Abstract
Rotavirus (RV) infects small intestinal epithelial cells, inducing severe diarrhea in children, resulting in over 500,000 deaths annually. Relatively little is known about how innate immunity contains acute infection and drives adaptive immune responses that afford complete clearance of RV and protection against future infection. Hence, we examined the consequence of the absence of MyD88, known to be central to innate immunity, in a mouse model of RV infection. The absence of MyD88, but not combined blockade of IL-1β and IL-18 signaling, resulted in greater infectivity, as reflected by levels of RV in feces, intestinal lysates and viremia. Such increased RV levels correlated with an increase in incidence and duration of diarrhea. Loss of MyD88 also impaired humoral immunity to RV. Specifically, MyD88 knockout generated less RV-specific IgA and exhibited profoundly reduced RV-specific IgG2c/IgG1 ratios suggesting that MyD88 signaling drives RV-induced Th1 responses. A study of MyD88 bone marrow chimeras indicated that MyD88-dependent control of acute RV infection was mediated by both hemopoietic and non-hemopoietic cells, while generation of RV-specific humoral immunity was driven by MyD88 signaling in hemopoietic cells, which reflected the loss of IL-1β and IL-18 expression by these cells. Thus, TLR signaling and inflammasome cytokines drive innate and adaptive immunity to RV.
Keywords: Rotavirus, MyD88, Toll-like receptors, antibody, inflammasome
Introduction
Rotavirus (RV) is a non-enveloped, double-stranded RNA virus that preferentially infects small intestinal mature enterocytes. RV is the leading cause of severe viral gastroenteritis amongst children worldwide and causes moderate intestinal distress in adults. Before the introduction of RV vaccines in the 2000s, RV universally infected children under the age of 5 years and caused 2–4 million hospitalizations and >0.5 million deaths per year globally.1 While the introduction of RV vaccines has markedly reduced the disease burden caused by RV, it nonetheless remains a major public health problem, especially in developing countries both because infections result in more disease manifestations, including death, and because current vaccines that have proven highly effective in North America and Europe do not elicit robust protective immune responses in many developing countries.2 While the reasons for these disparities remain unclear, the now well-appreciated role of innate immunity in both controlling initial infection and driving adaptive immunity suggest the possibility that innate immune responses may be involved. Better understanding of basic mechanisms that mediate innate immunity to RV might make it possible to investigate this possibility.
RV has been studied using a well-defined mouse model that recapitulates many aspects of RV disease and immunity in humans. Similar to humans, neonatal mice develop diarrhea with RV infection; however, they do not develop any features of severe disease, like fever, vomiting or dehydration. Because mice only develop diarrhea with infection, this is the only method to expunge the body of virus. One key facet of innate immunity to RV revealed by use of this model is that absence of type I and/or type II IFN has a significant effect on RV-induced clearance, but not pathogenicity, based upon mouse gene deletion; specifically, the deletion of STAT1, a signaling component for both type I and II IFN, renders mice unable to control virus shedding as does the deletion of MAVS, a protein downstream from RIG-I which is partially responsible for type I IFN induction in response to dsRNA exposure.3,4 The roles of IFNs during RV infection are further discussed by Holloway and Coulson.5 TLR3-mediated production of type III IFN helps limits RV infectivity in adults, but lack of expression of TLR3 in the neonate gut indicates it is not involved in protecting the most susceptible hosts.6,7 Moreover, adult mice remain readily infectable to RV, indicating that TLR3-mediated signaling is not sufficient to protect against this pathogen, suggesting the existence of additional means of innate immunity to RV. TLR7 signaling, endosomal acidification and IFN-α/β receptor was recently found to activate dendritic and B cells after exposure to RV, but the authors did not address the role of TLR7-mediated control of innate immunity or Ab generation following infection.8
One of the most central molecules in innate immunity in general is the signaling adaptor MyD88. MyD88 mediates signaling for all TLRs, except TLR3, as well as receptors for inflammasome cytokines IL-1 and IL-18. Indeed, MyD88 signaling plays a role in immune responses to a wide variety of pathogens, including viruses, bacteria, protozoa and fungi, both in terms of limiting initial infection and promoting pathogen-specific adaptive responses.9 One example of MyD88-mediated antiviral immunity is the case of influenza in which MyD88-mediated inflammasome cytokine signaling protects against influenza mortality and induces specific Ab production, while MyD88-mediated TLR7 signaling induces type I IFN, which contributes to resistance against infection.10 Hence, the goal of this study was to investigate the extent to which MyD88 signaling contributes to the initial control of RV infection and induction of RV-specific Ab responses. However, the role of MyD88 signaling during RV infection is understudied, although it has been presented that upon RV infection, MyD88 signaling, independent of B cell receptor, was able to induce activation of B cells and Ab generation.11 Adult mice, deficient in MyD88 and related signaling molecules, were employed in this study as a model of infection, and neonatal mice served as a model of RV-induced disease. Our results indicate that MyD88-mediated TLR signaling contributes to control of primary RV infection, while inflammasome cytokine signaling mediates a robust and properly polarized RV-specific Ab response.
Materials and methods
Animals
All experiments, excluding those using neonatal mice, utilized 6–8-wk-old male MyD88 knockout (KO) mice (gift of Jian-Dong Li, Georgia State University, Atlanta, GA, USA), IL-18 KO mice (Jackson Laboratories), IL-1R KO (Jackson Laboratories), NLRP3 KO mice (Jackson Laboratories, Bar Harbor, ME, USA) or C57BL/6 offspring from C57BL/6 mice originally purchased from Jackson Laboratories. Experiments involving neonatal mice used 6-d-old neonates bred in-house.
Virus and inoculations
Mouse RV (EC strain) was supplied by Mary Estes (Baylor College of Medicine, Houston, TX, USA). Adult mice received 1.33% (w/v) sodium bicarbonate (Sigma-Aldrich, St. Louis, MO, USA) by oral gavage, followed by 105 SD50 murine RV, also by oral gavage. Neonates received 1 DD50 murine RV.
Fecal and serum RV antigen detection
Supernatants, made from fecal homogenates (100mg feces/ml PBS), or serum was frozen or immediately analyzed by ELISA as described.12
RV genome qRT-PCR
Small intestinal and colonic samples were harvested, washed in PBS, homogenized in TRIzol (Ambion, Austin, TX, USA) and probed for RV genome as described.12 Blood was harvested, RNA from peripheral blood cells were isolated using QIAamp RNA Blood Mini Kit (Qiagen, Valencia, CA, USA) and RV genome was amplified.
Single-stranded qRT-PCR for RV replication
RV replication in small intestinal and colonic samples were determined by the (+):(−) RV strand ratio magnitude as previously described.13
Ab ELISAs
Relative serum RV-specific Ab production (measured by OD at a specified serum dilution) and titer (measured by the serum dilution at which OD was 0.2 over blank) was analyzed as described.12
Bone marrow chimeras
Recipient mice were irradiated with the equivalent of either 800–850 or 600 Rads (C57BL/6 or MyD88 KO mice, respectively) using an X-ray irradiator (Rad Source, Suwanee, GA, USA) and were administered bone marrow cells from either C57BL/6, MyD88 KO or IL-1/18 DKO mice as described.14 Bones used to generate cells from IL-1/18 DKO were provided by Gabriel Nunez (University of Michigan, Ann Arbor, MI, USA) who generated such mice by crossing IL-1β and IL-18 mice that had been backcrossed to C57BL/6 mice for more than six generations.
IL-1 β and IL-1 8 detection
IL-1β was measured in ex vivo small intestinal culture supernatants as described previously.15 IL-18 was captured using an unlabeled anti-mouse IL-18 Ab (MBL, Nagoya, Japan) and detected utilizing a biotinylated anti-mouse IL-18 Ab (MBL).
IL-1 β neutralization
IL-1β was neutralized with i.p. administration of human IL-1Ra, Anakinra (Kineret, Swedish Orphan Biovitrum, Stockholm, Sweden), at a concentration of 100 μg/g body mass once daily the day of inoculation and up to 3 d post-inoculation (PI). This dosing regimen was equal to or higher than several studies found effectively neutralized IL-1β activity.16,17
Statistics
Statistical significance was evaluated using the Student’s t-test. A Grubbs test was applied to identify and remove outliers in situations where patterns were observed but no statistical significance was achieved. The χ2test was also applied. Additionally, statistical significance was evaluated by the two-way ANOVA test. A hashtag (#) indicates P < 0.05.
Results
MyD88 signaling contributes to protection against RV infection, dissemination and diarrhea
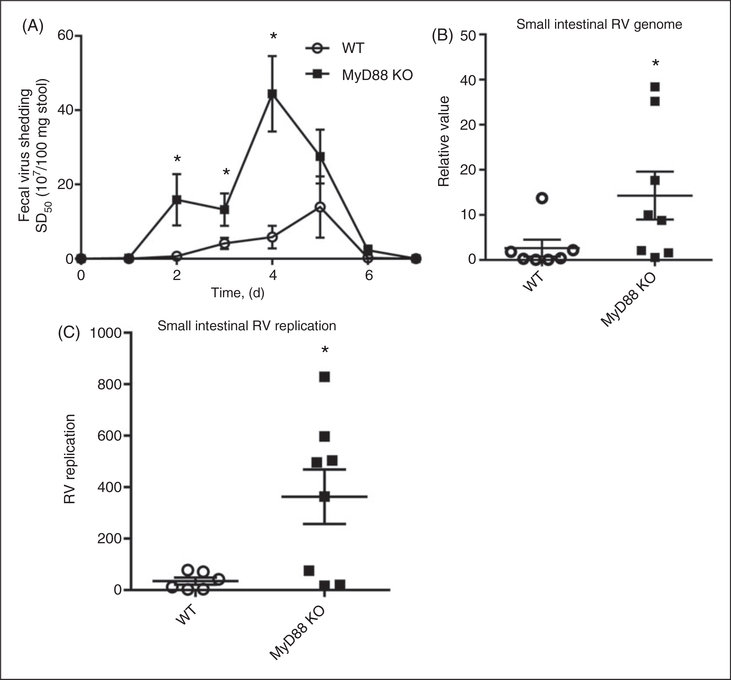
Infection of adult mice with RV serves as a well-defined infection model without causing severe manifestations of RV disease.1 An oral inoculation of a 6–8 wk-old C57BL/6 mouse with 105 SD50 of murine RV strain EC resulted in RV antigen becoming detectable in feces 1–2 d PI, with a peak at 2–5 d PI, and antigen shedding lasting 6–8 d PI. Such RV antigen shedding is proportional to infectivity, i.e. the level of viral genome in intestinal lysates. To elucidate the role of innate immunity in RV infection, we inoculated adult and neonatal mice lacking MyD88, an adaptor protein that mediates all signaling by inflammasome cytokine receptors (IL-1β and IL-18) and a major portion of the signaling by all TLRs, except for TLR3. In the absence of MyD88, mice displayed greater Ag shedding from d 2–7 PI (Figure 1A). To confirm that the increased fecal RV antigen shedding was, in fact, reflecting increased infection of RV, small intestinal lysates were assayed for levels of RV genomes by qRT-PCR at d 3 PI, which corresponds approximately to the peak of infection (Figure 1B). In the absence of MyD88, levels of RV virus genome increased more than fivefold relative to WT mice, thus verifying that the absence of MyD88 resulted in increased RV loads in intestinal epithelial cells (IECs). Increased RV loads could reflect that the absence of MyD88 enhanced RV replication ability and/or enhanced RV entry, with the latter possibility potentially reflecting reduced expression of extracellular antiviral mediators. To help distinguish between such possibilities, we assayed intestinal lysate for RV replication levels by measuring the relative ratios of (+):(−) RV strands by single-stranded qRT-PCR (Figure 1C). MyD88 KO mice exhibited a 10-fold enhancement of in the (+):(−) RV strand ratio, suggesting increased RV loads likely reflect an increased ability of RV to replicate in IECs of MyD88-deficient mice.
Figure 1.
MyD88 signaling contributes to the control of RV infection in adult mice. (A) Six–8-wk-old male mice were inoculated with 105 SD50 of murine RV, and feces were collected daily and probed for RV antigen by ELISA. (B) On d 3 PI, duodenal lysates were probed for RV genome by the presence of NSP3 mRNA. (C) Day 3 PI duodenal lysates were analyzed for RV replication [relative (+):(−) RV strand ratios] by ssqRT-PCR. Results in (A) are the mean ± SEM of an individual experiment (n = 5) and representative of five independent experiments. Results in (B) and (C) were generated from the combination of two independent experiments. Each point denotes an individual mouse, horizontal lines represent mean; error bars represent SEM; n = 6–8. * P < 0.05.
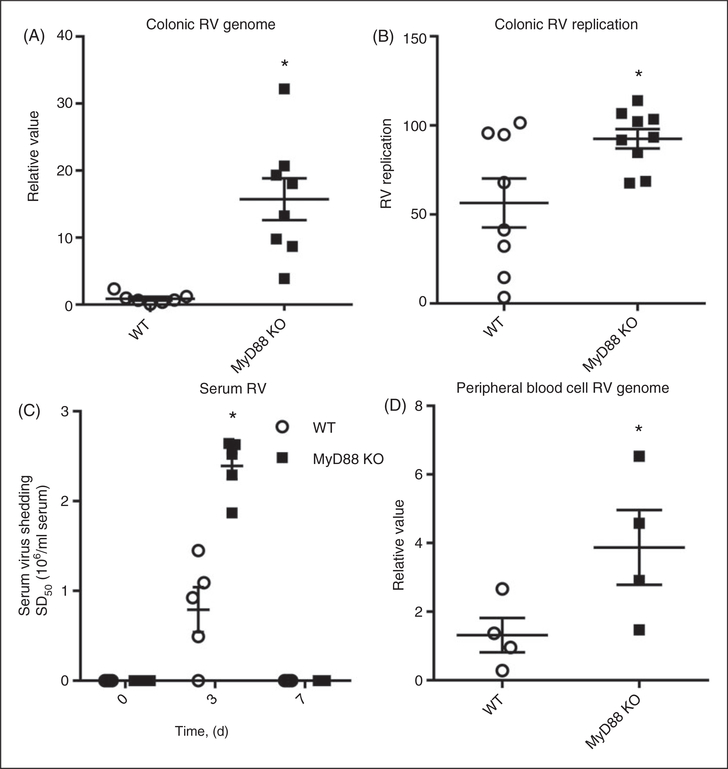
While RV infection normally predominates in the small intestine, it can disseminate more widely in immunocompromised mice. Thus, we next examined if the loss of MyD88 resulted in increased spread of RV beyond the small intestine. Loss of MyD88 resulted in a marked increase in the amount of viral genome in the colon (Figure 2A), indicating a role for MyD88 signaling RV in preventing RV spread within the intestinal tract. The (+):(−) RV strand ratio indicated that the virus present in colon lysates, in both WT and MyD88 KO mice, was actively replicating virus. Yet, the increased RV loads in MyD88 KO colon were associated with only a modest increase such ratios (Figure 2B), suggesting that enhanced colonic RV loads were, at least in part, driven by greater amounts of RV arriving from the small intestine. RV genomes were undetectable in the livers, spleens and lungs of both WT and MyD88 KO mice (data not shown). However, in the absence of MyD88, serum RV antigen levels were increased (Figure 2C), and peripheral blood cells exhibited increased levels of RV genomes at 3 d PI (Figure 2D), supporting a role for MyD88 signaling in limiting viral spread to the blood. Thus, MyD88 signaling plays a role in restricting RV to the small intestine, likely, at least in part, by limiting its replication within this organ.
Figure 2.
MyD88 limits RV spread to the colon and blood. (A) Day 3 PI colonic lysates were probed for RV genome by the presence of NSP3 mRNA. (B) Day 3 PI colonic lysates were analyzed for RV replication [relative (+):(−) RV strand ratios] by ssqRT-PCR. (C) Serum was harvested on d 0, 3 and 7 PI, and analyzed for the presence of RV by ELISA. (D) Mice were bled 3 d PI, RNA harvested as described and probed for RV genome by NSP3 mRNA qRT-PCR. Results in (A) and (B) are the combination of two independent experiments. (C) is the combination of two independent experiments where one group of mice was bled on d 0 and 7, and another was bled on d 3 PI. (D) is representative of one experiment. Each point in (A–D) denotes an individual mouse, horizontal lines represent the mean; error bars represent SEM; n = 4–8. *P < 0.05.
We next investigated the extent to which the impairment of MyD88 KO mice in controlling RV infection might reflect loss of TLR or inflammasome cytokine signaling. Loss of the best-studied inflammasome, NLRP3, which has been implicated in a broad array of innate immune recognition pathways, had no effect on the course of RV shedding suggesting lack of involvement in RV recognition (online Supplementary Figure 1A). Next, we determined if RV induced an increase in the expression of IL-1β and IL-18. While, in accordance with other studies, mature IL-1β and IL-18 were detectable in intestinal supernatants (duodenum and jujenum), their levels were not significantly increased following RV inoculation (online Supplementary Figures 1B,C). Moreover, neither groups of mice deficient in IL-18 or IL-1 receptor exhibited significant impairment in their ability to clear RV (data not shown). To address the possibility that perhaps both IL-1 and IL-18 might be required for efficient handling of RV, IL-18 KO mice were administered an IL-1R antagonist prior to and following inoculation with RV. Such combined blockade of IL-1 and IL-18 signaling did not alter the time course or extent of RV infectivity relative to that observed in WT mice (online Supplementary Figure 1D), indicating that the increased RV infectivity observed in the absence of MyD88 does not reflect loss of inflammasome cytokine signaling.
To further understand how MyD88 signaling was contributing to control of RV infection, we examined RV infectivity in MyD88 KO bone marrow-irradiated chimeric mice. While this method has proven useful in understanding what types of cells are responsible for specific phenotypes, conclusions drawn from experiments using MyD88 bone marrow chimeric mice bring caveats. Specifically, MyD88-deficient mice are extremely susceptible to irradiation, developing a variety of health problems, making it difficult to compare irradiated WT and MyD88 mice. Hence, we focused on comparing irradiated mice of the same host genotype that received WT or MyD88 KO bone marrow. Reconstituting WT-irradiated mice with MyD88 KO bone marrow, under conditions that give > 95% chimerism, did not impair clearance of RV relative to mice receiving WT bone marrow (online Supplementary Figure 2A). Yet, administering WT bone marrow to irradiated MyD88 resulted in a moderate reduction in RV infectivity (online Supplementary Figure 2B). Together, these results indicate that MyD88 in both hemopoietic and radio-resistant cells, likely epithelial cells, contributes to MyD88-mediated clearance of RV infection.
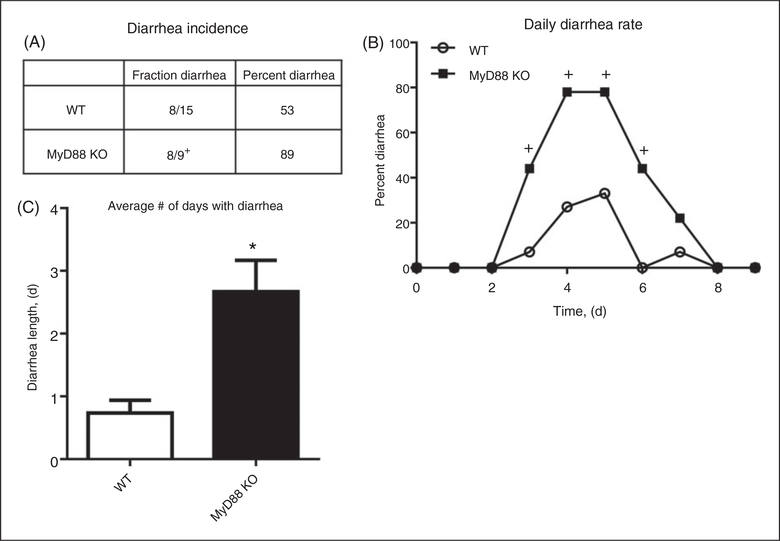
Analogous to the case for human infants, RV infection in neonatal mice results in diarrhea that begins 2–3 d after virus exposure and, depending on the initial dose, lasts for approximately 1 wk. To determine if increased production of RV antigens that resulted from the absence of MyD88 translates into greater disease in neonatal mice, we inoculated 6-d-old neonatal MyD88 KO mice with 1 DD50 RV and monitored mice for diarrhea, as indicated by the presence of profuse, yellow, runny feces upon application of light pressure to the abdomen.12 This relatively low dose of RV resulted in the incidence of diarrhea in 53% of WT and 89% of MyD88 KO neonatal mice (Figure 3A). Moreover, the absence of MyD88 resulted in a marked increase of the daily diarrhea rates from d 3 to d 7 PI (Figure 3B), which corresponded to a more than threefold increase in the average duration of diarrhea (Figure 3C). Thus, the increased RV replication within IECs, and concomitant increase in RV antigens in MyD88 KO mice, translated into greater disease.
Figure 3.
MyD88 signaling protects neonatal mice from RV disease. (A) Six-d-old mice were inoculated with 1 DD50 murine RV and observed daily for diarrhea symptoms, including the presence of yellow, runny and profuse feces with application of light pressure to the abdomen. Total RV diarrhea incidence amongst MyD88 KO and control mice were calculated. (B) The daily diarrhea rate, or the percentage of mice per d that displayed symptoms of diarrhea, was calculated. (C) The number of days each neonate experienced symptoms of diarrhea was averaged for MyD88 KO and control groups. Results in (A–C) represent the combination of two independent experiments where n = 9–15. In (C), bars denote the mean and error bars represent SEM. *P < 0.05; +P < 0.05.
MyD88 signaling drives RV humoral responses and controls proper subisotype switching
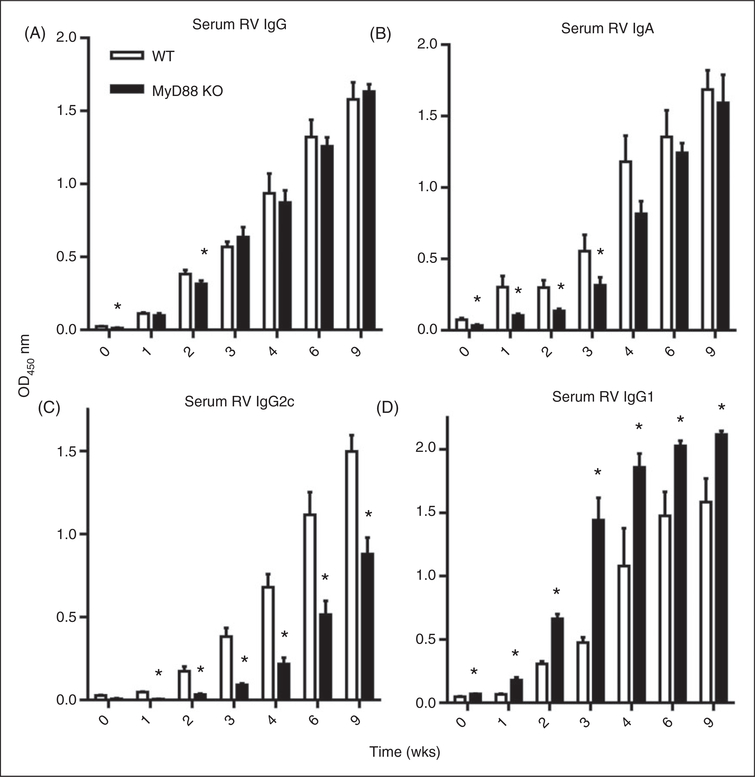
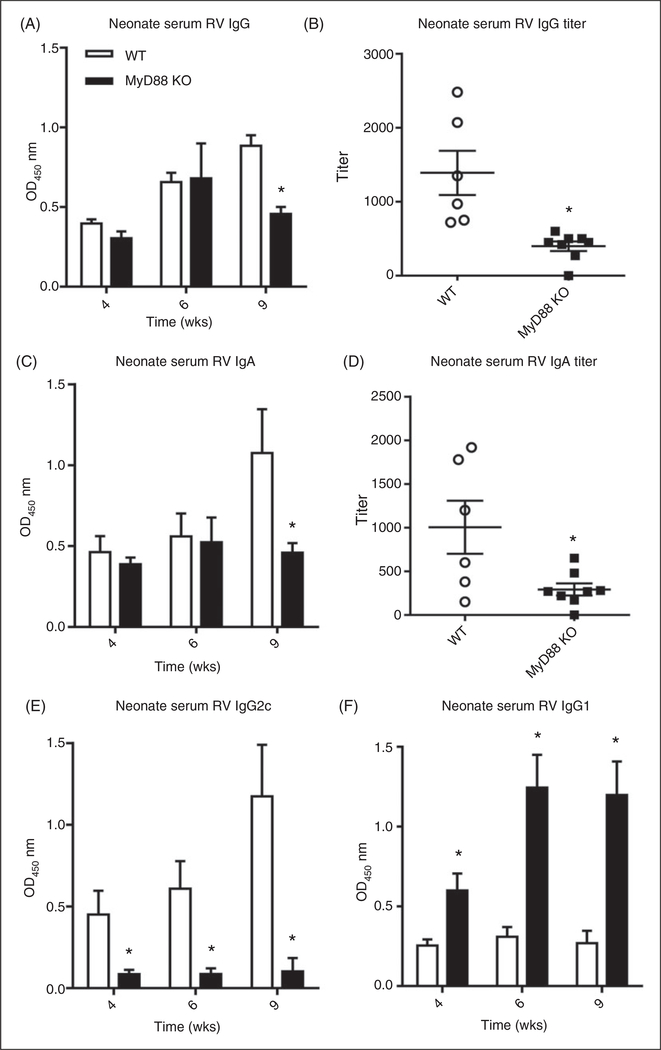
RV infection elicits a robust adaptive immune response that plays a critical role in clearing primary infection and confers protection against future infection. Such protective immunity correlates with and is largely mediated by the generation of RV-specific Abs.1 RV vaccines, which are live attenuated viruses, employ such protection and can be considered analogous to the adult model of RV infection in that both elicit Ab production by asymptomatic infection. It is now well appreciated that innate immune signaling is critical for initiation and regulation of adaptive immunity although the relative roles for MyD88 signaling is pathogen-dependent.18,19 Hence, to investigate the role of MyD88 in the induction of RV-specific humoral responses, we infected MyD88-deficient mice and measured anti-RV IgG and IgA responses. Although MyD88 KO mice were likely exposed to considerably more RV antigen, due to increased infectivity, the absence of MyD88 resulted in a modest reduction of anti-RV IgG and IgA, particularly within a few weeks of infection (Figure 4A,B). This modest deficit was overcome shortly thereafter and resulted in titers that did not significantly differ between WT and MyD88 KO mice at 9 wk PI (data not shown). Total serum IgA concentrations revealed that MyD88-deficient mice produce less serum IgA than WT mice (online Supplementary Figure 3), indicating that the weakened early RV-specific IgA response may be reflective a deficiency in total IgA. However, the absence of MyD88 resulted in a dramatic shift in IgG subtypes with MyD88 KO mice exhibiting a marked loss of IgG2c and increase in IgG1 (Figure 4C,D). Next, we examined Ab response in WT and MyD88 KO mice that had been infected as neonates. Somewhat similar to adult MyD88 KO mice, neonates exhibited a modest reduction in the anti-RV IgG and IgA response (Figure 5A,C), particularly at later time points when viewed as dilution titers (Figure 5B,D). Moreover, they exhibited a dramatic loss of RV-specific IgG2c and a corresponding marked increase in IgG1 (Figures 5E,F). These results indicate MyD88 signaling plays a key role in driving the Th1-mediated immune response normally expected with RV infection.
Figure 4.
MyD88 signaling in adult mice enhances anti-RV Ab generation and proper Ab subtype switch. (A) Adult MyD88 KO and control mice were inoculated with RV and bled until 9 wk PI. Serum anti-RV IgG production, as represented by the OD value at a specified serum dilution, was analyzed by ELISA. The same serum was also probed for RV-specific (B) IgA, (C) IgG2c and (D) IgG1 in the same manner. Results are from an individual experiment (n = 5) and representative of three separate experiments that gave a similar pattern of results. Bars denotes the mean; error bars represent SEM; n = 5. *P < 0.05.
Figure 5.
MyD88 signaling in neonatal mice enhances anti-RV Ab generation and proper Ab subtype switch. (A) Six-d-old mice were inoculated with 1 DD50 murine RV and bled at 4, 6 and 9 wk PI. Serum was probed for anti-RV IgG production, as represented by OD value at a specified serum dilution and (B) IgG titer, as measured by the sample dilution at which OD value = 0.2 over blank. The same serum was also probed for RV-specific (C) IgA production, (D) IgA titer, (E) IgG2c and (F) IgG1. Results are representative of two independent experiments, where the bars or horizontal lines represent the mean, error bars denote the SEM; n = 6–8. *P < 0.05.
Bone marrow-derived MyD88 signaling enhances RV humoral responses and proper Ab class switching
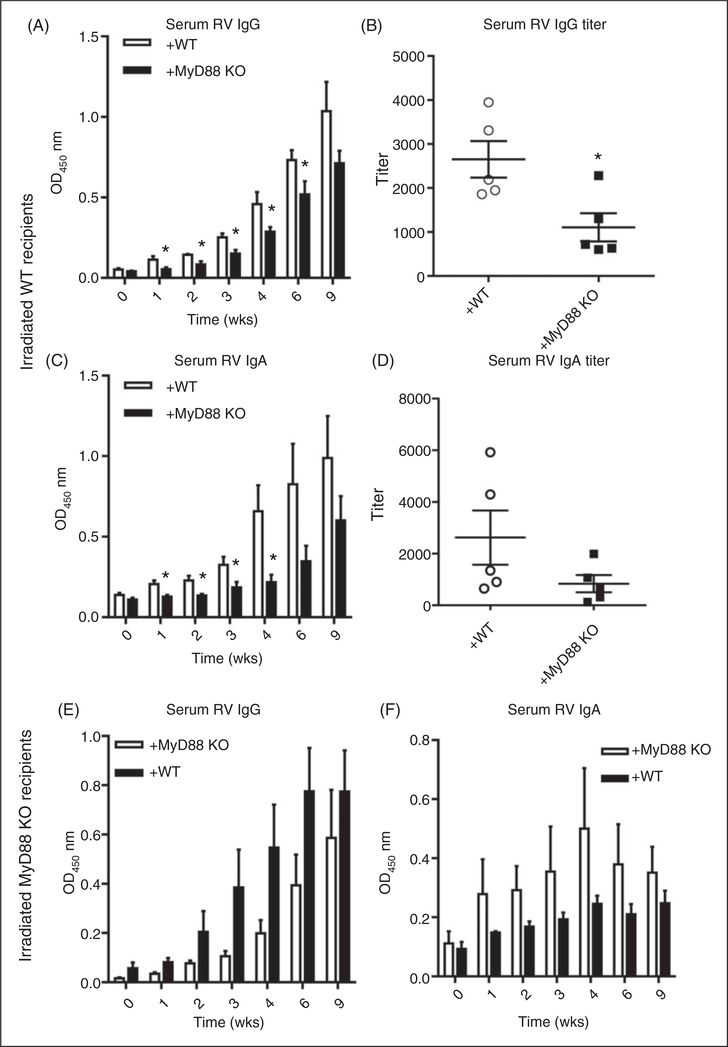
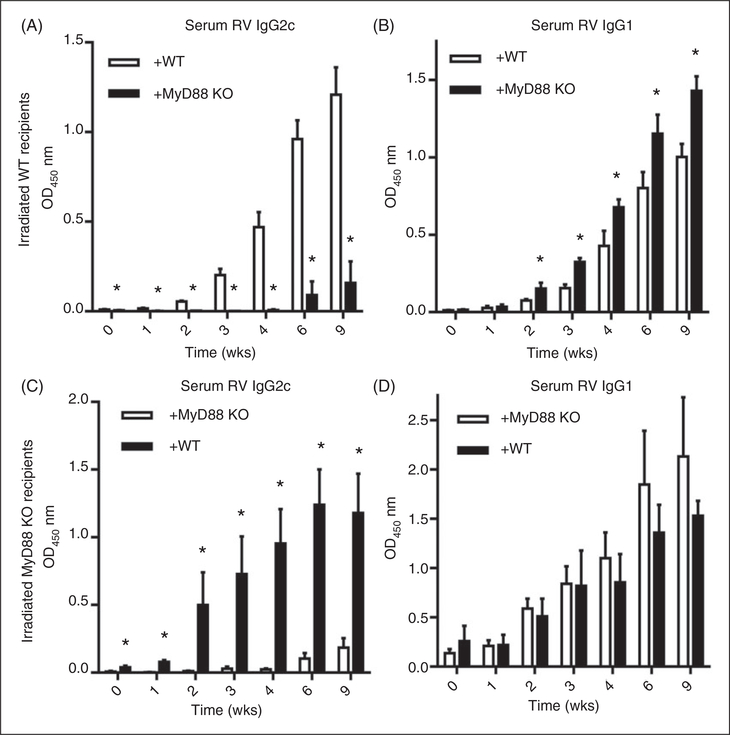
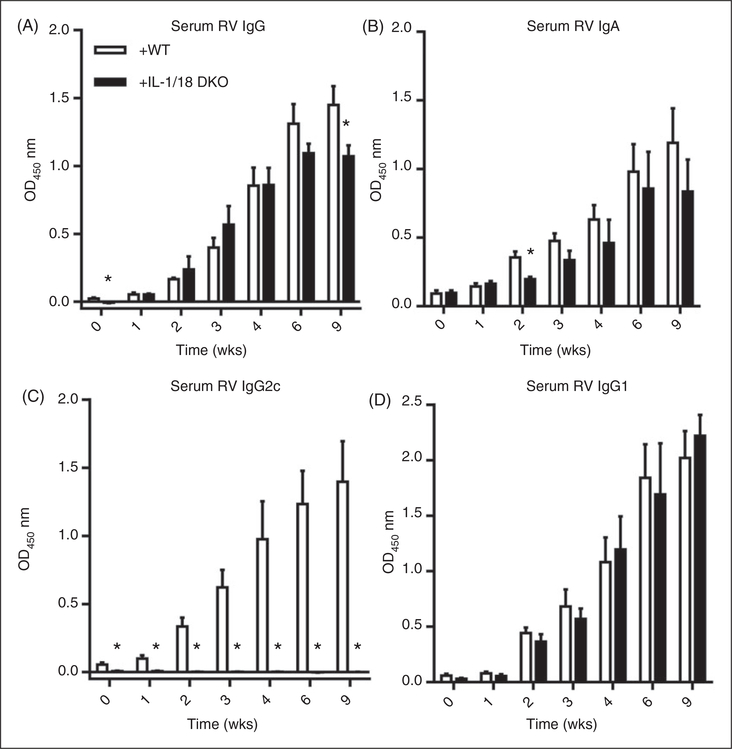
To determine in which cells (radio-resistant/epithelial vs. bone marrow-derived) MyD88 signaling contributes to RV-specific Ab responses, we utilized the above-described MyD88 KO bone marrow chimeric mice. In contrast to the case for controlling RV infectivity, proper anti-RV humoral response was largely a consequence of the absence of MyD88 in bone marrow-derived cells. Specifically, reconstitution of WT mice with MyD88 KO bone marrow resulted in a marked attenuation of anti-RV IgG and IgA (Figure 6A–D) while, conversely, administration of WT bone marrow to irradiated MyD88 KO enhanced anti-RV IgG (Figure 6E). Moreover, in such chimeric mice, regardless of whether the irradiated host was WT or MyD88 KO, the presence of MyD88 in bone marrow-derived cells was necessary and sufficient for a robust anti-RV IgG2 response, the absence of which correlated with an enhanced level of anti-RV IgG1 (Figure 7A–D). Thus, the Th1-polarized humoral immune response to RV was mediated by MyD88 in bone marrow-derived cells. To discern whether such a role of MyD88 reflected loss of TLR or inflammasome cytokine signaling, we next investigated if reconstitution of irradiated WT mice with bone marrow from IL-1β/IL-18 DKO mice would result in a similar phenotype. Indeed, the absence of IL-1β and IL-18 in bone marrow cells resulted in a modest reduction in anti-RV IgG and IgA (Figure 8A,B) and a dramatic shift in IgG subtypes, especially the complete loss of anti-RV IgG2c (Figure 8C). Such loss of anti-RV IgG2c was not observed in mice lacking only IL-1R or IL-18 (online Supplementary Figure 4A–D) and was not phenocopied in mice lacking the NLRP3 inflammasome (online Supplementary Figure 5A–D).Thus, the combination of bone marrow-derived IL-1β and IL-18 supports proper anti-RV Ab class switching that optimizes the immune response to RV.
Figure 6.
MyD88 signaling in radio-sensitive/bone marrow-derived cells but not radio-resistant cells contributes to the production of RV-specific IgG and IgA. (A) Bone marrow chimeric mice were made where WT mice reconstituted with MyD88 KO bone marrow. After rest and confirmation of chimerism, experimental and control mice were inoculated with 105 SD50 murine RV and bled until 9 wk PI. (A) Serum was probed for anti-RV IgG production and (B) IgG titer. The same serum was also probed for RV-specific (C) IgA production and (D) IgA titer. (E) Another group of bone marrow chimeric mice were made where MyD88 KO mice were reconstituted with WT bone marrow. Mice were treated as in (A–C), and serum was probed for anti-RV IgG and (F) IgA production. Results in (A–F) are from an individual experiment (n = 4–5) with bars or horizontal lines representing the mean and error bars denoting the SEM. *P < 0.05.
Figure 7.
MyD88 in radio-sensitive/bone marrow-derived cells but not radio-resistant cells assist in proper Ab sub-type switch. (A) WT mice reconstituted with MyD88 KO bone marrow were prepared as described in the Materials and methods, and serum was probed for RV-specific IgG2c and (B) IgG1 production. (C) MyD88 KO mice reconstituted with WT bone marrow were also made as described, and serum was probed for RV-specific IgG2c and (D) IgG1. (A) and (B) are representative of one independent experiment while (C) and (D) are representative of another independent experiment. Results are from an individual experiment (n = 4–5) with bars or horizontal lines representing the mean and error bars denoting the SEM. *P < 0.05.
Figure 8.
Anti-RV IgG and IgA is independent of bone marrow-derived IL-1 and IL-18; however, proper Ab subtype switch is dependent on both IL-1 and IL-18. (A) Bone marrow chimeric mice were made where WT mice were reconstituted with IL-1 and IL-18 DKO bone marrow. Mice were treated as described and serum RV-specific IgG and (B) IgA production was probed by ELISA. The same serum was also probed for the production of anti-RV (C) IgG2c and (D) IgG1. Results are from an individual experiment (n = 4–5) with bars or horizontal lines representing the mean and error bars denoting the SEM. *P < 0.05.
Discussion
RV remains a world-leading cause of severe diarrhea, resulting in substantial morbidity and death. Even though the vast majority of RV infections resolve without treatment and result in lasting protection against reinfection, adult/asymptomatic RV infections are an example of an effective immune response. Indeed, mimicking this response with live attenuated orally-administered RV has proven to be an effective vaccine strategy. Yet, the host determinants that mediate initial control of RV, including restricting it to the gut and drive the well-characterized Ab responses that mediate lasting protection have not been well defined. Herein, we investigated the potential involvement of MyD88 signaling pathways, which mediate signals by inflammasome cytokine receptors and TLRs, in mediating innate and/or adaptive immunity to RV. We observed that, indeed, MyD88 signaling plays a key role in both the initial containment of RV and in directing the generation of RV-specific Abs.
In adult mice, the absence of MyD88 enhanced RV infectivity and increased virus spread to the colon and blood. In neonate mice, loss of MyD88 prolonged and exacerbated RV-induced diarrhea. As diarrhea causes much of the mortality associated with RV infection and extra-intestinal spread of the virus correlates with more severe infections, we conclude that MyD88 signaling is a key part of the innate immune response that normally limits RV infection and disease. Such MyD88-mediated protection did not reflect a role for inflammasome cytokines as blockade of IL-1β and/or IL-18 signaling did not significantly impede containment and clearance of RV. Rather, it suggests a role for TLR signaling in the initial control of RV infection. The mechanism of how MyD88-mediated TLR signaling controls RV infection is unclear but may resemble other viruses where specific or groups of TLRs limit virus infection and spread. For example, mice deficient in endosomal TLR7 and TLR9 show higher DNA virus murine CMV (MCMV) titers at the peak of infection and are more susceptible to virus-induced death.20 As such, it can be hypothesized that MyD88-mediated TLR signaling, most likely endosomal TLRs, may recognize RV and provide signaling necessary to control RV infection and spread. Indeed, Deal et al.21 depleted pDCs, a dendritic cell (DC) subtype that secretes type I IFN production in a TLR7- and/or TLR9-dependent manner, and found that it limited fecal RV shedding.21 However, the authors did not identify which TLR or MyD88 was responsible for pDC-mediated control of virus infection. The contribution of nearby innate cells to the control of infection was evaluated but is not presented here; DCs, neutrophils, NK cells, or macrophages were not found to be involved in limiting infection by various depletion methods. Based on our results with MyD88 bone marrow radiation chimeras and depletions, we speculate that activation of such MyD88 signaling by endosomal TLRs might occur in infected epithelial and nearby immune cells, like pDCs.
The other major consequence of absence MyD88 observed in our study was alteration in the humoral immunity elicited by RV. Considering that the increased infection of RV observed in MyD88 KO mice, which would presumably result in RV antigens reaching more immune cells, one might have expected a greater Ab response in MyD88 mice. Rather, we observed a modest reduction in total levels of RV-specific Abs and a dramatic shift in IgG subtypes, including the near complete loss of IgG2c. This virus-specific skew in IgG profiles may be influenced by basal non-specific IgG profiles in the MyD88 KO mouse as Gavin et al.22 reported that mice lacking both MyD88 and Trif made less IgG2c and more IgG1 than control mice. Moreover, the absence of MyD88 may influence the differentiation of B cells into plasma cells, as was observed by Guay et al.19 with polyoma virus infection in MyD88-deficient mice.23 This trend in humoral responses may be virus specific, as many have observed that the induction of antibacterial adaptive responses, such as to Toxoplasmosis gondii and Citrobacter rodentium, seems to be unchanged by the absence of MyD88.18,24 While the alteration in the response observed in MyD88 KO mice did not render them susceptible to re-infection with the homologous RV strain (data not shown), it might reduce protective efficacy against infection by heterologous RV strains.
In contrast to the case for MyD88 signaling in initial RV containment, MyD88-mediated control of RV-specific Abs was mediated by MyD88 in bone marrow-derived cells and reflected a role for inflammasome cytokines rather than TLR signaling in that it was phenocopied by combined, but not individual, absence of IL-1 and IL-18. That neither IL-18 nor IL-1β expression was significantly induced by RV but yet seemed to direct the Ab response to RV is reminiscent of studies by Pierini et al.,25 wherein IL-18-dependent IFN-γ production drove immunity to Franciscella novicida.25
That, as observed herein and by others,25,26 inflammasome cytokine signaling was needed for the generation of IgG2c raises the question as to which inflammasome might be triggering inflammasome cytokine IL-1 and IL-18 maturation. Some inflammasomes have already been implicated in the recognition of various pathogens and consequent IL-1 and IL-18 maturation. For example, free cytosolic DNA from DNA viruses vaccinia virus and MCMV, and from bacteria Francisella tularensis and Listeria monocytogenes activate the AIM2 inflammasome, and in the case of MCMV, AIM2 activation is necessary for IFNγ production via IL-18.27 Like MCMV activation of AIM2, it seems possible that an inflammasome may recognize RV infection and is involved in maturation of inflammasome cytokines and subsequent RV-specific subtype switching. Indeed, Kanneganti et al.28 found that dsRNA analog poly(I:C) and dsRNA isolated from RV triggered NLRP3-mediated caspase-1 activation with poly(I:C) also capable of eliciting IL-1β and IL-18 secretion.28 However, we did not elicit measurable IL-1β or IL-18 with RV infection, and found NLRP3 dispensable for IgG2c induction. In addition to IL-1 and IL-18 signaling, MyD88 has recently been shown to control class switch recombination via TACI signaling in B cells.29 It can be hypothesized that MyD88-mediated TACI signaling in B cells is the reason why less RV-specific Ab and IgG subtype switching occurs in mice lacking MyD88, as shown by us and others.11 Further experimentation in TACI-deficient models needs to be done to define the role of MyD88-mediated TACI signaling in IgG class switch.
As MyD88-mediated TLR signaling limited RV infectivity and spread of infection, TLRs could serve as a potential target to control RV infection in those that are chronically infected or to prevent infection in those of danger of bioterrorism or viral epidemics. Since TLR7, TLR8 and TLR9 all recognize nucleic acids and induce potent antiviral cytokines, these TLRs could be a target for RV disease treatment and prevention. In fact, some pharmaceutical companies have already done this with other viruses. For example, the TLR7 agonist imiquimod is approved for the treatment of external genital and perianal warts from human papillomavirus infection.30 Other TLRs not canonically thought to be involved in antiviral immunity could also be targeted to treat and prevent RV infection. For instance, administration of the TLR5 agonist, flagellin, prior to RV inoculation inhibits viral infectivity in mice.31 Thorough understanding of the role of MyD88 during RV infection not only expands our limited knowledge of RV-specific innate immunity, but also serves as a doorway to potential therapeutics.
Supplementary Material
Acknowledgments
Funding
This work was supported by NIH grants AI107943 and DK083890.
Footnotes
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
References
- 1.Greenberg HB and Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology 2009; 136: 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babji S and Kang G. Rotavirus vaccination in developing countries. Curr Opin Virol 2012; 2: 443–448. [DOI] [PubMed] [Google Scholar]
- 3.Vancott JL, McNeal MM, Choi AH and Ward RL. The role of interferons in rotavirus infections and protection. J Interferon Cytokine Res 2003; 23: 163–170. [DOI] [PubMed] [Google Scholar]
- 4.Broquet AH, Hirata Y, McAllister CS and Kagnoff MF. RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J Immunol 2011; 186: 1618–1626. [DOI] [PubMed] [Google Scholar]
- 5.Holloway G and Coulson BS. Innate cellular responses to rotavirus infection. J Gen Virol 2013; 94: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 6.Pott J, Mahlakoiv T, Mordstein M, et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A 2011; 108: 7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pott J, Stockinger S, Torow N, et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog 2012; 8: e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pane JA, Webster NL and Coulson BS. Rotavirus activates lymphocytes from non-obese diabetic mice by triggering toll-like receptor 7 signaling and interferon production in plasmacytoid dendritic cells. PLoS Pathog 2014; 10: e1003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Bernuth H, Picard C, Puel A and Casanova JL. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur J Immunol 2012; 42: 3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichinohe T. Respective roles of TLR, RIG-I and NLRP3 in influenza virus infection and immunity: impact on vaccine design. Exp Rev Vaccines 2010; 9: 1315–1324. [DOI] [PubMed] [Google Scholar]
- 11.Blutt SE, Akira S, Dustin L and Conner ME. MyD88 is required for viral-induced B cell activation and intestinal IgA production. J Immunol 2009; 182: 44.20.19109133 [Google Scholar]
- 12.Uchiyama R, Chassaing B, Zhang B and Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis 2014; 210: 171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenaux M, Cuadras MA, Feng N, et al. Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice. J Virol 2006; 80: 5219–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders CJ, Moore DA 3rd, Williams IR and Gewirtz AT. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol 2008; 180: 7184–7192. [DOI] [PubMed] [Google Scholar]
- 15.Chassaing B, Srinivasan G, Delgado MA, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PloS One 2012; 7: e44328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho FA, Nalbantoglu I, Ortega-Fernandez S, et al. Interleukin-1beta (IL-1beta) promotes susceptibility of Toll-like receptor 5 (TLR5) deficient mice to colitis. Gut 2012; 61: 373–384. [DOI] [PubMed] [Google Scholar]
- 17.Stoffels B, Hupa KJ, Snoek SA, et al. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 2014; 146: 176–187 e1. [DOI] [PubMed] [Google Scholar]
- 18.Lebeis SL, Bommarius B, Parkos CA, et al. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol 2007; 179: 566–577. [DOI] [PubMed] [Google Scholar]
- 19.Guay HM, Andreyeva TA, Garcea RL, et al. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol 2007; 178: 5124–5131. [DOI] [PubMed] [Google Scholar]
- 20.Zucchini N, Bessou G, Traub S, et al. Cutting edge: Overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J Immunol 2008; 180: 5799–5803. [DOI] [PubMed] [Google Scholar]
- 21.Deal EM, Lahl K, Narvaez CF, et al. Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses. J Clin Invest 2013; 123: 2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavin AL, Hoebe K, Duong B, et al. Adjuvant-enhanced Ab responses in the absence of toll-like receptor signaling. Science 2006; 314: 1936–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang SM, Yoo DG, Kim MC, et al. MyD88 plays an essential role in inducing B cells capable of differentiating into Ab-secreting cells after vaccination. J Virol 2011; 85: 11391–11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukhumavasi W, Egan CE, Warren AL, et al. TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol 2008; 181: 3464–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierini R, Perret M, Djebali S, et al. ASC controls IFN-gamma levels in an IL-18-dependent manner in caspase-1-deficient mice infected with Francisella novicida. J Immunol 2013; 191: 3847–3857. [DOI] [PubMed] [Google Scholar]
- 26.Lewkowich IP, Rempel JD and HayGlass KT. Prevention of allergen-specific, Th2-biased immune responses in vivo: role of increased IL-12 and IL-18 responsiveness. J Immunol 2005; 175: 4956–4962. [DOI] [PubMed] [Google Scholar]
- 27.Bauernfeind F and Hornung V. Of inflammasomes and pathogens—sensing of microbes by the inflammasome. EMBO Mol Med 2013; 5: 814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanneganti TD, Body-Malapel M, Amer A, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 2006; 281: 36560–36568. [DOI] [PubMed] [Google Scholar]
- 29.He B, Santamaria R, Xu W, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol 2010; 11: 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Es-Saad S, Tremblay N, Baril M and Lamarre D. Regulators of innate immunity as novel targets for panviral therapeutics. Curr Opin Virol 2012; 2: 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijay-Kumar M, Aitken JD, Sanders CJ, et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol 2008; 180: 8280–8285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.