Abstract
Purpose:
To perform a pilot study to quantitatively assess cognitive, vestibular, and physiological function during and after exposure to a magnetic resonance imaging (MRI) system with a static field strength of 10.5 Tesla at multiple time scales.
Methods:
A total of 29 subjects were exposed to a 10.5T MRI field and underwent vestibular, cognitive, and physiological testing before, during, and after exposure; for 26 subjects, testing and exposure were repeated within 2-4 weeks of the first visit. Subjects also reported sensory perceptions after each exposure. Comparisons were made between short and long term time points in the study with respect to the parameters measured in the study; short term comparison included pre-vs-isocenter and pre-vs-post (1-24 hours), while long term compared pre-exposures 2-4 weeks apart.
Results:
Of the 79 comparisons, 73 parameters were unchanged or had small improvements after magnet exposure. The exceptions to this included lower scores on short term (i.e. same day) executive function testing, greater isocenter spontaneous eye movement during visit 1 (relative to pre-exposure), increased number of abnormalities on videonystagmography visit 2 versus visit 1 and a mix of small increases (short term visit 2) and decreases (short term visit 1) in blood pressure. In addition, more subjects reported metallic taste at 10.5T in comparison to similar data obtained in previous studies at 7T and 9.4T.
Conclusion:
Initial results of 10.5T static field exposure indicate that 1) cognitive performance is not compromised at isocenter, 2) subjects experience increased eye movement at isocenter, and 3) subjects experience small changes in vital signs but no field-induced increase in blood pressure. While small but significant differences were found in some comparisons, none were identified as compromising subject safety. A modified testing protocol informed by these results was devised with the goal of permitting increased enrollment while providing continued monitoring to evaluate field effects.
Keywords: MRI safety, ultra-high field MRI, static magnetic field, human vital signs, cognitive function, vestibular function, physiologic function
1. Introduction
While clinical magnetic resonance imaging (MRI) at field strengths up to 3T is a fundamental component of the modern radiological toolbox, the potential benefits of ultrahigh field (UHF) MRI drive the development of MRI systems with ever increasing static magnetic fields. The motivation for UHF imaging is driven primarily by sensitivity which increases with field strength (1-4). Additional advantages of UHF include increased parallel imaging performance (5-7), spectral dispersion (8) and quantification accuracy (9), and greatly improved contrast to noise ratios benefitting both susceptibility weighted anatomical imaging (10-13) and functional mapping in the brain (14,15). While many of these developments have been realized in the brain, advantages have also been well documented in both musculoskeletal and torso applications ((16,17) and references therein). While the potential advantages of UHF are many, the main driver for the developing of the 10.5T scanner in Minnesota was the potential for obtaining unprecedented functional MRI resolutions.
In addition to the known advantages of UHF MRI, there are both technical and safety issues related to imaging humans that need to be addressed. In this work we focus on the safety aspects of human exposure to the static field strength of 10.5T. The importance of this safety study is predicated on the fact that systems greater than 8 Tesla are considered significant risk by the United States Food and Drug Administration (FDA) since 2003 (18) and more recently in the 2015 amendment to the 2010 IEC 60601-2-33 guidelines (19). The threshold for significant risk has been increasing steadily as human safety data from increasingly higher field strength systems becomes available. This study builds upon the multiple previous reports at 8T (20-23) and 9.4T (24) which have demonstrated that exposure to these fields does not pose a risk to human health. While these findings on UHF systems are promising, vigilance is warranted as continued work is performed at 10.5T and with higher field scanners both in existence and in the planning stages. While the field effects are mostly believed to be temporary and little evidence exists for any long term impact of high field exposure in humans, renewed scrutiny has been focused on better understanding longer term effects.
1.1. Background
Qualitative reports of mild, transient vestibular and cognitive effects of high field exposure have been reported in the literature as early as 1995 by Erhard et al. (25). Widely reported experiences include dizziness or vertigo (including a sensation of rotation or falling), nystagmus, double vision, fatigue or sleepiness, metallic taste, visual phenomena, and overall warmth/cold. However, some discrepancy about these incidences exists. For example, Erhard et al. (25) found metallic taste to be the only experience unequivocally correlated with field strength (7.5% of 4T subjects versus 0% of 0T subjects), Vaughan et al. (26) found 32% of 9.4T subjects had metallic taste, and Patel et al. (27) reports metallic taste as the third most common experience (33-67% of 9.4T MRI employees); in contrast, Friebe et al. (28) found none of the subjects exposed to 7T experienced metallic taste. Supporting Information Table S1 briefly summarizes the sensory perception results of these studies.
Numerous quantitative studies have investigated vestibular, cognitive, and physiological effects at field strengths up to 9.4T. Glover et al. (29) investigated several proposed physiological mechanisms to explain dizziness, vertigo, and sensations of rotation or falling that are commonly reported and concluded that induced galvanic vestibular stimulation (rotation) and diamagnetic susceptibility (falling/swaying) fit best with static field and time-changing field data. Roberts et al. (30) found subjects experienced nystagmus that scaled with field strength and did not require head or body movement in the field; they proposed that Lorentz forces on the baseline Ionic current in the endolymph fluid in the semicircular canals was the cause of the vestibular disturbance (which manifested as nystagmus). Mian et al. (31) reported that onset of nystagmus occurs at 1.7T, while onset of perceived dizziness/vertigo occurs at 5.1T and concluded “apparent temporal and spatial discordance between magnetically induced vertigo and nystagmus in the cited experiments is not incompatible with a common mode of stimulation”. Otero-Millan et al. (32) investigated three-dimensional nystagmus; the additional information obtained from observing torsional (in addition to horizontal) nystagmus allowed them to conclude that the “pattern of nystagmus is consistent with combined activation of lateral and anterior semicircular canals”. Finally, Theysohn et al. (33) found that measurable sway path and body axis rotation effects after 7T exposure resolved within 15 minutes of leaving the magnet. Patel et al. (27) reported vestibular effects on six subjects who worked around a 9.4T magnet, including both perceptual and clinical videonystagmography (VNG) results at baseline, post-exposure, and 3 month follow-up. They found “no measurable deterioration or loss of peripheral vestibular function over the course of the study” but did find that a higher percentage of their participants had abnormal VNG results (at all stages of the study) than had previously been reported in normal, healthy adults. Previous quantitative vestibular studies are summarized in Supporting Information Table S2.
Despite common reports of fatigue and sleepiness in exit questionnaires, numerous studies that quantitatively investigated cognitive effects of isocenter magnet exposure above 3T have found no effect or only an improvement in performance presumed to be due to learning effects. These studies have looked at working memory (no effect (20,34,35); learning effect (24)), attention (no effect (20,34,35); learning effect (24)), and verbal fluency (no effect (20,34)) at field strengths up to 9.4T. Mixed results have been reported in studies of dexterity and motor control, with some seeing no effect (20,34,35) while one found lower performance with increasing field strength (36). Visual tracking/discrimination/acuity have also been reported to have no effect (35). Previous quantitative cognitive studies at isocenter are summarized in Supporting Information Table S3.
Physiological effects were historically of some concern, as early modeling studies suggested that the magnetohydrodynamic (MHD) effect (of moving a conductive fluid through a static magnetic field) could dangerously raise blood pressure (37). A later modeling study by Keltner (38) re-examined the simplifying assumptions made in those earlier studies and found that at 10T, the MHD predicted a change in vascular pressure of about 0.2%. More recently, Eryaman et al. (39) used a swine model to measure effects of 10.5T exposure on blood pressure and heart rate and found an increase of 2-4 mm Hg when moving from outside the scanner to isocenter, an effect on the same order as changing positions from lying prone to lying on one’s side (40). Human studies monitoring vital signs during and after exposure to fields up to 9.4T have found no effect on heart rate (20,24,41), oxygen saturation (24,41), blood pressure (20,24,41), respiration rate (20,24,41), temperature (20,24,41), or pre-vs-post ECG (20,24,41). Chakeres et al. (41) did find a 3.6 mm Hg increase in blood pressure at 8T, although they noted this was half the size of the change in blood pressure observed when subjects move from supine to sitting position. Previous physiological studies are summarized in Supporting Information Table S4.
The literature on exposure to fringe fields in the context of occupational health provides an additional window into the effects of exposure to magnetic fields. Studies have examined cognitive, vestibular, motor, and visual effects with a focus on the health and performance of those working near the magnet, including surgeons, MR technologists, etc. These studies investigate exposure to fringe fields up to approximately 1.6T with or without controlled head movements to investigate time varying fields in addition to the static fields. These studies have looked at working memory (no effect (36,42)), verbal working memory (worse (43)), attention and concentration (worse (42,43)), dexterity (worse (36)), visual tracking and acuity (worse (36,42,43)) and postural sway (worse (44)). Symptom diaries for workers at 1.5T, 3T, and 7T systems primarily report vertigo and metallic taste (45), although health related symptoms at 1.5T and 3T did not scale with field strength (46). Occupational exposure studies, while looking at lower fields, are also focused on much more nuanced results and can have more stringent study design (e.g., double blind and fully randomized test administration) than magnet isocenter studies designed for patient exposure. Occupational exposure studies are summarized in Supporting Information Table S5.
With these studies in mind, we designed our study to extend basic measurements of vestibular, cognitive, and physiological functioning in humans to 10.5T and to focus on three different time scales from the acute (i.e. outside to inside scanner within a given exam) to short term (same day) to long term (i.e. several weeks) with the goal of identifying potential safety concerns and to inform future monitoring strategies.
2. Methods
2.1. Study design
The first human studies at 10.5T were conducted under an FDA approved Investigational Device Exemption (IDE) in 2017 and with the approval of the Institutional Review Board at the University of Minnesota. The primary objective of the IDE protocol was to perform a safety study to assess cognitive, vestibular, and physiologic effects with a secondary objective of exploring the feasibility of imaging at 10.5T.
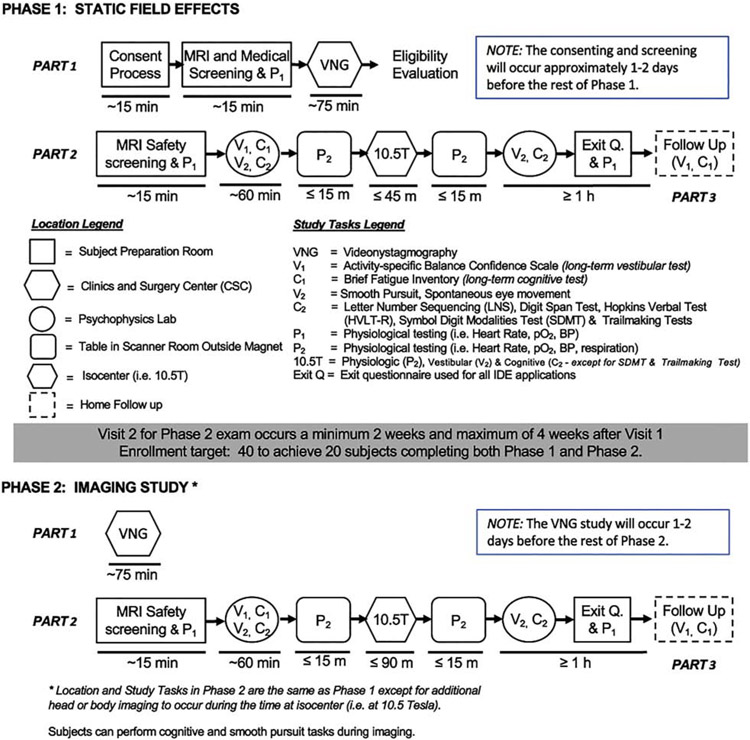
Our study design consisted of 5 encounters divided into two phases, shown schematically in Figure 1:
Figure 1.
Schematic diagram of the study which was accomplished in two phases. Phase 1 evaluated several physiologic, cognitive, and vestibular metrics that are known or could potentially be impacted when subjects are exposed to field strengths up to 10.5T. Along with repeating the tests performed in Phase 1, Phase 2 studied the ability to perform, and the quality of, a standard set of imaging acquisitions at 10.5T. This investigation also contained a temporal analysis which includes evaluation of both short term effects (within a given phase) and long term effects (between phases) of field exposure.
Consenting and screening visit
Vestibular function testing with VNG at the nearby University of Minnesota/M Health Fairview Otolaryngology clinic (phase 1, part 1)
First magnet visit, including cognitive, vestibular, and vital sign testing before (“pre”), during (“iso”, when the subject was at the magnet isocenter) and after (“post”) exposure. This visit took place 1-7 days after the VNG, and subjects spent approximately 20 minutes at isocenter (phase 1, part 2)
Repeat vestibular function testing with VNG at the clinic 2-4 weeks after first vestibular function visit (phase 2, part 1)
Second magnet visit, which was a repeat of the first but also included imaging while at isocenter. Subjects spent 90 minutes at isocenter, and this visit was 1-7 days after the second VNG (phase 2, part 2)
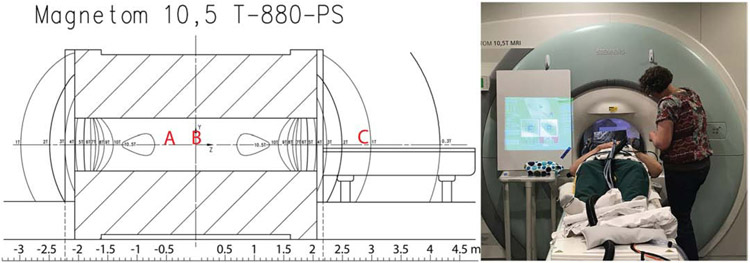
During both magnet visits, the target anatomy was at isocenter for the “iso” testing; for most of the studies this meant the torso was at isocenter, but the head was still experiencing a 10.5T field. Figure 2 shows approximate subject head position for body imaging, head imaging, and for pre and post-iso supine vital sign monitoring, along with a photo of a typical subject setup with eyetracking and vital signs equipment. Subject setup on the table took 10-30 minutes. For 22 subjects the torso was imaged and for 4 subjects the head was imaged; for torso imaging subjects, we sent their lower abdomen to isocenter for the first magnet visit and target anatomy for the second visit, and for head imaging subjects we sent their nasion to isocenter for the first magnet visit and nose or nasion for the second visit. For torso imaging subjects, the subject’s head was on a pillow for both magnet visits; for head imaging subjects, the subject’s head was on a pillow for the first visit and in the head coil for the second. Subjects were positioned using the laser positioner as described, then sent to isocenter at a constant speed of 1.9cm/s (travel time of approximately 105 s).
Figure 2.
Field plot for 10.5T system (left) and photograph of one subject before an imaging study (right). The field plot shows contour lines for 1T-10T; the isocenter is at 10.498T, and the small bubbles labeled 10.5T are above 10.5T. For body studies, the subject’s head was at approximately location A; for head studies the head was at isocenter in location B. Supine physiological monitoring was also done at “home”, with the table out; the subject’s head was approximately at position C, with a field of around 1T. The photograph shows the setup for a body imaging study; the white board to the left of the bore shows the eyetracking system setup screen to enable aiming the camera at the eyes and focusing the lens.
In this study, we were particularly interested in investigating three timescales of exposure effect: 1) acute (pre-exposure to isocenter in a given exam), 2) short term (pre-exposure to 10-60 minutes or 24 hours post-exposure), and 3) long term (pre-exposure phase 1 to preexposure phase 2).
Test scores were evaluated with paired student t-tests for: 1) isocenter effects, including a) visit 1 pre versus visit 1 iso, and b) visit 2 pre versus visit 2 iso; 2) short term effects, including a) visit 1 pre versus visit 1 post, and b) visit 2 pre versus visit 2 post; and 3) long term effects, visit 1 pre versus visit 2 pre. Inter-subject differences were accounted for by using paired tests. Some tests were not administered in all phases of the exam by design and, therefore, were evaluated over only the appropriate time scales. Significance was determined by a two-sided significance level of 0.05. Statistical tests were performed without correction for multiple comparisons; adjusted p-values were also calculated for each test with corrections for multiple comparisons using a resampling-based method that accounts for the high correlations between the tests (47). Since this is an initial (pilot) study with a small sample size, the results section discusses the unadjusted statistics to provide initial evidence of association for further investigation, even if each of the statistical results does not survive after adjustments for multiple testing. The figures and tables report both sets of statistics..
The secondary aim of the study was to explore the feasibility of head and body imaging at 10.5T. The 22 body studies included a range of target anatomies and imaging sequences (48,49), while the 4 head studies focused on T2 and T2* weighted imaging (50).
2.2. Tests
Cognitive Testing
Cognitive tests were chosen to assess fatigue, executive function (general attention and task switching), and working memory. Fatigue was assessed with the Brief Fatigue Inventory (51), a subjective self-assessment of fatigue that was administered before exposure and 24 hours post-exposure with a take-home survey. The questionnaire results in a mean score of overall fatigue ranging from 0-10.
Executive function was assessed with: 1) Symbol Digit Modalities Test (SDMT) (52), a symbol-decoding task that measures visual attention and tracking, divided attention, and processing speed; it was administered before and after exposure. The total number of correct answers and total number of trials answered in 90 s are reported. 2) the Trailmaking test (53), a pair of number and number-letter searches that measures visual attention and task switching and was administered before and after exposure. Time on part A (number search) and part B (1-A-2-B-3-C…. search) are reported.
Working memory was assessed with: 1) Hopkins Verbal Learning Test, Revised (HVLT-R) (54), consisting of a 12-word memory test with 3 immediate recalls, 1 delayed recall, and a false positive forced match recall, was administered before and after exposure. The scoring has seven direct measures (score on trials 1-4 [0-12 per trial], true positive forced choice [0-12], semantically related false positive forced choice [0-6], semantically unrelated false positive forced choice [0-6], total false positive [0-12]) and three derived measures (total recall raw score (sum of trials 1-3 [0-36]), retention raw score (100*T4/(higher of T2 or T3)) [percent], and recognition discrimination index (True positive – total false positive) [12-0]). 2) Digit span (DS) from WAIS-IV (55), where subjects recall increasingly longer spans of numbers forward, backwards, and sorted, was administered before, during, and after exposure. The raw scores include the number of correct forward, backward, and sorted trials (0-16 for each), the total raw score (0-48), and the longest correct sequence for each. 3) Letter Number Sequence (LNS) from WAIS-IV, where subjects sort and recall a mixed sequence of letters and numbers, was administered before, during, and after exposure. The raw score includes the number of correct trials (0-30) and the longest correct sequence.
Vestibular Testing
The study included three types of vestibular-related tests: 1) a clinical assessment of vestibular function, including VNG, 1-7 days before each field exposure, 2) eyetracking of smooth pursuit/spontaneous eye movements administered at our facility before, during, and after exposure, and 3) a balance confidence survey.
Videonystagmography (VNG) was performed at a nearby audiology clinic 1-7 days before each magnet exposure by a single trained audiologist for all exams (MK). VNG measures vestibular function via a series of eyetracking tasks, recordings of nystagmus during positional maneuvers, and bi-thermal caloric irrigations to look for asymmetry that would indicate hypofunction (56). The report generated included percent peripheral vestibular hypofunction, an indicator of normal/abnormal for the nystagmus tests based on lab normative values (Gaze-Horizontal, Gaze-Vertical, Gaze with Fixation Denied, High Hz Headshake, Right Dix-Hallpike, Left Dix-Hallpike, Roll Test, and Positional Body), Caloric Tests (Right Warm, Left Warm, Right Cool, Left Cool), and Oculomotor Tests (Saccade-Random, Anti-Saccade Test, Pursuit), as well as the quantitative measures used to determine if those results are normal/abnormal (slow phase velocity of nystagmus during calorics, positional, and positioning tests, smooth pursuit gain and accuracy, saccade overshoot and latency).
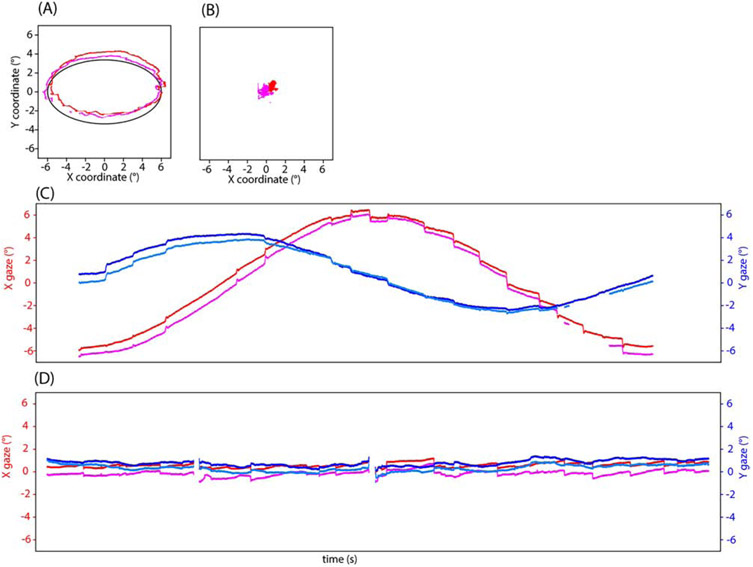
A separate Smooth Pursuit (SP) task (different from that included in the VNG battery) was performed as an eyetracking task at our facility where subjects followed an oscillating target on a computer screen as it moved in a vertical line, horizontal line, diagonal lines, clockwise, and counterclockwise. The pursuit stimulus reached a maximum of 6 degrees of visual angle with a base speed of 0.15 degrees/sec. This was administered before, during, and after exposure. The number of catch-up saccades (jumps in eye movement to stay on target when voluntary tracking lagged) for each trial type was recorded, and the average across trials is reported. Spontaneous eye movement (SEM) with and without a fixation cue was measured with eyetracking at our facility. For true SEM, the test must be administered in complete darkness, as the presence of visual cues allows subjects to suppress spontaneous nystagmus to some extent; when classic nystagmus is observed, the slow phase velocity is calculated. Due to the limitations of our experimental setup, we were unable to eyetrack in complete darkness; instead subjects viewed either a computer monitor in a normally lit room or a rear-projection screen via a mirror within the bore of the magnet with ambient room lighting on. Classic nystagmus allowing for the calculation of slow phase velocity was not observed, instead we counted the number of spontaneous eye movements (rapid jerks in eye movement) made by the subjects during each 30 s trial (one with fixation cue, one without). Subjects were instructed to fixate on the dot when present, and to keep their eyes “in the center of the screen where the dot was” when it was absent. This was administered before, during, and after exposure. The number of spontaneous eye movements for each trial type was recorded. Eyetracking at our facility was done with an infrared camera recording binocularly at 500Hz (Eyelink 1000; SR Research, Ottawa, Canada). Example eyetracking traces for an SP and SEM trial are shown in Figure 3.
Figure 3.
Example eyetracking at 10.5T isocenter for clockwise smooth pursuit (A, C) and spontaneous eye movement without fixation cue (B, D). A and B show target (solid line) and gaze (red and orange) in degrees of visual angle as x-vs-y plots. C and D show a timeseries of gaze in degrees of visual angle for the left and right eyes for x (reds) and y (blues). For each type of trial, the number of catch-up saccades (smooth pursuit) or jerks (SEM) was counted (18, 21 for these examples).
Activity Specific Balance Confidence Survey (ABC) (57) is a subjective self-assessment of balance confidence in performing everyday tasks. This was administered before exposure and 24 hours later with a take-home survey. The mean percent confidence on all measures is reported.
Physiological Testing
Physiological tests included typical vital sign monitoring; vital signs were measured in a sitting position in the lab before and after magnet exposure, and in the supine position on the magnet patient table before entering the magnet, at isocenter, and with the table out after magnet exposure. Heart rate, oxygen saturation, and blood pressure were measured both in the lab and on the patient table; respiration was measured only on the patient table. Lab vital signs were measured with Welch Allen 53NTO or Connex Spot Monitor. Patient table vital signs were measured with a Schiller MAGLIFE Serenity system.
Submeasures
Many of the tests have multiple, highly correlated submeasures. A complete list of submeasures recorded for the cognitive and vestibular tests is given in Supporting Information Table S6. For each test, a single measure was chosen to summarize the results for that test (see Supporting Information for details).
2.3. Subjects
Subjects were recruited from a general volunteer pool that included individuals from the general public and University students and staff. All subjects signed informed consent. In total, 29 subjects passed initial screening and 26 subjects completed the study (10 female, 16 male, mean age 36.2, range 20-64).
In addition to typical MRI exclusion criteria (presence of ferrous metal, active implants, etc.), we further excluded:
anyone with non-ferrous metallic implants (aside from dental fillings)
subjects with serious cognitive or vestibular issues in their medical history
women with pregnancy
subjects under 18 or over 70
subjects over 120 or under 43 kg (due to body imaging array validation (48))
subjects with vital signs out of normal range
where the weight criteria was defined by the weight range permitted with the validated body imaging array used in Phase 2 (48). Vital signs were measured after obtaining informed consent during the screening visit. At this visit subjects also filled out a medical history questionnaire that was used to screen for existing neurological or vestibular conditions that might be exacerbated by exposure to the 10.5T magnetic field. The form was comprised of vestibular questions derived from a screening questionnaire developed by Joel Goebel (58), screening questions for stroke and other relevant conditions derived from the NIH Stroke Scale, and from the University of Minnesota Otolaryngology and Neurology Clinics initial patient consultation questionnaires. The medical questionnaire was reviewed by the study neurologist (TH); affirmative answers to specific symptoms and diagnosis were cause for exclusion and affirmative answers to other questions required follow-up to ascertain whether or not to exclude or include a particular subject in the study.
Of the 39 subjects enrolled, 10 were excluded due to the additional MRI and medical screening criteria, 3 subjects withdrew after phase 1 was complete (one due to unpleasant, though transitory, nausea, and two for unrelated reasons), and 26 completed both phases of the study.
3. Results
For each of the 26 subjects during each phase (i.e. 1 VNG + 1 magnet visit), we recorded 332 data points across the cognitive, vestibular, and physiological testing. As described in the methods, some tests have multiple, highly correlated sub-measures, so a single representative measure was chosen. These tests were performed before, during (if feasible), and after each exposure to 10.5T to assess isocenter effect, short term effects, and long term effects. Long term effects were assessed by comparing the “before” administrations on the first and second phase magnet visits.
Main measures for each test, along with adjusted and unadjusted statistical significance for isocenter, short term, and long term effects are given in Tables 1, 2, and 3, respectively. Higher values in the main measures do not always indicate improved performance (i.e., shorter times on Trailmaking are better than longer times); potentially evaluative terms like “better” or “worse” are used to indicate higher or lower performance that may still be in the normal range and do not indicate an abnormality. Statistical test results for each submeasure are given in Supporting Information Tables S7-S9.
Table 1.
Isocenter effect: comparison between pre and isocenter results during visits 1 and 2; statistics are given without correction for multiple comparison (“P-value”) and adjusted using a resampling method (“P-value adj.”). is the mean, σ is the standard deviation, CI stands for confidence interval.
| Test | visit 1 Pre (σ) |
visit 2 During (σ) |
visit 1 difference Pre – During (95% CI) |
visit 1 P- value |
visit 1 P- value adj. |
visit 2 Pre (σ) |
visit 2 During (σ) |
visit 2 difference Pre – During (95% CI) |
visit 2 P-value |
visit 2 P-value adj. |
|---|---|---|---|---|---|---|---|---|---|---|
| Supine (magnet) heart rate | 66.4 (11.4) | 65.9 (11.1) | 0.5 (−1.1, 2.2) | 0.51 | 0.999 | 65.8 (8.4) | 64 (8.7) | 1.8 (0.1, 3.6) | 0.04 | 0.430 |
| Supine sp02 | 97.4 (1.2) | 97.4 (1.4) | 0 (−0.5, 0.5) | 0.876 | 1.000 | 96.9 (1.5) | 97.1 (1.5) | −0.2 (−0.8, 0.3) | 0.387 | 0.999 |
| Supine BP systolic | 120.2 (10.5) | 118.2 (10.7) | 2 (−0.4, 4.4) | 0.101 | 0.758 | 119 (9.4) | 115.9 (11.4) | 3.1 (0.8, 5.4) | 0.009 | 0.113 |
| Supine BP diastolic | 73 (8.4) | 73.5 (7.8) | −0.5 (−2.3, 1.3) | 0.543 | 0.999 | 74.7 (9) | 71.7 (8.4) | 3 (1, 5.1) | 0.006 | 0.073 · |
| Supine respiration rate | 14.2 (3.6) | 14.3 (3.1) | −0.2 (−1.5, 1.2) | 0.819 | 1.000 | 15.3 (3.7) | 15.5 (3.8) | −0.2 (−1.4, 1) | 0.738 | 1.000 |
| SP | 12.1 (5.6) | 13 (5.4) | −0.9 (−3.4, 1.7) | 0.485 | 0.999 | 12.7 (4.7) | 12.7 (3.6) | 0 (−2.5, 2.6) | 0.973 | 1.000 |
| SEM fixation | 23.2 (19.1) | 38.2 (20.2) | −15 (−31.1, 1.1) | 0.065 | 0.606 | 29.5 (20) | 34.5 (22.6) | −5 (−16.9, 6.9) | 0.389 | 0.999 |
| SEM no fixation | 21.9 (17.5) | 37.8 (21.7) | −15.8 (−24.9, −6.7) | 0.002 | 0.024 | 27.8 (22.7) | 31.8 (16.3) | −4 (−11.9, 3.9) | 0.304 | 0.993 |
| DS | 28.4 (4.3) | 28.4 (4.9) | 0 (−1.3, 1.2) | 0.948 | 1.000 | 30 (5.1) | 29.8 (6.3) | 0.2 (−1.9, 2.4) | 0.829 | 1.000 |
| LNS | 15.1 (2.7) | 14.7 (2.1) | 0.3 (−0.6, 1.3) | 0.459 | 0.999 | 15.4 (3) | 14.9 (2.5) | 0.5 (−0.5, 1.5) | 0.292 | 0.992 |
Table 2.
Short term effect: comparison between pre and post in visits 1 and 2; statistics are given without correction for multiple comparison (“P-value”) and adjusted using a resampling method (“P-value adj.”). is the mean, σ is the standard deviation, CI stands for confidence interval.
| Test | visit 1 Pre (σ) |
visit 1 Post (σ) |
visit 1 difference Pre – Post (95% CI) |
visit 1 P- value |
visit 1 P-value adj. |
visit 2 Pre (σ) |
visit 2 Post (σ) |
visit 2 difference Pre – Post (95% CI) |
visit 2 P- value |
visit 2 P-value adj. |
|---|---|---|---|---|---|---|---|---|---|---|
| Sitting (lab) heart rate | 75 (13.3) | 72.1 (11.1) | 3 (0.3, 5.6) | 0.03 | 0.412 | 72.9 (9.2) | 71 (10.5) | 2 (−1.3, 5.2) | 0.23 | 0.992 |
| Sitting Sp02 | 97.6 (1.8) | 98.1 (1.5) | −0.5 (−1, 0.1) | 0.09 | 0.818 | 97.5 (1.4) | 98.3 (1.1) | −0.8 (−1.3, −0.3) | 0.003 | 0.04 |
| Sitting BP systolic | 119.7 (11.4) | 121.5 (9.1) | −1.8 (−4.3, 0.6) | 0.13 | 0.926 | 117.6 (9.3) | 121.8 (9.6) | −4.2 (−8, −0.4) | 0.032 | 0.432 |
| Sitting BP diastolic | 76 (8.7) | 77.2 (8.2) | −1.2 (−2.8, 0.4) | 0.141 | 0.94 | 74.5 (6.5) | 77 (7.2) | −2.5 (−4.8, −0.3) | 0.029 | 0.396 |
| ABC | 97.7 (3.9) | 97.4 (4.2) | 0.3 (0, 0.7) | 0.066 | 0.691 | 97.6 (4) | 97.1 (4.8) | 0.5 (−0.4, 1.4) | 0.279 | 0.998 |
| SP | 13.1 (5.5) | 12.5 (4.9) | 0.6 (−0.9, 2) | 0.424 | 0.999 | 12.6 (4.6) | 12.4 (4.9) | 0.2 (−0.8, 1.1) | 0.692 | 1 |
| SEM fixation | 27.9 (21.8) | 30.3 (18.5) | −2.4 (−7.2, 2.3) | 0.301 | 0.999 | 29.9 (18.7) | 29.7 (18.2) | 0.2 (−6.5, 6.8) | 0.959 | 1 |
| SEM no fixation | 28.6 (22.1) | 27.3 (21.7) | 1.3 (−4.5, 7) | 0.655 | 1 | 28.2 (21.4) | 26.3 (20.7) | 2 (−2.6, 6.6) | 0.387 | 0.999 |
| BFI | 1.4 (1.3) | 0.9 (0.8) | 0.5 (0.2, 0.9) | 0.005 | 0.07 | 1.4 (1.3) | 1.2 (1.3) | 0.3 (−0.1, 0.7) | 0.157 | 0.955 |
| HVLT-R | 27.5 (3.8) | 26.5 (3.5) | 1 (−0.4, 2.3) | 0.152 | 0.952 | 27.2 (5.1) | 26.9 (4.3) | 0.3 (−1.7, 2.3) | 0.784 | 1 |
| DS | 28.4 (4.3) | 29.7 (5.2) | −1.3 (−2.7, 0) | 0.051 | 0.609 | 30 (5.1) | 29.9 (6.3) | 0.2 (−1.1, 1.4) | 0.804 | 1 |
| LNS | 15.1 (2.7) | 15.3 (2.8) | −0.2 (−1, 0.6) | 0.618 | 1 | 15.4 (3) | 15.8 (2.6) | −0.4 (−1.1, 0.3) | 0.284 | 0.998 |
| SDMT | 57.4 (10.2) | 57.1 (10.8) | 0.3 (−2, 2.5) | 0.809 | 1 | 64.7 (14.6) | 54.9 (9.5) | 9.8 (6, 13.6) | 0 | 0 |
| Trailmaking | 41.9 (13.5) | 40.3 (13.1) | 1.6 (−2.7, 6) | 0.451 | 0.999 | 39.3 (14.2) | 36.1 (13.4) | 3.2 (−0.2, 6.5) | 0.061 | 0.671 |
| Supine (magnet) heart rate | 66.4 (11.4) | 65.3 (9.8) | 1.2 (−0.9, 3.2) | 0.264 | 0.997 | 65.8 (8.4) | 67.7 (10.1) | −1.8 (−4.4, 0.8) | 0.164 | 0.964 |
| Supine Sp02 | 97.4 (1.2) | 96.7 (1.9) | 0.7 (−0.1, 1.4) | 0.071 | 0.718 | 96.9 (1.5) | 97.8 (1.4) | −0.9 (−1.7, −0.2) | 0.017 | 0.243 |
| Supine BP systolic | 120.2 (10.5) | 118.8 (9.5) | 1.4 (−0.5, 3.4) | 0.144 | 0.944 | 119 (9.6) | 123.8 (11.3) | −4.7 (−7.7, −1.7) | 0.004 | 0.054 |
| Supine BP diastolic | 73 (8.4) | 73.4 (8.4) | −0.4 (−2.4, 1.6) | 0.67 | 1 | 74.2 (8.8) | 76.7 (7.8) | −2.6 (−5.3, 0.1) | 0.062 | 0.679 |
| Supine respiration rate | 14.2 (3.6) | 14 (3.9) | 0.2 (−1.3, 1.6) | 0.832 | 1 | 15.3 (3.7) | 14.5 (3.6) | 0.8 (−0.6, 2.1) | 0.251 | 0.995 |
Table 3.
Long term effect: comparison between Visit 1 and Visit 2; statistics are given without correction for multiple comparison (“P-value”) and adjusted using a resampling method (“P-value adj.”). is the mean, σ is the standard deviation, CI stands for confidence interval.
| Test | Visit 1 (σ) |
Visit 2 (σ) |
difference visit 1 – visit 2 (95% CI) |
P-value | P-value adj. |
|---|---|---|---|---|---|
| Sitting (lab) heart rate | 75 (13.3) | 72.9 (9.2) | 2.1 (−2.5, 6.7) | 0.353 | 0.999 |
| Sitting Sp02 | 97.6 (1.8) | 97.5 (1.4) | 0.2 (−0.5, 0.8) | 0.609 | 1 |
| Sitting BP systolic | 119.7 (11.4) | 117.6 (9.3) | 2 (−1.8, 5.9) | 0.283 | 0.999 |
| Sitting BP diastolic | 76 (8.7) | 74.5 (6.5) | 1.5 (−1.1, 4.2) | 0.246 | 0.997 |
| VNG (# abnormals) | 1.6 (1.3) | 2.3 (2) | −0.7 (−1.2, −0.1) | 0.015 | 0.252 |
| VNG hypofunction | 16.1 (14.8) | 15.8 (16.2) | 0.3 (−1.9, 2.5) | 0.748 | 1 |
| ABC | 97.7 (3.9) | 97.6 (4) | 0.1 (−0.5, 0.6) | 0.792 | 1 |
| SP | 13.2 (5.6) | 13 (4.8) | 0.3 (−1.3, 1.8) | 0.742 | 1 |
| SEM fixation | 26.6 (18.7) | 29.9 (18.7) | −3.3 (−9.3, 2.7) | 0.265 | 0.998 |
| SEM no fixation | 27.7 (20.7) | 28.4 (20.9) | −0.7 (−5.6, 4.2) | 0.781 | 1 |
| BFI | 1.4 (1.3) | 1.4 (1.3) | −0.1 (−0.5, 0.3) | 0.726 | 1 |
| HVLT-R | 27.5 (3.8) | 27.2 (5.1) | 0.3 (−1.8, 2.4) | 0.768 | 1 |
| DS | 28.4 (4.3) | 30 (5.1) | −1.7 (−2.7, −0.6) | 0.003 | 0.052 |
| LNS | 15.1 (2.7) | 15.4 (3) | −0.3 (−1.3, 0.7) | 0.527 | 1 |
| SDMT | 57.4 (10.2) | 64.7 (14.6) | −7.3 (−10.5, −4.2) | 0 | 0.001 |
| Trailmaking | 41.9 (13.5) | 39.3 (14.2) | 2.6 (−1.5, 6.7) | 0.203 | 0.993 |
| Supine (magnet) heart rate | 66.4 (11.4) | 65.8 (8.4) | 0.6 (−3.4, 4.6) | 0.769 | 1 |
| Supine Sp02 | 97.4 (1.2) | 96.9 (1.5) | 0.5 (−0.1, 1.1) | 0.119 | 0.94 |
| Supine BP systolic | 120.2 (10.5) | 119 (9.4) | 1.2 (−1.7, 4.1) | 0.42 | 0.999 |
| Supine BP diastolic | 73 (8.4) | 74.7 (9) | −1.7 (−3.8, 0.5) | 0.117 | 0.932 |
| Supine respiration rate | 14.2 (3.7) | 15.3 (3.7) | −1.1 (−3.1, 0.9) | 0.263 | 0.998 |
3.1. Cognitive
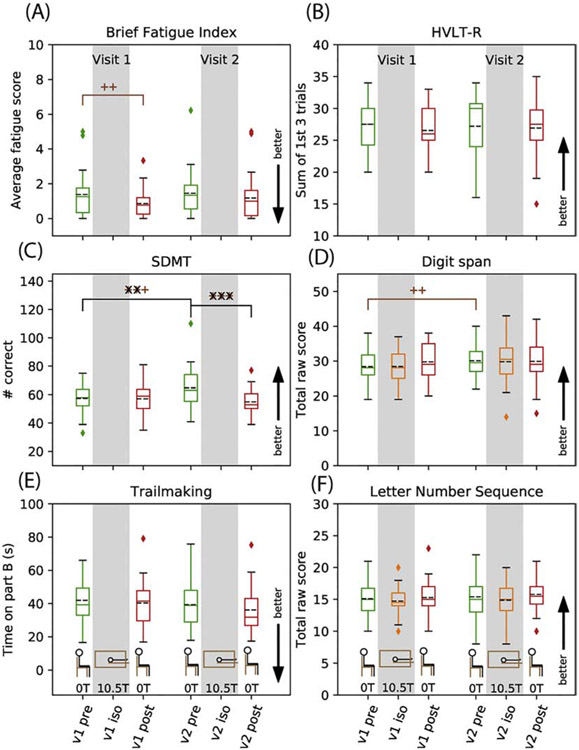
Results for fatigue and executive function (BFI, SDMT, and Trailmaking) and working memory (HVLT-R, DS, and LNS) are shown in Figure 4 (submeasures are shown in Supporting Information Figures S1-S5). Self-reports of fatigue on the BFI showed reduced short term fatigue, i.e., they were less tired the next day (p=0.005, Figure 4, Table 2). Subjects showed better performance on the SDMT in the long term (p<0.0001, Figure 4, Table 3), but worse performance in visit 2 short term (p<0.0001, Figure 4, Table 2). Trailmaking showed no effect. Working memory tests (digit span, letter number sequence, and HVLT-R) showed no effect except for a long term improvement in digit span scores (p=0.003, Figure 4, Table 3) driven specifically by an increase in the backward raw score (Supporting Information Figure S4).
Figure 4.
Cognitive test results for executive function and fatigue: Brief Fatigue Index (A), Symbol Digit Modalities Test (C) and Trailmaking Test (E) and for working memory: Hopkins Verbal Learning Test, Revised (B), WAIS-IV Digit Span (D) and WAIS-IV Letter Number Sequence (F). Data are shown as box and whisker plots: the dashed black line represents the mean, the solid line is the median, the box is the interquartile range, the whiskers are +/−2.698 σ, and the diamonds represent outlier points. Unadjusted statistical significance for isocenter, short term, and long term effects are indicated by p<0.05 (+), p<0.01(++), p<0.001(+++); resampling-method adjusted p-values are overlain as x for the same p-value cutoffs. Timepoint is indicated by color: pre (green), isocenter (orange), post (red).
3.2. Vestibular
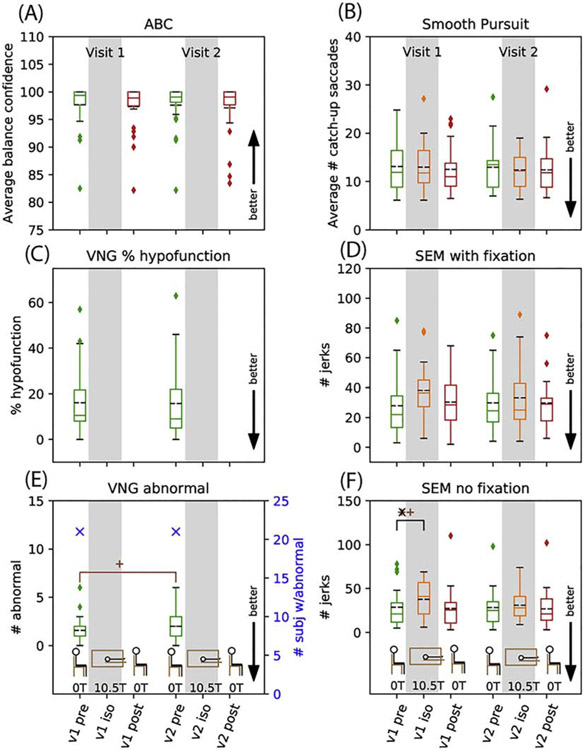
Results for vestibular tests are shown in Figure 5 for balance, VNG, and lab eyetracking. Self-reported balance via the ABC questionnaire showed no effect. No change was observed in VNG percent hypofunction or the total number of subjects with any abnormal results after the first exposure to 10.5T (21 with any abnormal result, Figure 5); of the 26 subjects, 3 were normal on both VNGs, 2 changed from normal to abnormal, 2 changed from abnormal to normal, and 19 were abnormal on both VNGs (where “abnormal” means “any abnormal result”). VNG submeasures showed no effect in: 1) directional preponderance, 2) fixation index, 3) caloric slow phase velocity for right/left and warm/cool tests, 4) saccade latencies and overshoot, and 5) smooth pursuit gain and asymmetry, except for an improvement in 0.4 and 0.6Hz gain on visit 2 (Supporting Information Figures S6-S11). The number of subjects with abnormal responses during phase 1 VNG (VNG1) and phase 2 VNG (VNG2) were, respectively: 1) any abnormal finding 21 and 21; 2) caloric response 7 and 7; and 3) positional nystagmus 14 and 17 (Supporting Information Figures S10-11). It should be noted that for 20 of the 31 abnormal positional nystagmus results, a note stating that “the effects of medication and/or caffeine should be ruled out” was made by the audiologist. Eyetracking at our facility showed no effect except for an increase in the number of involuntary eye jerks detected during SEM without fixation on visit 1 at isocenter relative to visit 1 pre (p=0.002, Figure 5, Table 1).
Figure 5.
Vestibular results: Activity Specific Balance Confidence (A), videonystagmography (VNG) caloric hypofunction (C) and VNG number of subjects with any abnormal results and average number of abnormal results (E), and for lab eyetracking: average number of catch-up saccades in 6 smooth pursuit trials (B), number of jerks in spontaneous eye movement with fixation (D) and without fixation (F). Data are shown as box and whisker plots with statistical significance as described in Figure 3.
3.3. Physiological
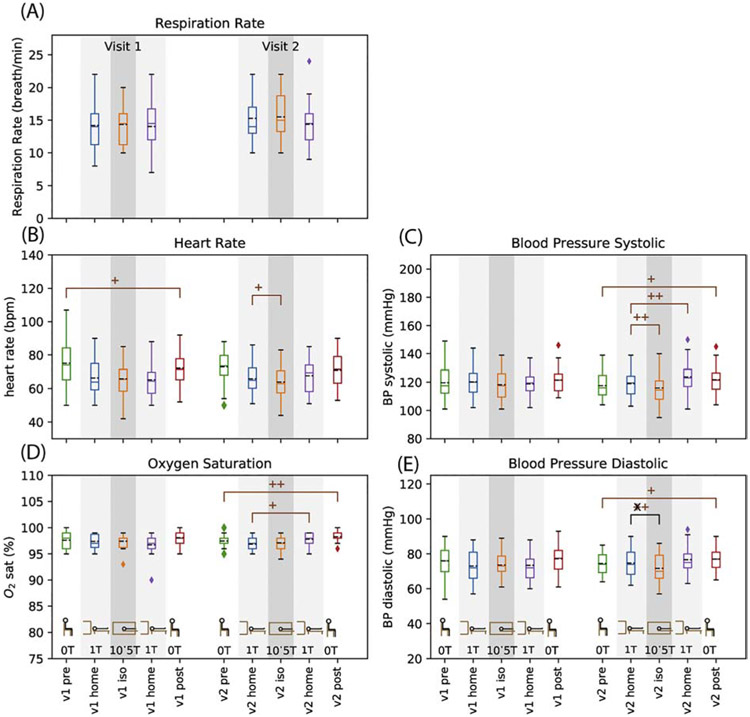
Results for physiological testing are shown in Figure 6. Sitting (lab) heart rate showed a short term decrease on visit 1 (p=0.03, Figure 6, Table 2). Supine (magnet) heart rate showed a decrease at isocenter on visit 2 (p=0.04, Figure 6, Table 1). Both lab and magnet oxygen saturation showed short term increase on visit 2 (p=0.003 and p=0.017, respectively, Figure 6, Table 2). Systolic and diastolic blood pressure in the lab had a short term increase on visit 2 (p=0.032 and p=0.029 respectively, Figure 6, Table 2). Magnet blood pressure decreased at isocenter on visit 2 for both systolic and diastolic (p=0.009 and p=0.006, respectively, Figure 6, Table 1) but systolic increased in the short term, i.e., by the time subjects left the magnet after 90 minutes of imaging (p=0.004, Figure 6, Table 2). The magnitude of the mean blood pressure changes ranged from 0.4 to 4.7 mm HG. Supporting information Figure S12 shows the differences for each physiological measurement for each subject. The long term individual difference for the lab blood pressure ranges from −23 to +23 mmHg for systolic and −12 to +14 mmHg for diastolic; the individual differences seen for isocenter and short term range from −24 to +17 for systolic and −13 to 15 for diastolic. For context, repeated measurements of blood pressure in individuals can show changes of −20 to 30 systolic and −40 to 40 diastolic, with supine measurements have less variability than sitting measurements (59).
Figure 6.
Vital signs: respiration rate (A), heart rate (B), peripheral digit oxygen saturation (D), and systolic (C) and diastolic (E) blood pressure. Note that the data contain a mix of sitting (no shading) and supine (light and dark grey shading) values. Data are shown as box and whisker plots with statistical significance as described in Figure 3.
3.4. Sensory Perception
Results of the exit questionnaire reporting sensory perceptions are shown in Figure 7 along with data previously acquired at our center at 0T, 4T, 7T, and 9.4T using the same exit questionnaire where subjects report “yes”, “no”, or “uncertain” for 10 specific items plus “other” and “unusual sensations” with space for free answers on the last two. The most common experiences were sleepiness (45%), metallic taste (33%), cold (16%), vertigo (16%), and lightheadedness (11%). The only sensory experience that unambiguously scaled with field strength was metallic taste (Fig 7), although vertigo has a weak trend with field strength (Supporting Information Figure S13). All other subjective self-reported effects of field exposure reduced in frequency with increasing field strengths. Reports from the first and second magnet exposure were similar (Supporting Information Figure S14). Comparison of objective measures on the VNG with subjective reports of dizziness in exit questionnaires did not point to any correlation (Supporting Information Figure S11).
Figure 7.
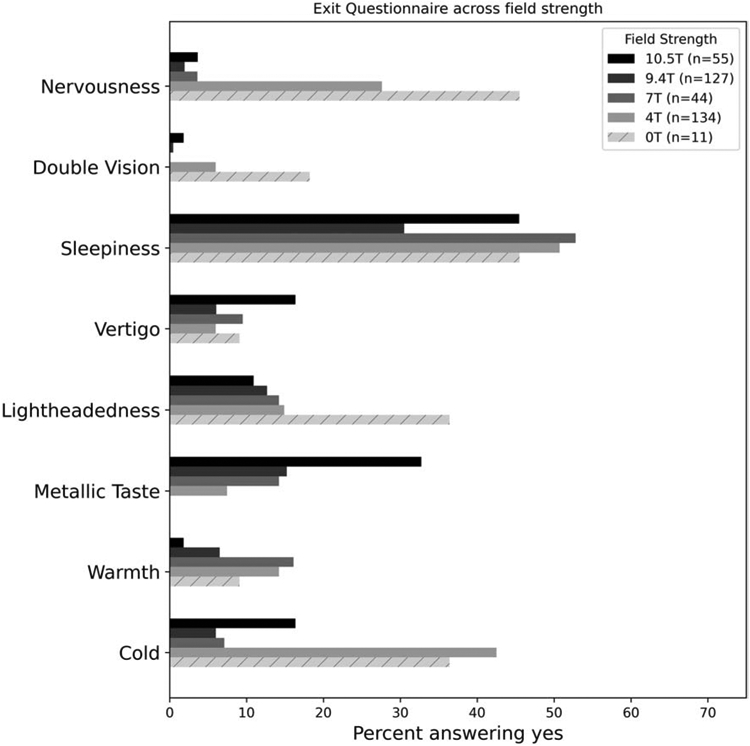
Exit questionnaire results from our facility for 0T (ramped down 4T), 4T, 7T, 9.4T, and 10.5T.
4. Discussion
With this unique investigation of multiple physiologic, cognitive, and vestibular markers over several time scales, with a large number of tests and a relatively small number of subjects, the interpretation of results can be challenging. In light of this, two main questions guide this discussion: 1) is there unambiguous evidence of an effect of B0 exposure that is so strong that it stands out despite the low power? 2) are there general trends that suggest which directions to investigate to tease out more nuanced results in a larger study?
The strongest findings in this study were worse short-term performance on the SDMT in visit 2 (i.e., lower score after magnet exposure relative to before), and an increase in jerks in SEM without fixation at isocenter during visit 1. Short term SDMT performance was also worse in visit 1, but did not reach significance, suggesting that the stronger effect on visit 2 is related to the duration of exposure, although it is unclear if it is a direct physiological effect of B0 exposure, or something else, such as fatigue from 3-4 hours of testing. Slightly more subjects reported in-magnet sleepiness on visit 2 than on visit 1 (45% vs 41%; Supporting Information Figure S14).
Vestibular (eyetracking) tests at our lab showed an increase in jerks in SEM without fixation at isocenter during visit 1 but not visit 2. True detection of spontaneous eye movement requires a dark room with no visual cues, since the presence of anything in the visual field of view allows subjects to voluntarily suppress some amount of spontaneous nystagmus (56); our eyetracking tests instead measured “jerks” which may be related to un-suppressed nystagmus, some difference in the ability to control eye movements related to feeling dizzy, or could be attributed to unrelated searching behavior in the absence of a visual target. The larger increase in jerks at isocenter on visit 1 compared to visit 2 could be explained by the timing of test administration: the eyetracking during visit 1 was done within approximately 5 minutes of reaching isocenter, whereas the isocenter eyetracking during visit 2 was performed 10-70 minutes after reaching isocenter during a convenient break in the imaging protocol. Whatever unregulated eye movement that was present was resolved, as the short term results show no effect for either visit. It is known that the vestibular system acclimates to continuous stimuli (30), which is consistent with this observation of decreased effect at longer exposures. Another potential confound is that the head imaging subjects’ heads were held more still on visit 2 compared to the body imaging subjects and head pitch can impact the vestibular-ocular response (30) and head movement can impact cognitive tests (42,43). Data segregated by head fixation (Supporting Information Figures S15-S18) don’t show any striking trends.
The other strongest findings included long term increases for DS and SDMT and a few changes to vital signs on visit 2 (increased short term lab SP02 and lower isocenter BP diastolic). The improved cognitive test scores are possibly due to learning effects since the same version of the test was administered “pre-magnet” on both visits. The vital sign improvements, while reassuring, are also small in scale and come amidst a mix of small increases and decreases to vital signs in general in both visit 1 and visit 2.
The remaining findings are now explored to look for either broad patterns or hints of effects that could be followed up with further study. Supporting Information Figure S19 gives a visual overview of all of the findings; marker size and distance from the axis are scaled by Cohen’s d (as a way of approximating the strength of the result, not as a strict quantification of effect size). A few broad trends are apparent in this figure: 1) there is consistent evidence pointing toward vestibular symptoms at isocenter, but with short and long term results being close to no effect, suggesting the vestibular impact is short lived; 2) isocenter vital signs are typically improved, while short and long term changes to vitals show an apparently random mix of increases and decreases that are fairly small, with the exception being a short term increase in blood pressure by the end of visit 2; 3) for cognitive tests, executive function and fatigue tests are generally positive (i.e., better EF performance and less fatigue), while memory tests show generally worse performance at isocenter and a mix of small increases and decreases in performance in the short and long term.
The lack of significant effect in working memory is consistent with previous studies (20,34-36), while our finding of worse short-term performance of executive function on visit 2 is new. It is somewhat puzzling that SDMT and Trailmaking don’t have consistency (i.e., SDMT has a statistically significant performance drop on visit 2, but Trailmaking has a not-significant small improvement). Although this study used alternative forms of the Trailmaking Test to minimize learning effects that have been reported (60), the test administrator observed many subjects had markedly improved search strategies on subsequent tests, which could account for a learning effect across different forms. The small effect and mixed results of the memory tasks along with the inconsistency in executive function tasks could indicate that no effect exists, or that these tests are either not sensitive or reproducible enough to detect them given the current sample size.
The VNG showed no change in percent peripheral vestibular hypofunction from bithermal caloric irrigation, including on individual submeasures, and no change in saccade overshoot/latencies or in smooth pursuit gain and asymmetry except for an improvement in SP gain for 0.4 and 0.6Hz, which may also be evidence of learning effects. The number of subjects with abnormal caloric function (hypofunction greater than 20%) was 7 for both the first and second phase VNGs. For both VNG1 and VNG2, there were 21 subjects with some finding of abnormality; 19 subjects had at least one abnormality on both VNGs, 2 subjects had at least one abnormality on the first VNG but none on the second, and 2 subjects had no abnormalities on the first VNG and had at least one on the second VNG. The number of subjects with “unqualified” abnormal results for positional nystagmus was 5 and 6, respectively, for the two VNG phases (14 and 17 if including those whose results may be compromised by medication or caffeine).
The literature on VNG abnormalities in healthy, asymptomatic adults (i.e. those recruited for this study) with no history of balance or vestibular issues is complex. For example, abnormal caloric findings range from 0/30 (0%) (61) to 3/49 (3%) (62) to 22/75 (30%) (63); our finding of 7/26 (27%) is within this range. For abnormal positional nystagmus, reports include 0/75 (0%) (63), 16/29 (55%) (64), 65/89 (73%) (65), and 66/75 (88%) (66); again, our finding of 6/26 (23%) is within the range. Zamyslowska-Szmytke and Silwinska-Kowalska (62) also reported 14/49 (28%) of their healthy subjects had at least one finding of any VNG abnormality. Our finding of 21/26 (81%) is higher, but may not include the same set of VNG submeasures in deriving that tally.
Physiological tests showed a number of effects, a few of which reached statistical significance but which are not believed to represent a safety concern. Supine (magnet) blood pressure and heart rate lowered at isocenter on visit 2, short term sitting (lab) heart rate lowered on visit 1, and short term sitting (lab) and supine (magnet) oxygen saturation increased on visit 2. Short term sitting (lab) and supine (magnet) blood pressure increased on visit 2 by a small amount (mean change up to 4.7mm Hg, individual change up to 14 mmHg); while this was statistically significant using uncorrected tests, it is smaller than the change that could be observed moving from supine to lateral (39,40), supine to standing (67), or even with change in arm position (68) and as such does not present a safety concern at 10.5T.
The subjective self-reported sensory perception effects that reduced in frequency with increasing field strength (all except vertigo and metallic taste) may be partly procedural (e.g., slower table motion and better subject comfort procedures) or cultural (MRI has become increasingly common since 1995, leading to less nervousness).
A number of subjects spontaneously remarked on how long and exhausting the study protocol was, particularly with respect to the extensive and repeated cognitive testing; this sentiment was shared by the first author, who administered the protocol a total of 55 times to all 29 subjects who passed the screening criteria. It is reasonable to speculate that some of the lower performance in cognitive testing at the end of the final visit (part 2, phase 2: post-magnet testing) was due to testing fatigue; one limitation of the study design is we are unable to distinguish between testing fatigue and self-reported B0-induced fatigue.
While the investigation of multiple physiologic, cognitive and vestibular markers over several time scales was a unique aspect of this study, its design resulted in several limitations. First, given the required scheduling and time commitment for participants along with strict exclusion criteria, it was known that the recruitment of subjects was going to be low. This was found to be acceptable as the study was meant to identify major potential safety issues and ultimately set the stage for future ongoing field exposure studies with larger recruitment goals. Further limitations, beyond the learning effects detailed above, include the use of fixed rather than randomized experimental conditions and the absence of a mock scanner control where subject responses could be assessed at zero Tesla isocenter conditions (i.e., single or double blinded). Randomized experimental tests were not possible due to the complex study design and timing requirements of some of the cognitive tests. The mock scanner control was difficult as the study was already quite time consuming, subject recruitment was already challenging, and the ability to simulate the 10.5T experience at zero field was questionable given the unique environment and size of the magnet. Given these factors, this study should not be presumed to be conclusive about exposure to main or fringe fields of 10.5T (69), but rather to assess overall safety at initial exposure and to guide future investigations.
In order to continue with in vivo studies at 10.5T while maintaining continuous safety assessments for participants, a revised IDE study protocol was submitted, and approved, by the FDA. The revised protocol was informed by the results of the current study but significantly modified make it feasible to include a larger number of subjects by reducing the testing burden and improving the workflow. In our revised protocol we removed the VNG and added static posturography using a balance plate assessment (e.g., (70-72)) and removed in-bore cognitive and vestibular testing. While we recognize the specificity of the VNG tests, it is not a practical exam to run for a larger number of subjects. Static posturography does not replace the VNG, but does provide a practical means to assess complex interactions and is a test that can be repeated pre and post exposure and used as a litmus test for release from our imaging center. In addition, by replacing the VNG with a balance plate, all evaluations can now be performed at the imaging center which avoids the delay, scheduling issues, and cost of using a clinical service. For the cognitive tests, pairwise correlations were performed as a guide to reducing the testing protocol along with practical considerations with respect to protocol administration. With these considerations in mind, a revised evaluation was developed removing LNS, HVLT-R, and Trailmaking from the protocol (LNS is more difficult to enunciate clearly than DS, HVLT-R favors native English speakers, and Trailmaking appears to have a more marked learning effect than SDMT). In total, the revised study is reduced to a single “phase”: once screened, subjects will be allowed to participate in 4 imaging visits per year with testing before and after each magnet exposure; we anticipate the pre and post testing will take appropriately 30 minutes to perform. In addition, while not specifically designed to evaluate behavioral or reported incidents between studies, the ability to repeatedly monitor subjects across multiple studies provides improved ability to identify potential adverse events.
5. Summary
Twenty-six of 29 subjects completed the multiple phase study and in general tolerated exposure to the 10.5T static magnetic field in this pilot study. Cognitive tests at isocenter and over the long term showed no effect or small increases in score, while SDMT had lower short term results on the second visit. Vestibular tests showed increased eye movement at isocenter on visit 1 with no short term effects, and no change in the total number of subjects with abnormal results on the VNG at 2-4 week follow-up. Vital sign monitoring showed a mix of small changes to heart rate, oxygen saturation, and blood pressure; notably, among the four blood pressure measurements at isocenter (systolic and diastolic during visits 1 and 2), there was either no change or a decrease at isocenter relative to pre-exposure. These initial results suggest that there are no major or unexpected field effects that would slow or halt enrollment of subjects for studies at 10.5T under the current IDE. However, vigilance is still warranted as the impact of field effects may be only assessed by performing large numbers of studies to reach statistically significant findings for those parameters that may have clinical significance. In order to recruit subjects to further our understanding of the impact of exposure to 10.5T, a modified protocol is proposed which is based on the strategic refinement of the current study and which supports continued observation of short term and long term field effects with the intention of ensuring subject safety during each exam. Monitoring subject performance and tolerance to increasingly higher magnetic field strengths is critical to ensure subject safety and for studies involving cognitive tasks and other applications which are increasingly driven to higher magnetic fields to increase SNR and resolution.
Supplementary Material
7. Acknowledgements
This work was supported by NIBIB P41 EB027061 and S10 RR029672.
Supported by: NIBIB P41 EB027061 and S10 RR029672
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6 References
- 1.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med 2001;46(1):24–30. [DOI] [PubMed] [Google Scholar]
- 2.Guerin B, Villena JF, Polimeridis AG, Adalsteinsson E, Daniel L, White JK, Wald LL. The ultimate signal-to-noise ratio in realistic body models. Magn Reson Med 2017;78(5):1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn Reson Med 2016;75(2):801–809. [DOI] [PubMed] [Google Scholar]
- 4.Wiesinger F, Boesiger P, Pruessmann KP. Electrodynamics and ultimate SNR in parallel MR imaging. Magn Reson Med 2004;52(2):376–390. [DOI] [PubMed] [Google Scholar]
- 5.Wiesinger F, Van de Moortele PF, Adriany G, De Zanche N, Ugurbil K, Pruessmann KP. Parallel imaging performance as a function of field strength--an experimental investigation using electrodynamic scaling. Magn Reson Med 2004;52(5):953–964. [DOI] [PubMed] [Google Scholar]
- 6.Ohliger MA, Grant AK, Sodickson DK. Ultimate intrinsic signal-to-noise ratio for parallel MRI: electromagnetic field considerations. Magn Reson Med 2003;50(5):1018–1030. [DOI] [PubMed] [Google Scholar]
- 7.Wiesinger F, Van de Moortele PF, Adriany G, De Zanche N, Ugurbil K, Pruessmann KP. Potential and feasibility of parallel MRI at high field. NMR Biomed 2006;19(3):368–378. [DOI] [PubMed] [Google Scholar]
- 8.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med 2001;46(3):451–456. [DOI] [PubMed] [Google Scholar]
- 9.Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med 2009;62(4):868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koopmans PJ, Manniesing R, Niessen WJ, Viergever MA, Barth M. MR venography of the human brain using susceptibility weighted imaging at very high field strength. MAGMA 2008;21(1-2):149–158. [DOI] [PubMed] [Google Scholar]
- 11.Abosch A, Yacoub E, Ugurbil K, Harel N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery 2010;67(6):1745–1756; discussion 1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bian W, Hess CP, Chang SM, Nelson SJ, Lupo JM. Susceptibility-weighted MR imaging of radiation therapy-induced cerebral microbleeds in patients with glioma: a comparison between 3T and 7T. Neuroradiology 2014;56(2):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duyn JH. Studying brain microstructure with magnetic susceptibility contrast at highfield. Neuroimage 2018;168:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugurbil K Imaging at ultrahigh magnetic fields: History, challenges, and solutions. Neuroimage 2018;168:7–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ugurbil K Magnetic resonance imaging at ultrahigh fields. IEEE Trans Biomed Eng 2014;61(5):1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erturk MA, Li X, Van de Moortele PF, Ugurbil K, Metzger GJ. Evolution of UHF Body Imaging in the Human Torso at 7T: Technology, Applications, and Future Directions. Top Magn Reson Imaging 2019;28(3):101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juras V, Mlynarik V, Szomolanyi P, Valkovic L, Trattnig S. Magnetic Resonance Imaging of the Musculoskeletal System at 7T: Morphological Imaging and Beyond. Top Magn Reson Imaging 2019;28(3):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Guidance for Magnetic Resonance Diagnostic Devices—Criteria for Significant Risk Investigations In: Services USDoHaH, editor. Maryland: FDA; 2003. [Google Scholar]
- 19.International Electrotechnical Commission. Medical electrical equipment - Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis. Geneva, Switzerland: IEC; IEC 60601-2-33:2010 + AMD1:2013 + AMD2:2015 CSV 2015. [Google Scholar]
- 20.Kangarlu A, Burgess RE, Zhu H, Nakayama T, Hamlin RL, Abduljalil AM, Robitaille PM. Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn Reson Imaging 1999;17(10):1407–1416. [DOI] [PubMed] [Google Scholar]
- 21.Miyakoshi J Effects of static magnetic fields at the cellular level. Prog Biophys Mol Biol 2005;87(2-3):213–223. [DOI] [PubMed] [Google Scholar]
- 22.Schenck JF. Safety of strong, static magnetic fields. J Magn Reson Imaging 2000;12(1):2–19. [DOI] [PubMed] [Google Scholar]
- 23.Schenck JF. Physical interactions of static magnetic fields with living tissues. Prog Biophys Mol Biol 2005;87(2-3):185–204. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson IC, Renteria L, Burd H, Pliskin NH, Thulborn KR. Safety of human MRI at static fields above the FDA 8 T guideline: sodium imaging at 9.4 T does not affect vital signs or cognitive ability. J Magn Reson Imaging 2007;26(5):1222–1227. [DOI] [PubMed] [Google Scholar]
- 25.Erhard P, Chen W, J-H L, Ugurbil K. A study of effects reported by subjects at high magnetic fields. Proceedings of the 3rd Annual Meeting of ISMRM 1995. [Google Scholar]
- 26.Vaughan T, DelaBarre L, Snyder C, Tian J, Akgun C, Shrivastava D, Liu W, Olson C, Adriany G, Strupp J, Andersen P, Gopinath A, van de Moortele PF, Garwood M, Ugurbil K. 9.4T human MRI: preliminary results. Magn Reson Med 2006;56(6):1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel M, Williamsom RA, Dorevitch S, Buchanan S. Pilot study investigating the effect of the static magnetic field from a 9.4-T MRI on the vestibular system. J Occup Environ Med 2008;50(5):576–583. [DOI] [PubMed] [Google Scholar]
- 28.Friebe B, Wollrab A, Thormann M, Fischbach K, Ricke J, Grueschow M, Kropf S, Fischbach F, Speck O. Sensory perceptions of individuals exposed to the static field of a 7T MRI: A controlled blinded study. J Magn Reson Imaging 2015;41(6):1675–1681. [DOI] [PubMed] [Google Scholar]
- 29.Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA. Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics 2007;28(5):349–361. [DOI] [PubMed] [Google Scholar]
- 30.Roberts DC, Marcelli V, Gillen JS, Carey JP, Della Santina CC, Zee DS. MRI magnetic field stimulates rotational sensors of the brain. Curr Biol 2011;21(19):1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mian OS, Glover PM, Day BL. Reconciling Magnetically Induced Vertigo and Nystagmus. Front Neurol 2015;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otero-Millan J, Zee DS, Schubert MC, Roberts DC, Ward BK. Three-dimensional eye movement recordings during magnetic vestibular stimulation. J Neurol 2017;264(Suppl 1):7–12. [DOI] [PubMed] [Google Scholar]
- 33.Theysohn JM, Kraff O, Eilers K, Andrade D, Gerwig M, Timmann D, Schmitt F, Ladd ME, Ladd SC, Bitz AK. Vestibular effects of a 7 Tesla MRI examination compared to 1.5 T and 0 T in healthy volunteers. PLoS One 2014;9(3):e92104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakeres DW, Bornstein R, Kangarlu A. Randomized comparison of cognitive function in humans at 0 and 8 Tesla. J Magn Reson Imaging 2003;18(3):342–345. [DOI] [PubMed] [Google Scholar]
- 35.Heinrich A, Szostek A, Meyer P, Nees F, Rauschenberg J, Grobner J, Gilles M, Paslakis G, Deuschle M, Semmler W, Flor H. Cognition and sensation in very high static magnetic fields: a randomized case-crossover study with different field strengths. Radiology 2013;266(1):236–245. [DOI] [PubMed] [Google Scholar]
- 36.de Vocht F, Stevens T, Glover P, Sunderland A, Gowland P, Kromhout H. Cognitive effects of head-movements in stray fields generated by a 7 Tesla whole-body MRI magnet. Bioelectromagnetics 2007;28(4):247–255. [DOI] [PubMed] [Google Scholar]
- 37.Vardanian VA. [Effect of a magnetic field on blood flow]. Biofizika 1973;18(3):491–496. [PubMed] [Google Scholar]
- 38.Keltner JR, Roos MS, Brakeman PR, Budinger TF. Magnetohydrodynamics of blood flow. Magn Reson Med 1990;16(1):139–149. [DOI] [PubMed] [Google Scholar]
- 39.Eryaman Y, Zhang P, Utecht L, Kose K, Lagore RL, DelaBarre L, Kulesa J, Eberly LE, Adriany G, Iles TL, Iaizzo PA, Vaughan JT, Ugurbil K. Investigating the physiological effects of 10.5 Tesla static field exposure on anesthetized swine. Magn Reson Med 2018;79(1):511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakao S, Come PC, Miller MJ, Momomura S, Sahagian P, Ransil BJ, Grossman W. Effects of supine and lateral positions on cardiac output and intracardiac pressures: an experimental study. Circulation 1986;73(3):579–585. [DOI] [PubMed] [Google Scholar]
- 41.Chakeres DW, Kangarlu A, Boudoulas H, Young DC. Effect of static magnetic field exposure of up to 8 Tesla on sequential human vital sign measurements. J Magn Reson Imaging 2003;18(3):346–352. [DOI] [PubMed] [Google Scholar]
- 42.van Nierop LE, Slottje P, van Zandvoort MJ, de Vocht F, Kromhout H. Effects of magnetic stray fields from a 7 tesla MRI scanner on neurocognition: a double-blind randomised crossover study. Occup Environ Med 2012;69(10):759–766. [DOI] [PubMed] [Google Scholar]
- 43.van Nierop LE, Slottje P, van Zandvoort M, Kromhout H. Simultaneous exposure to MRI-related static and low-frequency movement-induced time-varying magnetic fields affects neurocognitive performance: A double-blind randomized crossover study. Magn Reson Med 2015;74(3):840–849. [DOI] [PubMed] [Google Scholar]
- 44.van Nierop LE, Slottje P, Kingma H, Kromhout H. MRI-related static magnetic stray fields and postural body sway: a double-blind randomized crossover study. Magn Reson Med 2013;70(1):232–240. [DOI] [PubMed] [Google Scholar]
- 45.Schaap K, Christopher-de Vries Y, Mason CK, de Vocht F, Portengen L, Kromhout H. Occupational exposure of healthcare and research staff to static magnetic stray fields from 1.5-7 Tesla MRI scanners is associated with reporting of transient symptoms. Occup Environ Med 2014;71(6):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Vocht F, Batistatou E, Molter A, Kromhout H, Schaap K, van Tongeren M, Crozier S, Gowland P, Keevil S. Transient health symptoms of MRI staff working with 1.5 and 3.0 Tesla scanners in the UK. Eur Radiol 2015;25(9):2718–2726. [DOI] [PubMed] [Google Scholar]
- 47.Westfall PH, Young SS. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment. New York: John Wiley; 1993. [Google Scholar]
- 48.He X, Erturk MA, Grant A, Wu X, Lagore RL, DelaBarre L, Eryaman Y, Adriany G, Auerbach EJ, Van de Moortele PF, Ugurbil K, Metzger GJ. First in-vivo human imaging at 10.5T: Imaging the body at 447 MHz. Magn Reson Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steensma B, van de Moortele PF, Erturk A, Grant A, Adriany G, Luijten P, Klomp D, van den Berg N, Metzger G, Raaijmakers A. Introduction of the snake antenna array: Geometry optimization of a sinusoidal dipole antenna for 10.5T body imaging with lower peak SAR. Magn Reson Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadeghi-Tarakameh A, DelaBarre L, Lagore RL, Torrado-Carvajal A, Wu X, Grant A, Adriany G, Metzger GJ, Van de Moortele PF, Ugurbil K, Atalar E, Eryaman Y. In vivo human head MRI at 10.5T: A radiofrequency safety study and preliminary imaging results. Magn Reson Med 2020;84(1):484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- 52.Smith A Symbol digit modalities test : manual. Los Angeles: Western Psychological Services; 1982. 22 p. p. [Google Scholar]
- 53.Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery : theory and clinical interpretation. Tucson, Ariz: Neuropsychology Press; 1985. xv, 486 pages p. [Google Scholar]
- 54.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 1998;12(1):43–55. [Google Scholar]
- 55.Wechsler D, Psychological Corporation., PsychCorp (Firm). WAIS-IV technical and interpretive manual. San Antonio, Tex: Pearson; 2008. xvii, 218 pages p. [Google Scholar]
- 56.McCaslin DL. Electronystagmography/videonystagmography. San Diego: Plural Pub; 2013. vi, 213 pages p. [Google Scholar]
- 57.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 1995;50A(1):M28–34. [DOI] [PubMed] [Google Scholar]
- 58.Goebel JA. The ten-minute examination of the dizzy patient. Semin Neurol 2001;21(4):391–398. [DOI] [PubMed] [Google Scholar]
- 59.Jamieson MJ, Webster J, Philips S, Jeffers TA, Scott AK, Robb OJ, Lovell HG, Petrie JC. The measurement of blood pressure: sitting or supine, once or twice? J Hypertens 1990;8(7):635–640. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira RS, Trezza BM, Busse AL, Jacob-Filho W. Learning effect of computerized cognitive tests in older adults. Einstein (Sao Paulo) 2014;12(2):149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ila K, Soylemez E, Yilmaz N, Kayis SA, Eshraghi AA. Vestibular functions in patients with tinnitus only. Acta Otolaryngol 2019;139(2):162–166. [DOI] [PubMed] [Google Scholar]
- 62.Zamyslowska-Szmytke E, Sliwinska-Kowalska M. Vestibular and balance findings in nonsymptomatic workers exposed to styrene and dichloromethane. Int J Audiol 2011;50(11):815–822. [DOI] [PubMed] [Google Scholar]
- 63.Weaver LK, Wilson SH, Lindblad AS, Churchill S, Deru K, Price R, Williams CS, Orrison WW, Patel JB, Walker JM, Meehan A, Mirow S, Team NS. Comprehensive Evaluation of Healthy Volunteers Using Multi-Modality Brain Injury Assessments: An Exploratory, Observational Study. Front Neurol 2018;9:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geisler C, Bergenius J, Brantberg K. Nystagmus findings in healthy subjects examined with infrared videonystagmoscopy. ORL J Otorhinolaryngol Relat Spec 2000;62(5):266–269. [DOI] [PubMed] [Google Scholar]
- 65.Sunami K, Tochino R, Zushi T, Yamamoto H, Tokuhara Y, Iguchi H, Takayama M, Konishi K, Yamane H. Positional and positioning nystagmus in healthy subjects under videonystagmoscopy. Acta Otolaryngol Suppl 2004(554):35–37. [DOI] [PubMed] [Google Scholar]
- 66.Martens C, Goplen FK, Nordfalk KF, Aasen T, Nordahl SH. Prevalence and Characteristics of Positional Nystagmus in Normal Subjects. Otolaryngol Head Neck Surg 2016;154(5):861–867. [DOI] [PubMed] [Google Scholar]
- 67.Nardo CJ, Chambless LE, Light KC, Rosamond WD, Sharrett AR, Tell GS, Heiss G. Descriptive epidemiology of blood pressure response to change in body position. The ARIC study. Hypertension 1999;33(5):1123–1129. [DOI] [PubMed] [Google Scholar]
- 68.Netea RT, Lenders JW, Smits P, Thien T. Influence of body and arm position on blood pressure readings: an overview. J Hypertens 2003;21(2):237–241. [DOI] [PubMed] [Google Scholar]
- 69.de Vocht F, Kromhout H. Human MRI above the FDA 8 T guideline: can we conclude that it is safe? J Magn Reson Imaging 2008;27(4):938–939; author reply 939. [DOI] [PubMed] [Google Scholar]
- 70.Agrawal VB. Characteristics of keratoconus patients at a tertiary eye center in India. J Ophthalmic Vis Res 2011;6(2):87–91. [PMC free article] [PubMed] [Google Scholar]
- 71.Ickenstein GW, Ambach H, Kloditz A, Koch H, Isenmann S, Reichmann H, Ziemssen T. Static posturography in aging and Parkinson's disease. Front Aging Neurosci 2012;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Howcroft J, Kofman J, Lemaire ED. Prospective Fall-Risk Prediction Models for Older Adults Based on Wearable Sensors. IEEE Trans Neural Syst Rehabil Eng 2017;25(10):1812–1820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.