Abstract
Macadamia integrifolia is a representative of the large basal eudicot family Proteaceae and the main progenitor species of the Australian native nut crop macadamia. Since its commercialisation in Hawaii fewer than 100 years ago, global production has expanded rapidly. However, genomic resources are limited in comparison to other horticultural crops. The first draft assembly of M. integrifolia had good coverage of the functional gene space but its high fragmentation has restricted its use in comparative genomics and association studies. Here we have generated an improved assembly of cultivar HAES 741 (4,094 scaffolds, 745 Mb, N50 413 kb) using a combination of Illumina paired and PacBio long read sequences. Scaffolds were anchored to 14 pseudo-chromosomes using seven genetic linkage maps. This assembly has improved contiguity and coverage, with >120 Gb of additional sequence. Following annotation, 34,274 protein-coding genes were predicted, representing 90% of the expected gene content. Our results indicate that the macadamia genome is repetitive and heterozygous. The total repeat content was 55% and genome-wide heterozygosity, estimated by read mapping, was 0.98% or an average of one SNP per 102 bp. This is the first chromosome-scale genome assembly for macadamia and the Proteaceae. It is expected to be a valuable resource for breeding, gene discovery, conservation and evolutionary genomics.
Keywords: Proteaceae, genome, pseudo-chromosome, transcriptome, nut crop
The genomes of most crop species have now been sequenced and their availability is transforming breeding and agricultural productivity (Bevan et al. 2017, Chen et al. 2019). Macadamia is the first Australian native plant to become a global food crop. In 2018/2019, world production was valued at US$1.1 billion (59,307 MT/year, kernel basis), reflecting the most rapid increase in production of any nut crop over the past 10 years (International Nut and Dried Fruit Council, http://www.nutfruit.org). As a member of the Gondwanan family Proteaceae (83 genera, 1660 species) (Christenhusz and Byng 2016), Macadamia (F.Muell.) is a basal eudicot and is phylogenetically divergent from other tree crops (Nock et al. 2014). M. integrifolia, the main species used in cultivation (Hardner 2016), is a mid-storey tree endemic to the lowland rainforests of subtropical Australia (Powell et al. 2014) (Figure 1). Macadamia was commercialised as a nut crop from the 1920s in Hawaii and cultivated varieties are closely related to their wild ancestors in Australia (Nock et al. 2019).
Figure 1.
Macadamia integrifolia (a) orchard (b) nut in husk (c) racemes.
Despite the rapid expansion in production over the past 50 years and commercial cultivation in 18 countries, breeding is restricted by a paucity of information on the genes underlying important crop traits (Topp et al. 2019). There are few genomic resources for either macadamia or within the Proteaceae. Transcriptomic data are available for Macadamia (Chabika et al. 2020) and other Proteaceae genera including Banksia (Lim et al. 2017), Grevillea (Damerval et al. 2019), Protea (Akman et al. 2016) and Gevuina (Ostria-Gallardo et al. 2016), and have been utilized to help to understand the evolution of floral architecture (Damerval et al. 2019) and variation in locally adaptive traits (Akman et al. 2016). Recent genome wide association studies in macadamia have identified markers associated with commercially important traits (O’Connor et al. 2020) but their location and context in the genome is unknown. An earlier highly fragmented (193,493 scaffolds, N50 4,745) draft genome assembly of the widely grown M. integrifolia cultivar HAES 741 was constructed from Illumina short read sequence data (Nock et al. 2016).
Here we report on the first annotated and anchored genome assembly for macadamia and the Proteaceae. Long read PacBio and paired-end Illumina sequence data were generated and 187 Gb of data were used to construct an improved HAES 741 assembly (Table 1). Assembled sequence scaffolds from the new hybrid de novo assembly (745 Mb, 4094 scaffolds, N50 413 kb) were then anchored and oriented to 14 pseudo-chromosomes using methods to maximize colinearity across seven genetic linkage maps (Langdon et al. 2020). A contiguous genome sequence for macadamia is expected to facilitate the identification of candidate genes and support marker assisted selection to accelerate the development of new improved varieties.
Table 1. Data files and library information for Macadamia integrifolia genome sequencing. *Data deposited for draft assembly v1.1.
| Sequencing Platform | Library | Insert size (bp) | Read length (bp) | Sequence reads (Million) | Sequence bases (Gb) | Accession |
|---|---|---|---|---|---|---|
| Genomic data | ||||||
| Illumina | Pair end | 200 | 2 x 125 | 228.8 | 57.2 | SRR10896963 |
| Pair end | 350 | 2 x 125 | 119.0 | 29.8 | SRR10896962 | |
| Pair end | 550 | 2 x 125 | 107.6 | 26.9 | SRR10896961 | |
| Pair end | 480 | 2 x 150 | 58.7 | 17.7 | ERX1468522-23* | |
| Pair end | 700 | 2 x 150 | 28.7 | 8.7 | ERX1468524* | |
| Mate pair | 8000 | 2 x 100 | 200.3 | 40.5 | ERX1468525* | |
| PacBio | NA | 9769 | 1.0 | 6.4 | 10896960 | |
| Total gDNA | 744.1 | 187.2 | ||||
| Transcriptomic data | ||||||
| Illumina | young leaf | 300 | 2 x 100 | 77.9 | 15.6 | SRR10897159 |
| shoot | 300 | 2 x 100 | 71.6 | 14.3 | SRR10897158 | |
| flower | 300 | 2 x 100 | 83.3 | 16.7 | SRR10897157 | |
| Total RNA-seq | 232.8 | 46.6 | ||||
Materials and Methods
Sample collection and extraction
Leaf, shoot and flower tissue were collected from a single M. integrifolia cultivar HAES 741 individual located in the M2 Regional Variety Trial plot, Clunes, New South Wales, Australia (28°43.843’S; 153°23.702’E). An herbarium specimen was submitted to the Southern Cross Plant Science Herbarium [accession PHARM-13-0813]. On collection, samples were snap frozen in liquid nitrogen or placed on dry ice and stored at -80° prior to extraction. Previously described methods for and DNA/RNA extraction for Illumina (Nock et al. 2016) and PacBio (Furtado 2014) sequencing were followed.
Library preparation and sequencing
In addition to the original 480 bp and 700 bp insert and 8 kb mate pair libraries (51.6 Gb total), new libraries with 200, 350 and 550 bp insert sizes were prepared with TruSeq DNA PCR-free kits and sequenced with Illumina HiSeq 2500 producing 57.0, 29.8 and 29.6 Gb paired-end sequence data. A PacBio library (20-Kb) was prepared and sequenced across 10 SMRT cells on a PacBio RSII system (P6-C4 chemistry) generating 6.44 Gb data. Transcriptome sequencing of leaf, shoot and flower tissue followed previously described methods (Nock et al. 2016) and generated 44.6 Gb paired-end RNA-seq data in total (Table 1).
De novo assembly and anchoring
Illumina raw read data were initially assessed for quality using FastQC (Andrews 2010)· Low quality bases (Q < 20), adapters and chloroplast reads were removed from Illumina data using BBMaps (Bushnell 2020). PacBio reads were error-corrected using the Long Read DBG Error Correction method (LoRDEC) with over 170 × post-QC Illumina data (Salmela and Rivals 2014). The performance of hybrid error correction methods including LoRDEC is optimized with increasing coverage of more accurate short, rather than long read, sequence data since each long read is corrected independently (Fu et al. 2019). Hybrid de novo assembly, scaffolding and gap closing was performed using MaSuRCA (Zimin et al. 2013). Illumina paired-end and mate-pair reads were extended into super-reads of variable lengths, and combined with PacBio reads to generate the assembly. The MaSURCA output was further scaffolded in two rounds, first using SSPace (Boetzer et al. 2011) scaffolder with the Illumina mate pair reads, and second, using L_RNA_Scaffolder (Xue et al. 2013) with the long transcripts generated using a Trinity (Grabherr et al. 2011) pipeline and RNA-Seq reads. Scaffolds were anchored and oriented with 100 bp gap size between scaffolds using ALLMAPS (Tang et al. 2015) with 4,266 ordered sequence-based markers across seven genetic linkage maps generated from four mapping populations with HAES 741 parentage (Langdon et al. 2020).
Genome size and heterozygosity
For genome size and heterozygosity estimation, k-mer frequency was determined from cleaned short and long read data for k ranging from 15 to 33 at a step of two in Jellyfish (Marcais and Kingsford 2011). Genome size was estimated at the optimal k-mer of 25, with short read data only using findGSE because it is an optimal method for plants (Sun et al. 2018). Genome-wide heterozygosity using reads data were estimated with GenomeScope (Vurture et al. 2017) from the k-mer 25 histogram computed using Jellyfish. In addition, genome-based heterozygosity was determined by mapping post-QC short read data to the assembly using minimap2 (Li 2018) and heterozygous sites identified using GATK best practices (Van der Auwera et al. 2013) for SNP discovery.
Genome annotation and gene prediction
Repetitive elements were first identified in the final assembly by modeling repeats using RepeatModeler, and then quantified using RepeatMasker (Tarailo-Graovac and Chen 2009). Transcriptome assembly was performed using the Trinity pipeline (Grabherr et al. 2011). Following repeat masking, the final assembly was annotated using the MAKER gene model prediction pipeline (Campbell et al. 2014). Sources of evidence for gene prediction included the Trinity assembled transcripts and protein sequences of the taxonomically closest available genome sequence of the sacred lotus Nelumbo nucifera, and the model plant Arabidopsis thaliana.
Comparative analysis of orthologous eudicot genes
Orthologous gene clusters were identified and compared using OrthoVenn2 (Zu et al. 2019) and protein sequences from M. integrifolia, Nelumbo nucifera, Arabidopsis thaliana, Prunus persica (peach), Eucalpytus grandis and Coffea canephora (coffee) genomes. Pairwise sequence similarities were determined applying a BLASTP E-value cut-off of 1E-05 and an inflation value of 1.5 for OrthoMCL Markov clustering. Macadamia specific gene clusters were tested for GO enrichment using OrthoVenn2.
Quality assessment
The completeness of the genome assembly was evaluated by BUSCO (benchmarking universal single copy orthologs) (Simão et al. 2015) using 956 plant BUSCOs. To further assess the accuracy of the M. integrifolia genome assembly, short read and corrected long read data were independently mapped to the pseudo-genome using Minimap2 (Li 2018) and the number of mapped reads were computed using paftools.js. The final step of the LoRDEC error correction method applied involves trimming and splitting PacBio reads by extracting runs of solid bases as separate reads (Salmela and Rivals 2014). To test for chimerism, Pacbio reads > 100 bp in length we re-aligned to the assembly using cutoffs of 1/2 and 1/3 read length.
Data availability
The macadamia Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JAAEEG000000000. The version described in this paper is JAAEEG010000000. Datasets generated in this study have been deposited at NCBI under BioProject number PRJNA593881, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA593881 Raw genomic DNA and RNA-seq read files have been deposited in the NCBI Sequence Read Archive (Table 1). Datasets including protein, transcript and gff3 annotation files, and raw trinity transcriptome assembly have been deposited in the Southern Cross University data repository, https://doi.org/10.25918/5e320fd1e5f06.
Results and Discussion
Genome assembly
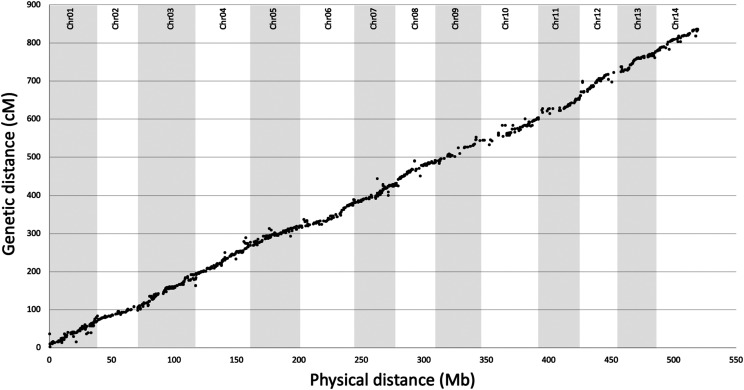
Hybrid de novo assembly using 180.8 Gb Illumina short read and 6.44 Gb PacBio long read data produced a 744.6 Mb genome assembly (v2) with 180 × coverage of the genome and a scaffold N50 of 413 kb (Table 1). In comparison to the v1.1 assembly of the same cultivar, this v2 assembly represents an ∼68 fold increase in contiguity and includes 120.3 Mb of new sequence (Table 2). Scaffolds were anchored and oriented to 14 pseudo-chromosomes using ALLMAPs to maximize the collinearity of ordered sequence-based markers across seven genetic linkage maps generated from mapping populations with HAES 741 parentage. Of 1,465 anchored scaffolds, 474 were N50 scaffolds and 856 containing more than one marker were oriented (Langdon et al. 2020). This anchored 69.7% of the assembly to 14 pseudo-chromosomal sequences ranging in size from 29.2 to 47.0 Mb (Table 3). Mapped markers were evenly distributed across the 14 pseudo-chromosomes with the exception of some gapped regions particularly on Chr10 and Chr12 (Figure 2).
Table 2. Comparison of the new Macadamia integrifolia cv. HAES 741 genome assembly with the previously published draft assembly. Coverage is based on k-mer estimated genome size of 895.7 Mb. Gaps are ambiguous base calls.
| Data | Scaffold | Assembly | Annotation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-QC Gb | Genome Coverage X | Number | N50 kb | Longest Mb | Assembled Genome Mb | Gaps Mb | Genome Coverage % | Repeats | Gene Models | Expected Gene content | |
| v1.1 | 51.6 | 58 | 193,493 | 4.7 | 0.64 | 518.49 | 70 | 58% | 37% | 35,337 | 77.4% |
| v2 | 161.5 | 180 | 4094 | 413.4 | 2.19 | 744.64 | 11 | 83% | 55% | 34,274 | 90.2% |
Table 3. Summary of the assembled chromosomes of macadamia.
| Chromosome | Length bp | scaffolds | genes |
|---|---|---|---|
| Chr01 | 36,399,236 | 126 | 1543 |
| Chr02 | 44,010,915 | 120 | 2214 |
| Chr03 | 37,831,390 | 112 | 2039 |
| Chr04 | 37,507,012 | 91 | 2133 |
| Chr05 | 46,991,412 | 110 | 2334 |
| Chr06 | 40,842,004 | 101 | 1941 |
| Chr07 | 36,789,931 | 112 | 1940 |
| Chr08 | 34,869,527 | 94 | 1975 |
| Chr09 | 42,247,492 | 109 | 2019 |
| Chr10 | 34,167,173 | 87 | 1701 |
| Chr11 | 33,967,801 | 107 | 1488 |
| Chr12 | 31,654,987 | 87 | 1523 |
| Chr13 | 29,219,948 | 100 | 1398 |
| Chr14 | 32,845,142 | 109 | 1531 |
| Anchored | 519,343,970 | 1465 | 25,779 |
| Unplaced | 225,433,603 | 2629 | 8495 |
| Total | 744,777,573 | 4094 | 34,274 |
Figure 2.
Genetic length (centimorgans, cM) vs. physical length (megabases, Mb) plotted for the Macadamia integrifolia cv. HAES 741 genome.
The quality and completeness of the assembly, assessed using BUSCO, indicates that the macadamia assembly contains 90.2% of the expected single copy gene content. In comparison to the previous assembly of the same cultivar, 16.5% compared to 39.2% of the plant BUSCOs searched were fragmented. The accuracy of the assembly was assessed by read mapping with 99% of short reads (98.97–99.15% per library) and 93.5% of corrected PacBio reads tagged as primary alignments by Minimap2 aligned reliably to the assembly. No Pacbio reads aligned to two different scaffolds at a cutoff of 1/2 read length. At 1/3 read length, 130 reads (mean length 7833 bp representing < 0.14% of the assembly) aligned to two scaffolds and no reads aligned to more than two scaffolds.
Genome size, heterozygosity and repetitive content
Genome size estimation of 896 Mb was 1.37 times larger than the only previous estimate for M. integrifolia of 652 Mb (600-700 Mb) that was based on 51.6 Gb Illumina short read data (Nock et al. 2016). The new k-mer based estimate is considered to be more accurate due to improved read coverage from an additional 114 Gb of high-quality Illumina data. Based on the revised genome size estimate, the v2 assembly covers 83% of the genome.
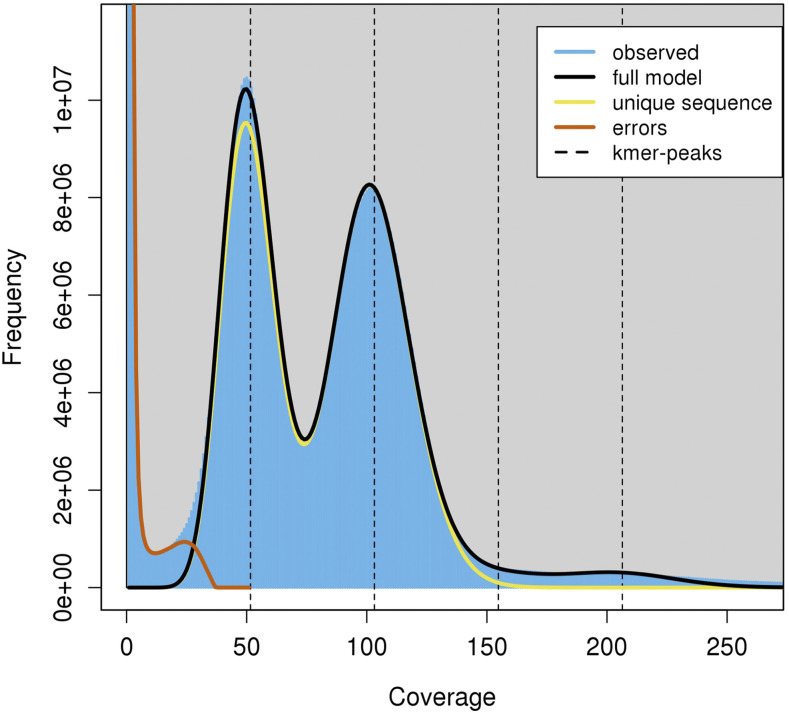
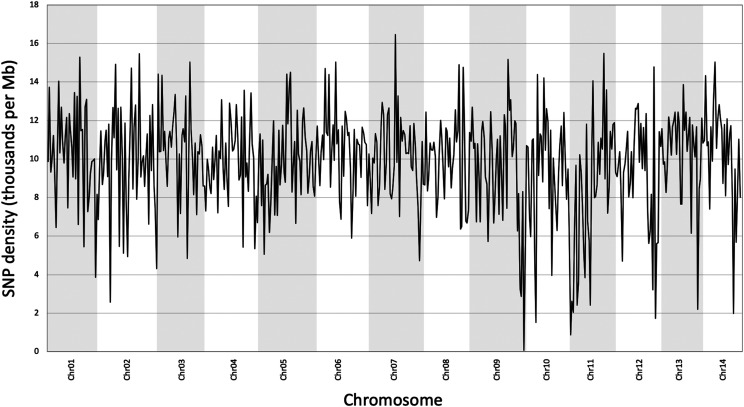
Average genome heterozygosity, determined using short read data from the k-mer 25 Jellyfish histogram, was 1.36% (Figure 3). Genome-wide heterozygosity was slightly lower at 0.98% for the assembly-based SNP analysis method (Li 2018, Van der Auwera et al. 2013). In total, 7,309,539 heterozygous sites were identified in the HAES 741 genome assembly representing one SNP per 102 bp and consistent with reports that macadamia is highly heterozygous and predominantly outcrossing (Topp et al. 2019). Heterozygous sites were unevenly distributed across chromosomes. On average, there were 367,467 SNPs per chromosome and SNP density ranged from 66 to 16,453 SNPs in one Mb windows of Chr07 and Chr10 respectively (Figure 4).
Figure 3.
The 25-mer distribution for estimation of genome heterozygosity and size. Peaks at approximately 50, 100 and 200 represent heterozygous, homozygous and repeated k-mers respectively.
Figure 4.
Genome wide SNP density. Thousands of SNPs per 1 Mb window, shown across each chromosome.
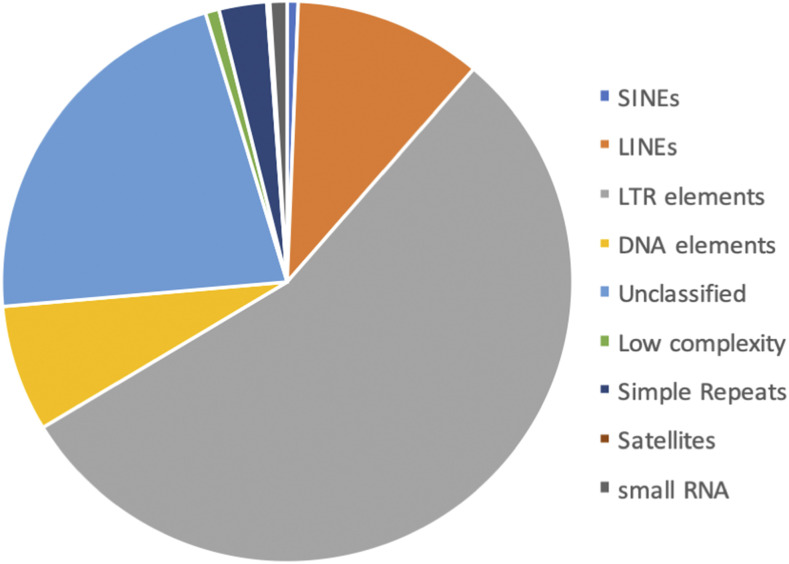
Interspersed repeats and low complexity elements accounted for 410.5Mb (55.1%) of the assembly. As reported for many other plant genomes, long terminal repeat (LTR) retrotransposons were the most abundant repeat type (30.5%). Small RNAs including pseudogenes and active small RNA genes including tRNA and snRNA accounted for 0.54% of the assembly (Figure 5).
Figure 5.
Interspersed repeats and low complexity elements representing 55.1% of the macadamia genome assembly.
Annotation and comparative analysis of orthologous genes
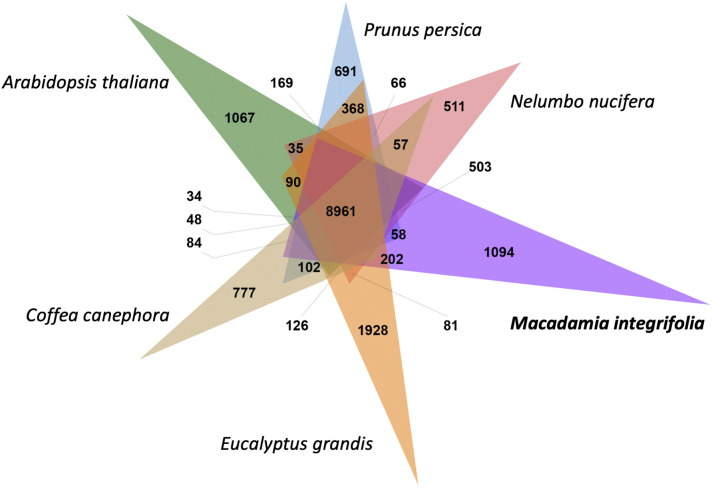
Annotation of the final assembly, identified 34,274 high-confidence gene models. Of these, 25,779 (75.2%) were anchored to pseudo-chromosomes and 8,495 were located on unanchored scaffolds (Table 3). Comparative analysis of the macadamia gene models with the proteins of N. nucifera, A. thaliana, P. persica, E. grandis and C. canephora identified 8,961 orthologous clusters of which 2,051 contained a single gene from each of the six eudicot species. M. integrifolia and N. nucifera both belong to the basal eudicot order Proteales. These species and contained similar numbers of clusters with 13,491 and 13,321 respectively and shared the largest number of clusters (503) of any species pair (Figure 6). Tests for gene ontology (GO) enrichment of 1,094 macadamia specific clusters identified five significant terms including those associated with the defense response (GO:0006952, P = 4.8E-05) and fruit ripening (GO:0009835, P = 5.9E-04).
Figure 6.
Venn diagram of orthologous gene clusters for six eudicot species including the basal eudicots M. integrifolia and N. nucifera (Proteales) and core eudicots P. persica (Rosales), A. thaliana (Brassicales), C. canephora (Gentianales) and E. grandis (Myrtales).
Conclusion
Here we present the first chromosome-scale genome assembly for the nut crop macadamia and the large Gondwanan plant family Proteaceae. This provides a platform for unraveling the genetics of macadamia and is expected to underpin future breeding, and comparative and horticultural genomics research.
Code availability
Versions and parameters of the tools implemented in data analysis are provided below:
BBMap version 36.62: ktrim = r k = 23 mink = 11 hdist = 1 tpe tbo maxns = 1 minlen = 50 maq = 8 qtrim = rl trimq = 20
LoRDEC version 0.4: lordec-correct -2 input_for_read_correction.fastq -k 19 -S out.stat.txt -s 3 -T 12 -i PacBio_filtered_reads.fasta -o out.pacbio.corrected.fasta
MaSuRCA version 3.2.1. Default parameters except: NUM_THREADS = 16, JF_SIZE = 20000000000 (jellyfish hash size)
L_RNA_scaffolder: blat -t = dna -q = dna scaffolds_gapClosed_min1000.fa Trinity.fasta transcript.vs.macaAssembly.psl -noHead -out = psl
ALLMAPS version 0.7.7: Default parameters
Jellyfish version 2.0: jellyfish count -t 14 -C -m 27 -s 8G -o 27mer_maca_illumOnly_out <all Illumina-only WGS fastqs > jellyfish histo -t14 27mer_maca_illumOnly_out> 27mer_maca_illumOnly_out.histo
findGSE: Default parameters
GenomeScope version 2.2.6:kmer length 27, read length 125bp, Max kmer coverage 1000
Trinity, version 2.0.3: Trinity–seqType fq–max_memory 100G –left reads_S_R1_clean.fq,reads_F2_R1_clean.fq,reads_YL_R1_clean.fq–right reads_S_R2_clean.fq,reads_F2_R2_clean.fq,reads_YL_R2_clean.fq–CPU 8
RepeatModeler version 2.0.1. Default parameters
RepeatMasker version 4.0.9. Default parameters
MAKER, version 2.31.10. Default parameters except: Gene prediction methods Augustus and SNAP (trained with previously generated macadamia gene models); AED score = 0.40; Minimum protein length: = 50 amino acids
BUSCO version 3.0.2: busco -i proteins.fasta -l viridiplantae_odb10 -m proteins -o output_name
Minimap2 version 2.17. Default parameters (paftool.js bundled with Minimap2): minimap2 -ax sr ref_assemblyfasta fastq1stReadPair fastq2ndReadPair > ref_readPairs_aln.paf; k8 paftools.js stat ref_readPairs_aln.paf > alignment_mapstats
GATK HaplotypeCaller version 4.1.4.1: gatk–java-options “-Xmx8g” HaplotypeCaller -R refGenome.fasta -I input.bam -O output.g.vcf.gz -ERC GVCF -heterozygosity 0.01
GATK GenotypeGVCFs version 4.1.4.1: gatk–java-options “-Xmx4g” GenotypeGVCFs -R refGenome.fasta -V input.g.vcf.gz -O output.vcf.gz–heterozygosity 0.01
GATK VariantsToTable version 4.1.4.1: gatk-4.1.4.1/gatk–java-options “-Xmx8g” VariantsToTable -V output.vcf.gz -F CHROM -F POS -F TYPE -F REF -F ALT -F HET -GF GT -GF AD -O output_genoGVCF.table.txt.
Acknowledgments
This research was supported by Horticulture Innovation Australia project MC15008 Establishing an open-source platform for unravelling the genetics of Macadamia: integration of linkage and genome maps. The project provided a PhD scholarship for K.L. with funding from Horticulture Innovation Australia, Knappick Foundation Ltd., Macadamia Conservation Trust, Australian Macadamia Society and Southern Cross University. Laboratory and horticultural support were provided by Asuka Kawamata, Tiffeny Byrnes and Alicia Hidden. Assistance with sample collection was provided by Dr Alam Mobashwer. We thank Kim Wilson and Alex Yong for providing access to the regional variety trial site in Clunes, NSW, Australia where the HAES 741 tree used for genome sequencing is located.
Footnotes
Communicating editor: I. Parkin
Literature Cited
- Akman M., Carlson J. E., Holsinger K. E., and Latimer A. M., 2016. Transcriptome sequencing reveals population differentiation in gene expression linked to functional traits and environmental gradients in the South African shrub Protea repens. New Phytol. 210: 295–309. 10.1111/nph.13761 [DOI] [PubMed] [Google Scholar]
- Andrews S., 2010. FastQC: a quality control tool for high throughput sequence data. Available online at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Bevan M. W., Uauy C., Wulff B. B., Zhou J., Krasileva K. et al. , 2017. Genomic innovation for crop improvement. Nature 543: 346–354. 10.1038/nature22011 [DOI] [PubMed] [Google Scholar]
- Boetzer M., Henkel C. V., Jansen H. J., Butler D., and Pirovano W., 2011. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27: 578–579. 10.1093/bioinformatics/btq683 [DOI] [PubMed] [Google Scholar]
- Bushnell, B., 2020 BBMap. https://sourceforge.net/projects/bbmap/
- Campbell M. S., Holt C., Moore B., and Yandell M., 2014. Genome annotation and curation using MAKER and MAKER‐P. Curr. Protoc. Bioinformatics 48: 4–11. 10.1002/0471250953.bi0411s48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabikwa T. G., Barbier F. F., Tanurdzic M., and Beveridge C. A., 2020. De novo transcriptome assembly and annotation for gene discovery in avocado, macadamia and mango. Sci. Data 7: 9 10.1038/s41597-019-0350-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Song Y., Li X., Chen J., Mo L. et al. , 2019. Genome sequences of horticultural plants: past, present, and future. Hortic. Res. 6: 112 10.1038/s41438-019-0195-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenhusz M. J., and Byng J. W., 2016. The number of known plants species in the world and its annual increase. Phytotaxa 261: 201–217. 10.11646/phytotaxa.261.3.1 [DOI] [Google Scholar]
- Damerval C., Citerne H., Conde e Silva N., Deveaux Y., Delannoy E. et al. , 2019. Unravelling the developmental and genetic mechanisms underpinning floral architecture in Proteaceae. Front. Plant Sci. 10: 18 10.3389/fpls.2019.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S., Wang A., and Au K. F., 2019. A comparative evaluation of hybrid error correction methods for error-prone long reads. Genome Biol. 20: 26 10.1186/s13059-018-1605-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado A., 2014. DNA Extraction from Vegetative Tissue for Next-Generation Sequencing, pp. 1–5 in Cereal Genomics. Methods in Molecular Biology (Methods and Protocols), Vol. 1099., Humana Press, Totowa, NJ: 10.1007/978-1-62703-715-0_1 [DOI] [PubMed] [Google Scholar]
- Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A. et al. , 2011. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 29: 644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardner C., 2016. Macadamia domestication in Hawai’i. Genet. Resour. Crop Evol. 63: 1411–1430. 10.1007/s10722-015-0328-1 [DOI] [Google Scholar]
- Lim S. L., D’Agui H. M., Enright N. J., and He T., 2017. Characterization of leaf transcriptome in Banksia hookeriana. Genom. Proteom. Bioinf. 15: 49–56. 10.1016/j.gpb.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor K., Hayes B., Hardner C., Nock C., Baten A. et al. , 2020. Genome-wide association studies for yield component traits in a macadamia breeding population. BMC Genomics 21: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostria‐Gallardo E., Ranjan A., Chitwood D. H., Kumar R., Townsley B. T. et al. , 2016. Transcriptomic analysis suggests a key role for SQUAMOSA PROMOTER BINDING PROTEIN LIKE, NAC and YUCCA genes in the heteroblastic development of the temperate rainforest tree Gevuina avellana (Proteaceae). New Phytol. 210: 694–708. 10.1111/nph.13776 [DOI] [PubMed] [Google Scholar]
- Langdon K. S., King G. J., Baten A., Mauleon R., Bundock P. C. et al. , 2020. Maximising recombination across macadamia populations to generate linkage maps for genome anchoring. Sci. Rep. 10: 5048 10.1038/s41598-020-61708-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., 2018. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 34: 3094–3100. 10.1093/bioinformatics/bty191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais G., and Kingsford C., 2011. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27: 764–770. 10.1093/bioinformatics/btr011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock C. J., Baten A., and King G. J., 2014. Complete chloroplast genome of Macadamia integrifoliaconfirms the position of the Gondwanan early-diverging eudicot family Proteaceae. BMC Genomics 15: S13 10.1186/1471-2164-15-S9-S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock C. J., Baten A., Barkla B. J., Furtado A., Henry R. J. et al. , 2016. Genome and transcriptome sequencing characterises the gene space of Macadamia integrifolia (Proteaceae). BMC Genomics 17: 937 10.1186/s12864-016-3272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock C. J., Hardner C. M., Montenegro J. D., Ahmad A. A., Termizi S. et al. , 2019. Wild origins of macadamia domestication identified through intraspecific chloroplast genome sequencing. Front. Plant Sci. 10: 334 10.3389/fpls.2019.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell M., Accad A., and Shapcott A., 2014. Where they are, why they are there, and where they are going: using niche models to assess impacts of disturbance on the distribution of three endemic rare subtropical rainforest trees of Macadamia (Proteaceae) species. Aust. J. Bot. 62: 322–334. 10.1071/BT14056 [DOI] [Google Scholar]
- Salmela L., and Rivals E., 2014. LoRDEC: accurate and efficient long read error correction. Bioinformatics 30: 3506–3514. 10.1093/bioinformatics/btu538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão F. A., Waterhouse R. M., Ioannidis P., Kriventseva E. V., and Zdobnov E. M., 2015. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31: 3210–3212. 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- Sun H., Ding J., Piednoël M., and Schneeberger K., 2018. findGSE: estimating genome size variation within human and Arabidopsis using k-mer frequencies. Bioinformatics 34: 550–557. 10.1093/bioinformatics/btx637 [DOI] [PubMed] [Google Scholar]
- Tang H., Zhang X., Miao C., Zhang J., Ming R. et al. , 2015. ALLMAPS: robust scaffold ordering based on multiple maps. Genome Biol. 16: 3 10.1186/s13059-014-0573-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarailo‐Graovac M., and Chen N., 2009. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinformatics 25: 4.10.1–4.10.14. 10.1002/0471250953.bi0410s25 [DOI] [PubMed] [Google Scholar]
- Topp B. L., Nock C. J., Hardner C. M., Alam M., and O’Connor K. M., 2019. Macadamia (Macadamia spp.) Breeding, pp. 221–251 in Advances in Plant Breeding Strategies: Nut and Beverage Crops, Springer, Cham: 10.1007/978-3-030-23112-5_7 [DOI] [Google Scholar]
- Van der Auwera G. A., Carneiro M. O., Hartl C., Poplin R., Del Angel G., et al. . 2013. From FastQ Data to High-Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Curr Protoc Bioinformatics 43: 11.10.11–11.10.33. 10.1002/0471250953.bi1110s43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurture G. W., Sedlazeck F. J., Nattestad M., Underwood C. J., Fang H. et al. , 2017. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics 33: 2202–2204. 10.1093/bioinformatics/btx153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W., Li J.-T., Zhu Y.-P., Hou G.-Y., Kong X.-F. et al. , 2013. L RNA scaffolder: scaffolding genomes with transcripts. BMC Genomics 14: 604 10.1186/1471-2164-14-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin A. V., Marçais G., Puiu D., Roberts M., Salzberg S. L. et al. , 2013. The MaSuRCA genome assembler. Bioinformatics 29: 2669–2677. 10.1093/bioinformatics/btt476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu L., Dong Z., Fang L., Luo Y., Wei Z. et al. , 2019. OrthoVenn2: a web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 47: W52–W58. 10.1093/nar/gkz333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The macadamia Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JAAEEG000000000. The version described in this paper is JAAEEG010000000. Datasets generated in this study have been deposited at NCBI under BioProject number PRJNA593881, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA593881 Raw genomic DNA and RNA-seq read files have been deposited in the NCBI Sequence Read Archive (Table 1). Datasets including protein, transcript and gff3 annotation files, and raw trinity transcriptome assembly have been deposited in the Southern Cross University data repository, https://doi.org/10.25918/5e320fd1e5f06.