ABSTRACT
The Northeast of Brazil has experienced a triple epidemic, with the simultaneous circulation of dengue virus (DENV), chikungunya virus (CHIKV) and Zika virus (ZIKV), which may have contributed to the observed increase across this region of atypical forms of disease and deaths. In view of this fact, non-congenital neurological disorders related to arboviruses were compared with other etiologies, mortality and survival rates of patients admitted to referral neurology hospitals in Pernambuco State, Northeast Brazil, from 2015 to 2018. Blood and cerebrospinal fluid samples were collected and tested using molecular and serological assays. The arbovirus-exposed groups were compared with respect to epidemiological, clinical and neurologic characteristics by using the Pearson’s chi-square test. For the survival analysis, the Kaplan-Meier and Hazard Ratio (HR) tests were used, with a 95% confidence interval (CI). Encephalitis and encephalomyelitis were more frequent in arboviruses, while myelitis predominated in the neurological disorders of other etiologies. Guillain-Barré Syndrome (GBS) was similarly distributed amongst the groups. Exposure to one of the arboviruses caused a six-fold increase in the risk of death (HR: 6.37; CI: 2.91 - 13.9). Amongst the arbovirus-exposed groups, infection (DENV/CHIKV) increased nine times the risk of death (HR: 9.07; CI: 3.67 - 22.4). The survival curve indicates that have been exposed to some arbovirus decreased the likelihood of survival compared to those with other etiologies (Log-Rank: p<0.001). Within this scenario, neurologic manifestations of DENV, CHIKV and ZIKV have the potential to increase mortality and decrease survival, and concomitant infection (DENV/CHIKV) is an aggravating factor in reducing the likelihood of survival when compared to monoinfections.
Keywords: Neuroinvasive arboviruses, Neurologic manifestations, Log rank and survival
INTRODUCTION
Emerging arbovirus infections are widespread worldwide, causing severe systemic and neurological disorders that are mediated by viral or immunological factors1. In French Polynesia and India, reported cases related to Guillain Barré Syndrome (GBS) and other neurological disorders were associated with ZIKV, while CHIKV and DENV are associated with cases of encephalitis and meningoencephalitis with sequelae and deaths2,3. In a Reunion Island cohort, there was a 16.6% encephalitis mortality rate associated to CHIKV, including a proportion of children, varying from 30-45%, who were discharged with persistent sequelae4. In Brazil, there are very few studies that associate neurological disorders with arboviruses mortality5. However, there are a number of cases that describe a high frequency of death6. Within this scenario, Pernambuco State, located in Northeast Brazil, experienced a triple epidemic with confirmed cases of neuro-invasive arboviral diseases caused by DENV, ZIKV in 2015, and afterwards, by CHIKV in 20167. One study with 41 cases in Pernambuco reported an association of encephalitis, GBS and myelitis with DENV8, as well as a cohort of 6 patients diagnosed with ZIKV-related disseminated encephalomyelitis (2) and GBS (4) in which patients had motor dysfunction, low visual acuity and cognitive decline9. Thus, determining the risk factors and the evolution of atypical forms of arboviruses is a major challenge, considering that the differential diagnosis among these three arboviruses has presented difficulties and clinical management has not been well established. In this study, we describe non-congenital neurological disorders related to DENV, CHIKV and ZIKV, analysing the clinical-epidemiological characteristics, death-related risk factors and the survival of hospitalized patients compared to the survival of patients with no arboviruses, at 3 referral neurology hospitals in Pernambuco State, Northeast Brazil.
MATERIALS AND METHODS
A cohort study with prospective and retrospective components was conducted, by monitoring patients seen at three reference hospitals in Pernambuco State, Northeast Brazil, from 2015 to 2018, to assess risk factors, epidemiological, clinical and neurological characteristics until evolution for discharge, sequelae or death. The prospective cohort began in 2016 and was extended until 2018. Patients hospitalized in 2015 were included and assessed retrospectively by analysing medical records and a description of neurological exams. The inclusion criteria were: patients with symptoms compatible with GBS, myelitis, encephalitis, meningoencephalitis or other central or peripheral syndromes with acute onset, presenting in the previous 90 days with three or more of the following symptoms: fever, nausea/vomiting, rash, myalgia, headache, retrorbital pain, petechiae or leukopenia. The exclusion criteria were: children ager under one year and individuals with a history of chronic neurological disease or symptoms probably related to other plausible causes, such as: bacterial infections, vascular disease, trauma, intoxication and metabolic diseases. The study population was selected through the spontaneous patient demand admitted to referral neurology hospitals in Pernambuco State. All patients admitted with clinical suspicion of acute neurological syndrome were included. After analyzing the RT-PCR tests and serological tests on CSF and blood, they were diagnosed with infection by neuroarbovirus or another etiology (comparison group). For the purposes of this study, we considered as suspected/confirmed cases of DENV, CHIKV or ZIKV those that met the definition of case and death according to the protocols of the Brazilian Ministry of Health10,11 and presented serology immunoglobulin M/Enzyme-Linked Immunosorbent Assay (IgM/ELISA) or positive reverse transcriptase polymerase chain reaction (RT-PCR) in serum or cerebrospinal fluid (CSF) samples, while deaths were confirmed by RT-PCR or positive visceral immunohistochemistry, according to laboratory protocol12-15. Neurological disorders were classified based on clinical, laboratory and imaging findings. The definition of a GBS case followed the criteria of the Pan American Health Organization16 and the definition of encephalitis cases followed the criteria suggested by the International Encephalitis Consortium17. The criteria used to define the other neurological disorders were: clinical assessment, report issued by the neurologist of each hospital unit, CSF analysis and neuroimaging tests (computed tomography and magnetic resonance imaging of the skull). Neurological syndromes that could not be defined by the neurologist or presented a specific number of cases were grouped as “others” which included: myositis, radiculopathies and undefined diagnoses. The study was authorized by the Ethics Committee of the Federal University of Pernambuco (UFPE) - CAAE Nº 55508216.0.0000.5208. Epidemiological, clinical, laboratory and neurological data were collected from patients’ medical records and obtained and transcribed to standardized forms by the responsible researcher. The most prevalent comorbidities and neurological manifestations were presented in the descriptive component, which assessed the duration of neurological symptoms and listed the main neurological disorders. In the analytical stage, the arbovirus exposure groups were compared according to epidemiological, clinical and neurologic characteristics by the Pearson’s chi-square test in the comparison of proportions, and the Kruskal-Wallis in the comparison of medians. The Komogorov-Smirnov normality test was applied, in which none of the variables presented a normal distribution. In the survival analysis, Kaplan-Meier’s curves were presented for each exposure to arbovirus, and mortality rates were estimated at 100 person-days, as well as the HR risk measure with a 95% confidence interval. The log-rank test was applied to compare survival functions. The statistical significance adopted was 5% (p <0.05) and p <0.001 for the log rank. The statistical software used in the analysis was Stata (version 14.0, StataCorp, College Station, Texas, USA).
RESULTS
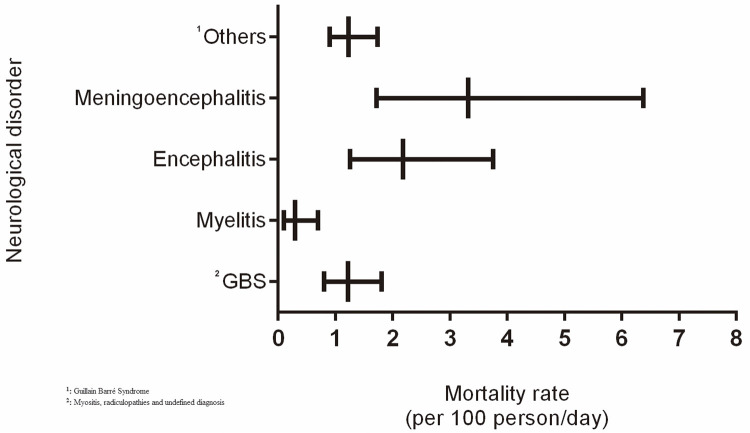
A total of 141 patients with neurological disorders met the inclusion criteria, 93 (66%) of which were associated with arboviruses, according to the laboratory result (IgM or RT-PCR) in CSF or blood (Table 1), and 48 (34%) with other etiologies: 18 cytomegalovirus (CMV), 10 herpes simplex and 20 with undefined diagnosis. Most cases of arboviruses, a total of 55 (59.1%), were associated with CHIKV, followed by 18 (19.3%) cases of DENV and 16 (17.2%) cases of DENV/CHIK. Only 33 (23.4%) were tested for ZIKV (4 positive and 29 negative). The epidemiological, clinical and neurological profile is described in Table 2. Regarding age, we observed that patients with DENV and DENV/CHIKV were younger when compared to CHIKV, ZIKV and other etiologies (p < 0.05). The most prevalent clinical manifestations prior to the neurological disorder were fever, myalgia and headache for the three arboviruses, highlighting severe arthralgia (58.2%) in patients with CHIKV and DENV/CHIKV. The most prevalent comorbidities were hypertension and diabetes in patients with and without arboviruses. In terms of neurological manifestations, reduced level of consciousness was more frequent in the arboviruses than in patients with other etiologies (p < 0.05), and disorientation was more prevalent in patients with CHIKV and in DENV/CHIKV coinfection (p < 0.05). A loss of strength in the lower limbs and changes in ambulation presented a similar prevalence in all groups. The median duration of neurological symptoms was greater than 10 days for all groups, with no differences among them. Regarding neurological disorders, GBS presented a similar frequency distribution between the groups, with around a quarter of cases in each group. Considering the cases of GBS associated with an arbovirus (n = 24), we obtained n = 8 (33.3%) cases that did not receive IV immunoglobulin. Encephalitis was more frequent in cases of CHIKV and DENV. Amongst the patients with CHIKV, GBS (29.1%) and encephalitis (21.8%) were predominate. The estimated mortality rate for meningoencephalitis was around 3.5 per 100 person/day, and for myelitis 2.5 per 100 person/day, while for GBS it was 1.8 per 100 person/day and in myelitis, 0.5 per 100 person/day. Although the graph suggests higher mortality rates in meningoencephalitis and encephalitis, confidence intervals overlapped in relation to other clinical disorders and GBS, however, a significant difference was observed when compared to the estimated mortality rate in myelitis (Figure 1).
Table 1. Positive laboratory results (IgM or RT-PCR) for dengue virus (DENV), chikungunya virus (CHIKV) e zika virus (ZIKV) in CSF or blood, according to the diagnosed neurological disorder (n = 93).
| Neurological disorder | CHIKV positive (n=55) | DENV positive (n=18) | DENV/CHIK positive (n=16) | ZIKV positive (n=4) | ||||
|---|---|---|---|---|---|---|---|---|
| IgM | RT-PCR | IgM | RT-PCR | IgM | RT-PCR | IgM | RT-PCR | |
| GBSa | 5 (9%) | 11 (20%) | 2 (11.1%) | 1 (5.5%) | 4 (25%) | - | - | 1 (25%) |
| Encephalitis | 6 (10.9%) | 6 (10.9%) | 2 (11.1%) | 2 (11.1%) | 1 (6.25%) | 1 (6.25%) | - | - |
| Myelitis | 2 (3.6%) | 1 (1.8%) | 3 (16.6%) | - | 2 (12.5%) | - | - | 1 (25%) |
| Meningoencephalitis | - | 6 (10.9%) | - | - | 2 (12.5%) | 1 (6.25%) | - | - |
| Othersb | 7 (12.7%) | 11 (20%) | 4 (22.2%) | 4 (22.2%) | 2 (12.5%) | 3 (18.7%) | - | 2 (50%) |
| Total | 20 (36.4%) | 35 (63.6%) | 11 (61.1%) | 7 (38.9%) | 11 (68.8%) | 5 (31.2%) | - | 4 (100%) |
aGuillain Barré Syndrome; bMyositis, radiculopathies and undefined diagnosis
Table 2. Epidemiological, clinical and neurological profile of patients with nonbacterial neurological disorders according to etiological diagnosis.
| Characteristics | Other etiology (n = 48) | Chikungunya (n = 55) | Dengue (n = 18) | Dengue & chikungunya (n = 16) | Zika (n = 4) | p-value |
|---|---|---|---|---|---|---|
| Ageb | 43 (17; 63) | 63 (42; 74) | 19 (3; 43) | 28 (17; 50) | 56 (49;61) | 0.002a |
| Sex: Female | 24 (50.0%) | 24 (43.6%) | 8 (44.4%) | 11 (68.7%) | 3 (75.0%) | 0.360 |
| Originating from | ||||||
| Recife | 9 (18.8%) | 13 (23.6%) | 3 (16.7%) | 9 (56.2%) | 0 (0%) | 0.036a |
| Metropolitan Region of Recife | 23 (47.9%) | 14 (25.5%) | 7 (38.9%) | 4 (25.0%) | 2 (50.0%) | |
| Interior | 16 (33.3%) | 28 (50.9%) | 8 (44.4%) | 3 (18.7%) | 2 (50.0%) | |
| Time spent in hospital (days)b | 13 (7; 24) | 15 (7; 23) | 11 (4; 23) | 11 (7; 34) | 8 (1; 16) | 0.828 |
| Symptoms | ||||||
| Fever | 40 (85.1%) | 51 (92.7%) | 18 (100%) | 12 (75.0%) | 4 (100%) | 0.123c |
| Myalgia | 27 (56.2%) | 32 (58.2%) | 3(72.2%) | 7 (43.7%) | 2 (50.0%) | 0.563 |
| headache | 26 (54.2%) | 25 (45.5%) | 6 (33.3%) | 9 (56.2%) | 1 (25.0%) | 0.448 |
| Strong Arthralgia | 15 (31.2%) | 32 (58.2%) | 4 (22.2%) | 9 (56.2%) | 3 (75.0%) | 0.053c |
| Comorbidities | ||||||
| SAH | 16 (33.3%) | 22 (40.0%) | 4 (22.2%) | 5 (31.2%) | 2 (50.0%) | 0.663c |
| Diabetes | 6 (12.5%) | 12 (21.8%) | 2 (11.1%) | 2 (2.7%) | 2 (50.0%) | 0.275c |
| Neurological Manifestations | ||||||
| Loss of strength in lower limbs | 30 (62.5%) | 29 (52.7%) | 9 (50.0%) | 12 (75.0%) | 2 (50.0%) | 0.477 |
| Changes in ambulation | 25 (52.1%) | 20 (36.4%) | 7 (38.9%) | 7 (43.7%) | 1 (25.0%) | 0.516 |
| Reduced level of conscientiousness | 13 (27.1%) | 31 (56.4%) | 9 (50.0%) | 7 (43.5%) | 2 (50.0%) | 0.040ac |
| Disorientation | 7 (14.6%) | 27 (49.1%) | 3 (16.7%) | 5 (31.2%) | 0 (0%) | 0.001c |
| Change in sensitivity | 6 (12.5%) | 6 (10.9%) | 3 (16.7%) | 3 (18.7%) | 3 (75.0%) | 0.042ac |
| Duration of neurologic symptoms (days)b | 19 (7; 34) | 14 (6; 28) | 13 (6; 40) | 13 (9; 41) | 17 (3; 33) | 0.765 |
| Initial time of symptoms and neurological disorders (days)b | 7 (3; 32) | 6 (3; 16) | 10 (3; 17) | 4.5 (2.5; 6) | 37 (3; 105) | 0.446 |
| Neurological disorder | ||||||
| Myelitis | 10 (20.8%) | 3 (5.4%) | 3 (16.7%) | 2 (12.5%) | 1 (25.0%) | 0.039c |
| GBS | 13 (27.1%) | 16 (29.1%) | 3 (16.7%) | 4 (25.0%) | 1 (25.0%) | |
| Encephalitis | 0 (0%) | 12 (21.8%) | 4 (22.2%) | 2 (12.5%) | 0 (0%) | |
| Meningoencephalitis | 1 (2.1%) | 6 (10.9%) | 0 (0%) | 3 (18.7%) | 0 (0%) | |
| Others | 24 (50.0%) | 18 (32.7%) | 8 (44.4%) | 5 (31.2%) | 2 (50.0%) |
aSignificant statistical difference; bMedian (P25; P75); cFischer Exact Test
Figure 1. The mortality rate due to neurological disorders in patients with a previous diagnosis of arbovirus infection.
The mortality rate in the other etiology group was 0.3 (0.1 - 0.5) per 100 person/day, while in patients exposed to DENV - 1.1 (0.7-2.0); CHIKV 2.0 (1.5-2.8), DENV/CHIKV 2.6 (1.6-4.3) and ZIKV 1.0 (0.3-3.2). When considering exposure to some arbovirus, the mortality rate was 1.8 (1.4 - 2.3) per 100 person/day. The HR adjusted calculation demonstrated that the patient’s risk of death was 6.46 (2.91 – 14.3) times greater (p <0.001) through exposure to some arbovirus. And amongst the arboviruses, the risk of death from a concomitance of infections (DENV/CHIKV) was 9.47 (3.80 – 23.6) times greater when compared to those not diagnosed with arbovirus (p <0.001) (Table 3).
Table 3. Mortality rate and Hazard Ratio of deaths of patients with neurological disorders according to etiological diagnosis.
| Diagnosis | Number of deaths | Mortality rate (100 person/day) | HRadjusted (IC95%) | p-value |
|---|---|---|---|---|
| Other etiology | 7 | 0.3 (0.1 – 0.5) | Reference | - |
| Dengue | 13 | 1.1 (0.7 – 2.0) | 4,90 (1,87 – 12,8) | 0,001 |
| Chikungunya | 37 | 2.0 (1.5 – 2.8) | 6,30 (2,72 – 14,6) | <0,001 |
| Dengue and Chikungunya | 15 | 2.6 (1.6 – 4.3) | 9,47 (3,80 – 23,6) | <0,001 |
| Zika | 3 | 1.0 (0.3 – 3.2) | 5,71 (1,43 – 22,8) | 0,013 |
| Some arbovirus | ||||
| No | 7 | 0.3 (0.1 – 0.5) | Reference | - |
| Yes | 68 | 1.8 (1.4 – 2.3) | 6,46 (2,91 – 14,3) | <0,001 |
HRadjusted = adjusted according to the neurological diagnosis; IC = confidence interval
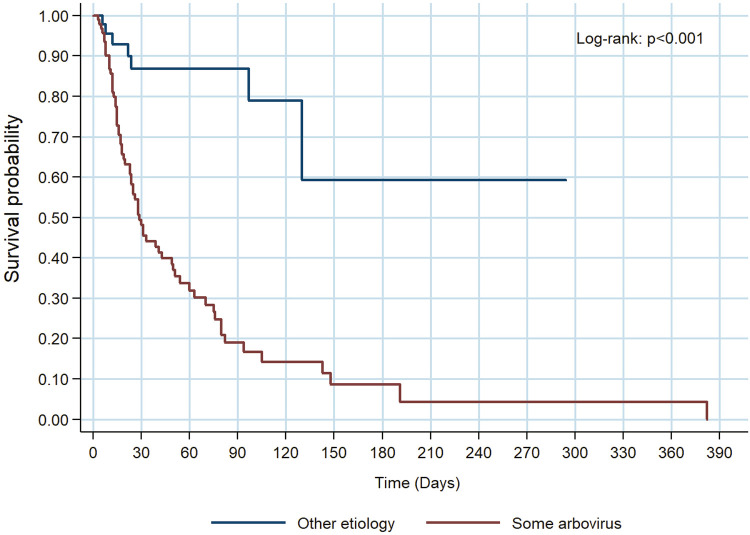
The survival curve indicates that having some arbovirus decreases the likelihood of survival compared to those diagnosed with a neurological disorder from other etiologies (Log-Rank: p <0.001) (Figure 2).
Figure 2. Survival of patients in the group diagnosed with some arbovirus infection compared to other etiologies.
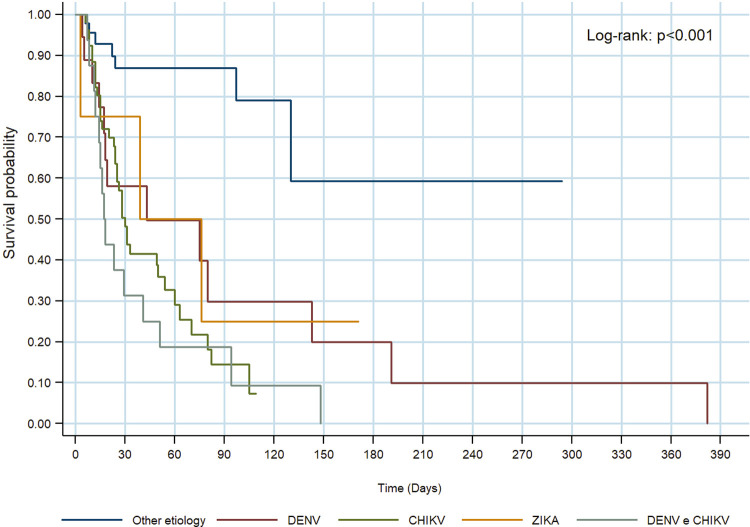
Survival curves of arboviruses are similar, showing no significant differences (Log-Rank: p = 0.464) (Figure 3)
Figure 3. Survival of patients diagnosed with DENV, CHIKV, ZIKAV, DENV/CHIKV compared to other etiologies.
DISCUSSION
Our data has demonstrated that the neurological disorders related to arboviruses presented a higher frequency of reduced levels of consciousness and disorientation than those unrelated to arboviruses. Encephalitis and encephalomyelitis were more frequent in arboviruses, while myelitis predominated in neurological disorders of other etiologies. The occurrence of GBS was similar among groups, about one quarter of all etiologies. CMV was the most prevalent etiology found in the non-arboviral group. These data corroborate the findings in the literature that demonstrate the importance of this virus as an antecedent viral infection associated with neurological syndromes, particularly a GBS18,19.
The mortality rate was higher in neurological disorders related to arboviruses and the probability of death amongst patients with neuro-invasive arbovirus was 6 times higher than in patients with other etiologies, and was even higher (9 times) in DENV/CHIKV coinfection. The survival analysis confirms the lower survival probability of patients with neurological complications caused by arboviruses. A comparison of the survival curves of different arboviruses demonstrated that there was no difference among them.
In the present study, patients diagnosed with DENV were younger, while the neurological cases of CHIKV occurred in the older age group, thereby corroborating reports in the literature stating that 64.52% of individuals infected by CHIKV are aged between 50 and 69 years20. Infection may result in a reduced life expectancy in cases of older people with CHIKV, as demonstrated in a previous study in which the importance of monitoring the progression of the disease in these cases was emphasized21.
Neurological complications have been reported to account for up to 25% of atypical cases and up to 60% of severe atypical cases of CHIKV infection, and include various disorders such as encephalitis, optic neuritis, myeloradiculitis and GBS6,22. In our study, we observed that the most frequent neurological complications in patients with CHIKV were GBS, encephalitis and meningoencephalitis, and a high proportion of deaths, as reported by several other authors, thereby suggesting that these complications may be more severe than previously recognized22-24. The cases of chikungunya stood out with high morbidity, mortality and incidence of serious diseases, including encephalitis and meningoencephalitis. On the other hand, we obtained no cases of encephalitis confirmed by other etiologies in our group. This corroborates the epidemiological situation experienced in Pernambuco State during this period and the awareness of hospitals and the Central Laboratory of Pernambuco (LACEN/ PE) in the notifications and collection of samples from suspected arboviruses cases, thereby contributing to an increase in the diagnoses of cases in the state. Before the epidemic period, neurological syndromes were less expressive than those we have reported.
We observed a high number of deaths, reaching 73.1% in patients with neurological symptoms associated with arboviruses, 67.3% in CHIKV, 93.7% in DENV/CHIK coinfections, as opposed to 14.6% in neurological symptoms unrelated to arboviruses. The mortality rate for CHIKV infection in outpatients is around 1 in 1000 cases or less. However, when analyzing the situation in a hospital environment, we observed a distinct behaviour of the disease. In Reunion Islands25, the authors described the evolution of 610 patients hospitalized with CHIKV, obtaining 10.6% of mortality and 36.4% of severe morbidity, while another study in India reported a 28% mortality rate26. However, these authors described the mortality rate amongst all patients diagnosed with CHIK and did not discriminate the mortality due to neurological disorders. Considering that the neurological form is amongst the most severe, it is likely that mortality rates would have been higher if the authors had selected only patients with neurological syndromes, as in our study. Our region was experiencing a triple epidemic, which reinforces the hypothesis of the high mortality rates found in our patients, as much as that we obtained a high mortality rate among the 16 (17.2%) confirmed cases of DENV/ CHIK coinfections. A further justification would be the fact that the study sites were public referral hospitals for neurology, thus, it is possible that the most severe cases were referred to us indicating a later access to the health system. We may also justify the difference by the fact that we monitored patients for up to 6 months, since neurological disorders persist for long periods due to their late complications.
Unlike our findings, a case-control study conducted in Bahia State, Northeast Brazil, indicated that GBS was strongly associated with ZIKV, with a 6% mortality rate in this study group, suggesting that prior ZIKV infection may result in severe neurological complications27. We observed a high proportion of patients with prior GBS-associated CHIKV infection, corroborating the findings of a study conducted in another Northeastern Brazilian state28. Thus, the presence of GBS does not necessarily indicate an association with ZIKV. This should be laboratory-investigated within the epidemiological context experienced at the particular site. Moreover, our finding of GBS diagnosed with other etiologies corroborates the findings of other authors, who have emphasized the importance of the differential diagnosis with other etiologies, such as cytomegalovirus, Epstein-Barr, and herpes simplex29 infections. In terms of coinfections, in recent years there have been reports of high rates of DENV/CHIKV coinfections in India30. CHIKV coinfection has also been reported with DENV and ZIKV in Colombia and Ecuador31,32. There was also a series of cases of combined arbovirus infection in Rio de Janeiro, Brazil, however, there were few data on neurological complications5. In a case study of neurological manifestations in patients with GBS in Pernambuco State, only 6.7% of prior DENV/CHIKV infections were reported and these cases did not evolve to death7. However, studies have demonstrated that individuals with coinfection appear to be more likely to present with complications and a higher risk of death than those with monoinfections33,34. Corroborating these data, our study has revealed that DENV/CHIKV coinfections showed a higher probability of death. The fact that many of our patients presented with evidence of dual infections may indicate that combined infections are responsible for more serious diseases, as observed in other contexts31. These data are significant, since there is no specific treatment for these infections, although early detection and timely access to supportive medical care decreases the mortality rate.
Our results should be interpreted within the context of the study limitations, as we did not test all patients for ZIKV, due to laboratory limitations at the time of this research. In our patients with evidence of concomitant infections (DENV/CHIKV), it is unclear whether the neurological disease was caused by one arbovirus or another, or a combination of the two. In addition, despite the use of advanced laboratory diagnostic tools, etiological diagnosis is achieved in less than 50% of presumed viral infections in the central nervous system32,35, thereby corroborating the 41.6% of undefined etiology ratio found in our control group, i.e., of cases that were not identified as being related to arboviruses.
In summary, neurological disorders related to DENV, CHIKV, and ZIKV infections have demonstrated the potential to increase the mortality, decrease the survival, and concomitant infections (DENV/CHIKV) were found as an aggravating factor in reducing the likelihood of survival compared to monoinfections. Surveillance and clinical management should be reinforced in patients with previous or concomitant arboviruses, followed by neurological manifestations such as a reduced level of consciousness and disorientation, as these patients may worsen and progress to severe forms of disease and death.
ACKNOWLEDGMENTS
The researchers would like to thank the hospitals involved in the research and the Pernambuco State Secretariat of Health (SES/PE) for enabling access to local epidemiological information, the Central Laboratory of Public Health of Pernambuco (LACEN/ PE) for the partnership in diagnosing cases and to the patients who were monitored by the research team.
ETHICS APPROVAL
This research was approved by the Ethics Committee of the Health Sciences Center of the Federal University of Pernambuco (UFPE) - CAAE Nº 55508216.0.0000.5208.
FUNDING
The research was funded by the Pernambuco Science and Technology Support Foundation (FACEPE).
REFERENCES
- 1.Pialoux G, Gaüzère BA, Jauréguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 2.Bonifay T, Prince C, Neyra C, Demar M, Rousset D, Kallel H, et al. Atypical and severe manifestations of chikungunya virus infection in French Guiana: a hospital-based study. PLoS One. 2018;13:e0207406. doi: 10.1371/journal.pone.0207406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taraphdar D, Roy BK, Chatterjee S. Chikungunya virus infection amongst the acute encephalitis syndrome cases in West Bengal, India. Indian J Med Microbiol. 2015;33(Suppl):153–156. doi: 10.4103/0255-0857.150946. [DOI] [PubMed] [Google Scholar]
- 4.Gérardin P, Couderc T, Bintner M, Tournebize P, Renouil M, Lémant J, et al. Chikungunya virus–associated encephalitis: a cohort study on La Réunion Island, 2005–2009. Neurology. 2016;86:94–102. doi: 10.1212/WNL.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 5.Mehta R, Soares CN, Medialdea-Carrera R, Ellul M, Silva MT, Rosala-Hallas A, et al. The spectrum of neurological disease associated with Zika and chikungunya viruses in adults in Rio de Janeiro, Brazil: a case series. PLoS Negl Trop Dis. 2018;12:e0006212. doi: 10.1371/journal.pntd.0006212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieira MA, Costa CH, Linhares AD, Borba AS, Henriques DF, Silva EV, et al. Potential role of dengue virus, chikungunya virus and Zika virus in neurological diseases. Mem Inst Oswaldo Cruz. 2018;113:e170538. doi: 10.1590/0074-02760170538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira JA, Firmino MF, Cavalcanti DB. Guillain-Barré syndrome associated with arboviruses in the state of Pernambuco in 2016. Fisioter Mov. 2019;32:e003225 [Google Scholar]
- 8.Ferreira ML, Cavalcanti CG, Coelho CA, Mesquita SD. Manifestações neurológicas de dengue: estudo de 41 casos. Arq Neuropsiquiatr. 2005;63:488–493. doi: 10.1590/s0004-282x2005000300023. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira ML. Neurologic manifestations of arboviruses in the epidemy of Pernambuco, Brazil. Neurology. 2016;87:e20 [Google Scholar]
- 10.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis . Dengue: diagnóstico e manejo clínico, adulto e criança. 5ª. Brasília: Ministério da Saúde; 2016. [cited 2020 Aug 26]. https://portalarquivos2.saude.gov.br/images/pdf/2016/janeiro/14/dengue-manejo-adulto-crianca-5d.pdf. [Google Scholar]
- 11.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis . Chikungunya: manejo clínico. Brasília: Ministério da Saúde; 2017. [cited 2020 Aug 26]. https://bvsms.saude.gov.br/bvs/publicacoes/chikungunya_manejo_clinico.pdf. [Google Scholar]
- 12.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked Immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38:1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties os Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, et al. Chikungunya virus in US travellers returning from India, 2006. Emerg Infect Dis. 2007;13:764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santiago GA, Vergne E, Quiles Y, Cosme J, Vazquez J, Medina JF, et al. Analytical and clinical performance of CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis. 2013;7:e2311. doi: 10.1371/journal.pntd.0002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Identification and management of Guillain-Barré syndrome in the context of Zika virus: interim guidance. Geneva: WHO; 2016. [cited 2020 Aug 26]. https://www.who.int/csr/resources/publications/zika/guillain-barre-syndrome/en/ [Google Scholar]
- 17.Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the International Encephalitis Consortium. Clin Infect Dis. 2013;57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nafissi S, Vahabi Z, Ghahar MS, Amirzargar AA, Naderi S. The role of cytomegalovirus, Haemophilus influenzae and Epstein Barr virus in Guillain Barre syndrome. Acta Med Iran. 2013;13:372–376. [PubMed] [Google Scholar]
- 19.Spagnoli C, Iodice A, Salerno GG, Frattini D, Bertani G, Pisani F, et al. CMV-associated axonal sensory-motor Guillain-Barré syndrome in a child: case report and review of the literature. Eur J Paediatr Neur. 2016;20:168–175. doi: 10.1016/j.ejpn.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Kohler LI, Azevedo J, Lima MA, Marinho RA, Souza LJ. Perfil epidemiológico dos pacientes com evolução subaguda e crônica de infecção por Chikungunya. Rev Soc Bras Clin Med. 2018;16:13–17. [Google Scholar]
- 21.Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 22.Cerny T, Schwarz M, Schwarz U, Lemant J, Gérarin P, Keller E. The range of neurological complications in chikungunya fever. Neurocritic Care. 2017;27:447–457. doi: 10.1007/s12028-017-0413-8. [DOI] [PubMed] [Google Scholar]
- 23.Chandak NH, Kashyap RS, Kabra D, Karandikah P, Sahar SS, Morey SH, et al. Neurological complications of Chikungunya virus infection. Neurol India. 2009;57:177–180. doi: 10.4103/0028-3886.51289. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal A, Vibha D, Srivastava AK, Shukla G, Prasad K. Guillain-Barre syndrome complicating chikungunya virus infection. J Neurovirol. 2017;23:504–507. doi: 10.1007/s13365-017-0516-1. [DOI] [PubMed] [Google Scholar]
- 25.Economopoulou A, Dominguez M, Helynck B, Sissoko D, Wichmann O, Quenel P, et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Réunion. Epidemiol Infect. 2009;137:534–541. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- 26.Tandale BV, Sathe PS, Arankalle VA, Wadia RS, Kulkarni R, Shah SV, et al. Systemic involvement sand fatalities during Chikungunya epidemic in India. J Clin Virol. 2009;46:145–149. doi: 10.1016/j.jcv.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Styczynski AR, Malta JM, Krow-Lucal ER, Percio J, Nóbrega ME, Vargas A, et al. Increased rates of Guillain-Barre syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl Trop Dis. 2017;11:e0005869. doi: 10.1371/journal.pntd.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matos AM, Carvalho FM, Malta DL, Rodrigues CL, Félix AC, Pannuti CS, et al. High proportion of Guillain-Barr´e syndrome associated with chikungunya in Northeast Brazil. Neurol Neuroimmunol Neuroinflamm. 2020;7:e833. doi: 10.1212/NXI.0000000000000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastos MS, Lessa N, Naveca FG, Monte RL, Braga WS, Figueiredo LT, et al. Detection of herpesvirus, enterovirus and arbovirus infection in patients with suspected central nervous system viral infection in the western Brazilian Amazon. J Med Virol. 2014;86:1522–1527. doi: 10.1002/jmv.23953. [DOI] [PubMed] [Google Scholar]
- 30.Saswat T, Kumar A, Kumar S, Mamidi P, Muduli S, Debata NK, et al. High rates of co-infection of Dengue and Chikungunya virus in Odisha and Maharashtra, India during 2013. Infect Genet Evol. 2015;35:134–141. doi: 10.1016/j.meegid.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Mallewa M, Vallely P, Faragher B, Banda D, Klapper P, Mukaka M, et al. Viral CNS infections in children from a malaria-endemic area of Malawi: a prospective cohort study. Lancet Glob Health. 2013;1:153–160. doi: 10.1016/S2214-109X(13)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bookstaver PB, Mohorn PL, Shah A, Tesh LD, Quidley AM, Kothari R, et al. Management of viral central nervous system infections: a primer for clinicians. J Cent Nerv Syst Dis. 2017;9 doi: 10.1177/1179573517703342. 1179573517703342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J Virol. 2013;87:8327–8341. doi: 10.1128/JVI.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi BS, Kulkarni K, Godbole M, Dole SS, Kapur S, Satpathy P, et al. Dengue and Chikungunya coinfection associated with more severe clinical disease than mono-infection. Int J Healthc Biomed Res. 2015;3:117–123. [Google Scholar]
- 35.Solomon T, Michael BD, Smith PE, Sanderson F, Davies NW, Hart IJ, et al. Management of suspected viral encephalitis in adults - Association of British Neurologists and British Infection Association National Guidelines. J Infect. 2012;64:347–373. doi: 10.1016/j.jinf.2011.11.014. [DOI] [PubMed] [Google Scholar]