Abstract
BACKGROUND
There are currently no published data directly comparing postoperative seizure incidence following endoscopic third ventriculostomy (ETV), with/without choroid plexus cauterization (CPC), to that for ventriculoperitoneal shunt (VPS) placement.
OBJECTIVE
To compare postoperative epilepsy incidence for ETV/CPC and VPS in Ugandan infants treated for postinfectious hydrocephalus (PIH).
METHODS
We performed an exploratory post hoc analysis of a randomized trial comparing VPS and ETV/CPC in 100 infants (<6 mo old) presenting with PIH. Minimum follow-up was 2 yr. Variables associated with and the incidence of postoperative epilepsy were compared (intention-to-treat) using a bivariate analysis. Time to first seizure was compared using the Kaplan–Meier method, and the relative risk for the 2 treatments was determined using Mantel-Haenszel hazard ratios.
RESULTS
Seizure incidence was not related to age (P = .075), weight (P = .768), sex (P = .151), head circumference (P = .281), time from illness to hydrocephalus onset (P = .973), or hydrocephalus onset to treatment (P = .074). Irritability (P = .027) and vision deficit (P = .04) were preoperative symptoms associated with postoperative seizures. Ten (10%) patients died, and 20 (20%) developed seizures over the follow-up period. Overall seizure incidence was 9.4 per 100 person-years (9.4 and 9.5 for ETV/CPC and VPS, respectively; P = .483), with no significant difference in seizure risk between groups (hazard ratio, 1.02; 95% CI: 0.42, 2.45; P = .966). Mean time to seizure onset was 8.5 mo for ETV/CPC and 11.2 mo for VPS (P = .464). As-treated, per-protocol, and attributable-intervention analyses yielded similar results.
CONCLUSION
Postoperative seizure incidence following treatment of PIH was 20% within 2 yr, regardless of treatment modality.
Keywords: Epilepsy, ETV/CPC, Global neurosurgery, Postinfectious hydrocephalus, Uganda, Ventriculoperitoneal shunt, VPS, Seizures
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ETV
endoscopic third ventriculostomy
- CCHU
CURE Children's Hospital of Uganda
- CPC
choroid plexus cauterization
- EEG
electroencephalogram
- VPS
ventriculoperitoneal shunt
- PIH
postinfectious hydrocephalus
- CSF
cerebrospinal fluid
- ITT
intention-to-treat
An estimated 400,000 children develop hydrocephalus worldwide each year, with nearly half of these cases occurring in sub-Saharan Africa.1 In Uganda, the single most common etiology is postinfectious hydrocephalus (PIH) arising from neonatal ventriculitis, accounting for 60% of cases.2,3 The risk of developing seizures among infants treated for PIH in Uganda has not previously been studied. Seizure disorders are relatively common among children treated for hydrocephalus, with retrospective studies reporting prevalence between 12% and 38%.4-10 For both congenital and acquired hydrocephalus, epilepsy may be associated with, but not caused by, hydrocephalus or its treatment. In such instances, epilepsy may result from the original brain infection or injury, or from primarily disordered brain development. Thus, hydrocephalus etiology influences the likelihood of associated seizures. Studies have suggested that myelomeningocele patients have a lower risk of developing seizures after surgery for hydrocephalus compared to those with other etiologies, particularly those with posthemorrhagic or PIH.4,7,9 One study demonstrated that epileptogenesis in patients with neural tube defects correlated with coexisting cerebral anomalies rather than the location of the ventricular shunt catheter.10 Relevant to the coincidence of seizures and PIH, it is notable that seizures are reported in 19% of all African children treated for bacterial meningitis following hospital discharge.11 Distinguishing association from causation in relation to hydrocephalus is difficult.
It has long been suggested that the hydrocephalus treatment may itself be a cause of epilepsy, with seizures resulting from cortical transgression or the implantation of a foreign body into the brain.12 One study of children undergoing ventriculoperitoneal shunt (VPS) placement prior to the age of 2 yr found the first seizure occurred at a median of 1.6 yr following shunt insertion.5 The risk of seizures and subsequent antiepileptic drug initiation was 2% per year in a different study of children following shunt placement,12 and another study found that this risk continues throughout life.13 Subsequent shunt infections or revision operations may further increase the risk of epilepsy.4 However, the seizure risk from placement of a ventricular shunt catheter in isolation from other causative factors has not been determined.
In recent years, endoscopic third ventriculostomy combined with choroid plexus cauterization (ETV/CPC) has emerged as a treatment option for infant hydrocephalus. This is particularly attractive in limited resource countries where access to urgent neurosurgical care for shunt malfunction is much more limited.2,3 Little is known regarding the relative risk for developing seizures after ETV/CPC placement compared to VPS. Given that ventriculoscopy likely results in a more substantial cortical insult than passage of a ventricular catheter, it is possible that the seizure risk could be greater for ETV (±CPC). But one retrospective, single-institution study reported that no patients developed seizures following ETV alone.14 Another larger, multicenter study has recently reported a seizure incidence of 5.1% following ETV/CPC.15
Objective
A previously reported randomized controlled trial of ETV/CPC vs VPS for PIH in infants <6 mo of age (clinicaltrials.gov, NCT01936272) found no significant difference in neurocognitive outcome, brain growth, treatment failure, or mortality at 1 yr.16 Now, with a minimum of 2 yr’ follow-up, this cohort provided an opportunity to study the seizure incidence in this specific population and to determine whether this might be affected by treatment modality. We performed a post hoc analysis to determine the postoperative seizure incidence in these infants with PIH and the time between surgery and first seizure for the 2 treatment arms.
METHODS
Trial Design, Participants, Interventions, Outcomes, and Analysis
Patient Selection
With appropriate institutional approvals, the authors carried out a post hoc analysis of a 100-patient cohort presenting with PIH to CURE Children's Hospital of Uganda (CCHU) that had been randomized to VPS placement (using a Chhabra shunt; G. Surgiwear, Shahjahanpur, India) or ETV/CPC using a 3.7-mm, flexible, steerable ventriculoscope (Karl Storz, Tuttlingen, Germany) between May 2013 and April 2015. All patients had a minimum follow-up of 2 yr, were less than 6 mo of age at treatment, resided in 1 of 31 predetermined districts of Eastern Uganda, and presented with PIH diagnosed according to previously published criteria.17 Patients had been excluded if there was hydrocephalus at birth, an active cerebrospinal fluid (CSF) infection noted on ventricular puncture at presentation, scalp erosion or infection that would prevent shunt implantation, any pre-existing congenital brain anomaly (myelomeningocele/Chiari II malformation, encephalocele, Dandy-Walker malformation, schizencephaly, or congenital arachnoid cyst), ventricular loculations, or absence of any visible cortical mantle on preoperative CT scan. All patients were determined to be appropriate candidates for either surgical procedure, and informed parental consent had been obtained. Fifty-one patients were randomly assigned to the ETV/CPC group and 49 to the VPS group. There was no difference at preoperative baseline between the treatment groups in regard to median age or weight, sex, developmental scores, or brain volume. There was a small difference in pretreatment CSF volume and head circumference. Nine patients who were randomized to ETV/CPC crossed over to shunt placement because a scarred prepontine cistern was encountered at the time of the ETV. Details of the trial have been published elsewhere.13,16
Data Collection
Upon enrollment into the cohort, data were collected on patient demographics as well as presence and duration of clinical symptoms and signs using REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, Tennessee).18 No identifying information was collected from study participants for this post hoc analysis. Patients enrolled in the randomized cohort were followed for at least 2 yr postoperatively to monitor for symptomatic improvement and development of complications, including postoperative seizures, treatment failure, and death. Postoperative seizures were defined as history or evidence of a clinically apparent, generalized seizure that occurred after operation, whether witnessed in the hospital or by caretakers after discharge. No electroencephalogram (EEG) was available, and therefore, the seizure focus could not be localized, and unrecognized subclinical seizures could not be excluded. In most cases, seizures were treated using age- and weight-appropriate dosing of phenobarbital for the duration of follow-up. For this analysis, variables of interest were extracted from the study database.
Data Analysis
Data were extracted from REDCap and analyzed in Stata 12.1 (StataCorp, College Station, Texas). Demographic, clinical characteristics, and outcomes of cohort patients were analyzed using descriptive statistics. Association between each possible seizure risk factor and the occurrence of postoperative seizures was determined using Student's t-test for continuous variables and χ2 test for categorical variables. To understand the difference in seizure occurrence by treatment group, intention-to-treat (ITT) analysis was used to compare patients randomized to ETV/CPC or VPS. An “as-treated” analysis and “per-protocol” analysis were also performed and used for comparison. The “as-treated” approach analyzed patients based on the actual procedure they had, not the procedure that they were initially assigned to. The “per-protocol” analysis excluded all patients who received a different surgical treatment from their original randomization, excluding any patient in whom both an endoscope had been passed and a shunt had been placed. An additional analysis was performed, henceforth referred to as “attributable-intervention” analysis, in which outcome was initially assigned to the “as-treated” surgical assignments, but for patients who later crossed over, subsequent outcome was assigned to the crossover treatment beginning at the time of the crossover surgery. This occurred exclusively in the setting of initial ETV/CPC, with subsequent crossover to VPS at ETV/CPC failure. The Mantel-Haenszel method was used to calculate hazard ratios of seizure development. Average time to seizure development was calculated for each treatment group. Incidence rate of postoperative seizures for each treatment group was calculated using the standard person-time denominator. Kaplan–Meier survival curves were used to further analyze time to postoperative seizure onset between ETV/CPC- and VPS-treated patients. A P value of <0.05 was considered statistically significant, recognizing that these were exploratory analyses given the post hoc nature of this study.
RESULTS
Baseline Data
Demographics
Average age of the 100-patient cohort was 3.25 mo at surgery. The mean weight was 5.96 kg. Of all patients, 39 (39%) were female. All patients in the cohort were diagnosed with PIH. The mean time from reported febrile illness to onset of hydrocephalus symptoms was 38 D. On average, there was a 46-D delay between the onset of hydrocephalus symptoms and presentation to CCHU. There were no significant differences for any of these parameters between patients who developed seizures postoperatively and those who did not (Table 1).
TABLE 1.
Background Characteristics and Clinical Findings of Study Cohort (n = 100)
| All patients (Percent of total patients) | Post-op seizures (Percent of subgroup) | No post-op seizures (Percent of subgroup) | P value* | |
|---|---|---|---|---|
| Age, mo (mean ± SD) | 3.25 ± .12 | 2.82 ± .20 | 3.35 ± .14 | .075 |
| Weight, kg (mean ± SD) | 5.96 ± .14 | 5.88 ± .23 | 5.99 ± .17 | .768 |
| Sex | ||||
| Female | 39 (39%) | 34 (87.2%) | 5 (12.8%) | .151 |
| Male | 61 (61%) | 46 (75.4%) | 15 (24.6%) | |
| Febrile illness to hydrocephalus, d (mean ± SD) | 37.7 ± 2.65 | 36.5 ± 5.30 | 37.7 ± 3.06 | .973 |
| Hydrocephalus onset to hospital presentation, d (mean ± SD) | 45.6 ± 2.71 | 36.0 ± 4.11 | 48.0 ± 3.17 | .074 |
| Head circumference, cm | 48.6 ± .44 | 47.6 ± .82 | 48.8 ± .51 | .281 |
| Presenting symptoms | ||||
| Macrocephaly | 25 (25%) | 2 (8%) | 23 (92%) | .083 |
| Full fontanel | 99 (99%) | 20 (20%) | 79 (80%) | .615 |
| Abnormal eye movements | 6 (6%) | 3 (50%) | 3 (50%) | .058 |
| Vomiting | 17 (17%) | 2 (12%) | 15 (88%) | .351 |
| Lethargy | 2 (2%) | 0 (0%) | 2 (100%) | .475 |
| Irritability | 18 (18%) | 7 (39%) | 11 (61%) | .027 |
| Vision deficit | 3 (3%) | 2 (66.7%) | 1 (33.3%) | .040 |
| Loss of developmental milestones | 1 (1%) | 0 (0%) | 1 (100%) | .615 |
| Presenting signs | ||||
| Bulging fontanel | 93 (93%) | 20 (22%) | 73 (88%) | .170 |
| Splaying of cranial sutures | 7 (7%) | 2 (28.6%) | 5 (71.4%) | .557 |
| Abnormally increasing head circumference | 52 (52%) | 8 (15%) | 44 (85%) | .230 |
| Upward gaze palsy | 44 (44%) | 9 (20%) | 35 (80%) | .920 |
| 6th Nerve palsy | 2 (2%) | 2 (100%) | 0 (0%) | – |
| Fever | 3 (3%) | 1 (33.3%) | 2 (66.7%) | .558 |
| Nuchal rigidity | 2 (2%) | 1 (50%) | 1 (50%) | .284 |
| Bradycardia | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Apnea | 0 (0%) | 0 (0%) | 0 (0%) | – |
Abbreviations: Post-op, postoperative; SD, standard deviation.
*Student's t-test was used for continuous variables; Chi-square test was used for categorical variables.
Clinical Presentation
The mean head circumference at presentation was 48.6 cm, with no significant differences noted between the seizure and nonseizure groups. Ninety-nine patients presented with a full fontanel, with 93 described as “bulging.” The next most common presenting signs and symptoms were accelerated head growth (52), upward gaze palsy (44), macrocephaly (25), irritability (18), and vomiting (17) (Table 1). Of all presenting signs and symptoms, irritability and vision deficit (defined as absence of visual fixation and tracking) were associated with the development of postoperative seizures (P = .027 and P = .04, respectively).
Outcomes
Effect of Treatment on Seizure Occurrence
Of the 100 PIH patients in this cohort, none of the patients’ caregivers reported seizures between the development of hydrocephalus and the operative intervention. Of 20 (20%) patients who developed postoperative seizures over the 2-yr follow-up period, 10 (50%) had randomized to ETV/CPC and 10 (50%) to shunt placement (P = .920). Using ITT analysis, the yearly postoperative seizure incidence rate was 9.4 per 100 person-years among all patients. Patients randomized to ETV/CPC had an incidence rate of 9.4 seizures per 100 person-years, whereas those randomized to VPS had an incidence rate of 9.5 seizures per 100 person-years. The difference was not statistically significant (P = .483). Average time to seizure onset was 8.5 and 11.2 mo for the ETV/CPC and VPS groups using ITT analysis, respectively, and the difference was not statistically significant (P = .464). For all patients, seizure-free survival at 6, 12, and 24 mo was 91%, 85%, 82%, respectively (Kaplan–Meier method), with a similar risk of seizures between treatment groups (HR, 1.02; 95% CI: 0.42, 2.45; P = .966) (Figure 1). There were also no statistically significant differences in risk, incidence rate, or time to postoperative seizure onset between groups for the “as-treated,” “per-protocol,” or “attributable-intervention” analyses (Figures 2–4).
FIGURE 1.
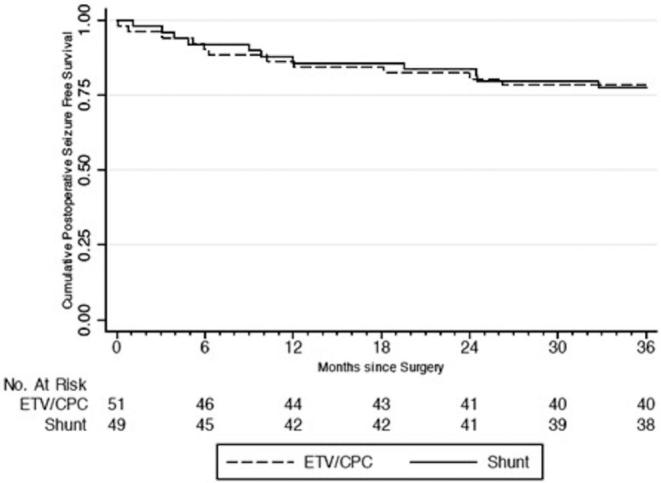
Time to seizure occurrence by intention-to-treat analysis.
FIGURE 2.
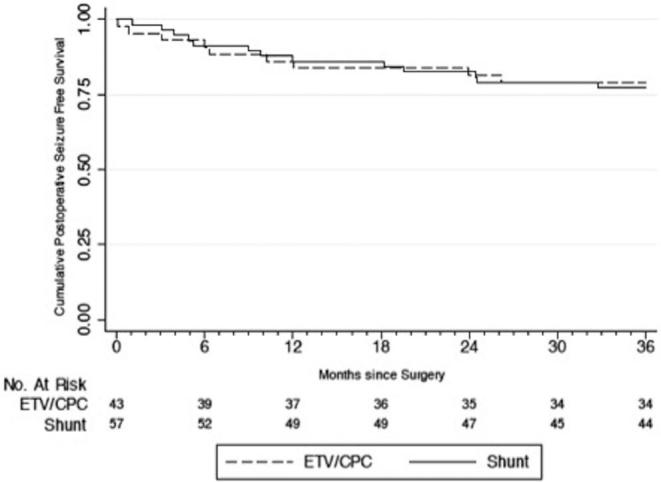
Time to seizure occurrence by “as-treated” analysis.
FIGURE 4.
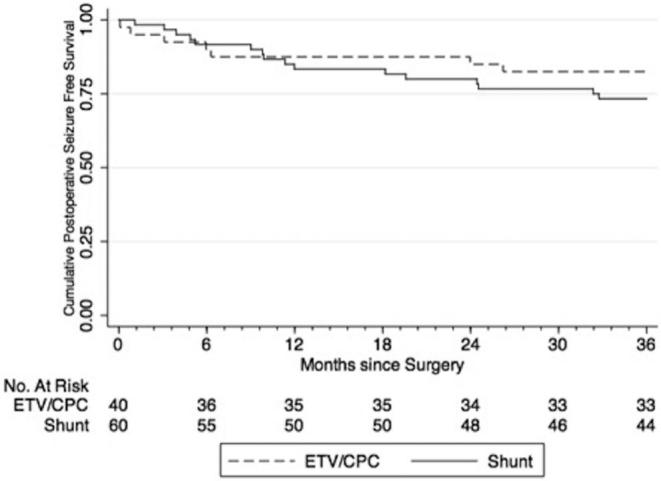
Time to seizure occurrence by “attributable-intervention” analysis.
FIGURE 3.
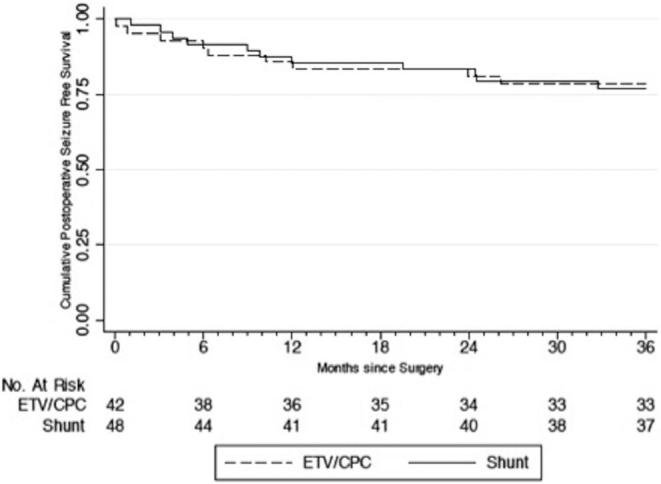
Time to seizure occurrence by “per-protocol” analysis.
Postoperative Outcomes
Postoperative seizures correlated with longer hospital stay (mean, 2.3 D; P = .017). There was no difference in postoperative head circumference between patients who developed postoperative seizures compared to those who did not. There were 30 first-time ETV/CPC or VPS failures (including 4 shunt infections, one of which resulted in death) within 2 yr of follow-up, with no association between treatment failure and seizure occurrence (P = .102) (Table 2).
TABLE 2.
Surgical Intervention and Outcomes for Study Cohort (n = 100)
| All patients (Percent of total patients) | Post-op seizures (Percent of subgroup) | No post-op seizures (Percent of subgroup) | P value* | |
|---|---|---|---|---|
| Surgical intervention | ||||
| ETV/CPC | 51 (51%) | 10 (19.6%) | 41 (80.4%) | .920 |
| VPS | 49 (49%) | 10 (20.4%) | 39 (79.6%) | |
| Outcome | ||||
| Length of hospitalization, d (mean ± SD) | 2.3 ± .07 | 2.68 ± .28 | 2.24 ± .06 | .017 |
| Post-op head circumference, cm (mean ± SD) | ||||
| 1 mo | 47.1 ± .36 | 46.6 ± .71 | 47.3 ± .42 | .474 |
| 3 mo | 47.7 ± .33 | 46.9 ± .69 | 47.8 ± .38 | .270 |
| 6 mo | 48.5 ± .34 | 47.8 ± .75 | 48.6 ± .38 | .400 |
| 12 mo | 48.9 ± .36 | 47.5 ± .69 | 49.2 ± .40 | .060 |
| 24 mo | 49.6 ± .38 | 48.4 ± .88 | 49.9 ± .42 | .116 |
| ETV or VPS failure | 30 (30%) | 9 (30%) | 21 (70%) | .102 |
| Mortality | 10 (10%) | 3 (30%) | 7 (70%) | .405 |
Abbreviations: CPC, choroid plexus cauterization; ETV, endoscopic third ventriculostomy; Post-op, postoperative; SD, standard deviation; VPS, ventriculoperitoneal shunt.
*Student's t-test was used for continuous variables; Chi-square test was used for categorical variables.
Mortality
There were 10 (10%) deaths within 2 yr of follow-up, with no association between mortality at 2 yr and seizure occurrence (P = .405). Two deaths were attributed to treatment failure (1 shunt infection and 1 ETV/CPC failure in the first year). The remaining 8 deaths were not directly related to hydrocephalus or its treatment: 3 from gastroenteritis, 3 from malnutrition, 1 from pneumonia, and 1 from measles.
DISCUSSION
Interpretation of Results
A direct comparison of postoperative seizure risk for shunt placement and ETV (with or without CPC) has not previously been reported. A randomized controlled trial of ETV/CPC vs VPS, designed to determine whether either was superior in regard to developmental outcome or brain growth, provided the opportunity to compare these treatments in regard to postoperative seizure development. Likewise, this study provided the first opportunity to prospectively observe the incidence of seizure development over time in Ugandan infants treated for PIH. This post hoc analysis found that 20% of the patients developed seizures within 2 yr of surgery, with no statistically significant difference between treatment modalities. There was no significant difference between the treatment groups (ITT, as-treated, per-protocol, or attributable-intervention analyses) in regard to hazard ratio of postoperative epilepsy or time from surgery to seizure onset. The lack of any difference between the per-protocol groups (ie, shunted patients had not undergone endoscopy, and patients undergoing endoscopy did not have a shunt placed) did not suggest that the presence of an indwelling catheter added additional risk, nor did it suggest any increased risk from the slightly larger transcortical tract created by placement and subsequent manipulation of a 3.7-mm, flexible ventriculoscope.
Previously, the gold standard for treating infant hydrocephalus has been the placement of a VPS. In recent years, ETV/CPC has gained traction as an alternative treatment that can avoid life-long shunt dependence.2,19,20 Previous estimates of postoperative epilepsy prevalence in patients with VPS have ranged from 9% to 65%.4-8,12,21-28,17,29 One study of 129 children shunted for nontumor-associated hydrocephalus before 2 yr of age found epilepsy had developed in 30% after at least 10 yr of follow-up, with an average time from surgery to seizure onset of 1.6 yr.5 In a more recent longitudinal study of 379 children treated for infant-onset hydrocephalus, 23% developed epilepsy at a mean age of 2.7 yr.9 These are similar to the results in this cohort of infants with PIH, 20% of which developed seizures over a 2-yr follow-up period regardless of treatment modality. Two prior studies have reported postoperative seizure incidence following ETV. In one, of 42 children undergoing ETV at average age of 7.6 years for non-postinfectious causes of hydrocephalus, none developed seizures over 32 mo follow-up.14 A larger multicenter study from the Hydrocephalus Clinical Research Network found a 5.1% postoperative seizure incidence following ETV/CPC for infant hydrocephalus.15
The risk or mechanism of postoperative epilepsy following shunt placement is uncertain. In one study of 40 shunted patients, EEG abnormalities were localized to the shunted side in patients with no neurological deficits traceable to focal damage of the parenchyma, suggesting that the presence of the ventricular catheter might itself be responsible for the epileptogenic focus.30 Brain implants, like ventricular catheters, have been associated with ongoing mechanical strain against the tissue, resulting in a damaging inflammatory cascade.31 Some have postulated that the absence of these factors in ETV patients could result in a lower risk of postoperative seizures.14 Conversely, it is thought that any corticotomy may potentially be epileptogenic and that limiting its size might decrease that risk.32 Injury from the passage and manipulation of an endoscope through the cortical mantle could be greater than that caused by the passage of a smaller ventricular catheter. The severity of fiber degeneration along brain electrode tracts, as demonstrated by modified Nauta silver staining, can be widespread because of Wallerian degeneration, and this could correlate with electrode diameter.33 Thus, whereas shunts might possibly have a higher seizure risk because of the presence of the indwelling ventricular catheter over time, ETV could likewise carry a greater initial risk by virtue of a larger transcortical tract. However, a recent study of postoperative epilepsy in children following surgery for brain tumor did not find corticotomy to be a risk factor.34
It is important to emphasize that this study cohort was comprised entirely of infants with PIH. A systematic literature review to determine the consequences of bacterial meningitis among children in Africa found a 7% to 19% incidence of seizure occurrence following hospital discharge.11 It is possible that the 20% incidence of seizure onset within 2 yr seen in the study cohort, comprised of infants with hydrocephalus following severe ventriculitis, was primarily the result of the infection and unassociated with hydrocephalus or its mode of treatment. That absence of visual tracking correlated with increased seizure risk may suggest that both resulted from primary parenchymal injury. Although common for these infants to have had a febrile convulsion witnessed at the time of the acute illness, none of these patients’ caregivers had reported an additional seizure between resolution of the illness and presentation for treatment of hydrocephalus. This is not surprising given the short time interval between the illness and presentation for treatment of hydrocephalus, which was at a mean age of 3.25 mo (mean time from illness to hydrocephalus, 38 D; mean time from hydrocephalus onset to treatment, 46 D).
Although we did not observe a difference in relative risk between VPS and ETV/CPC, it is important to note how the cohort size of our randomized study affects the power to have detected such differences. Based upon prior reports, if one assumed a 7% incidence of seizures in this PIH cohort (the lower postmeningitis seizure incidence from the African study cited above), a 4% incidence of seizure resulting from VP shunt placement (at 2% per year),12 and a 5% incidence of seizures resulting from ETV/CPC,15 the calculated sample size to detect a significant difference between groups of 11% for VPS and 12% for ETV/CPC (alpha = 0.05 and a power of 0.80) exceeds 32 000. This is assuming that the higher postmeningitis seizure incidence of 19% would require an even larger sample size to detect a difference between treatment modalities. Thus, although our results show the same seizure risk for both treatments of PIH (20%), a small difference between treatment modalities cannot be excluded, and the results should not be extrapolated to other etiologies of hydrocephalus. However, this study suggests that, for this population, risk at 2 yr is the same for both treatments. These results, then, fail to show any meaningful difference in seizure risk between the 2 modalities and do not suggest the existence of any difference, although the study does not exclude that possibility.
Limitations and Generalizability
Our study has several important limitations. This was a post hoc analysis, with multiple comparisons that were not defined a priori as part of the initial randomized trial protocol. We view these results, therefore, as only exploratory in nature and in need of further confirmation. The study was restricted to a relatively small cohort of infants with PIH, thus the findings may not be applicable to other groups. And it is possible that a difference in seizure risk between treatment groups could have been concealed by a high underlying risk of epilepsy secondary to the initial brain infection and injury. There was no access to continuous video EEG monitoring for conclusive seizure diagnosis, instead relying on clinical observation from caregivers. Such behavioral observations can underrepresent the true positive rate of epilepsy, because unobserved and subconvulsive seizures will not be accounted for. Conversely, the lack of EEG data consistent with seizure activity can also lead to an increase in false positive diagnoses. Because irritability was found to be positively associated with seizure occurrence, it is possible that this symptom may have represented presurgical seizure activity in some instances, but that would not have affected the comparison between treatment modalities. The lack of observed association between the development of postoperative seizures and mortality in the cohort is interesting given the known link between epilepsy and mortality.35 This could be explained by the high baseline mortality from non-neurological causes and the relatively brief follow-up period of 2 yr. For the general population in Uganda, the reported infant mortality (under 1 yr) approaches 9%, and the under-5 mortality rate is 16%.36 The 10% 2-yr mortality in our cohort is, therefore, not unexpected.
CONCLUSION
Among infants with PIH in this single-center randomized trial, our exploratory post hoc analysis showed no difference in postoperative seizure occurrence between VPS placement and ETV/CPC. The incidence of developing seizures within 2 yr following the treatment of PIH in this population of Ugandan infants less than 6 mo of age was 20%.
Disclosures
This work was supported by the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Fogarty International Center (project numbers R21TW009612 and R01HD085853). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
COMMENT
This is an excellently written manuscript addressing an important question. It is also timely because endoscopic third ventriculostomy/choroid plexus cauterization are gaining popularity. The data are from a randomized controlled trial, and the patient population is well defined. The authors fully acknowledge the ambiguity that clinicians often encounter when diagnosing epilepsy, which is true in their clinical setting, as well as anywhere else. My greatest concern, however, is that the study is underpowered. Because of the limited sample size, seizure incidence differences that were less than 20% would fall under the detection threshold. Equivalence in seizure incidence with endoscopic and shunt treatment modalities does fit my own clinical impression, and I look forward to future studies on this topic.
Joshua J. Chern
Atlanta, Georgia
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. Dewan M, Rattani A, Mekary R et al.. Global hydrocephalus: epidemiology and worldwide volume. J Neurosurg. published online: April 3, 2018 (doi: 10.3171/2017.11.JNS171500). [Google Scholar]
- 2. Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg. 2005;103(6 Suppl):475-481. [DOI] [PubMed] [Google Scholar]
- 3. Warf BC. Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg Pediatr. 2005;102(1):1-15. [DOI] [PubMed] [Google Scholar]
- 4. Bourgeois M, Sainte-Rose C, Cinalli G et al.. Epilepsy in children with shunted hydrocephalus. J Neurosurg. 1999;90(2):274-281. [DOI] [PubMed] [Google Scholar]
- 5. Hoppe-Hirsch E, Laroussinie F, Brunet L et al.. Late outcome of the surgical treatment of hydrocephalus. Childs Nerv Syst. 1998;14(3):97-99. [DOI] [PubMed] [Google Scholar]
- 6. Keene DL, Ventureyra ECG. Hydrocephalus and epileptic seizures. Childs Nerv Syst. 1999;15(4):158-162. [DOI] [PubMed] [Google Scholar]
- 7. Klepper J, Busse M, Straburg H, Sorensen N. Epilepsy in shunt-treated hydrocephalus. Dev Med Child Neurol. 1998;40(11):731-736. [DOI] [PubMed] [Google Scholar]
- 8. Piatt JH, Carlson CV. Hydrocephalus and epilepsy: An actuarial analysis. Neurosurgery. 1996;39(4):722-727. [DOI] [PubMed] [Google Scholar]
- 9. Tully HM, Kukull WA, Mueller BA. Clinical and Surgical Factors Associated with Increased Epilepsy Risk in Children with Hydrocephalus. Pediatr Neurol. 2016;59:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshida F, Morioka T, Hashiguchi K et al.. Epilepsy in patients with spina bifida in the lumbosacral region. Neurosurg Rev. 2006;29(4):327-332. [DOI] [PubMed] [Google Scholar]
- 11. Ramakrishnan M, Ulland AJ, Steinhardt LC, Moïsi JC, Were F, Levine OS. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med. 2009;7:47 doi: 10.1186/1741-7015-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sato O, Yamguchi T, Kittaka M, Toyama H. Hydrocephalus and epilepsy. Childs Nerv Syst. 2001;17(1-2):76-86. [DOI] [PubMed] [Google Scholar]
- 13. NIH. Neurocognitive outcomes and changes in brain and CSF volume after treatment of post-infectious hydrocephalus in Ugandan infants by shunting or ETV/CPC: a randomized prospective trial. Research Portfolio Online Reporting Tools. https://projectreporter.nih.gov/project_info_description.cfm?aid=8992100&icde=0. Published 2017. Accessed August 6, 2017. [Google Scholar]
- 14. Kramer U, Kanner AA, Siomin V, Harel S, Constantini S. No evidence of epilepsy following endoscopic third ventriculostomy: a short-term follow-up. Pediatr Neurosurg. 2001;34(3):121-123. [DOI] [PubMed] [Google Scholar]
- 15. Kulkarni AV, Riva-Cambrin J, Rozzelle CJ et al.. Endoscopic third ventriculostomy and choroid plexus cauterization in infant hydrocephalus: a prospective study by the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 2018;21(3):214-223. [DOI] [PubMed] [Google Scholar]
- 16. Kulkarni A, Schiff S, Mbabazi-Kabachelor E et al.. Endoscopic treatment versus shunting for infant hydrocephalus in Uganda. N Engl J Med. 2017;377(25):2456-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noetzel M, Blake J. Seizures in children with congenital hydrocephalus: long-term outcome. Neurology. 1992;42(7):1277-1277. [DOI] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kahle K, Kulkarni A, Limbrick D, Warf B. Hydrocephalus in children. Lancet North Am Ed. 2016;387(10020):788-799. [DOI] [PubMed] [Google Scholar]
- 20. Stone S, Warf B. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. J Neurosurg Pediatr. 2014;14(5):439-446. [DOI] [PubMed] [Google Scholar]
- 21. Johnson D, Conry J, O’Donnell R. Epileptic seizure as a sign of cerebrospinal fluid shunt malfunction. Pediatr Neurosurg. 1996;24(5):223-228. [DOI] [PubMed] [Google Scholar]
- 22. Al-Sulaiman AA, Ismail HM. Pattern of electroencephalographic abnormalities in children with hydrocephalus: a study of 68 patients. Childs Nerv Syst. 1998;14(3):124-126. [DOI] [PubMed] [Google Scholar]
- 23. Veggiotti P, Beccaria F, Papalia G, Termine C, Piazza F, Lanzi G. Continuous spikes and waves during sleep in children with shunted hydrocephalus. Childs Nerv Syst. 1998;14(4-5):188-194. [DOI] [PubMed] [Google Scholar]
- 24. Chadduck W, Adametz J. Incidence of seizures in patients with myelomeningocele: a multifactorial analysis. Surg Neurol. 1988;30(4):281-285. [DOI] [PubMed] [Google Scholar]
- 25. Copeland G, Foy P, Shaw M. The incidence of epilepsy after ventricular shunting operations. Surg Neurol. 1982;17(4):279-281. [DOI] [PubMed] [Google Scholar]
- 26. Ines DF, Markand ON. Epileptic seizures and abnormal electroencephalographic findings in hydrocephalus and their relation to the shunting procedures. Electroencephalogr Clin Neurophysiol. 1977;42(6):761-768. [DOI] [PubMed] [Google Scholar]
- 27. Stellman G, Bannister C, Hallier V. The incidence of seizure disorder in children with acquired and congenital hydrocephalus. Z Kinderchir. 1986;42(Suppl 1):38-41. [DOI] [PubMed] [Google Scholar]
- 28. Dan N, Wade M. The incidence of epilepsy after ventricular shunting procedures. J Neurosurg. 1986;65(1):19-21. [DOI] [PubMed] [Google Scholar]
- 29. Francisco De Amorim Júnior R, Emiliana S, Dantas C et al.. Screening of epileptic seizures in children and adolescents with hydrocephalus and ventriculoperitoneal shunt. J Epilepsy Clin Neurophysiol. 2009;15(3):106-109. [Google Scholar]
- 30. Liguori G, Abate M, Buono S, Pittore L. EEG findings in shunted hydrocephalic patients with epileptic seizures. Ital J Neuro Sci. 1986;7(2):243-247. [DOI] [PubMed] [Google Scholar]
- 31. Zhong Y, Bellamkonda R V. Biomaterials for the central nervous system. J R Soc Interface. 2008;5(26):957-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teo C. Chapter 29. Complications of endoscopic third ventriculostomy. In: Cinalli G, Sainte-Rose C, W Maixner, eds. Pediatric Hydrocephalus. Milano: Springer; 2005:411-420. [Google Scholar]
- 33. Schiff S. Unpublished Data (Personal Communication). 2017. [Google Scholar]
- 34. Massimi L, Battaglia D, Bianchi F, Peraio S, Peppucci E, Di Rocco C. Postoperative epileptic seizures in children: Is the brain incision a risk factor? Neurosurgery. 2018;82(4):465-472. [DOI] [PubMed] [Google Scholar]
- 35. Camfield CS, Camfield PR, Veugelers PJ. Death in children with epilepsy: a population-based study. Lancet North Am Ed. 2002;359(9321):1891-1895. [DOI] [PubMed] [Google Scholar]
- 36. Warf B, Wright E, Kulkarni A. Factors affecting survival of infants with myelomeningocele in southeastern Uganda. J Neurosurg Pediatr. 2011;7(2):127-133. [DOI] [PubMed] [Google Scholar]