Abstract
Background
This study aimed to establish a prediction model based on the maternal laboratory index score (Lab-score) for histologic chorioamnionitis (HCA) in patients with prelabor rupture of membranes (PROM) during late pregnancy.
Material/Methods
Sixty-nine cases of pregnant women with PROM were retrospectively analyzed. The general information and laboratory indicators were compared between the HCA (n=22) and non-HCA (n=47) groups. A multivariate logistic regression method was used to establish the prediction model. We plotted the receiver operating characteristic curve and calculated the area under the curve (AUC). The clinical effectiveness of each model was compared by decision curve analysis.
Results
Only C-reactive protein (CRP) in the laboratory index predicted HCA, but its diagnostic efficacy was not ideal (AUC=0.651). Then, we added CRP to the platelet/white blood cell count ratio and triglyceride level to construct the Lab-score. Based on the Lab-score, important clinical parameters, including body mass index, diastolic blood pressure, and preterm birth, were introduced to construct a complex joint prediction model. The AUC of this model was significantly larger than that of CRP (0.828 vs. 0.651, P=0.035), but not significantly different from that of Lab-score (0.828 vs. 0.724, P=0.120). Considering the purpose of HCA screening, the net benefit of the complex model was better than that of Lab-score and CRP.
Conclusions
The complex model based on Lab-score is useful in the clinical screening of high-risk populations with PROM and HCA during late pregnancy.
MeSH Keywords: C-Reactive Protein, Chorioamnionitis, Pregnancy
Background
Prelabor rupture of membranes (PROM), which is defined as the spontaneous rupture of the membranes before the onset of labor, causes a significant proportion of preterm births. It is divided into PROM (prelabor rupture of membranes) and PPROM (preterm PROM), based on the gestational stage when it occurs [1]. PROM frequently causes chorioamnionitis, one of the leading causes of preterm labor [2]. The delayed diagnosis and treatment of chorioamnionitis and its resulting infection are associated with an increased risk of maternal and infant inflammation, fetal hypoxia, and cerebral palsy. Therefore, the early detection of chorioamnionitis is critical in obstetrics [3,4]. Chorioamnionitis is divided into 2 types: clinical chorioamnionitis and histologic chorioamnionitis (HCA). The symptoms of clinical chorioamnionitis in pregnant women are usually fever or other symptoms of infection, such as tachycardia, elevated body temperature, and uterine tenderness [5]. However, HCA has no obvious early symptoms and is mainly diagnosed by pathological examination of the placenta. To date, there are no satisfactory clinical or laboratory indicators for the prenatal diagnosis of HCA [6–10].
Clinically, some maternal biomarkers have been used to predict chorioamnionitis, such as C-reactive protein (CRP), white blood cell count (WBC), procalcitonin (PCT), interleukin IL-6 (IL-6), interleukin IL-10, tumor necrosis factor-alpha, and granulocyte colony-stimulating factor [11,12]. Since WBC can be increased by pregnancy-associated physiological changes and is also affected by steroid administration, it has minimal value in HCA prediction [13,14]. PCT is an acute-phase reactant and marker for monocyte activity and is widely used to predict infection under various conditions [15], especially bacterial infection [16]. However, whether PCT can predict chorioamnionitis in pregnant women is controversial [17]. CRP is elevated in both infectious (viral and bacterial) and non-infectious diseases [18], but its value is limited because of its association with physiological changes during pregnancy [19]. Some studies have suggested that PCT is a better criterion for chorioamnionitis diagnosis compared to CRP and WBC [20,21], but some researchers believe maternal serum CRP is the criterion standard for the noninvasive identification of infection-related intraamniotic complications in cases of PROM [22,23].
We hypothesized that a prediction model of laboratory tests could accurately predict HCA caused by PROM in late pregnancy. To test this hypothesis, we retrospectively analyzed the general information and laboratory indicators of women with and without HCA and developed a prediction model. We evaluated the benefit of this prediction model in screening for PROM with HCA in late pregnancy to control delivery time and prevent complications.
Material and Methods
General information
This retrospective case-control study included 69 patients with PROM who were treated in the Department of Obstetrics and Gynecology of the Third Affiliated Hospital of Soochow University from March 2018 to June 2019. The clinical data of the patients are shown in Table 1. All patients included in this study had live births. The exclusion criteria were as follows: severe or chronic diseases including other pregnancy complications (such as gestational diabetes, preeclampsia, intrahepatic cholestasis, and placental abruption), cardiovascular disease, autoimmune disease, cancer, diabetes, kidney disease, infectious disease, hyperthyroidism, twin pregnancy, history of inherited diseases, and any structural abnormality of the heart, liver, lungs, and kidneys as found by ultrasound or electrocardiogram.
Table 1.
Comparison of admission general information and laboratory indicators between the two groups.
| Non-HCA group n=47 | HCA group n=22 | P-value | |
|---|---|---|---|
| Age (years) | 29.1±4.9 | 27.6±3.5 | 0.188 |
| BMI (kg/m2) | 27.9 (21.6–40.0) | 25.8 (20.1–29.7) | 0.008 |
| Systolic pressure (mmHg) | 122 (101–169) | 122 (100–128) | 0.169 |
| Diastolic pressure (mmHg) | 78 (60–106) | 73 (58–89) | 0.026 |
| Gestational week (weeks) | 38 (34+1–40+4) | 38 (35–40+2) | 0.400 |
| Premature delivery | 6 (13%) | 7 (32%) | 0.059 |
| Number of pregnancies | 2 (1–6) | 1 (1–7) | 0.244 |
| Number of deliveries | 0 (0–2) | 0 (0–2) | 0.698 |
| Membrane rupture time (hours) | 12 (2–68) | 10 (3–61) | 0.490 |
| Neonatal body weight (g) | 3261±458 | 3164±384 | 0.394 |
| Amniotic fluid volume (ml) | 500 (50–2000) | 500 (150–800) | 0.681 |
| Amniotic fluid status | 0.423 | ||
| Clear | 45 (96%) | 20 (91%) | |
| I–III | 2 (4%) | 2 (9%) | |
| Delivery method | 0.344 | ||
| Natural delivery | 37 (79%) | 15 (68%) | |
| Cesarean section | 10 (21%) | 7 (32%) | |
| Postpartum hemorrhage | 8 (17%) | 3 (14%) | 0.720 |
| Neonatal gender (male) | 22 (47%) | 13 (59%) | 0.342 |
| GBS* | 5 (14%) | 4 (25%) | 0.328 |
| Hb (g/L) | 115 (89–136) | 119 (92–134) | 0.435 |
| PLT (×109/L) | 204.0 (111.0–358.0) | 212.0 (99.0–278.0) | 0.701 |
| PLT/WBC | 23.2 (10.2–57.5) | 19.3 (12.4–41.8) | 0.105 |
| GLU (mmol/L) | 4.6 (2.9–8.4) | 4.5 (3.2–8.1) | 0.745 |
| TC (mmol/L) | 6.10 (4.19–9.03) | 6.40 (4.06–9.38) | 0.644 |
| TG (mmol/L) | 3.64 (1.62–9.02) | 3.43 (1.40–6.01) | 0.158 |
| CRP (mg/L) | 4.2 (0.6–11.3) | 4.7 (3.0–31.3) | 0.039 |
| WBC (×109/L) | 8.92 (4.54–13.77) | 8.75 (4.40–19.70) | 0.063 |
| N% (%) | 75.4 (62.6–86.4) | 76.7 (52.0–93.0) | 0.291 |
| PCT (ng/mL) | 0.040 (0.010–0.395) | 0.023 (0.010–0.089) | 0.098 |
The data are expressed as Mean±SD/Median (Min–Max)/N (%).
Indicates 17 missing cases.
GBS – group B Streptococcus; HB – hemoglobin; PLT – platelet count; WBC – white blood cell count; GLU – glucose; TC – total cholesterol; TG – triglycerides; CRP – C-reaction protein; N% – neutrophil percentage; PCT – procalcitonin.
The following indicators were recorded from all enrolled patients at admission: age, sex, gravidity, parity, blood pressure, weight, and vital signs including temperature, heart rate, and respiratory rate. All participants signed written informed consent, and the study was approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University.
Diagnosis
Most cases of PROM can be diagnosed based on patient medical history and physical examination. The diagnostic criteria for PROM [1] are as follows: patient complained of vaginal fluid or wet vulva; vaginal examination showed fluid flowing from the internal cervix or a liquid pool formed in the posterior fornix; ultrasound examination showed reduced amniotic fluid after the rupture of the membranes; and changes in vaginal pH (the normal vaginal pH ranges between 4.4 and 6.0, while the pH of amniotic fluid is 8.0 [24]).
No patients in our study received antibiotics prior to admission. After the PROM was confirmed, the patients were asked to rest in bed in a high hip position. Body temperature, pulse, heart rate, fetal heart rate, and vaginal secretions were measured, vaginal examinations were done, the uterus was checked for tenderness, and rectal contents were voided as necessary. After admission, patients were scrubbed daily to keep the vulva clean. If the time since membrane rupture was equal to 12 h or more, antibiotics were used to prevent infection. The antibiotic of choice was ampicillin, with a loading dose of 2 g IV, then 1 g IV every 4 h until delivery. For women with severe penicillin allergy, cefazolin was used, with a loading dose of 2 g IV, and then 1 g IV every 8 h until delivery. Most patients received rectal and vaginal tests for group B streptococci. Fetal heart rate monitoring and B-mode ultrasounds were performed regularly. Routine blood tests, CRP, and PCT were measured every 1 to 3 days.
The enrolled patients were pregnant women with PROM at ≥34 weeks of pregnancy; all patients were recommended to proceed with delivery. If the patient requested expectant treatment, we would carefully consider the options and discuss the benefits and risks with the patient. However, expectant management should not exceed 37 weeks [25]. If clinical chorioamnionitis and fetal distress were found, the recommendation was to immediately proceed with delivery, and the specific delivery method was determined by the obstetric situation.
The diagnostic criteria for clinical chorioamnionitis [6] are as follows: (1) maternal body temperature ≥38°C; (2) vaginal secretion with an abnormal smell; (3) increased fetal heart rate (≥160 beats/min) or increased maternal heart rate (≥100 beats/min); (4) maternal WBC ≥15×109/L; and (5) uterus irritation and tenderness. If the increased maternal body temperature was accompanied by any of the other symptoms (2–5), the patient was diagnosed with chorioamnionitis. The criterion standard for HCA diagnosis is the pathological examination of placental and fetal membrane showing ≥5 neutrophil infiltration in each high-magnification field under a microscope [6].
Laboratory testing
A Sysmex XN9000 analyzer (Hyogo, Japan) was used to perform routine prenatal blood examination, which included WBC (range, 5.8–20×109/L), neutrophil percentage (range, 64–89%), red blood cell count (range, 2.9–4.5×1012/L), hemoglobin (range, 83–136 g/L), and platelet count (PLT; range, 96–297×109/L). The Roche Cobas 8000 system (Indianapolis, IN, USA) was used to determine maternal serum PCT levels in the range of 0.020–100.00 ng/mL. CRP (0~10.0 mg/L) and other biochemical indicators, including total cholesterol (range, 4.09–8.63mmol/L) and triglycerides (TG; range, 1.34–4.03 mmol/L), were measured by a Beckman Coulter AU5800 analyzer (Brea, CA, USA). The criteria of the above laboratory indexes refer to the normal ranges of healthy late-term pregnant women [26,27]. Postnatal records included delivery method, neonatal sex, neonatal weight, Apgar scores at 1 min and 5 min, amniotic fluid volume, amniotic fluid status (clear, degree I, II, and III), and 24 h postnatal bleeding.
After delivery, samples of placenta and fetal membrane tissues measuring 3×3 cm were removed, fixed in 10% formaldehyde, embedded in paraffin, and stored at room temperature. The samples were sent to the pathology department for HCA diagnosis.
Statistical analysis
Continuous variables were expressed as mean±standard deviation (SD) or median (min–max), and categorical variables were expressed as frequencies (%). When appropriate, continuous variables were compared using unpaired t tests or Mann-Whitney nonparametric tests, and categorical variables were compared using the Pearson chi-square test and Fisher’s exact test. Multivariate logistic regression was used to establish the prediction model, and the minimum Akaike’s information criterion was used to select optimal model parameters and draw the nomogram and calibration curves. For each model, receiver operating characteristic (ROC) curves were drawn, and the area under the curve (AUC) of different models was compared using the DeLong method [28]. The Bootstrap resampling (times=500) method recommended by TRIPOD reporting specifications [12] was used to internally validate the model and calculate the 95% confidence interval of the AUC. Finally, a decision curve analysis was performed to quantify and compare the clinical effectiveness of all models. This method uses threshold probabilities to express the relative harm from false positives and false negatives. By subtracting the proportion of false positives from the proportion of true positives, and then weighing the relative harm of false positives and false negatives, we could get the quantification for net benefit. The following formula was used to calculate the net benefit of model-based decision:
Where n is the total number of patients in the study, and Pt is the given threshold of probability. All analyses were performed using R-3.4.3 (http://www.R-project.org; software packages: pROC, rms, dca.R). P<0.05 was considered statistically significant.
Results
A total of 69 pregnant women were included in the study, with ages ranging from 19 to 43 years (28.7±4.6), and a median gestational week of 38 (range, 34+1 to 40+4 weeks). Following delivery, 22 (31.9%) patients were diagnosed with HCA (HCA group), of which 8 (11.6%) were diagnosed with clinical chorioamnionitis. The remaining 47 (68.1%) patients did not have chorioamnionitis (non-HCA group). The comparison of general information and laboratory indicators between the 2 groups is shown in Table 1. The HCA group had a lower body mass index (BMI) and diastolic blood pressure (P=0.008 and 0.026, respectively), and higher CRP levels (P=0.039) compared with those of the non-HCA group.
We first drew ROC curves of HCA prediction based on the laboratory indicators. We found that only CRP was significant (AUC=0.651; 95% confidence interval, 0.501~0.802; sensitivity, 0.810; and specificity, 0.500) (Figure 1, Table 2). Next, we performed multivariate logistic regression analysis, which used HCA occurrence as the dependent variable and the laboratory indicators hemoglobin, WBC, PLT/WBC ratio, CRP, glucose, total cholesterol, TG, neutrophil percentage, and PCT as independent variables. From this, the Lab-score was calculated as follows: Lab-score=1.93388–0.09814×PLT/WBC–0.32428×TG+0.14708×CRP.
Figure 1.
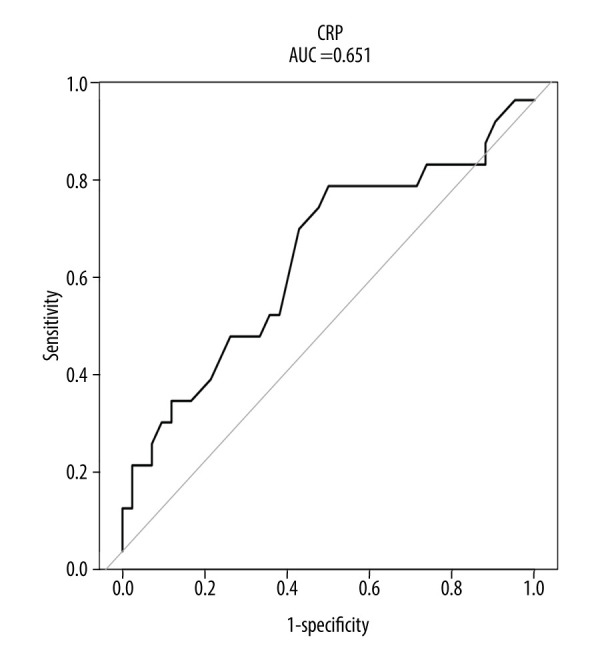
The receiver operating characteristic (ROC) curve of histologic chorioamnionitis (HCA) predicted by C-reactive protein (CRP).
Table 2.
Comparison of the diagnostic effects of the three models in predicting histological chorioamnionitis.
| Test | AUC | 95% CI low | 95% CI up | Best threshold | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| CRP | 0.651 | 0.501 | 0.802 | 4.150 | 0.500 | 0.810 |
| Lab-Score | 0.724 | 0.578 | 0.870 | −0.729 | 0.588 | 0.833 |
| Complex model | 0.828 | 0.701 | 0.956 | −0.030 | 0.882 | 0.722 |
AUC – area under the curve; CI – confidence interval; Lab-Score – laboratory index score.
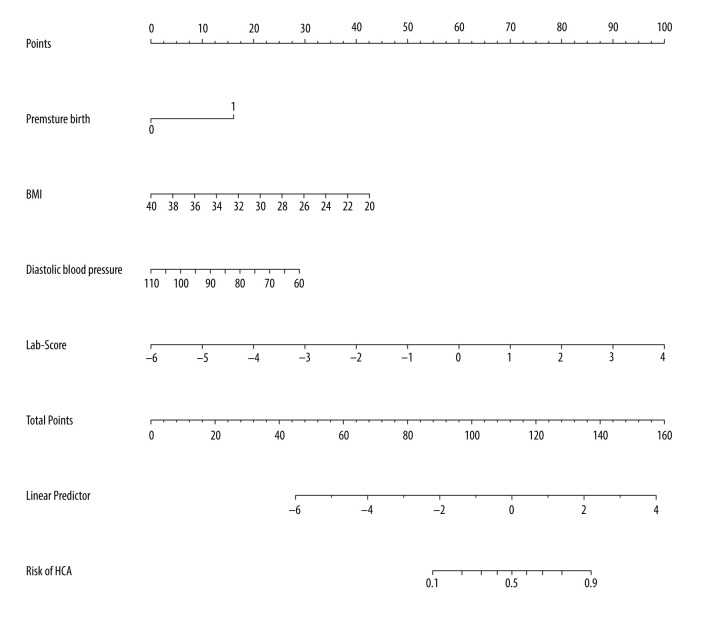
Based on the Lab-score, we introduced other clinical parameters including BMI, diastolic blood pressure, and premature delivery to conduct logistic regression analysis and build a complex joint prediction model (herein referred to as “complex model”). The AUC confidence interval and significance test of the complex model used a nonparametric repeated sampling method (Bootstrap resampling times=500). The complex model was calculated as follows: Logit (P)=15.72802+2.89525×(premature birth=1)–0.30453×BMI–0.10903×diastolic blood pressure+1.61265×Lab-score, where P is the probability of HCA. The nomogram and calibration curve of the complex model were drawn (Figures 2, 3), and there was a good consistency between the predicted and observed values. For patients with suspected HCA, we first calculated the respective points for premature birth, BMI, diastolic blood pressure, and Lab-score, and then added these points to obtain the total points, using the nomogram to assess the risk of HCA based on the total points. For example, a pregnant woman with PROM at 35 weeks of gestation (16 points), BMI of 26 (30 points), diastolic blood pressure of 75 mmHg (20 points), and Lab-score of 1 (70 points), would have 136 total points and an HCA risk of 0.88. The higher of risk value, the higher the probability of HCA.
Figure 2.
The nomogram of the complex model.
Figure 3.
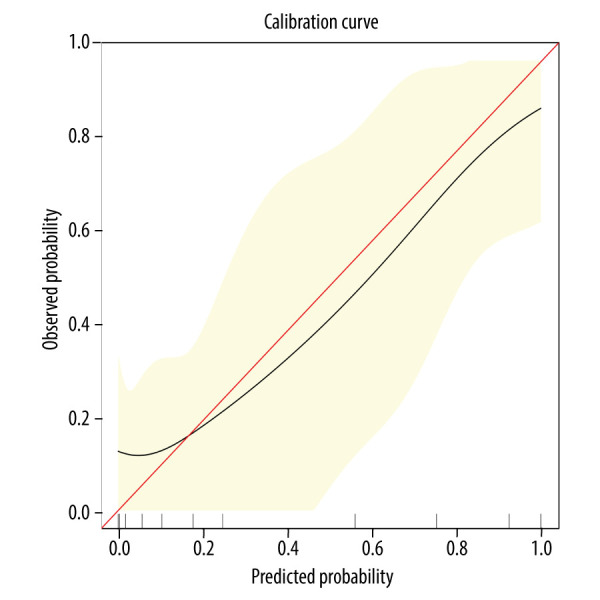
Calibration curve of the nomogram. The horizontal axis is the predicted incidence of histologic chorioamnionitis (HCA). The vertical axis is the observed incidence of HCA. The red diagonal line is the reference line, indicating that the predicted value is equal to the observed value. The black line is the calibration curve, and the yellow areas on both sides represent the 95% confidence interval.
The ROC curve was used to compare the diagnostic effect of the 3 models (Figure 4, Table 2). The AUC of the complex model was significantly greater than that of CRP (0.828 vs. 0.651, P=0.035); it was also greater than that of the Lab-score, but the difference was not significant (0.828 vs. 0.724, P=0.120). A further comparison of the sensitivity and specificity revealed that when the sensitivity of the complex model was the same as that of CRP (0.810) its specificity was better (0.735); and when the specificity of the complex model was the same as that of CRP (0.500), its sensitivity was better (0.912). Similarly, the complex model had better specificity (0.735) when its sensitivity was the same as that of the Lab-score (0.833) and better sensitivity (0.912) when its specificity was same as that of the Lab-score (0.588).
Figure 4.
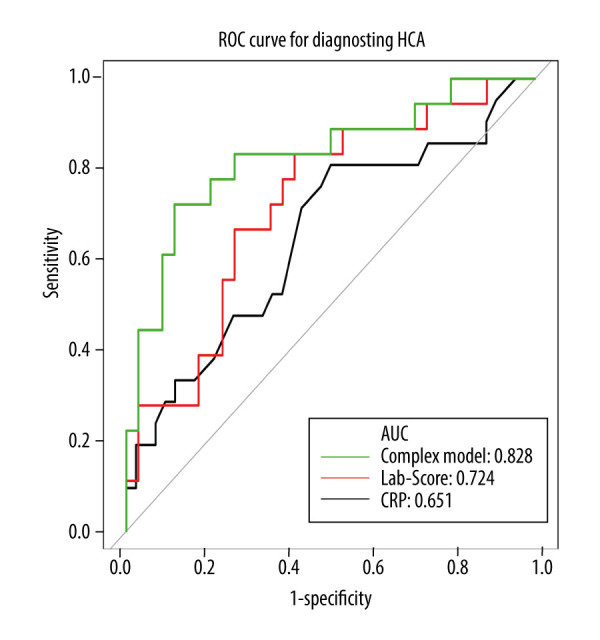
The receiver operating characteristic (ROC) curves of the 3 models in diagnosing histologic chorioamnionitis (HCA).
Since the AUC of the complex model was not significantly different from that of the Lab-score, we introduced a decision curve analysis method (Figure 5) to evaluate the performance of the different models. Considering the purpose of screening for HCA (sensitivity ≥0.833), the threshold probability was set between 0.35 and 0.75. As shown in Figure 5, the net benefit of the complex model was better than that of the Lab-score and CRP.
Figure 5.
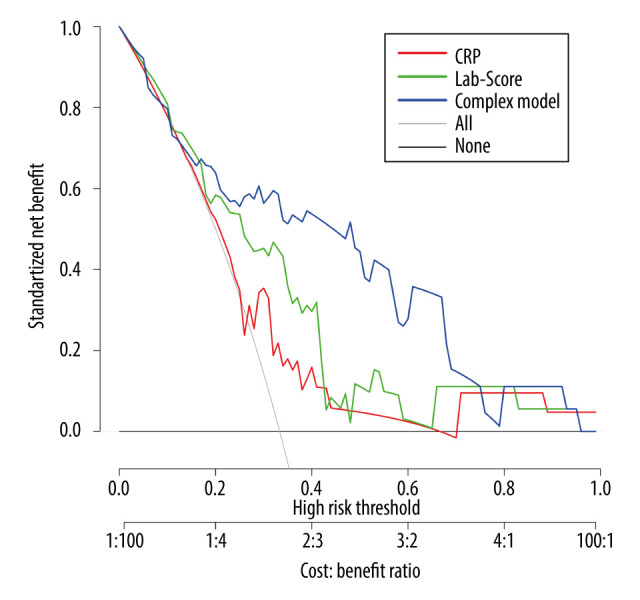
The decision curve analysis of the 3 models in predicting the correct diagnosis of histologic chorioamnionitis (HCA).
Discussion
Early diagnosis of chorioamnionitis and timely delivery can significantly reduce the complications of mother and child in cases of PROM. Chorioamnionitis is usually asymptomatic and lacks sensitivity or specificity in clinical symptoms. More than one-third of women with these symptoms do not have histological evidence of placental inflammation. Other diseases may also produce clinical signs similar to chorioamnionitis [29,30]. Therefore, it is important to find early identification markers of PROM with HCA to screen for high-risk patients, which can provide a basis for clinical decision-making.
Despite the advances in perinatal medicine, chorioamnionitis is still the leading cause of premature delivery, accounting for more than 70% of neonatal mortality in developed countries [31]. HCA can result in either premature delivery or PPROM [32]. Similarly, in our study, the premature delivery rate in the HCA group was significantly higher than that of the non-HCA group. Therefore, early detection of HCA in PROM is essential.
The weight gain during pregnancy reflects multiple characteristics including maternal fat accumulation, fluid swelling, and the development of the fetus, placenta, and uterus [33]. Proper weight gain during pregnancy is necessary for fetal health, and lower weight gain is associated with increased risk of adverse outcomes for the mother and newborn [34]. In the present study, the average BMI before delivery was lower in the HCA group than in the non-HCA group and it was a protective factor in the model, indicating that an appropriate increase in body weight before delivery was associated with a lower incidence of HCA.
We found that the diastolic blood pressure in the HCA group was significantly lower than that in the non-HCA group, and diastolic blood pressure was an independent protective factor in the complex model. Recent studies have suggested that infections related to anti-inflammatory reactions will lead to a reduction in diastolic blood pressure [35]. Compared to the babies born after idiopathic premature delivery or PROM, the babies born from hypertensive mothers have a lower risk of brain damage [36]. Our findings also suggest that the inflammation caused by PROM may cause vasodilation and reduced diastolic blood pressure.
Some studies have suggested that the PLT/WBC ratio and PLT level were significantly increased in HCA patients; therefore, PLT/WBC ratio and PLT are sensitive biomarkers that can distinguish HCA patients from non-HCA patients in premature delivery [2]. However, in the current study, the PLT level of the HCA group was higher than that of the non-HCA group, but the difference was not significant. Moreover, the PLT/WBC ratio was lower in the HCA group, which is contrary to the previous results. Because the population of the above-mentioned study included patients with preterm delivery, and our study included both preterm and full-term delivery, this discrepancy may have led to the difference in results.
The changes in progesterone and estrogen levels during pregnancy can cause changes in maternal blood lipid levels [27]. All lipid components increase gradually, and some components can increase by 2-fold. These metabolic changes are essential to support the development of the fetus [37]. Our results showed that the TG level was higher in the non-HCA group than in the HCA group and was an independent protective factor.
CRP is an acute reaction protein, which is produced and released into blood circulation after infection or tissue damage [38]. In the early stages of chorioamnionitis, infection and inflammation are limited to the chorion and amniotic membrane, and IL-6 is released from the membrane to the maternal blood circulation, which causes the mother to secrete CRP. Therefore, CRP is an indirect indicator of IL-6 secretion and can be used as a screening biomarker for chorioamnionitis [39]. Studies have shown that the maternal serum PCT and WBC of women with PPROM are not accurate predictors for chorioamnionitis, whereas CRP levels are more reliable and can be used for diagnosis [17]. In our study, CRP levels were higher in the HCA group than in the non-HCA group, and prenatal CRP was an independent risk factor for HCA.
In the present study, the diagnostic efficacy of CRP was not ideal. Therefore, we combined CRP, PLT/WBC, and TG level to construct the Lab-score, which included all commonly used laboratory indicators. Based on the Lab-score, we introduced other meaningful clinical parameters including BMI, diastolic blood pressure, and premature birth and built a complex model. The complex model was better at prediction than were the Lab-score and prenatal CRP, and its advantage was more significant for the purpose of HCA screening. The nomogram of the complex model can make it easy for clinicians to assess the risk of HCA.
This study had several limitations. First, the sample size was small, and subsequent studies are needed to increase the sample size and further explore the diagnostic values of different indicators. Second, the analysis on preterm membrane rupture and full-term membrane rupture were not stratified. Finally, this study is based on the Chinese Han population and therefore requires external validation to verify the applicability of the model.
Conclusions
CRP was the only laboratory indicator that could predict HCA, but its prediction efficacy was not ideal. The Lab-score improved the diagnostic performance; however, the complex model built on the Lab-score showed the most benefit for patient screening purposes. This prediction model can help screen the high-risk population with HCA caused by PROM in late pregnancy.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Changzhou Science and Technology Program [grant no. CJ20180022]
References
- 1.Kuba K, Bernstein PS. ACOG practice bulletin No. 188: Prelabor rupture of membranes. Obstet Gynecol. 2018;131(6):1163–64. doi: 10.1097/AOG.0000000000002663. [DOI] [PubMed] [Google Scholar]
- 2.Qiu L, Pan M, Zhang R, Ren K. Maternal peripheral blood platelet-to-white blood cell ratio and platelet count as potential diagnostic markers of histological chorioamnionitis-related spontaneous preterm birth. J Clin Lab Anal. 2019;33:e22840. doi: 10.1002/jcla.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilir F, Akdemir N, Ozden S, et al. Increased serum procalcitonin levels in pregnant patients with asymptomatic bacteriuria. Ann Clin Microbiol Antimicrob. 2013;12:25. doi: 10.1186/1476-0711-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canpolat FE, Yigit S, Korkmaz A, et al. Procalcitonin versus CRP as an early indicator of fetal infection in preterm premature rupture of membranes. Turk J Pediatr. 2011;53:180–86. [PubMed] [Google Scholar]
- 5.Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. 1993;36:795–808. doi: 10.1097/00003081-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: From pathogenesis to treatment. Clin Microbiol Infect. 2011;17:1304–11. doi: 10.1111/j.1469-0691.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- 7.Practice bulletins No. 139: premature rupture of membranes. Obstet Gynecol. 2013;122:918–30. doi: 10.1097/01.AOG.0000435415.21944.8f. [DOI] [PubMed] [Google Scholar]
- 8.Nasef N, Shabaan AE, Schurr P, et al. Effect of clinical and histological chorioamnionitis on the outcome of preterm infants. Am J Perinatol. 2013;30:59–68. doi: 10.1055/s-0032-1321501. [DOI] [PubMed] [Google Scholar]
- 9.Kim CJ, Romero R, Chaemsaithong P, et al. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29–52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishman SG, Gelber SE. Evidence for the clinical management of chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:46–50. doi: 10.1016/j.siny.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015;451:46–64. doi: 10.1016/j.cca.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Cetin O, Dokurel Cetin I, Uludag S, et al. Serial ultrasonographic examination of the fetal thymus in the prediction of early neonatal sepsis in preterm premature rupture of membranes. Gynecol Obstet Invest. 2014;78:201–7. doi: 10.1159/000364871. [DOI] [PubMed] [Google Scholar]
- 13.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–54. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang J, Streitman D. Physiologic adaptations to pregnancy. Neurol Clin. 2012;30:781–89. doi: 10.1016/j.ncl.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Bell SG. Procalcitonin and neonatal sepsis: Is this the biomarker we are looking for? Neonatal Netw. 2017;36:380–84. doi: 10.1891/0730-0832.36.6.380. [DOI] [PubMed] [Google Scholar]
- 16.Samsudin I, Vasikaran SD. Clinical utility and measurement of procalcitonin. Clin Biochem Rev. 2017;38:59–68. [PMC free article] [PubMed] [Google Scholar]
- 17.Thornburg LL, Queenan R, Brandt-Griffith B, Pressman EK. Procalcitonin for prediction of chorioamnionitis in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2016;29:2056–61. doi: 10.3109/14767058.2015.1077224. [DOI] [PubMed] [Google Scholar]
- 18.Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–17. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 19.Musilova I, Kacerovsky M, Stepan M, et al. Maternal serum C-reactive protein concentration and intra-amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One. 2017;12:e0182731. doi: 10.1371/journal.pone.0182731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asadi N, Faraji A, Keshavarzi A, et al. Predictive value of procalcitonin, C-reactive protein, and white blood cells for chorioamnionitis among women with preterm premature rupture of membranes. Int J Gynaecol Obstet. 2019;147:83–88. doi: 10.1002/ijgo.12907. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal A, Pahwa S. Evaluation of the role of CRP as an early predictor of chorioamnionitis in PPROM. Int J Reprod Contracept Obstet Gynecol. 2018;7:1351–56. [Google Scholar]
- 22.van de Laar R, van der Ham DP, Oei SG, et al. Accuracy of C-reactive protein determination in predicting chorioamnionitis and neonatal infection in pregnant women with premature rupture of membranes: A systematic review. Eur J Obstet Gynecol Reprod Biol. 2009;147:124–29. doi: 10.1016/j.ejogrb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Trochez-Martinez RD, Smith P, Lamont RF. Use of C-reactive protein as a predictor of chorioamnionitis in preterm prelabour rupture of membranes: A systematic review. BJOG. 2007;114:796–801. doi: 10.1111/j.1471-0528.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 24.Adama van Scheltema PN, In’t Anker PS, Vereecken A, et al. Biochemical composition of fluids for amnioinfusion during fetoscopy. Gynecol Obstet Invest. 2008;66:227–30. doi: 10.1159/000147168. [DOI] [PubMed] [Google Scholar]
- 25.Mercer BM, Crocker LG, Boe NM, Sibai BM. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: A randomized trial. Am J Obstet Gynecol. 1993;169:775–82. doi: 10.1016/0002-9378(93)90004-3. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Yang M, Zhou Y, et al. Establishment of reference intervals for procalcitonin in healthy pregnant women of Chinese population. Clin Biochem. 2017;50:150–54. doi: 10.1016/j.clinbiochem.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y, Lu J, Jin H, et al. Reference intervals for biochemical, haemostatic and haematological parameters in healthy Chinese women during early and late pregnancy. Clin Chem Lab Med. 2018;56:973–79. doi: 10.1515/cclm-2017-0804. [DOI] [PubMed] [Google Scholar]
- 28.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 29.Howman RA, Charles AK, Jacques A, et al. Inflammatory and haematological markers in the maternal, umbilical cord and infant circulation in histological chorioamnionitis. PLoS One. 2012;7:e51836. doi: 10.1371/journal.pone.0051836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su H, Chang SS, Han CM, et al. Inflammatory markers in cord blood or maternal serum for early detection of neonatal sepsis-a systemic review and meta-analysis. J Perinatol. 2014;34:268–74. doi: 10.1038/jp.2013.186. [DOI] [PubMed] [Google Scholar]
- 31.Galinsky R, Polglase GR, Hooper SB, et al. The consequences of chorioamnionitis: Preterm birth and effects on development. J Pregnancy. 2013;2013 doi: 10.1155/2013/412831. 412831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blume HK, Li CI, Loch CM, Koepsell TD. Intrapartum fever and chorioamnionitis as risks for encephalopathy in term newborns: A case-control study. Dev Med Child Neurol. 2008;50:19–24. doi: 10.1111/j.1469-8749.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 33.Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol. 2015;30:1141–52. doi: 10.1007/s10654-015-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voerman E, Santos S, Inskip H, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321:1702–15. doi: 10.1001/jama.2019.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González-Fernández D, Pons EDC, Rueda D, et al. Identification of high-risk pregnancies in a remote setting using ambulatory blood pressure: The MINDI cohort. Front Public Health. 2020;8:86. doi: 10.3389/fpubh.2020.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ancel PY, Marret S, Larroque B, et al. Are maternal hypertension and small-for-gestational age risk factors for severe intraventricular hemorrhage and cystic periventricular leukomalacia? Results of the EPIPAGE cohort study. Am J Obstet Gynecol. 2005;193:178–84. doi: 10.1016/j.ajog.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 37.Farias DR, Franco-Sena AB, Vilela A, et al. Lipid changes throughout pregnancy according to pre-pregnancy BMI: Results from a prospective cohort. BJOG. 2016;123:570–78. doi: 10.1111/1471-0528.13293. [DOI] [PubMed] [Google Scholar]
- 38.Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res. 2013;56:131–42. doi: 10.1007/s12026-013-8384-0. [DOI] [PubMed] [Google Scholar]
- 39.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7:169–77. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]