Abstract
Background
Contrast-enhanced spectral mammography (CESM) is digital mammography with contrast agent. This promising new breast imaging method can be used for planning surgical treatment. This study compared CESM versus digital mammography (MG) in evaluating tumor size in breast cancer.
Material/Methods
Comparison of tumor dimensions in CESM, MG, and histopathology was made. The correlation of these data was assessed by histopathological type, biological subtype, grading of the carcinoma, and patient age.
Results
The average difference in tumor size between CESM and histopathological examination was 5 mm. The differences in size measurement between CESM and MG were significant (p=0.00). The Pearson’s linear correlation coefficients of CESM versus HP and MG versus HP were −0.01 (p=0.79) and −0.25 (p=0.00), respectively, indicating no differences between CESM and HP based on the lesion size. A weak negative correlation between those values was observed on MG. No relationship was found between the tumor size in CESM and the biological subtype, carcinoma malignancy degree, or patient age.
Conclusions
CESM is a new diagnostic method in breast cancer. The accuracy of measurement of tumor size using CESM is independent of lesion size, but it overestimates the size by 5 mm on average. The difference is not dependent on grading, biological subtype of the carcinoma, or patient age. They concern the histopathological type, and values are significantly greater in pre-invasive carcinomas.
MeSH Keywords: Breast Neoplasms; Mammography; Pathology, Surgical
Background
Breast cancer is the most common malignant tumor diagnosed in women worldwide. In Poland, it accounts for 22.4% of new malignancy cases among women. Much concern is raised by the growing morbidity trend in premenopausal women (ages 20–49 years), which is responsible for an almost 2-fold increase in the morbidity rate in this age group over the last 3 decades [1,2].
Among numerous imaging methods, mammography is the most useful technique for detecting focal lesions in breast glands recommended by EUSOBI (European Society of Breast Imaging). However, the overall sensitivity and specificity of the examinations is only 62–68% in patients with dense breasts [3–7]. The recently developed contrast-enhanced spectral mammography (CESM) is intended to obtain a more visible image of the tumor through the injection of a contrast agent filling the neoplastic region and the surrounding intercellular space. CESM, also known as dual-energy contrast-enhanced spectral mammography, generates images of low and high energy during a single, short compression upon injection of a contrast agent [8]. The choice between the local or systemic treatment method for breast cancer in the particular stages of the disease is based on clinical and pathomorphological assessment, with consideration of the histological type and grading of carcinoma, ER/PgR and Ki67 expressions, HER2 status, progression of the primary tumor and regional lymph nodes, the presence and extent of metastases in distant organs, menopausal status, age, fitness status, past and concomitant disease and related treatment, and the patient’s preferences [9–12]. The choice between breast-conserving surgical treatment and mastectomy is largely dependent on the size of the tumor and exclusion of multifocality of the neoplastic lesions. Precise planning of the type of surgical procedures is of crucial importance for the treatment results and directly translates into a limited number of local recurrences. Besides magnetic resonance imaging (MRI), the most useful imaging procedure that best reflects the size of the tumor is mammography [13–15].
The objective of our study was to evaluate the relevance of contrast-enhanced spectral mammography (CESM) in comparison with conventional digital mammography (MG) in assessing the size of a neoplastic tumor by comparing the size of the neoplastic lesion estimated on MG and CESM with the size of this lesion measured in postoperative histopathological examination.
Material and Methods
The study protocol required no consent from the Committee for Bioethics at the Medical University of Silesia, which was confirmed in writing. All the test procedures were carried out in compliance with the ethical principles of the 1964 Helsinki Declaration and its subsequent amendments.
The retrospective study analyzed 668 patients with initially operable breast cancer who had been operated on between Jan 2013 and Jan 2019 in the Clinic of Oncological Surgery, Prof. Kornel Gibiński Independent Public Central Clinical Hospital, Medical University of Silesia in Katowice (Poland). The age distribution for the patients analyzed was not of a normal nature, with the minimum age in the sample being 29 years and the maximum 91 years (median, 65 years). The lesion sizes in the HP examination (whose distribution was not of a normal nature either) did not exceed 120 mm (median, 18 mm). All the patients included in the study underwent preoperative diagnostics at the Hospital Outpatient Clinic of Oncological Surgery, including: medical history, clinical examination, imaging tests, and core-needle biopsy (CNB). Each female patient with CNB-diagnosed breast cancer had digital mammography performed, and 661 women (661/668, 99%) additionally underwent contrast-enhanced spectral mammography (CESM). The lesion size was measured on MG and CESM and then compared with the lesion size measured on the basis of a postoperative histopathological examination.
An examination was made of the difference in lesion size between the MG results and the HP result for 668 patients, and 661 patients had this difference determined between the CESM result and the HP result.
These differences were also analyzed in the subgroups, including:
-
histopathological type of carcinoma diagnosed by core-needle biopsy (CNB):
No special type (NST) carcinoma
Infiltrating lobular carcinoma;
Special subtype of infiltrating carcinoma;
Infiltrating ducto-lobular carcinoma;
Ductal carcinoma in situ (DCIS) High Grade;
DCIS Low Grade;
Lobular carcinoma in situ (LCIS) – pleomorphic type.
grading (G): 1, 2, 3.
-
biological subtype:
Luminal A;
Luminal B (HER2-negative);
Luminal B (HER2-positive);
HER2-positive (non-luminal);
triple-negative (ductal).
Imaging procedures
All CESM examinations were performed and assessed in the Mammography Laboratory in our center. All the MGs performed in our center and those performed outside were assessed by consultant radiologists having at least 15 years of experience in the diagnosis of breast diseases. Prior to the qualification for CESM, all the patients filled out a survey, which was used as the basis for eliminating those with a risk of allergic reactions and potential pregnancy. Each patient also had her creatinine and GFR levels assessed. All CESM examinations were carried out with a digital mammography device dedicated to perform dualenergy CESM acquisitions (SenoBright, GE Healthcare).
An intravenous injection of 1.5 ml/kg of body mass of non-ionic contrast agent was performed using a power injector at a rate of 3 ml/s with a bolus chaser of 30 ml of saline. In CESM mode, the device automatically performed a pair of exposures (low- and high-energy) in each view. Specific image processing of low-energy and high-energy images was done to obtain subtraction images to highlight contrast enhancement and suppress structured noise due to fibroglandular breast tissue [16]. The total examination time was usually 10 min. On mammography (FFDM) and on CESM in CC and MLO projection, 3 tumor measurements were made, and only the largest tumor was included in the statistical analysis. After examination, the patients were observed for about 30 min for the appearance of any adverse reactions.
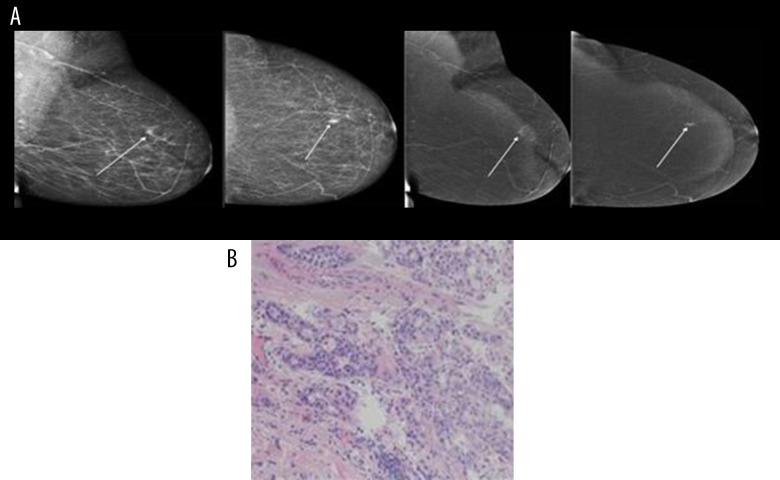
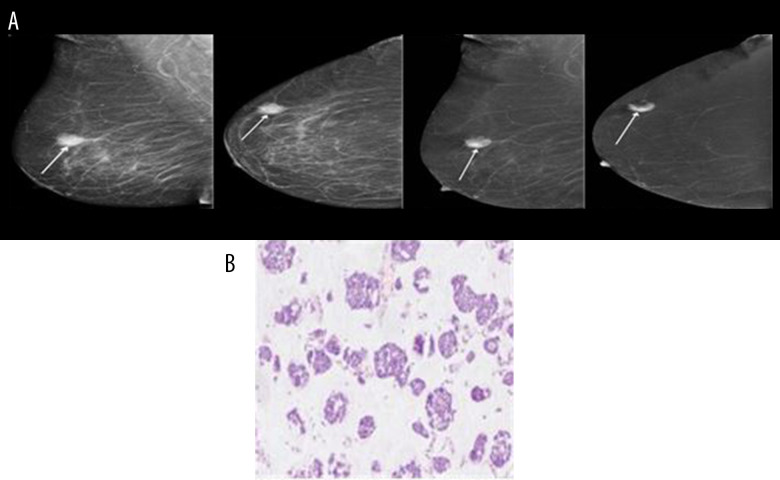
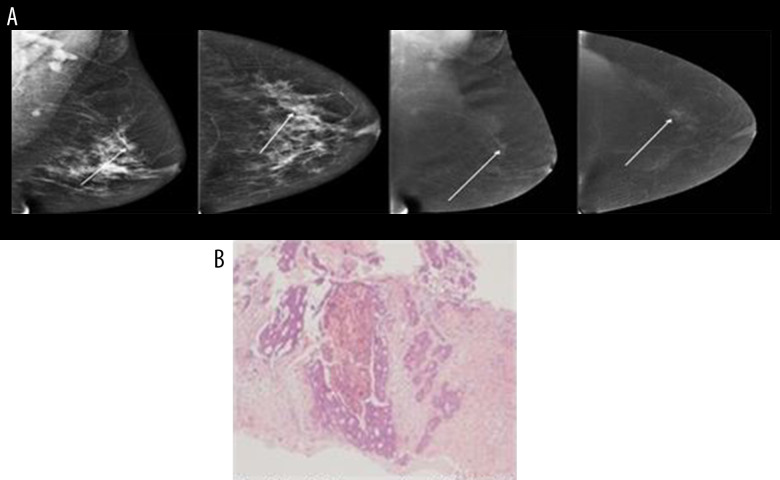
Below are shown images of carcinoma obtained from CESM along with postoperative histopathological verifications: NST GII (Figure 1A, 1B), mucinous carcinoma (Figure 2A, 2B) and DCIS high-grade (Figure 3A, 3B).
Figure 1.
(A, B) NST GII.
Figure 2.
(A, B) Mucinous carcinoma.
Figure 3.
(A, B) DCIS high grade.
Histopathological examination
The histopathological examination was conducted in the Histopathology Laboratory in our center. The greatest dimension of the tumor necessary for determining the T descriptor in the pTNM classification, besides the macroscopic measurement, was verified histopathologically by means of a microscope and use of cellSens Dimension® software by Olympus from 2013. Tumors up to 2 cm were excised in whole, serially, on a cross-sectional basis, with a margin of 0.2 to 0.4 cm and embedded in a paraffin block, after each cross-section. Tumors measuring over 2 cm and not fitting within a single paraffin block were divided into 2 or more parts by making parallel cuts of the lesion. Next, they were marked in pairs with ink of the same color and the individual layers were given numbers to allow finding the entire largest section of the tumor. The T value of the tumor was the total of the parallel measurements of the particular parts of the lesion. In the case of multifocal breast cancers, account was taken of the maximum dimension of the largest invasive tumor.
Surgical treatment
Surgeries were performed in a total of 668 patients and included the following procedures:
Madden-type radical mastectomy – 113 (17%);
Wide local excision (WLE) with sentinel lymph node biopsy (SLNB) – 299 (44.7%);
Wide local excision (WLE) with axillary lymph node dissection (ALND) – 66 (9.8%);
Total (simple) mastectomy – 9 (1.3%);
Total (simple) mastectomy with SLNB – 138 (20.6%);
Subcutaneous mastectomy with SLNB – 4 (0.6%);
WLE – 39 (5.3%).
Statistical analysis
The comparison of the neoplastic lesion size on MG and CESM with the postoperative HP examination was made for the total number of patients and in the subgroups divided by carcinoma subtype and grading (G). For all statistical tests conducted during the analysis, the level of significance was set at 5%. We also examined whether the average results obtained in each of the measurement methods differed significantly from one another. Next, the difference was determined for each female patient between the size of the neoplastic lesion measured by each tested method and it was examined whether the obtained values differ significantly. The next stage involved determining the average values of differences in the above-mentioned subgroups (CNB, grading, and biological subtype). After analyzing the results obtained in the classification subgroups, the dependence of these parameters on patient age and HP results was checked.
Results
In our study the tumor size in HP ranged from 2 to 120 mm (median, 18 mm), in MG from 4 to 121 mm (median, 19 mm), and in CESM from 5 to 123 mm (median, 22 mm).
The CNB performed in the study group diagnosed 406 NST carcinomas with tumor size 2 mm to 120 mm (median, 18 mm), 83 infiltrating lobular carcinomas (ILC) with tumor size 7 mm to 60 mm (median, 20 mm), 56 infiltrating carcinomas of special subtype (SSIC) with tumor size 7 mm to 75 mm (median, 20 mm), 25 infiltrating ductolobular carcinomas (ID-LC) with tumor size 4 mm to 80 mm (median, 22 mm), 67 DCIS-HG with tumor size 2 mm to 50 mm (median, 15 mm), 27 DCIS-LG with tumor size 6 mm to 60 mm (median, 15 mm), and 4 LCIS of pleomorphic subtype with tumor size 5 mm to 21 mm (median, 9 mm).
We obtained data on differences between the descriptive statistics for the differences in the measured values (Delta) between the HP measurement and those obtained from MG and CESM from raw values (mm) and percentage values.
The results of comparison of the average values indicate the presence of significant differences between them, which means that the size of the neoplastic lesions observed vary depending on the method used (MG, CESM, or HP measurement).
In the examined samples, the mean deviation in size from the HP dimension ranged from 0.56 mm for MG to 5.19 mm for CESM. The comparison of the average values and medians of these deviations indicates that the lesion size is overestimated in both of these measurement methods, with the overestimation value in CESM being significantly higher.
In accordance with the description of the method, the average values were determined for the differences between DeltaMG, DeltaCESM, DeltaMG%, and DeltaCESM% versus the HP measurement for the pre-defined subgroups (carcinoma type in CNB, grading, biological subtype). After conducting detailed tests, it was concluded that significant differences existed only for the DCIS-HG group (p<0.006). In the remaining cases no significant differences were found between the groups. The minimum values for the differences between HP and the mammography results were obtained in the special carcinoma subtypes group (Table 1).
Table 1.
Values of the differences between the HP measurement and the mammography results for the particular groups divided by the CNB-based histopathological type of carcinoma in mm and% of the HP value.
| CNB | DeltaMG | DeltaCESM | DeltaMG% | DeltaCESM% |
|---|---|---|---|---|
| NST | 0.10 | 4.66 | 5.05 | 29.52 |
| Infiltrating lobular carcinoma (ILC) | 0.24 | 5.71 | 6.71 | 34.35 |
| Special subtypes of infiltrating carcinoma (SSIC) | 0.02 | 1.31 | 1.71 | 5.63 |
| Infiltrating ducto-lobular carcinoma (ID-LC) | 0.32 | 3.63 | 9.62 | 23.41 |
| DCIS-HG | 5.00 | 11.18 | 51.40 | 87.59 |
| DCIS-LG | −1.38 | 6.93 | 6.91 | 47.45 |
| LCIS (pleomorphic type) | 4.25 | 2.75 | 97.74 | 36.13 |
The analysis of the data summarized in Table 2 show significant differences between the DCIS-HG group and the other groups, as well as, depending on the method (i.e., MG or CESM), between the DCIS-LG group for MG and the infiltrating ductolobular carcinoma group for CESM (Tables 2, 3).
Table 2.
The significance degree of the difference values between the average DeltaMG values in the particular carcinoma groups divided according to CNB.
| CNB | NST | ILC | SSIC | ID-LC | DCIS-HG | DCIS-LG | LCIS |
|---|---|---|---|---|---|---|---|
| NST | 1.0000 | 1.0000 | 1.0000 | 0.0001 | 0.8954 | 0.8704 | |
| ILC | 1.0000 | 1.0000 | 1.0000 | 0.0036 | 0.9472 | 0.8725 | |
| SSIC | 1.0000 | 1.0000 | 1.0000 | 0.0138 | 0.9518 | 0.8844 | |
| ID-LC | 1.0000 | 1.0000 | 1.0000 | 0.2028 | 0.9516 | 0.9311 | |
| DCIS-HG | 0.0001 | 0.0036 | 0.0138 | 0.2028 | 0.0042 | 1.0000 | |
| DCIS-LG | 0.8954 | 0.9472 | 0.9518 | 0.9516 | 0.0042 | 0.6631 | |
| LCIS | 0.8704 | 0.8725 | 0.8844 | 0.9311 | 1.0000 | 0.6631 |
Table 3.
The significance degree of the difference values between the average DeltaCESM values in the particular carcinoma groups divided according to CNB.
| CNB | NST | ILC | SSIC | ID-LC | DCIS-HG | DCIS-LG | LCIS |
|---|---|---|---|---|---|---|---|
| NST | 0.9543 | 0.3042 | 0.9997 | 0.0001 | 0.8287 | 0.9999 | |
| ILC | 0.9543 | 0.1617 | 0.9743 | 0.0446 | 0.9943 | 0.9975 | |
| SSIC | 0.3042 | 0.1617 | 0.9663 | 0.0000 | 0.1713 | 1.0000 | |
| ID-LC | 0.9997 | 0.9743 | 0.9663 | 0.0486 | 0.8688 | 1.0000 | |
| DCIS-HG | 0.0001 | 0.0446 | 0.0000 | 0.0486 | 0.7407 | 0.7192 | |
| DCIS-LG | 0.8287 | 0.9943 | 0.1713 | 0.8688 | 0.7407 | 0.9822 | |
| LCIS | 0.9999 | 0.9975 | 1.0000 | 1.0000 | 0.7192 | 0.9822 |
According to biological subtype of cancer (Luminal A vs. Luminal B vs. Non-Luminal vs. Triple-Negative) and according to grading G1 vs. G2 vs. G3) no significant differences were found between the groups. No significant relationship was found in verification as to whether the DeltaMG and DeltaCESM values correlate with the patient’s age.
To determine the causes of the differences found, Pearson’s linear correlation coefficient was calculated between the size of the pathological lesion measured in HP and the differences from this value for both mammography measurements. We found that the results differed depending on the mammography technique used (Table 4).
Table 4.
Value of Pearson’s linear correlation and the degree of its significance between the lesions measured in HP and the difference values for Delta MG and Delta CESM.
| N | Pearson | p-Value | |
|---|---|---|---|
| HP_W & DeltaMam | 668 | −0.2516 | 0.000000 |
| HP_W & DeltaCSEM | 661 | −0.0104 | 0.79 |
For the MG measurement, a clear relationship was found to exist in the difference between DeltaMG and HP values, and this relationship was statistically significant. The CESM measurement does not show a similar relationship.
The analysis shows that the deviation from the HP value with the CESM is not dependent on the actual size of this lesion and is not significant (the value of r2=0.0001, p=0.79). For all of the lesion sizes, the average overestimation of the result is approximately 5 mm.
For MG measurements and HP_W values lower than 25 mm, a decrease was observed in the trend line. When this value is exceeded, the trend line becomes stable. For the purposes of the analysis, the material examined was divided into 2 groups according to the HP_W value: first, with HP_W ≤25 mm and second including all the remaining values. For the second group, similarly to the CESM method, no statistically significant relationship was obtained between the DeltaMG values and the HP_W values, and, similar to the CESM method, the average overestimation of the result was about 4 mm, regardless of the size of the pathological lesion. On the other hand, the first group (HP_W ≤25 mm) was observed to manifest a statistically significant (p=0.002) relationship between the deviation and the size of the pathological lesion.
Discussion
While planning the surgical treatment of breast cancer, the scope of the surgery is, to a large extent, dependent on the size of the tumor and exclusion of the multifocality of the neoplastic lesions. The results of breast-conserving treatment in breast cancer are comparable to those of radical treatment. The extent of the surgery can be precisely planned, among others, by assessing the lesion size on imaging. Imaging methods, including digital mammography and ultrasonography, have several limitations and do not always allow for precise preoperative prediction of the tumor size, especially in women with dense glandular breast tissue or implants. CESM is a new technique that may improve the clinical efficacy of assessing the tumor size and support the surgical planning of treatment in the case of patients diagnosed with breast cancer. According to current reports, CESM can be compared to magnetic resonance imaging (MRI) in terms of assessing the tumor size [17,18].
The objective of our study was to compare the size of the neoplastic lesions assessed on MG and CESM with the size of this lesion in postoperative histopathological examination in patients with breast cancer who had initially received surgical treatment. To the best of our knowledge, there have been no studies on such a large group that analyzed the correlation of tumor size in CESM with histopathological examination in women diagnosed with breast cancer.
Based on the results summarized in Tables 1 and 4, it was found that neither of the examinations under analysis (i.e., MG and CESM) is able to precisely represent the actual size of the tumor measured in a histopathological examination. The average of the deviations in the samples examined ranged from 0.56 mm for MG to 5.19 mm for CESM. The comparison of the average values and medians of these deviations revealed that the lesion size was overestimated in both of the measurement methods in question, with the overestimation value in CESM being significantly higher (p=0.000) than that in MG. Interestingly, the representation of the average difference from the HP size in MG measurement for lesions below 20 mm converged with the results obtained in CESM. On the other hand, in the range of tumors measuring 20–26 mm in diameter, MG was more precise in visualizing the lesion size than the CESM method. Furthermore, the study revealed that the difference in assessing the lesion size on MG is significantly dependent on its size in HP (p-0.000). On the other hand, the CESM measurement did not show a similar relationship (p-0.79), thereby indicating that the CESM-measured size is not dependent on its actual size in HP examination. The results obtained in our study seem to be comparable to those presented by other authors. In their study, Jochelson et al. claim that the visualization of the lesion sizes was precise if the difference from the lesion size measured in postoperative HP examination was ≤0.5 cm. Luczyńska et al. demonstrated that the lesion sizes in CESM and MG were comparable to each other, yet overestimated, on average, by 3.0 mm for CESM and 3.3 mm for MG, compared with histopathological results [19,20]. Patel et al. proved that patients with
biopsy-proven malignancy, size measurements in CESM correlated well with histopathologic size and the difference between CESM and histopathological examination was less than 3 mm [21].
The MG and CESM imaging methods involve breast compression, which may be a factor overstating the lesion size compared to histopathological examination. Moreover, each breast cancer forms an autonomous structure partially based on its own specific vascularization. Despite being connected with the systemic circulation, this vascular network is poorly organized, and the endothelial wall of the capillary vessels is damaged, which changes the normal transportation function. Therefore, in the case of CESM, filling the neovascular network with a material that is non-transparent for radiation (i.e., an iodine-based contrast agent) may potentially increase the radiographic density on a mammogram and cause the contrast agent to permeate into the areas adjacent to the lesion, thereby increasing their visibility and potentially leading to overestimation of such lesions [22–24].
The histopathological processing of the postoperative material and measuring the infiltration upon specimen collection also carry a risk of error. It is difficult to assess macroscopically whether the visible borders of the tumor are the limits of tumor infiltration or the limits of the accompanying inflammatory infiltration and calcification. The borders described are corrected upon microscopic assessment, which is possible only when the entire neoplastic infiltration fits into the paraffin block and then on the microscope slide. Furthermore, the specimen must be surrounded by reagents and paraffin, which, in practice, makes it possible to embed a tumor of up to 2 mm in diameter into the cassette. Lesions measuring over 2 cm are assessed macroscopically and this method may carry a low risk of error [25].
In our study, no difference was observed in the lesion sizes on CESM and MG compared to HP depending on the grading, the biological subtype, and the patient’s age. However, significant differences occurred depending on the histopathological subtype of carcinoma.
The highest compliance of dimensions on MG and CESM with the histopathological examination was observed in our study in the group of special carcinoma subtypes. The average difference in MG in relation to HP amounted to only 0.02 mm, while in CESM it was 1.3 mm. Tumors with dense collagen stroma, or the so-called desmoplastic ones, have a tendency to capillary collapse and manifest lower perfusion than tumors with loose stroma. Special subtypes of carcinoma are quite often well-delineated tumors, with a clear border of infiltration and no strip of inflammatory tissues around the lesion, which could be mistaken for neoplastic infiltration on mammography, thereby overestimating the tumor diameter [26,27].
In our group, all of the DCIS cases manifested pathological contrast enhancement. However, a significant problem was observed in reassessing their size in CESM compared to other carcinomas. The average difference in MG in relation to HP amounted to 5 mm, while in CESM it was 11.18 mm. The lesion size in CESM was overestimated by more than 5 mm in the case of 44 (52%) out of the 84 DCISs under analysis. Breast conservation therapy (BCT) surgery with positive margins is reported to occur in 34% of DCIS cases compared to 3–7% in patients with invasive (ductal or lobular) breast cancers [28,29]. In the preoperative treatment planning, obtaining precise information on the extent and distribution of DCIS is important in determining the extent of surgery required.
Ductal carcinoma in situ represents a morphologically and biologically heterogeneous group of neoplastic proliferations. On X-ray mammography, it usually manifests as microcalcifications (72%) or microcalcifications with an accompanying abnormal tissue image (12%) [30]. Taking into consideration the fact that X-ray mammography has the disadvantage of being less sensitive in the case of highly glandular breasts, assessing the dimensions of focal asymmetries or architectural distortion that accompany carcinomas in situ with such breast tissue is very difficult and sometimes even impossible. Therefore, new methods are being searched for that would allow for more precise assessment of the tumor size. Ductal carcinoma in situ is limited by the basement membrane, does not infiltrate the stroma and, generally, manifests no angiogenetic capacity [31]. The question arises, therefore, as to why CESM subtraction images (upon injection of a contrast agent) can provide better visualization of these carcinomas.
Ductal carcinomas in situ (DCIS) may exhibit increased vascularization, despite the fact they do not cross the basement membrane, and the stromal microvessel density (MVD) between the ducts invaded by the DCIS may be higher than that in unremarkable tissue [32,33]. This explains why these carcinomas are visible at all on spectral mammography upon intravenous injection of a contrast agent. To the best of our knowledge, there are no publications concerning the issue of reassessing DCISs in CESM. There are, however, papers where the authors have similar experiences with the use of magnetic resonance imaging (MRI). Both spectral mammography and magnetic resonance imaging are based on contrast enhancement of pathological foci, i.e., an attempt to visualize angiogenesis. Therefore, the discussion refers to the publications that explore this issue in MR examination. In his publication, Jethava [34] states that the problem of overestimating the size of carcinomas on MRI amounts to 31.9%, and primarily concerns high-grade carcinomas and DCISs. The researchers noticed that the MRI-based measurement of the DCIS was overestimated by more than 0.5 cm in 43% of all pre-invasive ductal carcinomas. Similar observations can be seen in the papers by other authors [35,36]. The most plausible explanation for this situation is the fact that DCIS often coexists with other pathologies, such as benign proliferation around foci fibrocystic changes, atypical ductal hyperplasia, and inflammatory lesions.
The overestimation of the lesion sizes obtained on CESM images had no influence on the surgical treatment in our group of patients because it was insignificant, and the safety margins were always taken into consideration during surgical interventions.
In the study group of 668 surgical patients observed to date, local recurrence was noted in 2 cases, while nodular recurrence following breast-conserving treatment was found in 3 cases. In 48 (6.9%) cases, it was necessary to apply local radicalization due to microscopically non-radical margins (R1 resection), of which 28 (58%) were DCIS cases.
A separate, extremely significant feature of CESM observed in the study group of patients was the potential to identify additional neoplastic foci in the breast, which often translated into a changed scope of surgery. Careful scrutiny of these cases will be the subject of our next publication.
A limitation of examination was the fact that part of MG was performed outside our Department using lower-quality equipment, which could have caused less precise tumor limits imaging. Moreover, about 50% of patients had high-density glands, contributing to problems with finding further tumors in MG, which were later detected using CESM.
Conclusions
CESM is a new method relevant in the surgical planning of breast cancer therapy. The measurement accuracy of the tumor size using the CESM is independent from the size of the lesion. However, it overestimates the size by an average of about 5 mm. The differences in the representation of the lesion size in CESM are not dependent on the patient’s age, grading, or biological subtype of the carcinoma, but they concern the histopathological type and are significantly greater in pre-invasive carcinomas.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.International Agency for Research on Cancer. GLOBOCAN. 2018. Available from: http://globocan.iarc.fr.
- 2.Wojciechowska U, Didkowska J. Polish National Cancer Registry, Oncology Center – Maria Sklodowska-Curie. http://onkologia.org.pl [in Polish]
- 3.Pisano ED, Hendrick RE, Yaffe MJ, et al. Diagnostic accuracy of digital versus film mammography: Exploratory analysis of selected population subgroups in DMIST. Radiology. 2008;246:376–83. doi: 10.1148/radiol.2461070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jochelson M. Advanced imaging techniques for the detection of breast cancer. Am Soc Clin Oncol Educ Book. 2012:65–69. doi: 10.14694/EdBook_AM.2012.32.223. [DOI] [PubMed] [Google Scholar]
- 5.Fischer U, Baum F, Obenauer S, et al. Comparative study in patients with microcalcifications: full-field digital mammography vs. screen-film mammography. Eur Radiol. 2002;12:2679–83. doi: 10.1007/s00330-002-1354-x. [DOI] [PubMed] [Google Scholar]
- 6.Smith A. Fundamentals of digital mammography: physics, technology and practical considerations. Radiol Manage. 2003;25:18–24. 26–31. quiz 32–34. [PubMed] [Google Scholar]
- 7.Sardanelli F, Aase HS, Álvarez M, et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur Radiol. 2017;27(7):2737–43. doi: 10.1007/s00330-016-4612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dromain C, Thibault F, Diekmann F, et al. Dual-energy contrast-enhanced digital mammography: Initial clinical results of a multireader, multicase study. Breast Cancer Res. 2012;14:R94. doi: 10.1186/bcr3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curigliano G, Burstein HJ, Winer EP, et al. Panel Members of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017, St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700–12. doi: 10.1093/annonc/mdx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 12.Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J Clin Oncol. 2016;34(33):4040–46. doi: 10.1200/JCO.2016.68.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iacconi C. Diffusion and perfusion of the breast. Eur J Radiol. 2010;76(3):386–90. doi: 10.1016/j.ejrad.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Sardanelli F, Fallenberg EM, Clauser P, et al. European Society of Breast Imaging (EUSOBI), with language review by Europa Donna – The European Breast Cancer Coalition. Mammography: An update of the EUSOBI recommendations on information for women. Insights Imaging. 2017;8(1):11–18. doi: 10.1007/s13244-016-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhimani C, Matta D, Roth RG, et al. Contrast-enhanced spectral mammography: Technique, indications, and clinical applications. Acad Radiol. 2017;24(1):84–88. doi: 10.1016/j.acra.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Puong S, Bouchevreau X, Patoureaux F, et al. Dual-energy contrast enhanced digital mammography using a new approach for breast tissue canceling. Proc SPIE. 2007;6510:65102H–12. [Google Scholar]
- 17.Fallenberg EM, Dromain C, Diekmann F, et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumor size. Eur Radiol. 2014;24:256–64. doi: 10.1007/s00330-013-3007-7. [DOI] [PubMed] [Google Scholar]
- 18.Yousef AF, Khater HM, Jameel LM. Contrast-enhanced spectral mammography versus magnetic resonance imaging in the assessment of breast masses. Benha Med J. 2018;35:5–12. [Google Scholar]
- 19.Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: Feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology. 2013;266:743–51. doi: 10.1148/radiol.12121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luczyńska E, Heinze-Paluchowska S, Dyczek S, et al. Contrast-enhanced spectral mammography: Comparison with conventional mammography and histopathology in 152 women. Korean J Radiol. 2014;15(6):689–96. doi: 10.3348/kjr.2014.15.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel BK, Garza SA, Eversman S, et al. Assessing tumor extent on contrast-enhanced spectral mammography versus full-field digital mammography and ultrasound. Clin Imaging. 2017;46:78–84. doi: 10.1016/j.clinimag.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Dromain C, Balleyguier C, Muller S, et al. Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography. Am J Roentgenol. 2006;187:W528–37. doi: 10.2214/AJR.05.1944. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Lan L, Sennett C, Giger M. SU-E-J-248: contributions of tumor and stroma phenotyping in computer-aided diagnosis. Med Phys. 2015;42:3323. [Google Scholar]
- 24.Phamduy TB, Sweat RS, Azimi MS, et al. Printing cancer cells into intact microvascular networks: a model for investigating cancer cell dynamics during angiogenesis. Integr Biol (Camb) 2015;7:1068–78. doi: 10.1039/c5ib00151j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horn CL, Naugler C. Breast specimen shrinkage following formalin fixation. Dovepress. 2014;6:11–14. doi: 10.2147/PLMI.S59842. [DOI] [Google Scholar]
- 26.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker HE, Chang J, Cox TR, et al. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–72. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Esser S, Peters NHGM, van den Bosch MAAJ, et al. Surgical outcome of patients with core-biopsy-proven nonpalpable breast carcinoma: A large cohort follow-up study. Ann Surg Oncol. 2009;16:2252. doi: 10.1245/s10434-009-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobbes MB, Vriens IJ, Bommel ACV, et al. Breast MRI increases the number of mastectomies for ductal cancers, but decreases them for lobular cancers. Breast Cancer Res Treat. 2017;162:353–64. doi: 10.1007/s10549-017-4117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dziukowa J, Wesołowska E. Medipage, editor. Mammography in breast cancer diagnostics. Second edition. 2006. [Google Scholar]
- 31.Pattani N, Cutuli B, Mokbel K. Current management of DCIS; A review. Breast Cancer Res Treat. 2008;111:1–10. doi: 10.1007/s10549-007-9760-z. [DOI] [PubMed] [Google Scholar]
- 32.Raica M, Cimpean M, Ribatti M. Angiogenesis in pre-malignant conditions. Eur J Cancer. 2009;45(11):1924–34. doi: 10.1016/j.ejca.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Menakuru SR, Brown NJ, Staton CA, Reed MWR. Angiogenesis in pre-malignant conditions. Br J Cancer. 2008;99:1961–66. doi: 10.1038/sj.bjc.6604733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jethava A, Ali S, Wakefield D, et al. Diagnostic accuracy of MRI in predicting breast tumor size: Comparative analysis of MRI vs. histopathological asssessed breast tumor size. Conn Med. 2015;79(5):261–67. [PubMed] [Google Scholar]
- 35.Onesti JK, Mangus BE, Helmer SD, et al. Breast cancer tumor size: Correlation between magnetic resonance imaging and pathology measurements. Am J Surg. 2008;196:844–50. doi: 10.1016/j.amjsurg.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Grimsby GM, Gray R, Dueck A, et al. Is there concordance of invasive breast cancer pathologic tumor size with magnetic resonance imaging? Am J Surg. 2009;198:500–4. doi: 10.1016/j.amjsurg.2009.07.012. [DOI] [PubMed] [Google Scholar]