Patients with IBD are less likely to be placed on pharmacologic venous thromboembolism prophylaxis, and hematochezia seems to be the major driver. Additionally, prophylaxis is not associated with evidence of increased bleeding, regardless of whether it is given in the setting of hematochezia.
Keywords: ulcerative colitis, Crohn’s disease, deep vein thrombosis, pulmonary embolism
Abstract
Background
Despite increased risk of venous thromboembolism (VTE) among hospitalized patients with inflammatory bowel disease (IBD), pharmacologic prophylaxis rates remain low. We sought to understand the reasons for this by assessing factors associated with VTE prophylaxis in patients with IBD and the safety of its use.
Methods
This was a retrospective cohort study conducted among patients hospitalized between January 2013 and August 2018. The primary outcome was VTE prophylaxis, and exposures of interest included acute and chronic bleeding. Medical records were parsed electronically for covariables, and logistic regression was used to assess factors associated with VTE prophylaxis.
Results
There were 22,499 patients studied, including 474 (2%) with IBD. Patients with IBD were less likely to be placed on VTE prophylaxis (79% with IBD, 87% without IBD), particularly if hematochezia was present (57% with hematochezia, 86% without hematochezia). Among patients with IBD, admission to a medical service and hematochezia (adjusted odds ratio 0.27; 95% CI, 0.16–0.46) were among the strongest independent predictors of decreased VTE prophylaxis use. Neither hematochezia nor VTE prophylaxis was associated with increased blood transfusion rates or with a clinically significant decline in hemoglobin level during hospitalization.
Conclusion
Hospitalized patients are less likely to be placed on VTE prophylaxis if they have IBD, and hematochezia may drive this. Hematochezia appeared to be minor and was unaffected by VTE prophylaxis. Education related to the safety of VTE prophylaxis in the setting of minor hematochezia may be a high-yield way to increase VTE prophylaxis rates in patients with IBD.
Introduction
Hospitalized patients with inflammatory bowel disease (IBD) have a 3-fold increased risk of venous thromboembolism (VTE), with this risk increasing to 6-fold when IBD patients are hospitalized with active disease.1–4 Recent data have even suggested that this risk may persist for several weeks postdischarge.5–7 Given the significant morbidity and mortality associated with VTE among IBD patients8 and the rising prevalence of IBD in North America,9–11 much emphasis has been placed on VTE prevention. Despite this, prior data suggest an increasing prevalence of VTE events among IBD patients.12
To decrease the risk of VTE among hospitalized IBD patients, multisociety guidelines currently recommend the use of pharmacologic VTE prophylaxis for ulcerative colitis (UC) patients admitted with a disease flare who do not have severe bleeding.13–16 Given the increased overall risk of VTE in IBD patients, many guidelines and experts additionally recommend prophylaxis among all hospitalized patients with UC or Crohn’s disease (CD), regardless of whether admission is due to a disease-related flare.5, 17
Despite evidence regarding the risk of VTE among hospitalized IBD patients, adherence to these guidelines remains poor.18 The reasons for this are uncertain, and data examining risk factors related to pharmacologic VTE prophylaxis among IBD patients are limited. In this study, we aimed to understand why VTE prophylaxis was not prescribed in patients with IBD by identifying risk factors associated with lack of VTE prophylaxis. We also sought to provide data regarding the safety of VTE prophylaxis.
METHODS
Population
We conducted a retrospective cohort study among all unique patients 18 years of age or older who were admitted to the general medicine or colorectal surgery services at NewYork-Presbyterian Hospital/Columbia University Medical Center for ≥48 hours between January 2013 and August 2018. In order to ensure that patients admitted more frequently would not be disproportionately represented, only the patient’s first admission within the study period was selected for analysis. Patients admitted with an acute VTE were excluded, with acute VTE classified based on International Classification of Diseases, Clinical Modification codes (ICD-CM; see appendix).19 Within this larger cohort, patients with IBD were initially identified using ICD-9-CM and ICD-10-CM diagnosis codes for ulcerative colitis (UC: 556.x, K51.x) and Crohn’s disease (CD: 555.x, K50.x); these charts were then manually reviewed for accuracy. Patients with IBD were further subclassified as having UC or CD and as having or not having a flare based on endoscopic and clinical documentation. This study was approved by the institutional review board of Columbia University Medical Center.
Primary Outcome
The primary outcome was VTE prophylaxis, classified categorically. Prophylaxis was recorded as having been given if an active order for heparin, low molecular weight heparin, warfarin, apixiban, rivaroxaban, or dabigatran was placed within the electronic medical record (EMR) within 48 hours of presentation to the hospital. Of note, because patients with an acute VTE were excluded, those who were placed on agents such as warfarin, apixiban, rivaroxaban or dabigatran within 48 hours of presentation were likely being continued on their outpatient medications. We also classified patients whose international normalized ratio (INR) was ≥2 within the 48-hour period as being on prophylaxis, because the coagulative state of these patients would not warrant additional prophylaxis.
Exposures of Interest
Our a priori hypothesis was that lower gastrointestinal bleeding, even if trivial, would be a major driver of decreased VTE prophylaxis use. We therefore designed exposure variables that would capture both acute and chronic blood loss: admission hemoglobin as measured at presentation, classified categorically as <7 g/dL, 7 to 11 g/dL, >11 g/dL; delta hemoglobin, a continuous variable defined by change in hemoglobin from admission to nadir (of note, if hospitalization was >30 days, the nadir within the first 30 days of hospitalization was used); and hematochezia, identified by documentation of bright red blood per rectum on presentation based on manual chart review. To ensure accuracy, all charts of IBD patients with hematochezia were also reviewed to ensure no upper endoscopy was performed during hospitalization for suspected upper gastrointestinal bleeding. Last, we examined units of packed red blood cell transfusions (pRBCs) as a continuous variable throughout admission (if the hospitalization was >30 days, the total number of pRBCs within the first 30 days was used). To account for patients with large rather than small volume bleeding, we also included transfusions as a categorical variable, characterizing patients as having a major transfusion requirement if 3 or more pRBCs were transfused.
Covariables
Demographic and baseline information relating to age at admission, race/ethnicity, admitting service (medicine vs surgery), admission INR, and history of VTE (see appendix) were identified within the EMR using automated queries. Initial platelet value at presentation was also captured from the EMR and classified as a 3-level categorical variable (>150 × 109/L, 50–150 × 109/L, <50 × 109/L). A Charlson comorbidity index was calculated using the existing ICD-9-CM and ICD-10-CM codes associated with chronic health conditions at the time of admission.20 Patients were then categorized based on the number of underlying comorbid conditions (0, 1, ≥2 conditions). Medication data, including corticosteroid, immunomodulator, and biologic use during hospitalization, were also manually recorded for inclusion in our analysis.
Statistical Approach
Categorical variables were compared using a χ 2 test or Fisher exact test if the expected count was <5. Continuous variables were compared using a t test or Wilcoxon rank sum test if they had a non-normal distribution. Logistic regression modeling was performed first to evaluate factors associated with VTE prophylaxis among patients with IBD and second to evaluate factors associated with VTE prophylaxis among all patients. To build the final multivariable models for predictors of VTE prophylaxis, all variables with a P value ≤0.1 on univariable analysis were examined in a full model. The final, reduced model was then produced through stepwise subtraction, retaining variables with an independent relationship with VTE prophylaxis (P < 0.05). Charlson comorbidity index and history of VTE were included in the final model based on an a priori decision given their established association with VTE prophylaxis. Additional subgroup analyses compared UC with Crohn’s disease patients and those with and without disease flares. All analyses were performed on STATA 15 (College Station, TX) at the alpha 0.05 level of significance with 2-sided testing.
RESULTS
Population
A total of 22,499 patients were included in the study, 474 (2.1%) of whom had IBD. Among IBD patients, 43.7% had UC, 55.3% had CD, and 1.0% had indeterminate colitis. The majority (60%) of IBD patients were under 50 years old, with 26% between the ages of 18 and 30. Of the 474 IBD patients, 457 (96%) presented with a known history of IBD, 328 (69%) were admitted with an IBD flare, and 112 (24%) were admitted with hematochezia. Of those with hematochezia, 77 (69%) patients had UC, 34 (30%) had Crohn’s disease, and 1 (1%) had indeterminate colitis.
VTE Prophylaxis Rates Among Those With and Without IBD
Of the 22,025 patients without IBD, 19,182 (87%) were placed on pharmacologic VTE prophylaxis compared with 374 (79%) of patients with IBD (P < 0.01, Supplemental Table 1). On multivariable analysis of all patients, older age, multiple comorbidities, female sex, and history of a VTE were associated with an increased likelihood of VTE prophylaxis, whereas anemia, thrombocytopenia, and admission to a medical as compared with a surgical service were associated with a decreased likelihood of VTE prophylaxis (Supplemental Table 2). Tested in this multivariable model, the presence of IBD (adjusted odds ratio [OR] 0.57; 95% CI, 0.44–0.73) was also significantly associated with a decreased likelihood of VTE prophylaxis. However, when hematochezia was included in the final model, IBD was no longer independently related to VTE prophylaxis.
Predictors of VTE Prophylaxis Among Those With IBD
Among 474 patients with IBD, rates of VTE prophylaxis were lower among those with an IBD flare as compared with those without (76% vs 85% respectively, P = 0.03; Table 1). Additionally, on univariable analysis, hematochezia, admission to a medical service, and an initial hemoglobin <7 g/dL were also associated with a decreased likelihood of VTE prophylaxis. In the final multivariable model for VTE prophylaxis among those with IBD, the same predictors remained significantly associated with a decreased likelihood of VTE prophylaxis (Table 2). These results were similar after stratifying based on UC vs CD (Supplemental Table 3) and after including only those with a known history of IBD on presentation (Supplemental Table 4). Of note, although on univariable analysis, receiving corticosteroids during hospitalization was also significantly associated with lower odds of VTE prophylaxis use (Table 1); when adjusting for included variables such as hematochezia, corticosteroid use was no longer a significant predictor.
Table 1.
Univariable Analysis of Factors Associated With Receipt of Venous Thromboembolism Prophylaxis in 474 Patients With Inflammatory Bowel Disease
| VTE Prophylaxis N (%) | No VTE Prophylaxis N (%) | P | |
|---|---|---|---|
| Age in years | 0.11 | ||
| 18–40 | 164 (43.9%) | 54 (54.0%) | |
| 41–60 | 129 (34.5%) | 24 (24.0%) | |
| >61 | 81 (21.7%) | 22 (22.0%) | |
| Sex | 0.21 | ||
| Male | 206 (55.1%) | 48 (48.0%) | |
| Female | 168 (44.9%) | 52 (52.0%) | |
| Race/Ethnicity | 0.15 | ||
| White | 158 (42.3%) | 30 (30.0%) | |
| Hispanic | 40 (10.7%) | 14 (14.0%) | |
| Black | 24 (6.4%) | 9 (9.0%) | |
| Other/Not Listed | 152 (40.6%) | 47 (47.0%) | |
| Comorbidity Index | 0.68 | ||
| 0 | 288 (77.0%) | 75 (75.0%) | |
| 1 | 55 (14.7%) | 18 (18.0%) | |
| ≥2 | 31 (8.3%) | 7 (7.0%) | |
| History of VTE | 0.32 | ||
| No | 364 (97.3%) | 99 (99.0%) | |
| Yes | 10 (2.7%) | 1 (1.0%) | |
| Admission Service | <0.01 | ||
| Colorectal surgery | 174 (46.5%) | 16 (16.0%) | |
| Medicine housestaff | 101 (27.0%) | 44 (44.0%) | |
| Medicine hospitalist | 99 (26.5%) | 40 (40.0%) | |
| Hematochezia | <0.01 | ||
| No | 311 (83.2%) | 51 (51.0%) | |
| Yes | 63 (16.8%) | 49 (49.0%) | |
| Disease Flare | 0.03 | ||
| No | 124 (33.2%) | 22 (22.0%) | |
| Yes | 250 (66.8%) | 78 (78.0%) | |
| Corticosteroid Use During Hospitalization | 0.05 | ||
| No | 222 (59.4%) | 48 (48.0%) | |
| Yes | 152 (40.6%) | 52 (52.0%) | |
| Immunomodulator Use During Hospitalization | 0.15 | ||
| No | 343 (91.7%) | 87 (87.0%) | |
| Yes | 31 (8.3%) | 13 (13.0%) | |
| Biologic Use During Hospitalization | 0.87 | ||
| No | 335 (89.6%) | 89 (89.0%) | |
| Yes | 39 (10.4%) | 11 (11.0%) | |
| Initial Hgb (g/dL) | <0.01 | ||
| >11 | 239 (63.9%) | 54 (54.0%) | |
| 7–11 | 131 (35.0%) | 38 (38.0%) | |
| <7 | 4 (1.1%) | 8 (8.0%) | |
| Initial Platelet (x 10 9 /L) | 0.32 | ||
| >150 | 354 (94.7%) | 92 (92.0%) | |
| 50–150 | 18 (4.8%) | 6 (6.0%) | |
| <50 | 2 (0.5%) | 2 (2.0%) |
Table 2.
Multivariable Analysis of Factors Associated With Receipt of Venous Thromboembolism Prophylaxis in 474 Patients With Inflammatory Bowel Disease
| N (%) | Odds Ratio (95% CI) | |
|---|---|---|
| Comorbidity Index | ||
| 0 | 363 (76.6%) | Reference |
| 1 | 73 (15.4%) | 1.36 (0.70–2.63) |
| ≥2 | 38 (8.0%) | 2.15 (0.80–5.80) |
| History of VTE | ||
| No | 463 (97.7%) | Reference |
| Yes | 11 (2.3%) | 3.14 (0.36–27.2) |
| Hematochezia | ||
| No | 362 (76.4%) | Reference |
| Yes | 112 (23.6%) | 0.27 (0.16–0.46) |
| Admission Service | ||
| Colorectal surgery | 190 (40.1%) | Reference |
| Medicine housestaff | 145 (30.6%) | 0.24 (0.12–0.47) |
| Medicine hospitalist | 139 (29.3%) | 0.31 (0.15–0.61) |
| Initial Hemoglobin (g/dL) | ||
| >11 | 293 (61.8%) | Reference |
| 7–11 | 169 (35.7%) | 0.76 (0.46–1.27) |
| <7 | 12 (2.5%) | 0.13 (0.03–0.49) |
From our analysis, hematochezia was one of the strongest predictors of decreased likelihood of VTE prophylaxis (adjusted OR 0.27; 95% CI, 0.16–0.46). Among the 106 patients with hematochezia experiencing a disease flare, only 57% received VTE prophylaxis. On subgroup analysis, hematochezia was also significantly associated with decreased prophylaxis use regardless of whether a patient was experiencing a disease-related flare or not.
Significance of Hematochezia Among Those With IBD
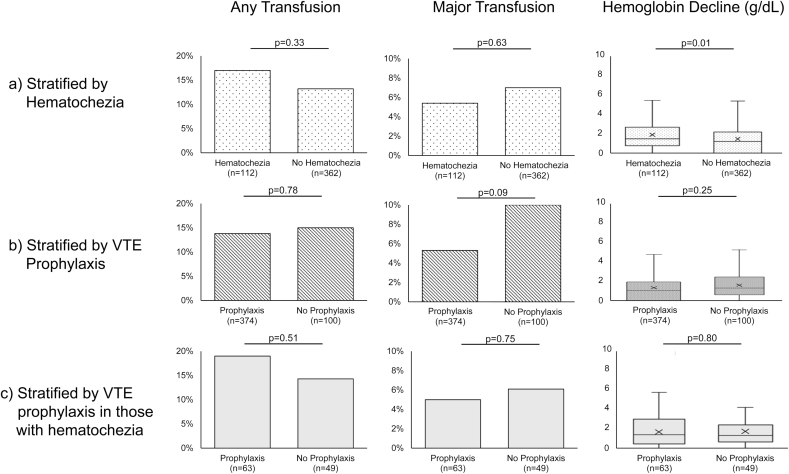
Because hematochezia was strongly associated with a decreased likelihood of VTE prophylaxis, we sought to evaluate its clinical significance in those with IBD. In examining transfusion requirements, no statistically significant differences were found between patients with or without hematochezia. Among those with hematochezia, 17% required any pRBC transfusion during hospitalization compared with 13% among those without hematochezia (P = 0.33) (Fig. 1A). Similarly, 5.4% of patients required ≥3 pRBC transfusions when hematochezia was present vs 6.6% when it was not (P = 0.63). There was, however, a significantly greater decline in hemoglobin level during hospitalization associated with hematochezia (1.50 g/dL median decrease during hospitalization if hematochezia was present vs 1.20 g/dL when hematochezia was not present; P = 0.01).
Figure 1.
Differences in pRBC transfusion requirements and median changes in hemoglobin stratified by (A) IBD patients with and without hematochezia, (B) IBD patients receiving and not receiving VTE prophylaxis, and(C) IBD patients with hematochezia receiving and not receiving VTE prophylaxis. The “x” within each boxplot represents the mean, whereas the dividing line within each boxplot represents the median delta hemoglobin value (g/dL).
Safety of VTE Prophylaxis Among Those With IBD
Several of the variables most strongly associated with decreased likelihood of VTE prophylaxis seemed to represent either chronic bleeding (initial hemoglobin level) or acute bleeding (hematochezia). Therefore, we next sought to evaluate the extent of bleeding during hospitalization and whether this depended on VTE prophylaxis. Among patients with IBD, 14% of those who received VTE prophylaxis required a pRBC transfusion, as compared with 15% in those who did not receive VTE prophylaxis (P = 0.78, Fig. 1B). For major transfusion requirement, there was also no difference between those who received VTE prophylaxis and those who did not (5.3% vs 10%, respectively; P = 0.09). When comparing changes in hemoglobin throughout hospitalization, there was also no difference (median hemoglobin decrease of 1.30 mg/dL in those who received VTE prophylaxis vs 1.45 mg/dL in those who did not receive VTE prophylaxis; P = 0.25).
Safety of VTE Prophylaxis Among Those With IBD Based on the Presence or Absence of Hematochezia
To measure whether hematochezia was associated with increased bleeding in IBD patients on prophylaxis, we last examined the 112 patients with both IBD and hematochezia and compared transfusion requirements and hemoglobin differences between those who received VTE prophylaxis and those who did not. Of note, there were no significant differences in initial hemoglobin values between those who did and did not receive VTE prophylaxis (P = 0.71). Among those who received VTE prophylaxis, 19% required a pRBC transfusion during hospitalization vs 14% who did not receive VTE prophylaxis (P = 0.51); 5.0% and 6.1% respectively required ≥3 pRBC transfusions (P = 0.75) (Fig. 1C). The delta hemoglobin values were also similar between the 2 groups (median decline of 1.60 g/dL with VTE prophylaxis vs 1.50 g/dL without; P = 0.80).
Discussion
Patients with IBD have a higher risk of VTE compared with the general population,21 and this risk has been shown to increase more than 6-fold when hospitalized with a flare.4 Given the increased risk of VTE among all IBD patients and the significant morbidity and mortality associated with a VTE, guidelines frequently recommend pharmacologic VTE prophylaxis for all hospitalized IBD patients.8, 17 Despite these guidelines, this study found that IBD patients had an 8% lower absolute risk of receiving VTE prophylaxis compared with the general inpatient population and were even less likely to receive prophylaxis when admitted with an IBD flare. Among IBD patients, the presence of hematochezia was associated with an almost 4-fold decreased likelihood of VTE prophylaxis. Yet, such hematochezia appeared to be relatively trivial because it was not accompanied by clinically important declines in hemoglobin. Moreover, VTE prophylaxis seemed to be safe because it was not associated with evidence of increased bleeding or greater transfusion requirements, regardless of whether it was given in the setting of hematochezia.
Previous studies, like this one, have demonstrated low rates of VTE prophylaxis among patients with IBD18, 22, 23 and have also suggested that this decreased use of prophylaxis may in part be due to concern over the presence of hematochezia.23, 24 In our study, hematochezia was strongly associated with failure to prescribe VTE prophylaxis. Importantly, our data suggest that the decreased rate of VTE prophylaxis observed in patients with IBD may have been driven primarily by the presence of hematochezia, as IBD was no longer significantly associated with decreased VTE prophylaxis when hematochezia was included in the final multivariable model for all inpatients.
Given that hematochezia was associated with a decreased use of VTE prophylaxis, we sought to assess its clinical significance. Among all IBD patients, we found that the presence of hematochezia was only associated with a 0.3 g/dL greater decrease in hemoglobin during hospitalization and was not associated with an increased pRBC transfusion requirement throughout hospitalization. Although the difference in hemoglobin decline between the two groups was statistically significant, a hemoglobin differential of 0.3 g/dL is unlikely to represent a clinically meaningful difference.
To address the safety concern of prophylaxis in IBD patients with hematochezia, we similarly measured differences in transfusion requirements and hemoglobin changes based on VTE prophylaxis. There were no differences in hemoglobin decline or in transfusion requirements. Similar results were seen in a study by Ra et al reviewing single-center data regarding IBD patient admissions from 2010 to 2012, where they found that the rates of both minor and major bleeding were independent of VTE prophylaxis use.23 Older studies examining the use of heparin as a treatment for UC also concluded that there was no significant increase in bleeding-related adverse events.25 This is consistent with the observation that rectal bleeding in UC is mostly low level and supports the conclusion that VTE prophylaxis is safe even in the setting of hematochezia. This finding is especially important because hematochezia often accompanies a flare of disease activity and thus a higher risk of VTE. Of the patients in our study with hematochezia, 95% were experiencing a disease flare, and only 57% of these received prophylaxis.
The possibility of reverse causality must be recognized when interpreting these findings (i.e., patients with more significant hematochezia were less likely to receive VTE prophylaxis, and therefore, bias is generated “favoring” prophylaxis). However, upon manual chart review, including assessment of initial hemoglobin values, we found no baseline differences in clinical presentation to explain why certain IBD patients with hematochezia received VTE prophylaxis and others did not, but we did find a difference based upon admitting service (surgery, 80% [16 of 20] with hematochezia received prophylaxis vs medicine, 51% [47 of 92]; P = 0.02). In accordance with prior literature, we found that among all IBD patients, admission to a medicine hospitalist or housestaff team resulted in a 69%–76% lower odds of prophylaxis use.23 This may reflect increased wariness among medical as opposed to surgical providers or may indicate more significant bleeding among medical as opposed to surgical patients.24, 26 Alternatively, this may reflect the use of different order sets between services; prior inpatient data examining both patients with and without IBD have demonstrated improved prophylaxis adherence rates with implementation of standardized order sets.27, 28
A strength of our study is that it evaluated the reasons why IBD patients may receive lower rates of VTE prophylaxis and then extended this by asking whether these reasons were justified (i.e., whether implied fear of inducing bleeding was warranted). We also adjusted for multiple relevant factors such as underlying comorbid status, admission team characteristics, and initial laboratory results. Our study also has certain limitations. Although we suspect that our results are likely generalizable to other institutions, this was a single-center study. Additionally, it was observational, and as in all observational studies, the possibility remains of baseline unmeasured differences between those who did and did not receive VTE prophylaxis. Last, our data were not powered to quantify the net risk-benefit of VTE prophylaxis in IBD, and this should be rigorously evaluated in future studies.
In summary, patients with IBD were less likely to be placed on pharmacologic VTE prophylaxis during hospitalization. Among those with IBD, hematochezia was one of the strongest factors associated with decreased prophylaxis, yet hematochezia in IBD was minor, and prophylaxis in those with hematochezia appeared to be safe by all measures examined. To limit the number of preventable VTE events, educational efforts should emphasize the importance and safety of prophylaxis in IBD patients, including those with hematochezia.
Supplementary Material
Glossary
Abbreviations
- CD
Crohn’s disease
- Hgb
hemoglobin
- IBD
inflammatory bowel disease
- ICD-CM
International Classification of Diseases Clinical Modification
- pRBC
packed red blood cell transfusion
- UC
ulcerative colitis
- VTE
venous thromboembolism
Appendix: International Classification of Diseases, 9th And 10th revision, Clinical Modification (Icd-9-CM And Icd-10-CM) Codes used in our study.
| Description | ICD-9-CM Codes | ICD-10-CM Codes |
|---|---|---|
| Acute VTE diagnosis (pulmonary embolism or deep vein thrombosis) | 415.1x, 451.1x, 453.4x, 453.8x, 453.9x, 673.2x, 673.8x, 671.3x, 671.4x, 671.5x | I82.4, I82.9, I26 |
| History of VTE | V12.51, V12.55 | Z86.718, Z86.711 |
| Ulcerative colitis | 556.x | K51.x |
| Crohn’s disease | 555.x | K50.x |
Author Contributions: ASF, KWH, JWB, ARP, BL, and DEF contributed to the study concept and design. ASF, KC, and JL contributed to the acquisition of data. ASF, KWH, KC, JWB, ASM, ARP, GL, BL, and DEF contributed to the analysis and interpretation of data. ASF and DEF contributed to the drafting of the manuscript. ASF, KWH, KC, JWB, ASM, ARP, JL, GL, BL, and DEF contributed to the critical revision of the manuscript for important intellectual content.
Supported by: ASF: NIH: T32DK083256
REFERENCES
- 1. Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol. 2011;106:713–718. [DOI] [PubMed] [Google Scholar]
- 2. Yuhara H, Steinmaus C, Corley D, et al. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:953–962. [DOI] [PubMed] [Google Scholar]
- 3. Solem CA, Loftus EV, Tremaine WJ, et al. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99:97–101. [DOI] [PubMed] [Google Scholar]
- 4. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657–663. [DOI] [PubMed] [Google Scholar]
- 5. Chu TPC, Grainge MJ, Card TR. The risk of venous thromboembolism during and after hospitalisation in patients with inflammatory bowel disease activity. Aliment Pharmacol Ther. 2018;48:1099–1108. [DOI] [PubMed] [Google Scholar]
- 6. McCurdy JD, Israel A, Hasan M, et al. A clinical predictive model for post-hospitalisation venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:1493–1501. [DOI] [PubMed] [Google Scholar]
- 7. Faye AS, Wen T, Ananthakrishnan AN, et al. Acute venous thromboembolism risk highest within 60 days after discharge from the hospital in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019.. pii: S1542-3565(19)30772-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrade AR, Barros LL, Azevedo MFC, et al. Risk of thrombosis and mortality in inflammatory bowel disease. Clin Transl Gastroenterol. 2018;9:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156:1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 10. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 11. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:2272–2280. [DOI] [PubMed] [Google Scholar]
- 13. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23; quiz 524. [DOI] [PubMed] [Google Scholar]
- 14. Mowat C, Cole A, Windsor A, et al. ; IBD Section of the British Society of Gastroenterology Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- 15. Bitton A, Buie D, Enns R, et al. ; Canadian Association of Gastroenterology Severe Ulcerative Colitis Consensus Group Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107:179–194; author reply 195. [DOI] [PubMed] [Google Scholar]
- 16. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 2: current management. J Crohns Colitis. 2017;11:769–784. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–848.e6. [DOI] [PubMed] [Google Scholar]
- 18. Tinsley A, Naymagon S, Enomoto LM, et al. Rates of pharmacologic venous thromboembolism prophylaxis in hospitalized patients with active ulcerative colitis: results from a tertiary care center. J Crohns Colitis. 2013;7:e635–e640. [DOI] [PubMed] [Google Scholar]
- 19. Wen T, Wright JD, Goffman D, et al. Postpartum venous thromboembolism readmissions in the United States. Am J Obstet Gynecol. 2018;219:401.e1–401.e14. [DOI] [PubMed] [Google Scholar]
- 20. Salmasian H, Freedberg DE, Friedman C. Deriving comorbidities from medical records using natural language processing. J Am Med Inform Assoc. 2013;20:e239–e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scoville EA, Konijeti GG, Nguyen DD, et al. Venous thromboembolism in patients with inflammatory bowel diseases: a case-control study of risk factors. Inflamm Bowel Dis. 2014;20:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen GC, Murthy SK, Bressler B, et al. ; CINERGI group Quality of care and outcomes among hospitalized inflammatory bowel disease patients: a multicenter retrospective study. Inflamm Bowel Dis. 2017;23:695–701. [DOI] [PubMed] [Google Scholar]
- 23. Ra G, Thanabalan R, Ratneswaran S, et al. Predictors and safety of venous thromboembolism prophylaxis among hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2013;7:e479–e485. [DOI] [PubMed] [Google Scholar]
- 24. Sam JJ, Bernstein CN, Razik R, et al. Physicians’ perceptions of risks and practices in venous thromboembolism prophylaxis in inflammatory bowel disease. Dig Dis Sci. 2013;58:46–52. [DOI] [PubMed] [Google Scholar]
- 25. Shen J, Ran ZH, Tong JL, et al. Meta-analysis: the utility and safety of heparin in the treatment of active ulcerative colitis. Aliment Pharmacol Ther. 2007;26:653–663. [DOI] [PubMed] [Google Scholar]
- 26. Papa A, Gerardi V, Marzo M, et al. Venous thromboembolism in patients with inflammatory bowel disease: focus on prevention and treatment. World J Gastroenterol. 2014;20:3173–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewin SM, McConnell RA, Patel R, et al. Improving the quality of inpatient ulcerative colitis management: promoting evidence-based practice and reducing care variation with an inpatient protocol. Inflamm Bowel Dis. 2019;25:1822–1827. [DOI] [PubMed] [Google Scholar]
- 28. Mayer RS, Streiff MB, Hobson DB, et al. Evidence-based venous thromboembolism prophylaxis is associated with a six-fold decrease in numbers of symptomatic venous thromboembolisms in rehabilitation inpatients. Pm R. 2011;3:1111–1115.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.