Genetically impaired plastid division leads to a burst of reactive oxygen species and lipid peroxidation, causing the activation of autoimmune responses.
Abstract
Chloroplasts mediate genetically controlled cell death via chloroplast-to-nucleus retrograde signaling. To decipher the mechanism, we examined chloroplast-linked lesion-mimic mutants of Arabidopsis (Arabidopsis thaliana) deficient in plastid division, thereby developing gigantic chloroplasts (GCs). These GC mutants, including crumpled leaf (crl), constitutively express immune-related genes and show light-dependent localized cell death (LCD), mirroring typical autoimmune responses. Our reverse genetic approach excludes any potential role of immune/stress hormones in triggering LCD. Instead, transcriptome and in silico analyses suggest that reactive electrophile species (RES) generated via oxidation of polyunsaturated fatty acids (PUFAs) or lipid peroxidation-driven signaling may induce LCD. Consistent with these results, the one of the suppressors of crl, dubbed spcrl4, contains a causative mutation in the nuclear gene encoding chloroplast-localized FATTY ACID DESATURASE5 (FAD5) that catalyzes the conversion of palmitic acid (16:0) to palmitoleic acid (16:1). The loss of FAD5 in the crl mutant might attenuate the levels of RES and/or lipid peroxidation due to the reduced levels of palmitic acid–driven PUFAs, which are prime targets of reactive oxygen species. The fact that fad5 also compromises the expression of immune-related genes and the development of LCD in other GC mutants substantiates the presence of an intrinsic retrograde signaling pathway, priming the autoimmune responses in a FAD5-dependent manner.
INTRODUCTION
Chloroplasts are responsive to a multitude of environmental factors such as light intensity and quality, drought, cold, heat, and the presence of microbial pathogens (Xiao et al., 2013; de Torres Zabala et al., 2015; Chan et al., 2016a; Kleine and Leister, 2016; Zhao et al., 2016). These stimuli disturb chloroplast homeostasis, causing the release or activation of distinct retrograde signals from the chloroplast to the nucleus. One of the earliest stress responses of chloroplasts is the increased production of reactive oxygen species (ROS) by the two photosystems (Apel and Hirt, 2004; Asada, 2006). The ROS burst excites other interrelated signaling components, including calcium and plant stress hormones such as salicylic acid (SA), which contributes to the activation of defense/immune responses (Nomura et al., 2012; Duan et al., 2019). As potent oxidizing agents, ROS alter the redox status of chloroplasts, which participates in chloroplast-to-nucleus retrograde signaling (RS; Chan et al., 2016a; Dietz et al., 2016; Dogra and Kim, 2020). Recent studies demonstrated that the accumulation of damaged proteins in chloroplasts induces a damaged protein response to restore proteostasis (Dogra et al., 2019a). The intensified damaged protein response appears to trigger RS via SA, which is mostly synthesized through the chloroplast isochorismate pathway (Dogra and Kim, 2019). In addition, chloroplast-produced secondary metabolites and volatile compounds also participate in RS pathways under certain stress conditions, such as high light, heat, and drought (Ramel et al., 2012; Xiao et al., 2013; Chan et al., 2016b).
Several studies have established the presence of ROS sensors in chloroplasts, and these sensors mediate distinct RS pathways (Ramel et al., 2012; Chan et al., 2016b; Dogra et al., 2019b). Among ROS, singlet oxygen (1O2) has been extensively investigated because of the identification of two Arabidopsis (Arabidopsis thaliana) mutants: the fluorescent (flu) mutant conditionally generates 1O2 in chloroplasts upon a dark-to-light shift (Meskauskiene et al., 2001), and the chlorina1 (ch1) mutant has enhanced levels of 1O2 in PSII under light stress due to the lack of the light-harvesting antenna complex in PSII (Ramel et al., 2013). Studies of these two mutants have unveiled that β-carotene and EXECUTER1 (EX1) serve as 1O2 sensors that are functionally and spatially separated in thylakoid membranes (Wang et al., 2016). These sensors undergo oxidation by 1O2, which is essential for sensing 1O2 and mediating RS (Ramel et al., 2012; Dogra et al., 2019b).
Inactivation of EX1 results in the abrogation of 1O2-triggered stress responses such as growth inhibition and cell death in the flu mutant plants, indicating that the observed cell death upon 1O2 release is mediated by a genetic program rather than resulting from the cytotoxicity (Wagner et al., 2004; Lee et al., 2007). Using this characteristic of the flu mutant, several 1O2-signaling mutants have been isolated by utilizing a transgenic flu line expressing a luciferase reporter gene driven by the promoter of a 1O2-responsive gene (Baruah et al., 2009). Among several mutant lines which constitutively express the reporter gene, the crumpled leaf (crl) mutant was found to exhibit multiple lesions such as an abnormal cell cycle, impaired plastid division, aplastidic guard cells, and light-dependent localized cell death (LCD; Asano et al., 2004; Šimková et al., 2012; Hudik et al., 2014).
CRL is an intrinsic membrane protein with a transmembrane domain followed by the cpcT domain, whose function is yet unknown (Asano et al., 2004). Whereas cyanobacterial cpcT lyase (or bilin lyase) functions in the biogenesis of the light-harvesting antenna complex (phycobilisome) in the thylakoid membrane, CRL proteins localize to the outer envelope membrane of chloroplasts, indicating that CRL has likely a divergent function from the bilin lyase (Asano et al., 2004; Sugita et al., 2012). Interestingly, several Arabidopsis mutants deficient in plastid division show LCD similar to the crl mutant (Šimková et al., 2012). Moreover, given the observed constant upregulation of nuclear genes encoding cell cycle inhibitors, it was suggested that the impaired plastid division might coordinately hinder the cell cycle via RS, leading to an early exit from the cell cycle at the G2/M stage and enhanced endoreduplication (Hudik et al., 2014).
Besides proteins and carotenoids, lipids are also prime targets of 1O2 (Dogra and Kim, 2020). The nonenzymatic oxidation of polyunsaturated fatty acids (PUFAs) by 1O2 in lipids produces lipid-derived reactive electrophile species (RES) in the thylakoid membrane (Alméras et al., 2003). 1O2-induced lipid peroxidation generates a variety of RES, including 12-oxo-phytodienoic acid, phytoprostanes, malonaldehyde, acrolein, and hexenal (Mano, 2012), all of which alter the expression of nuclear genes (Mueller et al., 2008; Farmer and Mueller, 2013). Although the signaling role of RES is apparent, the mechanisms underlying the perception of diverse RES and the activation of related signaling pathways are mostly unclear (Farmer and Davoine, 2007).
Here, we link RES- or lipid peroxidation-driven chloroplast-to-nucleus signaling to the crl-induced LCD. A forward genetic screen revealed that inactivation of FATTY ACID DESATURASE5 (FAD5), a chloroplast integral membrane protein, abrogates LCD. FAD5 catalyzes the desaturation of palmitic acid (16:0), facilitating the production of 16C unsaturated FAs, which are essential constituents of plastid lipids (Heilmann et al., 2004). Therefore, loss of FAD5 would primarily affect the lipid homeostasis (lipidostasis) in the chloroplast membrane. The enhanced levels of 1O2 and the light-dependent but EX1-independent cell death suggest that RES- or lipid peroxidation-induced signaling may prime LCD in the crl mutant. We also observed a similar impact of fad5 in other chloroplast-related lesion-mimic mutants (CpLMMs) deficient in plastid division. In summary, we demonstrate here that FAD5-driven accumulation of unsaturated fatty acids and their oxidation by ROS induces autoimmune responses in Arabidopsis mutants deficient in plastid division.
RESULTS
GCs Induce Stress-Related Nuclear Genes before the Onset of Cell Death
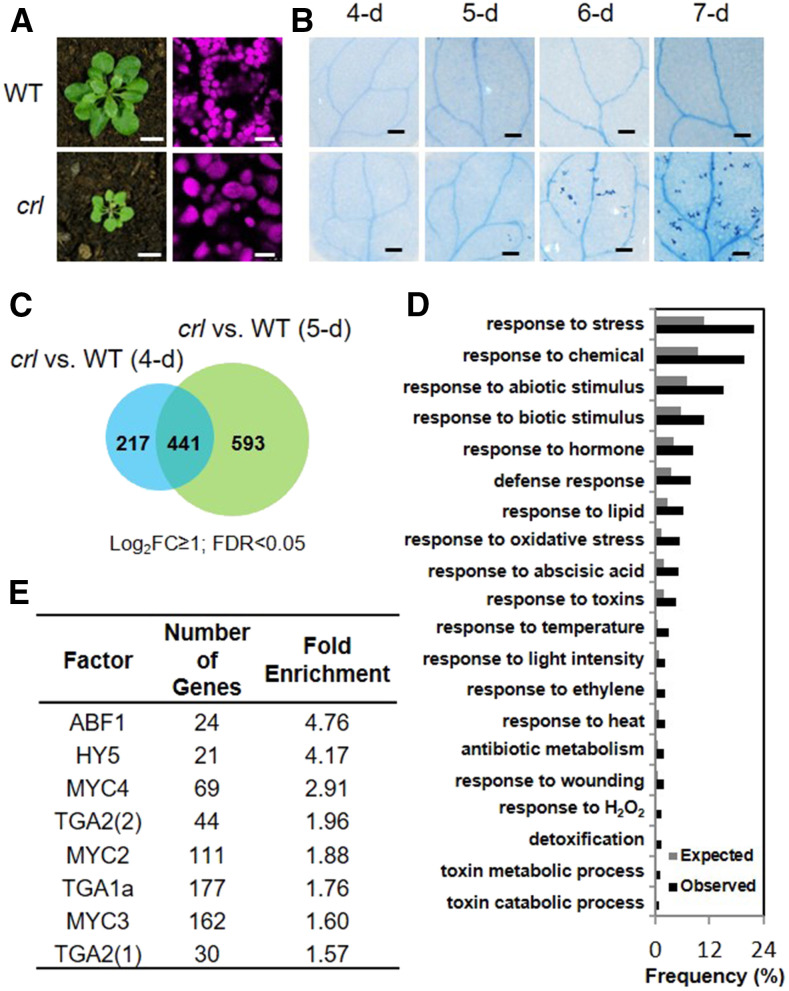
An earlier study demonstrated that Arabidopsis mutants deficient in plastid division exhibit a light-dependent foliar LCD. These plastid division mutants include crl (Figures 1A and 1B; Šimková et al., 2012), accumulation and replication of chloroplasts6 (arc6; Pyke et al., 1994), cell division protein FtsZ homolog1 (ftsz1; McAndrew et al., 2001), and plastid division2 (pdv2; Miyagishima et al., 2006). Whereas ARC6, FtsZ1, and PDV2 are part of the plastid division machinery, the precise function of CRL remains elusive. To investigate which signaling cascades become activated before the onset of cell death in GC mutants, we have chosen the crl mutant to conduct RNA sequencing (RNA-seq) and to gain insight into the molecular nature of CRL function. Because crl mutant seedlings grown under continuous light (CL; 100 µmol m−2 s−1) begin to exhibit cell death in cotyledons at 6 d following seed imbibition (Figure 1B), 4- and 5-d-old seedlings were used for RNA-seq analysis. Compared to the wild-type seedlings, crl mutant seedlings showed at least a twofold upregulation of 658 (4-d-old seedling) and 1034 (5-d-old seedling) genes, of which 441 genes overlapped (Figure 1C; Supplemental Data Set 1). Subsequent Gene Ontology (GO) analysis of these 1251 genes revealed that a total of 276 genes are related to stress responses (Figure 1D; Supplemental Data Sets 2 and 3). Next, we identified potential transcription factors (TFs) regulating the expression of these stress-associated nuclear genes using the AthaMap gene analysis tool (http://www.athamap.de/search_gene.php; Steffens et al., 2004). The promoter regions (defined as starting 1000 bp upstream of the ATG codon) revealed a significant enrichment of the binding sites of eight TFs including MYC (MYC2, MYC3, and MYC4) and TGA (class I and II; Figure 1E).
Figure 1.
Upregulation of Stress-Related Genes in crl before the Onset of Cell Death.
(A) Representative images of 21-d-old plants grown on soil under CL (left column). Chloroplasts in mesophyll cells were observed in the cotyledons of 5-d-old seedlings under a confocal laser scanning microscope (right column). Bars in left column = 0.8 cm; bars in right column = 15 μm.
(B) Representative images of cotyledons showing cell death detected by trypan blue staining in the 4- to 7-d-old wild-type (WT) and crl seedlings grown under CL. Bars = 0.2 mm.
(C) Venn diagram showing genes upregulated at least twofold in the 4- and 5-d-old crl versus wild-type (WT) seedlings. FDR, false discovery rate.
(D) GO enrichment analysis via Generic GO Term Finder. Observed, data from query; Expected, data from whole Arabidopsis genome.
(E) Transcription factors predicted to bind to the indicated cis-element in the regulatory regions of the stress-related genes.
Upregulation of JA-Responsive and Detoxification-Related Genes in crl
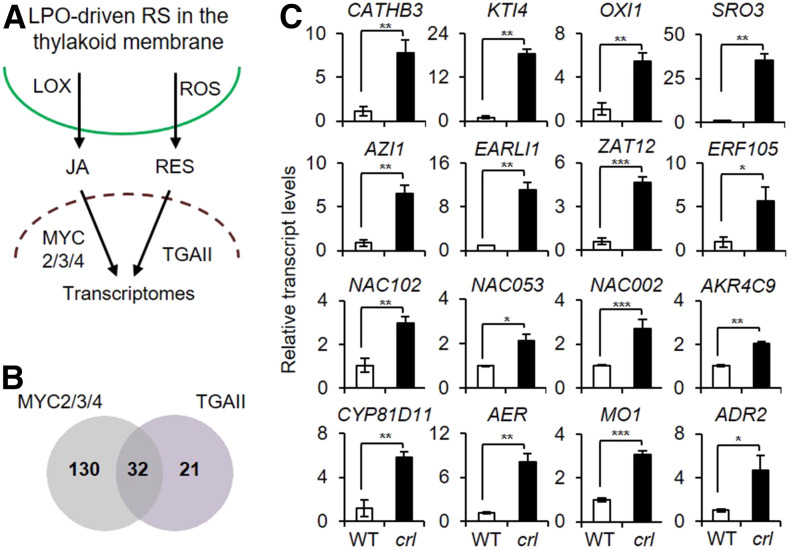
Among the eight identified TFs (Figure 1E), MYC2, MYC3, and MYC4 are well-known basic helix-loop-helix transcription activators for jasmonic acid (JA) signaling, while class II type TGA (TGAII) TFs regulate the expression of SA-responsive genes as well as detoxification-related genes (Turner et al., 2002; Chini et al., 2007; Mueller et al., 2008). Given that RES such as phytoprostane A1 and 12-oxo-phytodienoic acid can induce the expression of detoxification-related genes via TGAII TFs (Mueller et al., 2008), GCs may trigger both JA- and RES-dependent signaling pathways, most likely through lipid peroxidation (Figure 2A). In general, enzymatic lipid peroxidation (by lipoxygenases) induces JA signaling, while the nonenzymatic lipid peroxidation (by ROS) triggers the RES-dependent signaling pathways. There is also a group of genes induced by both signaling pathways (Farmer and Mueller, 2013). In accordance, among the stress-related genes upregulated in crl, 53 and 162 genes exhibit cis-elements for TGAII and MYC2/3/4, respectively (Supplemental Figures 1A and 1B; Supplemental Data Sets 4 and 5); 32 genes can be regulated by both TF types (Figure 2B). Many of these genes encode proteins that are implicated in defense and programmed cell death, including CATHEPSIN B-LIKE PROTEASE3 (CATHB3), OXIDATIVE INDUCIBLE1 (OXI1), KUNITZ TRYPSIN INHIBITOR4 (KTI4), EARLY ARABIDOPSIS ALUMINUM-INDUCED1 (EARLI1), AZELAIC ACID-INDUCED1 (AZI1), WRKY33, WRKY40, and the zinc finger proteins ZAT10 and ZAT12 (Li et al., 2008; Cecchini et al., 2015; Ge et al., 2016; Shumbe et al., 2016; Dogra et al., 2017).
Figure 2.
JA and RES Signaling Pathways in crl.
(A) Simplified scheme showing that lipid peroxidation-driven JA (LOX-dependent) and RES (ROS-dependent) signaling pathways alter the expression of nuclear genes via MYCs and TGAII TFs, respectively.
(B) Venn diagram showing the number of TGAII and MYC2/3/4 target genes upregulated in crl compared to wild-type seedlings.
(C) Relative expression levels of MYCs- and TGAII-driven selected defense- and detoxification-related genes were analyzed using RT-qPCR. ACT2 was used as an internal standard. Values represent means ± sd of three independent biological replicates. Asterisks indicate significant differences between mean values by Student’s t test (*P < 0.05; **P < 0.01; ***P < 0.001; Supplemental File). WT, wild type.
Also, NAC (NAM, ATAF, and CUC) family TFs such as ANAC102, ANAC002, ANAC032, and ANAC053 were also upregulated in crl (Supplemental Figures 1B and 2; Supplemental Data Sets 4 and 5). In particular, ANAC102 positively regulates the expression of detoxification-related genes (Fode et al., 2008; D’Alessandro et al., 2018), coinciding with the upregulation of a substantial number of genes encoding enzymes required for the detoxification of lipid peroxidation products in the crl mutant. These genes include CYP81D11, ALDEHYDE REDUCTASE1 (ADR1) and ADR2, MONOOXYGENASE1 (MO1), 2-ALKENAL REDUCTASE (AER), ALDO-KETO REDUCTASE FAMILY4 MEMBER C9 (AKR4C9), ALDEHYDE DEHYDROGENASE FAMILY7 MEMBER B4 (ALDH7B4), and ALDEHYDE DEHYDROGENASE1 (ADH1; Supplemental Figures 1A and 1B; Supplemental Data Sets 4 and 5). The RNA-seq data of these genes was further validated using RT-qPCR (Figure 2C).
Neither SA nor JA Participates in GCs-Mediated LCD
Because MYC2, MYC3, and MYC4 and TGAII TFs participate in signaling pathways mediated by the two major immune hormones JA and SA (Turner et al., 2002; Chini et al., 2007; Mueller et al., 2008), the crl-induced genes were compared with JA- and SA-responsive genes. From the previously published microarray and RNA-seq data, we obtained data for 223 SA- and 431 JA-responsive genes (Supplemental Figure 2A; Pauwels et al., 2008; Zhou et al., 2015; Duan et al., 2019). The resulting analysis revealed that, of these genes, 62 JA- and 50 SA-responsive genes were also induced in crl before the onset of cell death (Supplemental Figures 2A to 2C; Supplemental Data Sets 6 and 7). Accordingly, we reasoned that JA or SA might play a vital role in the crl-induced LCD.
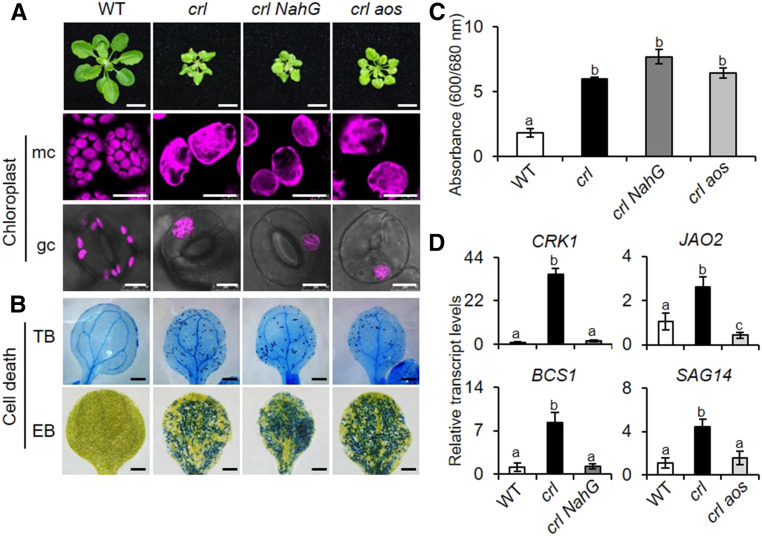
To explore this assumption, we generated JA- and SA-depleted crl mutants by genetically inactivating the allene oxide synthase (AOS) involved in JA synthesis pathway and by expressing the bacterial SA-hydrolyzing enzyme NahG under the control of the cauliflower mosaic virus 35S promoter (35Spro), respectively (Figure 3A). The ensuing macro- and microscopic phenotype analyses revealed that neither SA nor JA impacts plastid division and cell death in the crl mutant (Figures 3A to 3C) despite the apparent repression of SA- and JA-responsive genes in crl NahG and crl aos plants, respectively (Figure 3D). Interestingly, the loss of AOS slightly rescues the growth retardation phenotype of the crl mutant (Figures 3A), suggesting that JA may contribute to crl-conferred cell cycle inhibition. This correlates with the observation that exogenous JA inhibits the cell cycle in tobacco BY-2 cells (Swiatek et al., 2002; Pauwels et al., 2008). Our RT-qPCR analysis found an aos-driven reduction of cell cycle inhibitors, such as SIAMESE-RELATED5 (SMR5) and SMR7 (Supplemental Figure 3A), indicating that the retarded growth in the crl mutant could be partly attributable to an increase in cellular JA content. Indeed, we observed an increased level of JA in crl relative to the wild-type plants (Supplemental Figure 3B).
Figure 3.
SA- and JA-Independent Cell Death in crl.
(A) Macro- and microscopic phenotypes of the wild type (WT), crl, crl NahG, and crl aos. Images are representative of 21-d-old plants grown on soil under CL (bars in top row = 1 cm). Representative confocal images of chlorophyll autofluorescence in mesophyll cells (mc) and guard cells (gc) in 5-d-old seedlings grown on MS medium under CL (bars in middle and bottom rows = 20 μm for mc and 8 μm for gc).
(B) Cell death was visualized by TB and EB staining in the cotyledons of 10-d-old seedlings. Bars = 0.1 cm. WT, wild type.
(C) Dead cells visualized by EB in (B) were quantified by measuring relative EB absorbance as A600/A680. For EB staining, quantification was done using five cotyledons per genotype. Experiments were repeated at least three times, and the data represent means ± sd (Supplemental File). WT, wild type.
(D) Relative transcript levels of SA-responsive genes (CRK1, BSC1) and JA-responsive genes (JAO2, SAG14). Gene expression was determined in 5-d-old seedlings using RT-qPCR. ACT2 was used as an internal standard. Value represents means ± sd of three independent biological replicates. In (B) and (D), lowercase letters indicate statistically significant differences between mean values at each genotype (P < 0.05, one-way ANOVA with Tukey’s post hoc honestly significant difference (HSD) test; Supplemental File). WT, wild type.
EXECUTER-Independent Lesions in crl
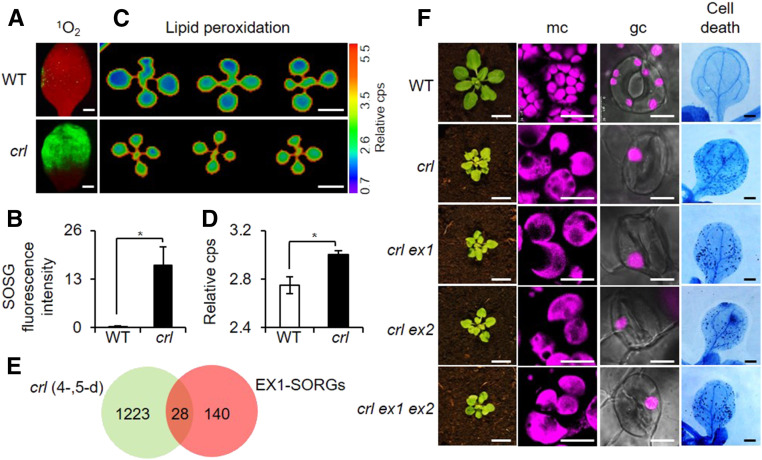
As aforementioned, the crl mutant was identified through a forward genetic screen as a potential 1O2-signaling mutant that continually expresses singlet oxygen-responsive genes (SORGs; Šimková et al., 2012). To understand the cause of this SORG expression, we measured 1O2 levels in crl and the wild-type seedlings grown under CL and discovered a considerably high level of 1O2 in the crl mutant as evidenced by the strong fluorescence of the singlet oxygen sensor green (SOSG; Figures 4A and 4B), a selective 1O2-detecting reagent, indicating that crl is not a 1O2-signaling mutant but is instead a 1O2-overproducing mutant. Consistent with the enhanced level of 1O2, an increase in foliar lipid peroxidation was seen, as manifested by the increased level of autoluminescence (Figures 4C and 4D). Foliar autoluminescence is visible due to the accumulation of light-emitting molecules, including 1O2 and triplet carbonyls, during lipid peroxidation (Havaux et al., 2006; Birtic et al., 2011). Consistently, we found that 28 SORGs (Dogra et al., 2017) are upregulated in crl versus the wild-type seedlings (Figure 4E; Supplemental Data Set 8). As EX1 mediates 1O2 signaling by directly sensing 1O2 via Trp-643 (Dogra et al., 2019b), loss of EX1 may attenuate cell death in crl mutants because of impaired 1O2 signaling. We then compared chloroplast sizes in both mesophyll and guard cells as well as cell death in crl ex1, crl ex2, and crl ex1 ex2 double and triple mutants relative to crl mutant plants (Figure 4F). We did not find any impact of ex1 in the crl-induced multiple lesions (Figure 4F). Because EX2, a close homolog of EX1, also acts as a modulator of 1O2 signaling (Lee et al., 2007; Kim et al., 2012), the crl-induced phenotypes were also examined in the crl ex2 and crl ex1 ex2 mutant plants. The results showed that EXs are not required to induce the multiple lesions in the crl mutant. Collectively, we could show that neither SA- nor JA- nor EXs-mediated 1O2 signaling is involved in the development of LCD in crl mutant.
Figure 4.
EXECUTER1-Independent LCD in crl Despite the Increased Level of 1O2.
(A) and (B) Cotyledons of 5-d-old seedlings grown under CL were treated with SOSG to detect 1O2-activated SOSG fluorescence. (A) Green SOSG fluorescence was recorded under the fluorescent microscope (using a GFP filter). The red fluorescence is the autofluorescence of chlorophyll in the chloroplasts. Bars = 0.7 mm. (B) Fluorescence intensity as shown in the confocal image was calculated with ImageJ software and normalized to per unit area of the cotyledons. WT, wild type.
(C) and (D) Peroxidation-derived autoluminescence represents the relative lipid peroxidation levels in the cotyledons of 10-d-old seedlings grown under CL. Bars = 0.4 cm. (C) Lipid peroxidation was quantified using the relative counts per second (cps) of the autoluminescence. (D) For both SOSG and lipid peroxidation experiments, quantification was done using five cotyledons per genotype. Experiments were repeated at least three times, and the representative data are shown. In (B) and (D), error bars indicate sd and the asterisks indicate significant differences between mean values by Student’s t test (*P < 0.05; Supplemental File). WT, wild type.
(E) Venn diagram showing the number of EX1-dependent SORGs (EX1-SORGs) in the crl-induced transcriptome of 4- and 5-d-old seedlings.
(F) Foliar phenotypes of 21-d-old wild-type (WT), crl, crl ex1, crl ex2, and crl ex1 ex2 seedlings. Bars in left column = 1 cm. Confocal images showing chlorophyll autofluorescence, which shows the chloroplast morphology in mesophyll cells (mc) and guard cells (gc) in 5-d-old seedlings. Bars in middle columns = 18 µm for mc and 10 µm for gc. Cell death was visualized by TB staining in the cotyledons of 10-d-old seedlings. Bars in right column = 0.1 cm.
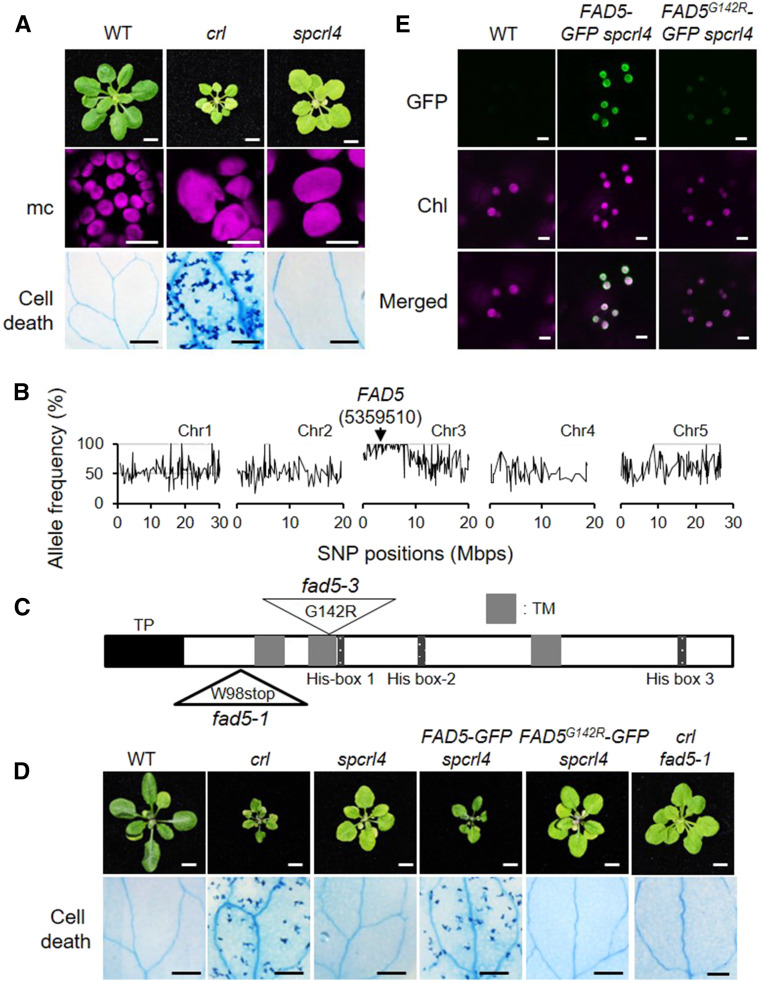
Inactivation of FAD5 Abrogates Cell Death in crl
To further characterize the crl mutant and to understand the function of the CRL protein, we isolated several suppressor mutants of crl (dubbed spcrl) through an ethyl methanesulfonate (EMS) mutagenesis-based forward genetic screen. Among them, we focused on spcrl4, which completely abrogates cell death while retaining the defect in plastid division. This mutation also altered the leaf morphology from crumpled to regular with a considerably expanded leaf size (Figure 5A). Despite the expanded leaf size, the average cell area in crl versus spcrl4 mutants was comparable (Supplemental Figures 4A and 4B), indicating substantially rescued cell proliferation by the spcrl4 mutation. Whole-genome sequencing allowed us to identify a causative missense mutation in a single genetic locus encoding FAD5 on chromosome 3 (Figure 5B). This mutation resulted in a Glu-142-to-Arg-142 substitution (G142R) in the second transmembrane domain (Figure 5C).
Figure 5.
Inactivation of FAD5 Abrogates Cell Death in crl.
(A) Representative images of 21-d-old plants grown on soil under CL are shown in the top row. Bars = 0.5 cm. Confocal images of chlorophyll fluorescence in mesophyll cells (mc) in 5-d-old seedlings are shown in the middle row. Bars = 15 µm. Cell death in cotyledons (10-d-old seedlings) was visualized by TB staining, and representative results are shown in the bottom row. Bars = 1 mm. WT, wild type.
(B) Whole-genome sequencing of spcrl4 in comparison with crl identified a mutation in FAD5 at position 5359510 in chromosome (Chr) 3. Mbps, million base pairs.
(C) Schematic illustration of FAD5 shows a transit peptide (TP, black), three transmembrane domains (TM, gray), and three His-box domains (dark gray). The mutation positions for fad5-1 and fad5-3 are indicated with triangles. G, Gly; R, Arg; stop, stop codon; W, Trp.
(D) Complementation assay. Images are representative of 21-d-old plants grown on soil under CL and shown at the same scale. Bars in top row = 0.5 cm. Cell death was visualized by TB staining in the cotyledons of 10-d-old seedlings grown under CL, and a representative image from each genotype is shown. Bars in bottom row = 1 mm. WT, wild type.
(E) Subcellular localization of FAD5-GFP and FAD5G142R in 5-d-old seedlings of stable spcrl4 transgenic lines grown on soil under CL. Bars = 5 μm. Chl, chlorophyll autofluorescence; WT, wild type.
Next, we conducted a complementation assay by constitutively expressing GFP-tagged FAD5 or FAD5G142R in spcrl4 plants (35Spro:FAD5-GFP and 35Spro:FAD5G142R-GFP). FAD5-GFP nicely complemented spcrl4, whereas FAD5G142R-GFP failed to reacquire crl phenotypes in the context of growth inhibition and cell death (Figure 5D). Next, we examined the transcript and protein levels of FAD5-GFP and FAD5G142R-GFP using RT-qPCR and immunoblot analysis. Despite the comparable transcript level of transgenes (Supplemental Figure 5A), the protein level of FAD5G142R-GFP was much lower than that of FAD5-GFP (Supplemental Figure 5B), coinciding with the difference in GFP signals monitored by confocal microscopy (Figure 5E). This result suggests that the G142R substitution in the transmembrane domain promotes FAD5 degradation. To further substantiate that inactivation of FAD5 abrogates cell death in crl, we generated the crl fad5-1 double mutant. The fad5-1 nonsense mutation causes premature termination of FAD5 translation (Heilmann et al., 2004). The crl fad5-1 double mutant showed a phenotype nearly comparable with that of spcrl4 (Figure 5D). As two mutant alleles already exist for fad5, we named fad5G142R as fad5-3.
We then examined FAD5 localization via the chloroplast fractionation assay. Owing to the difficulty of isolating gigantic chloroplasts for this assay, we transiently overexpressed MYC-tagged FAD5 and FAD5G142R in Nicotiana benthamiana leaves. Although the levels of transcripts were similar, we failed to detect FAD5G142R-MYC (Supplemental Figures 5C and 5D), corroborating the nature of the G142R mutation as aforementioned. Nevertheless, the fractionation results showed that FAD5-GFP was highly enriched in the envelope fraction with lesser accumulation in the thylakoids (Supplemental Figure 5E), suggesting the trafficking of the FAD-derived lipid (or PUFAs) from the inner envelope to the thylakoid membrane. The presence of a small amount of translocon TIC40 in the thylakoid membrane indicated a slight contamination between thylakoid and envelope fraction. Notably, the expression level of FAD5 in crl seedlings was slightly reduced relative to the wild-type seedlings (Supplemental Figure 5A).
Next, we compared the relative transcript abundances of the crl-induced stress-/detoxification-related nuclear genes in spcrl4 seedlings. The RT-qPCR analysis indicated that fad5 also rescues the molecular phenotype of crl (Supplemental Figure 6). The transcript levels of the stress-/detoxification-related genes in spcrl4 were either restored to the wild-type levels or significantly repressed compared to crl (Supplemental Figure 6). This result confirms that the altered expression of the nuclear transcriptome is likely associated with the FAD5 function.
Loss of FAD5 Reduces the Level of Palmitic Acid-Driven 16:1
Because FAD5 catalyzes the unsaturation of palmitic acid (16:0) to palmitoleic acid (16:1) and fad5 abrogates crl-induced cell death, we hypothesized that the 16:0-derived palmitoleic acid and subsequently formed PUFAs might contribute to the cell death upon oxidation by 1O2. This would account for the upregulation of the RES-responsive genes (Figure 2B). To this end, we profiled fatty acids in different genotypes using fatty acid methyl ester (FAME) analysis (Table 1). Our results clearly showed that the level of C18:0 and its unsaturated forms remain unchanged, whereas C16:0 significantly accumulated and C16:1 was downregulated in crl fad5 compared to the crl mutant. The notable reduction of C16:3, but not of C16:2, caused by the loss of FAD5 suggested that fatty acid synthesis pathways are more complex in the crl mutant than we anticipated. Nonetheless, given the increased levels of 1O2 in the crl mutant, it is reasonable to suggest that oxidized unsaturated C16:1 (or C16:3) may activate the autoimmune responses. Alternatively, it may be that the overall impaired lipid homeostasis in the chloroplasts of crl induces autoimmune responses. If this latter suggestion is correct, the loss of other FADs would also alter the crl phenotypes.
Table 1. Fatty Acid Compositions in Wild-Type, crl, and crl fad5 Plants.
| Fatty Acid | 16:0 | 16:1 | 16:2 | 16:3 | 18:0 | 18:1 | 18:2(n-6) | 18:3(n-6) | 18:3(n-3) |
|---|---|---|---|---|---|---|---|---|---|
| Wild type | 11.8 ± 0.37a | 0.92 ± 0.10a | 3.14 ± 0.13a | 0.91 ± 0.11a | 1.13 ± 0.02a | 0.54 ± 0a | 16.82 ± 0.70a | 0.32 ± 0.14a | 45.53 ± 1.97a |
| crl | 12.50 ± 0.24a | 0.99 ± 0.07a | 2.95 ± 0.16a | 0.66 ± 0.11b | 1.28 ± 0.03b | 3.59 ± 0.20b | 19.47 ± 0.09b | 0.51 ± 0.08a | 40.83 ± 0.29a |
| crl fad5 | 17.11 ± 0.31b | 0.29 ± 0.03b | 2.77 ± 0.01b | 0.16 ± 0.02c | 1.44 ± 0.01c | 3.16 ± 0.11c | 20.12 ± 0.40b | 0.42 ± 0.12a | 43.75 ± 0.99a |
FAME analyses were carried out using 5-d-old seedlings grown on half-strength MS agar medium as described in Methods. Values represent means ± sd of three independent biological replicates. Lowercase letters indicate statistically significant differences between mean values at each genotype (P < 0.05, one-way ANOVA with Tukey’s post hoc HSD test; Supplemental File).
To examine the specificity of FAD5, we generated a crl fad7 fad8 triple knockout mutant. FAD7 and FAD8 catalyze the production of both C16:3 and C18:3 PUFAs; thus, the fad7 fad8 double mutant plants exhibit altered fatty acid contents (e.g., notable decreased amounts of C16:3 and C18:3; Kachroo et al., 2005). In our analysis, we observed a persistent LCD in crl fad7 fad8 triple mutant plants relative to that in crl mutants despite the altered lipid profile (Supplemental Figures 7A and 7B), corroborating a direct causal relation between FAD5 and LCD in crl mutant plants. We also noticed that C16:3 was not decreased in the triple mutants even though this was anticipated (Supplemental Figure 7B). We speculate that the PUFA biosynthesis pathway is altered in gigantic chloroplasts to maintain membrane lipid homeostasis.
Chloroplasts deficient in division are also defective in photoprotection responses, such as chloroplast movement (Dutta et al., 2017). If this is true in our system, the loss of FAD5 may abrogate the crl-induced cell death by altering the responses related to photoprotection. However, we can exclude this possibility because photosynthetic activity (Fv/Fm) of crl versus crl fad5 mutant plants remained comparable even under photoinhibitory conditions (270 µmol m−2 s−1 and 10°C) wherein chloroplasts undergo photooxidative damage (Supplemental Figure 8). This result suggests that the development of LCD is not due to impaired photoprotection in the crl mutant. Besides, we did not observe any apparent alteration of chloroplast structure in crl fad5 versus crl mutant plants (Supplemental Figure 9).
FAD5 Is Required to Stimulate LCD in GC Mutants
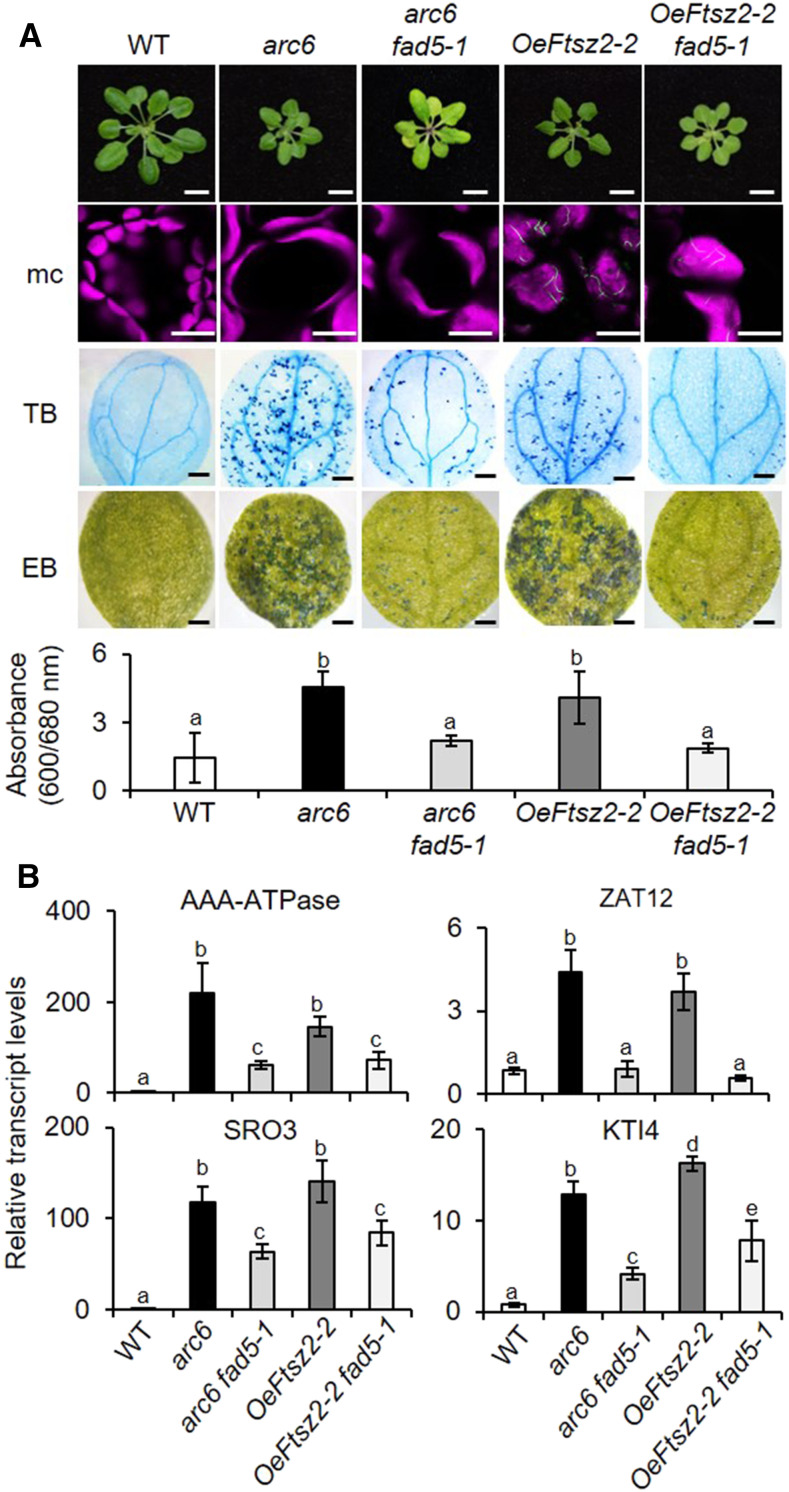
Because fad5 abrogates LCD in crl mutants, we examined the impact of fad5 in other GC mutants. An earlier study reported that several Arabidopsis mutants lacking the plastid-division machinery (such as ARC6, FtsZ1-1, and PDV2) develop a light-dependent LCD (Šimková et al., 2012). Besides, we found that GFP-tagged FtsZ2-2–overexpressing (OeFtsZ2-2) plants show a comparable phenotype: multiple lesions, including GCs, growth retardation, and LCD (Figure 6A). FtsZ2-2 is a constituent of the division machinery involved in the contractile ring formation prior to plastid division (McAndrew et al., 2001). Because the phenotypes are so similar between these GC mutants, we anticipated that fad5 might also compromise LCD in these plastid-division mutants. Thus, we crossed the arc6 mutant and OeFtsZ2-2 transgenic plants with fad5-1 to generate arc6 fad5-1 and OeFtsz2-2 fad5-1 mutant plants. In agreement with our expectations based on the aforementioned results, fad5 significantly repressed cell death in both arc6 and OeFtsz2-2 backgrounds (Figure 6A). As in spcrl4 plants, GCs remained unchanged in the double mutants (Figure 6A). However, unlike spcrl4 plants, though cell death in arc6 fad5-1 and OeFtsZ2-2 fad5-1 was drastically reduced, it was not entirely abrogated. The difference can be attributable either to the severity of cell death or because there is an additional factor contributing to cell death in arc6 and OeFtsZ2-2. Nonetheless, the inactivation of FAD5 attenuated not only cell death (Figure 6A) but also the expression of stress-related genes in these double mutants (Figure 6B). Collectively, we conclude that the depletion of C16 PUFAs significantly compromises GCs-mediated stress responses such as LCD.
Figure 6.
Loss of FAD5 Attenuates Cell Death in Plants Deficient in Chloroplast Division.
(A) Macro- and microscopic phenotypes of the wild type (WT), arc6, arc6 fad5-1, oeFTSZ2-2, and oeFTSZ2-2 fad5-1 are shown as described in Figures 3A to 3C. Bars = 0.5 cm in top row; 15 µm for mesophyll cells (mc) in second row; 0.7 mm for TB and EB in third and fourth row. For EB staining, quantification was done using five cotyledons per genotype. Experiments were repeated at least three times, and the data represent means ± sd (Supplemental File).
(B) Relative expression levels of selected stress-related genes were determined in 5-d-old seedlings using RT-qPCR. ACT2 was used as an internal standard. Values represent means ± sd of three independent biological replicates. Lowercase letters indicate statistically significant differences between mean values at each genotype (P < 0.05, one-way ANOVA with Tukey’s post hoc HSD test; Supplemental File).
Impaired Plastid Division and Lipidostasis Regulate the crl Transcriptome
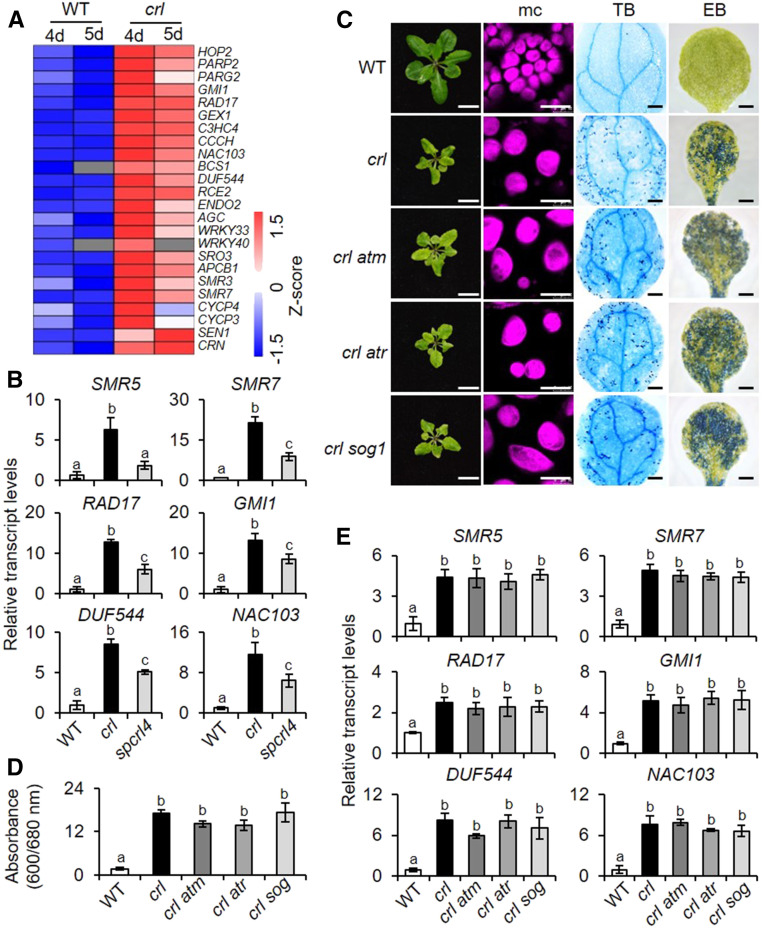
RNA-seq analysis revealed that a suite of DNA damage-responsive genes (DDRGs) was upregulated in the crl mutant (Figure 7A; Supplemental Data Set 9). Along with DDRGs, 24 transcripts of cell cycle inhibitors, including SMR5 and SMR7 (Hudik et al., 2014; Kumar et al., 2015), were also induced (Figures 7A and 7B). SMR5 and SMR7 are involved in the activation of a high light–dependent cell cycle checkpoint (Yi et al., 2014). The concurrent induction of the cell cycle inhibitors, together with DDRGs, implies a possible activation of the cell cycle checkpoint in crl before the onset of LCD. Moreover, the expression of the cell cycle inhibitors SMR5, SMR7, and the subset of DDRGs was repressed in spcrl4 (Figure 7B), suggesting that chloroplast lipid peroxidation may lead to their expression. This molecular phenotype supports the substantially improved stature of spcrl4 plants compared to that of crl (Figure 5A) and potentially implicates lipid peroxidation in the perturbations in the cell cycle.
Figure 7.
GCs Induce DDRGs in crl.
(A) Heatmap showing the relative expression of DDRGs and cell cycle inhibitors upregulated in crl compared to wild-type (WT) seedlings.
(B) Transcript abundance of selected cell cycle inhibitors (SMR5 and SMR7) and DDRGs were determined using RT-qPCR. Five-day-old seedlings grown under CL were used to extract total RNA. ACT2 was used as an internal standard. WT, wild type.
(C) to (E) The impact of the loss of ATM, ATR, or SOG1 in the crl-induced lesions. (C) and (D) Phenotypes are shown, as described in Figures 3A, 3C, and 3D. Bars in (C) = 1 cm in first column; 15 µm for mesophyll cells (mc) in second column, 0.1 cm for TB and EB in third and fourth columns. For EB staining and quantification was done using five cotyledons per genotype. Experiments were repeated at least three times, and the data represent means ± sd (Supplemental File). (E) Relative expression levels of selected cell cycle inhibitors/DDRGs were examined in 5-d-old seedlings of the wild type (WT), crl, crl atm, crl atr, and crl sog1 using RT-qPCR. (B) and (E) ACT2 was used as an internal standard. Values represents means ± sd of three independent biological replicates. Lowercase letters indicate statistically significant differences between mean values at each genotype (P < 0.05, one-way ANOVA with Tukey’s post hoc HSD test; Supplemental File).
Generally, the DNA damage response (DDR) is crucial for sustaining genome integrity in eukaryotic cells against DNA damage. To minimize genome damage, DDR results in DNA repair, gene expression changes, cell cycle arrest, and apoptosis (programmed cell death in plants). SUPPRESSOR OF GAMMA RESPONSE1 (SOG1), a plant-specific TF, is a major regulator of the DDR (Yoshiyama et al., 2009). When exposed to DNA-damaging reagents, sog1 mutant plants were defective in gene regulation, cell cycle arrest, and programmed cell death (Preuss and Britt, 2003; Yoshiyama et al., 2009; Furukawa et al., 2010). The DDR for double-strand and single-strand DNA breaks is mediated by ATAXIA TELANGIECTASIA MUTATED (ATM) and ATM AND RAD3-RELATED (ATR) kinases, respectively (Zhou and Elledge, 2000). Therefore, we speculated that the ATM and/or ATR pathway might regulate the expression of DDRGs and cell cycle inhibitors in crl plants, presumably through their shared downstream signaling component SOG1 (Yoshiyama et al., 2009). However, all of our macro- and microscopic phenotypes, including plant stature, cell death, and the transcript levels of DDRGs, remained unchanged in crl atm, crl atr, and crl sog1 plants compared to those in the crl mutant plants (Figures 7C to 7E). This result indicates that the canonical DDR pathways through SOG1, ATM, and ATR have no causal relations with the multiple crl-related lesions and that the induction of the DDR is instead a consequence of the crl mutation, which is likely directed by enhanced lipid peroxidation in a FAD5-dependent manner.
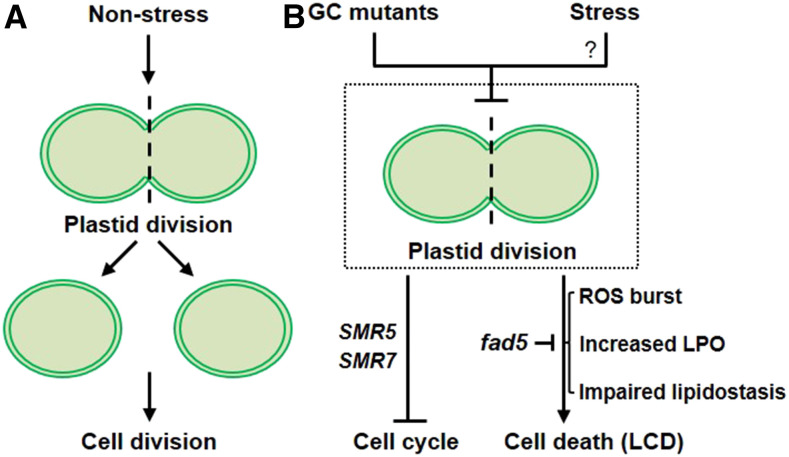
Under nonstressed conditions, plastid division precedes cell division, enabling an equal partitioning of plastids into the daughter cells (Figure 8A). The example reported here also suggests that impaired plastid division might repress the cell cycle via cell cycle inhibitors in GC mutants (Figure 8B). The role of SMR5 in crl mutant was demonstrated to be partly responsible for the cell cycle inhibition, whereas the impact of SMR7 in crl remains unclear. At present, it is not known whether there is any interrelation between cell cycle inhibition and LCD in GC mutants. However, it is clear that, as evidenced by the forward genetic screen, that FAD5 and its associated RS play a pivotal role in inducing autoimmune responses in GC mutants (Figure 8B).
Figure 8.
Impaired Plastid Division Triggers Cell Cycle Inhibition and Cell Death.
(A) Under nonstress conditions, plastid division precedes cell division, enabling equal partitioning of plastids into the daughter cells.
(B) In GC mutants, impaired plastid division seems to repress the cell cycle via cell cycle inhibitors such as SMR5 and SMR7 (Hudik et al., 2014). Whereas the role of SMR5 in crl mutant was previously demonstrated and shown to be partly responsible for the cell cycle inhibition, the impact of SMR7 induction remains unclear. Besides, GCs are largely defective in ROS and lipid homeostasis, leading to an increase in lipid peroxidation (LPO) and perhaps also RES. The abrogated and attenuated cell death caused by the loss of FAD5 in crl and other GC mutants, respectively, indicates the pivotal roles of LPO and RES in developing the multiple lesions. At present, it remains unknown whether any natural stress results in an arrest in plastid division, which leads to cell cycle arrest and controlled cell death.
DISCUSSION
In 2004, Apel and his research team reported on the genetic basis of chloroplast-mediated programmed cell death regulated by 1O2 and its sensor protein EX1 (Wagner et al., 2004). The failure of 1O2 sensing through the modification of the sensor domain of EX1 abolishes the expression of nuclear 1O2-responsive genes and cell death (Dogra et al., 2019b), further confirming that chloroplasts are involved in a genetically controlled cell death response via RS. Later, they also identified a peculiar phenotype in well-established Arabidopsis mutants defective in chloroplast binary fission (Šimková et al., 2012). These GC mutants, including crl, arc6, ftsz1-1, and pdv2, appear to develop a light-dependent LCD in photosynthetic tissue. Such an LCD phenotype mirrors that of typical lesion-mimic mutants, which have widely been used as bio-tools to unravel molecular mechanisms underlying cell death and immune responses in land plants (Lorrain et al., 2003; Bruggeman et al., 2015). Given the similarity regarding the autoimmune responses, we classified the GC mutants as CpLMMs.
Among these GC mutants, crl was initially designated as a potential 1O2-signaling mutant, constantly expressing 1O2-responsive genes (Baruah et al., 2009). Given the elevated level of 1O2 (Figure 4A), we concluded that crl is a 1O2-overproducing mutant rather than a mutant affected in 1O2 signaling. The constitutive expression of stress- and detoxification-related nuclear genes was observed in crl mutant seedlings prior to the onset of LCD (Figures 1 and 2). Therefore, we hypothesize that GCs produce these multiple phenotypes through a yet uncharacterized RS pathway(s). The increased levels of SA- and JA-responsive genes and 1O2 in crl seedlings relative to the wild-type seedlings prompted us to deplete either SA or JA or to inactivate EX1/EX2 in the crl mutant background to analyze potential impacts on the appearance of LCD. However, the resulting reverse genetic approach established that neither SA- nor JA- nor EX-mediated 1O2 signaling was responsible for the crl-induced LCD (Figures 3A, 3B, and F4F). Hence, we assumed that 1O2-driven lipid peroxidation and its derivative RES might prime LCD in crl. In agreement with this notion, a forward genetic screen revealed that inactivation of FAD5, an inherent fatty acid desaturase involved in the production of 16C PUFAs, abrogates and attenuates LCD in the crl and other GC mutants, respectively (Figures 5A, 5E, and 6A). Because oxidized PUFAs are central sources of RES, the significant impacts of the fad5 mutation in the expression of stress-related genes and LCD imply that RES or lipid peroxidation-associated RS is responsible for the observed LCD. Whereas C18 PUFAs-induced RES have been extensively studied (Mueller et al., 2008; Mano, 2012; Farmer and Mueller, 2013), the existence of C16 PUFAs-induced RES and their impact on intracellular signaling are yet to be discovered. Therefore, although it may be difficult to pinpoint one specific RES governing the GC-mediated multiple lesions, untargeted or targeted lipidomic analysis in GC versus FAD-lacking GC mutants (impairing chloroplast palmitic acid-derived PUFA biosynthesis in distinct steps) may enable us to identify differentially accumulated RES for further detailed investigation.
In addition to LCD, GC mutants (Figures 1A and 6A), including OeFtsZ2-2, show significant growth retardation, most likely because of cell cycle inhibition (Hudik et al., 2014). This finding indicates that the cell cycle and plastid division (or dysfunction) are tightly coordinated. Likewise, earlier studies found that plastid DNA replication (PDR) precedes nuclear DNA replication (NDR) in unicellular red algae of the Cyanidiales (Kobayashi et al., 2009, 2011). As PDR and NDR are indispensable for plastid division and mitosis, it is reasonable that plastid division is coordinated with the cell cycle. Mg-protoporphyrin IX (Mg-ProtoIX, a precursor of chlorophyll) was suggested to act as a retrograde signaling molecule that regulates NDR. Mg-ProtoIX that accumulated during or upon the completion of PDR appears to promote NDR by stabilizing a G1 cyclin, which otherwise undergoes E3 ubiquitin-mediated degradation (Kobayashi et al., 2011). Because Mg-ProtoIX is also synthesized in mitochondria, the authors suggested that mitochondrial DNA replication and PDR coordinate NDR via Mg-ProtoIX (Kobayashi et al., 2011). Whether Mg-ProtoIX functions in land plants to coordinate organelle DNA replication (mitochondrial DNA replication and PDR) and NDR remains to be investigated.
At present, we are unable to provide direct evidence that any specific external cue hinders plastid division, which consequently induces stress responses such as LCD via RS (Figure 8). However, it is appealing to presume that altered lipidostasis and/or enhanced lipid peroxidation via ROS may trigger FAD5-dependent RES-mediated immune/stress/detoxification responses in GC mutants (Figure 8). A recent study showed that both abiotic and biotic stresses, coupled with a ROS burst in chloroplasts, significantly affect chloroplast number and size as well as thylakoid organization (Zechmann, 2019). These changes in chloroplast ultrastructure in turn may potentiate ROS generation, leading to oxidative damage and cell death. Collectively, recent reports and our studies indicate that chloroplast-driven ROS and lipid peroxidation induce stress responses through changes in nuclear gene expression. Further investigation of CpLMMs should enable us to precisely decipher the role of chloroplasts in LCD and to find lipid-associated potential RS molecules.
METHODS
Plant Materials and Growth Conditions
All wild-type Arabidopsis (Arabidopsis thaliana) seeds used in this study were from the Columbia-0 (Col-0) ecotype and were harvested from plants grown under CL (100 µmol m−2 s−1) at 22 ± 2°C following a 2-d stratification in darkness at 4°C. All knockout mutant seeds, including crl, arc6, aos, atm, atr, fad5-1, fad7-1 fad8-1, and sog1, were obtained from the Nottingham Arabidopsis Stock Centre, and their genotypes were confirmed by PCR-based analyses. Primers used in this study are listed in the Supplemental Table. The ex1 null mutant used in this study was previously described by Šimková et al. (2012) and Dogra et al. (2019b). The ex2 mutant and ex1 ex2 double mutants were previously described by Lee et al. (2007). The crl NahG, crl aos, crl ex1, crl ex2, crl ex1 ex2, crl fad 5-1, crl fad5-1 fad8-1, crl atm, crl atr, crl sog1, arc6 fad5-1, and OeFtsZ2-2 fad5-1 mutants were generated by crossing the cognate homozygous plants. Seeds were soaked in a 20% hypochlorite solution followed by five washes with sterile water and were then sown on half-strength Murashige and Skoog (MS) medium (Duchefa Biochemie) with 0.5% (w/v) Suc and 0.7% (w/v) agar. All plants were grown under CL.
Four-week-old Nicotiana benthamiana plants grown in a controlled growth chamber under long-day condition (16-h-light/8-h-dark cycle) at 25°C were used for all transient assays (RT-qPCR, immunoblot analysis, and chloroplast fractionation assay).
Identification of spcrl4 and Whole-Genome Sequencing
Under nonstressed growth conditions, we screened the M2 progeny (grown on soil) of ∼12,000 M1 crl seeds that had been initially treated with 0.4% EMS for 8 h as previously described by Kim et al. (2006). The spcrl4 mutant plant was identified based on the increased biomass and a weakened crumpled leaf phenotype. To generate a mapping population, we genetically crossed spcrl4 (M3 homozygote line) with the crl parental line, and at least 50 F2 plants showing the typical spcrl4 phenotypes were selected to extract genomic DNA. A comparable number of crl mutant plants was also used to extract genomic DNA. Total genomic DNA was extracted using DNeasy plant mini kit (Qiagen) according to the manufacturer’s instructions. One microgram of genomic DNA was used for library construction using the Illumina TruSeq DNA PCR-free kit following the manufacturer’s protocol for 350-bp insert size. Genome sequencing was performed using a HiSeq2500 sequencer to generate 125-bp paired-end reads. Genome sequencing data were processed by SolexaQA (Cox et al., 2010) and Cutadapt (version 1.3) to remove low-quality regions and adapter sequences, respectively. Clean reads were mapped to The Arabidopsis Information Resource (TAIR) genome by bwa mem (Li and Durbin, 2009) with default parameters. Single-nucleotide polymorphisms (SNPs) were called by the mpileup function of samtools (Li et al., 2009). Poor-quality SNPs with mapping quality less than 60, or depth less than 3 or more than 200, were filtered out by vcftools (Danecek et al., 2011). SNPs that were found only in the spcrl214 mutant pool were retained with the assumption that these mutations were generated by EMS mutagenesis. Candidate causal mutations were identified using the SHOREmap method (Schneeberger et al., 2009). SHOREmap version 3.0 (Sun and Schneeberger, 2015) was used to draw allele frequency graphs and identify the region that contained the causal mutation surrounded by tightly linked SNPs. In the identified region, mutations that were predicted to have a large impact on open reading frame of target genes were prioritized as potential causative mutations.
The SNP analysis revealed potential causative mutations in chromosome 3. Each mutation in the potential candidate genes caused a missense mutation (Figure 5B; Supplemental Data Set 10). The mutations in the respective genes were confirmed by manual PCR-based sequencing using at least 40 individual F3 plants of spcrl4 backcrossed with crl. All 40 plants retained the identical mutation in the first exon of AT3G15850, leading to the G142R substitution in the plastid-localized protein FAD5. Next, we conducted a complementation assay by expressing the wild-type copy of FAD5 gene into spcrl4 for further analysis. The transgenic spcrl4 expressing FAD5-GFP driven by the CaMV 35S promoter largely restored the crl-conferred phenotypes.
FAME Analysis
For the FAME analyses, lipids were extracted and esterified from 150 mg of 5-d-old seedling. Briefly, the tissue samples were ground to fine powders in liquid nitrogen, and 0.3 g of the powder was used for each biological sample. The extraction and esterification were performed with 2 mL of sulfuric acid, methanol (5% [v/v]) and 300 μL of methylbenzene at 95°C for 1 h. Following this, solvents were extracted again using 2 mL of 0.9% (w/v) NaCl and 1 mL of n-hexane at room temperature. Afterward, solvents were centrifuged at 2250g for 5 min, and the supernatant was removed and dried under nitrogen and then reconstituted with 100 μL of n-hexane for the FAME analysis. FAME mixtures were analyzed using an Agilent 7890A gas chromatograph equipped with a flame ionization detector. Samples (2 µL) were injected with a split ratio of 10:1 and resolved on an HP-FFAP 30 m × 0.25 mm × 0.25-µm column (Agilent). The injection and detector temperature was set to 260 and 280°C, respectively. The initial temperature of the column was adjusted to 150°C and was increased to 210°C at a rate of 10°C/min and held for 8 min. Next, the temperature was increased to 230°C at a rate of 20°C/min and delayed for 6 min. The FAME contents were quantified using the gas chromatography with flame-ionization detection spectrum in Chemstation software (Agilent). The data obtained were corrected based on the retention time of the fatty acids mixture standard in the gas chromatography with flame-ionization detection spectrum and then the fatty acids content was quantified using the formula (S1/S2) × N/M* 100%, where S1 is total peak area of all fatty acids, S2 is peak area of internal standard, N is amount of the internal standard, and M is the sample mass (Supplemental Data Set 11).
JA Measurements
The endogenous JA levels in plant samples were measured by ultra-performance liquid chromatography (Shim-pack UFLC, SHIMADZU CBM30A)–tandem mass spectrometer (Quadrupole Trap 6500; AB SCIEX). Briefly, ∼0.5 g of plant tissue was homogenized to a fine powder using a sample grinder (MM 400 Retsch) for 1 min at 30 Hz. Following this, ∼25 mg of the fine powder was mixed with the internal standard (JA; Sigma-Aldrich) and extracted using methanol:water:formic acid (15:4:1, [v/v/v]). The combined extracts were then dried under nitrogen and reconstituted in 80% (v/v) methanol, followed by filtration (polytetrafluoroethylene, 0.22 μm; Anpel). For liquid chromatography–tandem mass spectrometry analysis, the sample was transferred to a fresh HPLC vial, and the chromatographic separations were conducted on a C18 column (1.8-μm particle diameter, 2.1 mm × 100 mm; Waters) before injection (volume = 2 µL) at 40°C. Water with 0.05% acetic acid (v/v) and acetonitrile with 0.05% (v/v) formic acid were used as mobile phase A and B, respectively. The elution profile after injection of each sample was as follows: 0 to 1 min, 95:5 (v/v); 8 to 9 min, 5:95 (v/v); 9 to 12 min, 95:5 (v/v) at a flow rate of 0.35 mL/min. The effluent was alternatively connected to an electrospray ionization–triple quadrupole-linear ion trap-mass spectrometer, equipped with an electrospray ionization Turbo Ion-Spray interface, operating in both positive and negative ion modes and controlled by Analyst 1.6 software (AB SCIEX). The JA content was determined using the multiple reaction monitoring mode of triple quadrupole mass spectrometry. JA concentrations were determined by measuring peak areas after integral correction between the corresponding peak in total ions current and extracted ion chromatogram.
Vector Construction and Generation of Transgenic Plants
The cDNA (minus the stop codons) of FAD5, FAD5G142R, and FtsZ2-2 were PCR amplified from the wild-type or spcrl4 cDNA and then cloned into the pDONR221 Gateway vector through Gateway BP reaction (Invitrogen). After verification by DNA sequencing, the entry vector was subsequently recombined into the Gateway-compatible plant binary vectors pGWB605 and pGWB517 (Nakagawa et al., 2007) for C-terminal superfolder GFP and 4×MYC fusion through the Gateway LR reaction (Invitrogen). Each vector was transformed into Agrobacterium tumefaciens strain GV3101. Arabidopsis transgenic plants were then generated using Agrobacterium-mediated transformation via the floral dip method (Clough and Bent, 1998). Transformants were selected on MS medium containing 20 mg/L glufosinate-ammonium (or BASTA; Sigma-Aldrich) until the T3 generation to screen for homozygous transgenic plants. For transient expression, the suspensions of agrobacteria carrying different constructs were infiltrated into healthy leaves of 4-week-old N. benthamiana plants and analyzed after 48 h.
RNA-Seq Library Construction and Data Analysis
RNA-seq analysis was performed as previously described by Dogra et al. (2017). Briefly, total RNA was extracted from three independent biological replicates of the 4- and 5-d-old crl and wild-type seedlings grown under CL. The RNA extracted using the RNeasy Plant Mini Kit (Qiagen) was subjected to on-column DNase digestion with RNase-free DNase Set (Qiagen) according to the manufacturer’s instructions. The purity of RNA was verified by a Nano Photometer spectrophotometer (IMPLEN). The Qubit RNA Assay Kit in Qubit 2.0 Fluorometer (Life Technologies) was used to measure RNA concentration. The RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies) was used to evaluate RNA integrity. RNA samples that passed the concentration, purity, and integrity checks were used for RNA-seq analyses. RNA-seq libraries were constructed using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs) following the manufacturer’s instructions. RNA-seq libraries were sequenced on an Illumina HiSeq 2500 platform to generate 100-bp paired-end reads. The raw sequencing data were processed by SolexaQA (version 2.2) to extract pair reads and to remove low quality reads. The clean reads obtained were mapped to the Arabidopsis genome (TAIR10) using TopHat (Trapnell et al., 2009). After mapping, Python software HTseq-count was used to extract raw counts of annotated genes. Differentially expressed genes were identified using R package edgeR, which uses counts per gene in different samples and performs data normalization using the trimmed mean of M-values method (Robinson et al., 2010). The gene expression data were normalized to transcripts per million according to the total number of mapped clean reads in each library. The genes with at least a twofold change in expression and a false discovery rate of less than 0.05 were considered to be differentially expressed. The transcripts per million values were used to build the expression matrix, and the subsequent clustering and visualization were done using Multi-Experiment Viewer 4.9.0. GO enrichment analysis of differentially expressed genes was performed using the Generic GO Term Finder tool (http://go.princeton.edu/cgi-bin/GOTermFinder; Katari et al., 2010), with a significance of P < 0.05.
RNA Extraction and RT-qPCR
Total RNA was extracted using the FastPure Plant Total RNA Isolation Kit (Vazyme). One microgram of RNA was treated with RQ1 RNase-Free DNase (Promega) and reverse transcribed using HiScript II Q RT SuperMix for qPCR (+gDNA wiper; Vazyme) according to the manufacturer’s recommendations. RT-qPCR was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme) on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). Relative transcript levels were calculated using the comparative delta-Ct method and normalized to the transcript levels of ACTIN2 (AT3G18780). The primers used in this study are listed in the Supplemental Table.
Microscopic Analyses
The GFP signals and chlorophyll autofluorescence were visualized using a confocal laser scanning microscope (TCS SP8; Leica Microsystems). Images were acquired and processed using LAS AF Lite software version 2.6.3 (Leica Microsystems). Unless otherwise stated, cotyledons of 5-d-old seedlings were used for imaging GFP and chlorophyll autofluorescence.
For transmission electron microscopy analysis, cotyledons of 5-d-old seedlings were harvested and pre-fixed in 2.5% (v/v) glutaraldehyde in 0.1 M PBS, pH 7.4, at 4°C for 3 d. The pre-fixed samples were then washed three times with 0.1 M PBS. Afterward, samples were post-fixed in 1% (v/v) osmic acid overnight at 4°C, followed by washing three times with 0.1 M PBS. Samples were then dehydrated using a gradient ethanol–acetone series and then embedded in Spurr resin. Ultrathin resin sections (70 nm) were cut by a diamond knife on a UC7 ultramicrotome (Leica Microsystems), mounted on copper grids, and stained with 2% (w/v) uranyl acetate and 0.5% (w/v) lead citrate. The sections were observed and photographed using a transmission electron microscope (H7700; Hitachi).
Subcellular Localization
Healthy leaves of 5-d-old transgenic spcrl4 plants expressing the GFP-tagged FAD5 or FAD5G142R were used to monitor GFP signals and chlorophyll autofluorescence by confocal microscopy.
Imaging 1O2
Measurement of the 1O2 accumulation was done as previously described by Dogra et al. (2019b). Briefly, 5-d-old seedlings were submerged in a solution of 260 µM SOSG (Thermo Fisher Scientific, Molecular Probes) in 50 mM phosphate buffer, pH 7.4. Leaves were vacuum infiltrated for 5 min and then incubated for 2 h in dark followed by imaging using the blue light GFP filter of an M205 FA fluorescence microscope (Leica Microsystems). 1O2-activated SOSG was visualized with excitation at 488 nm and emission at 530 nm. At least 10 cotyledons from each genotype were monitored, and representative images are shown. All the images were acquired and processed using LAS software version 4.2.0 (Leica Microsystems).
Lipid Peroxidation Imaging
Lipid peroxidation was visualized in whole plants by autoluminescence imaging (Birtic et al., 2011). Ten-day-old plants grown under CL were dark adapted for 5 min, and the luminescence emitted from the spontaneous decomposition of lipid peroxides was captured by a highly sensitive thermoelectric-cooled camera (NightShade LB985 Plant In Vivo Imaging System; Berthold Technologies) under luminescence emitted from a lamp on the bottom. Parameters were as follows: reillumination, 470 nm; spectral peak, 560 nm; lamp energy, 50%; exposure time, 0.1 s; camera readout, fast. Images were acquired and processed using IndiGo software version 2.0.5.0 (Berthold Technologies). Experiments were repeated at least three times.
Determination of Cell Death
Cell death was determined by trypan blue (TB) and Evans blue (EB) staining. For TB staining, plant cotyledons were submerged in TB staining solution (0.02 g of trypan blue and 10 g of phenol dissolved in 30 mL of glycerol, lactic acid, and water [1:1:1]; this solution was further diluted with ethanol in 1:2 [v/v]) and boiled for 2 min. After a 16-h incubation at room temperature, nonspecific staining was removed with the destaining solution (250 g of chloral hydrate dissolved in 100 mL of water, pH 1.2). Plant tissues were then kept in 10% (v/v) glycerol for imaging. Imaging was done using an S8APO microscope (Leica Microsystems). Images were acquired and processed using LAS software version 4.2.0.
For EB staining, cotyledons were submerged in 2 mL of a 0.1% (w/v) EB solution and incubated for 16 h at room temperature. After incubation, cotyledons were thoroughly rinsed three times with water to remove unbound dye. Imaging was done using an S8APO microscope. Images were acquired and processed using LAS software version 4.2.0. For quantification, the dye bound to dead cells was solubilized in 50% (v/v) methanol containing 1% (w/v) SDS for 8 h. Cell death was estimated spectrophotometrically by measuring the ratio of absorbance at 600 and 680 nm (A600/A680) of the resulting dye solution as previously described by Shumbe et al. (2016).
Protein Extraction and Immunoblot Analysis
Total proteins were isolated from 100 mg of plant tissue. The plant tissue was ground to a fine powder in liquid nitrogen and resuspended in protein extraction buffer (50 mM Tris-Cl, pH 6.8, 100 mM DTT, 2% [w/v] SDS, 10% [w/v] glycerol, and 1× Complete Protease Inhibitor Cocktail [Roche]) at a ratio of 1:5 (tissue:buffer). The supernatant was collected after two centrifugations at 13,000 rpm for 20 min in a tabletop centrifuge. Protein contents were quantified using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The desired amount of proteins was resuspended in 1× Laemmli SDS sample buffer (Laemmli, 1970) and denatured for 10 min at 95°C. Equal amounts of proteins were separated by 10% SDS-PAGE gels and blotted onto Immuno-Blot polyvinylidene fluoride membranes (Bio-Rad). The GFP fusion proteins were detected using mouse anti-GFP monoclonal antibody (1:5000; catalog no. 11814460001; Roche).
Chloroplast Isolation and Fractionation
Intact chloroplasts were isolated from healthy leaves of 4-week-old N. benthamiana leaves transiently expressing FAD5-GFP under the control of the 35S promoter as previously described by Kauss et al. (2012) and Dogra et al. (2019a). Isolated chloroplasts were fractionated into stroma, thylakoids, and envelopes following the method previously described by Flores-Pérez and Jarvis (2017). Briefly, intact chloroplasts were resuspended in prechilled hypotonic lysis buffer (25 mM HEPES-KOH, pH 8.0, supplemented with 1× Complete protease inhibitor cocktail [Roche]) and rotated at 20 rounds/min for 1 h in a cold room. The lysate was then loaded onto a prechilled Suc-HEPES gradient (containing 3.6 mL of 1.2 M Suc, 3.6 mL of 1.0 M Suc, and 3.6 mL of 0.46 M Suc) in a 12.5-mL ultracentrifuge tube. The gradient was then centrifuged in a swinging-bucket rotor (Sorval SW-41 Ti) at 200,000g for 1 h at 4°C, with medium speed acceleration and deceleration. The stroma-containing soluble fraction was collected from the top of the gradient. The envelope and thylakoid fractions separated at the interfaces of 0.46/1.0 M Suc and 1.0/1.2 M Suc were collected in prechilled tubes. The residual Suc remaining in the envelope and thylakoid fractions was removed by washing in hypotonic lysis buffer at 48,000g, for 1 h at 4°C, after which the pellets containing envelopes and thylakoids were collected.
The stroma and envelope fractions were directly resuspended in 1× Laemmli SDS sample buffer for gel and immunoblot analysis. For total chloroplast and thylakoid fractions, chlorophyll was removed using 100% acetone as previously described by Wang et al. (2016) and then the enriched proteins were resuspended in 1× Laemmli SDS sample buffer. Proteins were then denatured for 10 min at 95°C. Equal amounts of proteins were separated by 10% SDS-PAGE gels and blotted onto Immun-Blot polyvinylidene fluoride membrane. The MYC-fusion proteins were detected using mouse anti-MYC (1:10,000, catalog no. 2276; Cell Signaling Technology).
Accession Numbers
Sequences of the genes studied in this article can be found in the Arabidopsis TAIR database (https://www.arabidopsis.org) under the following accession numbers: AAA-ATPase (At3g28580), ACT2 (At3g18780), ADR2 (At3g04000), AER (At5g16970), AKR4C9 (At2g37770), AOS (At5g42650), ARC6 (At5g42480), ATM (At5g66130), ATR (At3g18780), AZI1 (At4g12470), BCS1 (At3g50930), CATHB3 (At4g01610), CRK13 (At4g23210), CYP81D11 (At3g28740), DUF544 (At4g22960), EARLI1 (At4g12480), ERF105 (At5g51190), EX1 (At4g33630), EX2 (At1g27510), FAD5 (At3g15850), GMI1 (At5g24280), JAO2 (At5g05600), KTI4 (At1g73260), MO1 (At4g15760), NAC102 (At5g63790), NAC053 (At3g10500), NAC002 (At1g01720), NAC103 (At5g64060), OXI1 (At3g25250), RAD17 (At5g66130), SAG14 (At5g20230), SMR5 (At1g07500), SMR7 (At3g27630), SOG1 (At3g18780), SRO3 (At1g70440), ZAT12 (At5g59820). Germplasm used included crl (GABI_714_E08), arc6 (SAIL_693_G04), aos (SALK_017756), atm (SALK_092606), atr (SALK_032841), fad5-1 (N206), fad7-1 fad8-1 (N8036), sog1 (SALK_094099), the ex1 null mutant (SALK_002088), and ex2 (SALK_012127).
Supplemental Data
Supplemental Figure 1. TGAII and MYC2/3/4 target genes are upregulated in crl versus wild-type seedlings.
Supplemental Figure 2. Subsets of JA- and SA-responsive genes upregulated in crl versus wild-type seedlings.
Supplemental Figure 3. crl-driven JA accumulation induces cell cycle inhibitors.
Supplemental Figure 4. The loss of FAD5 partially rescues cell proliferation in the crl mutant.
Supplemental Figure 5. FAD5 resides mainly in the envelope membrane, and G142R mutation renders FAD5 unstable.
Supplemental Figure 6. Inactivation of FAD5 results in a substantial repression of stress- and detoxification-related genes in crl.
Supplemental Figure 7. Macroscopic, microscopic, and molecular phenotypes of wild-type, crl, and crl fad7 fad8 mutant plants.
Supplemental Figure 8. PSII efficiency in crl and crl fad5 under photoinhibitory stress conditions.
Supplemental Figure 9. Chloroplast ultrastructure in crl, fad5, and spcrl4 mutant plants.
Supplemental Table. List of primers used in this study.
Supplemental File. Statistical analysis tables.
Supplemental Data Set 1. List of genes upregulated in 4- and 5-d-old seedlings of crl as compared to the wild type WT.
Supplemental Data Set 2. Gene Ontology (GO) enrichment analysis of genes upregulated in crl versus wild-type seedlings.
Supplemental Data Set 3. List of stress-related genes upregulated in crl versus wild-type seedlings.
Supplemental Data Set 4. List of TGAII target stress-related genes upregulated in crl versus wild-type seedlings.
Supplemental Data Set 5. List of MYC2, 3, and 4 target stress-related genes upregulated in crl versus wild-type seedlings.
Supplemental Data Set 6. List of MeJA-responsive genes upregulated in crl versus wild-type seedlings.
Supplemental Data Set 7. List of SA-responsive genes upregulated in crl versus wild-type seedlings.
Supplemental Data Set 8. List of EX1-Dependent 1O2-responsive genes (EX1-SORGs) upregulated in crl versus wild-type seedlings.
Supplemental Data Set 9. List of core DNA damage-responsive genes upregulated in crl versus wild-type seedlings.
Supplemental Data Set 10. Genome sequencing data showing chromosome-wide single nucleotide polymorphisms (SNPs) in various genes and their allele frequency in spcrl4.
Supplemental Data Set 11. FAME profiles in the wild type, crl, crl fad5, and crl fad7 fad8 mutant plants.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
FAD7 Gramene: NCBI Gene ID: 544203
FAD7 Araport: NCBI Gene ID: 544203
Acknowledgments
We thank the Core Facility of Genomics and Bioinformatics in Shanghai Center for Plant Stress Biology (PSC) for carrying out the RNA-seq and data mining. We also thank the Core Facility of Cell Biology in PSC for training students for the use of various microscopes. This research was supported by the Strategic Priority Research Program from the Chinese Academy of Sciences (grant XDB27040102), the 100-Talent Program of the Chinese Academy of Sciences, and the National Natural Science Foundation of China (grant 31871397 to C.K.).
AUTHOR CONTRIBUTIONS
V.D. and C.K. designed the research; B.L., J.F., R.M.S., and V.D. conducted the experiments; B.L., J.F., V.D., and C.K. analyzed the data; H.Z., S.L., and R.L. analyzed RNA-seq and whole-genome sequencing data; B.L., J.F., V.D., and C.K. wrote the article. All authors reviewed the article.
References
- Alméras, E, Stolz, S, Vollenweider, S, Reymond, P, Mène-Saffrané, L., Farmer, E.E(2003). Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34: 205–216. [DOI] [PubMed] [Google Scholar]
- Apel, K, Hirt, H .(2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55: 373–399. [DOI] [PubMed] [Google Scholar]
- Asada, K.(2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano, T, Yoshioka, Y, Kurei, S, Sakamoto, W, Machida, Y; Sodmergen. (2004). A mutation of the CRUMPLED LEAF gene that encodes a protein localized in the outer envelope membrane of plastids affects the pattern of cell division, cell differentiation, and plastid division in Arabidopsis. Plant J. 38: 448–459. [DOI] [PubMed] [Google Scholar]
- Baruah, A., Simková, K., Apel, K., Laloi, C.(2009). Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol. Biol. 70: 547–563. [DOI] [PubMed] [Google Scholar]
- Birtic, S., Ksas, B., Genty, B., Mueller, M.J., Triantaphylidès, C., Havaux, M.(2011). Using spontaneous photon emission to image lipid oxidation patterns in plant tissues. Plant J. 67: 1103–1115. [DOI] [PubMed] [Google Scholar]
- Bruggeman, Q., Raynaud, C., Benhamed, M., Delarue, M.(2015). To die or not to die? Lessons from lesion mimic mutants. Front. Plant Sci. 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini, N.M., Steffes, K., Schläppi, M.R., Gifford, A.N., Greenberg, J.T.(2015). Arabidopsis AZI1 family proteins mediate signal mobilization for systemic defence priming. Nat. Commun. 6: 7658. [DOI] [PubMed] [Google Scholar]
- Chan, K.X., Mabbitt, P.D., Phua, S.Y., Mueller, J.W., Nisar, N., Gigolashvili, T., Stroeher, E., Grassl, J., Arlt, W., Estavillo, G.M., Jackson, C.J., Pogson, B.J.(2016b). Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc. Natl. Acad. Sci. USA 113: E4567–E4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, K.X., Phua, S.Y., Crisp, P., McQuinn, R., Pogson, B.J.(2016a). Learning the languages of the chloroplast: Retrograde signaling and beyond. Annu. Rev. Plant Biol. 67: 25–53. [DOI] [PubMed] [Google Scholar]
- Chini, A., Fonseca, S., Fernández, G., Adie, B., Chico, J.M., Lorenzo, O., García-Casado, G., López-Vidriero, I., Lozano, F.M., Ponce, M.R., Micol, J.L., Solano, R.(2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., Bent, A.F.(1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Cox, M.P., Peterson, D.A., Biggs, P.J.(2010). SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics 11: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro, S., Ksas, B., Havaux, M.(2018). Decoding β-cyclocitral-mediated retrograde signaling reveals the role of a detoxification response controlled by SCL14 and ANAC102 in plant tolerance to photooxidative stress. Plant Cell 30: 2495–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek, P., Auton, A., Abecasis, G., Albers, C.A., Banks, E., DePristo, M.A., Handsaker, R.E., Lunter, G., Marth, G.T., Sherry, S.T., McVean, G., Durbin, R.; 1000 Genomes Project Analysis Group (2011). The variant call format and VCFtools. Bioinformatics 27: 2156–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres Zabala, M., et al. (2015). Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 1: 15074. [DOI] [PubMed] [Google Scholar]
- Dietz, K.J., Turkan, I., Krieger-Liszkay, A.(2016). Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 171: 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V., Kim, C.(2019). Chloroplast protein homeostasis is coupled with retrograde signaling. Plant Signal. Behav. 14: 1656037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V., Kim, C.(2020). Singlet oxygen metabolism: From genesis to signaling. Front. Plant Sci. 10: 1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V., Duan, J., Lee, K.P., Kim, C.(2019a). Impaired PSII proteostasis triggers a UPR-like response in the var2 mutant of Arabidopsis. J. Exp. Bot. 70: 3075–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V., Duan, J., Lee, K.P., Lv, S., Liu, R., Kim, C.(2017). FtsH2-dependent proteolysis of EXECUTER1 is essential in mediating singlet oxygen-triggered retrograde signaling in Arabidopsis thaliana. Front. Plant Sci. 8: 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V., Li, M., Singh, S., Li, M., Kim, C.(2019b). Oxidative post-translational modification of EXECUTER1 is required for singlet oxygen sensing in plastids. Nat. Commun. 10: 2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J., et al. (2019). Impaired PSII proteostasis promotes retrograde signaling via salicylic acid. Plant Physiol. 180: 2182–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, S., Cruz, J.A., Imran, S.M., Chen, J., Kramer, D.M., Osteryoung, K.W.(2017). Variations in chloroplast movement and chlorophyll fluorescence among chloroplast division mutants under light stress. J. Exp. Bot. 68: 3541–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, E.E., Davoine, C.(2007). Reactive electrophile species. Curr. Opin. Plant Biol. 10: 380–386. [DOI] [PubMed] [Google Scholar]
- Farmer, E.E., Mueller, M.J.(2013). ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 64: 429–450. [DOI] [PubMed] [Google Scholar]
- Flores-Pérez, Ú., Jarvis, P.(2017). Isolation and suborganellar fractionation of Arabidopsis chloroplasts. Methods Mol. Biol. 1511: 45–60. [DOI] [PubMed] [Google Scholar]
- Fode, B., Siemsen, T., Thurow, C., Weigel, R., Gatz, C.(2008). The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell 20: 3122–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa, T., Curtis, M.J., Tominey, C.M., Duong, Y.H., Wilcox, B.W., Aggoune, D., Hays, J.B., Britt, A.B.(2010). A shared DNA-damage-response pathway for induction of stem-cell death by UVB and by gamma irradiation. DNA Repair (Amst.) 9: 940–948. [DOI] [PubMed] [Google Scholar]
- Ge, Y., Cai, Y.M., Bonneau, L., Rotari, V., Danon, A., McKenzie, E.A., McLellan, H., Mach, L., Gallois, P.(2016). Inhibition of cathepsin B by caspase-3 inhibitors blocks programmed cell death in Arabidopsis. Cell Death Differ. 23: 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux, M., Triantaphylidès, C., Genty, B.(2006). Autoluminescence imaging: A non-invasive tool for mapping oxidative stress. Trends Plant Sci. 11: 480–484. [DOI] [PubMed] [Google Scholar]
- Heilmann, I., Mekhedov, S., King, B., Browse, J., Shanklin, J.(2004). Identification of the Arabidopsis palmitoyl-monogalactosyldiacylglycerol delta7-desaturase gene FAD5, and effects of plastidial retargeting of Arabidopsis desaturases on the fad5 mutant phenotype. Plant Physiol. 136: 4237–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudik, E., et al. (2014). Chloroplast dysfunction causes multiple defects in cell cycle progression in the Arabidopsis crumpled leaf mutant. Plant Physiol. 166: 152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo, P., Venugopal, S.C., Navarre, D.A., Lapchyk, L., Kachroo, A.(2005). Role of salicylic acid and fatty acid desaturation pathways in ssi2-mediated signaling. Plant Physiol. 139: 1717–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katari, M.S., Nowicki, S.D., Aceituno, F.F., Nero, D., Kelfer, J., Thompson, L.P., Cabello, J.M., Davidson, R.S., Goldberg, A.P., Shasha, D.E., Coruzzi, G.M., Gutiérrez, R.A.(2010). VirtualPlant: A software platform to support systems biology research. Plant Physiol. 152: 500–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss, D., Bischof, S., Steiner, S., Apel, K., Meskauskiene, R.(2012). FLU, a negative feedback regulator of tetrapyrrole biosynthesis, is physically linked to the final steps of the Mg(++)-branch of this pathway. FEBS Lett. 586: 211–216. [DOI] [PubMed] [Google Scholar]
- Kim, C., Meskauskiene, R., Zhang, S., Lee, K.P., Lakshmanan Ashok, M., Blajecka, K., Herrfurth, C., Feussner, I., Apel, K.(2012). Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 24: 3026–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y., Schumaker, K.S., Zhu, J.K.(2006). EMS mutagenesis of Arabidopsis. Methods Mol. Biol. 323: 101–103. [DOI] [PubMed] [Google Scholar]
- Kleine, T., Leister, D.(2016). Retrograde signaling: Organelles go networking. Biochim. Biophys. Acta 1857: 1313–1325. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y., Imamura, S., Hanaoka, M., Tanaka, K.(2011). A tetrapyrrole-regulated ubiquitin ligase controls algal nuclear DNA replication. Nat. Cell Biol. 13: 483–487. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y., Kanesaki, Y., Tanaka, A., Kuroiwa, H., Kuroiwa, T., Tanaka, K.(2009). Tetrapyrrole signal as a cell-cycle coordinator from organelle to nuclear DNA replication in plant cells. Proc. Natl. Acad. Sci. USA 106: 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, N., et al. (2015). Functional conservation in the SIAMESE-RELATED family of cyclin-dependent kinase inhibitors in land plants. Plant Cell 27: 3065–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K.(1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Lee, K.P., Kim, C., Landgraf, F., Apel, K.(2007). EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104: 10270–10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Durbin, R. .(2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., Marth, G., Abecasis, G., Durbin, R.; 1000 Genome Project Data Processing Subgroup (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Brader, G., Palva, E.T.(2008). Kunitz trypsin inhibitor: An antagonist of cell death triggered by phytopathogens and fumonisin b1 in Arabidopsis. Mol. Plant 1: 482–495. [DOI] [PubMed] [Google Scholar]
- Lorrain, S., Vailleau, F., Balagué, C., Roby, D.(2003). Lesion mimic mutants: Keys for deciphering cell death and defense pathways in plants? Trends Plant Sci. 8: 263–271. [DOI] [PubMed] [Google Scholar]
- Mano, J.(2012). Reactive carbonyl species: Their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 59: 90–97. [DOI] [PubMed] [Google Scholar]
- McAndrew, R.S., Froehlich, J.E., Vitha, S., Stokes, K.D., Osteryoung, K.W.(2001). Colocalization of plastid division proteins in the chloroplast stromal compartment establishes a new functional relationship between FtsZ1 and FtsZ2 in higher plants. Plant Physiol. 127: 1656–1666. [PMC free article] [PubMed] [Google Scholar]
- Meskauskiene, R., Nater, M., Goslings, D., Kessler, F., op den Camp, R., Apel, K.(2001). FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98: 12826–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima, S.Y., Froehlich, J.E., Osteryoung, K.W.(2006). PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. Plant Cell 18: 2517–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, S., Hilbert, B., Dueckershoff, K., Roitsch, T., Krischke, M., Mueller, M.J., Berger, S.(2008). General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 20: 768–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, T., et al. (2007). Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71: 2095–2100. [DOI] [PubMed] [Google Scholar]
- Nomura, H., et al. (2012). Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 3: 926. [DOI] [PubMed] [Google Scholar]
- Pauwels, L., Morreel, K., De Witte, E., Lammertyn, F., Van Montagu, M., Boerjan, W., Inzé, D., Goossens, A.(2008). Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 105: 1380–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss, S.B., Britt, A.B.(2003). A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics 164: 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke, K.A., Rutherford, S.M., Robertson, E.J., Leech, R.M.(1994). arc6, a fertile Arabidopsis mutant with Only Two Mesophyll Cell Chloroplasts. Plant Physiol. 106: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel, F., Birtic, S., Ginies, C., Soubigou-Taconnat, L., Triantaphylidès, C., Havaux, M.(2012). carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 109: 5535–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel, F., Ksas, B., Akkari, E., Mialoundama, A.S., Monnet, F., Krieger-Liszkay, A., Ravanat, J.L., Mueller, M.J., Bouvier, F., Havaux, M.(2013). Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell 25: 1445–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, M.D., McCarthy, D.J., Smyth, G.K.(2010). edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger, K., Ossowski, S., Lanz, C., Juul, T., Petersen, A.H., Nielsen, K.L., Jørgensen, J.E., Weigel, D., Andersen, S.U.(2009). SHOREmap: Simultaneous mapping and mutation identification by deep sequencing. Nat. Methods 6: 550–551. [DOI] [PubMed] [Google Scholar]
- Shumbe, L., Chevalier, A., Legeret, B., Taconnat, L., Monnet, F., Havaux, M.(2016). Singlet oxygen-induced cell death in Arabidopsis under high-light stress is controlled by OXI1 kinase. Plant Physiol. 170: 1757–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimková, K., Kim, C., Gacek, K., Baruah, A., Laloi, C., Apel, K.(2012). The chloroplast division mutant caa33 of Arabidopsis thaliana reveals the crucial impact of chloroplast homeostasis on stress acclimation and retrograde plastid-to-nucleus signaling. Plant J. 69: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens, N.O., Galuschka, C., Schindler, M., Bülow, L., Hehl, R.(2004). AthaMap: An online resource for in silico transcription factor binding sites in the Arabidopsis thaliana genome. Nucleic Acids Res. 32: D368–D372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita, C., Kato, Y., Yoshioka, Y., Tsurumi, N., Iida, Y., Machida, Y., Sugita, M.(2012). CRUMPLED LEAF (CRL) homologs of Physcomitrella patens are involved in the complete separation of dividing plastids. Plant Cell Physiol. 53: 1124–1133. [DOI] [PubMed] [Google Scholar]
- Sun, H., Schneeberger, K.(2015). SHOREmap v3.0: Fast and accurate identification of causal mutations from forward genetic screens. Methods Mol. Biol. 1284: 381–395. [DOI] [PubMed] [Google Scholar]
- Swiatek, A., Lenjou, M., Van Bockstaele, D., Inzé, D., Van Onckelen, H.(2002). Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol. 128: 201–211. [PMC free article] [PubMed] [Google Scholar]
- Trapnell, C., Pachter, L., Salzberg, S.L.(2009). TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, J.G., Ellis, C., Devoto, A.(2002). The jasmonate signal pathway. Plant Cell 14 (Suppl): S153–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, D., Przybyla, D., Op den Camp, R., Kim, C., Landgraf, F., Lee, K.P., Würsch, M., Laloi, C., Nater, M., Hideg, E., Apel, K.(2004). The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306: 1183–1185. [DOI] [PubMed] [Google Scholar]
- Wang, L., Kim, C., Xu, X., Piskurewicz, U., Dogra, V., Singh, S., Mahler, H., Apel, K.(2016). Singlet oxygen- and EXECUTER1-mediated signaling is initiated in grana margins and depends on the protease FtsH2. Proc. Natl. Acad. Sci. USA 113: E3792–E3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y., Wang, J., Dehesh, K.(2013). Review of stress specific organelles-to-nucleus metabolic signal molecules in plants. Plant Sci. 212: 102–107. [DOI] [PubMed] [Google Scholar]
- Yi, D., et al. (2014). The Arabidopsis SIAMESE-RELATED cyclin-dependent kinase inhibitors SMR5 and SMR7 regulate the DNA damage checkpoint in response to reactive oxygen species. Plant Cell 26: 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama, K., Conklin, P.A., Huefner, N.D., Britt, A.B.(2009). Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc. Natl. Acad. Sci. USA 106: 12843–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann, B.(2019). Ultrastructure of plastids serves as reliable abiotic and biotic stress marker. PLoS One 14: e0214811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J., Zhang, X., Hong, Y., Liu, Y.(2016). Chloroplast in plant-virus interaction. Front. Microbiol. 7: 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B.B., Elledge, S.J.(2000). The DNA damage response: Putting checkpoints in perspective. Nature 408: 433–439. [DOI] [PubMed] [Google Scholar]
- Zhou, M., Wang, W., Karapetyan, S., Mwimba, M., Marqués, J., Buchler, N.E., Dong, X.(2015). Redox rhythm reinforces the circadian clock to gate immune response. Nature 523: 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]