Abstract
Background
Compared with medical therapy, catheter ablation of atrial fibrillation (AF) in patients with heart failure (HF) improves cardiovascular outcomes. Risk scores (CAAP-AF and APPLE) have been developed to predict the likelihood of AF recurrence after ablation, have not been validated specifically in patients with AF and HF.
Methods
We analyzed baseline characteristics, risk scores, and rates of AF recurrence 12 months post-ablation in a cohort of 230 consecutive patients with AF and HF undergoing PVI in the Duke Center for Atrial Fibrillation registry from 2009–2013.
Results
During a follow-up period of 12 months, 76 of 230 (33%) patients with HF experienced recurrent AF after ablation. The median APPLE and CAAP-AF scores were 1.5 [(Q1, Q3): (1.0, 2.0)] and 4.0 [(Q1, Q3): (3.0, 5.0)], respectively and were not different from those patients with and without recurrent AF. Freedom from AF was not different according to APPLE and CAAP-AF scores. Discrimination for recurrent AF with the CAAP-AF score was modest with a C-statistic of 0.60 (95% CI 0.52 – 0.67). Discrimination with the APPLE score was similarly modest, with a c-statistic of 0.54 (95% CI: 0.47 – 0.62).
Conclusions
Validated predictive risk scores for recurrent AF after catheter ablation exhibit limited predictive ability in cohorts of AF and HF. Additional tools are needed to facilitate risk stratification and patient selection for AF ablation in patients with concomitant HF.
Keywords: atrial fibrillation, catheter ablation, congestive heart failure, pulmonary vein isolation
Introduction
AF and heart failure (HF) frequently co-exist due to shared risk factors and pathophysiology. Catheter ablation of AF in patients with HF improves quality of life, left ventricular ejection fraction, and survival compared to pharmacologic rhythm control. 1–3 Despite its benefits, catheter ablation is an invasive procedure with a small, but measurable risk of major adverse events. The proportion of patients with HF who maintain normal sinus rhythm after catheter ablation is variable. 4–7 The ability to select patients with HF and AF most likely to benefit from an invasive rhythm control strategy is therefore highly desirable. Some patient characteristics have been associated with lower ablation success, such as atrial fibrosis, left atrial (LA) size, persistent forms of AF, number of failed anti-arrhythmic drugs, hypertension, and obstructive sleep apnea (OSA). 4, 5
Several risk scores have been developed for the prediction of recurrent AF after catheter ablation. The CAAP-AF score was developed in a derivation cohort of 1,125 AF patients undergoing catheter ablation. CAAP-AF scores range from 0–13, incorporating a composite of known risk factors for AF recurrence after ablation including coronary artery disease, left atrial diameter, age, presence of persistent AF, number of anti-arrhythmic drugs failed, and female gender.8 CAAP-AF was subsequently verified in a 937 patient validation cohort.8 The APPLE risk score was derived from a 1,145 patient cohort and ranges from 0–5 and includes age over 65, persistent AF, impaired renal function, left atrial enlargement and left ventricular ejection fraction (LVEF) less than 50%.9 Despite the development of these risk scores, they are routinely implemented to stratify prospective ablation candidates in the setting of concomitant HF. Cohorts for both the APPLE and CAAP-AF risk scores contained only a small number of patients with clinical HF. The objective of this study was to assess the ability of the CAAP-AF and APPLE risk scores to predict recurrent AF following catheter ablation in the specific population of patients with concomitant HF.
Methods
Study design and population
The study was an observational, retrospective cohort analysis of patients with AF and HF undergoing de novo pulmonary vein isolation in the Duke Center for Atrial Fibrillation. The study was approved by the Duke University Institutional Review Board.10 Catheter ablation procedures performed from January 1, 2007 through June 30, 2013 were retrospectively reviewed for inclusion. Patients 18 years of age and older, undergoing their first catheter ablation procedure for AF with a clinical diagnosis of HF as determined by the Framingham criteria were included in the study. Patients with a baseline LVEF>50% were defined as having heart failure with preserved ejection fraction (HFpEF) and those with LVEF <50% as heart failure with reduced ejection fraction (HFrEF). Patients without a baseline measurement of LVEF or follow-up data and those with catheter ablation other than radiofrequency ablation (e.g. surgical ablation, cryoballoon or laser balloon) were excluded. Manual chart extraction was used to determine baseline demographics, medical history, laboratory data and medications (including antiarrhythmic drug use) prior to the index ablation.
APPPLE and CAAP-AF Score Assignment
CAAP-AF scores were calculated as demonstrated in Table 1 using baseline characteristics by assigning one point for coronary artery disease, 0–4 points for atrial diameter (0 for anterior-posterior diameter <1cm, 1 point for 4.0–4.5 cm, 2 points for 4.5–5.0 cm, 3 points for 5.0–5.5 cm, and 4 points for >5.5 cm), 0–3 points for age (0 for age <50, 1 point age 50–60, 2 points age 60–70, and 3 points age>70), 2 points for persistent AF, 0–2 points for prior failed anti-arrhythmic drugs (0 points for none, 1 point for one or two failed AAD, and 2 points for >2 failed AAD), and 1 point for female gender.8 The APPLE score was calculated using baseline characteristics as shown in Table 1 with each patient being assigning one point for each of the following factors: age > 65, persistent AF, impaired eGFR (<60mL/min/1.73m2), and LA diameter >43 mm, LVEF <50%.9
Table 1.
CAAP-AF and APPLE Risk Scores.
| CAAP-AF | Value | APPLE | Value |
|---|---|---|---|
| Coronary artery disease | 1 | Age > 65 | 1 |
| Left atrial diameter (cm) | Persistent AF | 1 | |
| <4 | 0 | eGFR < 60 | 1 |
| 4– <4.5 | 1 | Left atrial diameter > 43 mm | 1 |
| 4.5– <5.0 | 2 | LVEF <50% | 1 |
| 5– <5.5 | 3 | Range | 0–5 |
| >=5.5 | 4 | ||
| Age | |||
| <50 | 0 | ||
| 50– <60 | 1 | ||
| 60– <70 | 2 | ||
| >=70 | 3 | ||
| Persistent or longstanding AF | 2 | ||
| Antiarrhythmic drugs failed | |||
| None | 0 | ||
| 1 or 2 | 1 | ||
| >2 | 2 | ||
| Female Gender | 1 | ||
| Range | 0–13 | ||
AF = atrial fibrillation. eGFR = estimated glomerular filtration rate. LVEF = left ventricular ejection fraction.
Patient groups and outcomes
Arrhythmia recurrence was defined as atrial tachycardia, atrial flutter or atrial fibrillation (AT/AF/AFL) captured on 12-lead ECG, lasting >30 seconds on ambulatory monitoring or implantable device, or requiring cardioversion.10 Presence of AF symptoms was not required for the diagnosis of arrhythmia recurrence.
Radiofrequency ablation procedure
Our ablation protocol for this patient cohort has been previously described.10 Patients underwent pre-procedural axial imaging with computed tomography or magnetic resonance imaging to define left atrial and pulmonary vein anatomy. Pulmonary-vein isolation (PVI) was performed using point-to-point circumferential ablation with non-contact force sensing open-tipped irrigated catheters. Entrance and exit block was routinely confirmed with multi-electrode catheters, pacing, and adenosine. Additional ablation beyond PVI was performed at the operator’s discretion, as previously described.11 Electroanatomic mapping systems were utilized in all cases (CARTO (Biosense-Webster Inc, Diamond Bar, CA) or NavX, St Jude Medical, Inc, Minneapolis, MN).
Post-ablation management and ambulatory monitoring
In addition to in-office visits with 12-lead ECG at 3, 6, and 12 months, ambulatory ECG monitoring or device interrogations were obtained in the presence of symptoms to ascertain arrhythmia recurrence as we have previously described.11 Continuation of anti-arrhythmic drug therapy was left to the discretion of the primary electrophysiologist, with roughly half of patients remaining on anti-arrhythmic drug therapy at 12-month follow-up as previously described.10 Both New York Heart Association (NYHA) functional class and Mayo AF Symptom Inventory (MAFSI) were used to assess symptomatic status by standardized patient interviews conducted by the same clinician at baseline and structured follow-up.12
Statistical analysis
Continuous variables are reported using means with standard deviations for normally distributed variables and medians with interquartile 25th (Q1) and 75th (Q3) percentiles for variables that were not normally distributed. Univariable comparisons of continuous variables were performed using the Wilcoxon rank sum test if data were not normally distributed or student’s t-test if normally distributed. Categorical variables are described by counts and percentages for non-missing data, with chi-square test or Fisher’s exact test (expected cell counts < 5) used for univariable comparisons as appropriate.
To assess the predictive ability of the CAAP-AF and APPLE scores, logistic regression modeling was used with AF recurrence as the outcome and score (categorical) as the single predictor. A sensitivity test was also conducted to see if the predictive ability improved when each score was instead treated as a continuous ordinal variable. To assess and visualize model fit, calibration plots were constructed to compare the estimated probability of an event to the true probability. Additionally, receiver operating characteristic (ROC) curves with c-statistics were calculated to assess the ability of the model to discriminate. The predictive ability of each score to predict changes in symptom status by MAFSI or NYHA score was assessed using a generalized linear regression model, with change in symptom status as the outcome and baseline APPLE or CAAP-AF score as single predictor.
Arrhythmia-free recurrence stratified by CAAP-AF and APPLE score was analyzed using the Kaplan-Meier method with log-rank significance testing and a 30-day blanking period. A p-value < 0.05 indicated statistical significance, and all statistical analyses were performed by the Department of Biostatistics and Bioinformatics, Duke University Medical Center using SAS version 9.4 (SAS Institute, Cary NC).
Results
Patient population
A total of 230 patients with clinical HF underwent ablation during the study period, with 76 (33%) developing recurrent AF over the course of the one year follow-up period (Table 2). The proportion of patients HFrEF did not differ in those patients with and without AF recurrence (55.8% versus 56.6%, p=0.916; Table 2). Compared to patients with recurrent AF, patients free from AF had similar age (Median [Q1,Q3]: 66 [59.0, 74.0] vs. 67 [57.0, 73.0], p =0.73), LA diameter (Mean [SD]: 4.5 [0.8] vs. 4.7 [0.8], p=0.19), eGFR (Median [Q1,Q3]: 89.4 [68.9,115.3] vs. 94.6 [72.0, 137.4], p=0.28) body mass index (Median [Q1,Q3]: 31.0 [27.7, 36.7] vs. 32 [29.4, 37.4], p=0.33), and gender (31.8% vs. 27.6% female, p=0.52) (Table 2). The prevalance of relevant co-morbidities such as diabetes mellitus, coronary artery disease, and obstructive sleep apnea did not differ according to AF recurrence (Table 2). All patients (n=230) underwent pulmonary vein isolation, while a significant proportion of patients underwent ablation of non-pulmonary vein triggers including mitral isthmus line (n=26, 11%), left atrial roof line (n=93, 40%), ablation of complex fractionated electrograms (n=53, 23%), and coronary sinus ablation (n=30, 13%). Thirty-two (14%) patients underwent concomitant isolation of the cavotricuspid isthmus. Roughly three-quarters of patients received some form of ambulatory monitoring during the follow-up period in the form of either ambulatory Holter monitoring or implantable device interrogation (Table 3).
Table 2.
Patient Characteristics by AF Recurrence
| Characteristic | Overall (N=230) | AF Free (N=154) | AF Recurrence (N=76) | p value |
|---|---|---|---|---|
| Age (years) | 66.0 (59.0, 74.0) | 66.0 (59.0, 74.0) | 67.0 (57.0, 73.0) | 0.726 |
| Female | 70 (30.4%) | 49 (31.8%) | 21 (27.6%) | 0.516 |
| BMI (kg/m2) | 31.4(27.7, 37.1) | 31.0 (27.7, 36.7) | 32.2 (28.4, 37.4) | 0.331 |
| AF Type | ||||
| Paroxysmal | 80 (34.8%) | 54 (35.1%) | 26 (34.2%) | 0.484 |
| Persistent | 48 (20.9%) | 36 (23.4%) | 12 (15.8%) | |
| Long-standing | 54 (23.5%) | 35 (22.7%) | 19 (25.0%) | |
| Persistent | ||||
| Baseline NYHA Class | ||||
| I | 67 (33.3%) | 43 (32.3%) | 24 (35.3%) | 0.671 |
| II | 103 (51.2%) | 71 (53.4%) | 32 (47.1%) | |
| III/IV | 31 (15.4%) | 19 (14.3%) | 12 (17.6%) | |
| eGFR (mL/min) | 92.1 (69.8, 122.2) | 89.4 (68.9, 115.3) | 94.6 (72.0, 137.4) | 0.278 |
| Left atrial diameter (cm) | 4.6 (0.8) | 4.5 (0.8) | 4.7 (0.8) | 0.190 |
| LVEF <50% | 129 (56.1%) | 86 (55.8%) | 43 (56.6%) | 0.916 |
| Coronary artery disease | 103 (45.0%) | 74 (48.1%) | 29 (38.7%) | 0.180 |
| Hypertension | 192 (83.5%) | 133 (86.4%) | 59 (77.6%) | 0.094 |
| Diabetes mellitus | 58 (25.4%) | 38 (25.0%) | 20 (26.3%) | 0.830 |
| COPD | 26 (11.4%) | 17 (11.2%) | 9 (11.8%) | 0.883 |
| OSA | 0.934 | |||
| None | 133 (58.1%) | 90 (58.8%) | 43 (56.6%) | |
| Yes, untreated | 28 (12.2%) | 18 (11.8%) | 10 (13.2%) | |
| Yes, treated | 68 (29.7%) | 45 (29.4%) | 23 (30.3%) | |
| Stroke/TIA | 34 (15.0%) | 25 (16.6%) | 9 (12.0%) | 0.367 |
| Mitral Regurgitation | 32 (13.9%) | 21 (13.6%) | 11 (14.5%) | 0.863 |
| AAD failed | 0.545 | |||
| None | 81 (35.2%) | 57 (37.0%) | 24 (31.6%) | |
| 1–2 | 148 (64.3%) | 96 (62.3%) | 52 (68.4%) | |
| >2 | 1 (0.4%) | 1 (0.6%) | 0 (0.0%) |
Note: Categorical data presented as count (percentage) and continuous data as mean (standard deviation) or median (Q1, Q3). AAD = anti-arrhythmic drug. AF = atrial fibrillation. BMI = body mass index (kg/m2). COPD = chronic obstructive pulmonary disease. NYHA = New York Heart Association Functional Classification. LVEF = left ventriuclar ejection fraction. OSA = obstructive sleep apnea. TIA = transient ischemic attack.
Table 3.
Follow-up and anti-arrhythmic drug use.
| Monitoring | n (%) |
|---|---|
| Any Monitoring* | 167 (73%) |
| Ambulatory monitor# | 96 (42%) |
| 3 months | 59 (26%) |
| 6 months | 28 (12%) |
| 9 months | 9 (4%) |
| 12 months | 9 (4%) |
| 12-month outcome | |
| ECG recurrence | 74 (32%) |
| AAD use at 12 months | |
| None | 113 (49%) |
| Class IC | 10 (4%) |
| Class III | 107 47%) |
ECG recurrence = Electrocardiographic recurrence was defined as atrial tachycardia, atrial flutter or atrial fibrillation (AT/AF/AFL) captured on 12-lead ECG, lasting >30 seconds on ambulatory monitoring or implantable device, or requiring cardioversion. AAD = antiarrhythmic drug.
“Any Monitoring” includes patients with AF recurrence monitoring by device interrogation or ambulatory monitor.
Ambulatory monitor group includes use of 24-hour Holter monitoring, event monitors, and implantable loop recorders.
Freedom from AF Based on CAAP-AF and APPLE Scores
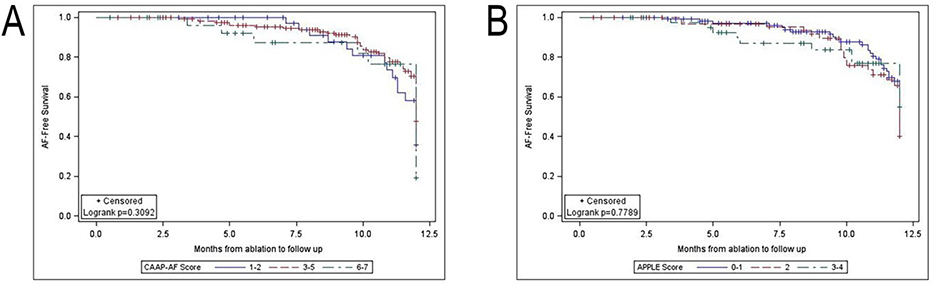
Freedom from recurrent AF according to the CAAP-AF and APPLE scores are reported in Table 4. There was no significant increase in the proportion of patients with recurrent AF at higher CAAP-AF or APPLE scores. As demonstrated in Figure 1A and B, AF-free survival was not significantly different in patients stratified by CAAP-AF or APPLE scores.
Table 4.
Recurrence Rates by APPLE and CAAP-AF Score.
| APPLE Score | Recurrent AF/Total n=76/230 | CAAP AF | Recurrent AF/Total n=76/230 |
|---|---|---|---|
| 0 | 11/37 (30%) | 1 | 4/13 (31%) |
| 1 | 27/78 (35%) | 2 | 13/29 (45%) |
| 2 | 26/71 (37%) | 3 | 19/55 (35%) |
| 3 | 10/35 (29%) | 4 | 15/47 (32%) |
| 4 | 2/9 (22%) | 5 | 14/58 (24%) |
| 6 | 11/25 (44%) | ||
| ≥7 | 0/3 (0%) |
Numerator = recurrent AF; Denominator = total patients. Recurrence rate in parenthesis. AF = atrial fibrillation.
Figure 1.
Kaplan-Meier curves for freedom from AF after ablation by CAAP-AF (A) and APPLE (B) scores. CAAP-AF scores grouped into 1–2, 3–5, and 6–7. APPLE scores grouped into 0–1, 2, and 3–4. AF recurrence was defined as electrocardiographic recurrence. 95% confidence intervals are denoted by error bars.
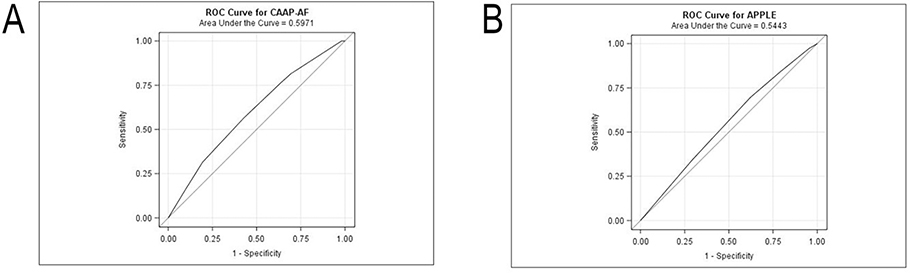
When evaluating score as a categorical predictor, discrimination with the CAAP-AF score was modest, with a c-statistic of 0.60 (95% CI: 0.52 – 0.67) (Figure 2A). Similarly, discrimination for recurrent AF with the APPLE score was modest with a c-statistic of 0.54 (95% CI: 0.47 – 0.62) (Figure 2B). The predictive ability of neither model was improved by treating score as an ordinal variable.
Figure 2.
Receiver operating curves for the CAAP-AF (A) and APPLE (B) scores. Sensitivity is displayed on y axis and 1-specificity on x axis.
Symptomatic Improvement after Ablation Based on CAAP-AF and APPLE Scores
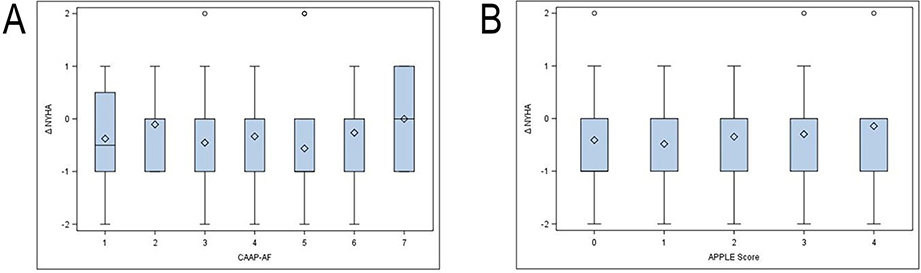
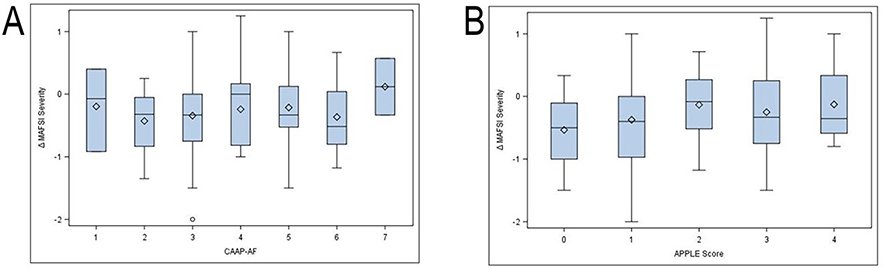
Patients experienced significant improvements NYHA functional classification and MAFSI symptom severity and frequency across the full spectrum of CAAP-AF and APPLE scores (Figures 3 and 4). Baseline CAAP-AF score did not predict the magnitude of symptomatic improvement by MAFSI or CAAP-AF score (Figure 3A and 4A). Similarly, baseline APPLE score was not predictive of symptomatic improvement at follow-up (Figure 3B and 4B).
Figure 3.
NYHA Class improvement by baseline CAAP-AF (A) and APPLE (B) score. Values are mean change in symptom score between baseline and follow-up. Error bars represent 95% confidence intervals. bars represent standard deviations. MAFSI symptom severity from 0 = mild, 2 = moderate, 4 = severe.
Figure 4.
MAFSI improvement by baseline CAAP-AF (A) and APPLE (B) score. Values are mean change in symptom score between baseline and follow-up. Error bars represent 95% confidence intervals. bars represent standard deviations. MAFSI symptom severity from 0 = mild, 2 = moderate, 4 = severe.
Discussion
Clinical trial data have shown that catheter ablation of AF leads to improved outcomes in patients with HF. 2, 7 However, very little is known about which HF patients with AF are most likely derive benefit from ablation. More specifically, there are few data available on risk stratification among patients with HF and AF undergoing ablation. There are 3 main findings in this observational cohort of patients with HF undergoing catheter ablation of AF. First, the CAAP-AF and APPLE risk scores demonstrated a modest ability to predict recurrent AF after catheter ablation. Second, there were a paucity of distinguishing characteristics between HF patients with and without AF recurrence after ablation. Finally, and perhaps most importantly, patients across all risk strata derived symptomatic benefit from AF ablation. These findings highlight an unmet need for additional tools to better understand which HF and AF most likely to benefit from catheter ablation from both a symptoms and quality of life standpoint and from a cardiovascular outcomes perspective.
Despite the cumulative evidence supporting improved outcomes with catheter ablation in patients with AF and HF, little is known about the heterogeneity of treatment effects. Moreover, there are no prospective studies of risk stratification for recurrent AF in this important patient population. We found the descriminative capacity for the CAAP-AF and APPLE scores had modest predictive capacity in patients with AF and HF, with c statistics of 0.60 and 0.54, respectively. By comparison, the c-statistic for the CAAP-AF in the original test cohort was 0.65 and the APPLE score c-statistic in the original derivation cohort was 0.63.8, 9 Simlarly, a validation cohort of patients with persistent and paroxysmal AF undergoing cryoablation found a c-statistic of 0.71 to predict AF recurrence for a CAAP-AF score >5.13 APPLE similarly outperformed CHADS2 in predicting arrythmia recurrence in an external validation cohort of patients undergoing repeat catheter ablation for recurrrent AF in The Leipzig Heart Center Ablation Registry, with a c statistic of 0.617, p=0.002.14
The traditional risk factors for recurrent AF incorporated into the CAAP-AF and APPLE scores such as gender, LA size, LVEF<50%, and body mass index did not differ between patients with and without AF recurrence. Considering that these risk factors were reproducibly derived from numerous cohorts of patients without HF undergoing ablation for paroxysmal and persistent AF, they clearly have prognostic value in broader populations of patients with AF undergoing catheter ablation. 8, 9 However, their predictive ability may be much more limited among patients with AF and HF.8, 9
Risk scores for post-ablation AF recurrence have been postulated to predict the extent of atrial fibrosis and electrical remodeling.8, 15 In support of these assertions, the APPLE score predicts the extent of atrial low voltage areas (LVA) on electroanatomic mapping.13, 14 Prior studies have also demonstrated that LA volume and persistent AF do not predict AF recurrence when adjusted for extent of left atrial fibrosis as determined by delayed gadolinium enhancement on cardiac magnetic resonance imaging (MRI).15,16 Potentially, a greater extent of background atrial remodeling in patients with AF and HF could limit the descriminative capacity of traditional risk factors for AF recurrence. In support of more advanced atrial remodeling in our cohort, the mean LA diameter was 4.6±0.8 cm, compared to 4.3±0.7 cm in CAAP-AF and 4.3±0.6 cm in APPLE derivation cohorts (for reference, the median left atrial diameter in CASTLE-AF was 4.8 cm). Although the multicenter prospective Delayed-Enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation (DECAAF) study demonstrated that greater degrees of atrial fibrosis conferred higher risk for atrial fibrillation recurrence, the cohort did not contain a significant number of patients with clinical HF.15 Another potential hypothesis to explain the limited discriminatory capacity of these scores is that recurrence in patients with AF and HF may be primarily due to a higher prevalence of non-PV triggers, with which the APPLE and CAAP-AF scores may have a poor or more limited association.
Considering the evidence supporting catheter ablation in appropriately selected patients with HF and AF, improved tools are needed to identify patients most likely to benefit. It is possible that improved phenotyping of the underlying atrial substrate in patients with AF and HF, perhaps with the use of cardiac MRI or echocardiographic strain imaging, could improve our ability to predict ablation success. Additional studies are needed to determine the impact of atrial fibrosis burden estimated by delayed enhancement MRI on ablation outcomes in patients with AF and HF.15,16 Assessment of ventricular scar burden may also assist in identifying HF patients most likely to benefit from catheter ablation of AF. In the CAMERA-MRI study, LVEF improvement and normalization at 6-month follow-up were more likely to occur in patients without ventricular late gadolinium enhnacement on pre-ablation cardiac MRI.17 In CASTLE-AF, patients with LVEF>25% were more likely to benefit from catheter ablation than those with LVEF<25%.2 It is also worthwhile considering if a binary definition of AF ablation procedural success (presence or absence of recurrent atrial fibrillation) remains valid considering data from CASTLE-AF demonstrating that reduction in AF burden was associated with improved outcomes, even in patients with recurrent AF.
It is also unclear if patient characteristics and comorbidities predict better post-ablation outcomes. There were no significant differences between post-ablation outcomes among patients with non-ischemic and ischemic cardiomyopathy in CASTLE-AF, and patients with NYHA class II HF symptoms benefitted more from catheter ablation than those with more advanced symptoms.2 The presence of diabetes mellitus appeared to attenuate the benefits of catheter ablation, although the effect did not reach statistical significance. 2 As more granular genetic assessement of HF susceptibility alleles is generated by genome-wide association studies, specific HF genotypes most likely to benefit from AF ablation could potentially be identified.18 New clinical variables associated with outcomes post-ablation in patients with HF and AF are also likely be identified from eagerly anticipated long-term follow-up data from patients enrolled in the CABANA study, the largest and longest prospective randomized trial of catheter ablation in AF to date.19
Another key finding in our analysis was the observation of similar symptomatic benefit across the spectrum of CAAP-AF and APPLE scores. This observation is reassuring as a primary motiviation to perform AF ablation is to reduce symptoms and improve quality of life. Whether or not improvements in cardiovascular outcomes are also independent of baseline risk remains unclear but should be a focus of future analyses.
Limitations
Our study has several important limitations. This was a single center observational study and therefore electrocardiographic or symptomatic AF recurrences that were detected at outside instutions may have been missed. T. Furthermore, although the majority of patients received some form of ambulatory monitoring, rhythm monitoring strategy was not used systematically in the study cohort.The retrospective design of the trial limits any causal inferences regarding the impact of any specific variables on ablation outcomes. The study period predates several several important technological advances in catheter ablation, limiting the generalizability of our findings to contemporary cohorts.The sample size of our study cohort and number of outcomes were modest. Finally, our study focused on validation of existing risk scores rather than identification of novel risk scores.
Conclusion
In conclusion, while the APPLE and CAAP-AF risk scores can identify patients undergoing AF ablation who are at high risk of recurrence, our findings suggest that these scores may have limited utility among patients with AF and HF. Importantly, patients with AF and HF appear to derive symptomatic improvement regardless of their baseline risk. Further studies are needed to help identify subgroups of patients with AF and HF most likely to benefit from AF catheter ablation.
Acknowledgments
Funding
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL143156 (to BAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
EBM, XR, CLG, ASB, NSR, SMA, CFM, AOG, DDH, KPJ, LRJ, JIK, RKL, AYS, and KLT have no relevant disclosures. BAS reports fellowship support from Boston Scientific, St. Jude Medical, Medtronic, Janssen Pharmaceuticals, and Bristol Meyers Squibb. BAD has received research funding from Abbott and Boston Scientific; has served on advisory boards for Abbott, Biotronik, and Medtronic; has served as a consultant for Abbott and Biosense Webster; and has served as a speaker for Medtronic.TBD has received research grants from Abbott, CardioFocus, the National Institutes of Health, and Boston Scientific; has served as a consultant for CardioFocus and Ventrix; is a named investigator on institutional clinical research grant support from Biosense Webster, Boston Scientific, and St. Jude Medical; and has performed case proctoring for CardioFocus Inc. JPD receives research funding from St Jude Medical. JPD reports receiving honaria and/or research support from Boston Scientific, Biosense Webster, and St Jude Medical. JPP receives grants for clinical research from Abbott, American Heart Association, Boston Scientific, NHLBI, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, Johnson & Johnson, LivaNova, Medtronic, Milestone, Oliver Wyman Health, Sanofi, Philips, and Up-to-Date. SDP as received research grants from Boston Scientific, Janssen Pharmaceuticals, Bristol-Myers Squibb, Pfizer, and Gilead; and has received consulting/advisory board support from Boston Scientific, Medtronic, Janssen Pharmaceuticals, Bristol-Myers Squibb, Pfizer, and Portola. DAF has received salary support from the National Institutes of Health; research support from the National Cardiovascular Data Registry, Boston Scientific, Biosense Webster, and Abbott; and educational grants from Boston Scientific, Medtronic, Abbott, and Biotronik.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. [DOI] [PubMed] [Google Scholar]
- 2.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Eng J Med. 2018;378(5):417–27. [DOI] [PubMed] [Google Scholar]
- 3.Turagam MK, Garg J, Whang W, Sartori S, Koruth JS, Miller MA, et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Annals of internal medicine. Ann Intern Med 2018. December 25. doi: 10.7326/M18-0992 [DOI] [PubMed] [Google Scholar]
- 4.Winkle RA, Mead RH, Engel G, Patrawala RA. Long-term results of atrial fibrillation ablation: the importance of all initial ablation failures undergoing a repeat ablation. Am Heart J. 2011;162(1):193–200. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava M, Di Biase L, Mohanty P, Prasad S, Martin DO, Williams-Andrews M, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009;6(10):1403–12. [DOI] [PubMed] [Google Scholar]
- 6.Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7(1):31–8. [DOI] [PubMed] [Google Scholar]
- 7.Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation. 2016;133(17):1637–44. [DOI] [PubMed] [Google Scholar]
- 8.Winkle RA, Jarman JW, Mead RH, Engel G, Kong MH, Fleming W, et al. Predicting atrial fibrillation ablation outcome: The CAAP-AF score. Heart Rhythm. 2016;13(11):2119–25. [DOI] [PubMed] [Google Scholar]
- 9.Kornej J, Hindricks G, Shoemaker MB, Husser D, Arya A, Sommer P, et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015;104(10):871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace. 2018;20(1):157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black-Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS, et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. 2018;15(5):651–7. [DOI] [PubMed] [Google Scholar]
- 12.Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55(21):2308–16. [DOI] [PubMed] [Google Scholar]
- 13.Sanhoury M, Moltrasio M, Tundo F, Riva S, Dello Russo A, Casella M, et al. Predictors of arrhythmia recurrence after balloon cryoablation of atrial fibrillation: the value of CAAP-AF risk scoring system. J Interv Card Electrophysiol. 2017;49(2):129–35. [DOI] [PubMed] [Google Scholar]
- 14.Kornej J, Hindricks G, Arya A, Sommer P, Husser D, Bollmann A. The APPLE Score - A Novel Score for the Prediction of Rhythm Outcomes after Repeat Catheter Ablation of Atrial Fibrillation. PloS One. 2017;12(1):e0169933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement mri and atrial fibrillation catheter ablation: The DECAAF study. JAMA. 2014;311(5):498–506. [DOI] [PubMed] [Google Scholar]
- 16.Kornej J, Buttner P, Sommer P, Dagres N, Dinov B, Schumacher K, et al. Prediction of electro-anatomical substrate using APPLE score and biomarkers. Europace. 2019;21(1):54–9. [DOI] [PubMed] [Google Scholar]
- 17.Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J Am Coll Cardiol. 2017;70(16):1949–61. [DOI] [PubMed] [Google Scholar]
- 18.Aragam KG, Chaffin M, Levinson RT, McDermott G, Choi SH, Shoemaker MB, et al. Phenotypic Refinement of Heart Failure in a National Biobank Facilitates Genetic Discovery. Circulation. 2018; doi:10.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Moretz K, et al. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design. Am Heart J. 2018;199:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]