Abstract
Background
Post-traumatic stress (PTS), or the psycho-physiological response to a traumatic or life-threatening event, is implicated in medical patient outcomes. Emerging evidence suggests a complex relationship between PTS, the brain–gut axis, the gut microbiome, and immune function. Inflammatory bowel disease (IBD) may be susceptible to PTS and its subsequent impacts. To date, no study has evaluated PTS in IBD in the United States.
Methods
Adult patients with IBD were recruited from an outpatient gastroenterology practice, via social media, and via a research recruitment website. Patients with irritable bowel syndrome (IBS) were recruited as a comparison group. Participants completed demographic and disease information, surgical and hospitalization history, and the PTSD Checklist–Civilian Version (PCL-C). Statistical analyses evaluated rates of PTS in IBD and IBS, including differences between groups for PTS severity. Regression analyses determined potential predictors of PTS.
Results
One hundred eighty-eight participants (131 IBD, 57 IBS) completed the study. Thirty-two percent of IBD and 26% of IBS patients met the criteria for significant PTS symptoms based on PCL-C cutoffs. Inflammatory bowel disease patients are more likely to attribute PTS to their disease than IBS patients. Crohn’s disease (CD) patients appear to be the most likely to experience PTS, including those being hospitalized or undergoing ileostomy surgery. Symptom severity is the greatest predictor of PTS for ulcerative colitis and IBS.
Conclusions
Although PTS is relevant in both IBS and IBD, IBD patients are seemingly more susceptible to PTS due their disease experiences, especially CD patients. The nature of PTS symptoms may contribute to IBD disease processes, most notably through sleep disturbance and ANS arousal. Clinicians should assess for PTS in IBD patients as standard of care, especially after a hospitalization or surgery.
Keywords: post-traumatic stress, PTSD, inflammatory bowel disease
Research into post-traumatic stress disorder (PTSD) in IBD is limited to one Swiss study. We evaluated PTSD in a US cohort and found one-third endorse clinically significant PTSD symptoms, with Crohn’s disease patients undergoing ileostomy or being hospitalized most at-risk.
INTRODUCTION
Patients with inflammatory bowel diseases (IBDs; Crohn’s disease [CD], ulcerative colitis [UC], indeterminate colitis [IC]) consistently exhibit a reciprocal relationship between their disease physiology and psychological state. Stressors contributing to IBD disease activity and symptom report include chronic life stress,1, 2 psychological disorders,3 and the disease experience itself. In turn, IBD degrades health-related quality of life in many patients,4, 5 whereas psychological distress arises from symptom burden, self-management stressors, social stigma, and treatment side effects.6, 7 Collectively, these factors are associated with poorer outcomes. The negative impact of stress on disease course, including the emerging understanding of the brain–gut–microbiome axis,8, 9 emphasizes that early attention to psychological distress is a vital part of IBD patient care.10
Repeated studies and systematic reviews find that pediatric and adult IBD patients are more likely to experience anxiety and depression compared with healthy controls.3, 11–13 However, to date, the potential impact of post-traumatic stress disorder (PTSD) has not been evaluated in patients with IBD in the United States. Post-traumatic stress disorder is a chronic reaction to a traumatic or life-threatening event; symptoms include recurrent frightening thoughts, difficulty concentrating, hypervigilance, dissociative feelings, and trouble sleeping.14 Before developing PTSD, affected persons experience an acute stress reaction with similar symptoms to PTSD. If symptoms do not resolve within 1 month, a diagnosis of PTSD is made.15 Estimated rates of PTSD in the US population are around 6% to 8%,16 whereas approximately 1 million US adults are diagnosed with illness-induced PTSD annually.17 In IBD, 1 previous study of a Swiss cohort of 468 CD patients found that 19% scored above the diagnostic cutoff for PTSD, whereas only 10% reported no evidence of PTSD symptoms.18 Importantly, they found that patients scoring above the cutoff had 4 times higher odds of disease exacerbation.
Assessment of PTSD in other medical populations utilizes the related construct, post-traumatic stress (PTS), mainly because a PTSD diagnosis requires a clinical interview not always feasible in research.19, 20 Post-traumatic stress, or partial PTSD, is a subset of PTSD symptoms, is not necessarily as severe as PTSD, may not evolve into PTSD in all people, and is more easily assessed.14, 21 Although PTS is not synonymous with PTSD, it is associated with similar decreases in functioning, impairments in quality of life, increased risk for psychopathology including developing PTSD, occupational impairment, and increased health care utilization.18, 22 The estimated rate of PTS in the general population is approximately 6%.16 Risk factors for PTS and PTSD include age, socioeconomic status, symptom severity, treatment approaches (surgery), intensive care unit hospitalization, availability of social support, and premorbid psychological distress.14, 21–23 Given the negative impact of PTS on health outcomes, more in depth investigation of PTS among IBD patients is warranted. Further, as PTSD can lead to alterations in brain–gut interactions,24 untreated PTS/PTSD could exacerbate the IBD disease course.
The purpose of this study is to evaluate PTS in patients with IBD. We aim to (1) characterize rates of PTS among patients with IBD based on standardized cutoff scores for diagnostic levels of PTS symptoms, (2) determine differences between IBD subtypes (CD and UC), as well as differences between IBD and IBS (gastrointestinal disease control) in the presentation of PTS, and (3) quantify correlates and determine possible predictive factors of PTS from common clinical and demographic characteristics.
We hypothesize that PTS will be present in a subset of IBD patients, CD patients will demonstrate greater PTS than UC patients, and differences will exist between IBD and IBS patients. Further, we hypothesize that PTS in IBD will be associated with clinical variables including symptom severity, surgical history, and hospitalization history.
METHODS
Adult patients (aged 18–80 years) diagnosed with IBD or IBS for a minimum of 6 months were recruited from a university-based outpatient clinic, with diagnoses confirmed via electronic medical record review based on established endoscopic and histological criteria for IBD25 and Rome IV criteria for IBS.26 Additional self-reported IBD and IBS patients were recruited via the online research website ResearchMatch.org and social media (Facebook, Twitter). Individuals interested in participating provided informed consent and completed a series of screening questions to assess for diagnosis and exclusion criteria. Individuals were excluded if they did not have a diagnosis of IBD or IBS, were not between 18 and 80 years of age, or did not have their respective diagnosis for at least 6 months. In addition, individuals with a diagnosis of PTSD before developing IBD or IBS were excluded from the study. All data were anonymously captured via the online system REDCap. The study was approved by the Northwestern University Institutional Review Board (IRB #00205234). Ethical considerations included providing access to mental health support (NU Mental Health hotline) for patients who may have experienced significant psychological distress as it related to the topics covered in the study.
Demographic and Clinical Information
Eligible participants provided demographic and clinical information via structured surveys. The demographic information included age, sex, race, ethnicity, marital status, employment status, education level, and household income. Clinical information included diagnosis type (CD, UC, IC), age at symptom onset (in years), and age at diagnosis (in years). In addition, IBD patients completed the Patient Harvey Bradshaw Index27 (HBI; CD) or Patient Simple Clinical Colitis Activity Index28 (SCCAI; UC) to assess symptom severity. For direct comparison between IBD and IBS, all participants also completed the Short Form IBD Questionnaire (SIBDQ), with symptom severity measured by the total score of the physical symptom (bowel and systemic) subscales.29
Outpatient, Hospitalization, and Surgical History
The number of disease-related outpatient visits per year, surgical history including number of prior surgeries, hospitalization history including number of prior hospitalizations, type of room (intensive care unit [ICU], general, both), and duration of stay (in days) were obtained. Participants rated the overall experience during their most difficult or stressful surgery and hospital stay on a scale of 0 (horrible) to 100 (excellent) and either endorsed or declined (yes/no) the following statement: “During your surgery/hospital stay, did you ever feel intense fear or anxiety about your treatment or risk to your life?”
Post-traumatic Stress
The PTSD Checklist–Civilian Version (PCL-C)30 assessed PTS symptoms. The PCL-C is a standardized 17-item self-report questionnaire designed to correspond with the diagnostic symptoms of PTSD defined in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).31 Respondents rate how much they have been bothered by PTSD symptoms over the past month on a 5-point Likert scale ranging from 1 (not at all) to 5 (extremely). Total severity scores are the sum of all items, with higher scores indicating more severity. Individual item responses can be coded as symptomatic or nonsymptomatic depending on the response category endorsed (3–5 = symptomatic, 1–2 = nonsymptomatic). The PCL-C has good psychometric properties32 and diagnostic efficacy.33 PTSD diagnosis cannot be confirmed by the PCL-C alone and requires a clinical interview with a trained professional. Therefore, the purpose of the PCL-C was to assess for symptoms of PTS that may or may not develop into PTSD depending on the severity of the symptoms. In addition to PTS symptom severity, we were interested in the number of participants diagnosed by a mental health professional with PTSD after being diagnosed with their medical condition. Thus, each participant provided information on self-reported PTSD diagnosis, reason for PTSD diagnosis, and PTSD treatment history subsequent to their IBD or IBS diagnosis.
Statistical Analyses
Data from the REDCap system were exported into SPSS v25 for Macintosh (Chicago, IL, USA) for analyses. Responses were reviewed for inclusion criteria, with those not meeting these requirements excluded. Initial assessments for normal distribution determined the need for nonparametric tests. Descriptive statistics (mean [SD], percentage [frequency]) were computed for demographic and clinical variables for each study diagnosis. Z scores for 2 population proportions were determined any between-diagnosis differences (eg, IBD vs IBS, UC vs CD) across categorical variables. Participants recruited online vs in the clinic were compared using independent-samples t tests to determine any significant differences by recruitment source. Due to the exploratory nature of this study, statistical significance was set to a P value <0.05 rather than adjusting for multiple comparisons via the Bonferroni method.
To address aim 1, the total score for the PCL-C was calculated and normally distributed, with a cut-point score of 50 used to identify patients exhibiting significant PTS symptoms and dichotomized as “yes/no” based on prior PTS research34 and PCL-C guidelines. For each item on the PCL-C, the percentage (frequency) of respondents endorsing 3 (moderately) or higher (ie, symptomatic) was calculated for each diagnosis. Participants self-reporting a clinical PTSD diagnosis, the reason for diagnosis, and PTSD treatment history are reported as the percentage (frequency).
For aim 2, independent-samples t tests determined between-group differences in PCL-C for CD and UC, and for IBD (pooled) and IBS.
For aim 3, Pearson’s correlations and Spearman’s rho assessed the relationship between the PCL-C with continuous variables. Variables identified as having significant correlations with the PCL-C were entered as predictor variables of PTS in 3 separate stepwise linear regression models (1 for CD, 1 for UC, 1 for IBS). The adjusted R2 for each significant predictor variable was converted to percent variance of PTS explained and reported (R2adj × 100).
RESULTS
Study Sample
Two hundred seventy participants consented to the study. Of these, 50 (18.5%) were excluded based on study criteria: 3 indicated they did not have IBS or IBD, 3 were not between 18 and 80 years of age, 12 did not have their diagnosis for at least 6 months, and 32 had PTSD before diagnosis of illness. Thirty-one (12%) were removed due to incomplete data, leaving a final study sample of 189 (132 IBD: 89 CD, 43 UC; 57 IBS); demographic characteristics are outlined in Table 1 and clinical characteristics in Table 2. Nine patients with IC were grouped with ulcerative colitis for the purposes of statistical analyses. The sample was mostly female, white non-Hispanic, and college educated. The SIBDQ had a large, direct correlation with the harvey bradshaw index (HBI)/simple colitis clinical activity index (SCCAI) scores in IBD (r = 0.74; P < 0.001), allowing for use of the SIBDQ to assess symptomatology for comparisons with IBS. No significant differences existed for symptom severity between IBS and IBD as measured via the SIBDQ Bowel and Systemic scales.
TABLE 1.
Demographic Characteristics of Study Sample by Diagnosis
| CD | UC | IBS | |
|---|---|---|---|
| n = 89 | n = 43 | n = 57 | |
| Age, mean ± SD, y | 37.5 ± 11.4 | 37.5 ± 13.1 | 40.1 ± 13.3 |
| Sex, % (No.) | |||
| Male | 27.6 (24) | 25.6 (11) | 26.3 (15) |
| Female | 72.4 (63) | 74.4 (32) | 73.7 (42) |
| Race, % (No.) | |||
| African American | 0 | 0 | 3.5 (2) |
| Asian | 0 | 0 | 1.8 (1) |
| Latino/a | 0 | 2.4 (1) | 1.8 (1) |
| White | 97.5 (85) | 95.2 (40) | 91.2 (52) |
| Native American | 0 | 0 | 0 |
| Multiracial | 1.1 (1) | 2.4 (1) | 0 |
| Other | 1.1 (1) | 0 | 1.8 (1) |
| Ethnicity, % (No.) | |||
| Non-Hispanic | 97.7 (86) | 90.7 (39) | 96.4 (54) |
| Hispanic | 2.3 (2) | 9.3 (4) | 3.6 (2) |
| Marital status, % (No.) | |||
| Single | 33 (29) | 32.6 (14) | 26.3 (15) |
| Married | 61.4 (54) | 62.8 (27) | 57.9 (33) |
| Divorced/separated | 5.7 (5) | 4.7 (2) | 15.8 (9) |
| Education, % (No.) | |||
| Less than college | 36.4 (32) | 20.9 (9) | 21.1 (12) |
| College or higher | 63.6 (56) | 79.1 (34) | 88.9 (45) |
| Recruitment source, % (No.) | |||
| Social media | 69.3 (61) | 60.5 (26) | 12.3 (7) |
| ResearchMatch | 8.0 (7) | 14.0 (6) | 64.9 (30) |
| Clinic | 21.6 (19) | 25.6 (11) | 19.3 (11) |
| Other | 1.1 (1) | 0 | 15.8 (9) |
TABLE 2.
Clinical Characteristics of Study Sample by Diagnosis
| CD | UC | IBS | |
|---|---|---|---|
| n = 89 | n = 43 | n = 57 | |
| Age at symptom onset, mean ± SD, y | 19.7 ± 10.6 | 25.0 ± 12.0 | 21.3 ± 12.4 |
| Age at diagnosis, mean ± SD, y | 24.1 ± 11.1 | 27.3 ± 11.1 | 29.1 ± 12.0 |
| No. outpatient office visits per year, mean ± SD | 4.3 ± 3.5 | 2.95 ± 2.3 | 2.3 ± 2.8 |
| Symptom severity, mean ± SD | |||
| SIBDQ (Bowel + Systemic scales) | 20.9 ± 6.2 | 22.5 ± 5.9 | 18.4 ± 5.3 |
| HBI or SCCAI, % (No.) | 10.3 ± 6.6 | 8.7 ± 5.4 | - |
| Prior surgery for disease, % (No.) | 75.3 (67) | 27.9 (12) | 7.0 (4) |
| Type of surgery, % (No.) | |||
| Bowel resection | 51.5 (34) | 0 | - |
| Ileostomy | 24.2 (16) | 9.1 (1) | - |
| Colectomy/proctocolectomy (IPAA) | 7.6 (5) | 81.8 (9) | - |
| Other | 16.7 (11) | 9.1 (1) | - |
| No. surgeries, mean ± SD | 4.8 ± 5.7 | 3.4 ± 3.8 | 1.8 ± 1.5 |
| Most recent surgery experience, mean ± SD | 54.02 ± 29.4 | 58.3 ± 23.8 | 80.5 ± 7.9 |
| Felt intense fear/anxiety/risk to life, % (No.) | 70.1 (47) | 83.3 (10) | 50.0 (2) |
| Prior hospitalization for disease, % (No.) | 86.5 (77) | 62.8 (27) | 19.3 (11) |
| No. hospitalizations, mean ± SD | 12.11 ± 19.5 | 5.1 ± 6.4 | 2.3 ± 1.3 |
| Length of most recent hospital stay, mean ± SD, d | 16.0 ± 24.8 | 11.6 ± 9.5 | 3.6 ± 2.3 |
| Type of hospitalization, % (No.) | |||
| General room | 74.0 (57) | 77.8 (21) | 100 (11) |
| Intensive care unit | 6.5 (5) | 3.7 (1) | 0 |
| Both | 19.5 (15) | 18.5 (5) | 0 |
| Reason for hospitalization, % (No.) | |||
| Disease complication | 26.0 (20) | 8.3 (2) | 9.1 (1) |
| Medication complication | 9.1 (7) | 12.5 (3) | 9.1 (1) |
| Severe disease flare | 36.4 (28) | 66.7 (16) | 54.5 (6) |
| Severe infection | 3.9 (3) | 0 | 0 |
| Surgery | 23.4 (18) | 8.3 (2) | 9.1 (1) |
| Other | 1.3 (1) | 4.2 (1) | 18.2 (2) |
| Most recent hospital experience, mean ± SD | 49.6 ± 30.9 | 59.0 ± 24.0 | 58.8 ± 22.6 |
| Felt intense fear/anxiety/risk to life, % (No.) | 74.0 (57) | 85.2 (23) | 72.7 (8) |
“Other” surgery includes fistula repair and strictureplasty. Hospital and surgery experiences are rated on a scale of 0 (horrible) to 100 (excellent).
Differences in PCL-C score existed for demographic variables by diagnosis. Female IBD patients scored higher on the PCL-C than males (mean, 44.2 vs 36.5; P = 0.002), and IBD patients recruited via social media scored higher on the PCL-C than those recruited in the clinic (46.5 vs 37.0; P = 0.002) or on ResearchMatch (46.5 vs 32.5; P = 0.001); IBS patients did not differ in PTS by sex or recruitment source. Although differences between ethnicities did not exist for the PCL-C when analyzed separately, pooling IBD and IBS diagnoses shows that Hispanic patients report higher scores on the PCL-C (55.4 vs 41.4; P = 0.007). These findings should be interpreted with caution due to the small number of Hispanic participants in the sample. For UC only, younger patients (r = –0.36) and those diagnosed at a younger age (r = –0.30; both P < 0.05) reported more PTS. No differences existed for any other demographic groupings. As such, sex and recruitment source were entered as predictor variables in the stepwise linear regression for all IBD, and age and age at symptom onset were entered for UC.
Characterizing PTS in IBD
In our sample, 32% of IBD patients (38% CD, 19% UC) and 26% of IBS patients met the criteria for significant PTS symptoms (cut-point of 50 or greater on the PCL-C) (Table 3), with a mean score of 42.85 ± 14.4 for IBD and 41.33 ± 14.2 for IBS. Although CD patients trended toward more PTS symptoms than UC, this difference did not reach statistical significance (CD, 44.38 ± 14.9; vs UC, 39.77 ± 13.0; P = 0.073). However, the percentage of CD patients meeting or exceeding the diagnostic cutoff was significantly higher than UC or IBS patients. Although 38% of CD patients met the diagnostic cutoff, only 24% of these patients in our sample reported having been diagnosed with PTSD by a mental health professional. Similar discrepancies existed for UC and IBS. Most patients reporting a clinical diagnosis of PTSD were receiving treatment, either via psychotherapy, medication, or a combination.
TABLE 3.
Post-traumatic Stress Disorder by Diagnosis
| CD | UC | IBS | |
|---|---|---|---|
| n = 89 | n = 43 | n = 57 | |
| PCL-C score, mean ± SD | 44.4 ± 14.9 | 39.8 ± 13.0 | 41.3 ± 14.2 |
| Range | 18–85 | 17–69 | 19–68 |
| Meet diagnostic cutoff (PCL-C > 50), % (No.) | 38.2 (34) | 18.6 (8) | 26.3 (15) |
| Self-reported PTSD diagnosis, % (No.) | 23.6 (21) | 7.0 (3) | 14.0 (8) |
| Due to illness | 19.1 (17) | 7.0 (3) | 5.3 (3) |
| Not due to illness | 4.5 (4) | 0 | 8.8 (5) |
| Self-reported PTSD receiving treatment, % (No.) | 81 (17) | 100 (3) | 87.5 (7) |
| Psychotherapy | 35.3 (6) | 33.3 (1) | 28.6 (2) |
| Medication | 11.8 (2) | 0 | 14.3 (1) |
| Both | 52.9 (9) | 66.7 (2) | 57.1 (4) |
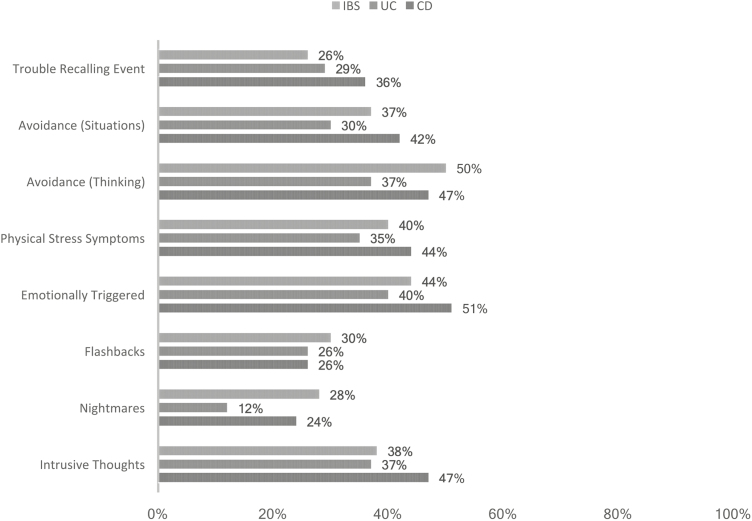
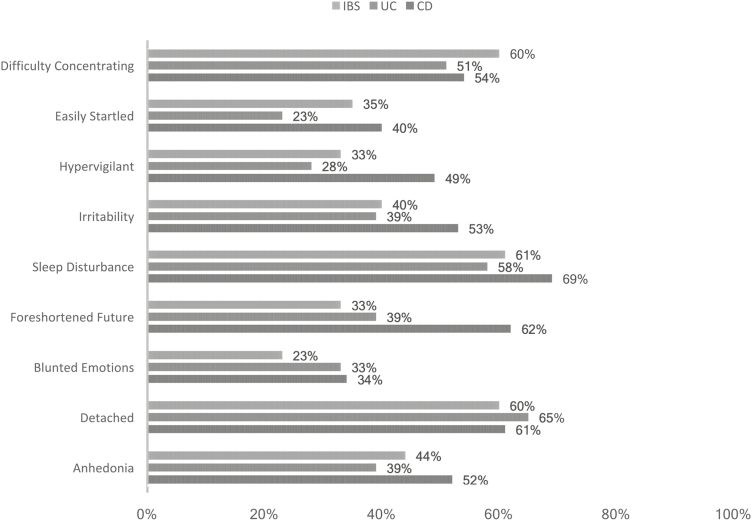
When evaluating separate PTS symptoms, CD patients reported being “symptomatic” across most items (Figs. 1 and 2). CD patients were twice as likely to endorse hypervigilance, nightmares, being easily startled, and a sense of foreshortened future than UC patients. The most common “re-experiencing” symptom of PTS reported was intrusive thoughts. CD patients reported similar levels of nightmares as IBS patients, and all groups showed similar rates of flashbacks (~30%). For autonomic nervous system arousal (eg, racing heartbeat, shallow breathing), CD patients reported the greatest levels of ANS symptoms.
FIGURE 1.
Percentage of patients endorsing “moderate” or greater re-experiencing and avoidance PTS symptoms.
FIGURE 2.
Percentage of patients endorsing “moderate” or greater ANS arousal and emotional PTS symptoms.
Disease Experiences and PTS
For all patients, symptom severity moderately correlated with PTS severity (Table 4). Significantly more CD patients reported prior surgery than UC or IBS patients, with the most common surgery being bowel resection. Crohn’s patients undergoing ileostomy surgery reported greater PTS symptoms than those undergoing resection (50.5 ± 14.6 vs 42.0 ± 13.6; P = 0.049) or fistula repair (31.2 ± 17.7; P = 0.016), but a similar amount of PTS symptoms as those undergoing ileal-pouch anal anastomosis (IPAA; 49.8 ± 11.6; P = 0.925). Both the number of surgeries and surgery experience significantly correlated with PTS severity for CD patients only.
TABLE 4.
Relationships of Demographic and Clinical Variables With PCL-C Score by Diagnosis
| CD | UC | IBS | |
|---|---|---|---|
| n = 89 | n = 43 | n = 57 | |
| Agea | –0.02 | –0.36* | 0.04 |
| Age at symptom onsetb | –0.09 | –0.30* | 0.13 |
| Age at diagnosisb | 0.01 | –0.22 | 0.10 |
| SIBDQ Symptom Severitya | 0.52*** | 0.47*** | 0.55*** |
| HBI/SCCAI Symptom Severity | 0.41*** | 0.28* | - |
| Outpatient visits per yearb | 0.25* | 0.15 | 0.26* |
| No. surgeriesb | 0.24* | –0.01 | - |
| Most recent surgery experiencea | –0.55*** | –0.09 | - |
| No. hospitalizationsb | 0.32** | 0.08 | 0.06 |
| Hospital stayb | 0.21 | 0.09 | 0.51* |
| Most recent hospitalization experiencea | –0.45*** | –0.19 | –0.38 |
SIBDQ Symptom Severity: IBD and IBS were measured via the Bowel and Systemic scales.
*P < 0.05; **P < 0.01; ***P < 0.001.
aPearson’s correlation.
bSpearman’s rho.
Similar trends existed for hospitalizations, with more CD patients reporting a prior hospital stay, a greater frequency of hospitalizations, and longer stays in days. The most common reason for hospitalizations in both CD and UC was a severe disease flare. Like surgical history, a greater number of hospitalizations and poor experiences were associated with more PTS symptoms only for CD patients. Approximately 20% of IBD patients required ICU services for at least part of their stay. Inflammatory bowel disease patients requiring ICU services reported more PTS than those only using a general floor room (ICU: mean [SD], 50.85 [10.9]; vs general: mean [SD], 41.98 [15.3]; P = 0.002). Rates of hospitalization were lower for IBS (19%), with no patients requiring ICU services.
Inflammatory bowel disease patients rated their most recent surgeries and hospitalizations relatively poorly, each approximately 50 out of a possible best score of 100. Three-quarters reported feeling intense fear, anxiety, or risk to life during either surgery (IBD) or hospitalization (IBD and IBS). Inflammatory bowel disease patients with more severe disease courses (eg, more surgeries and/or more hospitalizations) reported more PTS (Table 4). The number of IBS patients with a surgical history was too small to draw meaningful conclusions, and the number of hospitalizations was not associated with PTS.
Predictors of PTS Severity
Potential predictor variables identified in the previous analyses were entered into separate regression models for CD (sex, recruitment, SIBDQ symptom severity, number of surgeries, surgery experience, history of ileostomy surgery, hospitalizations, hospital experience, ICU), UC (sex, recruitment, age, age at symptom onset, SIBDQ symptom severity, ICU), and IBS (SIBDQ symptom severity, hospital stay in days) using a stepwise method. For CD patients, hospitalization experience was the largest predictor of PTS, accounting for 36% of the variance (P < 0.001), followed by history of ileostomy surgery at 9% of the variance (P = 0.01) and SIBDQ symptom severity predicting 7% (P = 0.02). All other variables were nonsignificant. For UC and IBS, only SIBDQ symptom severity predicted PTS (UC: 20%, P = 0.002; IBS: 39%, P = 0.024).
DISCUSSION
The present study is the first to examine PTS symptoms in a sample of IBD patients in the United States. Approximately one-third of IBD patients reported significant PTS symptoms, and one-quarter reported a clinical PTSD diagnosis since disease onset, with most stating that PTSD was due to their IBD experiences. Although a similar percentage of IBS patients reported being diagnosed with PTSD since their illness onset, considerably less attributed PTSD to their condition. Our findings are similar to the Swiss CD cohort,18 suggesting that 1 in 5 IBD patients may be affected by PTSD and that a greater proportion experience subclinical PTSD (ie, PTS). Also, although many IBD patients meet the diagnostic cutoff on the PCL-C, few have been diagnosed with PTSD or are receiving treatment. These results indicate an urgent need to more adequately assess and treat PTS and PTSD in patients with IBD.
Women with IBD were more likely to have more PTS symptoms, a finding consistent with PTS sex differences within the general population.35 Rates of PTS were higher in participants of Hispanic origin after IBD and IBS diagnoses were pooled to adjust for the small sample of ethnic minorities in our study. PTSD research, to date, has not clarified whether PTSD is more common among Hispanic/Latinos.36, 37 However, research among other groups provides evidence that minorities with IBS may be at an increased risk for PTS, including an independent association of PTSD with IBS among African Americans.38 Future studies should recruit a more racially and ethnically diverse sample to clarify this finding.
Online recruitment is a strategy increasingly utilized in the medical literature with the potential benefit of reaching broader patient populations than studies limited to university-based clinics and the possible drawbacks of biases in study samples or inability to confirm a diagnosis. Additionally, differences may exist between online and medical clinic recruits.39 In this study, IBD patients recruited via social media reported more PTS than those recruited in the clinic or via ResearchMatch; this difference was not seen in IBS. Differences in PTS appear to be related specifically to social media recruits and suggest that IBD patients with PTS may seek support or information via social media. A review of Internet and electronic resources in IBD found that support groups made up the larger proportion of the top 25 IBD-related Facebook pages.40 People with PTS also use social media to receive support and fight stigma.41 Future research is needed to understand the role of social media in IBD patients with PTS.
We found no differences between mean PCL-C score for IBD and IBS, and a nonsignificant trend toward more PTS among patients with CD. However, CD patients consistently reported more severe PTS symptoms than UC patients. Although it is generally accepted that IBS patients are more likely to experience early-life adversity than the general population,42 including childhood trauma, previous literature assessing an association between PTS and IBS is scarce, and to our knowledge is limited to 2 studies with significantly different PTS rates reported (36% and 7.8%).43, 44 Our study did find that IBD patients more often report a diagnosis of PTSD due to their illness than IBS patients, likely related to surgery or hospitalization experiences, which afford greater risk to traumatic experiences. Most IBD patients reported staying in a general room during their most stressful hospitalization, whereas less than 10% stayed in the ICU, and 20% stayed in both. One in 5 critical illness survivors have “clinically important” PTS symptoms in the first 12 months post-ICU.23 However, when controlling for other significant variables, ICU utilization did not predict PTS in our IBD sample. This may be an effect of a small sample size, even though the regression was adequately powered. Future studies should explore this association and potential risk factor.
Inflammatory bowel disease patients reported intense fear during surgery and hospitalizations and generally fair to poor surgery and hospital experiences. Hospital experience was the greatest predictor of PTS for IBD, followed by history of ileostomy and symptom severity. The prior Swiss study, along with the cancer literature, supports a positive relationship between symptom severity and PTS symptoms.34, 45 Our finding of a predictive nature of hospital experience and ileostomy for PTS in CD patients appears novel; however, the cross-sectional nature of this study precludes any definitive conclusions. It does underscore that proactive intervention in the hospital setting, via integrated behavioral services, could be helpful, even preventative, for PTS in IBD. Effective hospital-based interventions include expressive writing,46, 47 increased communication from clinicians,48, 49 and counseling as soon as possible after discharge.47, 50
The nature of PTS symptoms may contribute to IBD disease processes, most notably through autonomic nervous system (ANS) arousal via immune and brain–gut processes.51 Physical reactions, hypervigilance, and hyperarousal were most prevalent among CD patients, but many UC patients also endorsed ANS arousal. Evidence supports a complex relationship between brain–gut processes and PTS symptoms via dysregulation of the immune system, the hypothalamic–pituitary–adrenal axis, the vagus nerve, and the gut microbiome.24 Future studies should investigate how the presence of PTS symptoms could potentially modulate these mechanisms in IBD.
Our study has several limitations to consider. Although self-report measures are used to identify PTS symptoms and determine the likelihood of PTSD, a clinical interview must be used to diagnose PTSD. The PCL-C has been shown to overestimate the prevalence of PTSD among medical populations, which may be due to some items being confounded with symptoms or treatment side effects. As such, PTS rates may be overestimated in our study. An appropriate next step would be to use the PCL-C with a clinical interview (eg, Clinician Administered PTSD Scale-5) to more accurately assess prevalence rates of PTS and diagnose PTSD. The IBD sample was largely recruited from online sources, which may have introduced selection or voluntary bias and amplified PTS. Clinic samples are from an academic IBD specialty center and may limit generalizability.
Post-traumatic stress occurs in a significant proportion of IBD patients, and, compared with IBS, IBD patients, especially hospitalized CD patients or those undergoing ileostomy or IPAA surgery, may be more susceptible to PTS. Clinicians should assess for PTS in IBD as standard of care, especially in the days after hospitalization or surgery. The Primary Care PTSD Screen, which is a 5-item scale to assess for traumatic events and associated symptoms, is an easy-to-administer tool for clinicians to gauge PTS during routine visits. For patients exhibiting PTS symptoms, referral to a psychologist specifically trained in PTS/PTSD treatments is recommended. Two evidence-based treatments exist: cognitive processing therapy and prolonged exposure therapy.52 As these treatments extend beyond typical cognitive–behavioral therapies used for gastrointestinal diseases, IBD patients with PTS will require additional care to mitigate its potential detrimental effects.
Conflicts of interest: No relevant competing interests to report.
Supported by: L.G. and M.C. are supported by National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK: 3T32DK101363-05S1). T.T. is supported by NIH-NIDDK: 1P01DK117824-01.
Author contributions: T.T.: designed and conducted the study; collected, analyzed, and interpreted data; prepared manuscript. A.B.: data interpretation, manuscript preparation. M.C.: data interpretation, manuscript preparation. L.G.: data interpretation, manuscript preparation. S.Q.: study design, manuscript preparation. S.H.: study design, data interpretation, manuscript preparation. Guarantor of the article: Tiffany Taft, PsyD.
REFERENCES
- 1. Watanabe Y, Arase S, Nagaoka N, et al. Chronic psychological stress disrupted the composition of the murine colonic microbiota and accelerated a murine model of inflammatory bowel disease. PLoS One. 2016;11:e0150559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grzybowska-Chlebowczyk U, Wysocka-Wojakiewicz P, Jasielska M, et al. Oxidative and antioxidative stress status in children with inflammatory bowel disease as a result of a chronic inflammatory process. Mediators Inflamm. 2018;2018:4120973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neuendorf R, Harding A, Stello N, et al. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res. 2016;87:70–80. [DOI] [PubMed] [Google Scholar]
- 4. Knowles SR, Graff LA, Wilding H, et al. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part I. Inflamm Bowel Dis. 2018;24:742–751. [DOI] [PubMed] [Google Scholar]
- 5. Knowles SR, Keefer L, Wilding H, et al. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part II. Inflamm Bowel Dis. 2018;24:966–976. [DOI] [PubMed] [Google Scholar]
- 6. Mikocka-Walus A, Knowles SR, Keefer L, et al. Addressing psychological needs of individuals with inflammatory bowel disease is necessary. Inflamm Bowel Dis. 2016;22:E20–E21. [DOI] [PubMed] [Google Scholar]
- 7. Taft TH, Keefer L. A systematic review of disease-related stigmatization in patients living with inflammatory bowel disease. Clin Exp Gastroenterol. 2016;9:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin CR, Osadchiy V, Kalani A, et al. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Breit S, Kupferberg A, Rogler G, et al. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keefer L. Editorial: depression in the setting of inflammatory bowel disease means we have failed to provide early, effective, psychosocial care. Aliment Pharmacol Ther. 2017;46:553–554. [DOI] [PubMed] [Google Scholar]
- 11. Stapersma L, van den Brink G, Szigethy EM, et al. Systematic review with meta-analysis: anxiety and depression in children and adolescents with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48:496–506. [DOI] [PubMed] [Google Scholar]
- 12. Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105–1118. [DOI] [PubMed] [Google Scholar]
- 13. Mikocka-Walus A, Knowles SR, Keefer L, et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752–762. [DOI] [PubMed] [Google Scholar]
- 14. Supportive P, Board PCE.. Cancer-Related Post-traumatic Stress (PDQ®). National Cancer Institute (US), Bethesda, Maryland: 2015. [Google Scholar]
- 15. Soldatos CR, Paparrigopoulos TJ, Pappa DA, et al. Early post-traumatic stress disorder in relation to acute stress reaction: an ICD-10 study among help seekers following an earthquake. Psychiatry Res. 2006;143:245–253. [DOI] [PubMed] [Google Scholar]
- 16. Pietrzak RH, Goldstein RB, Southwick SM, et al. Prevalence and axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the National Epidemiologic Survey on alcohol and related conditions. J Anxiety Disord. 2011;25:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Gabalawy R, Mota N, Sommer JL, et al. Prevalence of illness-induced posttraumatic stress disorder in the United States. Psychosom Med. 2018;80:783–785. [DOI] [PubMed] [Google Scholar]
- 18. Cámara RJ, Gander ML, Begré S, et al. ; Swiss Inflammatory Bowel Disease Cohort Study Group. Post-traumatic stress in Crohn’s disease and its association with disease activity. Frontline Gastroenterol. 2011;2:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veterans Affairs. Understanding PTS(D): Adapt and Overcome Vol. 2018 National Center for PTSD, Washington, DC; 2015. https://www.ptsd.va.gov/public/PTSD-overview/basics/veteransdaystory.asp. Accessed June, 2018. [Google Scholar]
- 20. Azoulay E, Pochard F, Kentish-Barnes N, et al. ; FAMIREA Study Group. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171:987–994. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Veterans Affairs. FAQs About PTSD Assessment: For Professionals Vol. 2018 Veterans Affiars, Washington, DC; 2018. https://www.ptsd.va.gov/professional/assessment/overview/faq-ptsd-professionals.asp. Accessed June, 2018. [Google Scholar]
- 22. O’Connor M, Christensen S, Jensen AB, et al. How traumatic is breast cancer? Post-traumatic stress symptoms (PTSS) and risk factors for severe PTSS at 3 and 15 months after surgery in a nationwide cohort of Danish women treated for primary breast cancer. Br J Cancer. 2011;104:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parker AM, Sricharoenchai T, Raparla S, et al. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43:1121–1129. [DOI] [PubMed] [Google Scholar]
- 24. Leclercq S, Forsythe P, Bienenstock J. Posttraumatic stress disorder: does the gut microbiome hold the key? Can J Psychiatry. 2016;61:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization practice guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010;16:112–124. [DOI] [PubMed] [Google Scholar]
- 26. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407.e5. [DOI] [PubMed] [Google Scholar]
- 27. Bennebroek Evertsz’ F, Hoeks CC, Nieuwkerk PT, et al. Development of the patient Harvey Bradshaw Index and a comparison with a clinician-based Harvey Bradshaw Index assessment of Crohn’s disease activity. J Clin Gastroenterol. 2013;47:850–856. [DOI] [PubMed] [Google Scholar]
- 28. Bennebroek Evertsz’ F, Nieuwkerk PT, Stokkers PC, et al. The Patient Simple Clinical Colitis Activity Index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis. 2013;7:890–900. [DOI] [PubMed] [Google Scholar]
- 29. Jowett SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001;96:2921–2928. [DOI] [PubMed] [Google Scholar]
- 30. Weathers FW, Litz BT, Herman D, et al. The PTSD Checklist-Civilian Version (PCL-C). Boston, MA: National Center for PTSD; 1994. [Google Scholar]
- 31. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association, Washington DC: 2013. [Google Scholar]
- 32. Ruggiero KJ, Del Ben K, Scotti JR, et al. Psychometric properties of the PTSD Checklist-Civilian Version. J Trauma Stress. 2003;16:495–502. [DOI] [PubMed] [Google Scholar]
- 33. Blanchard EB, Jones-Alexander J, Buckley TC, et al. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34:669–673. [DOI] [PubMed] [Google Scholar]
- 34. Abbey G, Thompson SB, Hickish T, et al. A meta-analysis of prevalence rates and moderating factors for cancer-related post-traumatic stress disorder. Psychooncology. 2015;24:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–992. [DOI] [PubMed] [Google Scholar]
- 36. Alegría M, Fortuna LR, Lin JY, et al. Prevalence, risk, and correlates of posttraumatic stress disorder across ethnic and racial minority groups in the United States. Med Care. 2013;51:1114–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alcántara C, Casement MD, Lewis-Fernández R. Conditional risk for PTSD among Latinos: a systematic review of racial/ethnic differences and sociocultural explanations. Clin Psychol Rev. 2013;33:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iorio N, Makipour K, Palit A, et al. Post-traumatic stress disorder is associated with irritable bowel syndrome in African Americans. J Neurogastroenterol Motil. 2014;20:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones MP, Bratten J, Keefer L. Quality of life in patients with inflammatory bowel disease and irritable bowel syndrome differs between subjects recruited from clinic or the Internet. Am J Gastroenterol. 2007;102:2232–2237. [DOI] [PubMed] [Google Scholar]
- 40. Fortinsky KJ, Fournier MR, Benchimol EI. Internet and electronic resources for inflammatory bowel disease: a primer for providers and patients. Inflamm Bowel Dis. 2012;18:1156–1163. [DOI] [PubMed] [Google Scholar]
- 41. Salzmann-Erikson M, Hiçdurmaz D. Use of social media among individuals who suffer from post-traumatic stress: a qualitative analysis of narratives. Qual Health Res. 2017;27:285–294. [DOI] [PubMed] [Google Scholar]
- 42. Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–90.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Irwin C, Falsetti SA, Lydiard RB, et al. Comorbidity of posttraumatic stress disorder and irritable bowel syndrome. J Clin Psychiatry. 1996;57:576–578. [DOI] [PubMed] [Google Scholar]
- 44. Cohen H, Jotkowitz A, Buskila D, et al. Post-traumatic stress disorder and other co-morbidities in a sample population of patients with irritable bowel syndrome. Eur J Intern Med. 2006;17:567–571. [DOI] [PubMed] [Google Scholar]
- 45. Posluszny DM, Edwards RP, Dew MA, et al. Perceived threat and PTSD symptoms in women undergoing surgery for gynecologic cancer or benign conditions. Psychooncology. 2011;20:783–787. [DOI] [PubMed] [Google Scholar]
- 46. Jones C, Bäckman C, Capuzzo M, et al. ; RACHEL group. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Crit Care. 2010;14:R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Long AC, Kross EK, Davydow DS, et al. Posttraumatic stress disorder among survivors of critical illness: creation of a conceptual model addressing identification, prevention, and management. Intensive Care Med. 2014;40:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Partridge JS, Martin FC, Harari D, et al. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28:804–812. [DOI] [PubMed] [Google Scholar]
- 49. Walsh TS, Salisbury LG, Merriweather JL, et al. ; RECOVER Investigators. Increased hospital-based physical rehabilitation and information provision after intensive care unit discharge: the RECOVER randomized clinical trial. JAMA Intern Med. 2015;175:901–910. [DOI] [PubMed] [Google Scholar]
- 50. Holmes A, Hodgins G, Adey S, et al. Trial of interpersonal counselling after major physical trauma. Aust N Z J Psychiatry. 2007;41:926–933. [DOI] [PubMed] [Google Scholar]
- 51. Brzozowski B, Mazur-Bialy A, Pajdo R, et al. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain-gut axis. Curr Neuropharmacol. 2016;14:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Sci Public Interest. 2013;14:65–111. [DOI] [PMC free article] [PubMed] [Google Scholar]