Abstract
IMPORTANCE
The incidence of acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) in adolescent and young adult (AYA) patients (age range, 15–39 years) in the United States is increasing at a greater rate than in younger or older persons. Their optimal treatment has been increasingly debated as pediatric regimens have become more widely used in the age group. This review compares the basic features of pediatric and adult chemotherapy regimens for ALL and LBL, recognizes and describes the challenges of the pediatric regimen, and suggests strategies to facilitate its adoption for AYAs with ALL and LBL.
OBSERVATIONS
All but 2 of 25 published comparisons of outcomes with pediatric and adult regimens for ALL and LBL in AYAs and 1 meta-analysis favor the pediatric regimen. After more than a half-century of clinical trials of the pediatric regimens, including at least 160 phase 3 trials in the United States, the pediatric regimens have become far more complex than most adult regimens. Asparaginase, a critical component of the pediatric regimens, is more difficult to administer to AYAs (and older patients) but nonetheless has a favorable benefit to toxicity ratio for AYAs. A dramatic reduction in outcome of ALL and LBL during the AYA years (the “survival cliff”) is coincident with similar reductions in proportions of AYAs referred to academic centers and enrolled on clinical trials (the “accrual cliff” and “referral cliff”).
CONCLUSIONS AND RELEVANCE
The accumulating data increasingly support treating AYAs with ALL and LBL with a pediatric-inspired regimen or an approved institutional or national clinical trial tailored for this patient group. A need to develop clinical trials specifically for AYAs and to encourage their participation is paramount, with a goal to improve both the quantity and quality of survival.
Increasingly, adolescent and young adult (AYA) patients with acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) are being treated with pediatric-inspired regimens to improve both the quantity and quality of survival. In the United States, the cooperative groups sponsored by the National Cancer Institute (NCI) studying adult patients with cancer were able to successfully develop, enroll, and complete a trial focused on AYA patients with newly diagnosed ALL. Three adult cooperative groups were able to collaborate on this effort and double the survival of their AYA patients, as described herein. Now, through the National Cancer Treatment Network (NCTN), they have developed a successor trial (Alliance A0415011) that has opened as well.
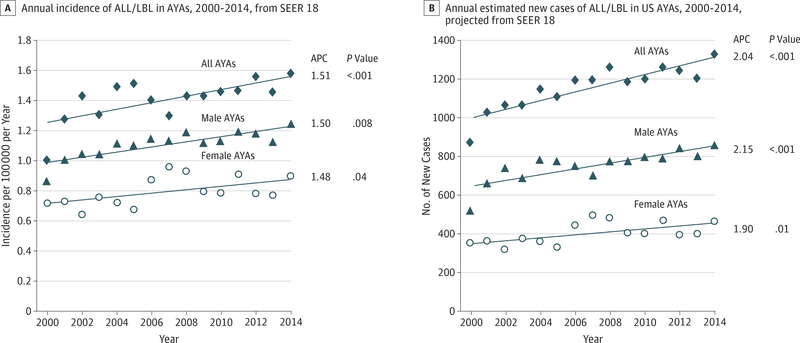
However, this remarkable accomplishment has not reached the vast majority of AYA patients with ALL and LBL who are not being treated with a pediatric type of regimen, despite the sentinel observation on this topic published a decade ago2 and numerous comparisons in favor of the pediatric regimen reviewed herein. This disparity is becoming increasingly important as the incidence of ALL and LBL in AYAs in the United States is increasing more rapidly in AYAs than in either younger or older persons,2 as noted by J.L. McNeer, MD (written communication, August 2017). By 2018, more than 1300 AYAs are expected to be diagnosed as having these lymphoid cancers (ALL and LBL) (Figure 1).
Figure 1. Annual Incidence and New Cases in the United States of Adolescents and Young Adults (AYAs) With Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma (ALL/LBL), 2000 to 2014.
A and B, Shown in A are incidence data from the Surveillance, Epidemiology, and End Results (SEER) 18 data3 on which the estimated numbers of new cases in B are based. The age range of the AYAs was 15 to 39 years. Average percentage change (APC) represents the mean percentage change of logarithmic values, with APC values and P values for incidence provided by SEER and calculated by us for new case numbers. The International Classification of Diseases–Oncology, Third Edition codes used for ALL and LBL are available in the eTable in the Supplement.
In this review, we compare the basic features of pediatric and adult chemotherapy regimens for ALL, describe the challenges of the pediatric regimen, and suggest strategies to facilitate adoption of the pediatric inspiration. We also review the contribution of clinical trials to the survival progress of ALL therapy and the need to develop clinical trials specifically for AYAs and to encourage their participation.
General Comparison of Pediatric and Adult Treatment Regimens in Young Adults
The Survival Cliff
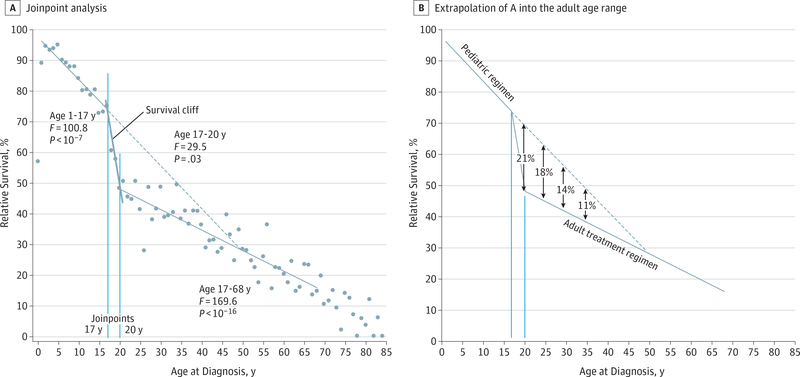
Figure 2 shows the 5-year relative survival of all patients with ALL in all US Surveillance, Epidemiology, and End Results program regions by single age at diagnosis. The regressions were generated by joinpoint analysis, a method that identifies when trends change, their statistical significance, and that of trends before and after the inflection.4 When analyzed as a function of age at diagnosis during 2000 to 2007, the survival of Americans with ALL is triphasic, with joinpoints at ages 17 and 20 years (Figure 2A). Given that the survival rates were 75% at age 17 years, 48% at age 20 years, and 15% at age 70 years, the drop during just 3 years from ages 17 to 20 years accounts for 45% of the total survival decrease between ages 17 and 70 years. Some of this “survival cliff” is due to the increasing incidence of poor prognostic ALL subtypes with age. For AYAs, the most important of these subtypes is “Ph-like” (subtype of Ph-negative B-cell precursor ALL with a gene expression profile similar to Ph-positive ALL) ALL because, of the adverse subtypes, it increases most rapidly during the AYA years and appears to peak in incidence between ages 20 and 40 years.5,6 The importance of Ph-like ALL is that an increasing number of tyrosine kinase inhibitors effective against the subtype are available and being added to pediatric regimens.5 The survival cliff between ages 17 and 21 years has also been attributed to the transition of patients from pediatric to adult treatment sites during this age span.7 Extending the slope of the pediatric linear survival trendforpatientsaged1to17years into the adult age range suggests that current pediatric regimens could increase the 5-year survival rate in those aged 20, 25, 30, and 35 years by absolute amounts of 21%, 18%, 14%, and 11%, respectively (Figure 2B).
Figure 2. Five-Year Relative Survival Rate of Patients With Acute Lymphoblastic Leukemia by Single Year of Age at Diagnosis, 2000 to 2007, From the Surveillance, Epidemiology, and End Results 18 Data3.
A, Included is joinpoint analysis4 that created linear regressions for ages 1 to 17 years and 20 to 68 years and associated statistical variables. B, The pediatric age-dependent survival trend in A is extrapolated into the adult age range. The solid lines are the regressions created by joinpoint analysis, and the vertical solid lines indicate the ages at which the joinpoints were identified. The diagonal dashed line in B is an extension of the survival regression of children.
Comparison of Outcomes
Concurrent outcome comparisons of pediatric and adult treatment regimens for ALL have consistently demonstrated, in 13 countries on 4 continents, the superiority of the pediatric regimen for AYAs (Table).2,8–21,23–33 Thirteen of 16 comparisons favor the pediatric regimen,2,8–21,23,24 albeit none are prospective randomized trials. In addition, all of 9 noncomparative reports have similar results for the pediatric regimen (Table).25–33
Table.
Pediatric and Adult Therapy Regimen Outcomes in AYAs With ALL or LBL
| Report | Clinical Trial | Age, y | No. of Patients | Follow-up, y | EFS, DFS, or CCR, % | Overall Survival, % |
|---|---|---|---|---|---|---|
| Comparative Reports of Pediatric and Adult Regimen Outcomes in AYAs | ||||||
| Stock et al,2 2008 | Pediatric Children’s Cancer Group | 16–20 | 197 | 7 | EFS 63 | 67 |
| Adult Cancer and Leukemia Group B | 16–20 | 124 | 7 | EFS 34 | 46 | |
| Boissel et al,8 2003 | Pediatric French Acute Lymphoblastic Leukemia Study Group-93 | 15–20 | 225 | 6 | DFS 68 | 78 |
| Adult Leucémie Aiguë Lymphoblastique de l’Adulte-94 | 15–20 | 712 | 6 | DFS 32 | 45 | |
| Haïat et al,9 2007 | Pediatric French Acute Lymphoblastic Leukemia Study Group-07 | 16–57 | 28 | 5 | DFS 91 | 85 |
| Adult European Organisation for Research and Treatment of Cancer ALL-4 | 16–57 | 20 | 5 | DFS 47 | 52 | |
| de Bont et al,10 2005 | Pediatric Dutch Childhood Oncology Group | 15–20 | 47 | 5 | EFS 69 | 79 |
| Adult Dutch Foundation for Adult Haemato-Oncology | 15–20 | 73 | 5 | EFS 34 | 38 | |
| Hallböök,11 2006 | Pediatric Nordic Society of Pediatric Haematology Oncology-92 | 15–18 | 36 | 10 | EFS 74 | NA |
| Adult Swedish Adult Leukemia Group | 15–20 | 23 | 10 | EFS 39 | NA | |
| Ramanujachar et al,12 2007 | Pediatric ALL-97 | 15–17 | 61 | 5 | EFS 65 | 71 |
| Adult United Kingdom Acute Lymphoblastic Leukemia XII | 15–17 | 67 | 5 | EFS 49 | 56 | |
| Alves et al,13 2008 | Pediatric Berlin-Frankfurt-Münster 90, 95 | 10–20 | 34 | 10 | EFS 69 | 69 |
| Adult Berlin-Frankfurt-Münster 84 | 10–20 | 11 | 10 | EFS 22 | 31 | |
| Usvasalo et al,14 2008 | Pediatric Nordic Society of Pediatric Haematology Oncology | 10–18 | 128 | 5 | EFS 60 | 76 |
| Adult (pediatric regimen) | 15–26 | 97 | 5 | EFS 57 | 68 | |
| Schroeder et al,15 2005 | Pediatric Nordic Society of Pediatric Haematology Oncology | 10–15 | 61 | 5 | EFS 60 | 67 |
| Adult Cancer and Leukemia Group B | 15–19 | 38 | 5 | EFS 38 | 47 | |
| Testi et al,16 2004 | Pediatric Italian Association of Pediatric Hematology and Oncology ALL-95 2000 | 14–18 | 150 | 2 | NA | 80 |
| Adult Gruppo Italiano Malattie Ematologiche dell’Adulto ALL-0496, 2000 | 14–18 | 95 | 2 | NA | 71 | |
| Hayakawa et al,17 2014 | Pediatric Japan Adult Leukemia Study Group ALL-202-U | 16–24 | 130 | 5 | DFS 67 | 73 |
| Adult Japan Adult Leukemia Study Group ALL-97 | 16–24 | 81 | 5 | DFS 44 | 45 | |
| López-Hernández et al,18 2008 | Pediatric LALIN | 15–25 | 20 | 2–3 | EFS 70 | ≈80 |
| Adult Leucémie Aiguë Lymphoblastique de l’Adulte | 15–25 | 20 | 2–3 | EFS 40 | ≈65 | |
| Ruiz-Delgado,19 2011 | Pediatric (simplified) Puebla | 18–86 | 80 | 12 | DFS 35 | 27 |
| Adult Mexico City hyper-CVAD | 18–86 | 36 | 5 | NA | 10 | |
| Alacacioglu et al,20 2014 | Pediatric Berlin-Frankfurt-Münster | 25a | 20 | 5 | NA | 59 |
| Adult hyper-CVAD | 31a | 30 | 5 | NA | 34 | |
| El-Cheikh et al,21 2017 | Pediatric Berlin-Frankfurt-Münster | 16–51 | 38 | 3 | DFS 77 | 76 |
| Adult hyper-CVAD±R | 21–73 | 24 | 3 | DFS 72 | 54 | |
| Rytting et al,23 2016 | Pediatric augmented Berlin-Frankfurt-Münster23 | 13–39 | 106 | 5 | CCR 53 | 60 |
| Adult hyper-CVAD±R23 | 15–40 | 102 | 5 | CCR 55 | 60 | |
| Douer et al,22 2014 | Adult hyper-CVAD22 | 16–29 | 64 | 5 | CCR 38 | 54 |
| Thomas et al,24 2009 | Adult hyper-CVAD±R24 | 13–21 | 83 | 3 | CCR 68 | 75 |
| Adult hyper-CVAD±R24 | 22–30 | 93 | 3 | CCR 60 | 66 | |
| Noncomparative Reports of Pediatric Regimen in AYAs | ||||||
| Nachman et al,25 2009 | Children’s Cancer Group-1961 | 16–21 | 262 | 5 | EFS 72 | 78 |
| De Angelo et al,26 2015 | Dana-Farber Cancer Institute 01-17526 | 18–60 | 92 | 4 | DFS 69 | 67 |
| De Angelo et al,27 2015 | Dana-Farber Cancer Institute 06-25427 | 18–50 | 110 | 3 | DFS 73 | 75 |
| Stock et al,28 2014 | C10403 | 16–30 | 318 | 2 | EFS 66 | 79 |
| Huguet et al,29 2009 | Group for Research in Adult Acute Lymphoblastic Leukemia (GRAALL) 200329 | 15–60 | 225 | 4 | EFS 55 | 58 |
| Haïat et al,30 2011 | Adult ALL group30 | 18–39 | 40 | 3 | DFS 67 | 86 |
| Huguet et al,31 2016 | Group for Research in Adult Acute Lymphoblastic Leukemia (GRAALL) 200531 | 18–34 | 372 | 4 | EFS 61 | NA |
| Ribera et al,32 2008 | Gruppo Italiano Malattie Ematologiche dell’Adulto ALL-96 | 14–18 | 35 | 6 | EFS 60 | 77 |
| 19–30 | 46 | 6 | EFS 63 | 63 | ||
| Ramanujachar et al,33 2006 | Medical Research Council/United Kingdom Acute Lymphoblastic Leukemia Xab | 15–19 | 200 | 5 | DFS 35 | 60 |
Abbreviations: ALL, acute lymphoblastic leukemia; AYA, adolescents and young adults; CCR, continuous complete remission; DFS, disease-free survival; EFS, event-free survival; hyper-CVAD, hyper–cyclophosphamide, vincristine, doxorubicin, and dexamethasone; LBL, lymphoblastic lymphoma; NA, not available; ±R, with or without rituximab.
Median age
Pediatric-like.
There are only 2 exceptions. The first exception is no reported difference in a comparison from The University of Texas MD Anderson Cancer Center24 that, other than a report from Mexico,18 is the only single-institution comparative study in the Table. In that comparison,24 the adult regimen (hyper–cyclophosphamide, vincristine, doxorubicin, and dexamethasone [hyper-CVAD]) included 6 patients who underwent allogeneic stem cell transplant after achieving remission, 5 of whom were alive at the time of analysis. For reasons not provided, 11 patients receiving the pediatric regimen also underwent stem cell transplant in first remission, 4 of whom died of transplant complications. Without censoring of the patients who received transplants, there was no significant difference in the continuous complete remission rate or overall survival. The greater number of deaths after transplant among the patients receiving the pediatric regimen was not addressed. The pediatric regimen also had a higher central nervous system (CNS) relapse rate (8.5% isolated and 14.2% isolated and concurrent with marrow relapse) than reported by others using a similar regimen.25–27,34,35 Also, the strong effect of asparaginase on CNS leukemia in all pediatric regimens is missing in hyper-CVAD.
The second exception is a comparison in Finland14 that had a similar event-free survival (EFS) for their pediatric and adult regimens but a better overall survival for their pediatric regimens. However, both the pediatric and adult regimens contained asparaginase, with the mean total dose of asparaginase actually higher in the adult regimens than in the pediatric regimens (50 000 vs 40 000 IU/m2).14
A meta-analysis36 of 11 of the above-cited reports of pediatric vs adult regimen comparisons,2,8–14,16,18,29 comprising 2489 patients, concluded that the pediatric regimens have statistically significant superior rates of complete remission and relapse-free, event-free, and overall survival rates. The relative risk of nonrelapse mortality was comparable.36
In the United States, the C10403 study28 was a national, Intergroup phase 2 trial of a pediatric regimen in 318 adults aged 17 to 39 years with either T-cell or B-precursor Ph chromosome–negative ALL, of whom 296 are fully evaluable. At a median follow-up of 28 months for surviving patients, the EFS was more than double that of the prior experience. The EFS of 59 months had a lower 95% CI of 38 months, which allowed rejection of the trial’s basic null hypothesis that, based on the prior Intergroup experience, the median EFS would have been at most 32 months.
As a result, pediatric regimens are increasingly being used to treat adults with ALL (Table).37–39 The largest pediatric regimen–based experience to date is of 1529 patients aged 15 to 35 years treated by the German Multicenter Group for Adult ALL.40 Their 5-year overall survival rates were 73%, 69%, and 60% for patients aged 15 to 17 years, 18 to 25 years, and 26 to 35 years, respectively. In Canada,41 there was a significant increase in survival during 1986 to 2009 among the patients with ALL aged 20 to 29 years, which was primarily attributed to pediatric regimens, in contrast to the same age group in the United States treated with adult regimens, in whom no increase occurred. In 51 adolescents aged 15 to 18 years, the Dana-Farber Cancer Institute (DFCI) Acute Lymphoblastic Leukemia Consortium42 reported a 5-year 78% EFS with a pediatric regimen, leading to the consortium’s adoption of this regimen for patients aged 18 to 50 years.
A 2013 meta-analysis43 concluded that in AYAs with ALL allogeneic hematopoietic stem cell transplant (HSCT) in first remission was superior to chemotherapy regimens without HSCT. However, the chemotherapy comparators in that report were limited to “traditional adult-intensity chemotherapy regimens,” for which results were published 20 to 30 years ago and not to current pediatric-inspired regimens.44 As concluded in a follow-up correspondence, “the more appropriate conclusion to be drawn is the importance of using more effective, conventional, pediatric-inspired ALL treatment regimens in the adolescent and young adult population”44(p5254) rather than the “regimens historically used for adults.”
In summary, all but 2 of 25 comparisons of outcomes with pediatric and adult regimens for ALL and LBL in AYAs and 1 meta-analysis favor the pediatric regimen. Why then, hasn’t the pediatric regimen been adopted more widely in the US?
Challenges of the Pediatric Regimen
Multiphasic Complexity and Intricacy
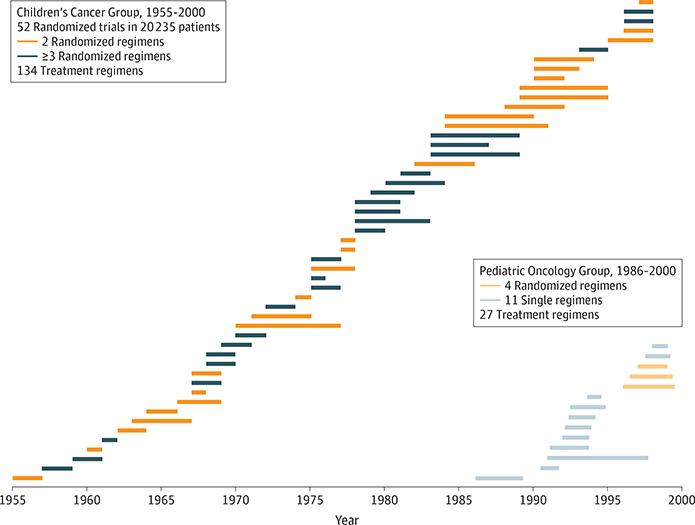
Considering the strong data on outcome, treatment-related mortality, and toxicity in general favoring pediatric-inspired regimens for AYAs, why have they not been more widely adopted in the medical oncology setting? Figure 3 shows the history of the pediatric regimen from the perspective of the national randomized clinical trials conducted in North American children with newly diagnosed ALL. In the United States, at least 160 regimens for ALL were evaluated during the last half-century in phase 3 trials conducted by the Children’s Cancer Group and the Pediatric Oncology Group. Since 2000, the Children’s Oncology Group has conducted 10 randomized controlled trials in patients newly diagnosed as having ALL. Not shown are regimens studied by the St Jude Children’s Research Hospital, the Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium, and in Europe by the following cooperative pediatric groups: International Berlin-Frankfurt-Münster (IBFM), United Kingdom Acute Lymphoblastic Leukemia (UKALL), French Acute Lymphoblastic Leukemia Study Group (FRALLE), Italian Association of Pediatric Hematology and Oncology (AEIOP), and Programa para el Tratamiento de Hemopatias Malignas Spanish Cooperative Group (PETHEMA). In contrast, less than 10 randomized controlled trials have been conducted to date in adult patients. Therefore, contemporary pediatric regimens have evolved into more complex, intricate, multiphasic, risk-based regimens. In contrast, adult treatment regimens have remained simple and easier to administer, with minor incorporation of risk or biological factors.
Figure 3. Children’s Cancer Group and Pediatric Oncology Group Phase 3 Randomized Trials in Adolescents and Young Adults With Acute Lymphoblastic Leukemia.
Each horizontal bar represents the patient accrual interval.
An element of particular importance on pediatric regimens is the phase of delayed intensification that was pioneered by the Berlin-Frankfurt-Münster Cooperative Group.45 It applies the Norton-Simon principle of cancer therapy46 by re-treating the patient with induction and consolidation therapy again (reinduction/reconsolidation) after an interim phase that allows recovery from the initial therapy. Delayed intensification was confirmed in a large phase 3 randomized trial to be a critical component of ALL therapy,47 substantiated in other trials,48,49 and found applicable to AYA patients with ALL,25 including the C10403 trial. Other than the intensive therapy enabled by HSCT after remission induction, no adult regimen to date has incorporated a delayed-intensification phase at a similar time after diagnosis.
Outpatient Management
The pediatric regimens were also designed to be delivered in the outpatient setting, allowing children and adolescents to be at home with their families as much as possible. This patient-centered strategy requires a robust clinic infrastructure to support the care of outpatients who require frequent interaction with the medical system. With the exception of a recent finding supporting the use of high-dose intravenous methotrexate in children with high-risk B-precursor ALL during an interim phase of treatment,34 none of the pediatric regimens require hospitalization after the initial admission for newly diagnosed cancer, staging, and initiation of therapy.28,37
Asparaginase
Asparaginase contributes more to the overall chemotherapy regimen benefit than its numerical value of “one in so many drugs” in combination chemotherapy regimens.22 For the pioneering prospective randomized trial of asparaginase in children with ALL, the asparaginase-containing regimen had a 10-year to 20-year overall survival rate that was 34% higher with asparaginase, despite it being the only difference in the regimen of 8 antileukemia drugs.50 A Pediatric Oncology Group study51 that also randomized asparaginase had an 8-year overall survival that was 53% greater with asparaginase compared with the control 9-drug regimen. Some in vitro experiments suggest that lymphoblasts from adult patients may be more resistant to asparaginase than those obtained from pediatric patients.52 No significant differences were observed between B-precursor and T-cell lymphoblasts.53
Asparaginase causes more hepatic dysfunction, pancreatitis, and coagulopathies in AYAs than in younger patients.23,27,53 In most cases, hyperbilirubinemia occurs with the first dose and not subsequent doses.54 A lower dose and longer intervals between doses of asparaginase prevent drug-limiting hyperbilirubinemia.27 Several review articles have addressed this challenge and offer practical guidelines for prevention and management of asparaginase toxicities in AYAs.53,55–58
In adult ALL, a lesser experience has nonetheless suggested significant benefit from asparaginase. In a Cancer and Leukemia Group B trial,59 the 22 patients who had less asparagine depletion had a lower overall survival (hazard ratio [HR], 2.37; 95% CI, 1.38–4.09; P = .002) and disease-free survival (HR, 2.21; 95% CI, 1.19–4.13; P = .01) than 63 patients who did achieve asparagine depletion. In a multi-institutional study60 of 95 adult patients with T-cell ALL or T-cell LBL with a median age at diagnosis of 32 years (age range, 17–75 years), those who received asparaginase had statistically improved relapse-free survival (HR, 2.65; P = .01) and overall survival (HR, 2.30; P = .02), differences that remained statistically significant after adjusting for covariates of age, sex, and white blood cell count at diagnosis. Overall survival was greater in asparaginase-treated patients younger than 40 years (HR, 3.4; 95% CI, 1.2–9.5) than in older adults. In another multi-institutional study,61 adults with early T-cell precursor ALL had a statistically significant better progression-free survival and overall survival if they received asparaginase. With regard to progression-free survival, only the inclusion of asparaginase with induction was associated with outcome, while all other covariates failed to show any significance, including cytogenetics status, histology, marrow or peripheral blast burden, chemotherapy choice, or allogeneic transplant in complete remission or at any time.61
Therefore, the benefit to toxicity ratio of asparaginase in AYAs with ALL or LBL is favorable. Learning how to prevent and manage its toxicity is a distinct challenge for oncologists who are not familiar with it. As experienced nationwide on the C10403 trial, in Europe by many of the adult-treating groups in the Table, and particularly by the Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium in the United States and Canada that uses prolonged intensive asparaginase,27 adult-treating oncologists have successfully managed asparaginase therapy in their patients.
Hematopoietic Stem Cell Transplant
With the notable exception of Ph chromosome–positive ALL, pediatric regimens have not required allogeneic HSCT.44 In contrast, many adult patients with ALL treated on an adult regimen receive HSCT during initial remission if they have a matched, available donor. Being able to avoid the toxicities, late adverse effects, and financial cost of HSCT substantially favors the pediatric regimen. Another factor favoring the pediatric regimen is that young AYA recipients are more susceptible to allogeneic HSCT-induced acute graft-vs-host disease than either younger or older patients.62
Collaboration
The challenge of the pediatric regimen lies in becoming knowledgeable and comfortable with its complexity. Adult-treating oncologists benefit from the collaboration with and support of pediatric oncologists and their staff in applying a pediatric regimen, as well as from organizational modifications of their ambulatory clinics to support effective and manageable delivery of the pediatric regimen.63 That the collaboration is critical is evidenced by the comparison of the mortality rate of pediatric and young AYA patients with that of patients having ALL at Children’s Oncology Group (COG) vs non-COG institutions.64 The mean death rates in the non-COG centers were clearly worse than those at COG institutions, with almost twice the death rate within 1 year after diagnosis and increasingly worse from 5 to 9 years after diagnosis. The AYAs treated at specialty or NCI-designated cancer centers likely have improved outcomes due to the familiarity of these centers with ALL management in this age group.
US ALL Treatment Trial Accruals—The Accrual Cliff
For NCI-supported clinical trials since 2000, Figure 4 shows the estimated accrual proportion of patients with ALL participating in clinical trials (blue curves) and its associated “accrual cliff” between ages 15 and 30 years. Since 2010, the accrual cliff has shifted upward in AYAs younger than 30 years (upward arrows), in contrast to a decreased proportion in older patients. The trend in AYA patients is a notable accomplishment for the age group that historically has had less than 10% of those diagnosed as having cancer referred to or initially seen at academic medical centers and the lowest referral rate of all ages up to 70 years.65
Figure 4. Estimated Accrual Proportion From 2000 to 2009 and 2010 to 2015 Onto National Cancer Institute–Sponsored National Treatment Acute Lymphoblastic Leukemia Trials.
AYAs indicates adolescents and young adults. Data by single year of patient age are from the National Cancer Institute Cancer Therapy Evaluation Program. The accrual proportion curves are 2-year running age means. The arrows signify trend changes from 2000–2009 to 2010–2015.
The NCI Community Clinical Oncology Program66 did not contribute to the improvement, with their AYA accruals decreasing during 2009 to 2013. The successor NCI Community Oncology Research Program67 is expected to reverse the trend. In the greater San Francisco Bay area of California, no adult patients treated before 2008 by adult-treating oncologists received a pediatric regimen.68 Between 2008 and 2012, while the C10403 protocol was open to accrual, 31% of AYA patients in the San Francisco Bay area treated by adult oncologists received pediatric regimens.68 Meanwhile, the national accrual cliff in those aged 17 to 21 years is just as steep since 2010 as it was during the prior decade (Figure 4).
The age-related survival cliff and accrual cliff, as well as a “referral cliff,” coincide (Figures 2 and 4). This overlap suggests a strong cause-effect relationship, with the lack of clinical trial activity likely representing a primary factor for the survival deficit.67 Strategies to improve clinical trial participation by AYAs with cancer include the following: increasing availability of clinical trials specifically designed for them, reducing clinical trial regulatory requirements, centralizing all national cancer clinical trial accruals and data management, optimizing the efficacy of central institutional review boards having reduced local review board management, liberalizing clinical trial eligibility criteria, using social media to inform patients with cancer and their families, increasing health insurance coverage of clinical trial expenses, and providing funds to offset patient travel expenses and meals and additional staff time for minority recruitment.69,70
National Comprehensive Cancer Network Guidelines
Since 2012, the National Comprehensive Cancer Network (NCCN) has recommended either a clinical trial or pediatric-inspired regimen for newly diagnosed Ph chromosome-negative ALL in AYAs.71 The clinical trial recommendation for AYAs was based in part on the likelihood that a clinical trial would be based on pediatric therapy.71
In 2016, the NCCN added hyper-CVAD plus rituximab to its AYA ALL guidelines but specified that it was for CD20-positive ALL only and that the pediatric regimens for all forms of Ph chromosome-negative ALL were “preferred.”72 In 2017, the guidelines expanded hyper-CVAD to all Ph chromosome-negative AYAs and added a pediatric-inspired University of Southern California regimen, with the specification that both were based on data from single institutions as opposed to the pediatric regimens that were based on data from multi-institutional or cooperative group studies.73
Where Should an AYA With ALL Be Treated?
Optimally, for the reasons stated herein and as recommended up front by the NCCN, AYAs with ALL should be referred to a center with an available clinical trial. As described in the Challenges of the Pediatric Regimen section, the challenges faced by adult-treating oncologists in transitioning to a pediatric regimen require pediatric oncologists and their staffs and the cooperative groups to educate, train, and provide close support to their medical oncology colleagues. Ideally, an AYA patient with ALL should be comanaged by the pediatric and adult services and, in certain circumstances, be transferred to a pediatric or AYA oncology service. Ultimately, an AYA oncology discipline with specific training, including fellowship programs, may provide a sufficient number of AYA oncologists to optimize management of a complex pediatric regimen.
Conclusions and Recommendations
The progress in treating AYAs with ALL and LBL is due to multiple factors. These include the following: the change that has occurred with recognition of this patient population, the knowledge and application of biological underpinnings of AYA ALL and LBL, the collaboration between the cooperative groups in the NCTN, and the development of protocols to address important treatment issues and subgroups. The survival cliff and accrual cliff and other data presented herein provide the rationale to treat AYAs with newly diagnosed ALL on either a pediatric-inspired regimen or an approved national clinical trial designed for this patient group, such as the Alliance A041501 trial.1 If not available in the AYA’s community, referral to a specialized center with access to these trials should be arranged.73 For the survival of AYAs with ALL and LBL to continue to improve, clinical trial development and accrual for this age group will need continued improvement.74 The new trials for Ph-like ALL, such as the AALL1131 trial,75 are particularly promising because this form of ALL predominates in AYAs.
Not included in this narrative review is a description of the better quality of life during and after therapy on the pediatric regimen than on the hyper-CVAD regimen, as indicated by hospitalization time, readmission for treatment complications, and late adverse effects, such as infertility and second malignant neoplasms. This quality-of-life advantage will be the subject of another review article.
Supplementary Material
Acknowledgments
Conflict of Interest Disclosures: Dr Stock reported consulting for Pfizer, Amgen, and Sigma-Tau Pharmaceuticals. Dr Johnson reported serving as a consultant to and on the speaker bureau for Shire Pharmaceuticals and Jazz Pharmaceuticals, which produce asparaginase products. Dr Advani reported consulting for and receiving honoraria from Enzon, Sigma-Tau Pharmaceuticals, and Pfizer. Dr Muffly reported having research support from Shire Pharmaceuticals. Dr Douer reported having research support from and serving as a consultant to Shire Pharmaceuticals. Dr Shah reported having research support from Incyte, Rosetta Genomics, and the DeBartolo Family Personalized Medicine Institute and reported receiving consulting reimbursement from Amgen, Baxalta, Bayer, Celgene, clonoSEQ, Jazz Pharmaceuticals, and Pfizer. Dr Luger reported consulting for Pfizer and Sigma-Tau Pharmaceuticals. Dr Hayes-Lattin reported serving on a speaker bureau for Sigma-Tau Pharmaceuticals. Dr DeAngelo reported receiving funding from Sigma-Tau Pharmaceuticals. Dr Bleyer reported serving as a consultant to Enzon and Sigma-Tau Pharmaceuticals regarding an asparaginase product. No other disclosures were reported.
Footnotes
Additional Contributions: Data in Figure 4 were provided by Shanda Finnegan, MPH, RN, CCRC, Michael Montello, PharmD, MBA, Steven Friedman, MHSA, and Nita L. Seibel, MD, of the National Cancer Institute Cancer Therapy Evaluation Program. None received compensation.
Contributor Information
Stuart E. Siegel, Critical Mass Young Adult Cancer Alliance.
Wendy Stock, Alliance for Clinical Trials in Oncology (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Section of Hematology/Oncology, University of Chicago Comprehensive Cancer Center, Chicago, Illinois.
Rebecca H. Johnson, SWOG (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Children’s Oncology Group (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); National Clinical Oncology Research Program (All in the National Cancer Institute National Clinical Trials Network); Pediatric Hematology/Oncology, Mary Bridge Children’s Hospital and Health Center and Tacoma General Hospital, Tacoma, Washington.
Anjali Advani, SWOG (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Hematology/Oncology, Cleveland Clinic Foundation, Cleveland, Ohio.
Lori Muffly, SWOG (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Blood and Marrow Transplantation, Department of Medicine, Stanford University, Palo Alto, California.
Dan Douer, ECOG-ACRIN Cancer Research Group (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Keck Medicine, University of Southern California Norris Comprehensive Cancer Center, Los Angeles.
Damon Reed, National Pediatric Cancer Foundation, Tampa, Florida; Moffitt Cancer Center, Tampa, Florida.
Mark Lewis, SWOG (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Hematology/ Oncology, Intermountain Healthcare, Salt Lake City, Utah.
David R. Freyer, Children’s Oncology Group (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Keck Medicine, University of Southern California Norris Comprehensive Cancer Center, Los Angeles.
Bijal Shah, SWOG (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Moffitt Cancer Center, Tampa, Florida; National Comprehensive Cancer Network.
Selina Luger, ECOG-ACRIN Cancer Research Group (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Brandon Hayes-Lattin, SWOG (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Department of Radiation Medicine, Oregon Health and Science University, Portland.
Jerry J. Jaboin, Department of Radiation Medicine, Oregon Health and Science University, Portland; NRG Oncology (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group), National Cancer Institute, Bethesda, Maryland.
Peter F. Coccia, Children’s Oncology Group (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); National Comprehensive Cancer Network; Department of Pediatrics, University of Nebraska Medical Center, Omaha.
Daniel J. DeAngelo, Alliance for Clinical Trials in Oncology (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts; Dana-Farber Cancer Institute, Boston, Massachusetts.
Nita Seibel, SWOG (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, Maryland.
Archie Bleyer, SWOG (National Cancer Institute–Sponsored National Clinical Trials Network Cooperative Group); Department of Radiation Medicine, Oregon Health and Science University, Portland.
REFERENCES
- 1.Alliance for Clinical Trials in Oncology. A Phase 3 Trial to Evaluate the Efficacy of the Addition of Inotuzumab Ozogamicin to Frontline Therapy in Young Adults (Ages 18 to 39 Years) With Newly Diagnosed Precursor B-cell ALL. Alliance A041501 https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=/Public/News-ActivatedTrials-June2017. Accessed September 5, 2017. [Google Scholar]
- 2.Stock W, La M, Sanford B, et al. ; Children’s Cancer Group; Cancer and Leukemia Group B Studies. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? a comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5): 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute. SEER*Stat Database: Incidence SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (2000–2014) <Katrina/Rita Population Adjustment> Linked To County Attributes Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission. [Google Scholar]
- 4.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. [published correction appears in Stat Med. 2001;20(4):655]. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 5.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371 (11):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold T, Baldus CD, Gökbuget N. Ph-like acute lymphoblastic leukemia in older adults. N Engl J Med. 2014;371(23):2235. [DOI] [PubMed] [Google Scholar]
- 7.Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymphoma. 2011;52(12):2237–2253. [DOI] [PubMed] [Google Scholar]
- 8.Boissel N, Auclerc MF, Lhéritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21(5):774–780. [DOI] [PubMed] [Google Scholar]
- 9.Haïat S, Vekhoff A, Marzac C, et al. Improved outcome of adult acute lymphoblastic leukemia treated with a pediatric protocol: results of a pilot study. Blood. 2007:110 Abstract 2822. [Google Scholar]
- 10.de Bont JM, van der Holt B, Dekker AW, van der Does-van den Berg A, Sonneveld P, Pieters R. [Adolescents with acute lymphatic leukaemia achieve significantly better results when treated following Dutch paediatric oncology protocols than with adult protocols] [in Dutch]. Ned Tijdschr Geneeskd. 2005;149(8):400–406. [PubMed] [Google Scholar]
- 11.Hallböök H, Gustafsson G, Smedmyr B, Söderhäll S, Heyman M; Swedish Adult Acute Lymphocytic Leukemia Group; Swedish Childhood Leukemia Group. Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer. 2006;107(7):1551–1561. [DOI] [PubMed] [Google Scholar]
- 12.Ramanujachar R, Richards S, Hann I, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48(3):254–261. [DOI] [PubMed] [Google Scholar]
- 13.Alves M, Daudt L, Mazzucco KLM, et al. Is it better to treat adolescents with acute lymphoblastic leukemia as old children or as young adults? Am Soc Hematol Annu Meeting Abstracts. 2008;112:3968 Abstract 3968. [Google Scholar]
- 14.Usvasalo A, Räty R, Knuutila S, et al. Acute lymphoblastic leukemia in adolescents and young adults in Finland. Haematologica. 2008;93(8):1161–1168. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder H, Kjeldahl M, Boesen AM, et al. Prognosis of ALL in 10–20 year old patients in Denmark 1992–2001. In: Supplement: 37th Annual Conference of the International Society of Paediatric Oncology SIOP 2005; September 21–24, 2005; Vancouver, Canada. Pediatr Blood Cancer. 2005;45(4):578 Abstract P.S.001. [Google Scholar]
- 16.Testi AM, Valsecchi MG, Conter V, et al. Difference in outcome of adolescents with acute lymphoblastic leukemia (ALL) enrolled in pediatric (AIEOP) and adult (GIMEMA) protocols. Blood. 2004;104:1954 Abstract. [Google Scholar]
- 17.Hayakawa F, Sakura T, Yujiri T, et al. ; Japan Adult Leukemia Study Group (JALSG). Markedly improved outcomes and acceptable toxicity in adolescents and young adults with acute lymphoblastic leukemia following treatment with a pediatric protocol: a phase II study by the Japan Adult Leukemia Study Group. Blood Cancer J. 2014; 4(10):e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Hernández MA, Alvarado-Ibarra M, Jiménez-Alvarado RM, De Diego-Flores JE, González-Avante CM. Adolescents with de novo acute lymphoblastic leukemia: efficacy and safety of a pediatric vs adult treatment protocol [in Spanish]. Gac Med Mex. 2008;144(6):485–489. [PubMed] [Google Scholar]
- 19.Ruiz-Delgado GJ, Macías-Gallardo J, Lutz-Presno JA, Montes-Montiel M, Ruiz-Argüelles GJ. Outcome of adults with acute lymphoblastic leukemia treated with a pediatric-inspired therapy: a single institution experience. Leuk Lymphoma. 2011;52(2):314–316. [DOI] [PubMed] [Google Scholar]
- 20.Alacacioglu I, Medeni SS, Ozsan GH, et al. Is the BFM regimen feasible for the treatment of adult acute lymphoblastic leukemia? a retrospective analysis of the outcomes of BFM and hyper-CVAD chemotherapy in two centers. Chemotherapy. 2014;60(4):219–223. [DOI] [PubMed] [Google Scholar]
- 21.El-Cheikh J, El Dika I, Massoud R, et al. Hyper-CVAD compared with BFM-like chemotherapy for the treatment of adult acute lymphoblastic leukemia: a retrospective single-center analysis. Clin Lymphoma Myeloma Leuk. 2017;17(3):179–185. [DOI] [PubMed] [Google Scholar]
- 22.Douer D, DeAngelo DJ, Advani A, et al. Applying pediatric therapeutic strategies to adults with acute lymphoblastic leukemia and lymphoma, II: comparison with adult treatment regimens, including hyper-CVAD. Am Oncol Hematol Rev. 2014;2014:47–53. [Google Scholar]
- 23.Rytting ME, Jabbour EJ, Jorgensen JL, et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Münster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91(8):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas DA, Rytting ME, O’Brien SM, et al. Outcome for adolescents and young adults with the hyper-CVAD (with or without rituximab) regimens for de novo acute lymphoblastic leukemia or lymphoblastic lymphoma. Blood. 2009;114:2037 Abstract 3084.19567878 [Google Scholar]
- 25.Nachman JB, La MK, Hunger SP, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children’s oncology group. J Clin Oncol. 2009;27(31):5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29(3):526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeAngelo DJ, Stevenson K, Neuberg DS, et al. A multicenter phase II study using a dose intensified pegylated-asparaginase pediatric regimen in adults with untreated acute lymphoblastic leukemia: a DFCI ALL Consortium trial. Blood. 2015;126(23):80 Abstract 80.25838348 [Google Scholar]
- 28.Stock W, Luger SM, Advani AS, et al. Favorable outcomes for older adolescents and young adults with acute lymphoblastic leukemia: early results of U.S. Intergroup trial C10403. Blood. 2014;124(21):796 Abstract 796. [Google Scholar]
- 29.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–918. [DOI] [PubMed] [Google Scholar]
- 30.Haïat S, Marjanovic Z, Lapusan S, et al. Outcome of 40 adults aged from 18 to 55 years with acute lymphoblastic leukemia treated with double-delayed intensification pediatric protocol. Leuk Res. 2011;35(1):66–72. [DOI] [PubMed] [Google Scholar]
- 31.Huguet F, Leguay T, Thomas X, et al. The upper age limit for a pediatric-inspired therapy in younger adults with Ph-negative acute lymphoblastic leukemia? analysis of the GRAALL-2005 study. Blood. 2016;128(12):762 Abstract 762. [Google Scholar]
- 32.Ribera JM, Oriol A, Sanz MA, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26(11):1843–1849. [DOI] [PubMed] [Google Scholar]
- 33.Ramanujachar R, Richards S, Hann I, Webb D. Adolescents with acute lymphoblastic leukaemia: emerging from the shadow of paediatric and adult treatment protocols. Pediatr Blood Cancer. 2006; 47(6):748–756. [DOI] [PubMed] [Google Scholar]
- 34.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group study AALL0232. J Clin Oncol. 2016;34(20):2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang K, Chen X, Wuxiao Z, et al. Long-term outcomes of modified Berlin-Frankfurt-Münster-90 regimen in adults with T-lymphoblastic lymphoma: a single-center experience. Leuk Lymphoma. 2014;55(8):1800–1805. [DOI] [PubMed] [Google Scholar]
- 36.Ram R, Wolach O, Vidal L, Gafter-Gvili A, Shpilberg O, Raanani P. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am J Hematol. 2012;87(5):472–478. [DOI] [PubMed] [Google Scholar]
- 37.Muffly L, Petit K, Stock W. Treating the younger adult with acute lymphoblastic leukemia. Clin Pract. 2012;9(4):439–449. http://www.openaccessjournals.com/articles/treating-the-younger-adult-with-acute-lymphoblastic-leukemia.pdf. Accessed December 21, 2017. [Google Scholar]
- 38.Douer D, Aldoss I, Lunning MA, et al. Pharmacokinetics-based integration of multiple doses of intravenous pegaspargase in a pediatric regimen for adults with newly diagnosed acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9): 905–911. [DOI] [PubMed] [Google Scholar]
- 39.Advani AS, Sanford B, Luger S, et al. Frontline-treatment of acute lymphoblastic leukemia in older adolescents and young adults using a pediatric regimen is feasible: toxicity results of the prospective US Intergroup trial C10403 (Alliance). Blood. 2013;122(121). Abstract 3903. [Google Scholar]
- 40.Gökbuget N, Beck J, Brandt K, et al. Significant improvement of outcome in adolescents and young adults (AYAs) aged 15–35 years with acute lymphoblastic leukemia (ALL) with a pediatric derived adult ALL protocol: results of 1529 AYAs in 2 consecutive trials of the German Multicenter Study Group for Adult ALL (GMALL). Blood. 2013; 122(121). Abstract 839. [Google Scholar]
- 41.Pole JD, Darmawikarta D, Alibhai SM, Brandwein JM, Sung L. Differential survival improvement for patients 20–29 years of age with acute lymphoblastic leukemia. Leuk Res. 2013;37 (10):1258–1264. [DOI] [PubMed] [Google Scholar]
- 42.Barry E, DeAngelo DJ, Neuberg D, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25(7):813–819. [DOI] [PubMed] [Google Scholar]
- 43.Gupta V, Richards S, Rowe J; Acute Leukemia Stem Cell Transplantation Trialists’ Collaborative Group. Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood. 2013; 121(2):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isakoff MS, Freyer DR, Bleyer A. Young adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen do not need a bone marrow transplant in first remission. Blood. 2013;121 (26):5253–5255. [DOI] [PubMed] [Google Scholar]
- 45.Henze G, Langermann HJ, Fengler R, et al. Acute lymphoblastic leukemia therapy study BFM 79/81 in children and adolescents: intensified reinduction therapy for patients with different risk for relapse [in German]. Klin Padiatr. 1982;194(4): 195–203. [DOI] [PubMed] [Google Scholar]
- 46.Simon R, Norton L. The Norton-Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Clin Pract Oncol. 2006;3(8):406–407. [DOI] [PubMed] [Google Scholar]
- 47.Tubergen DG, Gilchrist GS, O’Brien RT, et al. Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Childrens Cancer Group phase III trial. J Clin Oncol. 1993;11(3): 527–537. [DOI] [PubMed] [Google Scholar]
- 48.Li CK, Chik KW, Ha SY, et al. Improved outcome of acute lymphoblastic leukaemia treated by delayed intensification in Hong Kong children: HKALL97 study. Hong Kong Med J. 2006;12(1):33–39. [PubMed] [Google Scholar]
- 49.Hutchinson RJ, Gaynon PS, Sather H, et al. ; Children’s Cancer Group/Children’s Oncology Group. Intensification of therapy for children with lower-risk acute lymphoblastic leukemia: long-term follow-up of patients treated on Children’s Cancer Group trial 1881. J Clin Oncol. 2003;21(9):1790–1797. [DOI] [PubMed] [Google Scholar]
- 50.Sallan SE, Hitchcock-Bryan S, Gelber R, Cassady JR, Frei E III, Nathan DG. Influence of intensive asparaginase in the treatment of childhood non–T-cell acute lymphoblastic leukemia. Cancer Res. 1983;43(11):5601–5607. [PubMed] [Google Scholar]
- 51.Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13(3):335–342. [DOI] [PubMed] [Google Scholar]
- 52.Styczynski J, Pieters R, Huismans DR, Schuurhuis GJ, Wysocki M, Veerman AJ. In vitro drug resistance profiles of adult versus childhood acute lymphoblastic leukaemia. Br J Haematol. 2000;110(4):813–818. [DOI] [PubMed] [Google Scholar]
- 53.Rizzari C, Putti MC, Colombini A, et al. Rationale for a pediatric-inspired approach in the adolescent and young adult population with acute lymphoblastic leukemia, with a focus on asparaginase treatment. Hematol Rep. 2014;6(3): 5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park JH, Ritchie EK, Rao AV, et al. A pediatric-inspired regimen containing multiple doses of intravenous pegylated asparaginase appears safe and effective in newly diagnosed adult patients with Ph-negative acute lymphoblastic leukemia in adults up to age 60: results of a multi-center phase II clinical trial. Blood. 2016;128: 1629 Abstract 1629. [Google Scholar]
- 55.Stock W, DeAngelo DJ, Douer D, et al. Applying pediatric therapeutic strategies to adults with acute lymphoblastic leukemia and lymphoma, I: role of asparaginase. Am Oncol Hematol Rev. 2014;2014: 38–46. [Google Scholar]
- 56.Kawedia JD, Rytting ME. Asparaginase in acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2014;14(suppl):S14–S17. [DOI] [PubMed] [Google Scholar]
- 57.Douer D, Thomas DA. New developments in acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2014;12(6)(suppl 12):13–22. [PubMed] [Google Scholar]
- 58.Alshiekh-Nasany R, Douer D. L-carnitine for treatment of pegasparaginase-induced hepatotoxicity. Acta Haematol. 2016;135(4):208–210. [DOI] [PubMed] [Google Scholar]
- 59.Wetzler M, Sanford BL, Kurtzberg J, et al. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B study 9511. Blood. 2007;109(10): 4164–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borate U, Hathaway AR, Peker P, et al. Early allogeneic stem cell transplantation and use of asparaginase during induction chemotherapy appear to improve otherwise poor outcomes in adult T-cell acute lymphoblastic leukemia/ lymphoma (T-ALL/T-LBL) patients: a multi-institutional review. Blood. 2015;126:4869 Abstract 4869. [Google Scholar]
- 61.Shah BD, Borate U, Kota VK, et al. Multi-institution review of adult early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL). Blood. 2015;126:3715 Abstract 3715. [Google Scholar]
- 62.Vignon M, Andreoli A, Dhédin N, et al. Graft-versus-host disease in adolescents and young adults (15–24 years old) after allogeneic hematopoietic stem cell transplantation for acute leukemia in first complete remission. J Adolesc Young Adult Oncol. 2017;6(2):299–306. [DOI] [PubMed] [Google Scholar]
- 63.Lukenbill J, Advani AS. The treatment of adolescents and young adults with acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2013;8(2):91–97. [DOI] [PubMed] [Google Scholar]
- 64.Bleyer A, Siegel SE, Coccia PF, Stock W, Seibel NL. Children, adolescents, and young adults with leukemia: the empty half of the glass is growing. J Clin Oncol. 2012;30(32):4037–4038. [DOI] [PubMed] [Google Scholar]
- 65.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107(7) (suppl):1645–1655. [DOI] [PubMed] [Google Scholar]
- 66.Roth ME, O’Mara AM, Seibel NL, et al. Low enrollment of adolescents and young adults onto cancer trials: insights from the Community Clinical Oncology Program. J Oncol Pract. 2016;12(4):e388–e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bleyer A. Adolescents and young adult cancer trial participation: will the National Community Oncology Research Program also fail? and what about the rest of us? J Oncol Pract. 2016;12(5):398–402. [DOI] [PubMed] [Google Scholar]
- 68.Muffly L, Lichtensztajn D, Shiraz P, et al. Adoption of pediatric-inspired acute lymphoblastic leukemia regimens by adult oncologists treating adolescents and young adults: a population-based study. Cancer. 2017;123(1):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albritton KH, Coccia P. Influencing referral of adolescents and young adults with cancer to sites with higher rates of trial enrollment. Pediatrics. 2014;133(suppl 3):S104–S108. [DOI] [PubMed] [Google Scholar]
- 71.Bleyer A How NCCN guidelines can help young adults and older adolescents with cancer and the professionals who care for them. J Natl Compr Canc Netw. 2012;10(9):1065–1071. [DOI] [PubMed] [Google Scholar]
- 72.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): acute lymphoblastic leukemia. Version 1. https://www.nccn.org/. Published 2016. Accessed December 21, 2017.
- 73.NCCN Clinical Practice Guidelines in Oncology. NCCN guidelines: acute lymphoblastic leukemia guidelines. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site Accessed August 1, 2017.
- 74.Aldoss IT, Marcucci G, Pullarkat V. Treatment of acute lymphoblastic leukemia in adults: applying lessons learned in children. Oncology (Williston Park). 2016;30(12):1080–1091. [PubMed] [Google Scholar]
- 75.ClinicalTrials.gov A Phase III Randomized Trial for Newly Diagnosed High Risk B-Lymphoblastic Leukemia (B-ALL) Including a Stratum Evaluating Dasatinib (IND#73789, NSC#732517) in Patients with Ph-like Tyrosine Kinase Inhibitor (TKI) Sensitive Mutations. AALL1131. https://www.clinicaltrials.gov/ct2/show/NCT02883049?term=AALL1131&rank=1. Accessed September 5, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.