Abstract
Context
Acetylshikonin, a naphthoquinone derivative, is mainly extracted from some species of the family Boraginaceae, such as Lithospermum erythrorhizon Sieb. et Zucc., Arnebia euchroma (Royle) Johnst., and Arnebia guttata Bunge. As a bioactive compound, acetylshikonin has attracted much attention because of its broad pharmacological properties.
Objective
This review provides a comprehensive summary of the pharmacology, toxicity, and pharmacokinetics of acetylshikonin focussing on its mechanisms on the basis of currently available literature.
Methods
The information of acetylshikonin from 1977 to 2020 was collected using major databases including Elsevier, Scholar, PubMed, Springer, Web of Science, and CNKI. Acetylshikonin, pharmacology, toxicity, pharmacokinetics, and naphthoquinone derivative were used as key words.
Results
According to emerging evidence, acetylshikonin exerts a wide spectrum of pharmacological effects such as anticancer, anti-inflammatory, lipid-regulatory, antidiabetic, antibacterial, antifungal, antioxidative, neuroprotective, and antiviral properties. However, only a few studies have reported the adverse effects of acetylshikonin, with respect to reproductive toxicity and genotoxicity. Pharmacokinetic studies demonstrate that acetylshikonin is associated with a wide distribution and poor absorption.
Conclusions
Although experimental data supports the beneficial effects of this compound, acetylshikonin cannot be considered as a therapy drug without further investigations, especially, on the toxicity and pharmacokinetics.
Keywords: Naphthoquinone derivative, bioactive compound, pharmacological effects, mechanism
Introduction
Zicao, a Chinese Materia Medica, is the root of Lithospermum erythrorhizon Sieb. et Zucc. (Boraginaceae), Arnebia euchroma (Royle) Johnst. (Boraginaceae), and Arnebia guttata Bunge (Boraginaceae) (Chen et al. 2002; Zhang et al. 2019). Furthermore, there are other plants of the genus Onosma (Boraginaceae) also named Zicao in some areas of China (Table 1) (Hu et al. 2006). Zicao has been widely used for the treatment of burns, carbuncles, measles, and macular eruptions in traditional Chinese medicine (Chen et al. 2002; Guo et al. 2019). For example, Shiunko, a clinical ointment made of Zicao and Angelica sinensis (Oliv.) Diels (Apiaceae), has been used in treating wounded skin caused by cuts, burns, and abrasions (Sakaguchi et al. 2001; Huang et al. 2004; Lu et al. 2008).
Table 1.
Quantification of acetylshikonin in nine species of Zicao.
| Plant ( family Boraginaceae) | Content (mg/g) | Source (provinces of China) | Reference |
|---|---|---|---|
| Arnebia euchroma (Royle) Johnst. | 19.7 ± 0.66 | Xinjiang | Hu et al. 2006 |
| Arnebia guttata Bunge | 1.36 ± 0.04 | Xinjiang | Hu et al. 2006 |
| Lithospermum erythrorhizon Sieb. et Zucc. | 7.49 ± 0.11 | Jilin | Hu et al. 2006 |
| Onosma paniculatum Bur. et Franch. | 2.86 ± 0.03 | Yunnan | Hu et al. 2006 |
| Onosma waltonii Duthic | 2.60 ± 0.13 | Tibet | Hu et al. 2006 |
| Onosma hookerii Clarke | 0.747 ± 0.01 | Tibet | Hu et al. 2006 |
| Onosma exsertum Hemsl. | 0.485 ± 0.01 | Yunnan | Hu et al. 2006 |
| Onosma hookerii Clarke var. longiflorum Duthie | 0.248 ± 0.01 | Tibet | Hu et al. 2006 |
| Onosma confertum W.W. Smith | 0.123 ± 0.002 | Yunnan | Hu et al. 2006 |
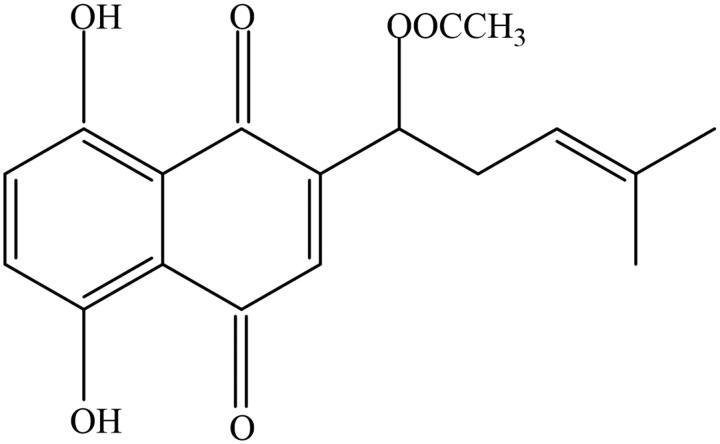
Acetylshikonin (Figure 1) is a naphthoquinone derivative mainly isolated from Zicao. However, there are obvious differences in the content of acetylshikonin in different species (Hayashi 1977; Fu et al. 1984; Yazdinezhad et al. 2009). As shown in Table 1, the content of acetylshikonin is 19.7 ± 0.66 mg/g and 0.123 ± 0.002 mg/g in Arnebia euchroma and Onosma confertum W.W. Smith (Boraginaceae), respectively (Hu et al. 2006). Apart from zicao, there are other plants containing acetylshikonin, such as Alkanna frigida Boiss. (Boraginaceae), Echium italicum L. (Boraginaceae), or Onosma visianii Clem (Boraginaceae) (Albreht et al. 2009; Yazdinezhad et al. 2009; Vukic et al. 2017). Currently, acetylshikonin has attracted much attention for its broad pharmacological effects such as anticancer, anti-inflammatory, antioxidative, antibacterial, antifungal, lipid-regulatory, antidiabetic, neuroprotective, and antiviral properties (Sasaki et al. 2000; Cheng et al. 2008; Kim et al. 2009; Gwon et al. 2012; Li et al. 2012; Wang et al. 2013; Xue and Li 2017; Huang et al. 2019; Liao et al. 2019). However, previous studies have reported the toxicity of acetylshikonin with respect to genotoxicity and reproductive toxicity (Li et al. 2015; He et al. 2016). In addition, acetylshikonin exhibits low water solubility and poor absorption rate after oral administration, which may affect its efficacy (Sun et al. 2008a; Peng et al. 2014). Overall, this review discusses the pharmacology, toxicity, and pharmacokinetics of acetylshikonin with an emphasis on its mechanisms, and provides future research opportunities.
Figure 1.
Chemical structure of acetylshikonin [(+)-acetic acid 1-(5,8dihydroxy-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)-4-methyl-pent-3-enyl ester].
Pharmacology
Anticancer effect of acetylshikonin
Colorectal cancer
Vukic et al. (2017) reported that treatment with acetylshikonin arrested the cell cycle at the G2/M phase, and induced apoptosis and necrosis in HCT116 cells. Furthermore, acetylshikonin suppressed the invasion of HCT116 cells via inhibition of epithelial-mesenchymal transition (EMT) process. Upregulation of the expression of E-cadherin mRNA and downregulation of N-cadherin, vimentin mRNA, and fibronectin were stated to be involved (Xue and Li 2017). Acetylshikonin/β-cyclodextrin inclusion complex decreased Bcl-2/Bax ratio and activated caspase-3, thereby inducing apoptosis in HCT116 cells. Moreover, this inclusion complex exerted long-term cytotoxicity via suppression of autophagy and excess accumulation of intracellular reactive oxygen species (ROS) (Vukic et al. 2020). Acetylshikonin suppressed the proliferation of colon cancers (HT29, DLD-1, and Caco-2 cells) in a dose-dependent manner. The inhibitory effects were related to induction of cell cycle arrest at G0/G1 phase and early apoptosis via inhibition of PI3K/Akt/mTOR pathway (Zhu et al. 2018). Besides, acetylshikonin downregulated the expressions of matrix metalloproteinase (MMP)-2 and MMP-9 to inhibit the invasion of HT29 cells, and suppressed the nuclear factor-κB (NF-κB) pathway to inhibit its proliferation (Zhao and Yang 2019).
Liver cancer
Zhao et al. (2008) reported that acetylshikonin injection showed inhibitory effect on H22 hepatocarcinoma cells, with little adverse effects on immune organs such as thymus and spleen. Co-treatment with acetylshikonin and β,β-dimethylacrylshikonin concentration- and time-dependently suppressed the growth of SMMC-7721 cells through induction of apoptosis and cell cycle arrest at G2/M phase (Wu et al. 2011). In another study, acetylshikonin was shown to suppress the proliferation of hepatitis B virus X protein (HBX)-expressing Hep3B cells by inducing apoptosis. The apoptotic mechanisms were associated with upregulation of orphan nuclear receptor 77 (Nur77), activation of Jun-N-terminal kinase (JNK) and endoplasmic reticulum stress (Moon et al. 2014). Park et al. (2017) indicated that acetylshikonin-induced apoptotic cell death of HepG2 cells was mediated via downregulation of Bcl-2 and upregulation of p53, Bax, and caspase-3. In addition, co-treatment with acetylshikonin and tumour necrosis factor-related apoptosis-inducing ligand increased intracellular ROS production, thereby inducing DNA damage and activating p53/PUMA/Bax signalling. These resulted in activation of caspases and sensitisation of the HepG2 cells to apoptosis (Hong et al. 2019).
Lung cancer
Previous studies demonstrated that the inhibition of acetylshikonin on LLC cells was involved in induction of apoptosis via release of cytochrome c and activation of caspase-3 (Miao et al. 2007; Xiong et al. 2009). Furthermore, acetylshikonin inhibited the metastasis and angiogenesis of LLC cells via suppression of vascular endothelial growth factor (VEGF) production and downregulation of urokinase-type plasminogen activator (uPA) (Lee et al. 2008). In NIH-H460 cells, acetylshikonin activated Nur77 pathway through regulation of Nur77 expression, nuclear export, and mitochondrial targeting to induce apoptosis (Liu et al. 2008). To enhance the antiproliferative property, acetylshikonin was incorporated into a solid-lipid nanoparticle. This compound showed a higher anticancer potential against A549 cells than intact acetylshikonin in terms of DNA damage and IC50 (Eskandani and Nazemiyeh 2014).
Melanoma
Chen et al. (2016) reported that co-treatment with acetylshikonin and pterostilbene showed marked synergistic effect on the proliferation of B16F10 cells. The anticancer activity was attributed to induction of apoptosis and cell cycle arrest via activation of p53 pathway. Jiang et al. (2019) demonstrated that acetylshikonin acted on A375 cells and induced apoptosis. The suppressions of migration and invasion of A375 cells also occurred through reversal of EMT process via PI3K/Akt/mTOR pathway. In addition, cyclopropylacetylshikonin, a structural analogue of acetylshikonin, suppressed the proliferation of melanoma cell lines (WM164 and MUG-Mel2). The mechanisms were associated with inducing caspase-dependent apoptosis, leading to double-stranded DNA breaks, and increasing the protein levels of cleaved Poly ADP-ribose polymerase (Durchschein et al. 2018).
Leukaemia
Studies confirmed that acetylshikonin produced antiproliferative effect on HL-60 cells by suppressing the extension of telomeres (Lu et al. 2002). Co-treatment with acetylshikonin and β,β-dimethylacrylshikonin loaded with jacalin-capped silver nanoparticles resulted in inhibitory effect on K562 cells. The mechanisms were related to excess production of ROS, upregulation of tumour necrosis factor-α (TNF-α), loss of mitochondrial membrane potential, and DNA damage (Ahmed et al. 2016). Furthermore, acetylshikonin activated the mitochondria-regulated intrinsic apoptotic pathway through regulation of ROS accumulation, inhibition of the NF-κB pathway and Bcr-Abl fusion protein expression in K562 cells (Hao et al. 2020).
Oral cancer
It is known that oral squamous cell carcinoma (OSCC) is one of the most common malignant neoplasms in the oral cavity (Greenlee et al. 2001). Acetylshikonin-induced apoptotic cell death of OSCC was mediated through G2 cell cycle arrest and excess ROS via JNK and p38 mitogen-activated protein kinase (MAPK) pathway (Kim et al. 2016). Treatment with acetylshikonin suppressed the invasion of Porphyromonas gingivalis-infected YD10B cells by inhibition of interleukin (IL)-8 release and IL-8-dependent MMP release (Cho et al. 2018). In another study, the antiproliferative effect of acetylshikonin against cisplatin-resistant KB-R5 cells could be due to induction of apoptosis and activation of the mTOR/PI3K/Akt pathway (Wang et al. 2019).
Other cancers
It was shown that acetylshikonin inhibited L-1 sarcoma-induced cutaneous angiogenesis in Balb/c mice (Pietrosiuk et al. 2004a). Treatment with acetylshikonin injection could lead to the inhibition of S180 sarcoma by inducing cell necrosis, while there were no abnormal changes in other organs of the mice (Zeng et al. 2008). Zeng et al. (2009) demonstrated that the suppression of acetylshikonin on SGC-7901 cells was associated with inducing apoptosis through upregulation of Bax and downregulation of Bcl-2. Acetylshikonin suppressed the proliferation of MDA-MB-231 cells through cell cycle arrest at G0/G1 phases, autophagy inhibition, and accumulation of intracellular ROS (Vukic et al. 2020). NF-κB-dependent productions of cytokines and MMP-9 were found to be decreased after acetylshikonin treatment, which led to the inhibition of the proliferation and invasion of PANC-1 cells (Cho and Choi 2015). In another study, acetylshikonin induced cell cycle arrest at S phase and apoptosis by activation of caspase-3 and caspase-8 in Siha cells (Sun et al. 2014). He et al. (2019) reported that acetylshikonin-induced suppression of SKOV3 cells was associated with apoptosis, followed by cell cycle arrest at G0/G1 phase. Acetylshikonin and other shikonin analogues could bypass cancer drug resistance mediated by P-gp, Bcl-2, Bcl-xL, MRP1, and BCRP1, through induction of necroptosis (Xuan and Hu 2009). A study was conducted on acetylshikonin-induced broad blocking of multidrug resistance (MDR) efflux pumps in parental and their drug resistant cell lines (EPG85.257, EPG85.257RDB, MCF7, MCF7MX, A2780, A2780RCIS). The results revealed that acetylshikonin increased drug accumulation inside these cells via reduction of the pump activities of BCRP, MDR1, and MRP2 (Mirzaei et al. 2018).
In general, acetylshikonin has a significant inhibitory effect on a variety of cancers, and regulates genes and proteins involved in cell apoptosis, metastasis, cell invasion, DNA damage, and cell cycle arrest. The synergistic anticancer effect of acetylshikonin in combination with other drugs has attracted attention as a treatment avenue.
Anti-inflammatory effect of acetylshikonin
It was reported that acetylshikonin possessed inhibitory effects on local vascular hyperpermeability and rat foot edoema, but this had no association with corticotropin-like effect (Hayashi 1977; Lin et al. 1980). Wang et al. (1994) found that acetylshikonin suppressed the biosynthesis of leukotriene B4 in leukocytes. In addition, acetylshikonin displayed anti-edematous effects via inhibition of preformed chemical mediators release and protection of the vasculature from mediator challenge (Wang et al. 1995). Inositol phosphate formation and phospholipase C (PLC) activity were suppressed by acetylshikonin in crude cytosolic preparations. Moreover, acetylshikonin blocked Ca2+ release from internal neutrophils, suggesting that the inhibition of phosphatidylinositol signalling may be involved (Wang and Kuo 1997). Acetylshikonin attenuated the carrageenan-induced acute inflammation, and relieved paw edoema by inhibiting the activation of TNF-α promoter (Kundakovic et al. 2006). Cheng et al. (2008) suggested an association between the anti-inflammatory properties of acetylshikonin and suppression of nitric oxide (NO), prostaglandin E2 (PGE2) and inducible NO synthase (iNOS) protein via NF-κB pathway in RAW 264.7 cells. Further study showed that acetylshikonin inhibited TNF-α and IL-1 in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells (Wei et al. 2011). In rat neutrophils, acetylshikonin inhibited the production of thromboxane B2 and leukotriene B4 through reduction in [Ca2+]i, which in turn attenuated membrane-associated cytosolic phospholipase A2, and blocked cyclooxygenase and 5-lipoxygenase activity (Hsu et al. 2009).
Acetylshikonin produced a strong chondro-protective effect in OA rats, probably through mechanisms related to anti-inflammation and suppression of MMPs production (Kim et al. 2013). Acetylshikonin also downregulated the expression of NO and PGE2 by inhibiting ROS/PI3K/Akt-dependent NF-κB activation, and mediating haem oxygenase-1 (HO-1) expression in LPS-stimulated BV2 microglial cells (Jayasooriya et al. 2015). Macrophages play an important part in inflammatory responses. Acetylshikonin showed cytotoxic effect on LPS-stimulated macrophages through caspase-dependent apoptotic cell death pathway (Koike et al. 2016). Zorman et al. (2016) demonstrated that acetylshikonin suppressed IL-1β release and the second step of Nod-like receptor protein 3 (NLRP3) inflammasome activation in C57BL/6 mice. In another study, acetylshikonin suppressed atherogenesis, vascular inflammation, and blood lipid levels via suppression of NF-κB pathway in apolipoprotein E-deficient mice (Cui et al. 2018). Acetylshikonin had the potential to ameliorate non-alcoholic steatohepatitis by inhibiting hepatic levels of IL-1β and TNF-α, and downregulating the expressions of fibronectin and fibrotic markers (a-SMA, collagen I, and collagen III). Hepatocyte autophagy was also induced via AMPK/mTOR pathway (Zeng et al. 2018). Acetylshikonin attenuated cigarette smoke-induced pathological changes and lung tissue damage, and downregulated expressions of TNF-α, IL-6, IL-1β, and monocyte chemoattractant protein-1 (MCP-1) in mice. These effects were produced via upregulation of nuclear factor-erythroid 2-related factor 2 (Nrf2) and Nur77-mediated dcyclooxygenase-2 (COX-2) (Zhang et al. 2019). In addition, Fan et al. (2019) observed that ovalbumin-induced allergic rhinitis was ameliorated by acetylshikonin due to its inhibitory effects on the production of IgE, IgG1, IL-4, IL-5, and IL-13.
In summary, the anti-inflammatory effect of acetylshikonin is associated with upregulation of HO-1 and Nrf2, and downregulation of TNF-α, leukotriene B4, NO, PGE2, iNOS, IL-1, IL-4, IL-5, IL-6, IL-13, IL-1β, MMPs, and MCP-1. In addition, acetylshikonin is shown to regulate NF-κB and Nur77/COX-2 pathway.
Lipid-lowering effect of acetylshikonin
Acyl-CoA: cholesterol acyltransferase (ACAT) is responsible for the intracellular esterification of free cholesterol with fatty acyl-CoA to produce cholesterol esters (Chang et al. 2006). An et al. (2007) demonstrated that acetylshikonin was found to weakly suppress human ACAT-1 and ACAT-2, but its analogue propanoylshikonin exerted strong inhibitory effect on ACAT, indicating that the inhibitory potency was associated with the length of the acyl group. In 3T3-L1 cells, acetylshikonin suppressed adipocyte differentiation and the expression of adipogenic transcription factors (PPARγ and C/EBPα) (Gwon et al. 2012). Furthermore, treatment of high fat diet (HFD)-induced obese rats with acetylshikonin led to reductions in body weight, white adipose tissue content, serum triglycerides, and free fatty acid levels. The lipid-regulatory effect was attributed to triacylglycerol hydrolysis via regulation of protein kinase A (PKA) signalling in 3T3-L1 cells (Su et al. 2016a). Acetylshikonin had an excellent therapeutic effect on obesity and non-alcoholic fatty liver disease in spontaneous obese db/db mice. The mechanisms were involved in marked suppression of alanine aminotransferase, aspartate aminotransferase, and pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β), as well as downregulation of sterol regulatory element-bindingprotein-1 (SREBP-1), fatty acid synthetase (FAS), and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) (Su et al. 2016b).
These studies indicate that acetylshikonin may exhibit beneficial effects on obesity diseases, and the mechanisms involved in the suppression of obesity are associated with regulation of related factors including ACAT, PPARγ, C/EBPα, PKA, SREBP-1, FAS, and HMGCR.
Antidiabetic effect of acetylshikonin
Protein-tyrosine phosphatase 1B (PTP1B) is an effective target for the treatment of type 2 diabetes (Goldstein 2001). Wang et al. (2016) reported that acetylshikonin and other shikonin derivatives showed significant inhibitory effects on PTP1B due to 2-substituted long lipo-chain of naphthoquinone. Acetylshikonin treatment could ameliorate diabetic nephropathy (DN) in mice through attenuation of renal inflammation, decrease in kidney fibrosis, and inhibition of the activation of TGF-β1/Smad pathway (Li et al. 2018). In another study, acetylshikonin enhanced glucose transporter 4 translocation and induced glucose uptake in L6 myotubes via the PLC-β3/PKCδ pathway to reduce blood glucose (Huang et al. 2019). Free fatty acid receptor (FFA) 4 is a member of the G-protein coupled receptors, which may play a role in diabetes treatment and other diseases (Milligan et al. 2017). Acetylshikonin induced dynamic mass redistribution (DMR) response in CHO-FFA1 and CHO-FFA4 cells. It was noteworthy that the agonist response of acetylshikonin was comparable to that of α-linolenic acid, indicating that acetylshikonin could be a novel FFA4 agonist (Xu et al. 2019).
Summarising the beneficial effect of acetylshikonin on diabetes, these reports prove the role of acetylshikonin in the amelioration of DN via TGF-β1/Smad pathway and the regulation of blood glucose via PLC-β3/PKCδ pathways.
Antioxidant properties of acetylshikonin
Acetylshikonin reduced superoxide anion generation in guinea pig polymorphonuclear leukocytes by suppressing the production of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, but it had no inhibitory effect on already generated NADPH oxidase (Kawakami et al. 1996). Weng et al. (2000) and Jiang et al. (2002) reported that acetylshikonin exerted antioxidant property in lard containing Fe3+, and showed synergistic effects with d,l-α-tocopherol. Another study proved that acetylshikonin suppressed Cu2+-induced oxidation of human low-density lipoprotein (Kim et al. 2009). Skrzypczak et al. (2015) found that acetylshikonin exhibited DPPH radical-scavenging property by using electron paramagnetic resonance spectroscopy.
In general, acetylshikonin plays a potential role in antioxidation by inhibiting the production of NADPH oxidase complex and DPPH radical. Further research is still needed to investigate other antioxidative properties of acetylshikonin and clarify its mechanisms.
Antibacterial and antifungal effects of acetylshikonin
Studies demonstrated that acetylshikonin exhibited a wide antifungal spectrum against Cladosporium herbarum, Candida albicans, Saccharomyces cerevisiae, Cryptococcus neoformans, Trichosporon cutaneum, and Aspergillus fumigatus (Li et al. 1998; Sasaki et al. 2000, 2002). Acetylshikonin not only showed inhibitory effects on some Gram-positive bacteria such as Staphylococcus aureus, Bacillus megaterium, Microbacterium arborescens, and Micrococcus luteus, but also suppressed the proliferation of some Gram-negative bacteria such as Escherichia coli, Pseudomonas aeruginosa, Hafnia alvei, Yersinia intermedia, and Citrobacter koseri (Damianakos et al. 2012; Vukic et al. 2017). Moreover, acetylshikonin produced antibacterial effects against different species of oral bacteria such as Fusobacterium nucleatum, Porphyromonas gingivalis, and Streptococcus mutans, and the minimum inhibitory concentration (MIC) values were 0.08, 0.04, and 0.08 mg/mL, respectively (Li et al. 2012). Further investigations revealed that acetylshikonin suppressed the activities of trypsin-like protease and glycosidase enzyme of Porphyromonas gingivalis (Li et al. 2014).
Although acetylshikonin exhibits extensive spectrum of antibacterial and antifungal effects, there are currently very few studies on the mechanisms underlying these effects. Therefore, the mechanisms involved in the antibacterial and antifungal effects of acetylshikonin require further investigation.
Neuroprotective effect of acetylshikonin
The flavin-containing enzymes monoamine oxidase (MAO)-A and MAO-B are involved in the treatment of Alzheimer’s disease (Wu et al. 2007; Schedin-Weiss et al. 2017). Choi et al. (2005) demonstrated that acetylshikonin inhibited mouse brain MAO-A and MAO-B activity in a competitive manner assayed by a modification of Kraml’s fluorometric method. Acetylshikonin could be used as a novel acetylcholinesterase inhibitor to suppress the loss of mitochondria membrane potential and the generation of ROS, thereby possessing anti-apoptotic effect on neuronal SH-SY5Y and PC12 cells. Besides, acetylshikonin-induced increase in HO-1 also played an important role in mediating anti-apoptosis in SH-SY5Y cells under oxidative stress (Wang et al. 2013). Acetylshikonin attenuated d-galactose-induced cognitive impairment and hippocampal senescence in mice, through reduction in oxidative stress and neuroinflammation, and inhibition of the activation of SIRT1/p53/p21 pathway (Li et al. 2018).
Acetylshikonin possesses powerful anti-apoptotic properties in the neuronal cells and attenuates brain aging in mice. Its neuroprotective effect was due to upregulation of HO-1, attenuation of oxidative stress and regulation of SIRT1/p53/p21 pathway.
Other properties of acetylshikonin
Chang et al. (1993) demonstrated that acetylshikonin suppressed platelet aggregation induced by platelet-activating factor, collagen, arachidonic acid, or thrombin in washed rabbit platelets. Another study proved that acetylshikonin inhibited the generation of [3H]inositol monophosphate and blocked increase in intracellular [Ca2+] via suppression of phosphoinositide breakdown, thereby inhibiting platelet activation (Ko et al. 1995). Acetylshikonin attenuated protein tyrosine phosphorylation, and influenced the assembly of a functional NADPH oxidase complex via suppression of respiratory burst in neutrophils (Wang et al. 1997). Pietrosiuk et al. (2004b) reported that splenocytes treated with acetylshikonin had high proliferative potential in vivo and active migratory potential in vitro. In addition, acetylshikonin promoted the proliferation of pellet-induced granulation tissue, and enhanced re-epithelialization and angiogenesis in wounded skin (Ozaki et al. 1994; Lu et al. 2008). Acetylshikonin suppressed acetylcholine and histamine-induced relaxation in PE pre-contracted aorta, suggesting that acetylshikonin might be an inhibitor of NO synthesis in endothelium (Hu et al. 2004). Liao et al. (2019) demonstrated that acetylshikonin exerted anti-replicative effect on HCV virus through autophagy pathway by downregulation of autophagy marker LC3. Acetylshikonin protected cells from coxsackievirus A16 (CVA16) via inactivation of viral particles and blockage of cellular entry. It also reduced CVA16-induced diseases in neonatal mouse model, decreased viral loads in internal organs, and alleviated inflammation via downregulation of interferon-γ and IL-6 (Liu et al. 2019). Shon et al. (2017) reported that acetylshikonin could act as a novel non-selective cytochrome P450 inhibitor to produce significant inhibitory effects on nine cytochrome P450 isoforms (i.e., CYP 1A2, CYP 2A6, CYP 2B6, CYP 2C8, CYP 2C9, CYP 2C19, CYP 2D6, CYP 2E1, and CYP 3 A). In another study, the proliferation and migration of angiotensin II-induced cerebrovascular smooth muscle cells were inhibited by acetylshikonin through suppression of Wnt/β-catenin pathway (Li et al. 2018).
In general, acetylshikonin plays a potential role in anticoagulation through inhibition of phosphoinositide breakdown, and shows antiviral activity via autophagy pathway and inactivation of viral particles. Acetylshikonin also possesses the properties of wound healing and immunoregulation, but further studies are needed to make clear of its mechanisms of action.
Toxicity of acetylshikonin
Acetylshikonin exerted toxic effects on DNA by binding to the minor groove of DNA and slightly intercalating into the DNA base pairs by using the fluorescence spectrometry with two kinds of probes and resonance light scattering spectroscopy (Li et al. 2015). In another study, acetylshikonin increased methanesulfonate-induced genotoxicity, and had cytotoxic effect on a normal cell line (V79 cells; EC50 = 0.49 mg/L), based on LDH assay (Figat et al. 2018). In acetylshikonin-treated Sprague-Dawley rats, reproductive toxicity effect was observed due to decrease in the populations of developing and mature follicles. The mechanisms involved in the reproductive toxicity effect were related to downregulation of follicle stimulating hormone and luteinizing hormone through inhibition of the secretion of gonadotropic hormone (GTH) (He et al. 2016).
Based on these data, acetylshikonin exhibits genotoxicity by binding to the minor groove of DNA, and induces reproductive toxicity through affecting the secretion of GTH. However, since the risk of human exposure to acetylshikonin remains unclear, more investigations are needed to evaluate its toxicity.
Pharmacokinetics of acetylshikonin
Lin et al. (1981) reported that radioactivity could be detected in the mice plasma after oral administration of [3H]-acetylshikonin for 15 min, and the radioactive intensity reached a peak after 1 h. Furthermore, [3H]-acetylshikonin was mainly distributed in the stomach and intestine, secondarily in the gallbladder, liver, kidneys, lungs, and least in the brain and spinal cord. The cumulative excretion rate of faeces and urine was nearly 80% within 48 h, mainly through faecal excretion. These results indicated that acetylshikonin had a wide distribution and poor absorption in mice. The binding rate of acetylshikonin to human plasma proteins was high, and no prototype of it could be detected in mice blood, which blocked its further content determination in vivo (Sun et al. 2008a). To solve this problem, an LC-ESI-MS/MS method after precolumn derivatization with 2-mercaptoethanol was developed for determining the pharmacokinetics of acetylshikonin after oral administration to macaque monkeys at the dose of 0.40 mg/kg. The pharmacokinetic parameters obtained from the non-compartmental analysis showed that the terminal elimination half-life (t1/2), the mean residual time (MRT), and the area under the concentration-time curve to the last measurable concentration (AUC0−t) were 12.3 ± 1.6 h, 10.2 ± 0.7 h, and 615.4 ± 206.5 ng/mL/h, respectively (Sun et al. 2008b).
In general, acetylshikonin has a wide distribution, poor absorption, and high binding rate to human plasma proteins. More attention should be paid to possible competition for binding to plasma proteins between acetylshikonin and any other conjunctive chemical agent. There is need to study the mechanisms involved in these interactions. In fact, previous researches on the pharmacokinetics of acetylshikonin are far from enough to make clear of its metabolic mechanisms. Thus, further studies are needed to clarify its metabolic pathway and investigate acetylshikonin metabolites in different kinds of experimental models.
Conclusion and future perspectives
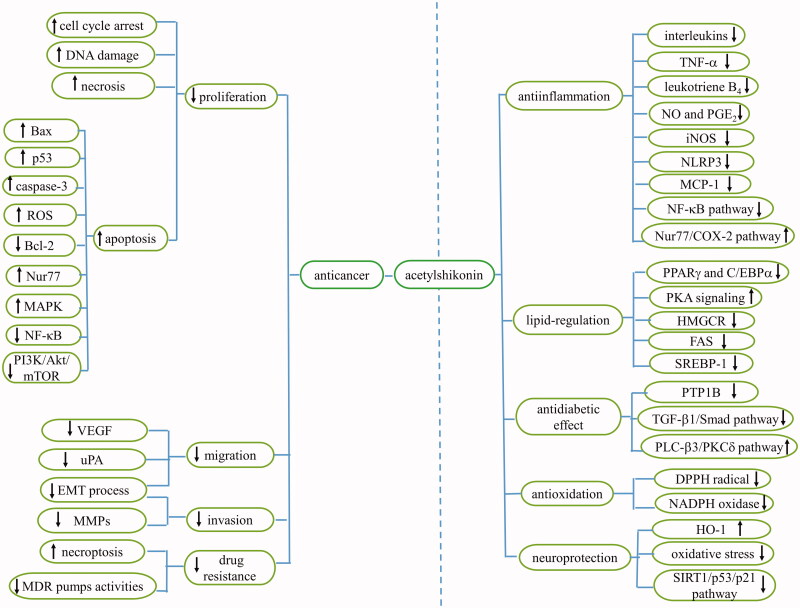
Acetylshikonin is a naphthoquinone derivative with broad pharmacological benefits, including anticancer, anti-inflammatory, lipid-regulatory, antidiabetic, antibacterial, antifungal, antioxidant, neuroprotective, and antiviral properties (Table 2). The various pharmacological effects of acetylshikonin are strongly associated with regulation of the expression of proteins and genes such as upregulation of Bax, Bak, Nur77, caspase-3, caspase-8, p53, p21, HO-1, as well as COX-2; and downregulation of Bcl-2, IL-10, IL-6, IL-1β, MCP-1, IgG1, uPA, MMP, and interferon-γ. Moreover, acetylshikonin was shown to regulate the PI3K/Akt/mTOR, NF-κB, MAPK, AMPK/mTOR, PLC-β3/PKCδ, Wnt/β-catenin, and SIRT1/p53/p21 pathways (Figure 2).
Table 2.
Pharmacology of acetylshikonin.
| Pharmacological effect | Cell lines/model | Activity/Mechanism(s) of action | Application | Reference |
|---|---|---|---|---|
| Anticancer activity | HCT116 cells | Inhibition of EMT and autophagy; Upregulation of ROS; Induction of apoptosis and necrosis | In vitro | Xue & Li 2017; Vukic et al. 2017; Vukic et al. 2020 |
| HT29 cells | Suppression of invasion; Regulation of NF-κB and PI3K/Akt/mTOR pathway | In vitro | Zhu et al. 2018; Zhao and Yang 2019 | |
| SMMC-7721 cells | Induction of apoptosis; Cell cycle arrested at G2/M phase | In vitro | Wu et al. 2011 | |
| Hep3B cells | Upregulation and export of Nur77; Regulation of JNK pathway; Induction of ER stress | In vitro | Moon et al. 2014 | |
| HepG2 cells | Induction of apoptosis; Regulation of p53/PUMA/Bax pathway; Upregulation of ROS | In vitro | Park et al. 2017; Hong et al. 2019 | |
| LLC cells | Downregulation of VEGF and uPA; Induction of apoptosis | In vivo and in vitro | Lee et al. 2008; Xiong et al. 2009 | |
| NIH-H460 cells | Regulation of Nur77 pathway | In vitro | Liu et al. 2008 | |
| A549 cells | DNA fragmentation | In vitro | Eskandani and Nazemiyeh 2014 | |
| B16F10 cells | Regulation of p53 pathway | In vitro | Chen et al. 2016 | |
| A375 cells | Inhibition of EMT process; Regulation of PI3K/Akt/mTOR pathway | In vitro | Jiang et al. 2019 | |
| HL-60 cells | Inhibition of the extension of telomeres | In vitro | Lu et al. 2002 | |
| K562 cells | Induction of apoptosis; Upregulation of ROS and TNF-α; DAN damage; Regulation of NF-κB pathway | In vitro | Ahmed et al. 2016; Hao et al. 2020 | |
| OSCC cells | Regulation of JNK and p38 MAPK pathway | in vitro | Kim et al. 2016 | |
| YD10B cells | Downregulation of IL-8 and MMP | in vitro | Cho et al. 2018 | |
| KB-R5 cells | Regulation of m-TOR/PI3K/Akt pathway | In vitro | Wang et al. 2019 | |
| L-1 sarcoma cells | Inhibition of cutaneous angiogenesis | In vivo | Pietrosiuk et al. 2004a | |
| S180 sarcoma | Induction of necrosis | In vivo | Zeng et al. 2008 | |
| SGC-7901 cells | Induction of apoptosis | In vitro | Zeng et al. 2009 | |
| MDA-MB-231 cells | Cell cycle arrest at G0/G1; Inhibition of autophagy; Upregulation of ROS | In vitro | Vukic et al. 2020 | |
| PANC-1 cells | Regulation of NF-κB pathway | In vitro | Cho and Choi 2015 | |
| Siha cells | Activation of caspase-3 and caspase-8 | In vitro | Sun et al. 2014 | |
| SKOV3 cells | Induction of apoptosis | In vitro | He et al. 2019 | |
| EPG85.257RDB, MCF7MX, A2780RCIS cells | Reduction of BCRP, MDR1, and MRP2 pump activity | In vitro | Mirzaei et al. 2018 | |
| Anti-inflammatory activity | Leukocyte cells | Inhibition of leukotriene B4 | In vitro | Wang et al. 1994 |
| Carrageenan-induced paw edoema | Inhibition of TNF-α activation | In vivo | Kundakovic et al. 2006 | |
| RAW 264.7 cells | Downregulation of NF-κB activation; Inhibition of TNF-α, IL-1, and NO | In vitro | Cheng et al. 2008; Wei et al. 2011 | |
| Rat neutrophils | Suppression of phosphatidylinositol signalling; Inhibition of cyclooxygenase and 5-lipoxygenase activity | In vitro | Wang and Kuo 1997; Hsu et al. 2009 | |
| BV2 microglial cells | Regulation of NF-κB pathway; Upregulation of HO-1 | In vitro | Jayasooriya et al. 2015 | |
| Macrophages | Induction of apoptosis | In vitro | Koike et al. 2016 | |
| C57BL/6 mice | Suppression of IL-1β release and NLRP3 activation | In vitro | Zorman et al. 2016 | |
| Apolipoprotein E deficient mice | Regulation of NF-κB pathway | In vivo | Cui et al. 2018 | |
| MCD diet-induced mice | Regulation of AMPK/mTOR pathway | In vivo | Zeng et al. 2018 | |
| Smoke-induced mice | Downregulation of TNF-α, IL-6, IL-1β, and MCP-1; Upregulation of COX-2 and Nrf2 | In vivo | Zhang et al. 2019 | |
| TH2 cells | Downregulation of lgE, lgG1, IL-4, IL-5, and IL-13 | In vivo | Fan et al. 2019 | |
| Lipid-regulatory activity | AC-29 cells | Inhibition of ACAT-1 and ACAT-2 | In vitro | An et al. 2007 |
| 3T3-L1 cells | Inhibition of adipocyte differentiation and adipogenic transcription factor; Regulation of PKA signalling | In vitro | Gwon et al. 2012; Su et al. 2016a | |
| Spontaneous obese db/db mice | regulation of lipid metabolism | In vivo | Su et al. 2016b | |
| Antidiabetic activity | Diabetic mice | Regulation of TGF-β1/Smad pathway | In vivo | Li et al. 2018 |
| Alloxan-induced mice | Regulation of PLC-β3/PKCδ pathway | In vivo | Huang et al. 2019 | |
| CHO cells | Induction of DMR response | In vitro | Xu et al. 2019 | |
| Antioxidative activity | leukocytes | Reduction of NADPH oxidase | In vitro | Kawakami et al. 1996 |
| Neuroprotective effect | SH-SY5Y and PC12 cells | Suppression of loss of mitochondria membrane potential and the generation of ROS | In vitro | Wang et al. 2013 |
| d-galactose-induced mice | Regulation of SIRT1/p53/p21 pathway | In vivo | Li et al. 2018 |
Figure 2.
Pharmacological activities and the mechanisms of acetylshikonin.
Available data indicates that combination treatment with acetylshikonin and other chemotherapeutic agents has attracted attention because of its good pharmacological effect in cancer treatment. However, there is need to determine the effect of acetylshikonin on the metabolism of other drugs. This is important for the safe use of drugs. Besides, some analogues of acetylshikonin also show effective, or even more effective inhibitory effects against a variety of cancers, than acetylshikonin. This provides new opportunities for future research.
However, the toxicity of acetylshikonin should not be neglected. Previous studies have reported the adverse effects of acetylshikonin, with respect to reproductive toxicity and genotoxicity. In particular, the reproductive toxicity of acetylshikonin should be paid more attention to, especially when it is used in the treatment of diseases in women of childbearing age and pregnant women. Overall, studies on the toxicity of acetylshikonin are far from sufficient. Thus, further studies are needed before acetylshikonin can be safely used for therapeutic purposes.
Funding Statement
This work was financially supported by the Beijing Natural Science Foundation [7194289], the National Natural Science Foundation of China [81703715].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ahmed KBA, Mahapatra SK, Charan Raja MR, Subramaniam S, Sengan M, Rajendran N, Das SK, Haldar K, Roy S, Sivasubramanian A, et al. 2016. Jacalin-capped silver nanoparticles minimize the dosage use of the anticancer drug, shikonin derivatives, against human chronic myeloid leukemia. RSC Adv. 6(23):18980–18989. [Google Scholar]
- Albreht A, Vovk I, Simonovska B, Srbinoska M.. 2009. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. J Chromatogr A. 1216(15):3156–3162. [DOI] [PubMed] [Google Scholar]
- An S, Park YD, Paik YK, Jeong TS, Lee WS.. 2007. Human ACAT inhibitory effects of shikonin derivatives from Lithospermum erythrorhizon. Bioorg Med Chem Lett. 17(4):1112–1116. [DOI] [PubMed] [Google Scholar]
- Chang C, Dong R, Miyazaki A, Sakashita N, Zhang Y, Liu J, Guo M, Li BL, Chang TY.. 2006. Human acyl-CoA:cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim Biophys Sin. 38(3):151–156. [DOI] [PubMed] [Google Scholar]
- Chang YS, Kuo SC, Weng SH, Jan SC, Ko FN, Teng CM.. 1993. Inhibition of platelet aggregation by shikonin derivatives isolated from Arnebia euchroma. Planta Med. 59(5):401–404. [DOI] [PubMed] [Google Scholar]
- Chen X, Yang L, Oppenheim JJ, Zack Howard OM.. 2002. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 16(3):199–209. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang S, Dong J, Zhong J, Wang X, Zhang B.. 2016. The synergistic antiproliferative effect of pterostilbene and acetylshikonin on B16F10 cells. Chinese Pharmacol Bull. 32:818–824. [Google Scholar]
- Cheng YW, Chang CY, Lin KL, Hu CM, Lin CH, Kang JJ.. 2008. Shikonin derivatives inhibited LPS-induced NOS in RAW 264.7 cells via downregulation of MAPK/NF-kappaB signaling. J Ethnopharmacol. 120(2):264–271. [DOI] [PubMed] [Google Scholar]
- Cho SC, Choi BY.. 2015. Acetylshikonin inhibits human pancreatic PANC-1 cancer cell proliferation by suppressing the NF-κB activity. Biomol Ther. 23(5):428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BH, Jung YH, Kim DJ, Woo BH, Jung JE, Lee JH, Choi YW, Park HR.. 2018. Acetylshikonin suppresses invasion of Porphyromonas gingivalis-infected YD10B oral cancer cells by modulating the interleukin-8/matrix metalloproteinase axis. Mol. Med. Rep. 17:2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WH, Hong SS, Lee SA, Han XH, Lee KS, Lee MK, Hwang BY, Ro JS.. 2005. Monoamine oxidase inhibitory naphthoquinones from the roots of Lithospermum erythrorhizon. Arch Pharm Res. 28(4):400–404. [DOI] [PubMed] [Google Scholar]
- Cui L, Yan Y, Zhang M, Wu J, Tang X, Yang J, Li L, Yao K.. 2018. Acetylshikonin suppresses atherogenesis by attenuating vascular inflammation in apolipoprotein E-deficient mice. Int. J Clin Exp Med. 11:1882–1890. [Google Scholar]
- Damianakos H, Kretschmer N, Sykłowska-Baranek K, Pietrosiuk A, Bauer R, Chinou I.. 2012. Antimicrobial and cytotoxic isohexenylnaphthazarins from Arnebia euchroma (Royle) Jonst. (Boraginaceae) callus and cell suspension culture. Molecules. 17(12):14310–14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durchschein C, Hufner A, Rinner B, Stallinger A, Deutsch A, Lohberger B, Bauer R, Kretschmer N.. 2018. Synthesis of novel shikonin derivatives and pharmacological effects of cyclopropylacetylshikonin on melanoma cells. Molecules. 23(11):2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandani M, Nazemiyeh H.. 2014. Self-reporter shikonin-Act-loaded solid lipid nanoparticle: Formulation, physicochemical characterization and geno/cytotoxicity evaluation. Eur J Pharm Sci. 59:49–57. [DOI] [PubMed] [Google Scholar]
- Fan XH, Cheng L, Yan AH.. 2019. Ameliorative effect of acetylshikonin on ovalbumin (OVA)-induced allergic rhinitis in mice through the inhibition of Th2 cytokine production and mast cell histamine release. J Pathol Microbiol Immunol. 127(10):688–695. [DOI] [PubMed] [Google Scholar]
- Figat R, Zgadzaj A, Geschke S, Sieczka P, Pietrosiuk A, Sommer S, Skrzypczak A.. 2018. Cytotoxicity and antigenotoxicity evaluation of acetylshikonin and shikonin. Drug Chem Toxicol. 10.1080/01480545.2018.1536710. [DOI] [PubMed] [Google Scholar]
- Fu SL, Shang TM, Xiao PG.. 1984. Analysis of naphthaquinone pigments in some Chinese medicinal “Zicao”, Arnebia euchroma. Acta Pharm Sin. 19:921–925. [PubMed] [Google Scholar]
- Goldstein BJ. 2001. Protein-tyrosine phosphatase 1B (PTP1B): a novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr Drug Targets Immune Endocr Metabol Disord. 1(3):265–275. [DOI] [PubMed] [Google Scholar]
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M.. 2001. Cancer statistics, 2001. CA. Cancer. CA Cancer J Clin. 51(1):15–36. [DOI] [PubMed] [Google Scholar]
- Guo C, He J, Song X, Tan L, Wang M, Jiang P, Li Y, Cao Z, Peng C.. 2019. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol Res. 149:104463. [DOI] [PubMed] [Google Scholar]
- Gwon SY, Ahn JY, Chung CH, Moon B, Ha TY.. 2012. Lithospermum erythrorhizon suppresses high-fat diet-induced obesity, and acetylshikonin, a main compound of Lithospermum erythrorhizon, inhibits adipocyte differentiation. J Agric Food Chem. 60(36):9089–9096. [DOI] [PubMed] [Google Scholar]
- Hao G, Zhai J, Jiang H, Zhang Y, Wu M, Qiu Y, Fan C, Yu L, Bai S, Sun L, et al. 2020. Acetylshikonin induces apoptosis of human leukemia cell line K562 by inducing S phase cell cycle arrest, modulating ROS accumulation, depleting Bcr-Abl and blocking NF-κB signaling. Biomed Pharmacother. 122:109677. [DOI] [PubMed] [Google Scholar]
- Hayashi M. 1977. Pharmacological studies on crude plant drugs, shikon and tooki (II) shikonin and acetylshikonin. Folia Pharmacol Jpn. 73(2):193–203. [PubMed] [Google Scholar]
- He M, Han L, Wang P, Fu L, Pei Y, Liu Y, Sun S.. 2019. Experimental study of acetylshikonin-induced apoptosis in ovarian cancer SKOV3 cells. Chinese J Lab Diagnosis. 23:1632–1633. [Google Scholar]
- He Y, Li Q, Su M, Huang W, Zhu B.. 2016. Acetylshikonin from Zicao exerts antifertility effects at high dose in rats by suppressing the secretion of GTH. Biochem Biophys Res Commun. 476(4):560–565. [DOI] [PubMed] [Google Scholar]
- Hong M, Li J, Li S, Almutairi MM.. 2019. Acetylshikonin sensitizes hepatocellular carcinoma cells to apoptosis through ROS-mediated caspase activation. Cells. 8(11):1466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hsu MF, Chang LC, Huang LJ, Kuo SC, Lee HY, Lu MC, Wang JP.. 2009. The influence of acetylshikonin, a natural naphthoquinone, on the production of leukotriene B4 and thromboxane A2 in rat neutrophils. Eur J Pharmacol. 607(1–3):234–243. [DOI] [PubMed] [Google Scholar]
- Hu CM, Cheng YW, Cheng HW, Kang JJ.. 2004. Impairment of vascular function of rat thoracic aorta in an endothelium-dependent manner by shikonin/alkannin and derivatives isolated from roots of Macrotomia euchroma. Planta Med. 70:23–28. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jiang Z, Leung KSY, Zhao Z.. 2006. Simultaneous determination of naphthoquinone derivatives in Boraginaceous herbs by high-performance liquid chromatography. Anal Chim Acta. 577(1):26–31. [DOI] [PubMed] [Google Scholar]
- Huang KF, Hsu YC, Lin CN, Tzeng JI, Chen YW, Wang JJ.. 2004. Shiunko promotes epithelization of wounded skin. Am J Chin Med. 32(03):389–396. [DOI] [PubMed] [Google Scholar]
- Huang W, Zeng J, Liu Z, Su M, Li Q, Zhu B.. 2019. Acetylshikonin stimulates glucose uptake in L6 myotubes via a PLC-β3/PKCδ-dependent pathway. Biomed Pharmacother. 112:108588. [DOI] [PubMed] [Google Scholar]
- Jayasooriya RGPT, Lee KT, Choi YH, Moon SK, Kim WJ, Kim GY.. 2015. Antagonistic effects of acetylshikonin on LPS-induced NO and PGE2 production in BV2 microglial cells via inhibition of ROS/PI3K/Akt-mediated NF-κB signaling and activation of Nrf2-dependent HO-1. In vitro Cell Dev Biol Anim. 51(9):975–986. [DOI] [PubMed] [Google Scholar]
- Jiang A, Sun L, Liu Y.. 2002. Extraction of antioxidant compositions from Lithospermum erythrorhizon and study of their antioxidant activity. Fine Chem. 19:51–54. [Google Scholar]
- Jiang S, Wang H, Guo Y, Liu Z, Song W.. 2019. Acetylshikonin inhibits the migration and invasion of A375 cells by reversing EMT process via the PI3K/Akt/mTOR pathway. Biotechnol Biotechnol Equip. 33(1):699–706. [Google Scholar]
- Kawakami N, Koyama Y, Tanaka J, Ohara A, Hayakawa T, Fujimoto S.. 1996. Inhibitory effect of acetylshikonin on the activation of NADPH oxidase in polymorphonuclear leukocytes in both whole cell and cell-free systems. Biol Pharm Bull. 19(10):1266–1270. [DOI] [PubMed] [Google Scholar]
- Kim G-S, Jeong T-S, Kwon B-M, Kim Y-O, Cha S-W, Song K-S, Bek N-I.. 2009. Inhibitory effect of acetylshikonin from roots of Lithospermum erythrorhizon on LDL oxidation and FPTase activity. J Appl Biol Chem. 52(4):221–225. [Google Scholar]
- Kim GS, Kim HJ, Lee DY, Choi SM, Lee SE, Noh HJ, Choi JG, Choi SI.. 2013. Effects of supercritical fluid extract, shikonin and acetylshikonin from Lithospermum erythrorhizon on chondrocytes and MIA-induced osteoarthritis in rats. Korean J Med Crop Sci. 21(6):466–473. [Google Scholar]
- Kim DJ, Lee JH, Park HR, Choi YW.. 2016. Acetylshikonin inhibits growth of oral squamous cell carcinoma by inducing apoptosis. Arch Oral Biol. 70:149–157. [DOI] [PubMed] [Google Scholar]
- Ko FN, Lee YS, Kuo SC, Chang YS, Teng CM.. 1995. Inhibition on platelet activation by shikonin derivatives isolated from Arnebia euchroma. Biochim Biophys Acta. 1268(3):329–334. [DOI] [PubMed] [Google Scholar]
- Koike A, Shibano M, Mori H, Kohama K, Fujimori K, Amano F.. 2016. Simultaneous addition of shikonin and its derivatives with lipopolysaccharide induces rapid macrophage death. Biol Pharm Bull. 39(6):969–976. [DOI] [PubMed] [Google Scholar]
- Kundakovic T, Fokialakis N, Dobric S, Pratsinis H, Kletsas D, Kovacevic N, Chinou I.. 2006. Evaluation of the anti-inflammatory and cytotoxic activities of naphthazarine derivatives from Onosma leptantha. Phytomedicine. 13(4):290–294. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Lee H-J, Magesh V, Nam D, Lee E-O, Ahn KS, Jung M-H, Ahn K-S, Kim D-K, Kim J-Y, et al. 2008. Shikonin, acetylshikonin, and isobutyroylshikonin inhibit VEGF-induced angiogenesis and suppress tumor growth in Lewis lung carcinoma-bearing mice. Yakugaku Zasshi. 128(11):1681–1688. [DOI] [PubMed] [Google Scholar]
- Li C, Fukushi Y, Kawabata J, Tahara S, Mizutani J, Uyeda I.. 1998. Antiviral and antifungal activities of some naphthoquinones isolated from the roots of Lithospermum erythrorhizon. J Pestic Sci. 23(1):54–57. [Google Scholar]
- Li ZZ, Hong Z, Peng ZQ, Zhao YC, Shao RS.. 2018. Acetylshikonin from Zicao ameliorates renal dysfunction and fibrosis in diabetic mice by inhibiting TGF-β1/Smad pathway. Hum Cell. 31(3):199–209. [DOI] [PubMed] [Google Scholar]
- Li W, Li J, Huang G, Yan L, Wang Q, Wang W.. 2015. Study and assessment of the potential toxicity of acetylshikonin. Food Ind. 36:174–178. [Google Scholar]
- Li M, Xu Z, Zhu C, Wang J.. 2012. Effect of different derivatives of shikonin from Lithospermum erythrorhizon against the pathogenic dental bacteria. Curr Pharm Anal. 8(3):255–260. [Google Scholar]
- Li ZQ, Yan ZY, Xu CB, Dong YQ, Xiong Y, Dai YY.. 2018. Acetylshikonin attenuates angiotensin II-induced proliferation and motility of human brain smooth muscle cells by inhibiting Wnt/β-catenin signaling. Hum Cell. 31(3):242–250. [DOI] [PubMed] [Google Scholar]
- Li Q, Zeng J, Su M, He Y, Zhu B.. 2018. Acetylshikonin from Zicao attenuates cognitive impairment and hippocampus senescence in d-galactose-induced aging mouse model via upregulating the expression of SIRT1. Brain Res Bull. 137:311–318. [DOI] [PubMed] [Google Scholar]
- Li M-Y, Zhu L, Lai G-Y, Mo S, Wang J.. 2014. The inhibition of acetylshikonin on bacteria and its trypsin-like protease and glycosidic enzyme may be essential to conquer periodontal ecological niche. Lett Drug Des Discov. 11(10):1162–1166. [Google Scholar]
- Liao W, Lu H, Liang H, Ye L, Chen H.. 2019. Research on the effects of acetylshikonin on HCV replication and its mechanism(s). Intern Med. 14:137–141. [Google Scholar]
- Lin Z, Chai B, Wang P, Guo Q, Lu F, Xiang G.. 1980. Studies on the anti-inflammatory effect of principle of Zicao. J Beijing Med. 12:101–106. [Google Scholar]
- Lin Z, Zhang J, Wang P.. 1981. Absorption, distribution and excretion of [3H]-acetylshikonin in mice. J Beijing Med Coll. 13(4):364–371. [Google Scholar]
- Liu X, Zhang X, Li J, Zhou H, Carr MJ, Xing W, Zhang Z, Shi W.. 2019. Effects of acetylshikonin on the infection and replication of coxsackievirus A16 in vitro and in vivo. J Nat Prod. 82(5):1089–1097. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou W, Li SS, Sun Z, Lin B, Lang YY, He JY, Cao X, Yan T, Wang L, et al. 2008. Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues. Cancer Res. 68(21):8871–8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Liu W, Ding J, Cai J, Duan W.. 2002. Shikonin derivatives: synthesis and inhibition of human telomerase. Bioorganic Med Chem Lett. 12(10):1375–1378. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Yang C, Lin CN, Li CF, Chu CC, Wang JJ, Chen JY.. 2008. Shiunko and acetylshikonin promote reepithelialization, angiogenesis, and granulation tissue formation in wounded skin. Am J Chin Med. 36(01):115–123. [DOI] [PubMed] [Google Scholar]
- Miao S, Luo G, Zhou L.. 2007. Antitumor effects of acetylshikonin injection on Lewis lung cancer in vivo. Sichuan J Physiol Sci. 29:154–155. [Google Scholar]
- Milligan G, Alvarez-Curto E, Hudson BD, Prihandoko R, Tobin AB.. 2017. FFA4/GPR120: pharmacology and therapeutic opportunities. Trends Pharmacol Sci. 38(9):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei SA, Reiisi S, Ghiasi Tabari P, Shekari A, Aliakbari F, Azadfallah E, Elahian F.. 2018. Broad blocking of MDR efflux pumps by acetylshikonin and acetoxyisovalerylshikonin to generate hypersensitive phenotype of malignant carcinoma cells. Sci Rep. 8(1):3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Koh SS, Malilas W, Cho IR, Kaewpiboon C, Kaowinn S, Lee K, Jhun BH, Choi YW, Chung YH.. 2014. Acetylshikonin induces apoptosis of hepatitis B virus X protein-expressing human hepatocellular carcinoma cells via endoplasmic reticulum stress. Eur J Pharmacol. 735:132–140. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Ohno A, Saito Y, Satake M.. 1994. Accelerative effect of shikonin, alkannin and acetylshikonin on the proliferation of granulation tissue in rats. Biol Pharm Bull. 17(8):1075–1078. [DOI] [PubMed] [Google Scholar]
- Park S-H, Phuc NM, Lee J, Wu Z, Kim J, Kim H, Kim ND, Lee T, Song K-S, Liu K-H.. 2017. Identification of acetylshikonin as the novel CYP2J2 inhibitor with anti-cancer activity in HepG2 cells. Phytomedicine. 24:134–140. [DOI] [PubMed] [Google Scholar]
- Peng J, Zhou W, Xia X, Qi X, Sun L, Wang M, Wu Z, Li Z.. 2014. Encapsulation of acetylshikonin by polyamidoamine dendrimers for preparing prominent nanoparticles. AAPS PharmSciTech. 15(2):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrosiuk A, Furmanowa M, Skopinska-Rozewska E, Sommer E, Skurzak H, Bany J.. 2004a. The effect of acetylshikonin isolated from Lithospermum canescens roots on tumor-induced cutaneous angiogenesis. Acta Pol Pharm – Drug Res. 61:379–382. [PubMed] [Google Scholar]
- Pietrosiuk A, Skopińska-Rózewska E, Furmanowa M, Wiedenfeld H, Sommer E, Sokolnicka I, Rogala E, Radomska-Leśniewska D, Bany J, Malinowski M.. 2004b. Immunomodulatory effect of shikonin derivatives isolated from Lithospermum canescens on cellular and humoral immunity in Balb/c mice. Pharmazie. 59(8):640–642. [PubMed] [Google Scholar]
- Sakaguchi I, Tsujimura M, Ikeda N, Minamino M, Kato Y, Watabe K, Yano I, Kaneda K.. 2001. Granulomatous tissue formation of shikon and shikonin by air pouch method. Biol Pharm Bull. 24(6):650–655. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Abe H, Yoshizaki F.. 2002. In vitro antifungal activity of naphthoquinone derivatives. Biol Pharm Bull. 25(5):669–670. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Yoshizaki F, Abe HS.. 2000. [The anti-Candida activity of shikon]. Yakugaku Zasshi. 120(6):587–589. [DOI] [PubMed] [Google Scholar]
- Schedin-Weiss S, Inoue M, Hromadkova L, Teranishi Y, Yamamoto NG, Wiehager B, Bogdanovic N, Winblad B, Sandebring-Matton A, Frykman S.. 2017. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimer’s Res Ther. 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon JC, Phuc NM, Kim WC, Heo JK, Wu Z, Lee H, Liu KH.. 2017. Acetylshikonin is a novel non-selective cytochrome P450 inhibitor. Biopharm Drug Dispos. 38(9):553–556. [DOI] [PubMed] [Google Scholar]
- Skrzypczak A, Przystupa N, Zgadzaj A, Parzonko A, Sykłowska-Baranek K, Paradowska K, Nałęcz-Jawecki G.. 2015. Antigenotoxic, anti-photogenotoxic and antioxidant activities of natural naphthoquinone shikonin and acetylshikonin and Arnebia euchroma callus extracts evaluated by the umu-test and EPR method. Toxicol in Vitro. 30(1 Pt B):364–372. [DOI] [PubMed] [Google Scholar]
- Su ML, He Y, Li QS, Zhu BH.. 2016b. Efficacy of acetylshikonin in preventing obesity and hepatic steatosis in db/db mice. Molecules. 21(8):976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ML, Huang WD, Zhu BH.. 2016a. Acetylshikonin from Zicao prevents obesity in rats on a high-fat diet by inhibiting lipid accumulation and inducing lipolysis. PLoS One. 11(1):e0146884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun DX, Tian HF, Meng ZY, Du A, Yuan D, Gu RL, Wu ZN, Dou GF.. 2008b. Quantitative determination of acetylshikonin in macaque monkey blood by LC-ESI-MS/MS after precolumn derivatization with 2-mercaptoethanol and its application in pharmacokinetic study. Acta Pharmacol Sin. 29(12):1499–1506. [DOI] [PubMed] [Google Scholar]
- Sun DX, Tian HF, Meng ZY, Zhou JY, Ding JG, Wu GR, Dou GF.. 2008a. Tissue distribution, excretion and blood distribution of [3H]-acetylshikonin in mice by sample oxidizer. Bull Acad Mil Med Sci. 32:348–352. [Google Scholar]
- Sun H, Xu H, Zhang A, Wang P, Han Y, Wang X.. 2014. In vitro anticancer activity of acetylshikonin action against cervical cancer. Plant Sci Today. 1(2):39–45. [Google Scholar]
- Vukic MD, Vukovic NL, Djelic GT, Popovic SL, Zaric MM, Baskic DD, Krstic GB, Tesevic VV, Kacaniova MM.. 2017. Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem. Excli J. 16:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukic MD, Vukovic NL, Popovic SL, Todorovic DV, Djurdjevic PM, Matic SD, Mitrovic MM, Popovic AM, Kacaniova MM, Baskic DD.. 2020. Effect of β-cyclodextrin encapsulation on cytotoxic activity of acetylshikonin against HCT-116 and MDA-MB-231 cancer cell lines. Saudi Pharm J. 28(1):136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Bai J, Liu D, Xue L, Zhu X.. 1994. The anti-inflammatory activity of shikonin and its inhibitory effect on leukotriene B4 biosynthesis. Acta Pharm Sin. 29:161–165. [PubMed] [Google Scholar]
- Wang P, Gao W, Wang Y, Wang J.. 2019. Acetylshikonin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant oral cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J Balk Union Oncol. 24:2062–2067. [PubMed] [Google Scholar]
- Wang JP, Kuo SC.. 1997. Impairment of phosphatidylinositol signaling in acetylshikonin-treated neutrophils. Biochem Pharmacol. 53(8):1173–1177. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pan WL, Liang WC, Law WK, Tsz-Ming Ip D, Ng TB, Miu-Yee Waye M, Chi-Cheong Wan D.. 2013. Acetylshikonin, a novel ache inhibitor, inhibits apoptosis via upregulation of heme oxygenase-1 expression in sh-sy5y cells. Evid-Based Comple Altern Med. 2013:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Raung SL, Chang LC, Kuo SC.. 1995. Inhibition of hind-paw edema and cutaneous vascular plasma extravasation in mice by acetylshikonin. Eur J Pharmacol. 272(1):87–95. [DOI] [PubMed] [Google Scholar]
- Wang X, Tang Z, Nan Y, Yue Z.. 2016. Protein tyrosine phosphatase 1B (PTP1B) inhibitors from Arnebia euchroma. Chinese Pharm J. 51:1120–1123. [Google Scholar]
- Wang JP, Tsao LT, Raung SL, Hsu MF, Kuo SC.. 1997. Investigation of the inhibition by acetylshikonin of the respiratory burst in rat neutrophils. Br J Pharmacol. 121(3):409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Zhao H, Shao M, Fan H, Liu S, Liu K.. 2011. Effects of hydroxynaphthoquinone derivatives from Arnebia euchroma on modulation of pro-inflammatory cytokines. Chinese J Inf Tradit Chinese Med. 18:40–41. [Google Scholar]
- Weng XC, Xiang GQ, Jiang AL, Liu YP, Wu LL, Dong XW, Duan S.. 2000. Antioxidant properties of components extracted from puccoon (Lithospermum erythrorhizon Sieb. et Zucc.). Food Chem. 69(2):143–146. [Google Scholar]
- Wu YH, Fischer DF, Swaab DF.. 2007. A promoter polymorphism in the monoamine oxidase A gene is associated with the pineal MAOA activity in Alzheimer’s disease patients. Brain Res. 1167:13–19. [DOI] [PubMed] [Google Scholar]
- Wu YY, Zhu L, Ma XY, Shao ZJ, Chen J, Chen XJ, Wan LH, Zhou LM.. 2011. The anti-proliferation effect of Aikete injection on hepatocellular carcinoma in vitro and in vivo. Pharm Biol. 49(5):531–538. [DOI] [PubMed] [Google Scholar]
- Xiong W, Luo G, Zhou L, Zeng Y, Yang W.. 2009. In vitro and in vivo antitumor effects of acetylshikonin isolated from Arnebia euchroma (Royle) Johnst (Ruanzicao) cell suspension cultures. Chin Med. 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Zhou H, Liu X, Zhang X, Wang Z, Hou T, Wang J, Qu L, Zhang P, Piao H, et al. 2019. Label-free cell phenotypic study of FFA4 and FFA1 and discovery of novel agonists of FFA4 from natural products. RSC Adv. 9(26):15073–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Y, Hu X.. 2009. Naturally-occurring shikonin analogues-a class of necroptotic inducers that circumvent cancer drug resistance. Cancer Lett. 274(2):233–242. [DOI] [PubMed] [Google Scholar]
- Xue D, Li R.. 2017. Effects of acetylshikonin on human colon cancer HCT116 cells proliferation, apoptosis and epithelial-mesenchymal transition. Chin Tradit Pat Med. 39:401–404. [Google Scholar]
- Yazdinezhad A, Monsef-Esfahani HR, Amanzadeh YS, Ebrahimi SE, Ghahremani MH, Ostad SN.. 2009. Naphthazarin derivatives from Alkanna frigida. Eur J Sci Res. 27:29–33. [Google Scholar]
- Zeng Y, Liu G, Zhou LM.. 2009. Inhibitory effect of acetylshikonin on human gastric carcinoma cell line SGC-7901 in vitro and in vivo. World J Gastroenterol. 15(15):1816–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Luo G, Yang W, Zhou L.. 2008. Inhibitory effect of acetylshikonin in injection on mouse S180 sarcoma. Pharmacol Clin Chinese Mater Medica. 24:22–23. [Google Scholar]
- Zeng J, Zhu B, Su M.. 2018. Autophagy is involved in acetylshikonin ameliorating non-alcoholic steatohepatitis through AMPK/mTOR pathway. Biochem Biophys Res Commun. 503(3):1645–1650. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Jia Y, Zhao Q, Wang W, Zhang Z, Li W.Sun L.. 2019. Ameliorative effect of acetylshikonin on cigarette smoke-induced lung inflammation in mice. J Asian Nat Prod Res. 5(4):44. https://www.researchgate.net/publication/337795243. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li J, Li G, Fu J, Zhang P, Huang Y.. 2019. Herbal textual analysis medicinal plant Arnebia. J Anhui Agr Sci. 47:199–202. [Google Scholar]
- Zhao J, Yang T.. 2019. Effects of acetylshikonin on proliferation, apoptosis, invasion and NF-κB pathway of colon cancer cells. J Chin Med Mat. 42:2168–2172. [Google Scholar]
- Zhao Y, Zheng X, Shao Z, Zhou L.. 2008. Inhibitory effect of acetylshikonin on mouse H22 hepatocarcinoma. Sichuan J Physiol Sci. 30:120–121. [Google Scholar]
- Zhu Y, Zhong Y, Zhou Y, Liu Y, Huang Q, Huang Z, Wang Y, Ye H, Zeng X, Zheng X.. 2018. Acetylshikonin inhibits colorectal cancer growth via PI3K/Akt/mTOR signaling pathway. Chin Med. 09(03):126–143. [Google Scholar]
- Zorman J, Sušjan P, Hafner-Bratkovič I.. 2016. Shikonin suppresses NLRP3 and AIM2 inflammasomes by direct inhibition of caspase-1. PLoS One. 11(7):e0159826. [DOI] [PMC free article] [PubMed] [Google Scholar]