ABSTRACT
Ten specimens of coral reefs were collected from the Red Sea in the Ein El-Sukhna region. Fungal isolation was done using two media, Dextrose Yeast Extract Agar (DYA) and Rose Bengal Agar (RBA). The morphological traits identified 18 fungal isolates belonging to the phyla Ascomycota, Mucoromycota and Deuteromycota. Five genera in three orders have been isolated: Eutrotiales (Aspergillus, Penicillium and Byssochlamys), Mucorales (Rhizopus) and Moniliales (Curvularia). The heat mapping clustering of the isolated fungi declared that Aspergillus and Penicillium were the most frequently isolate fungi in coral reefs. It was found that A. fumigatus colonised eight coral samples with 80% colonisation rate. Moreover, about 50% of the isolated fungal species were specific to one coral reef only such as A.candidus and A.carneus isolated from Isophyllastrea rigida only, A.japonicus and A.ochraceopetaliformis from Glaxaea fascicularis, A.niger van Tieghem from Porites astreoides, A.sydowii, A.terreus and P.waksmanii from Cladocora arbuscula, P.janthinellum from Pterogorgia guadalupensis and Curvularia tuberculata, Byssochlamys spectabilis and Rhizopus oryzae from Acropora humilis. Biological activities (antimicrobial, antioxidant antiradical and cytotoxicity) of the most predominant fungal species were investigated. The antimicrobial activity of coral fungal filtrates were investigated against six pathogenic bacteria including Escherichia coli ATCC11775, Neisseria gonorrhoeae ATCC19424, Pseudomonas aeruginosa ATCC10145, Streptococcus faecalis ATCC19433, Staphylococcus aureus subsp. aureus ATCC25923, Bacillus subtilis subsp. spizizenii ATCC6633 and two pathogenic yeast including Candida albicans ATCC7102 and Candida parapsilosis ATCC22019. Most of these fungal filtrates exhibited moderate to high antibacterial activities against both gram positive and gram negative bacteria, however it showed relatively low bioactivity towards the pathogenic Candida species. Investigating the free radical scavenging activity using DPPH reagent showed low to moderate bioactivities. The highest cytotoxic activity against liver cancer cell line Hep-G2 with an IC50 values of 18.8 µg/ml was exhibited by Aspergillus ochraceopetaliformis MN083316 and a metabolomics study was done on the ethyl acetate extract of this strain using LC-ESI-MS fingerprints leading to the isolation and purification of compound 1. Using 1D and 2D NMR techniques compound 1 was identified as ditryptophenaline. Compound 1 exhibited a strong antimicrobial, antioxidant activities as well as cytotoxic activities against MCF-7 and HEPG2 with IC50 values of 5.8 and 7.6 mmole, respectively.
The objective of this study, isolation of Coral-reef associated fungi and studying their biological activities to produce the most active secondary metabolite which might possess a novel biological activity.
KEYWORDS: Coral reefs, Red Sea, fungal variability, antimicrobial activity, free radical scavenging activity, cytotoxic activity
Introduction
More than 70% of the earth’s surface occupying by the oceans that support large habitats of living organisms which are considered as a source of a vast groups of structurally unique natural products. These natural products are mainly isolated from invertebrates common to ecosystems of coral reefs, such as tunicates, sponges, soft corals, molluscs and bryozoans (Putri et al. 2015).
Approximately 70,000 fungal species have been described as symbiotic microbes (John et al. 2009), among them 1500 species of marine-derived fungi were mentioned largely from coastal ecosystems (John et al. 2009). They are associated with abundant forms of marine organisms including sponges (Harvell et al. 1999), scleractinian corals and gorgonians.
Although the microbial communities (especially fungi) associated with coral reefs possessed a little attention, coral reefs are known as one of the most varied ecosystems on earth. These microbial communities are recognised to be very important members of many ecosystems especially that of coral reefs (Balser et al. 2006; Gutnecht et al. 2006; Schimel et al. 2007). Actually, the coral reef supporting broad microbial diversity facilitating structurally and environmentally complex array of habitats. This microbial diversity affects both host physiology and ultimately ecosystem processes (Ainsworth et al. 2009). Novel bioactive natural products that are not found in terrestrial strains have been produced by marine microbes (Jensen and Fenical 2002; Konig et al. 2006; Blunt et al. 2008). The actual producers or participants in the biosynthesis of secondary metabolites isolated from the marine hosts are the symbiotic microorganisms (Proksch et al. 2003; Li 2009). The symbiotic microbes in corals are believed to be a promising source of new drugs that are still unexplored (Radjasa 2004; Bhatnagar and Kim 2010).
Marine-derived fungi have been known as a source of structurally novel metabolites of potent bioactivity (Bugni and Ireland 2004; Blunt et al. 2006; Saleem et al. 2007). The secondary metabolites produced by coral-associated fungi are of great interest (Putri et al. 2015). The extract produced by many Aspergillus and Penicillium species are known to produce a large variety of active compounds, such as: Aspergillus versicolor isolated from soft coral Xestospongia exigua was found to be rich source of novel polyketides (Putri et al. 2015).
Marine-derived fungi (MDF) live in a symbiotic relationship with marine invertebrates and produce a huge numbers of novel bioactive metabolites including antibiotics, antioxidants, antitumors, antifungals, antialgals, antiinsects and acetylcholine esterase inhibitors (Kjer et al. 2010; Lee et al. 2010; Almeida et al. 2011; Chu et al. 2011; Thirunavukkarasu et al. 2012). Marine derived Aspergillus species produce many non-ribosomal peptides, polyketides, lipopeptides and isoprenoids of pharmaceutical importance (Mayer and Hamann 2004). Aspergillus versicolor produce a novel lipopeptide (Lee et al. 2010) and Aspergillus niger synthesises seven new diterpenoides (Hiort et al. 2004).
In the present work, the culturable diversity of fungi isolated from coral reefs, collected from Ein El-Sukhna region, Red Sea, Egypt were investigated. The antimicrobial, free radical scavenging and cytotoxic activities of fungal symbionts were also assayed. Moreover, the LC-MS fingerprints of the ethyl acetate extract of the most potent fungus, Aspergillus ochraceopetaliformis MN083316 were studied.
Materials and methods
Collection of coral reef materials
Ten specimens of coral reefs belonging to nine families (Acroporide, Faviidae, Euphylliidae, Scleractinia incertae sedis, Gorgoniidae, Mussidae, Poritidae, Pocilloporidae and Agariciidae) were collected from Ein El-Sukhna-Zafarana Rd 65 KM, Red Sea, Egypt at depth of 2–5 m by SCUBA diving and preserved under aseptic conditions until transported to the laboratory in ice box.
Culture media
Dextrose yeast agar (DYA) (g/l): [dextrose (10), yeast extract (10), agar (20)] and Rose bengal agar (RBA) (g/l):[peptone (5), glucose (10), KH2PO4 (1), MgSO4.7H2O (0.5), Rose Bengal (0.33), Agar (20)] were prepared using sea water supplied with the antibiotic benzyl penicillin (150 mg/l) to prevent bacterial growth.
Isolation of coral reef symbiotic fungi
Coral reef samples were rinsed with sterile distilled water (3 times) prior to isolation processes.
Impression method
A small pieces of approximately 1 cm3 and 2 cm3 of the inner tissues of each coral species (soft and hard, respectively) were excised under sterile conditions with scalp and forceps and directly spread onto petri plates containing different culture media (Koh et al. 2000; Cao et al. 2015).
Dilution method
Pieces of soft coral (1 cm3) were crushed using a sterile morter and pestle. The homogenised tissues were serially diluted (10−2 and 10−3) with sea water. One ml/dish from each dilution were plated on petri dishes containing different culture media (Putri et al. 2015).
Blender method
The specimens of stony coral were cut into small pieces and homogenised using a blender containing 20 ml sterile sea water under aseptic conditions. 100 µl of the resulting homogenate were directly plated onto petri dishes containing different culture media (Wang et al. 2011).
The plates were incubated at 25–27 °C for one week. Each developed fungal isolate was individually picked and transferred onto a new fresh medium and incubated at 25–27 °C for one week for purification and identification purposes. Stock cultures of the purified fungal isolates were sub-cultured on slants and preserved in refrigerator. The identification of fungal species takes place using the available identification references and molecular identification while the identification of coral reef species was performed with the aid of Marine Species Identification Portal. They were found to be Acropora humilis, Favia speciosa, Glaxaea fascicularis, Acropora cervicornis, Cladocora arbuscula, Pterogorgia guadalupensis, Isophyllastrea rigida, Porites astreoides, Stylophora pistillata and Povona clavus.
Extraction of the secondary metabolites from isolated symbiotic fungi
Pure fungal species were inoculated into 500 ml Erlenmeyer flasks containing 200 ml of both DY and RB broths. After incubation at 27–30 °C under shaking at 120 rpm for 12 days, the mycelia were separated from the culture filtrate by filtration. The supernatants were extracted with ethyl acetate (EtOAc) three successive times. The EtOAc phase was evaporated under vacuum using rotary evaporator to give solid or oily extract. On the other hand, mycelia were extracted with EtOAc three times to yield EtOAc extract. The obtained extracts were lyophilised and stored freeze dry to be used for bioassays (Wang et al. 2011).
Biological activity of the isolated symbiotic fungi
Assay of antimicrobial activity
Agar disc diffusion method was used. Paper discs were impregnated with 20 mg of the extracts dissolved in 1 ml DMSO. The discs were placed upon agar plates inoculated with bacterial and fungal pathogens including Streptococcus faecalis ATCC19433, Staphylococcus aureus subsp. aureus ATCC25923, Escherichia coli ATCC11775, Neisseria gonorrhoeae ATCC19424, Pseudomonas aeruginosa ATCC10145, Bacillus subtilis subsp. spizizenii ATCC6633 and two pathogenic fungi including Candida albicans ATCC7102 and Candida parapsilosis ATCC22019. Plates incubated at 37 °C and after 24 hr incubation, the inhibition zone diameters (mm) around discs were measured. Negative controls were only treated with DMSO. Relative activity was determined in response to the standard antibiotics used. Augmentin and fluconazole were used as positive controls for antibacterial and antifungal, respectively. Relative activity of the test extract = (x-y/z-y) × 100 (Gaurav et al. 2010). Where, (x): total area of inhibition of the test extract, (y): total area of inhibition of the solvent, (z):total area of inhibition of the standard drug.
Assay of free radical scavenging activity
It was performed using free radical scavenging (FRS) model according to (Hamed 2009). One mg of EtOAc extract of each of the fungal species were dissolved in 1 ml DMSO to prepare stock solution of 1000 µg/ml. 0.0035 g DPPH (2,2-diphenyl-1-picrylhydrazyl radical) was dissolved in 100 ml of methanol HPLC grade to prepare 0.0035% solution and stored in dark until use. 0.1 ml of stock solution was added to 0.9 ml of methanolic DPPH solution to reach the maximum concentration of tested samples 100 µg/ml. The reaction mixture was incubated for 30 min, then measured at wave length 540 nm. Blank using DMSO was measured. The free radicals scavenging activity of fungal extracts were calculated from the following equation:
Where, Ablank (Absorbance of reaction mixture without test sample “DPPH” only).
Asample (Absorbance of reaction mixture in presence of test samples).
Assay of cytotoxic activity
Assay of cytotoxicity of symbiotic fungal extracts against human cancer cell lines
Human liver carcinoma (HEPG 2) and breast cancer (MCF7) cell lines used in this study were obtained from the American Type Culture Collection (ATCC, Minnesota, USA). Different concentrations of the extract were prepared (5, 12.5, 25, 50 µg/ml) using DMSO. Cells were seeded in 96-well microtiter plates at initial concentration of 3 × 103 cell/well in 150 µl fresh medium and left for 24 hr to attach to the plates. Different concentrations 0, 5, 12.5, 25, 50 µg/ml of sample were added. For each sample concentration 3 wells were used. The plates were incubated for 48 hr. The cells were fixed with 50 μl cold trichloroacetic acid 10% for 1 hr at 4 C. Then the plates were washed with distilled water using automatic washer Tecan, Germany and stained with 50 μl 0.4% SRB dissolved in 1 % acetic acid for 30 min at room temperature and washed with 1 % acetic acid and air-dried. The dye was solubilised with 100 μl/well of 10 M tris base (pH 10.5) and optical density (O.D.) of each well was measured spectrophotometrically at 570 nm with an ELISA microplate reader (Sunrise Tecan reader, Germany). The mean background absorbance was automatically subtracted and mean values of each sample concentration was calculated.
Calculation
The percentage of cell survival was calculated as follows:
Surviving fraction = O.D. (treated cells)/O.D. (control cells).
The IC50 values (the concentrations of resveratrol required to produce 50% inhibition of cell growth) were also calculated.
The LC-MS fingerprints of the A.ochraceopetaliformis EtOAc extract
The fungual species, A. ochraceopetaliformis MN083316 was cultured on RBA medium (2 L, 250 mL medium/500 mL Erlenmeyer flask) at 120 rpm, 27ºC for 12 days. The culture filtrate was extracted thrice with EtOAc and the resulting EtOAc extract was evaporated under vacuum to give EtOAc extract for future analysis.
LC-ESI-MS spectrum was measured with an Agilent Technologies (Santa Clara, USA) HPLC1260 series coupled to Agilent Technologies (Santa Clara, USA) 6420 Triple quad LC-MS ion trap mass spectrometer fitted with an ESI source (Toyama University, Toyama, Japan). The samples were dissolved in methanol for HPLC (1 mg/mL) for injection into the HPLC-ESI-MS system. Separation through LC-column was performed using gradient separation from 20-100% methanol/water over a period of 20 min. with a flow rate 1 ml/min and monitored by absorption at 254 and 210 nm with a photodiode array detector. Positive and negative ionisation modes were detected with mass scan range 50–1500 amu. NMR spectra were recorded at 500 MHz (1H NMR) and 125 MHz (13C NMR) on a JEOL ECA500II spectrometer with chemical shift values expressed in δ (ppm) downfield from TMS, as an internal standard. Column chromatography was performed with Cosmosil 5C18-Ar-II column, Nacalai Tesque INC., 10 × 250 mm, Agilent, USA).
Statistical analysis
Was performed by SPSS 15.0 for windows evaluation version. (SPSS Inc. Released 2007. SPSS for Windows, Version 15.0. Chicago, SPSS Inc.)
Results and discussion
Variability of coral reef symbiotic fungi
Three techniques and two nutrient media were used for isolation of coral reef associated fungi. A total of 137 fungal isolates constituting 18 species were screened from the ten coral reef samples. Hard corals attained 80 isolates with frequency 58.4% while soft corals recorded 57 isolates with frequency 41.6% of the total isolates (Table 1). Impression and blender techniques were used for hard corals. Impression technique revealed 16 isolates representing 20% of the total taxa, whereas blender technique recorded 64 isolates representing 80% of the total count of hard coral. Dilution and impression techniques were used for soft corals. The dilution technique recorded higher count than the impression technique where 52 isolates with frequency 91.2 % while 5 isolates with 8.8 % frequency were detected, respectively (Table 1).
Table 1.
Total isolates and frequency percentage of coral Reefs symbiotic fungi isolated on four cultural techniques.
| Isolation techniques |
||||||
|---|---|---|---|---|---|---|
| Coral species |
Impression |
Blender |
||||
| Hard coral | TI (cfu/plate) |
Freq. % | TI (cfu/plate) |
Freq. % | Total isolates/survay |
Freq. % |
| Acropora humilis | 3 | 18.75 | 14 | 21.88 | 17 | 21.25 |
| Favia speciosa | 4 | 25.00 | 1 | 1.56 | 5 | 6.25 |
| Glaxaea fascicularis | 2 | 12.50 | 6 | 9.40 | 8 | 10.00 |
| Acropora cervicornis | 2 | 12.50 | 8 | 12.50 | 10 | 12.50 |
| Cladocora arbuscula | 1 | 6.25 | 11 | 17.20 | 12 | 15.00 |
| Stylophora pistillata | 2 | 12.50 | 1 | 1.56 | 3 | 3.75 |
| Povona clavus | 2 | 12.50 | 23 | 36.00 | 25 | 31.25 |
| Total |
16 |
20.00 |
64 |
80.00 |
80 |
58.40 |
|
Impression |
Dilution |
|||||
|
Soft coral |
TI (cfu/ plate) |
Freq. % |
TI (cfu/ plate) |
Freq. % |
Total isolates/survay |
Freq. % |
| Pterogorgia guadalupensis | 1 | 20.00 | 5 | 9.60 | 6 | 10.53 |
| Isophyllastrea rigida | 2 | 40.00 | 45 | 86.54 | 47 | 82.46 |
| Porites astreoides | 2 | 40.00 | 2 | 3.85 | 4 | 7.02 |
| Total | 5 | 8.77 | 52 | 91.23 | 57 | 41.60 |
| - TI: Total isolates (cfu/coral specimens plated), Freq. % = No. of isolated species/Total isolates × 100 | 137 | 100 | ||||
Medium DYA proved to be the most suitable in blender technique for isolation of symbiotic fungi from hard coral reef with frequency 41.3 % (Table 2). Meanwhile, medium RBA was the most suitable in soft coral using dilution technique with frequency 87.7%. Consequently the former media and techniques were used in the next experiments.
Table 2.
Fungal isolates screened from ten coral species collected from Red Sea, El-Ein El- Soukhna region using two growth media.
| Isolation techniques |
|||||
|---|---|---|---|---|---|
| Impression |
Blender |
||||
| Hard coral |
Media |
Media |
|||
| Coral species | DYA | RBA | DYA | RBA | |
| Acropora humilis | 2 | 1 | 11 | 3 | |
| Favia speciosa | 4 | - | - | 1 | |
| Glaxaea fascicularis | 1 | 1 | - | 6 | |
| Acropora cervicornis | - | 2 | 8 | - | |
| Cladocora arbuscula | 1 | - | 10 | 1 | |
| Stylophora pistillata | 1 | 1 | 1 | - | |
| Povona clavus | 2 | - | 3 | 20 | |
| Total Isolates (CFU) | 11 | 5 | 33 | 31 | |
| Freq. % |
13.75 |
6.30 |
41.30 |
38.80 |
|
|
Impression |
Dilution |
||||
|
Media |
Media |
||||
|
Soft coral |
DYA |
RBA |
DYA |
RBA |
|
| Pterogorgia guadalupensis | 1 | - | 1 | 4 | |
| Isophyllastrea rigida | - | 2 | 1 | 44 | |
| Porites astreoides | 2 | - | - | 2 | |
| Total Isolates (CFU) | 3 | 2 | 2 | 50 | |
| Freq. % | 5.30 | 3.50 | 3.50 | 87.70 | |
| Total Isolates (CFU) in both Hard and Soft corals | DYA | RMA | 137 | ||
| 49 | 88 | ||||
| Freq. % | 35.70 | 64.30 | 100 | ||
DYA: Dextrose Yeast Extract Agar, RBA: Rose Bengal Agar.
Venn diagram (Figure 1), showed that a number of overlapped fungal species were isolated from both hard and soft coral samples when cultured on DYA and RBA media. On the DYA medium 33 fungal isolates were overlapped between both coral reef species, while 15 and 1 isolates were specific to hard and soft coral species, respectively. Also in RBA medium 34 fungal isolates overlapped between both coral reef species, whereas a large number of fungal isolates were specific to hard (11 isolates) and soft (43 isolates) coral reef.
Figure 1.

Venn diagram of the total number and the overlap fungal species isolated from coral reefs using different growth media (a) DYA and (b) RBA.
The heat map clustering (Figure 2(a,b)) of total fungal count declared that Aspergilli were the most frequent genera in both hard and soft corals. Aspergillus aculeatus, A. parasiticus, A. carneus and A. fumigatus being the highly colonised species to coral reefs. Genus Penicillium followed in density of occurrence in coral reefs. It was found that two infrequent genera, Curvularia tuberculata and Rhizopus oryzae, were detected once with 2 and 1 colonies from coral tissues, respectively.
Figure 2.

(a) and (b) Heatmap clustering of the total number of symbiotic fungal species isolated from Coral reefs.
Concerning the variation in coral reefs, heat map Figure 2(a,b) indicates that the soft coral reef species Isophyllastrea rigida was highly colonised with fungal isolates representing 34.31% of total isolates in the ten samples. Followed by the hard coral reefs Povona clavus and Acropora humilis representing 19% and 12.4% of the total fungal count, respectively.
In close relation, limited information is known about the microbial diversity associated with marine coral reefs, despite the vital role that microorganisms may play in coral reef ecosystems (Wang et al. 2011). Fifty three isolates belong to 18 fungal species were isolated from the tissue of the gorgonian coral Echinogorgia rebekka from the Weizhou coral reef in the South China Sea. They are all belongs to the family Ascomycota and were distributed among seven genera in five orders Eurotiales (Aspergillus, Penicillium), Capnodiales (Cladosporium), Trichosphaerial (Nigrospora), Pleosporales (Alternaria) and Hypocreales (Hypocrea and Nectria) (Wang et al. 2011). The diversity of coral reef associated microorganisms has been poorly investigated in remote geographical areas like Red Sea (Strobel and Daisy 2003; Huang et al. 2007). Meanwhile (Koh et al. 2000) isolated 51 isolates of deuteromycetes from 18 fungal taxa, 2 types of yeasts and several isolates of sterile filamentous fungi. Penicillium spp. from the P. janthinellum series appear to be ubiquitous to gorgonian coral reef species.
Biological activities of coral reef symbiotic fungi
The biological activities were carried out on the all of the isolated symbiotic fungi then the most active and frequent fungal genera present were chosen, Aspergillus ochraceopetaliformis MN083316, A. aculeatus, A. fumigatus, A. carneus, A. parasiticus and B. spectabilis MN093943.
Antimicrobial activity of coral reef symbiotic fungi
Antimicrobial metabolites in coral reef fungal extracts are one of the most promising resources due to the increasing demand to novel antibiotic drugs with new mechanism of action to overcome bacterial drug resistance. A large number of symbiotic microorganisms in corals are considered to be a promising source of these novel drugs which have been largely unexplored (Radjasa 2004; Bhatnagar and Kim 2010).
The present data (Figure 3) declared high antimicrobial potentiality of most of the fungal filtrate extracts against the tested pathogenic bacteria and yeast. The most active fungal filtrate extract was Aspergillus ochraceopetaliformis MN083316 grown on both media DYA and RBA. It exhibited antimicrobial activity against pathogenic bacteria and yeast with relative activity ranging (37–110%) and (25-30%) in DYA medium, respectively. While in RBA medium, the antimicrobial activities of A. ochraceopetaliformis MN083316, A. fumigatus and B. spectabilis were much higher than the other fungal species. A. ochraceopetaliformis MN083316 showed relative activity ranging from 39–85% against bacteria and from 25 − 35% against yeast, A. fumigatus exhibited relative activity from 30- 70% against bacteria and from 25 − 28% against yeast while, B. spectabilis has relative activity ranging from 30–70% against bacteria and from 22 − 45% against yeast.
Figure 3.
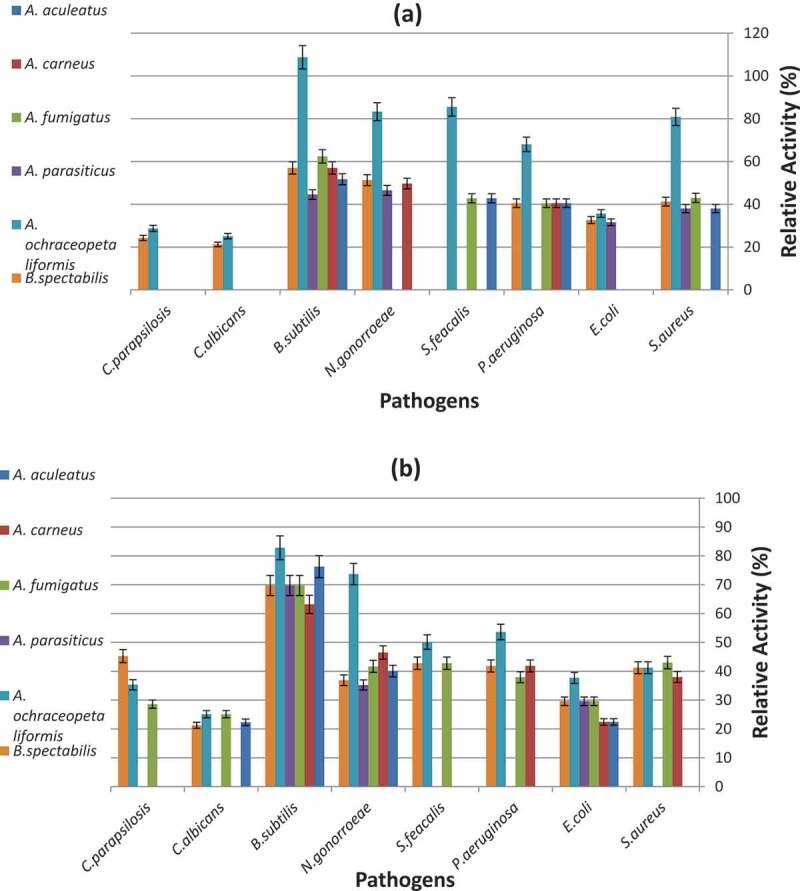
Antimicrobial activity of the most frequent Coral reefs symbiotic fungal species (a) Cultured on DYA, (b) Cultured on RBA.
Candida albicans and C. parapsilosis showed high resistance to the fungal filtrate extracts compared to bacterial pathogens, where the fungal isolates cultured on DYA media showed no activity against both candida except in cased A. ochraceopetaliformis MN083316 and B. spectabilis with relative activity 23-30% and 21-25%, respectively. Among bacteria, E. coli ATCC11775 and S. feacalis ATCC19433 were the most resistant pathogens treated with fungal filtrate extracts (DYA medium) as 3 only of the 6 fungal extracts showed activity. The highly susceptible pathogen was B. subtilis ATCC6633 treated with A. ochraceopetaliformis MN083316 (DYA medium) as it exhibited the highest relative activity (110%) in the assay compared to other pathogens used.
Similar results were obtained by Wang et al (Wang et al. 2011) who claimed that out of 18 fungal strains isolated from Gorgonian coral Echinogorgia rebekka collected from the Weizhou coral reef in the South China Sea, 12 were found to show moderate to high level of antibacterial activities against S. aureus, while 9 had moderate to very high activities against M. tetragenus. (Wang et al. 2017) further reported that the compounds isolated from coral-derived Aspergillus tritici which isolated from the coral Galaxea fascicularis collected at Port Dickson, Malaysia, showed high antibacterial activity against Methicillin-resistant Staphylococcus aureus (MRSA), Vibrio vulnificus, Vibrio rotiferianus and Vibrio campbellii. (Sabdaningsih et al. 2016) tested the crude extracts of marine derived fungi associated with soft corals isolated from Panjang Island against MDR-Staphylococcus haemolyticus and found that it exhibited strong antibacterial activity. (Abd El-Hady et al. 2014) examined the supernatant and mycelial extracts from the static culture of Emericella unguis isolated from the soft coral Sinularia sp. collected from Hurghada coast, Red Sea, Egypt, and found that it showed high antimicrobial activity against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans.
As a result, we can assume that there is a symbiotic relationship between coral reef and its associated fungi enabling fungi to be an alternative to produce antibacterial compounds to overcome the multidrug resistant pathogens by producing novel secondary metabolites, Mabrouk et al (Mabrouk et al. 2008).
Free radical scavenging activity of coral reef associated fungi
According to results shown in Figure 4, the growth medium have a role in the antioxidant activity of the coral reef symbiotic fungi. A. aculeatus and A. parasiticus grown on DYA showed higher antioxidant activity than those grown on RBA. Whereas A. carneus, A. fumigatus, A. ochraceopetaliformis MN083316 and B. spectabilis exhibited higher antioxidant activities when grown on RBA than on DYA. The descending arrangement of the most potent antioxidant fungal species grown on DYA was A. ochraceopetaliformis (19.5%) ˃ A. aculeatus (15.6%) ˃ B. spectabilis (14.2%) ˃ A. carneus (11.3%) ˃ A. parasiticus (11%) ˃ A. fumigatus (10%). However, A. ochraceopetaliformis (22%) ˃ B. spectabilis (16.6%) ˃ A. carneus (13%) ˃ A. fumigatus (11%) ˃ A. aculeatus (9.6%) ˃ A. parasiticus (9%) were descendly arranged in case of RBA. From the previous results, we declare that A. ochraceopetaliformis MN083316 is the most active antioxidant isolate when grown on both media DYA and RBA. Similar results were obtained by (Mabrouk et al. 2008), who reported that fungi associated with the soft coral Sinularia sp. collected from Hurghada coast, Red Sea, Egypt, showed antioxidant and antimicrobial activities.
Figure 4.
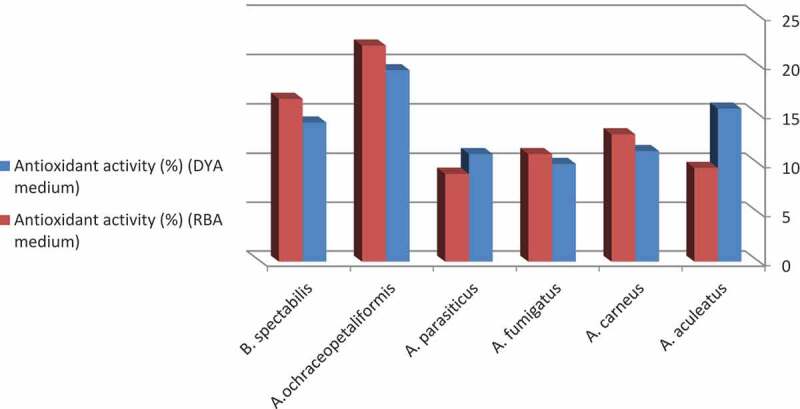
Antioxidant activity of the filtrate extract of the most frequent fungal species isolated from Red Sea Coral reefs.
Cytotoxicity of coral reef symbiotic fungi against tumour cell line
The cytotoxic activity was assayed in the filtrate extract of the most active coral reef symbiotic fungal species (A. ochraceopetaliformis MN083316) which exhibited also the highest antimicrobial and antioxidant activities (Figure 5). The fungal species showed high cytotoxic activity against human liver carcinoma cell line (HEPG-2) with IC50 value 18.8 µg/ml. In close relation, the culture filtrate extract of A. tritici isolated from the coral Galaxea fascicularis collected at Port Dickson, Malaysia, showed cytotoxic and antimicrobial activities (Sabdaningsih et al. 2016). (Hou et al. 2017) isolated cytotoxic compound Aspersymmetide A from A. versicolor isolated from a Gorgonian Carijoa sp. collected from Weizhou coral reefs in South China Sea. This compound displayed cytotoxic activity against NCI-H292 and A431 cell lines. Chondrosterins A isolated from soft coral associated fungus Chondrostereum sp. showed potent cytotoxicity against human lung cancer cell line A549, human nasopharyngeal carcinoma cell line CNE2 and human colon cancer cell line Lovo (Li et al. 2012).
Figure 5.

Cytotoxic activity of the filtrate extract of Aspergillus ochraceopetaliformis MN083316 isolated from Red Sea Coral species against human liver carcinoma cell line (HEPG-2).
The LC-MS fingerprints of the A. ochraceopetaliformis EtOAc extract
In order to correlate the promising biological activities to the active constituents of the ethyl acetate extract of A. ochraceopetaliformis MN083316 grown on RBA medium, the LC–MS of the ethyl acetate extract revealed the presence of several metabolites. From the ESI mass spectrum (Figure 6), we could expect the mass of compound 1 appearing at retention time 17.75 min to be 692 deduced from the two molecular ion peaks [M]+ at m/z 693 and [M + Na]+ at m/z 715 (Figure 7). Isolation and purification of compound 1 from the crude EtOAc extract was done with RP-HPLC using 70% MeOH/water for 60 min at flow rate 2 ml/min at UV 254 nm to afford compound 1 (6.3 mg). From 1H (Figure 8), 13C (Figure 9), COSY and HMQC NMR spectra and comparison of this data with similarly isolated compounds we could deduce the structure of 1 to be the diketopiperazine compound ditryptophenaline (Figure 10).
Figure 6.

Metabolomic profiling using LC-MS, chromatogram of ethyl acetate extract of A.ochraceopetaliformis showing (a) Total ion chromatogram, (b) UV 210 nm and c) UV 254 nm.
Figure 7.
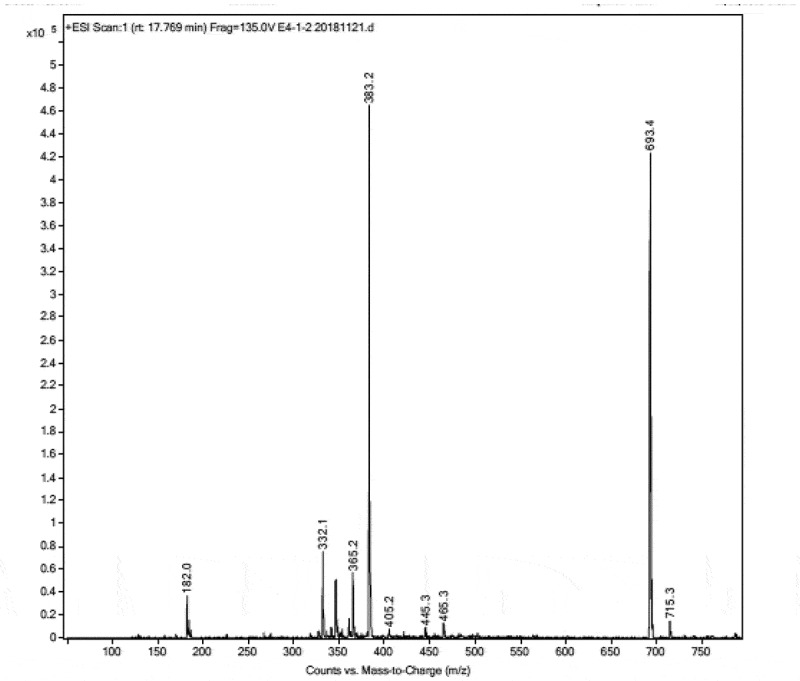
ESI-MS of ditryptophenaline 1.
Figure 8.

1H NMR spectrum of 1 in CDCl3 500 MHz.
Figure 9.

13C NMR spectrum of 1 in CDCl3 500 MHz.
Figure 10.

Chemical structure of 1 ditryptophenaline.
To confirm that compound 1 is the responsible for the promising biological activities of the EtOAc extract of A. ochraceopetaliformis MN083316. The antimicrobial, antioxidant and cytotoxic activities of compound 1 was done.
Compound 1, ditryptophenaline, showed a strong antimicrobial activity against E. coli, B. subtilis and C. parapsilosis with inhibition zone ranging from 8 to 9 mm at concentration 50 mmole. Also, 1 showed a strong antioxidant activity of about 14.3% at concentration 5 mmole. The cytotoxic activity of 1 against human breast carcinoma (MCF-7) and human liver carcinoma (HEPG2) cell lines was with IC50 values of 5.8 and 7.6 mmole, respectively.
Ditryptophenaline was previously isolated for the first time from Aspergillus flavus var. columnaris (Maes et al. 1986). (Shaaban et al. 2014) tested the crude extract isolated from A. oryzae MMAO1 for their antimicrobial and cytotoxic activities and related the antimicrobial activity to ditryptophenaline with other compounds (as constituents of the extract). The extract showed antimicrobial activity against a set of microorganisms include Bacillus subtilis, Staphylococcus aureus, Streptomyces viridochromogenes (Tü 57), Escherichia coli, Candida albicans and Mucor miehi, also showed cytotoxic activity against Brine shrimp.
(Yang et al. 2013b) reported that ditryptophenaline was isolated from mangrove endophytic fungus No.Gx-3a in the sea of South China exhibited strong inhibitory activity on KB and KBv200 cell lines with LD50 values of 8.0 and 12.0 µm, respectively.
In this paper, we report the heat map of symbiotic fungal species in relation to their variability in the Red sea coral reefs. The antimicrobial, antioxidant and cytotoxic activities of the culture filtrates of the isolated fungi were studied. The major compound ditryptophenaline was isolated from the most active fungus, A. ochraceopetaliformis MN083316 and found to highly contribute to the biological activities observed.
Conclusion
Coral reefs either hard or soft represent a host for different groups of microorganisms with biological activities such as antimicrobial, antioxidant and anticancer. These microorganisms can produce active secondary metabolites which might represent a new skeleton or possess a new biological activity which face no resistance from pathogens.
Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Abd El-Hady FK, Abdel-Aziz MS, Shaker KH, El-Shahid ZA, Ghani MA.. 2014. Tyrosinase, acetylcholinesterase inhibitory potential, antioxidant and antimicrobial activities of sponge derived fungi with correlation to their GC/MS analysis. Int J Pharm Sci Rev Res. 51:301–308. [Google Scholar]
- Ainsworth TD, Thurber RV, Gates RD.. 2009. The future of coral reefs: A microbial perspective. Trends Ecol Evol. 25(4):233–240. doi: 10.1016/j.tree.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Almeida C, Part N, Bouhired S, Kehraus S, Onig GMK.. 2011. Stachylines A−D from the Sponge-derived fungus Stachylidium sp. J Nat Prod. 24(1):21–26. doi: 10.1021/np1005345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balser TC, Mcmahon KD, Bart D, Bronson D, Coyle DR, Craig N, Flores-Mangual ML, Forshay K, Jones SE, Kent AE. 2006. Bridging the gap between micro- and macro-scale perspectives on the role of microbial communities in global change ecology. Plant Soil. 289(1–2):59–70. doi: 10.1007/s11104-006-9104-5. [DOI] [Google Scholar]
- Bhatnagar I, Kim S. 2010. Immense essence of excellence: marine microbial bioactive compounds. Mar Drugs. 8(10):2673–2701. doi: 10.3390/md8102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Hu WP, Munro MHG, Northcote PT, Prinsep MR. 2008. Marine natural products. Nat Prod Rep. 25:35–94. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. 2006. Marine natural products. Nat Prod Rep. 23:26–78. [DOI] [PubMed] [Google Scholar]
- Bugni TS, Ireland CM. 2004. Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Nat Prod Rep. 21(1):143–163. doi: 10.1039/b301926h. [DOI] [PubMed] [Google Scholar]
- Cao F, Yang Q, Shao CL, Kong CJ, Zheng JJ, Liu YF, Wang CY. 2015. Bioactive 7-Oxabicyclic [6.3.0] lactam and 12-membered macrolides from a Gorgonian-derived Cladosporium sp. fungus. Mar Drugs. 13(7):4171–4178. doi: 10.3390/md13074171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Peng C, Ding B, Liu F, Zhang F, Lin H, Li Z. 2011. Biological active metabolite cyclo (L-Trp-L-Phe) produced by South China Sea sponge Holoxea sp. associated fungus Aspergillus versicolor strain TS08. Bioprocess Biosys Eng. 34(2):223–229. doi: 10.1007/s00449-010-0464-0. [DOI] [PubMed] [Google Scholar]
- Gaurav K, Karthik L, Bhaskara Rao KV. 2010. In vitro anti-candida activity of Calotropis gigantean. J Pharm Res. 3:539–542. [Google Scholar]
- Gutnecht JLM, Goodman RM, Balser TC. 2006. Linking soil process and microbial ecology in fresh water wetland ecosystems. Plant Soil. 289(1–2):17–34. doi: 10.1007/s11104-006-9105-4. [DOI] [Google Scholar]
- Hamed A. 2009. Investigation of multiple cytoprotective actions of some individual phytochemical and plant extracts. United Kingdom: Nottingham University. [Google Scholar]
- Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhans ADME, Overstreet RM, et al. 1999. Emerging marine diseases-climate links and anthropogenic factors. Science. 285(5433):1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- Hiort J, Maksimenka K, Reichert M, Preovi c-Ottstadt S, Lin WH, Wary V, Steube K, Schaumann K, Weber H, Proksch P, et al. 2004. New natural products from the sponge-derived fungus Aspergillus niger. J Nat Prod. 67(9):1532–1543. doi: 10.1021/np030551d. [DOI] [PubMed] [Google Scholar]
- Hou XM, Zhang YH, Hai Y, Zheng JY, Gu YC, Wang CY, Shao CL. 2017. Aspersymmetide A, a new centrosymmetric cyclohexapeptide from the marine-derived fungus Aspergillus versicolor. Mar Drugs. 15(11):363–370. doi: 10.3390/md15110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WY, Cai YZ, Xing J, Corke H, Sun M. 2007. Potential antioxidant resource: endophytic fungi isolated from traditional Chinese medicinal plants. Econ Bot. 61(1):14–30. doi: 10.1663/0013-0001(2007)61[14:APAREF]2.0.CO;2. [DOI] [Google Scholar]
- Jensen PR, Fenical W. 2002. Secondary metabolites from marine fungi. Fungi in marine environments. In: Hyde KD, University of Hong Kong. Centre for Research in Fungal D , editor. Hong Kong: Fungal Diversity Press; p. 293–315. [Google Scholar]
- John WB, Brent RC, Hu WP, Murray HGM, Peter TN, Michele RP. 2009. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Nat Prod Rep. 26:170–224.19177222 [Google Scholar]
- Kjer J, Debbab A, Aly AH, Proksch P. 2010. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat Protoc. 5(3):479–490. doi: 10.1038/nprot.2009.233. [DOI] [PubMed] [Google Scholar]
- Koh LL, Tan TK, Chou LM, Goh NKC (2000) Fungi associated with gorgonians in Singapora. Proceedings 9th International Coral Reef Symposium, Bali, Indonesia 23-27 October. [Google Scholar]
- Konig GM, Kehraus S, Seiber SF, Abdel-lateff A, Muller D. 2006. Natural products from marine organisms and their associated microbes. Chem Biochem. 7:229–238. [DOI] [PubMed] [Google Scholar]
- Lee YM, Dang HT, Hong J, Lee CO, Bae KS, Kim DK, Jung JH. 2010. A cytotoxic lipopeptide from the sponge-derived fungus Aspergillus versicolor. Bull Kor Chem Soc. 31(1):205–208. doi: 10.5012/bkcs.2010.31.01.205. [DOI] [Google Scholar]
- Li HJ, Xie YL, Xie ZL, Chen Y, Lam CK, Lan WJ. 2012. Chondrosterins A-E, Triquinane-type Sesquiterpenoids from soft coral-associated fungus Chondrostereum sp. Mar Drugs. 10(12):627–638. doi: 10.3390/md10030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. 2009. Advances in marine microbial symbionts in the China Sea and related pharmaceutical metabolites. Mar Drugs. 7(2):113–129. doi: 10.3390/md7020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk AM, Kheiralla ZH, Hamed ER, Youssry AA, Aty AE. 2008. Production of some biologically active secondary metabolites from marine-derived. Malay J Micro. 4(1):14–24. [Google Scholar]
- Maes CM, Potgieter M, Steyn PS. 1986. N.m.r. assignments, conformation, and absolute configuration of ditryptophenaline and model dioxopiperazines. J Chem Soc Perkin Trans. 1:861–866. doi: 10.1039/p19860000861. [DOI] [Google Scholar]
- Mayer AMS, Hamann MT. 2004. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, antiinflammatroy, antimalarial, antiplatelet, antitubercolosis and antiviral activities: affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol (NY). 6(1):37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proksch P, Edrada-Ebel R, Ebel R. 2003. Drugs from the sea-opportunities and obstacles. Mar Drugs. 1(1):5–17. doi: 10.3390/md101005. [DOI] [Google Scholar]
- Putri DA, Radjasa OK, Pringgenies D. 2015. Effectiveness of marine fungal symbiont isolated from soft coral Sinularia sp. from Panjang Island as antifungal. Procedia Environ Sci. 23:351–357. doi: 10.1016/j.proenv.2015.01.051. [DOI] [Google Scholar]
- Radjasa OK. 2004. Marine invertebrate-associated bacteria in Coral reef ecosystems as a new source of bioactive compounds. J Coast Dev. 7:65–70. [Google Scholar]
- Sabdaningsih A, Cristianawati O, Sibero MT, Nuryadi H, Radjasa OK, Sabdono A, Trianto A (2016) Screening antibacterial agent from crude extract of marine-derived fungi associated with Soft Corals against MDR-Staphylococcus haemolyticus. 2nd International conference on Tropical and Coastal Region Eco Development 2016, Bali, Indonesia. [Google Scholar]
- Saleem M, Ali MSS, Hussain JA, Ashraf M, Lee YS. 2007. Marine natural products of fungal origin. Nat Prod Rep. 24(5):1142–1152. doi: 10.1039/b607254m. [DOI] [PubMed] [Google Scholar]
- Schimel J, Balser T, Wallenstein M. 2007. Microbial stress-response physiology and its implications for ecosystem function. Ecology. 88(6):1386–1394. doi: 10.1890/06-0219. [DOI] [PubMed] [Google Scholar]
- Shaaban M, El-Metwally MM, Nasr H. 2014. A new diketopiperazine alkaloid from Aspergillus oryzae. Nat Prod Res. 28(2):86–94. doi: 10.1080/14786419.2013.841687. [DOI] [PubMed] [Google Scholar]
- Strobel G, Daisy B. 2003. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 67(4):491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirunavukkarasu N, Suryanarayanan TS, Girivasan KP, Venkatachalam A, Geetha V, Ravishankar JP, Doble M. 2012. Fungal symbionts of marine sponges from Rameswaram, Southern India: species composition and bioactive metabolites. Fungal Divers. 55(1):37–46. doi: 10.1007/s13225-011-0137-6. [DOI] [Google Scholar]
- Wang W, Liao Y, Tang C, Huang X, Luo Z, Chen J, Cai P. 2017. Cytotoxic and antibacterial compounds from the Coral-derived fungus Aspergillus tritici SP2-8-1. Mar Drugs. 15(11):348–357. doi: 10.3390/md15110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YN, Shao CL, Zheng CJ, Chen YY, Wang CY. 2011. Diversity and antibacterial activities of fungi derived from the Gorgonian Echinogorgia rebekka from the South China Sea. Mar Drugs. 9(8):1379–1390. doi: 10.3390/md9081379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JX, Qiu SX, She ZG, Lin YC. 2013b. Metabolites of mangrove endophytic fungus Gx-3a from the south china sea. Guangxi Kexue. 20:168–170. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


