapies, thus examination of efficacy for EAW as a component of SCI-AIR should be a priority moving forward.
Circumstantial factors can affect session number, duration, compliance, and intensity of EAW sessions. Circumstantial factors include device malfunction, availability of certified personnel, and scheduling conflicts. The most consequential mechanical/electrical issue during the course of the study was a result from device breakdown. The breakdown occurred during an EAW session and prohibited the participant from taking sequential steps – ultimately determined to be a result of a foot sensor malfunction which was later replaced by the research team with guidance from the manufacturer. The risk to the participant was minimal due to safety measures that are built into the device along with the extensive training which is required to operate the exoskeleton with a participant.12 Scheduled EAW sessions were canceled for two participants who were active in the protocol at the time of the device breakdown, which took 5 days to correct. Another limiting factor was having had only two of the 6 PTs in the SCI-AIR facility certified to use the device. Consequently, EAW participants may have missed sessions due to scheduling conflicts resulting from paid time off for the PTs (vacation or sick days) or management decisions (e.g. patient census at capacity where certified PTs might not be able to see an unassigned patient for EAW). Although compliance could improve with more certified PTs, the time and expense to certifying PTs remains a significant barrier.
The manufacturer’s intention was to provide a device which makes the therapists more efficient. However, that could only be clinically feasible if the device is easy to learn which would translate to less effort by the therapist. This study demonstrated that most participants (77%) were able to use the device with at least moderate assistance – meaning, participants were highly engaged in the stepping process. In general, the study device is anecdotally thought to be easier to learn than other devices17,22,31 – this theoretically allows the PT to focus on achieving therapy goals. From a feasibility standpoint, utilizing a device which is exhausting for the PTs and is difficult to learn would not be beneficial for any SCI-AIR facility; even if there are possible improvements to discharge lower extremity motor scores.45
Limitations
The results of this study are not generalizable to the larger population of persons with SCI, primarily due to the small sample size and single group design. Eligibility criteria and screening protocol are subject to selection bias and confounding by indication. There is potential for bias toward positive results for participants and clinician-rated outcomes as a result of the convenience sample without randomization and blinding during the intervention. Furthermore, this study, in no way, tested the efficacy of EAW in SCI-AIR.
This study attempted to incorporate EAW within clinical locomotor training time – as opposed to providing additional therapy. Thus, performing the study within clinical hours heavily relied on clinical recommendation for recruitment rather than implement a rigorous pre-screening process – possibly biasing enrollment. Moreover, patients assigned to the exoskeleton-certified PTs were more likely to get the device versus those who were not assigned those PTs. Consequently, study eligibility could be artificially low (7% of all inpatients were enrolled) which impacts EAW feasibility; studies which have a more defined screening process are required. If the study had implemented EAW training outside clinical hours, this would have changed both the sample and research question.
Another consequence of relying on clinical judgment for study inclusion was the introduction of selection for determining which individuals were most appropriate for the device. For example, if a therapist deemed that a patient would likely outperform the device (e.g. central cord syndrome) then the potential participant was not deemed appropriate and should be given to individuals who are more severely impaired. However, this opinion changed over time and throughout the study therapists began to utilize this device in different ways which could improve postural stability, trunk balance, head control, and muscle strengthening in differing injury levels and completeness.12 In short, as the PTs continued to gain mastery of the device and how it could be incorporated into their practice, therapists were more willing to try EAW with more complex clinical cases (e.g. C2 SCI) while still maintaining safety. Not having a strict and defined inclusion criterion indeed affected the study’s interval validity, however, the participant’s therapy goals superseded some aspects of the study. Lastly, gait speeds calculated from 6MWT probably underestimate compared to 10MWT, but implementing the latter would probably not have produced a different result in regard to ambulation status (home versus limited community) and would add burden.
Clinical considerations
For SCI-AIR centers that have, or plan to implement EAW programs, the following general considerations may serve as a foundation for implementation:
Clinicians should always monitor for exoskeleton-specific AEs as defined by the FDA (e.g. hypotension and skin integrity issues21,22,24,25) and for any other unknown AEs. Specifically, active monitoring of symptomatic hypotension is strongly recommended primarily for patient safety. Monitoring may not prevent symptoms; however, it may guide clinical decisions which could be used to potentially addresshypotension preemptively (e.g. support stockings, binders, Alpha-1-Agonists, etc.).38–40
Scheduled EAW sessions should accommodate at least 30 min of up-time, which can maximize standing time, walk time, and step count. Increased upright time during EAW has recently been shown to improve lower extremity motor scores in acute SCI.31,45
The SA function should be used progressively (‘adaptive’ then ‘fixed’ – see Methods section) to increase the RPE component of EAW intensity.12 Skill level and proficiency with the device should be especially considered if SA is to be utilized.
Conclusions
The results from this preliminary study suggest that EAW can be both safe and feasible for SCI-AIR who have ambulation goals, when used as a supplemental locomotor training activity (outside the 3-hour therapy requirement) with appropriate monitoring. We provide recommendations to promote safe and feasible EAW for SCI inpatients; however, this study did not assess efficacy, so we are unable to comment on the clinical value of EAW in SCI-AIR. Efficacy aside, service delivery requirements, such as inpatient length of stay and clinician training, may also constrain implementation. Alternatively, providing EAW in SCI-AIR could serve as an early introduction to a mobility strategy that may be available after discharge.
Suppliers
a. Ekso GT, Ekso Bionics.
b. Indego; Parker Hannifin
c. ReWalk Robotics.
d. REDCap; REDCap Consortium.
e. SPSS version 20; IBM
f. Microsoft Excel version 14.0; Microsoft.
Disclaimer statements
Contributors None.
Funding This work was fully supported by the Department of Rehabilitation and Human Performance at Mount Sinai.
Conflicts of interest Dr. Kozlowski was a scientific advisory panel member for ReWalk Robotics during development and implementation of the study protocol. The other authors declare no conflicts of interest.
Acknowledgments
We acknowledge the contributions made to this work by the study participants and clinical staff who made this research possible. This body of work represents a portion of the Master’s thesis of Andrew D. Delgado as partial requirement for the fulfillment of the MS degree in Biomedical Sciences offered by the Graduate School of Biomedical Sciences at Mount Sinai.
References
- 1.Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, et al. . Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–93. doi: 10.1212/01.wnl.0000202600.72018.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morawietz C, Moffat F.. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2013;94:2297–308. doi: 10.1016/j.apmr.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 3.Ada L, Dean CM, Vargas J, Ennis S.. Mechanically assisted walking with body weight support results in more independent walking than assisted overground walking in non-ambulatory patients early after stroke: a systematic review. J Physiother. 2010;56:153–61. doi: 10.1016/S1836-9553(10)70020-5 [DOI] [PubMed] [Google Scholar]
- 4.Swinnen E, Duerinck S, Baeyens JP, Meeusen R, Kerckhofs E.. Effectiveness of robot-assisted gait training in persons with spinal cord injury: a systematic review. J Rehabil Med. 2010;42:520–6. doi: 10.2340/16501977-0538 [DOI] [PubMed] [Google Scholar]
- 5.Forrest GF, Sisto SA, Barbeau H, Kirshblum SC, Wilen J, Bond Q, et al. . Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J Spinal Cord Med. 2008;31:509–21. doi: 10.1080/10790268.2008.11753646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coupaud S, Jack LP, Hunt KJ, Allan DB.. Muscle and bone adaptations after treadmill training in incomplete spinal cord injury: a case study using peripheral Quantitative Computed Tomography. J Musculoskeletal Neuronal Interact. 2009;9:288–97. [PubMed] [Google Scholar]
- 7.Turiel M, Sitia S, Cicala S, Magagnin V, Bo I, Porta A, et al. . Robotic treadmill training improves cardiovascular function in spinal cord injury patients. Int J Cardiol. 2011;149:323–9. doi: 10.1016/j.ijcard.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 8.Adams MM, Hicks AL.. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med. 2011;34:488–94. doi: 10.1179/2045772311Y.0000000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginis KA M, Latimer AE.. The effects of single bouts of body-weight supported treadmill training on the feeling states of people with spinal cord injury. Spinal Cord. 2007;45:112–5. doi: 10.1038/sj.sc.3101911 [DOI] [PubMed] [Google Scholar]
- 10.Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM, et al. . Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab. 2006;31:283–91. doi: 10.1139/h05-036 [DOI] [PubMed] [Google Scholar]
- 11.Huang Q, Yu L, Gu R, Zhou Y, Hu C.. Effects of robot training on bowel function in patients with spinal cord injury. J Phys Ther Sci. 2015;27:1377–8. doi: 10.1589/jpts.27.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekso B. Ekso Bionics. 2017.
- 13.Parker HC. Indego – Powering People Forward | Parker Indego. 2017.
- 14.ReWalk Robotics I . ReWalk – More Than Walking. 2016.
- 15.Rex Bionics L . Rex Bionics – Step into the future. 2017.
- 16.What’s HAL®? The world’s first cyborg-type robot “HAL®” (Hybrid Assistive Limb). Cyberdyne Inc. 2018 [cited 2018 Nov 10]. Available from https://www.cyberdyne.jp/english/products/HAL/index.html.).
- 17.Food and Drug Administration HHS . Medical devices; physical medicine devices; classification of the powered lower extremity exoskeleton; republication. Final order; republication. Fed Regist. 2015;80:25226–30. [PubMed] [Google Scholar]
- 18.Asselin P, Knezevic S, Kornfeld S, Cirnigliaro C, Agranova-Breyter I, Bauman WA.. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J Rehabil Res Dev. 2015;52:147–58. doi: 10.1682/JRRD.2014.02.0060 [DOI] [PubMed] [Google Scholar]
- 19.Evans N, Hartigan C, Kandilakis C, Pharo E, Clesson I.. Acute cardiorespiratory and metabolic responses during exoskeleton-assisted walking overground among persons with chronic spinal cord injury. Top Spinal Cord Inj Rehabil. 2015;21:122–32. doi: 10.1310/sci2102-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fineberg DB, Asselin P, Harel NY, Agranova-Breyter I, Kornfeld SD, Bauman WA, et al. . Vertical ground reaction force-based analysis of powered exoskeleton-assisted walking in persons with motor-complete paraplegia. J Spinal Cord Med. 2013;36:313–21. doi: 10.1179/2045772313Y.0000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolakowsky-Hayner SA, Crew J, Moran S, Shah A.. Safety and feasibility of using the EksoTM bionic exoskeleton to aid ambulation after spinal cord injury. J Spine. 2013;S4:003. doi: 10.4172/2165-7939.S4-003 [DOI] [Google Scholar]
- 22.Kozlowski AJ, Bryce TN, Dijkers MP.. Time and effort required by persons with spinal cord injury to learn to Use a powered exoskeleton for assisted walking. Top Spinal Cord Inj Rehabil. 2015;21:110–21. doi: 10.1310/sci2102-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sale P, Russo EF, Russo M, Masiero S, Piccione F, Calabrò, RS, et al. . Effects on mobility training and de-adaptations in subjects with spinal cord injury due to a Wearable robot: a preliminary report. BMC Neurol. 2016;16:12-016-0536-0. doi: 10.1186/s12883-016-0536-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen O, Grasmuecke D, Meindl RC, Tegenthoff M, Schwenkreis P, Sczesny-Kaiser M, et al. . Hybrid Assistive limb exoskeleton HAL in the rehabilitation of chronic spinal cord injury: Proof of concept; the results in 21 patients. World Neurosurg. 2018;110:e73–e8. doi: 10.1016/j.wneu.2017.10.080 [DOI] [PubMed] [Google Scholar]
- 25.Platz T, Gillner A, Borgwaldt N, Kroll S, Roschka S.. Device-Training for individuals with thoracic and lumbar spinal cord injury using a powered exoskeleton for technically assisted mobility: achievements and user satisfaction. Biomed Res Int. 2016;2016:8459018. doi: 10.1155/2016/8459018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spungen AM, Asselin P, Fineberg DB, Kornfeld SD, Harel NY.. Exoskeletal-assisted walking for persons with motor-complete paraplegia. Research and Technology Organization. Human Factors, and Medicine Panel: North Atlantic Treaty Organization. 2013.
- 27.Asselin PK, Avedissian M, Knezevic S, Kornfeld S, Spungen AM.. Training persons with spinal cord injury to ambulate using a powered exoskeleton. J Visual Exp. 2016;112. doi: 10.3791/54071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scivoletto G, Tamburella F, Laurenza L, Foti C, Ditunno JF, Molinari M.. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord. 2011;49:736–40. doi: 10.1038/sc.2010.180 [DOI] [PubMed] [Google Scholar]
- 29.Kozlowski AJ, Fabian M, Lad D, Delgado AD.. Feasibility and safety of a powered exoskeleton for assisted walking for persons with Multiple Sclerosis: A single-group preliminary study. Arch Phys Med Rehabil. 2017;98:1300–7. doi: 10.1016/j.apmr.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 30.Rehabilitation Institute of C . Rehabilitation measures database. 2010.
- 31.Chang SH, Afzal T, Berliner J, Francisco GE, Group TSCE.. Exoskeleton-assisted gait training to improve gait in individuals with spinal cord injury: a pilot randomized study. Pilot Feasibility Stud. 2018;4:62. doi: 10.1186/s40814-018-0247-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middleton A, Fritz SL, Lusardi M.. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23:314–22. doi: 10.1123/japa.2013-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swank C, Sikka S, Driver S, Bennett M, Callender L.. Feasibility of integrating robotic exoskeleton gait training in inpatient rehabilitation. Disabil Rehabil Assist Technol. 2019;17:1–9. [DOI] [PubMed] [Google Scholar]
- 35.Baunsgaard CB, Nissen UV, Brust AK, Frotzler A, Ribeill C, Kalke YB, et al. . Gait training after spinal cord injury: safety, feasibility and gait function following 8 weeks of training with the exoskeletons from Ekso Bionics. Spinal Cord. 2018;56:106–16. doi: 10.1038/s41393-017-0013-7 [DOI] [PubMed] [Google Scholar]
- 36.He Y, Eguren D, Luu TP, Contreras-Vidal JL.. Risk management and regulations for lower limb medical exoskeletons: a review. Med Devices (Auckl). 2017;10:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorgey AS. Robotic exoskeletons: The current pros and cons. World J Orthop. 2018;9:112–9. doi: 10.5312/wjo.v9.i9.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagen EM. Acute complications of spinal cord injuries. World J Orthoped. 2015;6:17–23. doi: 10.5312/wjo.v6.i1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Partida E, Mironets E, Hou S, Tom VJ.. Cardiovascular dysfunction following spinal cord injury. Neural Regen Res. 2016;11:189–94. doi: 10.4103/1673-5374.177707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witiw CD, Fehlings MG.. Acute spinal cord injury. J Spinal Disord Techniques. 2015;28:202–10. doi: 10.1097/BSD.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 41.Ueba T, Hamada O, Ogata T, Inoue T, Shiota E, Sankai Y.. Feasibility and safety of acute phase rehabilitation after stroke using the hybrid assistive limb robot suit. Neurol Med Chir (Tokyo). 2013;53:287–90. doi: 10.2176/nmc.53.287 [DOI] [PubMed] [Google Scholar]
- 42.Cheung EYY, Ng TKW, Yu KKK, Kwan RLC, Cheing GLY.. Robot-Assisted training for People with spinal cord injury: A Meta-analysis. Arch Phys Med Rehabil. 2017;98:2320-31.e12. doi: 10.1016/j.apmr.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 43.National Spinal Cord Injury Statistical C . Spinal cord injury Facts and Figures at a Glance. J Spinal Cord Med. 2019;37:355–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey LA. Physiotherapy rehabilitation for people with spinal cord injuries. J Physiother. 2016;62:4–11. doi: 10.1016/j.jphys.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 45.Tsai C, Delgado A, Weinrauch W, Manente N, Levy I, Escalon M, et al. . The effects of exoskeletal-assisted walking during acute inpatient rehabilitation for people with spinal cord injury: a Pilot Study. Waikiki, Hawaii: Poster presented at the American Spinal Injury Association (ASIA) 2019 Annual Scientific Meeting; 2019.