Abstract
Background: Spinal cord injury (SCI) results in significant loss in pulmonary function secondary to respiratory muscle paralysis. Retention of secretions and atelectasis and, recurrent respiratory tract infections may also impact pulmonary function.
Objective: To determine whether usage of lower thoracic spinal cord stimulation (SCS) to restore cough may improve spontaneous pulmonary function in individuals with chronic SCI.
Design/Methods: 10 tetraplegics utilized SCS system on a regular daily basis. Spontaneous inspiratory capacity (IC), maximum inspiratory pressure (MIP) and maximum expiratory pressure (MEP) were measured at baseline prior to usage of the device and repeated every 4–5 weeks over a 20-week period. Maximum airway pressure generation (P) during SCS (40 V, 50 Hz, 0.2 ms) at total lung capacity (TLC) with subject maximal expiratory effort, at the same timepoints were determined, as well.
Results: Following daily use of SCS, mean IC improved from 1636 ± 229 to 1932 ± 239 ml (127 ± 8% of baseline values) after 20 weeks (P < 0.05). Mean MIP increased from 40 ± 7, to 50 ± 8 cmH2O (127 ± 6% of baseline values) after 20 weeks, respectively (P < 0.05). MEP also improved from 27 ± 3.7 to 33 ± 5 (127 ± 14% of baseline values) (NS). During SCS, P increased from baseline in all participants from mean 87 ± 8 cmH2O to 117 ± 14 cmH2O at weeks 20, during TLC with subject maximal expiratory effort, respectively (P < 0.05). Each subject stated that they experienced much greater ease in raising secretions with use of SCS.
Conclusion: Our findings indicate that use of SCS not only improves expiratory muscle function to restore cough but also results in improvement inspiratory function, as well.
Keywords: Cough, Spinal cord stimulation, Pulmonary function, Rehabilitation
Introduction
Lower thoracic spinal cord stimulation (SCS) is a useful method to restore an effective cough in subjects with cervical spinal cord injury (SCI).1–6 This device involves the minimally invasive placement of wire electrodes on the dorsal epidural surface of the spinal cord at the T9 and T11 levels. These wires are connected to a radiofrequency receiver which is implanted over the anterior chest wall. Bipolar stimulation is applied via an external stimulator connected to an antenna which is placed directly over the receiver. The user or caregiver can apply stimulation by depressing a button on the external stimulation, on demand. The level of electrical stimulation can be adjusted between 10 and 40 V to allow different levels of stimulation, based upon need. We have previously demonstrated that use of this system results in airway pressures and peak expiratory flow rates of 7.1 l/s and 103 cmH2O, respectively which are in the range of that generated by normal individuals.1 With regular use of this system, subjects have described the ability to effectively clear airway secretions and elimination of the need for other methods to clear secretions, including the commonly used insufflator/exsufflator device. Importantly, use of SCS is also associated with a reduced incidence of respiratory tract infections. The SCS system is a currently an investigational device (IDE: G980267) undergoing Clinical Trials Registry: NCT01659541.
During the course of the clinical trial to restore cough in the SCI population, subjects and/or their caregivers often anecdotally related the ability to take deeper breaths, breathe more easily and/or improved vocalization following chronic use of SCS. We hypothesized therefore, that SCS to restore cough may also improve pulmonary function. In the present study therefore, we compared inspiratory capacity and maximal inspiratory and expiratory pressures, before and after 20 weeks of daily use of the SCS to restore cough. We report that use of the SCS, in addition to restoring expiratory muscle function, also results in improvement in inspiratory function, as well.
Methods
This investigation was approved by the Institutional Review Board at MetroHealth Medical Center, the National Institute of Neurological Disorders and Stroke and the Food and Drug Administration. Written informed consent was obtained from each subject prior to enrollment in the study. All 10 subjects suffered a cervical SCI secondary to trauma (Table 1) except for one subject who suffered a spinal abscess. Each subject had marked paresis of their expiratory muscles, as demonstrated by markedly reduced maximum expiratory pressure generation (Table 1) which was associated with difficulty mobilizing secretions. Each subject underwent implantation of the SCS system to restore cough. The maximum pressures and spirometry were expressed as percentage predicted using the values of Black and Hyatt7 and Crapo et al.,8 respectively.
Table 1. Clinical data of the subjects.
| Subject | Sex | Age (y) | Cause of Injury | Level of Injury | Elapsed Time Since Injury (y) | Spontaneous Inspiratory Capacity (L) (% predicted) | Maximum Expiratory Pressure (cmH2O) (% predicted) | Peak Expiratory Airflow (L/s) (% predicted) |
|---|---|---|---|---|---|---|---|---|
| #1 | M | 50 | GSW | C4 | 2 | 0.9 (28) | 20 (29) | 2.0 (22) |
| #2 | M | 27 | Fall | C3/C4 | 5 | 1.3 (35) | 18 (8) | 2.2 (20) |
| #3 | M | 28 | Fall | C2 | 9 | 1.3 (35) | 41 (18) | 1.2 (11) |
| #4 | M | 57 | Equipment Accident | C4 | 37 | 1.2 (34) | 15 (7) | 2.8 (28) |
| #5 | M | 58 | MVA | C5–C7 | 4 | 1.6 (51) | 29 (14) | 4.2 (48) |
| #6 | M | 50 | Equipment Accident | C4–C6 | 3 | 3.1 (92) | 59 (25) | 5.9 (60) |
| #7 | M | 36 | Spinal Cord Abscess | C3–C6 | 3 | 1.4 (39) | 21 (9) | 2.7 (25) |
| #8 | M | 35 | Fall | C5–C7 | 4 | 2.5 (80) | 21 (9) | 2.7 (29) |
| #9 | M | 30 | Diving Accident | C3 | 2 | 1.9 (61) | 33 (14) | 2.6 (28) |
| #10 | M | 33 | MVA | C7–T1 | 2 | 1.0 (29) | 16 (7) | 2.6 (26) |
| Mean | 40 | 7 | 1.6 (49) | 27 (12) | 2.9 (30) | |||
Abbreviations: GSW, gunshot wound; M, male; MVA, motor vehicle accident.
Prior to implantation of SCS, each subject underwent baseline pulmonary function testing. In the seated posture, airway pressure was measured with a pressure transducer to assess the force of contraction of the inspiratory and expiratory muscles during maximal efforts. Maximum inspiratory pressure (MIP) was measured at residual volume while maximum expiratory pressure generation was measured at total lung capacity (TLC). Inspiratory capacity was measured with a heated pneumotach. Measurements were made via application of a tight fitting full face mask or through a tracheostomy tube, when present. Subjects with tracheostomies had cuffless endotracheal tubes. To minimize air leak, dressings were applied around the tracheostomy. During SCS, expiratory airway pressure measurements were made under conditions of airway occlusion at TLC with subject maximal expiratory effort. Cheek pressure was maintained manually during SCS. ATS criteria for reproducibility of test results were followed. If the initial 2 measurements were not within 10% of each other, additional measurements were made.
Details of the surgical procedure to implant the SCS system has been presented in detail in recent prior publications.1,2 Briefly, the procedure consisted of surgical implantation of 2 parallel wire electrodes on the dorsal epidural surface of the lower thoracic spinal cord to achieve bipolar stimulation (T9–T11 levels). Electrical stimulation was applied by activating a small portable external transmitter connected to an antenna, which was secured to the skin directly over the implanted receiver. The transmitter was activated by pushing a small button on the device.
Four to eight weeks following implantation, subjects underwent initial activation of SCS to activate the expiratory muscles. During the initial phase of stimulation, blood pressure, pulse rate and oxygen saturation were monitored. If absolute blood pressure exceeded 140 mmHg or 100 mmHg diastolic, stimulation was applied less frequently. Blood pressure elevation was observed during initial stimulation in 5 of the 10 subjects which resolved completely with continued stimulation over several weeks. Repeated muscle stimulation over several weeks was necessary to restore strength to the atrophied expiratory muscles. Subjects were instructed to use SCS every ∼30 s for 5–10 min, 2–3 times/day and as needed to clear airway secretions. Based upon previous studies,1 stimulus parameters were set at values (30–40 V, 50 Hz, pulse width 0.2 ms) which resulted in near maximal positive airway pressure generation. Repeat measurements of airway pressure and inspiratory capacity were made during out-patient visits every 4–5 weeks over a 20-week period. Study participants maintained a home stimulation log to record usage of the SCS system.
A BIOPAC Data Acquisition and Analysis System with AcqKnowledge software, MP150 system with TSD 160C pressure transducer and TSD117 pneumotach airflow transducer interfaces with the DA 100C transducer amplifiers (Biopac Systems Inc, 42 Aero Camino, CA) was used to monitor online P, IC, MIP and MEP.
Baseline data was obtained prior to usage of the device and compared to data obtained following daily usage of SCS over a 20-week period. Statistical analysis was performed using the Freidman Test, a non-parametric analogue to the repeated measures Analysis of Variance (ANOVA). Planned comparisons were tested using Dunnett’s method where values at each time point were compared to baseline values. Since subjects did not have follow-up visits at equal intervals graphs were constructed relating time to each variable for each subject. Mean data was obtained from interpolation of these graphs. Statistical significance was taken as P values <0.05.
Results
The clinical data of the subjects participating in this study are provided in Table 1. There were a variety of causes of traumatic insult resulting in cervical SCI with most subjects having suffered a recent injury within the past 5 years. Spontaneous inspiratory capacity ranged between 29% and 92% of predicted values. Spontaneous maximum expiratory pressures and peak expiratory airflows were significantly reduced to between mean 7% and 25%, and 11% and 60% predicted, respectively.
Based upon home stimulation logs, each study participant used SCS on a regular, daily basis. During the first, ten and twenty-week period of usage, the mean number of daily stimulations were 25.6 ± 1.3 (range 20–32); 28.1 ± 2.1 (range 20–38) and 24.4 ± 1.1 (range 20–32) respectively.
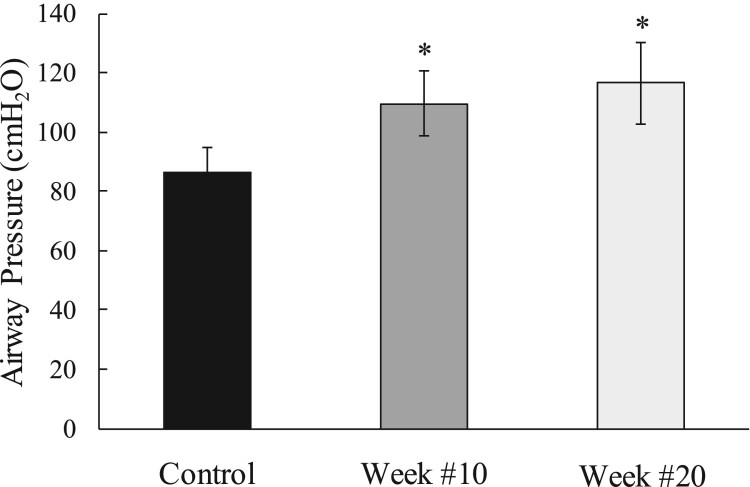
Consistent with our prior studies and the effects of reversal of muscle atrophy, regular use of SCS resulted in an increase in positive expiratory pressure generation from 87 ± 8 cmH2O during the initial stimulation visit to 110 ± 11 and 117 ± 14 cmH2O at weeks #10 and 20, respectively (P < 0.05 for each comparison) (Fig. 1).
Figure 1.
Mean changes in airway pressure generation during use of SCS (40 V, 50 Hz, 0.2 ms) at TLC with subject maximal expiratory effort during baseline and after 10 and 20 weeks of daily of use of SCS. Regular usage of SCS resulted in an increase in positive expiratory pressure generation overtime. *P < 0.05 compared to baseline values.
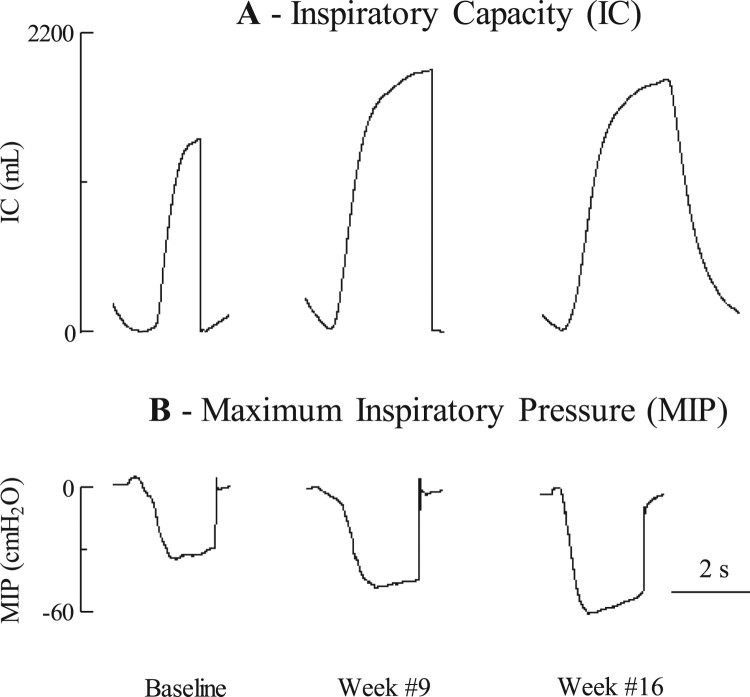
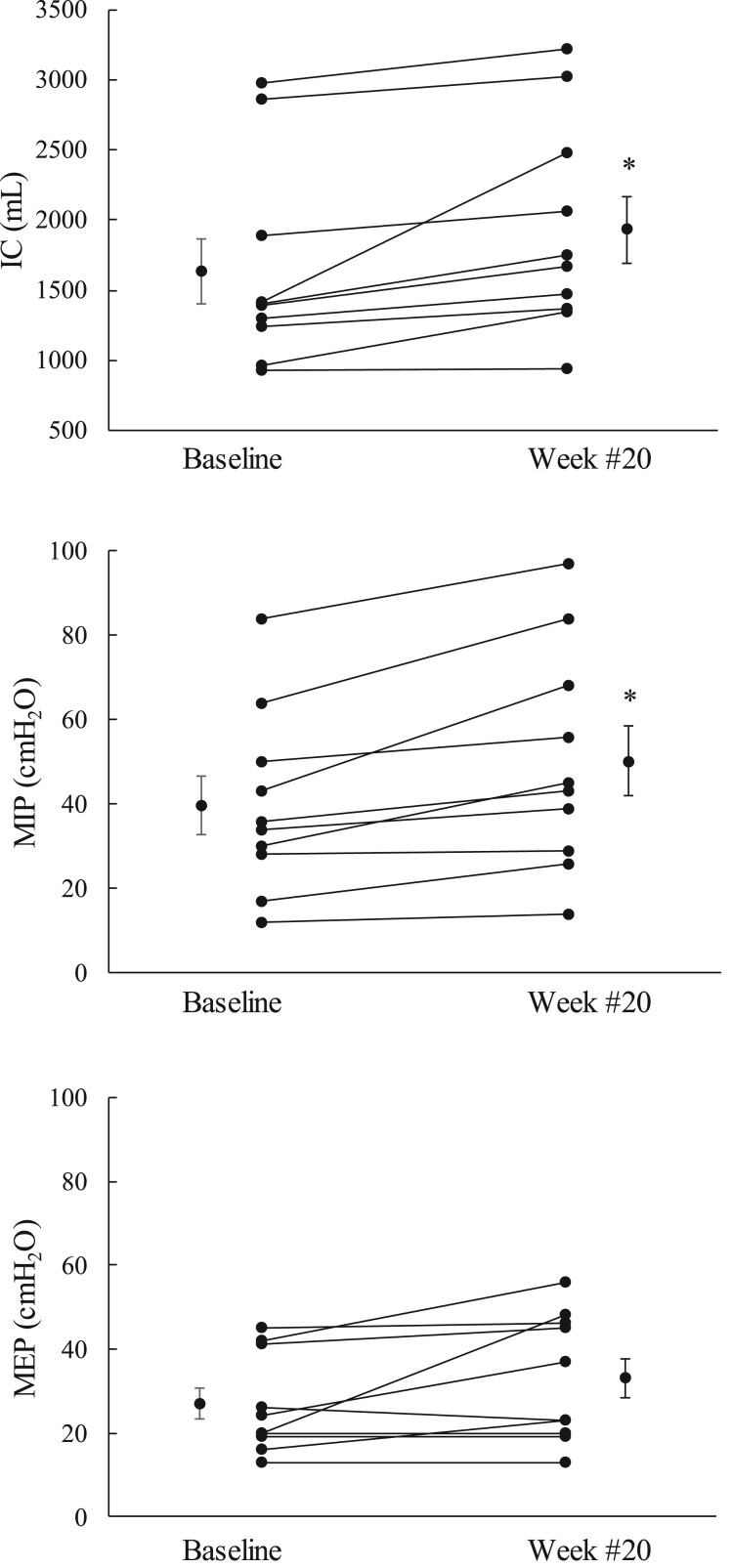
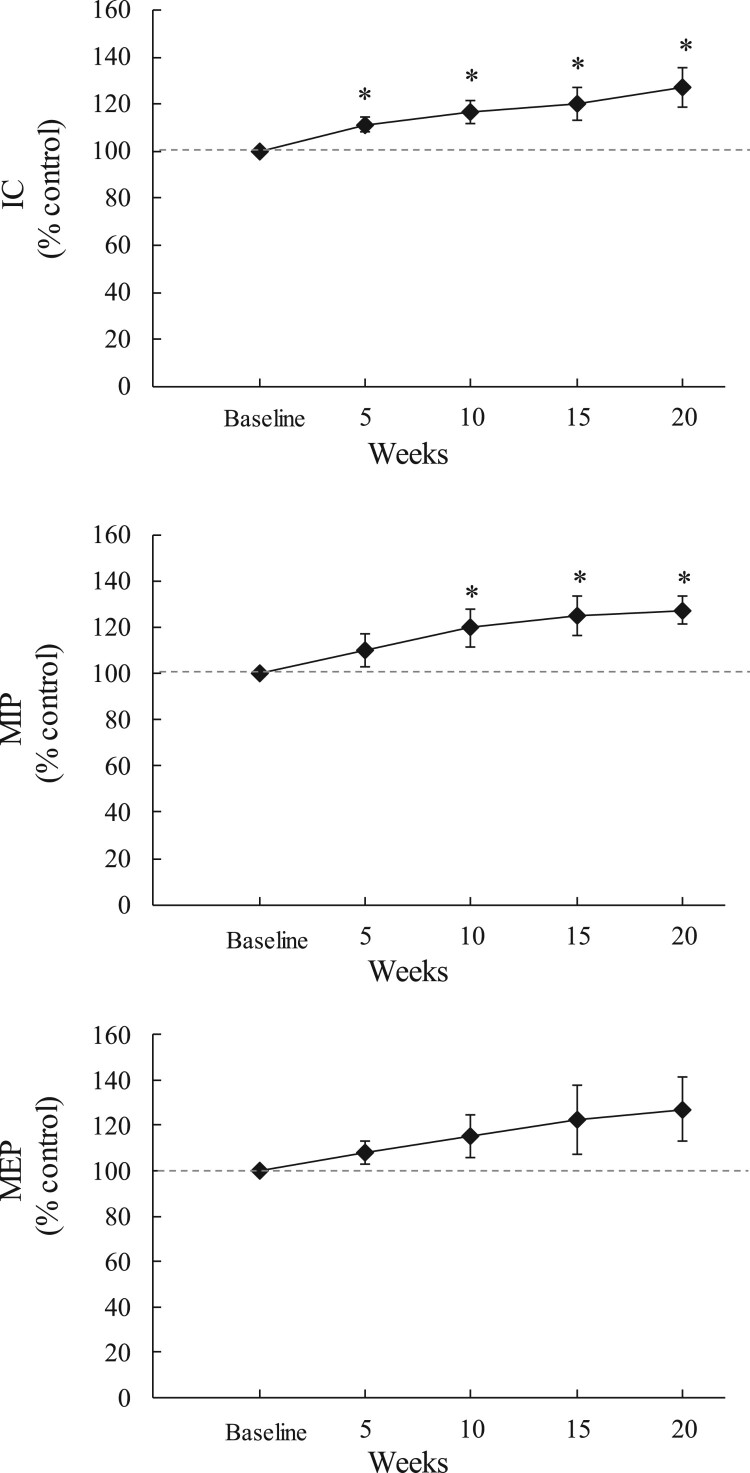
The individual data of the effects of chronic use of SCS on IC and MIP is shown for one representative subject in Fig. 2. Spontaneous IC and MIP were 1411 ml and 30 cmH2O at the time of study entrance and, 1977 ml and 57 cmH2O after 16 weeks of daily use of SCS. The changes in IC, MIP and spontaneous MEP after 20 weeks of daily use of SCS are shown graphically for each subject in Fig. 3. IC increased by 100 ml or more in 9 of the 10 subjects. Mean IC increased from 1636 ± 229 to 1932 ± 239 ml (P < 0.05). MIP increased by 5 cmH2O or more in 8 of the 10 subjects. Mean MIP increased from 40 ± 7 to 50 ± 8 cmH2O (P < 0.05). Spontaneous MEP increased by 5 cm H2O or more in 4 of the 10 subjects. Mean spontaneous MEP increased from 27 ± 4 to 33 ± 5 cmH2O (NS). Mean changes in IC, MIP and spontaneous MEP (expressed as a percentage of baseline values) over the course of the study are shown in Fig. 4. Each parameter increased gradually from over the course of the study. By week #20, mean IC, MIP and spontaneous MEP had increased by 127 ± 8% (P < 0.05), 127 ± 6%, (P < 0.05), and 127 ± 14% of baseline values (NS), respectively.
Figure 2.
Raw data of changes in inspiratory capacity (A) and maximum inspiratory pressure (B) during baseline and at the 9- and 16-week time points in one subject.
Figure 3.
Mean changes in IC, MIP and spontaneous MEP after 20 weeks of daily use of SCS. *P < 0.05 compared to baseline. See text for further explanation.
Figure 4.
Mean changes in inspiratory capacity (IC; top panel), maximum inspiratory pressure (MIP; middle panel) and maximum expiratory pressure (MEP; lower panel) expressed as a percentage of baseline values at the 5-, 10-, 15- and 20-week time points. *P < 0.05.
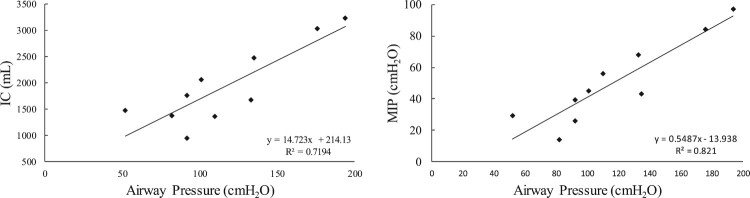
At week #20, the magnitude of airway pressure generation during SCS with patient effort at TLC was linearly related to IC and MIP with correlation coefficients of 0.72 (P < 0.05) and 0.82 (P < 0.05), respectively (Fig. 5).
Figure 5.
Relationship between airway pressure generation during use of SCS at TLC with subject effort and, inspiratory capacity (IC; left panel panel) and maximum inspiratory pressure (MIP; right panel) at week #20. There was a highly significant linear correlation between these parameters (P < 0.05, for each).
Discussion
This investigation demonstrates that routine use of SCS over the course of 20 weeks results in significant improvements in lung function i.e. inspiratory capacity and maximum inspiratory pressure generating capacity. Interestingly, there was no significant change in spontaneous expiratory muscle generating capacity, i.e. the muscles to which SCS was directly applied.
Potential mechanisms
It is well known that the application of volitional physical exercise and/or electrical stimulation to skeletal muscle results in significant improvements in strength and endurance.9–15 This has been demonstrated following various training paradigms for both respiratory and non-respiratory muscles. In fact, the expiratory muscles which were electrically stimulated in the present study demonstrated marked improvements in their capacity to generate large positive airway pressures following SCS consistent with improved expiratory muscle strength. However, due their spinal cord lesion, these muscles were not under volitional control and therefore, it is not entirely surprising that spontaneous maximal expiratory pressure generation did not improve significantly.
In a previous animal study,16 we demonstrated that dorsal lower thoracic epidural SCS results in activation of spinal cord pathways with connections to the phrenic motoneuron pools. Consequently, SCS not only results in activation of the expiratory muscles, but also co-activation of the diaphragm. The magnitude of diaphragm activation was significant in animals in which the spinal cord was intact but substantially greater following cervical spinal cord section, which may correlate with the subjects in the present study. It is possible therefore, that the improvement in MIP and IC observed in the present study resulted from co-incident diaphragm activation and associated training effects over the course of the study.
Atelectasis, obvious by routine radiographic testing, or micro atelectasis which may not be observable without CT scanning is a very common occurrence in patients with SCI.17,18 Symptoms of atelectasis include difficulty breathing, rapid shallow breathing and cough and may be associated with hypoxemia. A common cause of atelectasis in this person population includes retained secretions. This is not surprising given the fact that SCI patients lack an adequate cough mechanism.18 We speculate that routine daily use of SCS which restores a normal cough based upon the magnitude of airway pressures and peak airflow rates generated with use of this system, reduces or eliminates retained secretions and thereby reduces or eliminates atelectasis. Elimination of atelectasis would be expected to increase the volume of lung tissue available for gas exchange. This factor may have also played a role in the observed increase in IC. Unfortunately, these patients have not undergone routine radiographic studies or CT scanning before and after use of SCS to verify any changes in the degree of atelectasis.
The increase in IC and MIP may also be related to increases in diaphragm length which may have occurred over the course of the study. First, it is well known that individuals with cervical SCI experience less dyspnea in the supine compared to the sitting posture.19–23 This is related to the increase in diaphragm length consequent to the cephalad force of the abdominal wall in the supine posture. Since the force of inspiratory muscle contraction is directly related to resting inspiratory muscle length, any increase in diaphragm length would result in an increase in force development and less dyspnea. In the sitting posture, gravitational effects on the flaccid abdominal wall results in diaphragm shortening. Since SCS resulted in a marked increase in expiratory pressure generating capacity, it is likely that resting abdominal muscle tone improved substantially which would act to reduce the shortening effect on the diaphragm in the sitting posture and thereby result in increases in MIP.
Finally, although the increases in spontaneous MEP were not statistically significant, there was some observed increases in this parameter. This improvement would be expected to also improve expiratory reserve volume resulting in lower residual volume and consequently greater diaphragm length. Consequently, MIP may have improved by this mechanism, as well.
Study limitations
Pulmonary function testing including IC and maximal respiratory pressures are dependent upon subject effort. For this reason, each measurement was repeated three times to ensure accuracy. Improved effort or learning effect over the course of the study however must be considered a possibility to explain the observed improvements in pulmonary function. However, if this were the case, one would have expected increases in all the measured parameters. The fact that there were no significant changes in MEP however would argue against this possibility. It appears likely therefore that the improvements in IC and MIP are valid.
While the changes in IC and respiratory pressures improved in most subjects, it did not improve in every participant. The reason for this observation is not clear. Importantly, there were no instances in which there was a decline in these parameters. There was no apparent relationship between amount of use of the device and changes in pulmonary function.
Clinical implications
The lack of an effective cough was long been known to increase the risk of respiratory tract infections, cause difficulty in raising secretions and increase the risk of atelectasis. We previously demonstrated that restoration of an effective cough has demonstrable beneficial clinical benefits including much greater ease in raising secretions, reduction in the incidence of respiratory tract infections, less dependence on caregiver support and improved quality of life.1–6 SCS may also result in improvements in bowel function.24 The results of the present study which demonstrates improvement in pulmonary function suggests that the level of dyspnea and degree of pulmonary reserve may also be improved via use of SCS. The previously demonstrated improvements in quality of life may also be secondary, in part, to the observed improvements in pulmonary function.1–6
Acknowledgements
The valuable assistance of a statistician, Charles Thomas, BS is appreciated.
Disclaimer statements
Contributors None.
Funding This work was supported by the National Institute of Neurological Disorders and Stroke [grant number U01 NS083696].
Conflicts of interest Dr. DiMarco holds two United States Patents for technology related to the content of this paper: Method and Apparatus for Electrical Activation of the Expiratory Muscles to Restore Cough (5,999,855); Bipolar Spinal Cord Stimulation to Activate the Expiratory Muscles to Restore Cough (8,751,004).
References
- 1.DiMarco AF, Geertman RT, Tabbaa K, Polito RR, Kowalski KE.. Minimally invasive method to activate the expiratory muscles to restore cough. J Spinal Cord Med. 2017;41:562–6. doi: 10.1080/10790268.2017.1357916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiMarco AF, Geertman RT, Tabbaa K, Kowalski KE.. Complete restoration of respiratory muscle function in three subjects with spinal cord injury. Pilot interventional clinical trial. Am J Phys Med Rehabil. 2019;98:43–50. doi: 10.1097/PHM.0000000000001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR.. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part I: methodology and effectiveness of expiratory muscle activation. Arch Phys Med Rehabil. 2009;90:717–25. doi: 10.1016/j.apmr.2008.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR, Frost FS, Creasey GH, Nemunaitis GA.. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part II: clinical outcomes. Arch Phys Med Rehabil. 2009;90:726–32. doi: 10.1016/j.apmr.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR.. Spinal cord stimulation: a new method to produce cough in patients with spinal cord injury. Am J Respir Crit Care Med. 2006;173:1386–9. doi: 10.1164/rccm.200601-097CR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMarco AF, Kowalski KE, Hromyak DR, Geertman RT.. Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury. J Spinal Cord Med. 2014;37:380–8. doi: 10.1179/2045772313Y.0000000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black LF, Hyatt RE.. Maximal respiratory pressures, normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. [DOI] [PubMed] [Google Scholar]
- 8.Crapo RO, Morris AH, Gardner RM.. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. [DOI] [PubMed] [Google Scholar]
- 9.Cruickshank TM, Reyes AR, Ziman MR.. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or Parkinson disease. Medicine (Baltimore). 2015;94(4):1–15. doi: 10.1097/MD.0000000000000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi SJ, Kim JS.. The effects of respiratory muscle strengthening exercise using a sling on the amount of respiration. J Phys Ther Sci. 2015;27(7):2121–4. doi: 10.1589/jpts.27.2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illi SK, Held U, Frank I, Spengler CM.. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med. 2012;42(8):707–24. doi: 10.1007/BF03262290 [DOI] [PubMed] [Google Scholar]
- 12.Menezes KK, Nascimento LR, Avelino PR, Alvarenga MTM, Teixeira-Salmela LF.. Efficacy of interventions to improve respiratory function after stroke. Respir Care. 2018;63(7):920–33. doi: 10.4187/respcare.06000 [DOI] [PubMed] [Google Scholar]
- 13.Leith DE, Bradley M.. Ventilatory muscle strength and endurance training. J Appl Physiol. 1976;41(4):508–16. doi: 10.1152/jappl.1976.41.4.508 [DOI] [PubMed] [Google Scholar]
- 14.Hoffman M, Augusto VM, Eduardo DS, Silveira BMF, Lemos MD, Parreira VF.. Inspiratory muscle training reduces dyspnea during activities of daily living and improves inspiratory muscle function and quality of life in patients with advanced lung disease. Physiother Theory Pract. 2019;20:1–11. doi: 10.1080/09593985.2019.1656314 [DOI] [PubMed] [Google Scholar]
- 15.Gong H, Jiang Q, Shen D, Gao J.. Neuromuscular electrical stimulation improves exercise capacity in adult patients with chronic lung disease: a meta-analysis of English studies. J Thorac Dis. 2018;10(12):6722–32. doi: 10.21037/jtd.2018.11.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMarco AF, Romaniuk JR, Kowalski KE.. Effects of diaphragm activation on airway pressure generation during lower thoracic spinal cord stimulation. Respir Physiol Neurobiol. 2007;159:102–7. doi: 10.1016/j.resp.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Vázquez R G, Sedes P R, Fariña M M, Marqués A M, Velasco ME F.. Respiratory management in the patient with spinal cord injury. Biomed Res Int. 2013;2013:168757. doi: 10.1155/2013/168757. Epub 2013 Sep 9. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown R, DiMarco AF, Hoit JD, Garshick E.. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51(8):853–68. [PMC free article] [PubMed] [Google Scholar]
- 19.Miccinilli S, Morrone M, Bastianini F, Molinari M, Scivoletto G, Silvestri S, Ranieri F, Sterzi S.. Optoelectronic plethysmography to evaluate the effect of posture on breathing kinematics in spinal cord injury: a cross sectional study. Eur J Phys Rehabil Med. 2016;52(1):36–47. [PubMed] [Google Scholar]
- 20.Kim SH, Shin YB, Yoon JA, Lee JS, Lee BJ, Park HE.. Revisiting respiratory muscle strength and pulmonary function in spinal cord injury: the effect of body positions. Neuro Endocrinol Lett. 2018;39(3):189–95. [PubMed] [Google Scholar]
- 21.Chen CF, Lien IN, Wu MC.. Respiratory function in patients with spinal cord injuries: effects of posture. Paraplegia. 1990;28:81–6. [DOI] [PubMed] [Google Scholar]
- 22.Baydur A, Adkins RH, Milic-Emili J.. Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol. 2001;90(2):405–11. doi: 10.1152/jappl.2001.90.2.405 [DOI] [PubMed] [Google Scholar]
- 23.de Paleville DG T, Sayenko DG, Aslan SC, Folz RJ, McKay WB, Ovechkin AV.. Respiratory motor function in seated and supine positions in individuals with chronic spinal cord injury. Respir Physiol Neurobiol. 2014;203:9–14. doi: 10.1016/j.resp.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiMarco AF, Geertman RT, Tabbaa K, Nemunaitis GA, Kowalski KE.. Case report: effects of lower thoracic spinal cord stimulation on bowel management in a person with spinal cord injury. J Neuro Neurobiol. 2019;5(1). doi: 10.16966/2379-7150.156. [DOI] [PMC free article] [PubMed] [Google Scholar]