Abstract
Background
Microscopic colitis (MC) has been described as 1 pattern of injury in immune checkpoint inhibitor (ICPI)–induced colitis. The main objective of this study was to characterize ICPI-induced MC by exploring the differences in risk factors, colitis treatments, endoscopic features, and clinical outcomes between cancer and noncancer patients with MC with and without exposure to ICPIs.
Methods
A retrospective chart review was conducted among patients diagnosed with MC from our institutional pathology database from January 2012 to January 2018. Patients were categorized into MC in cancer patients with or without ICPI exposure and in noncancer patients. Risk factors (use of tobacco and certain medications), colitis treatments (antidiarrheals and immunosuppressants), endoscopic features (with or without mucosal abnormality), and clinical outcomes (diarrhea recurrence, hospitalization, mortality) were collected and compared among the 3 groups.
Results
Of the 65 eligible patients with MC, 15 cancer patients had exposure to ICPI, 39 cancer patients had no exposure to ICPI, and 11 had no cancer diagnosis. Among the risk factors, proton pump inhibitor was more frequently used in the ICPI-induced MC cohort (P = 0.040). Furthermore, in this population, mucosal abnormality was the most common endoscopic feature compared with normal findings in the non-ICPI-induced MC groups (P = 0.106). Patients with ICPI-induced MC required more treatments with oral and intravenous steroids and nonsteroidal immunosuppressive agents (all P < 0.001) and had a higher rate of hospitalization (P < 0.001).
Conclusion
This study suggests that despite some similarities between MC with and without exposure to ICPIs, ICPI-induced MC has a more aggressive disease course that requires more potent immunosuppressive treatment regimens and greater need for hospitalization.
Keywords: microscopic colitis, immune checkpoint inhibitors, lymphocytic colitis, collagenous colitis
INTRODUCTION
Immune checkpoint inhibitors (ICPIs) bear substantial promise for patients with advanced malignancies, as these novel agents have been found to prolong survival. Initially approved in 2011 for use in metastatic melanoma, ICPIs are now prescribed for non–small cell lung carcinoma, renal cell carcinoma, Hodgkin lymphoma, urothelial carcinoma, and solid tumors such as colorectal cancer with microsatellite instability.1–3 Research is still ongoing as to their therapeutic benefit in other cancer types. Immune checkpoint inhibitors’ unique mechanism of utilizing the immune system instead of directly attacking tumor cells, as with traditional antineoplastic treatments, allows for its therapeutic use in various malignancies. Immune checkpoint inhibitors target cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) found on T cells, and programmed cell death ligand 1 (PD-L1) expressed on cancer cells. In addition to serving as downregulators of effector T cells, which are key to tumor eradication, these immune checkpoint proteins are also promoters of regulatory T cells responsible for anti-inflammatory activities.1, 3 Inhibition of checkpoint proteins on effector T cells by ICPIs results in continued T-cell activation and sustained antitumor responses. However, ICPIs’ blockade of checkpoint proteins on regulatory T cells leads to reduced anti-inflammatory effects. This disruption of immune homeostasis, secondary to persistent T-cell activation and a shift to a more pro-inflammatory state, induces undesirable autoimmune-type manifestations known collectively as immunotherapy-related adverse events (irAEs). Immunotherapy-related adverse events can occur in almost every organ system, most notably the dermatologic, gastrointestinal, endocrine, and hepatic systems.2–4 After comparing the severity of irAEs by organ systems, Michot et al. reported that more severe irAEs are observed in the gastrointestinal tract.5 These gastrointestinal irAEs range from benign transient diarrhea to severe colitis that can potentially lead to colonic perforation.3, 6 ICPI-induced colitis (ICPIC) has a clinical presentation similar to that of other colitides; however, histologic evaluation reveals varying mechanisms of toxicity ranging from those similar to inflammatory bowel disease to microscopic colitis (MC).1, 2, 7–9
Traditional MC encompasses lymphocytic colitis (LC) and collagenous colitis (CC), both of which have a grossly normal endoscopic appearance and are differentiated by histology.10, 11 The pathophysiology of MC remains unclear. In addition to having an underlying autoimmune component, MC is thought to develop in genetically susceptible individuals following an aberrant immune response to a specific antigen.10–12 Triggers that have been associated with its development include nonsteroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), histamine type 2 receptor antagonists (H2 blockers), serotoninergic agents such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and tobacco.11, 12 Autoimmunity is hypothesized to play a role in MC given its association with other autoimmune conditions and its responsiveness to immunosuppressants.11, 12 In light of the similarity in pathophysiology of immune dysregulation between ICPIC and MC, it is not surprising that the latter has been described as 1 pattern of injury in ICPIC.2, 8, 9 Chen et al. suggested that although MC can be 1 mechanism of ICPI toxicity, it is also possible that ICPIs unmask MC in patients with genetic predisposition.8
ICPIC can contribute to poor quality of life, drug interruption or discontinuation, and even death.6, 13 Therefore, further characterization of ICPIC is warranted. Given the limited literature available and the anticipated increase in the use of ICPIs in the near future, we conducted the first large-scale retrospective chart review study to characterize ICPI-induced MC. We explored the differences in risk factors, treatment modalities, endoscopic and histological features, and clinical outcomes of patients with MC in noncancer and cancer cohorts with and without exposure to ICPIs. Furthermore, we examined the differences in clinical presentation, types of ICPIs used, histological features additional to standard MC criteria, and colitis treatment regimens between ICPI-induced LC and ICPI-induced CC.
METHODS
Patients
A retrospective chart review study approved by the institutional review board was conducted among patients treated at The University of Texas MD Anderson Cancer Center. Patients at least 18 years of age who met the standard MC criteria on colon histology between January 2012 and January 2018 from an institutional pathology database were eligible. Standard MC criteria used in this study were histologic features of >20 intra-epithelial lymphocytes per 100 surface epithelial cells for lymphocytic colitis and thickened subepithelial collagen layer of >10 micrometers for collagenous colitis.10 Patients with prior diagnosis of inflammatory bowel disease, mesenteric ischemia, sepsis, or with lack of follow-up were excluded. Those who met the inclusion criteria were categorized into 3 groups: cancer patients with ICPI-induced MC, cancer patients with non-ICPI-induced MC, and noncancer patients with traditional MC. The cohort of cancer patients with ICPI-induced MC was subsequently grouped into LC and CC.
Data Collection
In addition to baseline demographics, cancer characteristics such as type and stage of malignancy, cancer treatments since initial diagnosis of malignancy, and chemotherapy agent used 1 month before MC diagnosis were obtained. Also identified were risk factors associated with the development of MC, including smoking history and use of medications such as NSAIDs, PPIs, H2 blockers, SSRIs, and SNRIs within 3 months of MC diagnosis. Clinical symptoms were summarized based on the severity of diarrhea, measured by daily frequency of bowel movements (BMs; categorized as ≤6 BMs and >6 BMs per day). Colitis treatments including antidiarrheal medications, aminosalicylates, budesonide, other oral steroids such as prednisone or methylprednisolone, intravenous steroids, and nonsteroidal immunosuppressive agents such as infliximab and vedolizumab were also collected. Endoscopic features were noted for normal findings, tubular adenomas, and/or presence of mucosal abnormality characterized by erythema and edema. Clinical outcomes gathered included diarrhea recurrence, need for hospitalization, and mortality. Response was defined as either a return to baseline or a decrease in the daily frequency of BMs after initiation of colitis treatment. Recurrence was considered if there was an increase in the daily frequency of BMs after a response.
More variables were obtained in patients with ICPI-induced MC. Depending on the histologic features from pathology review of all 15 cases, ICPI-induced MC was categorized into LC and CC together with coexisting features of acute and chronic inflammation. Acute inflammation revealed in our pathology samples included the presence of intra-epithelial neutrophil and/or eosinophil infiltration, cryptitis, crypt abscesses, epithelial apoptosis, and mucin loss; features of chronic inflammation were paneth cell metaplasia and basal lymphoplasmocytosis. Additional data recorded were the presence of colitis symptoms, defined as abdominal pain and blood or mucus in the stool; types of ICPIs used such as anti-CTLA-4, anti-PD-1, anti-PD-L1, or combination therapy; and the duration from ICPI initiation to MC diagnosis. In this population, instead of measuring the daily frequency of BMs, the severity of diarrhea was scored using Common Terminology Criteria for Adverse Events (CTCAE; version 5.0).14 This scale designates grades largely on the basis of the increase in daily stools from baseline. Respectively, diarrhea grades 1 to 5 were defined as an increase of <4 daily stools, an increase of 4–6 daily stools, an increase of ≥7 daily stools, life-threatening consequences requiring urgent intervention, and death.
Statistical Analysis
Data analysis was performed using the SPSS software program (version 24.0; IBM Corporation, Armonk, NY, USA). Descriptive statistical analysis was performed. The distribution of continuous variables was summarized using means and standard deviations and compared between groups using the Wilcoxon rank-sum test. The distribution of categorical variables was summarized using frequencies and percentages, and associations of these variables were evaluated using the Fisher exact test or chi-square test. All statistical evaluations were 2-sided, and P values of less than 0.05 were considered statistically significant.
RESULTS
Patient Characteristics
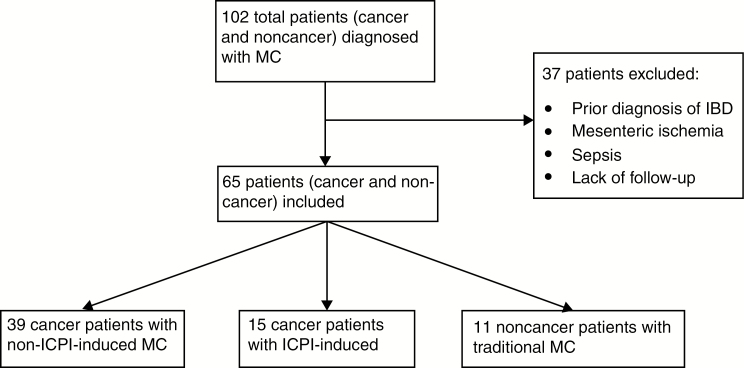
Out of the 102 cancer and noncancer patients in the pathology database who were diagnosed with MC within the specified time frame, 65 met the inclusion criteria. Of those, 39 cancer patients had non-ICPI-induced MC, 15 cancer patients had ICPI-induced MC, and 11 noncancer patients had traditional MC. The detailed selection and distribution of patients in our sample are shown in Figure 1. In the cancer and noncancer cohorts with non-ICPI-induced MC, the majority were Caucasian and in their sixth decade. Similarly, among patients with ICPI-induced MC, 93% were Caucasian, and the mean age was 64 years. However, a statistical difference in sex was noted, with a predominance in females in the non-ICPI-induced MC groups. Only 23% of men with cancer and 27% without cancer were observed in the MC groups that were not related to ICPI use, whereas the ICPI-induced MC cohort consisted of 80% men (P < 0.001). These baseline demographics are outlined in Table 1. With regards to cancer types, breast and/or ovarian cancer was seen in 41% of the non-ICPI-induced MC group and none in the ICPI-induced MC group. Conversely, prostate cancer was found in 29% of patients with ICPI-induced MC but only 3% of patients with non-ICPI-induced MC. In both groups, as seen in Table 2, solid tumors that had been treated with multiple regimens were more common than hematologic malignancies. Of the 37 cancer patients with non-ICPI-induced MC who had received cancer treatments, only 4 (11%) were found to have chemotherapy (all hormonal) within 1 month before MC diagnosis. Furthermore, 18 of 27 (67%) solid tumors in patients with non-ICPI-induced MC had cancer stages no higher than stage II. In contrast, patients with ICPI-induced MC had more advanced disease, with all cases at stage III or IV (P < 0.001).
FIGURE 1.
Flow diagram for selection of patients in the study.
TABLE 1:
Comparison of Demographics and Risk Factors Among Non-ICPI-Induced MC in Cancer Patients, ICPI-Induced MC in Cancer Patients, and Traditional MC in Noncancer Patients
| Characteristic | No. Patients (%)a | P | ||
|---|---|---|---|---|
| Non-ICPI-Induced MC With Cancer | ICPI-Induced MC With Cancer | Traditional MC Without Cancer | ||
| n = 39 | n = 15 | n = 11 | ||
| Mean age (SD), y | 61.9 (10) | 63.5 (7) | 62.4 (11) | 0.854 |
| Male sex | 9 (23) | 12 (80) | 3 (27.3) | <0.001 |
| Caucasian race | 32 (82) | 14 (93) | 11 (100) | 0.495 |
| Risk factors | ||||
| Tobacco | 17 (44) | 10 (67) | 7 (64) | 0.224 |
| NSAIDs | 10 (26) | 6 (40) | 4 (36) | 0.537 |
| PPIs | 8 (21) | 8 (53) | 2 (18) | 0.040 |
| H2 blockers | 2 (5) | 3 (20) | 1 (9) | 0.239 |
| SSRIs/SNRIs | 8 (21) | 5 (33) | 4 (36) | 0.441 |
aExcept where otherwise indicated.
TABLE 2:
Comparison of Cancer Characteristics Between Non-ICPI-Induced MC and ICPI-Induced MC in Cancer Patients
| Characteristic | No. Patients (%) | P | |
|---|---|---|---|
| Non-ICPI-Induced MC With Cancer | ICPI-Induced MC With Cancer | ||
| n = 39 | n = 15 | ||
| Cancer | 0.171 | ||
| Solid | 32 (82) | 15 (100) | |
| Hematologic | 7 (18) | 0 (0) | |
| Cancer stage for solid tumorsa | <0.001 | ||
| ≤II | 18 (67) | 0 (0) | |
| III/IV | 9 (33) | 15 (100) | |
| Cancer treatments | 0.714 | ||
| Single regimen | 7 (19) | 0 (0) | |
| Multiple regimens | 30 (81) | 15 (100) | |
aCancer stage is available only for 27 of 32 non-ICPI-induced MC cancer patients.
Risk Factors
Table 1 also shows the comparison of risk factors for the development of MC among cancer and noncancer patients with and without ICPI exposure. Although the ICPI-induced MC cohort had increased use of tobacco, NSAIDs, PPIs, and H2 blockers compared with its counterparts, this difference was not statistically significant, except for PPIs (P = 0.040).
Colitis Treatments
As seen in Table 3, most patients with ICPI-induced MC had more than 6 BMs per day, and 93% received colitis treatment. On the other hand, most patients in the other 2 groups had 6 or fewer BMs per day, with 64% and 91% receiving colitis treatment, respectively. Of the treated patients, budesonide was the most commonly used therapy in 84% of cancer and 90% of noncancer patients with non-ICPI-induced MC, but in only 43% of those with ICPI-induced MC (P = 0.008). However, patients with ICPI-induced MC required more treatments with oral steroids such as prednisone and methylprednisolone, intravenous steroids, and nonsteroidal immunosuppressive agents such as infliximab and vedolizumab (all P < 0.001). Specifically, among the 8 patients with ICPI-induced MC who were treated with nonsteroidal immunosuppressive agents, 3 received infliximab, 3 received vedolizumab, and 2 received infliximab followed by vedolizumab.
TABLE 3:
Comparison of Daily Frequency of Bowel Movements, Treatment Modalities, Endoscopic Features, and Clinical Outcomes Among Non-ICPI-Induced MC in Cancer Patients, ICPI-Induced MC in Cancer Patients, and Traditional MC in Noncancer Patients
| Characteristic | No. Patients (%) | P | ||
|---|---|---|---|---|
| Non-ICPI-Induced MC With Cancer | ICPI-Induced MC With Cancer | Traditional MC Without Cancer | ||
| n = 39 | n = 15 | n = 11 | ||
| Daily frequency of bowel movements | 0.185 | |||
| ≤6 | 23 (59) | 5 (33) | 7 (64) | |
| >6 | 16 (41) | 10 (67) | 4 (36) | |
| Colitis treatments | 0.035 | |||
| Yes | 25 (64) | 14 (93) | 10 (91) | |
| No | 14 (36) | 1 (7) | 1 (9) | |
| Colitis treatmentsa | ||||
| Antidiarrheals | 5 (20) | 7 (50) | 1 (10) | 0.052 |
| Aminosalicylates | 4 (16) | 4 (27) | 4 (40) | 0.310 |
| Budesonide | 21 (84) | 6 (43) | 9 (90) | 0.008 |
| Other oral steroids | 0 (0) | 12 (86) | 1 (10) | <0.001 |
| Intravenous steroids | 0 (0) | 5 (36) | 0 (0) | <0.001 |
| Infliximab/vedolizumab | 1 (4) | 8 (57) | 0 (0) | <0.001 |
| Endoscopic featuresb | 0.106 | |||
| Mucosal abnormality | 5 (13) | 7 (47) | 3 (27) | |
| Tubular adenoma | 6 (15) | 4 (27) | 0 (0) | |
| Both | 2 (5) | 0 (0) | 1 (9) | |
| Normal | 25 (64) | 4 (27) | 7 (64) | |
| Clinical outcomes | ||||
| Recurrence | 12 (31) | 6 (40) | 5 (46) | 0.502 |
| Hospitalization | 0 (0) | 6 (40) | 0 (0) | <0.001 |
| Death | 0 (0) | 2 (13) | 0 (0) | 0.032 |
aSome patients required multiple colitis treatments. Percentages are out of the number treated.
bEndoscopic features are available for 38 of 39 non-ICPI-induced MC cancer patients.
Endoscopic Features
Of the 39 colonoscopies in cancer patients with non-ICPI-induced MC, 25 (64%) showed normal results and 7 (18%) showed mucosal abnormality characterized by erythema and edema. Similarly, the majority of colonoscopies in noncancer patients with traditional MC were normal (64%), with mucosal abnormality observed in 36%. This is in comparison with patients with ICPI-induced MC where only 27% of colonoscopies had normal findings and 47% demonstrated mucosal abnormality (Fig. 2). Also, a higher incidence of tubular adenomas was found in this population.
FIGURE 2.
Endoscopic features of immune checkpoint inhibitor–induced microscopic colitis. A, Normal mucosa. B, Diffuse edema. C and D, Patchy erythema.
Clinical Outcomes
Despite similar rates of diarrhea recurrence in all groups, there was no hospitalization or death in the non-ICPI-induced MC cohorts. On the contrary, 6 of 15 patients (40%) with ICPI-induced MC required hospitalization, and 2 (13%) died (P < 0.001 and P = 0.032, respectively). The reasons for death were hypoxic respiratory failure in 1 patient and cancer progression in the other patient.
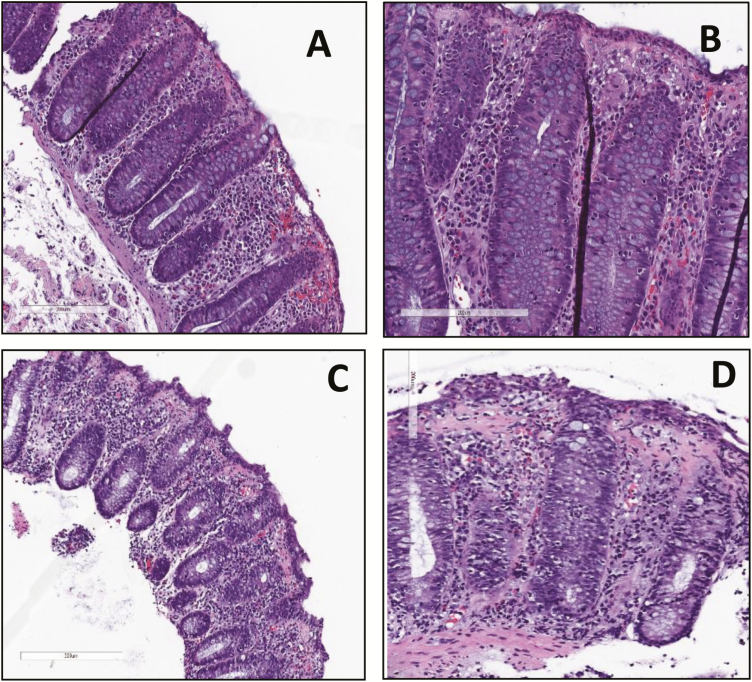
ICPI-Induced LC vs ICPI-Induced CC
Among the 15 patients with ICPI-induced MC, 13 had LC, whereas 2 had CC (Table 4, Fig. 3). In the LC group, most malignancies were of genitourinary origin, accounting for 62%, followed by head, neck, and chest cancers in 23% and melanoma in 15%. This population was exposed to various ICPIs, including combinations of ICPIs in 3 patients (23%). A higher severity of diarrhea based on the CTCAE was observed in 62% of the group, and colitis symptoms were present in 23%. The mean duration from the initiation of ICPIs to the colitis diagnosis was 126 days. With regards to other concurrent histologic features, 6 of 13 (46%) showed evidence of acute inflammation along with LC, 2 of which had coexisting chronic inflammation. A host of colitis treatments were used, including oral steroids other than budesonide in 83% of the patients, infliximab and/or vedolizumab in 58%, intravenous steroids in 42%, budesonide in 42%, antidiarrheal medications in 42%, and aminosalicylates in 33%.
TABLE 4:
Comparison of Clinical Presentations, Types of ICPIs, and Colitis Treatments Between Cancer Patients With ICPI-Induced Lymphocytic and Collagenous Colitis
| Characteristic | No. Patients (%)a | |
|---|---|---|
| Lymphocytic Colitis | Collagenous Colitis | |
| n = 13 | n = 2 | |
| Grade of diarrhea | ||
| 1 | 5 (38) | 0 (0) |
| 2–4 | 8 (62) | 2 (100) |
| Colitis symptoms | 3 (23) | 1 (50) |
| Cancer | ||
| Melanoma | 2 (15) | 0 (0) |
| Head, neck, and chest | 3 (23) | 2 (100) |
| Genitourinary | 8 (62) | 0 (0) |
| Type of ICPIs | ||
| Anti-CTLA-4 | 4 (31) | 0 (0) |
| Anti-PD-1 | 5 (38) | 2 (100) |
| Anti-PD-L1 | 1 (8) | 0 (0) |
| Combination | 3 (23) | 0 (0) |
| Mean duration from ICPI initiation to colitis diagnosis (SD), d | 126 (96) | 142 (108) |
| Concurrent histologic features | ||
| Chronic | 2 (15) | 1 (50) |
| Acute | 6 (46) | 0 (0) |
| Colitis treatmentsb | ||
| Antidiarrheals | 5 (42) | 2 (100) |
| Aminosalicylates | 4 (33) | 0 (0) |
| Budesonide | 5 (42) | 1 (50) |
| Other oral steroids | 10 (83) | 2 (100) |
| Intravenous steroids | 5 (42) | 0 (0) |
| Infliximab/vedolizumab | 7 (58) | 1 (50) |
aExcept where otherwise indicated.
bSome patients required multiple colitis treatments. Percentages are out of the number treated, 12 in LC and 2 in CC.
FIGURE 3.
Histology (hematoxylin and eosin) features of immune checkpoint inhibitor–induced microscopic colitis. A and B, Increased surface intra-epithelial lymphocytes consistent with lymphocytic colitis. C and D, Thickened subepithelial collagen band consistent with collagenous colitis. A and C, 20X magnification. B and D, 40X magnification.
In contrast, among the 2 patients with ICPI-induced CC, both received anti-PD-1 agents for head, neck, and chest cancer. Both also experienced more severe diarrhea based on the CTCAE, but only 1 of the 2 developed colitis symptoms (50%). The mean duration from the initiation of ICPIs to colitis diagnosis was 142 days. One of the 2 patients (50%) also demonstrated features of chronic inflammation on histology. Both patients were treated with antidiarrheal medications and oral steroids such as prednisone and methylprednisolone. One patient received budesonide, 1 required vedolizumab, and neither was treated with intravenous steroids or aminosalicylates.
DISCUSSION
Our study is the first attempt to characterize ICPI-induced MC and its variants, LC and CC. The findings of this study suggest a more aggressive disease course requiring more intensive immunosuppressant treatments and higher need for hospitalization in patients with ICPI-induced MC.
In comparing our patients in the ICPI-induced MC and non-ICPI-induced MC groups, our data showed substantially more women in the non-ICPI-induced MC cohorts. Although women are at higher risk than men to develop traditional MC based on the literature, the discrepancy in sex between the 2 cancer groups can be explained by our patients’ cancer characteristics.12, 15 Prostate cancer was seen in 29% of patients in the ICPI-induced MC group but only 3% of patients in the non-ICPI-induced MC group. On the other hand, breast and/or ovarian cancer was seen in 41% of patients with non-ICPI-induced MC and 0% of the ICPI-induced MC group. These differences in sex and cancer types could be driven by the approved indications for ICPI use during the time frame of our study.
Other risk factors associated with traditional MC include the use of tobacco, NSAIDs, PPIs, H2 blockers, and serotoninergic agents. A meta-analysis conducted by Tong et al. revealed that the use of SSRIs and PPIs significantly increased MC risk.15 After studying these risk factors in our patients with MC, we found a statistically significantly greater usage of PPIs in patients with ICPI-induced MC than in those with non-ICPI-induced MC. The reason behind the increased use of PPIs in this cohort is not entirely clear but could be related to nonspecific upper gastrointestinal symptoms secondary to ICPI use. However, information related to concurrent upper gastrointestinal symptoms or upper endoscopic findings within 3 months of MC diagnosis was not collected in our study; therefore, no clear conclusion can be drawn. Based on these findings, early discontinuation of PPIs as previously suggested by Law et al. is probably beneficial, particularly if indications for PPI use are not met.16 Although the underlying pathophysiology remains uncertain, PPI-induced MC is theorized to occur after aberrant immune responses given the intraluminal electrolyte and acid-base imbalances from inhibition of proton pumps in the colon.10, 16
In addition to risk factor elimination, antidiarrheal medications such as loperamide and immunosuppressants are mainstay therapies used to treat traditional MC. Well-studied `and effective, budesonide is the firstline treatment for traditional MC.11 Our data reinforce this concept by showing that a substantial proportion of our patients in the non-ICPI-induced MC cohorts were treated with budesonide. Some of our patients also received aminosalicylates such as mesalamine, although some studies have argued against its benefits.17, 18 One cancer patient in the non-ICPI-induced MC group received a nonsteroidal immunosuppressive agent, infliximab, which has been shown to be effective in case reports and case series and can be used in rare instances when MC is steroid-refractory.19, 20
In this study, we found differences in clinical presentations, disease courses, and treatments between non-ICPI-induced MC and ICPI-induced MC. Because the CTCAE classification system was not designed for use in the non-ICPI-induced MC groups, daily frequency of BMs was used to compare disease severity. Although without statistical significance, there was a larger proportion of patients with more than 6 daily BMs in the ICPI-induced MC cohort compared with its counterparts, suggesting greater symptom severity. This population also had a statistically significantly higher requirement for hospitalization and treatments with oral and intravenous steroids and nonsteroidal immunosuppressive agents such as infliximab and vedolizumab. These findings suggest that despite some similarities between MC with and without exposure to ICPIs, ICPI-induced MC has greater symptom severity and a more aggressive disease course that requires more potent immunosuppressive treatment regimens. This result also coincides with the overall picture of ICPIC’s behavior, clinical outcomes, and management guidelines, as illustrated by the Society of Immunotherapy of Cancer.21
Our data also showed that most patients with non-ICPI-induced MC had normal lower endoscopic findings, whereas mucosal abnormality was most common in patients who were exposed to ICPIs. Traditional MC is known for its grossly normal-appearing colonic mucosa, although erythema and edema have been noted occasionally.11, 12 In patients with ICPIC, nonspecific inflammatory mucosal abnormalities are visualized in lower endoscopies. These abnormalities include but are not limited to erythema, edema, friability, granularity, exudates, erosions, and ulcerations.2, 22–24 In contrast to the wide range of mucosal abnormalities described in ICPIC, the endoscopic features of ICPI-induced MC are limited to sparse case series and case reports. Left colonic congestion has been reported in ICPI-induced LC, and decreased vascularity has been reported in the rectum in ICPI-induced CC.8, 9 As demonstrated in Table 3 and Figure 2, abnormal colonic mucosa was noted in 47% of patients with ICPI-induced MC, as opposed to 18% of cancer patients and 36% of noncancer patients with non-ICPI-induced MC. We suspect that the presence of endoscopic mucosal inflammation can be a good indicator of a higher level of inflammation in cases of MC and can predict a more aggressive treatment requirement for effective disease control. We also found a lower incidence of tubular adenomas in patients with non-ICPI-induced MC. This finding parallels the inverse association between tubular adenomas and MC observed in Sonnenberg and Genta’s case–control study.25 The significance and incidence of tubular adenomas in ICPI-induced MC are unknown and yet to be explored.
In addition to exploring ICPI-induced MC, this study is the first to characterize ICPI-induced LC and CC. Statistical analysis comparing the 2 groups was not performed given the discrepancy in sample sizes. Notably, the 2 patients with CC were treated with anti-PD-1 agents. Lymphocytic colitis has been described after exposure to anti-PD-1 agents, but anti-PD-1-induced CC has been reported only once in the literature.8, 9 In a case report, a patient with anti-PD-1-induced CC was treated with budesonide, whereas both of our patients with anti-PD-1-induced CC required oral steroids apart from budesonide, and 1 even received vedolizumab.9 Our data also highlight a relatively long duration to MC diagnosis from ICPI initiation. Although delayed diagnosis may have been a confounding factor, these results reinforce the possible late presentation of ICPI-induced MC.8, 9 This delayed onset of MC compared with other histologic subtypes of ICPIC may suggest a different underlying immunomodulatory mechanism. Chen et al. reported a range of 50 to 350 days from ICPI initiation to diarrhea onset in patients with ICPI-induced LC.8 The previously reported patient with ICPI-induced CC developed diarrhea more than 280 days after starting ICPI.9 Therefore, suspicion for ICPI-induced MC should remain high even months after ICPI initiation. Aside from key histologic characteristics of LC or CC, evidence of inflammation can concomitantly be observed in at least half of the colonic biopsies of our patients with ICPI-induced MC. This emphasizes the importance of clinical history in correlation with pathology findings given the remarkable differences in colitis treatments and clinical outcomes between traditional MC and ICPI-induced MC.
Prompt recognition and treatment are the cornerstones of the management of ICPI-induced MC. Pernot et al. reported that dramatic symptom improvement occurs if treatments are initiated within 5 days of onset.24 Adopting a multidisciplinary approach to the management of ICPI-induced MC among pathologists, oncologists, and gastroenterologists is crucial given the novelty and complexity of this disease.
The biggest limitation in our study was the overall small sample size. Some patients at our institution may have been diagnosed with MC at outside facilities and therefore would not have been included in our pathology database. Also, the use of daily frequency of bowel movements can be an inaccurate measure for comparing symptom severity between non-ICPI-induced and ICPI-induced MC, as this can be confounded by multiple factors. Although only 4 cancer patients with non-ICPI-induced MC received hormonal chemotherapy within 1 month of MC diagnosis, certain chemotherapy agents beyond the 1-month window may still increase or decrease the number of daily bowel movements. Similarly, antidiarrheal agents, which can be obtained over the counter and are often underreported, can also affect this result. Other potential confounding factors include the differences in demographics and cancer characteristics between our cancer groups, particularly the sex discrepancy that can be accounted for by the types of malignancy, and the cancer stages. Unfortunately, a multivariate analysis adjusting for these confounding factors could not be performed due to the small sample size. Furthermore, because MC is described as one of the mechanisms of ICPI toxicity, our findings are not generalizable to all patients with ICPIC. As our study examined patients over the span of 6 years, there was a lack of uniformity in pathologists who reviewed the biopsy and provided the diagnosis of MC and its variants. Finally, due to the nature of a retrospective chart review, diarrhea recurrence rates may be an over- or underestimation as complete remission or partial response after initial treatments could not be accurately determined based on the available chart documentation.
CONCLUSION
Despite some similarities between non-ICPI-induced MC and ICPI-induced MC, our findings suggest a more aggressive disease course requiring more intensive immunosuppressant treatments and a greater need for hospitalization in the ICPI-induced MC cohort. High suspicion and timely diagnosis and treatment are key to improving patient outcomes in this population. In light of the limited literature available and the growing use of ICPIs, further research is required to gain a better understanding of this new adverse effect.
Conflicts of interest: The authors have no conflicts of interest to report.
Medical writing support for development of this paper was provided by the Office of Scientific Publications at the MD Anderson Cancer Center.
REFERENCES
- 1. Cramer P, Bresalier RS. Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep. 2017;19:3. [DOI] [PubMed] [Google Scholar]
- 2. Assarzadegan N, Montgomery E, Anders R. Immune checkpoint inhibitor colitis: the flip side of the wonder drugs. Virchow Arch. 2018;472:125–133. [DOI] [PubMed] [Google Scholar]
- 3. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. [DOI] [PubMed] [Google Scholar]
- 4. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11:e0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez-Cao M, Boada A, Teixidó C, et al. Fatal gastrointestinal toxicity with ipilimumab after BRAF/MEK inhibitor combination in a melanoma patient achieving pathological complete response. Oncotarget. 2016;7:56619–56627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42:406–417. [DOI] [PubMed] [Google Scholar]
- 8. Chen JH, Pezhouh MK, Lauwers GY, Masia R. Histopathologic features of colitis due to immunotherapy with anti-PD-1 antibodies. Am J Surg Pathol. 2017;41:643–654. [DOI] [PubMed] [Google Scholar]
- 9. Baroudjian B, Lourenco N, Pagès C, et al. Anti-PD1-induced collagenous colitis in a melanoma patient. Melanoma Res. 2016;26:308–311. [DOI] [PubMed] [Google Scholar]
- 10. Pisani LF, Tontini GE, Vecchi M, Pastorelli L. Microscopic colitis: what do we know about pathogenesis?Inflamm Bowel Dis. 2016;22:450–458. [DOI] [PubMed] [Google Scholar]
- 11. Cotter TG, Pardi DS. Current approach to the evaluation and management of microscopic colitis. Curr Gastroenterol Rep. 2017;19:8. [DOI] [PubMed] [Google Scholar]
- 12. Pardi DS. Diagnosis and management of microscopic colitis. Am J Gastroenterol. 2017;112:78–85. [DOI] [PubMed] [Google Scholar]
- 13. Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (27 November 2017, date last accessed).
- 15. Tong J, Zheng Q, Zheng Q, et al. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:265–276; quiz 277. [DOI] [PubMed] [Google Scholar]
- 16. Law EH, Badowski M, Hung YT, et al. Association between proton pump inhibitors and microscopic colitis. Ann Pharmacother. 2017;51:253–263. [DOI] [PubMed] [Google Scholar]
- 17. Miehlke S, Madisch A, Kupcinskas L, et al. Double-blind, double-dummy, randomized, placebo-controlled, multicenter trial of budesonide and mesalamine in collagenous colitis. Gastroenterology. 2014;146:1222–1230.24440672 [Google Scholar]
- 18. Pardi DS, Ramnath VR, Loftus EV Jr, et al. Lymphocytic colitis: clinical features, treatment, and outcomes. Am J Gastroenterol. 2002;97:2829–2833. [DOI] [PubMed] [Google Scholar]
- 19. Pola S, Fahmy M, Evans E, et al. Successful use of infliximab in the treatment of corticosteroid dependent collagenous colitis. Am J Gastroenterol. 2013;108:857–858. [DOI] [PubMed] [Google Scholar]
- 20. Esteve M, Mahadevan U, Sainz E, et al. Efficacy of anti-TNF therapies in refractory severe microscopic colitis. J Crohns Colitis. 2011;5:612–618. [DOI] [PubMed] [Google Scholar]
- 21. Puzanov I, Diab A, Abdallah K, et al. ; Society for Immunotherapy of Cancer Toxicity Management Working Group Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prieux-Klotz C, Dior M, Damotte D, et al. Immune checkpoint inhibitor-induced colitis: diagnosis and management. Target Oncol. 2017;12:301–308. [DOI] [PubMed] [Google Scholar]
- 23. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42:406–417. [DOI] [PubMed] [Google Scholar]
- 24. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28:264–268. [DOI] [PubMed] [Google Scholar]
- 25. Sonnenberg A, Genta RM. Low prevalence of colon polyps in chronic inflammatory conditions of the colon. Am J Gastroenterol. 2015;110:1056–1061. [DOI] [PubMed] [Google Scholar]