Data about the incidence of inflammatory bowel disease in the US for patients at the extremes of age are limited. This study showed that, in the commercially-insured and Medicare-enrollee population, the incidence rate in the elderly group in the US is similar to the adult group.
Keywords: inflammatory bowel disease, epidemiology, aging
Abstract
Background
Data on the incidence of inflammatory bowel disease (IBD) by age group are available in countries outside of the United States or localized populations within the United States. We aimed to estimate the incidence rates (IRs) of IBD by age group using a US multiregional data set.
Methods
We used the Optum Research Database to identify incident IBD patients with a disease-free interval of 1.5 years between 2005 and 2015. Overall and age-specific IRs were calculated for 4 different age groups: pediatric (0–17 years), young adult (18–25 years), adult (26–59 years), elderly (>60 years). Time trends of incidence were evaluated in each age group. Perianal phenotype (in Crohn’s disease [CD]) was also compared.
Results
The mean IR for the cohort (n = 60,247) from 2005 to 2015 was 37.5/100,000. The IR was highest in adult and elderly cohorts (36.4 and 36.7/100,000 respectively). In the adult and elderly groups, the IR for UC was higher than that for CD, whereas the opposite was true in the pediatric and young adult groups. The IR increased over the 10-year study period for all age groups (time trends P < 0.001). The elderly group had less perianal disease than the adult group (20.8 vs 22.3%, respectively; P < 0.05).
Conclusions
In one of the most comprehensive evaluations of the incidence of IBD in the United States, we found an incidence rate similar to those of other national populations. We also confirmed differences of specific IBD phenotypes based on age groups, with lower rates of perianal disease in the elderly.
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are inflammatory bowel diseases (IBDs) that are most commonly diagnosed between the ages of 20 and 40.1 Population-based studies have generally supported a worldwide increase in the overall incidence of IBD.2–7 Epidemiological data have further characterized adult patients with respect to disease type, extent, and phenotype.
Nonetheless, epidemiologic data about patients with IBD at the extremes of age are more limited. Few data exist about common disease phenotypes, such as concomitant perianal disease, in the pediatric and elderly populations. Many of the studies that address the epidemiology of IBD in the elderly population are from a single center or region.8–10 The few population-based studies that have characterized the older IBD population are limited to other countries.11, 12 The specific social systems, access to universal health care, and clinician reimbursement models noted in these studies may not be generalizable to the population in the United States.
Existing US data on the incidence, phenotype, and complications of IBD in elderly and pediatric cohorts come from single tertiary care centers, regional data sets, and localized populations.3, 13–15 Robust regional population-based data come from Olmsted County, but generalizability to the US population is limited, particularly for larger metropolitan areas outside the Midwest region. No population-based studies in the United States have comprehensively explored the incidence of pediatric and elderly populations, as has been done in European cohorts. Evaluating the epidemiology of the aging population in the United States is particularly relevant as 25% of Americans will be over the age of 65 by 2060.16 Given the gap in the current literature, we sought to estimate the overall incidence rates (IRs) of IBD in different age groups using a data set of commercially insured and Medicare patients; to assess time trends in IRs; and to further differentiate the demographics and phenotypes of IBD, particularly perianal CD, in different age groups.
METHODS
Data Source
We performed a longitudinal retrospective analysis of IBD patients in the Optum Research Database from the years 2005 to 2015. For this decade, Optum contains de-identified outpatient, inpatient, pharmaceutical claims, and lab data for approximately 56 million unique, privately insured patients nationwide. Optum also contains one of the largest populations of Medicare Part D participants. Pediatric and young adult enrollees have their own patient identification number in Optum while categorized under their parents’ insurance, allowing longitudinal assignment of their health services. The database includes information on patient characteristics and socioeconomic characteristics (sex, age, race, region, income), pharmaceutical claims data (day supply, strength, provider type), outpatient and inpatient claims data (hospital admissions, procedures), cost data, and select lab results (result value, unit, description).
Cohort Identification
Patients included in the sample met the following inclusion criteria: (1) at least 2 distinct IBD diagnoses (defined by the International Classification of Diseases, Ninth Revision [ICD-9] as 555.xx for CD and 556.xx for UC; the ICD-10 codes are K50.xx and K51.xx, respectively) and (2) 12 months’ minimum continuous enrollment. As previously described, to classify each patient as CD or UC, all distinct encounters including CD and UC ICD-9 and ICD-10 codes were summed (555.xx and K50.xx for Crohn’s disease, 556.xx and K51.xx for ulcerative colitis).17 If 80% or more of the ICD codes were 555.xx/K50.xx or 556.xx/K51.xx, the patient was classified as CD or UC, respectively. The patient was classified as having inflammatory bowel disease unclassified (IBDU) if neither the CD nor UC conditions were fulfilled. As a supplementary analysis, to define perianal phenotype in CD, patients were required to have at least 2 ICD-9 or ICD-10 codes (Supplementary Table 1) after the IBD diagnosis. The date of the diagnosis was defined as the date of the first IBD ICD-9 or ICD-10 inpatient or outpatient claim.
Incident IBD cases were defined as those with no IBD diagnosis code in the 1.5 years of continuous enrollment preceding the first IBD claim. Though several previous studies have subjectively utilized a 1- or 2-year disease-free interval to define incidence, we chose the approach previously described to (1) minimize inclusion of prevalent cases while (2) maximizing the sample size for the study.2, 18, 19 We conducted a sensitivity analysis varying the length of the disease-free interval to assess the effect on overestimation. We plotted the overestimation of the incidence every additional consecutive quarter compared with a 2-year disease-free period and selected 1.5 years (Supplementary Fig. 1).
Statistical Analysis
Discrete data were expressed as frequencies with confidence intervals and were compared using χ2 testing. Continuous data were expressed as means with standard deviations and compared using analysis of variance and Student t testing. Statistical significance was defined as a P value <0.05. To evaluate time trends, the IRs for CD and UC were calculated by multiplying the number of incident cases by 100,000, divided by the number of patients in Optum in each age group at the midpoint of each calendar year from 2005 to 2015. Age was defined as age at diagnosis and was broken up into the following groups: pediatric (0–17 years), young adult (18–25 years), adult (26–59 years), elderly (>60 years). The overall IR per 100,000 person-years was calculated, along with age-specific IRs for 4 different age groups. A Cuzick nonparametric test was utilized to detect the time trends in incidence of UC and CD from 2005 to 2015.
RESULTS
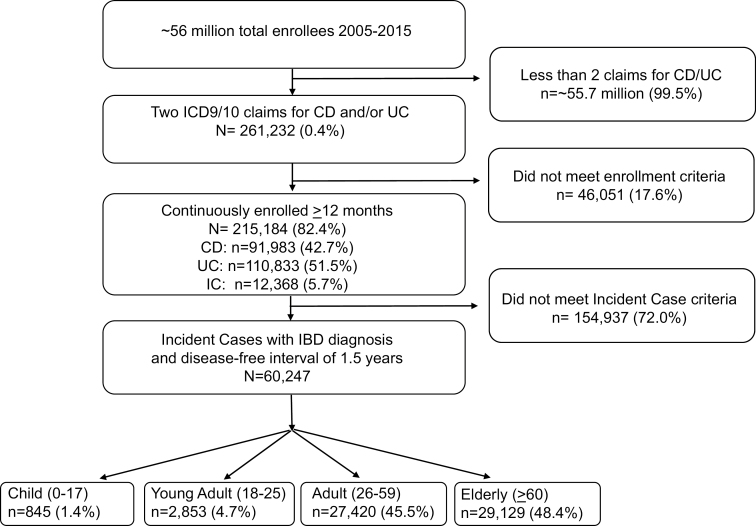
Between 2005 and 2015, more than 56 million distinct members were enrolled in the Optum Research Database. After application of inclusion criteria, 60,247 enrollees were included in the study, with the large majority being adult or elderly (Fig. 1). Table 1 lists the characteristics of incident cases in the different age groups. The mean age at diagnosis for the entire cohort was 57.1 years. A majority of the included population was white and from the South, Midwest, or West. The overall cohort’s mean Charlson comorbidity index was low (2.4), with the elderly population having the highest value (3.9).
FIGURE 1.
Flow diagram of enrollees in the study.
TABLE 1.
Characteristics of Incident Cases in Different Age Cohorts
| Entire Cohort (n = 60,247) | Pediatric Cohort (0–17 y) (n = 845) | Young Adult Cohort (18–25 y) (n = 2853) | Adult Cohort (26–59 y) (n = 27,420) | Elderly Cohort (>60 y) (n = 29,129) | P | |
|---|---|---|---|---|---|---|
| Mean age at diagnosis, y | 57.1 | 14.1 | 22.1 | 44.7 | 73.3 | <0.001 |
| Sex, female, No. (%) | 32,830 (54.5) | 399 (47.2) | 1325 (46.4) | 14,270 (52.0) | 16,836 (57.8) | <0.001 |
| Race, No. (%) | ||||||
| White | 44,736 (74.3) | 634 (75.0) | 2168 (76.0) | 20,065 (73.2) | 21,869 (75.1) | 0.002 |
| Black or African American | 4695 (7.8) | 47 (5.6) | 216 (7.6) | 2166 (7.9) | 2266 (7.8) | |
| Hispanic or Latino | 4313 (7.2) | 78 (9.2) | 231 (8.1) | 2295 (8.4) | 1709 (5.9) | |
| Asian | 1580 (2.6) | 31 (3.7) | 60 (2.1) | 957 (3.5) | 532 (1.8) | |
| Unknown | 4923 (8.2) | 55 (6.5) | 178 (6.2) | 1937 (7.1) | 2753 (9.5) | |
| Geographic region, No. (%) | ||||||
| Northeast | 7896 (13.1) | 134 (15.9) | 358 (12.6) | 3540 (12.9) | 3864 (13.3) | <0.001 |
| Midwest | 13,721 (22.8) | 227 (26.9) | 767 (26.9) | 6580 (24.0) | 6147 (21.1) | |
| South | 26,092 (43.3) | 346 (41.0) | 1241 (43.5) | 12,418 (45.3) | 12,087 (41.5) | |
| West | 12,096 (20.1) | 137 (16.2) | 474 (16.6) | 4824 (17.6) | 6661 (22.9) | |
| Unknown | 442 (0.7) | 1 (0.1) | 13 (0.5) | 58 (0.2) | 370 (1.3) | |
| Disease type | ||||||
| Ulcerative colitis, No. (%) | 33,517 (55.6) | 291 (34.4) | 1070 (37.5) | 14,325 (52.2) | 17,831 (61.2) | <0.001 |
| Crohn’s disease, No. (%) | 22,750 (37.8) | 506 (59.9) | 1597 (56.0) | 11,097 (40.5) | 9550 (32.8) | |
| Inflammatory bowel disease unclassified, No. (%) | 3980 (6.6) | 48 (5.7) | 186 (6.5) | 1998 (7.3) | 1748 (6.0) | |
| Charlson comorbidity index, mean, median | 2.4, 1.0 | 0.7, 0 | 0.6, 0 | 1.1, 0 | 3.9, 3 | <0.001 |
Incident Rate
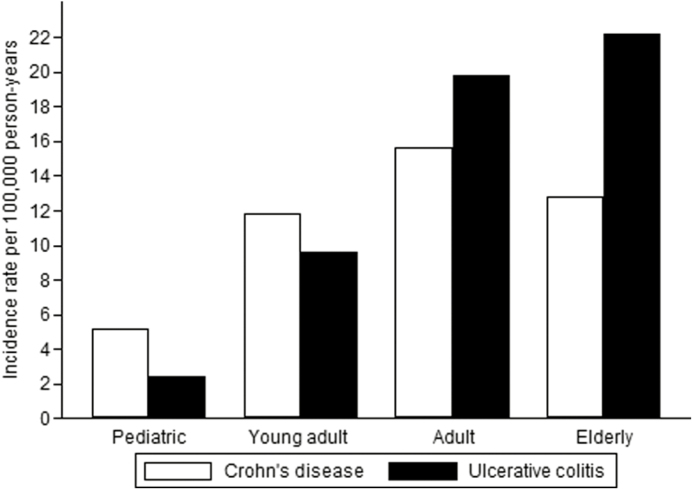
The mean IR for the cohort from 2005 to 2015 was 37.5/100,000. In the elderly cohort, the IR was 22.9/100,000 for UC and 12.8/100,000 for CD, whereas in the pediatric cohort it was 2.4/100,000 for UC and 5.2/100,000 for CD (Fig. 2). In the adult and elderly groups, the IR for UC was higher than that for CD, whereas the opposite was true in the pediatric and young adult groups.
FIGURE 2.
Distribution of IRs for IBD in 4 different age cohorts.
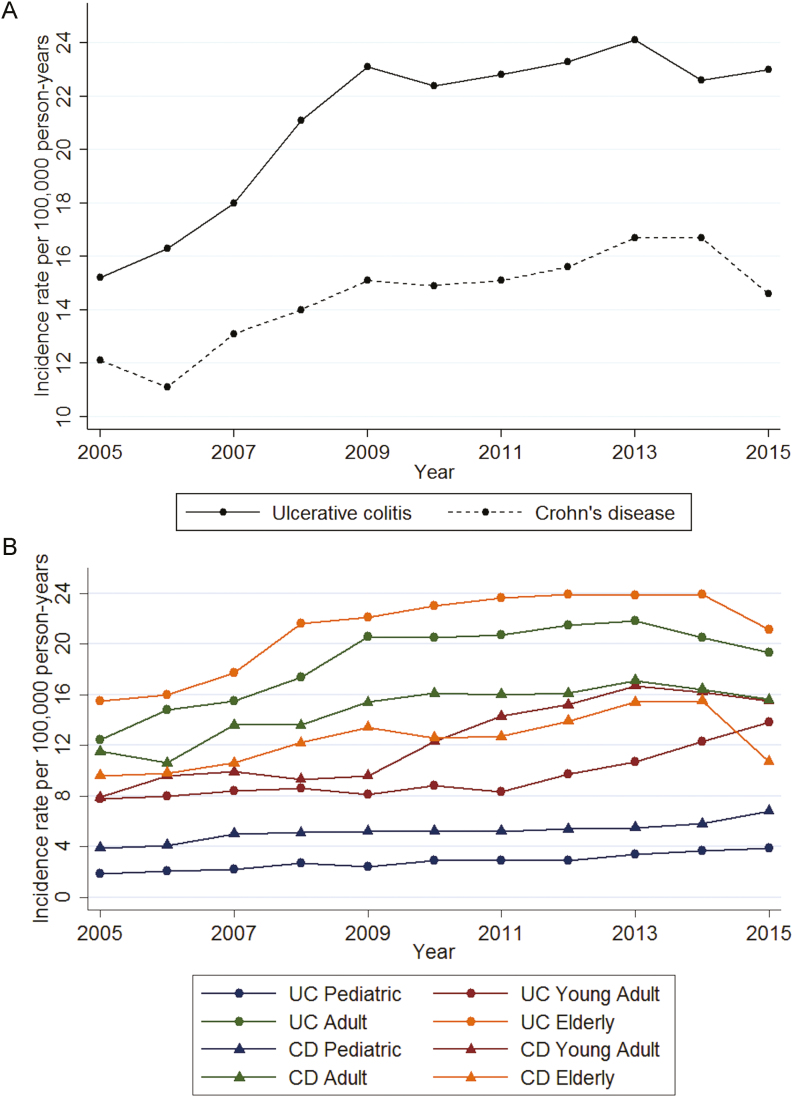
Figure 3 presents the time trends in IR in the different age cohorts between 2005 and 2015 for UC and CD. The IR increased throughout the study period for all age cohorts (time trends P < 0.001); the increase was most pronounced between 2005 and 2009.
FIGURE 3.
Time trends in incidence in (a) the entire cohort with IBD and (b) different age cohorts.
Perianal Disease Phenotype in CD
Perianal disease was found in 21.2% of the cohort with CD. The pediatric cohort had a lower rate of perianal disease (14.8%), whereas the elderly population had a slightly lower rate of perianal disease compared with the adult cohort (20.8% vs 22.3%, P = 0.011).
DISCUSSION
In this comprehensive epidemiological evaluation of IBD in the commercially insured and Medicare population in the United States, we observed an overall incidence rate of 37.5/100,000 person-years and found an increase in IBD incidence between 2005 and 2015. We found a similar incidence rate of IBD in the elderly population as compared with the adult population but found lower rates of perianal CD in the elderly. In the pediatric and young adult population, we found higher rates of CD than UC.
To our knowledge, this is the largest privately insured population study assessing the incidence of IBD in the United States with longitudinal data from enrollees in all 50 states. Previous studies from the United States have reported on the incidence of IBD in selected populations, such as enrollees in a managed care organization in Northern California, residents of Olmstead County, veterans, and patients at a single academic center.3, 13–15 The available national claims-based research within the United States has focused largely on prevalence rather than incidence.20–23 Outside of the United States, population-based studies have been published more recently in Europe.12 In the current study, incidence rates of IBD in the adult and elderly populations were consistent with previously described rates in adult and elderly European populations. Also comparable to the most recent European population study, our study found similar IRs between the adult and elderly cohorts.11 Finally, congruent with available European studies, the incidence of UC was higher than that of CD in the elderly population.11 The IRs in the pediatric and young adult cohorts, however, were lower than expected based on data from other populations, though interestingly a higher incidence of CD has previously been noted.7, 19, 24, 25 Such a finding might suggest that IBD onset occurs more commonly after age 25 in the US population compared with other countries, though further studies are needed to confirm this finding given the low number of pediatric and young adult enrollees.
Between 2005 and 2015, the incidence of both UC and CD increased. This increase was most notable in the years 2005–2009. This increase contrasts with the findings of a recent systematic review of worldwide IBD population trends,6 which suggested that the incidence and prevalence of inflammatory bowel disease in North America and Europe were largely stable between 1990 and 2015. This recent review incorporated data from studies reporting on regional populations such as California or Olmsted County. Our current study suggests that in the larger multiregional population of the United States, the incidence is actually increasing.
In patients with CD, perianal CD was noted in 21.2% of the overall cohort, which is slightly higher than previously reported rates.26, 27 This may relate to the comprehensive list of ICD codes used to define perianal disease in our study. However, we chose this comprehensive list given the applicability of all codes to documenting actual clinical complications. Perianal disease was found significantly more in adults compared with the elderly population. This phenotype was found less frequently in the pediatric and young adult population. However, given the small size of the pediatric and young adult populations, few conclusions can be drawn regarding these groups.
The strengths of this study include the utilization of a national insurance claims US database. The longitudinal nature of the Optum Research Database allows time trend evaluation of IR—one of the first studies doing so using US data. An added strength is the inclusion of Medicare Part D participants, allowing evaluation of an older IBD population. Very few US data sets exist that allow a multiregional comparison of the elderly vs a control adult population; Medicare data sets are largely weighted to the elderly, whereas other data sets may exclude the Medicare population. Finally, evaluation of perianal CD phenotypes through claims coding is novel and provides one of the first glimpses into this rarely described area in different age cohorts. Although conclusions regarding IBD disease extent and phenotype may be limited due to undercoding, we believe that severe complications (such as perianal disease) are truly captured in a claims database based on our own practice. By using a broad definition of perianal disease capturing multiple different codes, we believe that this captures the phenotype. However, validation studies will be needed.
A particular strength of our study was the methodological approach used to define incidence in our patient population.19 Studies utilizing claims-based data have traditionally used 1–2-year disease-free intervals to define incidence.28 Using a 1-year disease-free interval may overestimate incidence, as prevalent cases may be captured in that period. Using a 2-year disease-free interval results in significant loss of sample size to provide a meaningful estimate; furthermore, clinically, it would be unlikely that a patient diagnosed with IBD would go 2 years without a medical claim for the diagnosis. By using 2 years as the standard and comparing overestimation with shorter disease-free intervals, we chose 1.5 years as the optimal interval. Based on our evaluation, if we had used a 1-year disease-free interval to define incidence, we would have overestimated the “standard” incidence by more than 50%. By contrast, if we had used a 2-year disease-free interval to define incidence, we would have enrolled only 49,172 individuals, losing 11,075 enrollees. We concluded that 1.5 years minimally overestimated the calculated IR at 2 years while providing a large cohort to address IR. Furthermore, this interval was clinically relevant as patients would likely come into contact with providers within 1.5 years of diagnosis of their IBD. As is the case with any study-defining incidence, patients with milder disease may not be captured if they do not seek medical attention for more than 1.5–2 years.
The weaknesses of this study are related to the inherent limitations of an insurance claims data set. Optum does not include a Medicaid or uninsured population and underrepresents nonwhite populations in the United States, limiting generalization of the results to these populations. Furthermore, our cases had a higher representation of patients in the South and less inclusion of those in the Northeast; we did not have information on whether this geographic distribution is true of Optum in general, but, as previous studies point out, classifying geography based on insurance coverage is difficult.29 The diagnosis of IBD and phenotype of disease assume that providers and billers code accurately for such diagnoses. Accordingly, we minimized misclassification by requiring 2 separate claims of the diagnosis and perianal phenotype. In addition, although 12 months of follow-up was required to allow time for manifestation of perianal complications in Crohn’s disease, this exclusion could remove some patients with IBD from the analysis. Based on our clinical experience, we assume that providers are likely to accurately code for severe complications—particularly with perianal complications of CD. In claims-based research, true incidence of disease requires assumptions, with the goal of minimizing overestimation while including a large sample size; CD, for example, may be asymptomatic for years before the onset of symptoms or development of a complication. We attempted to address this weakness by assessing the overestimation in our analysis (Supplementary Fig. 1). Finally, although the actual IR among pediatric and young adult patients may be lower, Optum may include disproportionately low numbers of pediatric and young adult patients; this is reflected in the mean age of diagnosis (57 years) in our population, with 94% of the population being over age 26; the reason for this discrepancy is not clear but is not related to inclusion in the parents’ plan, as these patients include their own unique identifier.
CONCLUSIONS
Our study confirms that that the incidence of IBD in a multiregional privately insured and Medicare-enrolled US population is similar to the incidence rates of other populations outside of the United States, with an increase in incidence between 2005 and 2015. Our study confirms that a new diagnosis of IBD is common in the elderly patient population. Data shown here also support the concept that the phenotype of elderly CD is less aggressive than that in adults, particularly with respect to perianal disease.
Supplementary Material
Supported by: Support for this paper comes from an institutional grant from Stanford University School of Medicine (Department of Pediatrics), the Bacher-English Family Fund, and NIDDK094868 (K.T.P.). Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core, supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Amber Trickey, Jason Bentley, Lu Tian, Chris Cartwright, Rachel Bensen, and Aida Habtezion.
Conflicts of interest: K.T.P. has received research support from Janssen, Takeda, and AbbVie Inc. unrelated to this work and is supported by NIDDK094868 for this research. The remaining authors have no conflicts of interest to disclose. No form of payment or honorarium was given to any contributor for manuscript preparation or production.
Author contributions: K.K.: study concept and design, supporting analysis, drafting/editing manuscript. M.D.: study design, supporting and performing the analysis, drafting/editing the manuscript. L.S.: study design, supporting the analysis, drafting/editing the manuscript. C.K.: study design, supporting the analysis, editing the manuscript. B.N.L.: study design, supporting the analysis, editing the manuscript. C.C.: drafting/editing the manuscript. K.T.P.: obtaining funding, study concept and design, supporting the analysis, drafting/editing the manuscript.
REFERENCES
- 1. Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 2. Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559–1568. [DOI] [PubMed] [Google Scholar]
- 3. Herrinton LJ, Liu L, Lewis JD, et al. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996–2002. Am J Gastroenterol. 2008;103:1998–2006. [DOI] [PubMed] [Google Scholar]
- 4. Loftus CG, Loftus EV, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. [DOI] [PubMed] [Google Scholar]
- 5. Lophaven SN, Lynge E, Burisch J. The incidence of inflammatory bowel disease in Denmark 1980–2013: a nationwide cohort study. Aliment Pharmacol Ther. 2017;45:961–972. [DOI] [PubMed] [Google Scholar]
- 6. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 7. Sýkora J, Pomahačová R, Kreslová M, et al. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24(25):2741–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lakatos PL, David G, Pandur T, et al. IBD in the elderly population: results from a population-based study in Western Hungary, 1977–2008. J Crohns Colitis. 2011;5:5–13. [DOI] [PubMed] [Google Scholar]
- 9. Jeuring SF, van den Heuvel TR, Zeegers MP, et al. Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age—an increasing distinct entity? Inflamm Bowel Dis. 2016;22:1425–1434. [DOI] [PubMed] [Google Scholar]
- 10. Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423–432. [DOI] [PubMed] [Google Scholar]
- 11. Everhov ÅH, Halfvarson J, Myrelid P, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 years or older in Sweden. Gastroenterology. 2018;154:518–528.e15. [DOI] [PubMed] [Google Scholar]
- 12. Mañosa M, Calafat M, de Francisco R, et al. ; GETECCU Phenotype and natural history of elderly onset inflammatory bowel disease: a multicentre, case-control study. Aliment Pharmacol Ther. 2018;47:605–614. [DOI] [PubMed] [Google Scholar]
- 13. Saad AM, Czul F, Sakuraba A, et al. Age of diagnosis is associated with disease presentation and therapeutic complications in patients with Crohn’s disease. Inflamm Bowel Dis. 2016;22:1027–1031. [DOI] [PubMed] [Google Scholar]
- 14. Shivashankar R, Tremaine WJ, Harmsen WS, et al. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hou JK, Feagins LA, Waljee AK. Characteristics and behavior of elderly-onset inflammatory bowel disease: a multi-center US study. Inflamm Bowel Dis. 2016;22:2200–2205. [DOI] [PubMed] [Google Scholar]
- 16. Colby SL, Ortman JM.. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Current Population Reports Washington, DC: US Census Bureau; 2014. [Google Scholar]
- 17. Wren AA, Bensen R, Sceats L, et al. Starting young: trends in opioid therapy among US adolescents and young adults with inflammatory bowel disease in the Truven Marketscan database between 2007 and 2015. Inflamm Bowel Dis. 2018;24:2093–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abbas S, Ihle P, Köster I, et al. Estimation of disease incidence in claims data dependent on the length of follow-up: a methodological approach. Health Serv Res. 2012;47:746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benchimol EI, Bernstein CN, Bitton A, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. 2017;112:1120–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. [DOI] [PubMed] [Google Scholar]
- 22. Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of inflammatory bowel disease among adults aged ≥18 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166–1169. [DOI] [PubMed] [Google Scholar]
- 23. Bähler C, Vavricka SR, Schoepfer AM, et al. Trends in prevalence, mortality, health care utilization and health care costs of Swiss IBD patients: a claims data based study of the years 2010, 2012 and 2014. BMC Gastroenterol. 2017;17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benchimol EI, Fortinsky KJ, Gozdyra P, et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. [DOI] [PubMed] [Google Scholar]
- 25. Turunen P, Kolho KL, Auvinen A, et al. Incidence of inflammatory bowel disease in Finnish children, 1987-2003. Inflamm Bowel Dis. 2006;12:677–683. [DOI] [PubMed] [Google Scholar]
- 26. Adler J, Dong S, Eder SJ, et al. ; ImproveCareNow Pediatric IBD Learning Health System Perianal Crohn disease in a large multicenter pediatric collaborative. J Pediatr Gastroenterol Nutr. 2017;64:e117–e124. [DOI] [PubMed] [Google Scholar]
- 27. Singh B, McC Mortensen NJ, Jewell DP, et al. Perianal Crohn’s disease. Br J Surg. 2004;91:801–814. [DOI] [PubMed] [Google Scholar]
- 28. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 29. Claxton JS, Lutsey PL, MacLehose RF, et al. Geographic disparities in the incidence of stroke among patients with atrial fibrillation in the United States. J Stroke Cerebrovasc Dis. 2019;28:890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.