BES1, a key transcription factor involved in brassinosteroid signaling, coordinates plant growth and stress responses under ever-changing UV-B conditions by modulating flavonol biosynthesis.
Abstract
UV-B light is a potential stress factor in plants, but how plants coordinate growth and UV-B stress responses is not well understood. Here, we report that brassinosteroid (BR) signaling inhibits UV-B stress responses in Arabidopsis (Arabidopsis thaliana) and various crops by controlling flavonol biosynthesis. We further demonstrate that BRI1-EMS-SUPPRESSOR 1 (BES1) mediates the tradeoff between plant growth and UV-B defense responses. BES1, a master transcription factor involved in BR signaling, represses the expression of transcription factor genes MYB11, MYB12, and MYB111, which activate flavonol biosynthesis. BES1 directly binds to the promoters of these MYBs in a BR-enhanced manner to repress their expression, thereby reducing flavonol accumulation. However, exposure to broadband UV-B down-regulates BES1 expression, thus promoting flavonol accumulation. These findings demonstrate that BR-activated BES1 not only promotes growth but also inhibits flavonoid biosynthesis. UV-B stress suppresses the expression of BES1 to allocate energy to flavonoid biosynthesis and UV-B stress responses, allowing plants to switch from growth to UV-B stress responses in a timely manner.
INTRODUCTION
UV-B light is an intrinsic part of sunlight with significant effects on plant development and acclimation (Heijde and Ulm, 2012). UV RESISTANCE LOCUS 8 (UVR8) is the long-sought UV-B photoreceptor that is required for UV-B responses (Rizzini et al., 2011; Tilbrook et al., 2013; Jenkins, 2014). UV-B irradiation does not affect UVR8 protein abundance but rather induces its monomerization and nuclear accumulation (Kaiserli and Jenkins, 2007; Rizzini et al., 2011). CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1), a central regulator of light signaling (Yi and Deng, 2005), interacts with UVR8 in a UV-B-dependent manner to mediate UV-B signaling (Favory et al., 2009; Rizzini et al., 2011; Huang et al., 2014; Qian et al., 2016; Yin et al., 2016; Podolec and Ulm, 2018; Ren et al., 2019). The WD40-repeat proteins REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and RUP2 directly interact with UVR8 to mediate its redimerization, UV-B-induced photomorphogenesis, and flowering (Gruber et al., 2010; Heijde and Ulm, 2013; Arongaus et al., 2018). The basic leucine-zipper transcription factor ELONGATED HYPOCOTYL 5 (HY5) and its homolog HYH mediate UV-B-induced changes in gene expression downstream of UVR8 (Ulm et al., 2004; Brown et al., 2005; Oravecz et al., 2006; Brown and Jenkins, 2008; Stracke et al., 2010; Fehér et al., 2011; Huang et al., 2012). UVR8 also physically interacts with the transcription factors BRI1-EMS-SUPPRESSOR 1 (BES1), BES1-INTERACTING MYC-LIKE 1 (BIM1), WRKY DNA BINDING PROTEIN 36 (WRKY36), MYB DOMAIN PROTEIN 73/77 (MYB73/MYB77), and MYB13 to directly regulate transcription and photomorphogenesis (Liang et al., 2018, 2019; Yang et al., 2018; Qian et al., 2020; Yang et al., 2020). There are also some UVR8-independent UV-B responses, suggesting the existence of new or different UV-B photoreceptors (Brown and Jenkins, 2008).
UV-B can damage DNA, trigger the accumulation of reactive oxygen species, and impair photosynthesis (Demarsy et al., 2018). Plants have evolved efficient mechanisms to prevent or limit UV-B-induced damage by accumulating “sunscreen” flavonoids, including flavonol, anthocyanins, and proanthocyanidins. UV-B induces the expression of flavonoid biosynthesis genes, and Arabidopsis (Arabidopsis thaliana) mutants deficient in flavonoids are UV-B sensitive (Li et al., 1993; Stracke et al., 2010; Clayton et al., 2018). PRODUCTION OF FLAVONOL GLYCOSIDES (PFG1)/MYB12, PFG2/MYB11, and PFG3/MYB111 are R2R3-MYB transcription factors that are specifically involved in regulating flavonol biosynthesis (Stracke et al., 2007). Notably, PFG MYBs are also required for UV-B protection: the myb11 myb12 myb111 triple mutant is hypersensitive to UV-B stress (Stracke et al., 2010). UVR8 and HY5 induce the transcription of these PFG MYB genes in response to UV-B light, and the PFG MYBs work together with HY5 to regulate the expression of downstream target genes, including CHALCONE SYNTHASE (CHS), leading to flavonol biosynthesis (Cloix and Jenkins, 2008; Favory et al., 2009; Stracke et al., 2010). However, besides HY5, additional factors involved in regulating PFG MYB gene expression in response to UV-B have not yet been identified.
Brassinosteroids (BRs) are a class of steroidal hormones that are essential for plant development, including skotomorphogenesis, photomorphogenesis, thermomorphogenesis, cell elongation and proliferation, and flowering (Yang et al., 2005; Li et al., 2010; Clouse, 2011; Ye et al., 2011; Bai et al., 2012; Zhang et al., 2013; Anne et al., 2015; Chaiwanon et al., 2016; Ibanez et al., 2018; Ruan et al., 2018). BRs are also involved in plant responses to biotic and abiotic stress, such as drought stress responses and plant immunity (Ryu et al., 2014; Yang et al., 2016; Nolan et al., 2017a, 2017b; Yang et al., 2017). The surface receptor kinase complex including BRASSINOSTEROID INSENSITIVE 1 (BRI1) and BRI1 ASSOCIATED PROTEIN KINASE 1 (BAK1) is responsible for perceiving BRs (Li et al., 2002; Nam and Li, 2002; Tang et al., 2010). The phosphatase BR-SUPPRESSOR 1 (BSU1) is thought to be activated by BRI1 (Tang et al., 2008; Kim et al., 2009), which triggers the inactivation of the GSK3-like kinase BR-INSENSITIVE 2 (BIN2). The inactivation of BIN2 allows the unphosphorylated transcription factors BES1 and BRASSINAZOLE RESISTANT 1 (BZR1) to accumulate in the nucleus. There, BES1 and BZR1 function as master regulators of the BR signal transduction pathway by regulating the expression of BR target genes (Wang et al., 2002; Yin et al., 2002; He et al., 2005).
We previously showed that UVR8 physically interacts with BIM1 and the functional dephosphorylated BES1, which is promoted by UV-B, inhibits the DNA binding activities of BIM1 and BES1, thereby inhibiting the transcription of growth-related genes and BR-promoted growth (Liang et al., 2018). However, whether BR signaling is involved in UV-B stress responses remains unclear. The levels of UV-B that reach the Earth’s surface are highly dynamic and are determined by the time of day, season, latitude, altitude, shade, and many other factors. How plants adapt to changing UV-B levels and coordinate growth and UV-B stress responses is not well understood.
Here, we report that BRs negatively affect plant tolerance to UV-B stress. Our results demonstrate that BR-deficient and BR signaling mutants have altered UV-B stress tolerance, and BES1 acts downstream of BRI1 to inhibit UV-B stress tolerance. We show that BES1 binds to the promoters of PFG MYBs in a BR-enhanced manner to inhibit gene transcription, thus inhibiting flavonol biosynthesis and UV-B tolerance. Broadband UV-B represses the expression of BES1 in a UVR8-independent manner, thereby promoting flavonol accumulation and UV-B stress tolerance. Our findings indicate that, in addition to its known role in promoting growth, BES1 also inhibits flavonoid accumulation in the absence of UV-B, while UV-B stress suppresses the expression of BES1 to inhibit growth and promote flavonoid accumulation. Therefore, we conclude that BES1 mediates the tradeoff between plant growth and UV-B stress responses.
RESULTS
BRs Negatively Regulate UV-B Stress Tolerance
UV-B-induced photomorphogenesis helps plants prevent UV-B stress. BR is involved in the UVR8-regulated inhibition of hypocotyl growth (Liang et al., 2018), but whether BR signaling regulates plant responses to UV-B stress is unclear. Therefore, we analyzed the responses of Arabidopsis BR biosynthesis and signaling mutants to UV-B stress. Since UV-B damages PSII (Demarsy et al., 2018), we measured the maximum quantum yield of PSII (Fv/Fm) as a proxy for UV-B stress. The BR-deficient det2 (Li et al., 1996) and cpd (Szekeres et al., 1996) mutants were more tolerant to UV-B stress than wild-type Arabidopsis ecotype Columbia -0 (Col-0) or Enkheim-2 (En2; Supplemental Figure 1A). In the absence of UV-B, there was no significant difference in Fv/Fm between BR-deficient mutants and the wild type. However, following UV-B stress treatment, the BR mutants had higher Fv/Fm values than wild-type Col-0 or En2 (Supplemental Figure 1A). In addition, the BR receptor mutants bri1-301 and bri1-1 were more tolerant to UV-B stress than Col-0 (Figures 1A and 1B; Supplemental Figure 1A), and bri1-9 was more tolerant to UV-B stress than Ws-2 (Supplemental Figure 1A).
Figure 1.
BRs Negatively Regulate UV-B Stress Tolerance.
(A) and (B) BR mutants show increased tolerance to UV-B stress. (A) Analysis of UV-B stress tolerance in Arabidopsis. Plants of the indicated genotypes were grown in soil under long-day (16/8) conditions in white light for 10 d and irradiated with (+UV) or without (−UV) broadband UV-B plus white light for 8 h and allowed to recover for 3 d in white light. (B) Measurement of PSII maximal quantum yield (Fv/Fm). The indicated genotypes were irradiated with broadband UV-B plus white light for 5 h and recovered for 1 d, and Fv/Fm was measured and quantified with an imaging fluorometer.
(C) and (D) The bes1-D mutant and bes1-D-OX are sensitive to UV-B stress. (C) Analysis of UV-B stress tolerance in Arabidopsis. The indicated genotypes were grown in half-strength MS medium with 1 μM BRZ under long-day conditions in white light for 10 d and treated (or not) with broadband UV-B for 8 h. (D) measurement of Fv/Fm. The indicated genotypes were grown in half-strength MS medium with 1 μM BRZ and treated with broadband UV-B for 5 h. Fv/Fm was measured and quantified.
(E) and (F) BES1 functions downstream of BRI1 to regulate UV-B defense responses. (E) Analysis of UV-B stress tolerance. The indicated genotypes were grown in soil for 10 d and treated (or not) with broadband UV-B for 8 h. (F) Measurement of Fv/Fm.
In (B), (D), and (F), data are means ± SD (n > 15). Statistically significant differences were determined by one-way ANOVA, followed by Tukey’s test (P < 0.05). Letters “A” and “B” indicate the Fv/Fm of the indicated seedlings without UV-B treatment, while “a” to “d” indicate the Fv/Fm of seedlings subjected to UV-B treatment. At least three independent experiments were performed with similar results.
We investigated the effects of exogenous application of BRZ (a triazole-type BR biosynthesis inhibitor that inhibits cytochrome P450 enzymes) and BR on UV-B stress tolerance in the mutants. BR treatment had little effect on UV-B tolerance in Col-0 or bri1-301 plants but suppressed the UV-B tolerance of the BR-deficient mutant det2 (Supplemental Figure 1B). BRZ treatment strongly promoted UV-B stress tolerance in wild-type Col-0 (Supplemental Figures 1C and 1D). These results indicate that BRs negatively regulate UV-B stress tolerance in Arabidopsis.
We also analyzed the UV-B stress tolerance of BR mutants in various crops. The BR-deficient maize mutant na1 (Hartwig et al., 2011), which carries a loss-of-function mutation in a homolog of the BR biosynthesis gene DET2, and the BR receptor rice (Oryza sativa) mutant d61 (Yamamuro et al., 2000), which harbors a mutation in BRI1 homolog, were more tolerant to UV-B stress and had better photosynthetic performance than wild type in the presence of UV-B stress (Supplemental Figures 1E to 1H). Therefore, the inhibitory effect of BRs on UV-B stress tolerance is conserved in Arabidopsis and various crops.
UVR8 physically interacts with BES1, the master transcription factor of the BR signaling pathway, to inhibit hypocotyl elongation (Liang et al., 2018). We therefore analyzed whether BES1 is involved in regulating UV-B stress tolerance. BES1 has five homologue genes and we found that the single mutants of each gene had a subtle phenotype but were significantly more tolerant to UV-B stress than the wild type (Supplemental Figure 2A). The bes1 bzr1 beh1 beh2 beh3 beh4 hextuple mutant (Chen et al., 2019b) and BES1-RNAi (Yin et al., 2005) plants, in which BES1 and its homologue genes were down-regulated, showed a more dramatic phenotype than the single mutants (Supplemental Figure 2A). We also analyzed uvr8 BES1-RNAi plants and found that they were more tolerant to UV-B stress than the uvr8 mutant (Figures 1A and 1B). Consistent with the bes1 mutant, bes1-D (a dominant gain-of-function mutation) in the Col-0 or En2 background and transgenic plants overexpressing BES1 (bes1-D-OX) were more sensitive to UV-B stress than the wild type in the presence of BRZ (Figures 1C and 1D; Supplemental Figures 2B to 2D). In addition, overexpression of bes1-D in the bri1-301 mutant background suppressed the UV-B tolerant phenotype of bri1-301 (Figures 1E and 1F; Supplemental Figure 2D), indicating that BES1 functions downstream of BRI1 to regulate UV-B defense responses.
BIN2 kinase phosphorylates BES1 and inhibits its activity (Mora-García et al., 2004). The heterozygous BIN2 dominant mutant BIN2/bin2 (Li and Nam, 2002) was more tolerant to UV-B stress than the wild type (Supplemental Figures 2E and 2F). BIM1 functions together with BES1 to regulate UV-B-controlled hypocotyl elongation (Liang et al., 2018). However, the bim1 bim2 bim3 triple mutant had no obvious UV-B tolerance phenotype (Supplemental Figures 2E and 2F). These results indicate that BES1 is the key component of the BR signaling pathway that regulates UV-B stress tolerance, whereas BIM1 is not involved in this process. Therefore, the molecular mechanism underlying the role of BES1 in regulating UV-B stress tolerance appears to differ from that regulating hypocotyl elongation.
BES1 Represses PFG MYB Gene Expression and Inhibits Flavonol Biosynthesis
BES1 is a key transcription factor in the BR signaling pathway that controls the expression of numerous genes. An analysis of previously reported microarray data sets of bes1-D (Yu et al., 2011) indicated that cell-wall biogenesis-related genes are significantly enriched among genes up-regulated by BES1 (Figure 2A), accounting for the role of BES1 in promoting plant growth. Interestingly, flavonoid biosynthesis-related genes were the most significantly enriched among genes down-regulated by BES1 (Figure 2B). These flavonoid biosynthesis genes were down-regulated by BES1 but markedly induced by UV-B (Figure 2C), including FLAVONOL SYNTHASE 1 (FLS1), CHS, FLAVANONE 3-HYDROXYLASE (F3H), and MYB12, suggesting that UV-B and BES1 antagonistically regulate the expression of flavonoid biosynthesis genes.
Figure 2.
BES1 Inhibits PFG MYB Gene Expression and Flavonol Biosynthesis.
(A) to (C) Transcriptomic analysis of BES1- and UV-B-regulated gene expression. The microarray data sets of bes1-D (Yu et al., 2011) were reanalyzed to characterize BES1-regulated genes. The plants used for microarrays were grown in a greenhouse made of glass so the plants received sunlight including UV-B. Gene ontology analysis of genes up-regulated (A) or down-regulated (B) by BES1. (C) The fold changes of flavonoid-related genes down-regulated by BES1 were analyzed in response to UV-B light using the reanalyzed microarray data sets of Favory et al. (2009). Log fold change values are shown.
(D) RT-qPCR analysis of PFG MYB gene expression in wild type (Col-0) and BES1-RNAi mutants. Col-0 and BES1-RNAi were grown in soil under constant white light for 8 d and transferred to broadband UV-B for a 3-h time course. The ACT7 gene was analyzed as an internal control, and the expression level of Col-0 at 0 h was set to 1. Error bars, SDs of three biological replicates.
(E) Quantification of flavonol levels in seedlings grown with or without UV-B stress using HPLC. Error bars are SDs of three biological replicates. “A” and “B” indicate statistically significant differences between the flavonol contents of the indicated seedlings without UV-B treatment, as determined by one-way ANOVA, followed by Tukey’s least significant difference (LSD) test (P < 0.05), and “a” to “d” indicate those with UV-B treatment.
BRs regulate the expression of CHS and anthocyanin accumulation (Chory et al., 1991; Sävenstrand et al., 2004), but whether BRs control flavonol biosynthesis is unclear. Flavonoid biosynthesis involves a multitude of enzymatic and regulatory proteins. Three R2R3-MYB transcription factors, PRODUCTION OF FLAVONOL GLYCOSIDES (PFG1)/MYB12, PFG2/MYB11, and PFG3/MYB111, are master regulators that activate the expression of flavonol biosynthetic genes. RT-quantitative PCR (RT-qPCR) showed that these three PFG MYB genes were enhanced up-regulated in BES1-RNAi plants versus the wild type (Figure 2D), which is consistent with the results of transcriptome analysis. Furthermore, these three PFG MYB genes were less up-regulated in bes1-D-OX transgenic plants compared to the wild type after treatment with the BR-biosynthesis inhibitor brassinazole (BRZ, which promotes the formation of physiologically inactive phosphorylated BES1; Supplemental Figures 3A and 3B). These results indicate that BES1 represses the transcription of PFG MYBs.
The finding that BES1 represses the transcription of PFG MYB genes suggests that BES1 might affect endogenous flavonol levels. We therefore analyzed flavonol levels in the plants by high performance liquid chromatography (HPLC). Specifically, we measured the total flavonol contents in Col-0, uvr8, and BR mutant plants before and after UV-B stress treatment. As previously reported, uvr8 had markedly reduced flavonol levels (Kliebenstein et al., 2002; Favory et al., 2009), whereas BES1-RNAi, bri1-301, and det2 had higher flavonol levels than the wild type after UV-B treatment (Figure 2E). We performed diphenylboric acid 2-aminoethyl (DPBA) staining to analyze flavonol contents in situ. Consistent with the HPLC results, the flavonol levels were lower in uvr8 and higher in BES1-RNAi and bri1-301 compared to Col-0 after broadband UV-B treatment, whereas uvr8 BES1-RNAi and uvr8 bri1 plants accumulated more flavonol than the uvr8 mutant in response to this treatment (Supplemental Figure 3C). These results indicate that BES1 down-regulates the expression of PFG MYBs and represses flavonol accumulation.
BES1 Directly Binds to the Promoters of PFG MYBs in a BR-Enhanced Manner
Since BES1 inhibits the expression of PFG MYB genes, we asked whether BES1 directly regulates the transcription of these genes. BRRE elements (CGTGT/CG) and G-box elements (CACGTG) are frequently enriched in the promoters of BR-repressed target genes (Sun et al., 2010). We analyzed the promoters of PFG MYBs (2 kb upstream of the translational start site) and found several G-boxes and BRRE elements, with markedly more G-box elements than BRRE elements (Figures 3B to 3D). Therefore, we examined whether BES1 could bind the promoters of PFG MYBs. We conducted electrophoretic mobility shift assays (EMSAs) using recombinant BES1 protein purified from Escherichia coli in vitro. As shown in Figure 3A, BES1 bound to the promoters of MYB11, MYB12, and MYB111 but had much lower binding activity with promoters harboring a mutated G-box.
Figure 3.
BES1 Binds to the Promoters of PFG MYB Genes in a BR-Enhanced and UVR8-Independent Manner.
(A) EMSAs showing that BES1 binds to the PFG MYB promoters in vitro. The probes (∼40 bp; MYB11, −1171 bp to −1131 bp; MYB12, −468 bp to −428 bp; MYB111, −469 bp to −429 bp) were labeled with Cy5. BES1 expressed in E. coli could bind to the MYB promoters, while Trigger Factor (empty vector expressed) could not. Two arrows indicate that different BES1 complexes bind to the probes. Unlabeled probe was added as a competitor. pMYB12m is a mutant probe with mutations in the G-box (CACGTG mutated to AAAAAA).
(B) to (D) Promoter analysis of PFG MYBs and ChIP-qPCR. Red circles indicate G-box elements, and blue circles indicate BRRE elements in the MYB promoters. The letters indicate primer pairs used for ChIP-qPCR. ChIP results showing that BES1 binds to the promoters of PFG MYBs in vivo. Ten-day-old Col-0 and BES1-Flag transgenic plants were treated with 1 μM BR for 2 h before harvesting samples. Chromatin fragments (∼500 bp) were immunoprecipitated by anti-Flag-agarose beads (IP). The precipitated DNA was analyzed by RT-qPCR using primer pairs based on the promoters of MYBs and SAUR-AC as positive controls. The level of binding was calculated as the ratio between IP and input, normalized to that of ACT as an internal control, and the binding level of Col-0 was set to 1. Error bars, SDs of three biological replicates.
(E) ChIP-qPCR assays performed using Col-0 and BES1-RNAi transgenic plants. Chromatin fragments were immunoprecipitated by BES1 antibody-coupled agarose beads (IP). The precipitated DNA was analyzed by qPCR using the representative primer pairs based on the MYB promoters and SAUR-AC as positive controls. The level of binding was calculated as described above. Error bars, SDs of three biological replicates.
(F) BR treatment promotes the interaction of BES1 with the promoters of PFG MYBs. ChIP-qPCR assays were performed using Col-0 and BES1-Flag transgenic plants with phosphorylated or dephosphorylated BES1. BES1-Flag transgenic plants were grown in half-strength MS medium with or without 1 μM BRZ for 10 d and treated with 1 μM BR for 2 h before harvesting samples. Chromatin fragments were immunoprecipitated by anti-Flag-agarose beads (IP). The level of binding was calculated and analyzed as described above. Error bars, SDs of three biological replicates.
(G) Narrow-band UV-B treatment does not affect the interaction of BES1 with the PFG MYB promoters. ChIP-qPCR assays were performed using Col-0 and BES1-Flag treated with or without narrow-band UV-B for 3 h. Chromatin fragments were immunoprecipitated by anti-Flag-agarose beads (IP). The level of binding was calculated and analyzed as described above. Error bars, SDs of three biological replicates.
(H) Structure of the PFG MYB promoter-driven dual-LUC reporter plasmids and two effector plasmids. For the reporter construct, 35S promoter, MYB gene promoters, REN luciferase (REN), firefly luciferase (LUC) are indicated. For the effector construct BES1 and bes1-D were driven by the 35S promoter.
(I) and (J) BES1 inhibits the expression of PFG MYBs. Leaf epidermal cells of N. benthamiana were transfected with reporter DNA (Mock) or reporter DNA together with the empty effector DNA (Flag), BES1, or bes1-D. After 3 d of transfection, LUC and REN activity was measured and quantified with a luminometer. LUC activity values normalized to REN are shown in (I). Error bars, SDs of three biological replicates. Statistically significant differences were determined by one-way ANOVA, followed by Tukey’s test (P < 0.05). LUC activity was also captured and imaged by cold-CCD; representative images are shown in (J), above is bright field imaging, and below is LUC. At least three independent experiments were performed with similar results.
To further confirm that BES1 binds to the promoters of these MYB genes, we performed chromatin immunoprecipitation (ChIP) assays. First, we performed the ChIP assays using transgenic plants expressing Pro35S::BES1-Flag. Following immunoprecipitation of protein-DNA complexes with Flag antibody, enriched DNA sequences were amplified by RT-qPCR using primer pairs covering the promoter regions of PFG MYB genes. As shown in Figures 3B to 3D, BES1 bound to these PFG MYB promoters; some binding sites contained G-boxes, while some did not. We also performed ChIP assays using a BES1 antibody to immunoprecipitate BES1-DNA complexes from wild-type Col-0 and confirmed that the native BES1 indeed bound to the promoters of PFG MYBs (Figure 3E).
According to the classical BR signaling pathway, BES1 is dephosphorylated in response to BRs, and dephosphorylated BES1 then binds to growth-related genes to activate their expression (Vert and Chory, 2006). Therefore, we hypothesized that BRs also affect the binding of BES1 to the promoters of PFG MYB genes. To test this hypothesis, we grew transgenic plants expressing Pro35S::BES1-Flag in the presence of BRZ to promote the formation of phosphorylated BES1 or in the presence of BRs to promote BES1 dephosphorylation and performed ChIP assays. Compared to phosphorylated BES1, dephosphorylated BES1 had a higher binding affinity for PFG MYBs. Similar results were obtained using SAUR-AC, a positive control that is a well-established BES1 target gene (Figure 3F). These findings indicate that BRs promote the binding of BES1 to the promoters of PFG MYBs. Therefore, the binding of BES1 to PFG MYBs is enhanced by BR signaling.
We then performed transient transcription assays to confirm the effect of BES1 on MYB expression. We used dual-luciferase (LUC) reporter plasmids containing the firefly luciferase (LUC) gene driven by each PFG MYB gene promoter and the Renilla luciferase gene driven by the constitutive 35S promoter in the assays. The effector plasmids expressing BES1 or bes1-D driven by the 35S promoter were highly expressed in Nicotiana benthamiana leaves (Figure 3H; Supplemental Figure 4A). The three reporters were separately expressed in N. benthamiana leaves together with BES1-Flag, bes1-D-Flag, or Flag tag only. Compared to the control and Flag tag, BES1 or bes1-D significantly repressed the expression of all three PFG MYB genes (Figures 3I and 3J), indicating that BES1 inhibits the transcription of PFG MYB genes. Like BES1, BZR1 and other BES1 homologs also repressed the expression of MYB12 (Supplemental Figures 4B and 4C), indicating that BES1 and its homologs function redundantly to repress the expression of PFG MYBs, which may account for the subtle phenotype of the BES1 single mutant. Taken together, these findings indicate that BES1 directly binds to the PFG MYB promoters in a BR-enhanced manner to repress the transcription of PFG MYBs.
We previously reported that UVR8 interacts with BES1 to inhibit its binding to plant growth-related genes (such as SAUR-AC), thus repressing their expression and BR-promoted hypocotyl elongation (Liang et al., 2018). Here, we investigated the effect of UV-B on the binding of BES1 to the promoters of PFG MYBs. Interestingly, neither broadband nor narrow-band UV-B affected the DNA binding activity of BES1 to the PFG MYB promoters (Figure 3G; Supplemental Figure 4D). EMSAs (Supplemental Figure 4E) showed that the overall amount of BES1-MYB12 complex did not decrease in response to the addition of photo-activated UVR8W285A (although the level of the low-molecular-weight BES1-MYB12 complex slightly decreased with the addition of UVR8W285A, but the level of the high-molecular-weight complex increased). Taken together, these findings indicate that UV-B and UVR8 do not affect the DNA binding activity of BES1 to PFG MYBs, suggesting that UV-B light affects the regulatory activity of BES1 on PFG MYB gene expression via a different mechanism.
PFG MYBs Act Downstream of BES1 to Mediate UV-B Stress Tolerance
Since BES1 directly represses PFG MYB gene expression and inhibits flavonol biosynthesis, we speculated that PFG MYBs act downstream of BES1 to regulate UV-B stress responses. We crossed the myb11 myb12 myb111 triple mutant (abbreviated as mybt) with BES1-RNAi and bri1-301 to produce BES1-RNAi mybt and bri1 mybt (Supplemental Figures 5A to 5C). The flavonol content was significantly reduced in mybt versus the wild type, as previously reported (Stracke et al., 2007). The flavonol level was higher in BES1-RNAi than Col-0 but markedly lower in BES1-RNAi mybt than BES1-RNAi (Figure 4A). These results indicate that MYBs function downstream of BES1 to control flavonol accumulation. Consistent with flavonol levels, BES1-RNAi mybt was sensitive to UV-B stress and suppressed the UV-B-tolerant phenotype of BES1-RNAi (Figures 4B and 4C).
Figure 4.
PFG MYBs Act Downstream of BES1 to Regulate UV-B Stress Tolerance.
(A) PFG MYBs act downstream of BES1 to control flavonol accumulation. The PFG MYB triple mutant myb11 myb12 myb111 (abbreviated as mybt) was crossed with BES1-RNAi to obtain the multiple mutant BES1-RNAi mybt. Flavonols in cotyledons were stained with DPBA and imaged by epifluorescence microscopy. Photographs of representative seedlings are shown; bars represent 0.5 mm. Fluorescence intensities were quantified by ImageJ. Data are means ± sd (n > 15). The asterisks indicate significant difference by one-way ANOVA, followed by Tukey’s test (P < 0.05). At least three independent experiments were performed with similar results.
(B) and (C) The UV-B tolerant phenotype of BES1-RNAi is dependent on PFG MYBs. (B) Analysis of UV-B stress tolerance. (C) Measurement of Fv/Fm. Data are means ± SD (n > 15). “A” indicates statistically significant differences between the Fv/Fm of the indicated seedlings without UV-B treatment, as determined by one-way ANOVA, followed by Tukey’s test (P < 0.05), and “a” to “c” indicate the Fv/Fm of seedlings subjected to UV-B treatment. At least three independent experiments were performed with similar results.
bri1 mybt had much lower flavonol levels than bri1-301 in the presence of UV-B treatment (Supplemental Figure 5D), and bri1 mybt was sensitive to UV-B stress (Supplemental Figures 5E and 5F), indicating that the UV-B-tolerant phenotype of bri1-301 was at least partially dependent on MYBs. These results indicate that PFG MYBs act downstream of BRs to regulate UV-B stress tolerance. Together, the results of genetic and gene expression analysis indicate that BES1 inhibits PFG MYB gene expression to inhibit flavonoid biosynthesis and reduce UV-B stress tolerance. The observation that BES1-RNAi mybt and bri1 mybt plants were small but sensitive to UV-B stress (Figure 4B; Supplemental Figure 5E) indicates that the UV-B-tolerant phenotype of the BR mutants was not caused by their smaller size but primarily depended on flavonoid accumulation.
BES1 Is Down-Regulated by UV-B Stress
UV-B treatment had little effect on the DNA binding activity of BES1 to PFG MYB gene promoters. To explore the mechanism underlying how UV-B light controls the activity of BES1 in regulating PFG MYBs, we investigated whether broadband UV-B affects BR biosynthesis. We analyzed the effect of UV-B stress on the expression of BR biosynthesis genes DWF4, CPD, and BR6OX2 and observed that their expression changed little upon treatment, with only a slight decrease, followed by recovery (Supplemental Figure 6A). We then we measured the level of endogenous BRs including castasterone (CS), typhasterol (TY), and 6-deoxoCS in plants upon broadband UV-B treatment. This treatment did not significantly affect 6-deoxo CS or TY levels, but CS levels decreased slightly after broadband UV-B treatment (Supplemental Figure 6B). Finally, we examined BR responses to broadband UV-B treatment using hypocotyl growth as a proxy. Broadband UV-B inhibited plant growth, and supplementation with 24-epibrassinolide did not reverse this inhibition (Supplemental Figures 6C and 6D). Taken together, these results suggest that broadband UV-B does not significantly affect BR levels.
Broadband UV-B (280 to 315 nm) contains low-wavelength and high-energy UV-B light and is more likely to cause stress than narrow-band UV-B (311 to 313 nm). We previously demonstrated that narrow-band UV-B has no significant effect on BES1 transcription or BES1 protein stability (Liang et al., 2018). However, in this study, the levels of native BES1 protein decreased in response to broadband UV-B in both Col-0 and uvr8 plants (Figure 5A). The levels of both phosphorylated and dephosphorylated BES1 decreased in response to broadband UV-B treatment, although BR-activated dephosphorylated BES1 had a much higher binding affinity to the PFG MYBs than phosphorylated BES1. RT-qPCR assays showed that BES1 transcript levels in both Col-0 and uvr8 decreased after UV-B treatment (Figure 5B). These results indicate that UV-B stress inhibits the transcription of BES1 in a UVR8-independent manner. UVR8 neither inhibits BES1 expression nor affects the DNA binding activity of BES1 to PFG MYBs, which also accounts for the finding that uvr8 BES1-RNAi plants were more tolerant to UV-B stress compared to uvr8 plants (Figures 1A and 1B). We measured the transcript levels of BES1 homologs in response to UV-B treatment. BES1, BEH1, and BZR1 transcript levels decreased in response to UV-B stress, but BEH2 and BEH4 transcript levels barely changed and BEH3 was induced by UV-B stress (Supplemental Figure 7A). These results suggest that UV-B light regulates BES1 and its homologs via different mechanisms.
Figure 5.
BES1 Is Down-Regulated by UV-B Stress.
(A) Immunoblots showing the decrease in native BES1 protein levels in response to broadband UV-B. Col-0 and uvr8 seedlings were grown in constant white light for 7 d and transferred to broadband UV-B for 4, 8, 10, 12, or 24 h before sample collection. BES1-RNAi was collected under 0 h UV-B and served as a negative control. All samples were fractionated by 10% SDS-PAGE, blotted, and probed with anti-BES1 (gift from Yanhai Yin, 1:3000 dilution for immunoblot) or anti-ACTIN (D195301, Sangon Biotech, 1:3000 dilution for immunoblot) antibody. P-BES1 indicates phosphorylated BES1. The numbers below the bands are the intensity of the bands quantified by ImageJ. The value of Col-0 at 0 h was set to 1.
(B) RT-qPCR showing that broadband UV-B represses the transcription of BES1. Col-0 and uvr8 plants were grown in white light and transferred to broadband UV-B for an 8-h time course or kept in white light. The ACT7 gene was analyzed as an internal control, and the expression level in Col-0 at 0 h was set to 1. Error bars are SDs of three biological replicates.
(C) to (E) Low-wavelength UV-B inhibits the activation of the BES1 promoter. Transgenic pBES1::LUC plants were grown in constant white light for 7 d and treated with narrow-band UV-B (NUV), broadband UV-B (BUV), broadband UV-B with 300-nm transmission cutoff filter (BUV+ZJB300), and broadband UV-B with 340-nm transmission cutoff filter (BUV+ZJB340) for an 8-h time course. The UV-B light irradiances of NUV, BUV, and BUV+ZJB300 were equivalent to 5 μmol/m2/s. (C) LUC signals were captured and imaged by cold-CCD, and representative images are shown. (D) The light spectra of the four light treatments measured with a spectrograph. (E) LUC signals quantified by ImageJ. Error bars, SDs of three biological replicates.
To confirm the effect of UV-B stress on the transcription of BES1, we generated transgenic plants expressing pBES1::LUC in which the luciferase gene (LUC) was driven by the BES1 promoter (−2079 to −39) and analyzed LUC signals in the plants. Cutoff filters were used to modulate UV-B wavelength, ZJB300 to filter out < 300 nm UV-B light, and ZJB340 to filter out all UV-B (Figures 5C to 5E). Broadband UV-B (BUV) strongly decreased LUC signals, while narrow-band UV-B or BUV+ZJB300 or BUV+ZJB340 barely affected LUC signals (Figure 5C). We used the same UV-B light intensity (5 µmol/m2/s) for narrow-band UV-B, BUV, and BUV+ZJB300, so the effect of the broadband UV lamp on the expression of BES1 is due to irradiation below 300 nm. Furthermore, broadband UV-B repressed BES1 transcription in a fluence-response manner, as BES1 transcript levels were lower after higher-intensity broadband UV-B treatment (Supplemental Figures 7B and 7C).
We also measured the protein level and phosphorylation status of constitutively expressed BES1 in response to UV-B stress in Pro35S::BES1-Flag transgenic plants. The protein stability of BES1 and BR-induced dephosphorylation of BES1 did not change in response to broadband UV-B (Supplemental Figure 7D), indicating that UV-B stress primarily inhibits BES1 transcription rather than the protein stability or phosphorylation of BES1.
To dissect the mechanism underlying how broadband UV-B represses BES1 transcription, we tested some known factors of UV-B stress signaling and regulators of BES1. According to the classical UVR8-mediated UV-B signaling pathway, HY5 is the master transcription factor that functions downstream of UVR8. Therefore, we investigated whether HY5 affects the repression of BES1 transcription by broadband UV-B. As shown in Supplemental Figure 7E, HY5 did not affect UV-B stress-induced inhibition of BES1 transcription. We also tested MPK3 and MPK6, which are involved in regulating UV-B stress responses (González Besteiro et al., 2011) and found that the down-regulation of BES1 was not dependent on these MPKs (Supplemental Figure 7F). BR signaling and BES1 activate BES1 expression, as BES1 is expressed at higher levels in bes1-D than in wild-type En2 (Yu et al., 2011; Jiang et al., 2015). BES1 expression decreased in both bes1-D and En2 at a similar rate in response to broadband UV-B (Supplemental Figures 7G and 7H). Finally, BRZ treatment inhibited BES1 expression in the absence of UV-B but did not affect the rate of the response of BES1 to broadband UV-B (Supplemental Figures 7I and 7J). Therefore, BES1 protein is not involved in down-regulating BES1 transcription in response to broadband UV-B.
DISCUSSION
BR-Activated BES1 Mediates the Tradeoff between Plant Growth and UV-B Stress Tolerance
BRs are an important group of steroid hormones involved in plant development and various stresses responses (Nolan et al., 2017a). BRs coordinate plant growth and drought stress responses. For example, BES1 is degraded by selective autophagy during drought stress (Nolan et al., 2017b; Yang et al., 2017); BES1 and the drought-induced transcription factor RD26 antagonize each other (Ye et al., 2017), and BES1 and BZR1 repress the transcription of abscisic acid-regulated transcription factor genes (Ryu et al., 2014; Yang et al., 2016). UV-B light is an important environmental signal that regulates plant growth and inhibits the shade avoidance response and thermomorphogenesis (Heijde and Ulm, 2012; Hayes et al., 2014, 2017). We previously demonstrated that UV-B signals inhibit plant growth by repressing BR-promoted plant growth. UVR8 physically interacts with BIM1 and the functional dephosphorylated BES1 transcription factor, which mediates BR-regulated gene expression and plant growth to inhibit their DNA binding activities (Liang et al., 2018). Here, we showed that BRs are also involved in UV-B stress tolerance. BR-deficient and signaling mutants are tolerant to UV-B stress, and BES1 acts downstream of BRI1 to inhibit UV-B stress tolerance, since bes1-D suppressed the increased UV-B tolerance of the bri1 mutant (Figures 1E and 1F). BES1 directly binds to the promoters of PFG MYBs in a BR-enhanced manner to inhibit gene expression, thus inhibiting flavonoid accumulation, while UV-B stress represses the transcription of BES1 to suppress the inhibition of flavonoid accumulation. BR-activated BES1 promotes plant growth and inhibits flavonoid biosynthesis in the absence of UV-B. By contrast, UV-B stress represses the transcription of BES1 in a UVR8-independent manner to restrain its activity, thus inhibiting the expression of growth-related genes and suppressing the inhibition of PFG MYB expression. UV-B also activates HY5 signaling to induce PFG MYBs, allowing plants to allocate energy from growth to flavonoid biosynthesis and activate defense responses.
BR-deficient and signaling mutants such as det2 are small and have high flavonoid levels and enhanced UV-B stress tolerance. Perhaps these phenotypes are due to the dual functions of BES1, as BR-activated BES1 not only promotes growth but also inhibits the transcription of PFG MYBs. The BR mutants show increased UV-B stress tolerance not due to their small size, but due to their altered levels of PFG MYBs, as bri1 mybt and BES1-RNAi mybt have similar morphological phenotypes to bri1 and BES1-RNAi but reduced flavonoid contents and weak UV-B stress tolerance (Figure 4; Supplemental Figures 5D to 5F). In addition, there is a tradeoff between growth and flavonoid accumulation in plants, as the overaccumulation of flavonoids inhibits plant growth (Besseau et al., 2007). Indeed, mybt had larger leaf area than wild-type Col-0, and treatment with kaempferol (a natural flavonol) suppressed this phenotype (Supplemental Figure 8A). In addition, mybt had longer roots than Col-0 under normal conditions, while kaempferol treatment repressed root elongation and suppressed the longer root phenotype of mybt (Supplemental Figure 8B). However, PFG MYBs had little effect on BR-controlled hypocotyl responses, since mybt showed no obvious hypocotyl phenotype in the presence of BR or BRZ (Supplemental Figures 8C and 8D).
The BES1-PFG MYB module is regulated by BRs and UV-B stress at different levels. BR signaling promotes the dephosphorylation of BES1 to promote its binding to PFG MYBs (Figure 3F), indicating that the role of BES1 in UV-B stress tolerance is regulated by BRs. Although UV-B stress does not affect BR contents in Arabidopsis (Supplemental Figure 6) or the dephosphorylation of BES1 (Supplemental Figure 7D), it inhibits the transcription of BES1 in a UVR8-independent manner via an unknown mechanism. Taken together, these findings indicate that BES1 is a key factor mediating the tradeoff between plant growth and UV-B stress tolerance.
Narrow-Band UV-B and UVR8 Inhibit the DNA Binding Activity of BES1 on Growth-Related genes, while Broadband UV-B Inhibits BES1 Expression in a UVR8-Independent Manner
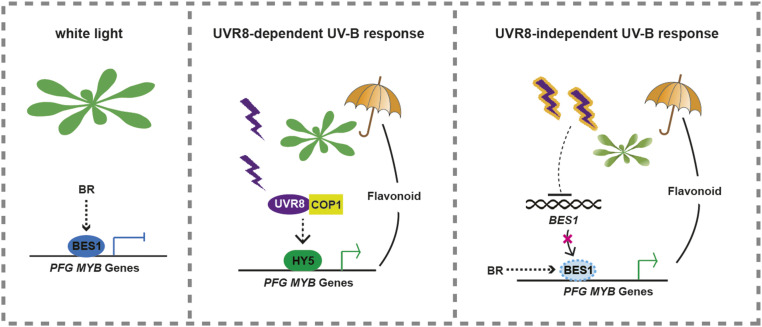
BES1 physically interacts with BIM1. These proteins bind synergistically to the E-box (CANNTG) sequence in BR-responsive growth-related genes to promote their expression (Yin et al., 2005; Li et al., 2009). BES1 binds to both BRRE (CGTG T/C G) and E-boxes. E-boxes are present in both BR-repressed and BR-induced genes, while BRRE and G-boxes (CACGTG, which contains two inverted repeats of the BRRE core sequence CGTG and is also a type of E-box) are dominant in BR-repressed genes (Sun et al., 2010; Yu et al., 2011). We previously demonstrated that UVR8 physically interacts with BR-activated BES1 and BIM1 to inhibit their DNA binding activity on BR-induced genes involved in cell elongation and that narrow-band UV-B does not affect BES1 expression (Liang et al., 2018). Here, we demonstrated that MYB11, MYB12, and MYB111 are direct targets of BES1. BR-activated BES1 directly binds to the promoters of these genes (partially via their G-boxes) to inhibit their transcription. Interestingly, neither narrow-band nor broadband UV-B affects the DNA binding activity of BES1 on these PFG MYBs. It is likely that BES1 forms complexes with different proteins to promote or repress gene expression, as BIM1 is not involved in UV-B stress tolerance or the regulation of PFG MYB gene expression (Supplemental Figures 2E, 2F, 4B, and 4C). Broadband UV-B represses the transcription of BES1 in a UVR8-independent manner, although UVR8 is involved in UV-B induced PFG MYB expression and flavonol accumulation. The role of BES1 in UV-B stress tolerance is UVR8 independent, which is different from the previous hypothesis that UVR8 interacts with BES1 and inhibits its binding to growth-related genes (Figure 6). There are several UVR8-independent UV-B responses; for example, genetically distinct UVR8-independent pathways that stimulate different sets of genes in mature Arabidopsis leaf tissue have been defined (Brown and Jenkins, 2008). UV-B-induced CPD photolyase gene expression is regulated by not only UVR8-dependent but also UVR8-independent pathways (Li et al., 2015). Nonstress, low-fluence-rate UV-B treatments can activate gene expression independently of UVR8 (O’Hara et al., 2019). We speculate that broadband UV-B inhibits the transcription of BES1 via an unknown mechanism, because we excluded the possibility that factors such as HY5, MPK3/6, or the self-activation of BES1 inhibits BES1 transcription (Supplemental Figures 7E to 7J). More effort is needed to elucidate the mechanism of broadband UV-B-induced inhibition of BES1 expression.
Figure 6.
A Working Model Describing UVR8-Dependent and UVR8-Independent Stress Responses.
When plants are grown without UV-B, BR signaling activates BES1 to repress the expression of PFG MYBs controlling flavonoid biosynthesis. When plants are irradiated with narrow-band UV-B, photo-activated UVR8 interacts with COP1 to promote HY5 accumulation, thus initiating UV-B photomorphogenesis, including activating the expression of PFG MYBs. When plants are irradiated with broadband UV-B, in addition to activating UVR8-COP1-HY5 signaling, UV-B stress inhibits the expression of BES1 in an UVR8-independent manner to suppress the inhibition of PFG MYBs. Flavonoids act as sunscreen compounds to protect plant cells from UV-B stress. BR-activated BES1 mediates the tradeoff between plant growth and UV-B stress responses.
UV-B light is highly dynamic during the day: not only is the light intensity highly dynamic, but the wavelength is as well (Supplemental Figures 9A and 9B). Here, we demonstrated that low-wavelength UV-B light is required to inhibit the expression of BES1 (Figures 5C to 5E). Therefore, plants may employ specific, complex mechanisms to adapt to dynamic changes in UV-B light.
UV-B and BRs Are Important for Flavonoid Biosynthesis
The accumulation of “sunscreen” flavonoids, including flavonol, anthocyanins, and proanthocyanidins is induced to protect plant cells from UV-B irradiation. HY5 plays a key role in the induction of MYBs. In the hy5 hyh and uvr8 mutants, PFG MYBs were only slightly induced by broadband rather than narrow-band UV-B (Supplemental Figure 9C), and uvr8 BES1-RNAi showed an enhanced induction compared with uvr8 mutant under broadband UV-B (Supplemental Figure 9D), indicating that plants employ multiple mechanisms to induce the expression of PFG MYBs in response to UV-B stress.
BES1 has both direct and indirect effects in UV-B stress responses. There was no significant difference in PFG MYB expression between Col and BES1-RNAi without UV-B treatment; with UV-B treatment, the expression of PFG MYBs was higher in BES1-RNAi lines than in the wild type (Figure 2D). However, there was no difference in the expression of PFG MYBs between the wild type and bes1-D-OX with or without UV-B treatment in the absence of BRZ (Supplemental Figure 3A). It is possible that there is already enough BES1 protein in the wild type to repress the expression of PFG MYBs, so there is not more severe repression in bes1-D-OX. Accordingly, we added BRZ to repress endogenous BES1 in the wild type (BRZ is commonly used in checking the hypocotyl phenotype of bes1-D and bzr1-1D). With BRZ treatment, the expression of PFG MYBs was lower in bes1-D-OX than in the wild type even without UV-B, indicating that bes1-D constitutively affects PFG MYB expression (Supplemental Figure 3A). From the ratio of +UV/−UV, we can tell that with higher concentration of BRZ, the UV-B induction of PFG MYBs expression was more dramatic, probably because of the inhibition of endogenous BES1, while there was always less induction of PFG MYBs in bes1-D-OX than in the wild type (Supplemental Figure 3B), indicating that bes1-D affects the UV-B-induced PFG MYB expression.
BR-deficient mutants, like det2, are dark green and dwarf when grown in the light, (Chory et al., 1991; Sävenstrand et al., 2004). Sävenstrand et al. (2004) reported that BR mutants are defective in UV-B-regulated defense gene expression, such that CHS was down-regulated in the bri1 mutant (Sävenstrand et al., 2004). The difference between our results and those of Sävenstrand et al. (2004) is likely due to the different ages of the plants used in the experiments. They used 5-week-old plants, while we used 7-d-old seedlings. We repeated these two experimental conditions, and our results indicate that in 5-week-old plants, the expression of CHS and PFG MYBs is lower in bri1 mutants than in the wild type under both white light and white light plus UV-B conditions (Supplemental Figure 9E), consistent with the published results. By contrast, in 7-d-old seedlings, the expression of CHS and MYB12 is higher in bri1 mutants than in the wild type both with and without UV-B treatment (Supplemental Figure 9F). It seems that developmental stage or age is quite important for BR-regulated flavonoid-biosynthesis gene expression. In addition, the BR-induced transcription factor BRASSINOSTEROID ENHANCED EXPRESSION1 (BEE1) negatively controls low-temperature-regulated anthocyanin accumulation (Petridis et al., 2016).
In summary, BES1 controls the expression of both growth-related genes and PFG MYBs to mediate the tradeoff between growth and UV-B stress tolerance. A narrow-band UV-B light-promoted, BR-dependent UVR8-BES1 interaction makes it possible for light and BR signaling to coordinately regulate plant growth and development (Liang et al., 2018), while broadband UV-B inhibits BES1 expression but induces HY5 expression to promote flavonol biosynthesis and UV-B stress tolerance (Figure 6).
METHODS
Plant Materials
The Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) was used in most experiments except as noted. The uvr8-6, mybt (myb11 myb12 myb111), BES1-RNAi, bri1-1, cpd, bri1-301, det2-1, BIN2/bin2, bim123, pBES1::BES1-GFP, hy5 hyh, mpk3, XVE-mapk6/3, beh2-CR, beh3-3, beh4-2, bzr1-2, and bes1 bzr1 beh1 beh2 beh3 beh4 lines were previously described by Li et al. (1996), Szekeres et al. (1996), Noguchi et al. (1999b), Li and Nam (2002), Yin et al. (2005), Stracke et al. (2007), Favory et al. (2009), Cheng et al. (2015), Qian et al., (2016), and Chen et al. (2019a, 2019b). Arabidopsis mutants bes1-D (En2 background), bes1-D (Col-0 background), and bri1-9 (Ws-2), the maize (Zea mays) mutant na1, and the rice (Oryza sativa) mutant d61 were also used (Noguchi et al., 1999a; Yamamuro et al., 2000; Hartwig et al., 2011). More information about the mutants can be found in Supplemental Table 1. uvr8 BES1-RNAi, uvr8 bri1-301, BES1-RNAi mybt, bri1 mybt were prepared by genetic crossing, and their identities were verified by genotyping. The full-length coding sequence of BES1 (short isoform) was cloned into pCambia1300 (Cambia), driven by the cauliflower mosaic virus 35S promoter, to generate Pro35S::BES1-Flag. The coding sequence of bes1-D was obtained by PCR with primers (Supplemental Table 2) containing the point mutation and cloned into pCambia1300 and transformed to Col-0 or bri1-301 to generate bes1-D-OX and bes1-D-OX/bri1. The BES1 promoter (−2079 to −39) was cloned into pBI101-LUC to generate pBES1::LUC. For every transformation, more than 10 independent transgenic lines with a single copy of the transgene were generated. The phenotypes of transgenic plants were verified in at least three independent transgenic lines. Immunoblot analysis was performed to verify overexpression of the transgenes.
Arabidopsis Plant Growth
To analyze UV-B stress tolerance in Arabidopsis, plants were grown in soil under long-day (16 h light/8 h dark) conditions in white light (100 μmol/m2/s, measured by an ILT1400 Radiometer Photometer) for 10 d, treated with white light (18 μmol/m2/s) plus broadband UV-B (5 μmol/m2/s, measured by a UV Meter 3414F [LightScout]) for 8 h, and allowed to recover for 3 d in white light (100 μmol/m2/s) prior to phenotypic analysis. To analyze the maximum quantum yield of PSII (Fv/Fm) in Arabidopsis, 10-d-old plants were treated with white light (18 μmol/m2/s) plus broadband UV-B (5 μmol/m2/s) for 5 h and analyzed using an imaging fluorometer the following day. White light was provided by TLD18W/54-765 fluorescent cool daylight tubes (Philips); broadband UV-B was provided by TL 40W/12 UV fluorescent tubes (Philips); and narrow-band UV-B was provided by TL20W/01RS narrow-band UV-B tubes (Philips). All UV-B treatments were provided as white light (18 μmol/m2/s) plus broadband UV-B (5 μmol/m2/s) except when noted in the figure legends. The photosynthetic active radiation value of white light at plant height (including Col-0 and BR mutants) is 18 μmol/m2/s, while the photosynthetic active radiation value of white light plus UV-B is 22 μmol/m2/s.
Most phenotypic analysis of Arabidopsis was performed as described above unless otherwise stated in the figure legends.
Crop Plant Growth
For stress analysis in maize, plants were grown at 28°C, long-day (16/8) conditions in white light (100 μmol/m2/s) for 8 d, treated with white light (18 μmol/m2/s) plus broadband UV-B (5 μmol/m2/s) for 2 h per day for 3 d, and allowed to recover for 2 d prior to phenotypic analysis. For Fv/Fm analysis in maize, 8-d-old plants were treated with broadband UV-B for 2 h per day for 2 d, and images were captured using an imaging fluorometer the following day.
For stress analysis in rice, plants were grown at 28°C under long-day (16/8) conditions in white light (100 μmol/m2/s) for 25 d, treated with white light (18 μmol/m2/s) plus broadband UV-B (5 μmol/m2/s) for 12 h per day for 3 d, and allowed to recover for 2 da prior to phenotypic analysis. For Fv/Fm analysis in rice, plants grown for 25 da were treated with broadband UV-B for 12 h per day for 2 d, and images were captured using an imaging fluorometer the following day.
The light sources used for crop analysis are the same as those used for Arabidopsis analysis.
HPLC Analysis
For HPLC assays, plants were grown on one-half strength Murashige and Skoog (MS) plates in continuous white light (18 μmol/m2/s) for 6 d and irradiated with white light plus broadband UV-B for 2 d. One-tenth grams seedling tissue was harvested, ground to a powder, dissolved in 80% (v/v) acetonitrile, vortexed for 1 min, and incubated overnight at 4°C. After centrifugation at 12,000g, the supernatants were subjected to HPLC/diode array detector (DAD; Agilent 1260) to quantify flavonol contents. HPLC was performed on a C18 column (2.7 μm, 4.6 mm × 100 mm Agilent) at a flow rate of 0.8 mL min−1 and an elution gradient of solvent A (water), solvent B (methanol), and solvent D (0.5% [v/v] formic acid) and the following elution profile: 20 to 40% B and 80 to 60% D from 0 to 10 min, 40 to 70% B and 60 to 30% D from 10 to 15 min, 70 to 90% B and 30 to 10% D from 15 to 20 min. DAD was used to detect UV-visible light absorption of 320 nm. For flavonol quantification, significant peaks collected by the fraction collector were subjected to liquid chromatography-electrospray ionization/quadrupole-time of flight (LC-ESI/Q-TOF)/mass spectrometry as described by Sun et al. (2012). Naringenin was used as an external standard; the naringenin was diluted in 80% (v/v) acetonitrile to 0.1 μg μL−1, 0.2 μg μL−1, 0.5 μg μL−1, and 1 μg μL−1. Following HPLC/DAD, a standard curve was constructed with concentration on the x axis and peak area on the y axis. Flavonol contents were quantified based on the standard curve.
DPBA Staining
DPBA staining was used to analyze in situ flavonol contents. Plants were grown under constant white light (18 μmol/m2/s) for 6 d and irradiated with white light plus broadband UV-B for 1 d. The seedlings were stained to saturation for at least 1.5 h in ethanol solution containing 0.25% (w/v) DPBA and 0.01% (v/v) Triton X-100 (ethanol works to destain chlorophyll). Fluorescence was visualized by epifluorescence microscopy with an excitation wavelength of 458 nm.
Luciferase Imaging
Transgenic plants expressing pBES1::LUC were grown on one-half strength MS medium containing 0.7% (w/v) agar and 1% (w/v) Suc under constant white light for 7 d. The plants were kept under white light or subjected to the indicated UV-B treatment for an 8-h time course. The treated transgenic plants were sprayed with 2.5 mM luciferin and imaged with a cold charge-coupled device (CCD) at the same time.
Determination of Endogenous BR Levels
The levels of endogenous BRs including CS, TY, and 6-deoxoCS were quantified with some modifications to simplify sample pretreatment as previously reported by Xin et al. (2013). The harvested plant materials were frozen in liquid nitrogen and ground to a fine powder. Two hundred milligrams of the powder were extracted with 90% (v/v) aqueous methanol in an ultrasonic bath for 1 h. Simultaneously, D3-BL, D3-CS, and D3-6-deoxo-CS were added to the extract as internal standards for BR content measurements. After the mixed-mode cation-exchange sorbent cartridge was activated and equilibrated with methanol, water, and 40% (v/v) methanol in sequence, the crude extracts (reconstructed in 40% [v/v] methanol) were loaded onto the cartridge. The mixed-mode cation-exchange cartridge was washed with 40% (v/v) methanol, and BRs were eluted with methanol. After drying under a N2 stream, the eluent was redissolved in acetonitrile and derivatized with 3-(dimethylamino)-phenylboronic acid prior to ultra-performance liquid chromatography (UPLC)-tandem mass spectrometry analysis. BR content analysis was performed on a quadrupole linear ion trap hybrid mass spectrometry (QTRAP 5500, AB SCIEX) equipped with an electrospray ionization source coupled with a UPLC (Waters). The UPLC inlet method, ESI source parameters, multiple reaction monitoring transitions, and related compound-dependent parameters were set as reported by Xin et al. (2013). For 6-deoxo-CS or D3-6-deoxo-CS, the multiple reaction monitoring transition 580.4 > 176.1 or 583.4 > 176.1 was used for quantification and 580.4 > 190.1 or 583.4 > 190.1 for qualification.
Transient Transcription Assay
Transient transcription dual-LUC assays were performed using Nicotiana benthamiana as described by Liu et al. (2013) and Ma et al. (2016). The effector and reporter plasmids were transformed into Agrobacterium strain GV3101 (containing the helper plasmid pSOUP+P19). Overnight cultures of Agrobacteria were collected by centrifugation, resuspended in MES buffer to 0.6 OD600, mixed with GV3101 expressing pSoup-P19, and incubated at room temperature for 2 h before infiltration. The Agrobacterium suspension was manually press-infiltrated into the healthy leaves of 3-week-old N. benthamiana plants using a 1-mL needleless syringe. The plants were grown under long-day conditions in white light for 3 d after infiltration. Luciferase activity was imaged with a CCD camera or quantified with a luminometer (Promega 20/20) using commercial LUC reaction reagents according to the manufacturer’s instructions (Promega).
ChIP assay
ChIP experiments were performed as described by Liu et al. (2008). Four grams of seedling tissue from BES1-RNAi and Col-0 plants and 2 g of tissue from Col-0 and BES1-Flag transgenic plants was harvested and cross-linked with 1% (v/v) formaldehyde (Sigma-Aldrich) for 15 min under a vacuum. Cross-linking was stopped by adding Gly to a final concentration of 0.125 M. The seedlings were rinsed with water, frozen in liquid nitrogen, and ground to a fine powder. The powder was homogenized in nuclear extraction buffer 1 (10 mM Tris-HCl [pH 8.0], 0.4 M Suc, 10 mM MgCl2, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], and protease inhibitor cocktail tablets [Roche]). Nuclei were precipitated by centrifugation at 2000g for 20 min, washed with nuclear extraction buffer 2 (10 mM Tris-HCl [pH 8.0], 0.25 M Suc, 10 mM MgCl2, 1% [v/v] Triton X-100, 0.1 mM PMSF, complete protease inhibitor cocktail tablets [Roche]), and lysed in nuclei lysis buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA, 1% [w/v] SDS, 0.1 mM PMSF, and protease inhibitor cocktail tablets [Roche]). The chromatin was sheared by sonication to ∼500 bp. The chromatin solution was diluted 10-fold with ChIP dilution buffer (16.7 mM Tris-HCl [pH 8.0], 167 mM NaCl, 1.1% [v/v] Triton X-100, 1.2 mM EDTA, 0.1 mM PMSF, complete protease inhibitor cocktail tablets [Roche]). Anti-Flag antibody (cat. no. F3165, Sigma-Aldrich, 2 μL per reaction) or anti-BES1 antibody (gift from Yanhai Yin, 2 μL per reaction) prebound to protein A/G sepharose was mixed with the chromatin solution and incubated overnight at 4°C. Immunocomplexes were precipitated and washed with four different buffers: low-salt buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.2% [w/v] SDS, 0.5% [v/v] Triton X-100, 2 mM EDTA), high-salt buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 0.2% [w/v] SDS, 0.5% [v/v] Triton X-100, 2 mM EDTA), LiCl washing buffer (20 mM Tris-HCl [pH 8.0], 0.25 M LiCl, 1% [v/v] Nonidet P-40, 1% [w/v] sodium deoxycholate, 1 mM EDTA), and transposable element washing buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). The bound chromatin fragments were eluted with elution buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% [w/v] SDS), and the cross-linking was reversed by incubating overnight in a final concentration of 200 mM NaCl at 65°C. The mixture was treated with Proteinase-K to remove proteins. The genomic DNA was purified with an equal volume of phenol/chloroform/isoamyl alcohol and precipitated with 2 vol. of 100% ethanol at -70°C for 2 h to overnight. The sample was centrifuged at 12,000g for 20 min at 4°C to recover the DNA, which was dissolved in 60 μL of transposable element buffer. 2 μL of each DNA sample was used for qPCR. The primer pairs used in the ChIP experiments are listed in Supplemental Table 2.
More information about the antibodies used can be found in Supplemental Table 3.
EMSA
For the probe, the synthetic complementary oligonucleotides of MYB11, MYB12 (or pMYB12m with G-box mutation), and the MYB111 promoter were annealed and cloned into the T-vector. The probes were PCR amplified using Cy5-labeled M13 primer pairs (Supplemental Table 2). For proteins, the sequence encoding BES1 lacking the N-terminal 22 amino acids was cloned into the pCold-TF vector (Takara), expressed, and purified with nickel-charged affinity resin agarose (Invitrogen). The binding reaction was performed in 20 μL binding buffer (25 mM HEPES [pH 7.5], 40 mM KCl, 3 mM DTT, 10% [v/v] glycerol, 0.1 mM EDTA, 0.5 mg mL−1 BSA, 0.5 mg/ml poly-glutamate) using 15 ng probe and 200 ng proteins. After 30 min of incubation on ice, the reactions were resolved by electrophoresis in a 6% (v/v) native polyacrylamide gel at 4°C. Cy5-labeled DNA in the gel was detected with a Starion FLA-9000 scanner (FujiFilm).
Transcriptome Analysis
Microarray data sets for bes1-D were a gift from Yanhai Yin, and microarray data from UV-B-treated Arabidopsis were obtained from the ArrayExpress database (accession number E-MEXP-1957). We re-evaluated the data with Affymetrix Expression Console ver. 1.3.0.187 using the analysis algorithm RMA. The differentially expressed genes were analyzed by moderated t-statistics using the Limma package in R, and fold change values were also calculated (significance was determined by P value 0.05 and log fold change 0.5); the results are shown in Supplemental Data Set 1. Genes that were up-regulated or down-regulated by BES1 were subjected to Gene Ontology analysis and functional annotation clustering.
RT-qPCR
For RT-qPCR, total RNA was isolated from the samples using RNAiso Plus (Takara). cDNA was synthesized from 500 ng of total RNA using a PrimeScript RT reagent kit with gDNA Eraser (Takara). SYBR Premix Ex Tag (Takara) was used for RT-qPCR on the MX3000 System (Stratagene). The level of ACTIN7 mRNA accumulation (AT5G09810) was used as an internal control. RT-qPCR data for each sample were normalized to the respective ACT7 expression level. The cDNA was amplified following denaturation via 40 cycles of PCR (95°C, 5 s; 60°C, 20 s per cycle). Three biological replicates and two technical replicates were performed per experiment. Primers are listed in Supplemental Table 2.
Statistical Analysis
All t test analysis were calculated using Excel and ANOVA were using SPSS software. To determine statistical significance, we employed independent t tests with two-tail distribution between two groups and one-way ANOVA Tukey’s test among various genotypes. A value of P < 0.05 was considered to be statistically significant. The results of statistical analyses are shown in Supplemental Data Set 2.
Accession Numbers
Sequence data for genes described in this article can be found in the Arabidopsis Information Resource under the following accession numbers: MYB12 (AT2G47460), MYB11 (AT3G62610), MYB111 (AT5G49330), CHS (AT5G13930), FLS1 (AT5G08640), BES1 (AT1G19350), ACT7 (AT5G09810), BZR1 (AT1G75080), BEH1 (AT3G50750), BEH2 (AT4G36780), BEH3 (AT4G36780), BEH4 (AT4G36780), BRI1 (AT4G39400), DET2 (AT2G38050), BIM1 (AT5G08130), HY5 (AT5G11260), HYH (AT3G17609), MPK3 (AT3G45640), MPK6 (AT2G43790), CPD (AT5G05690), DWF4 (AT3G50660), and BR6OX2 (AT3G30180).
Supplemental Data
Supplemental Figure 1. BRs inhibit UV-B stress tolerance.
Supplemental Figure 2. BES1 mediates UV-B stress tolerance.
Supplemental Figure 3. BES1 represses the expression of flavonol biosynthesis genes.
Supplemental Figure 4. BES1 binds to promoters of the PFG MYBs.
Supplemental Figure 5. PFG MYBs act downstream of BR signaling to regulate UV-B stress resistance.
Supplemental Figure 6. Broadband UV-B does not affect the BR biosynthesis significantly.
Supplemental Figure 7. The expression of BES1 is down-regulated by UV-B stress.
Supplemental Figure 8. Phenotypic analysis of the effect of flavonol on plant growth.
Supplemental Figure 9. Plants employ multiple mechanisms to adapt to the dynamic UV-B light.
Supplemental Table 1. Mutant list.
Supplemental Table 2. Primer list.
Supplemental Table 3. Key resources.
Supplemental Data Set 1. Transcriptomic analysis of BES1 and UV-B regulated genes.
Supplemental Data Set 2. Statistical analysis of t test and ANOVA results for the data shown in figures.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
The authors thank Xue-Lu Wang, Zhi-Yong Wang, Jia Li, Wen-Qiang Tang, Trevor C. McMorris, Joanne Chory, Burkhard Schulz, Kyoung H Nam, Frans E Tax, Bernd Weisshaar, Csaba Koncz, Yonghong Wang, Makoto Matsuoka, Hong-Quan Yang, Shu-Hua Yang, Yan Xiong, Jie Zhang, and Cheng-Cai Chu for materials and technical assistances. This work was supported by grants from the National Key Research and Development Program of China (grant 2016YFD0100404), the National Natural Science Foundation of China (grants 31825004, 31721001, 31730009, 31670282, 31670307, and 31470433), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant XDB27030000), the Program of Shanghai Academic Research Leader, and the National Institute of General Medical Sciences (to Y.Y.).
AUTHOR CONTRIBUTIONS
T.L., C.S., and H.L. conceived the project; T.L. and C.S. performed most experiments; Y.P. and Yu Y. made some constructs; J.C. and P.X. measured endogenous BR levels, Ya.Y., J.H., X.L., F.W., and H.T. provided materials; T.L., C.S., and H.L. analyzed data and wrote the article.
References
- Anne, P., Azzopardi, M., Gissot, L., Beaubiat, S., Hématy, K., Palauqui, J.C.(2015). OCTOPUS Negatively regulates BIN2 to control phloem differentiation in Arabidopsis thaliana. Curr. Biol. 25: 2584–2590. [DOI] [PubMed] [Google Scholar]
- Arongaus, A.B., Chen, S., Pireyre, M., Glöckner, N., Galvão, V.C., Albert, A., Winkler, J.B., Fankhauser, C., Harter, K., Ulm, R.(2018). Arabidopsis RUP2 represses UVR8-mediated flowering in noninductive photoperiods. Genes Dev. 32: 1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, M.Y., Shang, J.X., Oh, E., Fan, M., Bai, Y., Zentella, R., Sun, T.P., Wang, Z.Y.(2012). Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 14: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseau, S., Hoffmann, L., Geoffroy, P., Lapierre, C., Pollet, B., Legrand, M.(2007). Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19: 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B.A., Cloix, C., Jiang, G.H., Kaiserli, E., Herzyk, P., Kliebenstein, D.J., Jenkins, G.I.(2005). A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 102: 18225–18230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B.A., Jenkins, G.I.(2008). UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 146: 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiwanon, J., Wang, W., Zhu, J.Y., Oh, E., Wang, Z.Y.(2016). Information integration and communication in plant growth regulation. Cell 164: 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L.G., Gao, Z., Zhao, Z., Liu, X., Li, Y., Zhang, Y., Liu, X., Sun, Y., Tang, W.(2019a). BZR1 family transcription factors function redundantly and indispensably in BR signaling but exhibit BRI1-independent function in regulating anther development in Arabidopsis. Mol. Plant 12: 1408–1415. [DOI] [PubMed] [Google Scholar]
- Chen, W., Lv, M., Wang, Y., Wang, P.A., Cui, Y., Li, M., Wang, R., Gou, X., Li, J.(2019b). BES1 is activated by EMS1-TPD1-SERK1/2-mediated signaling to control tapetum development in Arabidopsis thaliana. Nat. Commun. 10: 4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z., Li, J.F., Niu, Y., Zhang, X.C., Woody, O.Z., Xiong, Y., Djonović, S., Millet, Y., Bush, J., McConkey, B.J., Sheen, J., Ausubel, F.M.(2015). Pathogen-secreted proteases activate a novel plant immune pathway. Nature 521: 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory, J., Nagpal, P., Peto, C.A.(1991). Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell 3: 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, W.A., Albert, N.W., Thrimawithana, A.H., McGhie, T.K., Deroles, S.C., Schwinn, K.E., Warren, B.A., McLachlan, A.R.G., Bowman, J.L., Jordan, B.R., Davies, K.M.(2018). UVR8-mediated induction of flavonoid biosynthesis for UVB tolerance is conserved between the liverwort Marchantia polymorpha and flowering plants. Plant J. 96: 503–517. [DOI] [PubMed] [Google Scholar]
- Cloix, C., Jenkins, G.I.(2008). Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin. Mol. Plant 1: 118–128. [DOI] [PubMed] [Google Scholar]
- Clouse, S.D.(2011). Brassinosteroids. Arabidopsis Book 9: e0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarsy, E., Goldschmidt-Clermont, M., Ulm, R.(2018). Coping with ‘dark sides of the sun’ through photoreceptor signaling. Trends Plant Sci. 23: 260–271. [DOI] [PubMed] [Google Scholar]
- Favory, J.J., et al. (2009). Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 28: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehér, B., Kozma-Bognár, L., Kevei, E., Hajdu, A., Binkert, M., Davis, S.J., Schäfer, E., Ulm, R., Nagy, F.(2011). Functional interaction of the circadian clock and UV RESISTANCE LOCUS 8-controlled UV-B signaling pathways in Arabidopsis thaliana. Plant J. 67: 37–48. [DOI] [PubMed] [Google Scholar]
- González Besteiro, M.A., Bartels, S., Albert, A., Ulm, R.(2011). Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J. 68: 727–737. [DOI] [PubMed] [Google Scholar]
- Gruber, H., Heijde, M., Heller, W., Albert, A., Seidlitz, H.K., Ulm, R.(2010). Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc. Natl. Acad. Sci. USA 107: 20132–20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig, T., Chuck, G.S., Fujioka, S., Klempien, A., Weizbauer, R., Potluri, D.P., Choe, S., Johal, G.S., Schulz, B.(2011). Brassinosteroid control of sex determination in maize. Proc. Natl. Acad. Sci. USA 108: 19814–19819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, S., Sharma, A., Fraser, D.P., Trevisan, M., Cragg-Barber, C.K., Tavridou, E., Fankhauser, C., Jenkins, G.I., Franklin, K.A.(2017). UV-B perceived by the UVR8 photoreceptor inhibits plant thermomorphogenesis. Curr. Biol. 27: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, S., Velanis, C.N., Jenkins, G.I., Franklin, K.A.(2014). UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc. Natl. Acad. Sci. USA 111: 11894–11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J.X., Gendron, J.M., Sun, Y., Gampala, S.S., Gendron, N., Sun, C.Q., Wang, Z.Y.(2005). BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijde, M., Ulm, R.(2012). UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 17: 230–237. [DOI] [PubMed] [Google Scholar]
- Heijde, M., Ulm, R.(2013). Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc. Natl. Acad. Sci. USA 110: 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., Ouyang, X., Yang, P., Lau, O.S., Li, G., Li, J., Chen, H., Deng, X.W.(2012). Arabidopsis FHY3 and HY5 positively mediate induction of COP1 transcription in response to photomorphogenic UV-B light. Plant Cell 24: 4590–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., Yang, P., Ouyang, X., Chen, L., Deng, X.W.(2014). Photoactivated UVR8-COP1 module determines photomorphogenic UV-B signaling output in Arabidopsis. PLoS Genet. 10: e1004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez, C., et al. (2018). Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr. Biol. 28: 303–310.e3. [DOI] [PubMed] [Google Scholar]
- Jenkins, G.I.(2014). Structure and function of the UV-B photoreceptor UVR8. Curr. Opin. Struct. Biol. 29: 52–57. [DOI] [PubMed] [Google Scholar]
- Jiang, J., Zhang, C., Wang, X.(2015). A recently evolved isoform of the transcription factor BES1 promotes brassinosteroid signaling and development in Arabidopsis thaliana. Plant Cell 27: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserli, E., Jenkins, G.I.(2007). UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B specific signaling component UVR8 and activates its function in the nucleus. Plant Cell 19: 2662–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T.W., Guan, S., Sun, Y., Deng, Z., Tang, W., Shang, J.X., Sun, Y., Burlingame, A.L., Wang, Z.Y.(2009). Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 11: 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein, D.J., Lim, J.E., Landry, L.G., Last, R.L.(2002). Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol. 130: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Li, Y., Chen, S., An, L.(2010). Involvement of brassinosteroid signals in the floral-induction network of Arabidopsis. J. Exp. Bot. 61: 4221–4230. [DOI] [PubMed] [Google Scholar]
- Li, J., Nagpal, P., Vitart, V., McMorris, T.C., Chory, J.(1996). A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272: 398–401. [DOI] [PubMed] [Google Scholar]
- Li, J., Nam, K.H.(2002). Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295: 1299–1301. [DOI] [PubMed] [Google Scholar]
- Li, J., Ou-Lee, T.M., Raba, R., Amundson, R.G., Last, R.L.(1993). Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Wen, J., Lease, K.A., Doke, J.T., Tax, F.E., Walker, J.C.(2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222. [DOI] [PubMed] [Google Scholar]
- Li, L., Yu, X., Thompson, A., Guo, M., Yoshida, S., Asami, T., Chory, J., Yin, Y.(2009). Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant J. 58: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N., Teranishi, M., Yamaguchi, H., Matsushita, T., Watahiki, M.K., Tsuge, T., Li, S.S., Hidema, J.(2015). UV-B-induced CPD photolyase gene expression is regulated by UVR8-dependent and -independent pathways in Arabidopsis. Plant Cell Physiol. 56: 2014–2023. [DOI] [PubMed] [Google Scholar]
- Liang, T., Mei, S., Shi, C., Yang, Y., Peng, Y., Ma, L., Wang, F., Li, X., Huang, X., Yin, Y., Liu, H.(2018). UVR8 interacts with BES1 and BIM1 to regulate transcription and photomorphogenesis in Arabidopsis. Dev. Cell 44: 512–523.e5. [DOI] [PubMed] [Google Scholar]
- Liang, T., Yang, Y., Liu, H.(2019). Signal transduction mediated by the plant UV-B photoreceptor UVR8. New Phytol. 221: 1247–1252. [DOI] [PubMed] [Google Scholar]
- Liu, H., Yu, X., Li, K., Klejnot, J., Yang, H., Lisiero, D., Lin, C.(2008). Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322: 1535–1539. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Li, X., Li, K., Liu, H., Lin, C.(2013). Multiple bHLH proteins form heterodimers to mediate CRY2-dependent regulation of flowering-time in Arabidopsis. PLoS Genet. 9: e1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, D., Li, X., Guo, Y., Chu, J., Fang, S., Yan, C., Noel, J.P., Liu, H.(2016). Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl. Acad. Sci. USA 113: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-García, S., Vert, G., Yin, Y., Caño-Delgado, A., Cheong, H., Chory, J.(2004). Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 18: 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, K.H., Li, J.(2002). BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212. [DOI] [PubMed] [Google Scholar]
- Noguchi, T., Fujioka, S., Choe, S., Takatsuto, S., Yoshida, S., Yuan, H., Feldmann, K.A., Tax, F.E.(1999a). Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 121: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, T., Fujioka, S., Takatsuto, S., Sakurai, A., Yoshida, S., Li, J., Chory, J.(1999b). Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-En-3-one to (24R)-24-methyl-5alpha-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiol. 120: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan, T., Chen, J., Yin, Y.(2017a). Cross-talk of brassinosteroid signaling in controlling growth and stress responses. Biochem. J. 474: 2641–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan, T.M., Brennan, B., Yang, M., Chen, J., Zhang, M., Li, Z., Wang, X., Bassham, D.C., Walley, J., Yin, Y.(2017b). Selective autophagy of BES1 mediated by DSK2 balances plant growth and survival. Dev Cell 41: 33–46.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara, A., Headland, L.R., Díaz-Ramos, L.A., Morales, L.O., Strid, Å., Jenkins, G.I.(2019). Regulation of Arabidopsis gene expression by low fluence rate UV-B independently of UVR8 and stress signaling. Photochem. Photobiol. Sci. 18: 1675–1684. [DOI] [PubMed] [Google Scholar]
- Oravecz, A., Baumann, A., Máté, Z., Brzezinska, A., Molinier, J., Oakeley, E.J., Adám, E., Schäfer, E., Nagy, F., Ulm, R.(2006). CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18: 1975–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridis, A., Döll, S., Nichelmann, L., Bilger, W., Mock, H.P.(2016). Arabidopsis thaliana G2-LIKE FLAVONOID REGULATOR and BRASSINOSTEROID ENHANCED EXPRESSION1 are low-temperature regulators of flavonoid accumulation. New Phytol. 211: 912–925. [DOI] [PubMed] [Google Scholar]
- Podolec, R., Ulm, R.(2018). Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr. Opin. Plant Biol. 45 (Pt A): 18–25. [DOI] [PubMed] [Google Scholar]
- Qian, C., Chen, Z., Liu, Q., Mao, W., Chen, Y., Tian, W., Liu, Y., Han, J., Ouyang, X., Huang, X.(2020). Coordinated transcriptional regulation by the UV-B photoreceptor and multiple transcription factors for plant UV-B responses. Mol. Plant 13: 777–792. [DOI] [PubMed] [Google Scholar]
- Qian, C., Mao, W., Liu, Y., Ren, H., Lau, O.S., Ouyang, X., Huang, X.(2016). Dual-source nuclear monomers of UV-B light receptor direct photomorphogenesis in Arabidopsis. Mol. Plant 9: 1671–1674. [DOI] [PubMed] [Google Scholar]
- Ren, H., et al. (2019). Two E3 ligases antagonistically regulate the UV-B response in Arabidopsis. Proc. Natl. Acad. Sci. USA 116: 4722–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini, L., Favory, J.J., Cloix, C., Faggionato, D., O’Hara, A., Kaiserli, E., Baumeister, R., Schäfer, E., Nagy, F., Jenkins, G.I., Ulm, R.(2011). Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106. [DOI] [PubMed] [Google Scholar]
- Ruan, Y., Halat, L.S., Khan, D., Jancowski, S., Ambrose, C., Belmonte, M.F., Wasteneys, G.O.(2018). The microtubule-associated protein CLASP sustains cell proliferation through a brassinosteroid signaling negative feedback loop. Curr. Biol. 28: 2718–2729.e5. [DOI] [PubMed] [Google Scholar]
- Ryu, H., Cho, H., Bae, W., Hwang, I.(2014). Control of early seedling development by BES1/TPL/HDA19-mediated epigenetic regulation of ABI3. Nat. Commun. 5: 4138. [DOI] [PubMed] [Google Scholar]
- Sävenstrand, H., Brosché, M., Strid, A.(2004). Ultraviolet-B signalling: Arabidopsis brassinosteroid mutants are defective in UV-B regulated defence gene expression. Plant Physiol. Biochem. 42: 687–694. [DOI] [PubMed] [Google Scholar]
- Stracke, R., Favory, J.J., Gruber, H., Bartelniewoehner, L., Bartels, S., Binkert, M., Funk, M., Weisshaar, B., Ulm, R.(2010). The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 33: 88–103. [DOI] [PubMed] [Google Scholar]
- Stracke, R., Ishihara, H., Huep, G., Barsch, A., Mehrtens, F., Niehaus, K., Weisshaar, B.(2007). Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 50: 660–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y., et al. (2010). Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 19: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y., Li, H., Huang, J.R.(2012). Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol. Plant 5: 387–400. [DOI] [PubMed] [Google Scholar]
- Szekeres, M., Németh, K., Koncz-Kálmán, Z., Mathur, J., Kauschmann, A., Altmann, T., Rédei, G.P., Nagy, F., Schell, J., Koncz, C.(1996). Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85: 171–182. [DOI] [PubMed] [Google Scholar]
- Tang, W., Deng, Z., Wang, Z.Y.(2010). Proteomics shed light on the brassinosteroid signaling mechanisms. Curr. Opin. Plant Biol. 13: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W., Kim, T.W., Oses-Prieto, J.A., Sun, Y., Deng, Z., Zhu, S., Wang, R., Burlingame, A.L., Wang, Z.Y.(2008). BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook, K., Arongaus, A.B., Binkert, M., Heijde, M., Yin, R., Ulm, R.(2013). The UVR8 UV-B photoreceptor: Perception, signaling and response. Arabidopsis Book 11: e0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm, R., Baumann, A., Oravecz, A., Máté, Z., Adám, E., Oakeley, E.J., Schäfer, E., Nagy, F.(2004). Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert, G., Chory, J.(2006). Downstream nuclear events in brassinosteroid signalling. Nature 441: 96–100. [DOI] [PubMed] [Google Scholar]
- Wang, Z.Y., Nakano, T., Gendron, J., He, J., Chen, M., Vafeados, D., Yang, Y., Fujioka, S., Yoshida, S., Asami, T., Chory, J.(2002). Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2: 505–513. [DOI] [PubMed] [Google Scholar]
- Xin, P., Yan, J., Fan, J., Chu, J., Yan, C.(2013). An improved simplified high-sensitivity quantification method for determining brassinosteroids in different tissues of rice and Arabidopsis. Plant Physiol. 162: 2056–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro, C., Ihara, Y., Wu, X., Noguchi, T., Fujioka, S., Takatsuto, S., Ashikari, M., Kitano, H., Matsuoka, M.(2000). Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12: 1591–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M., Li, C., Cai, Z., Hu, Y., Nolan, T., Yu, F., Yin, Y., Xie, Q., Tang, G., Wang, X.(2017). SINAT E3 ligases control the light-mediated stability of the brassinosteroid-activated transcription factor BES1 in Arabidopsis. Dev. Cell 41: 47–58.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Bai, Y., Shang, J., Xin, R., Tang, W.(2016). The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of ABSCISIC ACID INSENSITIVE 5 expression by BRASSINAZOLE RESISTANT 1. Plant Cell Environ. 39: 1994–2003. [DOI] [PubMed] [Google Scholar]
- Yang, X.H., Xu, Z.H., Xue, H.W.(2005). Arabidopsis membrane steroid binding protein 1 is involved in inhibition of cell elongation. Plant Cell 17: 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Liang, T., Zhang, L., Shao, K., Gu, X., Shang, R., Shi, N., Li, X., Zhang, P., Liu, H.(2018). UVR8 interacts with WRKY36 to regulate HY5 transcription and hypocotyl elongation in Arabidopsis. Nat. Plants 4: 98–107. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Zhang, L., Chen, P., Liang, T., Li, X., Liu, H.(2020). UV-B photoreceptor UVR8 interacts with MYB73/MYB77 to regulate auxin responses and lateral root development. EMBO J. 39: e101928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, H., Li, L., Yin, Y.(2011). Recent advances in the regulation of brassinosteroid signaling and biosynthesis pathways. J. Integr. Plant Biol. 53: 455–468. [DOI] [PubMed] [Google Scholar]
- Ye, H., et al. (2017). RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat. Commun. 8: 14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, C., Deng, X.W.(2005). COP1: From plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 15: 618–625. [DOI] [PubMed] [Google Scholar]
- Yin, R., Skvortsova, M.Y., Loubéry, S., Ulm, R.(2016). COP1 is required for UV-B-induced nuclear accumulation of the UVR8 photoreceptor. Proc. Natl. Acad. Sci. USA 113: E4415–E4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X., Li, L., Zola, J., Aluru, M., Ye, H., Foudree, A., Guo, H., Anderson, S., Aluru, S., Liu, P., Rodermel, S., Yin, Y.(2011). A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 65: 634–646. [DOI] [PubMed] [Google Scholar]
- Yin, Y., Vafeados, D., Tao, Y., Yoshida, S., Asami, T., Chory, J.(2005). A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120: 249–259. [DOI] [PubMed] [Google Scholar]
- Yin, Y., Wang, Z.Y., Mora-Garcia, S., Li, J., Yoshida, S., Asami, T., Chory, J.(2002). BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109: 181–191. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Li, B., Xu, Y., Li, H., Li, S., Zhang, D., Mao, Z., Guo, S., Yang, C., Weng, Y., Chong, K.(2013). The cyclophilin CYP20-2 modulates the conformation of BRASSINAZOLE-RESISTANT1, which binds the promoter of FLOWERING LOCUS D to regulate flowering in Arabidopsis. Plant Cell 25: 2504–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]