Abstract
Prenatal Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Trichomonas vaginalis (TV) infections are associated with adverse birth outcomes. As rapid diagnostic tests become available, it is important to evaluate prenatal sexually transmitted infection (STI) prevalence, as well as the acceptability and feasibility of prenatal screening programs. We recruited 371 pregnant women from four clinics in Kisantu Health Zone, Democratic Republic of Congo (DRC) from October 2016 to March 2017. Trained clinicians collected cervical swabs, and samples were tested by nucleic acid amplification for CT, NG, and TV using a GeneXpert® system. Those testing positive for an STI were treated and asked to return after 4–8 weeks for tests-of-cure. Screening for STIs was widely accepted (99%). STI prevalence at baseline was: CT, 3.2%; NG, 1.5%; and TV, 14%; treatment completion was 97%. Symptoms were reported among 34% of STI-positive women at baseline, compared with 37% of STI-negative women. Upon first test-of-cure, 100% of returning women were cured of CT (n= 10) and NG (n= 5), but only 47% were cured of TV. This study demonstrates the feasibility of implementing diagnostic STI testing for case detection and treatment among expectant mothers in DRC, with implications for maternal and birth outcomes.
Introduction
Every year, Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Trichomonas vaginalis (TV) account for approximately 349 million new sexually transmitted infections (STIs) worldwide.1 While STI rates vary by country and demographic group, STI rates in pregnant women are higher than in the general population, as young adults typically carry the highest burden of STIs.2 In pregnant women, STIs may contribute to serious health consequences including adverse birth outcomes for offspring. Maternal CT, NG, and TV infections have been associated with premature labor and low birth weight infants.3–5 Furthermore, untreated CT or NG infection can be passed to the infant during birth. Neonatal CT infection can lead to chlamydial opthalmia neonatorum and chlamydial pneumonia, while neonatal NG infection can cause gonococcal opthalmia neonatorum and blindness.6–8 In the mother, long-term untreated CT, NG, or TV infection can lead to pelvic inflammatory disease, resulting in future ectopic pregnancy, spontaneous abortion, or infertility.9 Finally, maternal CT, NG, or TV infection is associated with higher risk of acquiring human immunodeficiency virus (HIV) infection, as well as an increased chance of mother-to-child HIV transmission.10–11
In the Democratic Republic of Congo (DRC), there are no population-level data on the prevalence of CT, NG, and TV among pregnant women. Despite documented adverse outcomes of prenatal CT, NG, and TV infection, the World Health Organization (WHO) currently provides no recommendations to screen pregnant women for those infections. In place of prenatal screening, WHO currently recommends symptom-based management of CT, NG, and TV infection.12 Given the frequently asymptomatic nature of those infections, that approach likely leads to many undiagnosed and untreated infections, and may contribute to a large number of adverse birth outcomes. The lack of WHO recommendation is due to the high cost of laboratory testing, poor infrastructure for testing in resource-limited settings, the need for highly trained laboratory personnel, and insufficient data supporting the cost-effectiveness of prenatal STI screening programs. As a result, most countries, including the DRC, do not currently screen pregnant women for CT, NG, or TV infection.13
Point-of-care, rapid diagnostic tests are becoming increasingly available for CT, NG, and TV infection.14 With those tests, prenatal STI screening is quicker, simpler, and less expensive. Screening can be conducted in 90 minutes, and minimal laboratory training and equipment are required. Furthermore, women can receive their results at the clinic and, if they screen positive, can be treated in the same day. Because CT and NG are bacterial and TV is protozoan parasite, infections can typically be cured with antibiotics. Antibiotic treatment is safe for pregnant women, and has been shown to prevent many of the adverse outcomes associated with maternal CT, NG, or TV infection.15–16 Therefore, new diagnostic technology creates an opportunity for prenatal screening programs that could help prevent adverse birth outcomes, both for the mother and the infant. 7–22
To understand the need for prenatal CT, NG, and TV screening in the DRC, it is necessary to understand the prevalence of these infections among pregnant women. Furthermore, it is important to understand the acceptability and feasibility of screening antenatal care settings. Hence, we evaluated the frequency of maternal CT, NG, and TV infection among pregnant women in the DRC, as well as the sociodemographic and behavioral correlates associated with infection. We also sought to determine screening acceptability, STI cure rates, and treatment feasibility among both direct participants and their sex partners.
Methods
Study design and population
We conducted a cross-sectional study of prenatal CT, NG, and TV infection with longitudinal follow-up for infected participants between October 2016 and September 2017. Between October 2016 to March 2017, a convenience sample of pregnant women was recruited from three antenatal clinics and one HIV clinic in Kisantu Health Zone (HZ), Kongo Central province (formerly, Bas Congo province), Democratic Republic of Congo. All three antenatal clinics are located at varying distance from the central health zone office in Kisantu city (3, 17, and 34 km away) and were selected from a list of urban (Kintanu Etat clinic), peri-urban (Ngeba clinic), or rural (Lemfu clinic) maternity facilities in order to ensure enrollment of women from a diverse spectrum of catchment areas; the HIV clinic is centrally located in Kisantu town and services patients across the entire health zone.
Women were recruited while attending any of the four clinics described and screened in a private space; all eligible participants were offered enrollment in the study. Women eligible for participation were 18 years or older, less than 35 weeks pregnant at the time of clinic visit, and agreed to be contacted for follow-up. Women with an AIDS-defining illness were excluded from participation. In total, 371 women met the eligibility criteria and were enrolled in the study. Women partook in a face-to-face survey and had two cervical swabs collected by a clinician for STI testing. Those positive for an STI at study baseline were followed up to three times to assess for clearance of infection, with the final participant follow-up activity ending in September 2017. Those women negative for all three tested STIs at baseline were not followed further.
Prior to study initiation, local clinic staff (nurses and doctors) were trained in study procedures. Study authors led all trainings and supervised study procedures throughout its entirety. IRB approval was obtained from the Kinshasa School of Public Health in DRC (ref. ESP/CE/034/2016) and the University of California Los Angeles (ref. 14-000830-AM-00006).
Questionnaire administration
Those providing informed consent proceeded with their intended clinical care (original reason for clinic contact), and were then administered a baseline questionnaire collecting sociodemographic, sexual practice, and clinical history information. The HIV status of each woman was also collected by self-report, based on serological testing provided free of charge by the national government as a part of routine antenatal care throughout DRC. Participants were also asked about any current urogenital symptoms they were experiencing, specifically about genital lesions, genital warts, vaginal itching or discomfort, abnormal vaginal discharge, and dyspareunia (painful intercourse) in the past month. For those testing negative for all STIs, this was the only survey administered. For those women testing positive for any STI, short follow-up surveys were conducted at each clinic visit for up to three additional visits, each 4–8 weeks following the prior visit.
Biological specimen collection and laboratory analysis:
Following survey completion, trained clinicians (local physicians and nurses) collected two sets of cervical swabs from each participant: one for CT/NG and one for TV (Xpert ® CT/NG and TV test kits; Cepheid, Sunnyvale, California). After excess cervical mucus removal with an initial cleaning swab, sample collection swabs were placed in the endocervical canal of consenting participants and turned clockwise for 10 – 30 seconds before being placed in a reagent tube for storage. All cervical swab specimens were then analyzed for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis via DNA assay (also known as nucleic acid amplification test, or NAAT) by trained study personnel using a GeneXpert® machine (Cepheid, Sunnyvale, CA). This specimen collection procedure was repeated successively 4–8 weeks following study baseline for those women testing positive for any STI at baseline as a test of cure.
Treatment and follow up testing:
Screening results were communicated to participants within 24 hours of swab collection, either in person at the clinic or over the phone. Women testing positive for CT, NG, or TV were provided treatment for themselves and their untested sex partners (expedited partner therapy) free of charge. Those presenting with CT or NG infections were administered a single dose of 1 g oral azithromycin, per local practice.i Women presenting with active TV infection were treated with 2 g oral metronidazole. In persistent trichomonal infection (failing tests of clearance for TV at the second or third follow-up visit), clinic staff treated patients with a single course of 2 g oral tinidazole.
Statistical analysis:
Acceptability of screening procedures were expressed as the proportion of eligible women who agreed to and underwent cervical specimen collection over the course of their baseline clinic visit. Amongst those who accepted screening, the prevalence of CT, NG, and TV was calculated from a sub-group with available laboratory test results. Basic sociodemographic characteristics of all study participants were then tabulated and expressed according to participant STI status. Chi-square analysis was conducted to test for demographic differences between STI- positive and negative women. Treatment feasibility was measured as the proportion of women who accepted treatment for their infections following a positive test.
Next, follow-up and infection clearance frequencies were calculated for up to three return clinic visits among women STI-positive at study baseline. Demographic characteristics were compared for those returning versus missing from the initial follow-up visit using chi-square tests of proportions. Partner treatment feasibility was also calculated amongst the group returning for first follow-up as the proportion of women reporting treatment completion by their partners. Finally, urogenital symptom frequency was measured for STI positive and negative women. Complete case analysis was utilized in all analyses to account for missing data. All statistical procedures were performed using SAS version 9.4 (Cary, NC).
Results
Baseline results
In total, 371 pregnant women were enrolled following recruitment from HIV (n=4) and antenatal care (n=367) clinics in Kisantu Health Zone. Ninety-nine percent of enrolled women (n= 366) accepted prenatal STI screening, of whom 9 had invalid CT/NG test results, and 3 had invalid TV test results. Only one woman had to be excluded from all further analyses due to invalid results for both tests, leaving a final test result sample of 365 women.
Half of women in our study fell between ages 18 – 24 years, and only 12% of mothers enrolled were 35 years or older. Most women were in their second or third trimester at the time of enrollment (91%), had had at least one previous pregnancy (69%), and were married to or otherwise cohabitating with a primary partner (71%). Only four pregnant women were recruited from Kisantu’s central HIV clinic; among those recruited at one of the antenatal care clinics, a majority (61%) came through urban Kintanu Etat. No significant demographic differences were noted between those infected versus uninfected with CT, NG, or TV at baseline, although younger women and those attending the rural Lemfu antenatal clinic had higher rates of STIs than older women and those attending the other clinics (Table 1).
Table 1.
Demographic characteristics of pregnant women in Kisantu Health Zone, Democratic Republic of Congo at study baseline by tested STI status (October 2016 – March 2017).
| STI Status† | |||
|---|---|---|---|
| Positive (%) n= 65 | Negative (%) n= 300 | p-value* | |
| Age group | |||
| 18–24 | 41 (23) | 141 (77) | 0.06 |
| 25–34 | 17 (12) | 122 (88) | |
| 35+ | 7 (16) | 36 (84) | |
| Education | |||
| None - some primary | 10 (14) | 59 (86) | 0.53 |
| Finished primary | 35 (20) | 139 (80) | |
| Secondary & beyond | 20 (17) | 100 (83) | |
| Civil status | |||
| Married/ cohabitating | 47 (18) | 211 (82) | 0.78 |
| Not in a union | 18 (17) | 88 (83) | |
| Pregnancy history | |||
| Primigravid | 21 (20) | 85 (80) | 0.62 |
| Multigravid | 42 (18) | 197 (82) | |
| Current pregnancy** | |||
| First trimester | 3 (9.4) | 29 (91) | 0.26 |
| Second trimester | 31 (21) | 117 (79) | |
| Third trimester | 31 (17) | 154 (83) | |
| Clinic visited | |||
| Kintanu Etat ANC | 40 (18) | 179 (82) | 0.05 |
| Ngeba ANC | 5 (8.1) | 57 (92) | |
| Lemfu ANC | 20 (25) | 60 (75) | |
| Nkandu HIVC | 0 | 4 (100) | |
STI infections include: CT, NG, and TV only.
p-values calculated using Wald chi-square tests.
First trimester defined as 0–12 weeks; second trimester 13–27 weeks; third trimester 28+ weeks.
Abbreviations: STI, sexually transmitted infection; ANC, antenatal clinic; HIVC, HIV clinic.
In total, 6 women were identified as HIV positive (1.6%), and 65 women tested positive for at least one STI following swab collection (18%). Across the 357 women with a valid CT/NG test, 11 were positive for CT (3.1%) and 5 were positive for NG (1.4%). There were no women positive for both CT and NG. Cumulative prevalence for CT/NG was 16/357 (4.5%). Of the 363 women with valid TV test, 52 were positive (14%); 3 TV positive women were also positive for CT, although no TV-infected women were concurrently infected with NG. None of the six pregnant participants with HIV—4 visiting the HIV clinic and 2 visiting ANC clinics—were positive for any other STI (CT/NG/TV). Almost all infected women (n= 63) accepted treatment, resulting in a treatment feasibility of 97% in our cohort. Of the demographic characteristics assessed, only participant age and clinic site were moderately associated with infection status, with a higher proportion of STI positivity seen in those of the youngest age category (p= 0.06) and those attending the rural Lemfu clinic (p= 0.05).
Follow-up results and tests of clearance
Of the 65 women screening positive for an STI at baseline enrollment, 58 returned for at least one follow-up test of clearance, 55 (85%) of whom also filled out a follow-up survey regarding their current symptoms and partner treatment. No significant demographic differences in age (p= 0.30), education level (p= 0.43), civil/ partnership status (p= 0.96), pregnancy history (p= 0.57), current trimester of pregnancy (p= 0.76), or clinic site visited (indicative of patient’s residence within Kisantu Health Zone; p= 0.61) were noted between STI positive women who returned or were missing from follow-up. Of the 55 women who completed the first follow-up survey, 67% reported their partners accepted treatment, 3.6% had offered treatment but were not sure if their partners had taken it, and 29% declined to answer (Table 2).
Table 2.
Partner treatment feasibility and participant STI clearance during each follow-up period, by infection type.
| Infection status | ||||
|---|---|---|---|---|
| Positives returning | Partner treatment accepted | Positive | Negative | |
| First follow-up | CT (n= 10) | Yes | 0 | 9 |
| No | 0 | 0 | ||
| Unknown | 0 | 0 | ||
| Declined | 0 | 1 | ||
| NG (n= 5) | Yes | 0 | 4 | |
| No | 0 | 0 | ||
| Unknown | 0 | 0 | ||
| Declined | 0 | 1 | ||
| TV (n= 45) | Yes | 15 | 10 | |
| No | 0 | 0 | ||
| Unknown | 2 | 0 | ||
| Declined | 7 | 12 | ||
| Second follow-up | TV (n= 5) | Yes | 0 | 0 |
| No | 1 | 0 | ||
| Unknown | 1 | 0 | ||
| Declined | 2 | 1 | ||
| Third follow-up | TV (n= 2) | Yes | 0 | 2 |
| No | 0 | 0 | ||
| Unknown | 0 | 0 | ||
| Declined | 0 | 0 | ||
Unknown signifies that the participant was not sure if her partner had taken the treatment provided by the study team. Declined indicates the participant did not answer the survey question about partner therapy, or did not complete a follow-up survey at all. Abbreviations: CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae; TV, Trichomonas vaginalis.
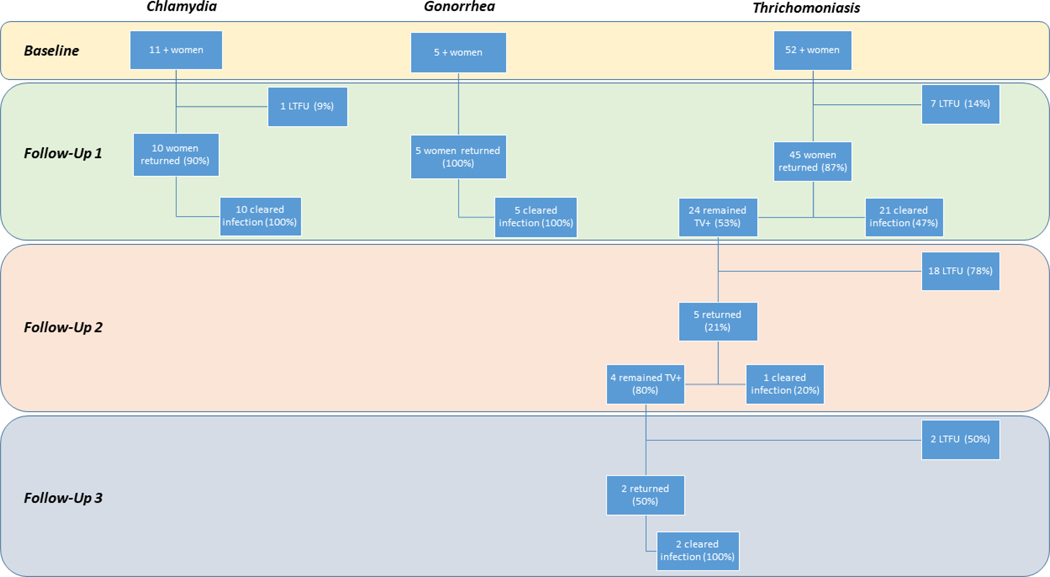
Among 11 women with CT at baseline, ten (91%) returned for follow up testing and survey; all 10 cleared infection at first follow-up. Of the five women with NG at baseline, all returned for follow up testing and survey, and all had cleared infection by first follow-up. Of the 52 women with TV at baseline, 45 (87%) returned for at least one follow-up test, and 42 (81%) also completed at least one follow-up survey. At initial follow-up, 24 women remained TV-positive (53%), and 21 had cleared infection (47%). Only 5/24 positives at first follow-up returned again for a second follow up, 80% of whom remained positive. By third follow-up, only 2/4 women TV+ at second follow-up returned again to the clinic and filled out a final survey; both had cleared infection by their third visit and were not followed further (Figure 1). Notably, a majority of women clearing CT and NG infections by the first follow-up period had reported successful partner treatment (90% and 100%, respectively), whereas only 40% of participants positive for TV at baseline and reporting successful partner treatment had cleared their infections (Table 2). At second follow-up, not a single returning participant reported successful partner treatment, but by third follow-up the two remaining women reported partner treatment acceptance and tested negative for their infections.
Figure 1. Study progression and testing results from baseline to third follow-up, by STI of interest. Kisantu, Democratic Republic of Congo (October 2016 to September 2017).
LTFU: lost to follow-up; STI: sexually transmitted infection.
Symptom reports
In total, 132 participants (36%) reported at least one symptom during the baseline interview. The proportion of women reporting at least one symptom at baseline was similar across infection types, and was also comparable between STI-infected (34%) and uninfected (37%) women. Among CT/NG cases, abnormal discharge and dyspareunia were the most common symptoms reported (n= 2, 13%); among TV cases, abnormal discharge and genital itching/ discomfort were most prevalent, occurring in 8 of 52 cases (15%). STI-uninfected women were more symptomatic than others, and comprised the only group to report genital warts (n= 10, 3.3%). At first follow up—the only return period to include women positive at baseline for all three tested STIs—twenty of 55 (36%) women who completed a follow-up survey reported some current symptom; as at baseline, less than half of them were STI positive at that visit (all for TV; Table 3). Using syndromic management strategies alone, more than 65% of true positive STI cases may have been missed in this cohort; additionally, 110 DNA-negative women may have been empirically treated for an STI based on their symptoms.
Table 3.
Clinical symptom reports by infection status.
| Baseline | First Follow-Up | |||||
|---|---|---|---|---|---|---|
| CT/NG+(n= 16) | TV+(n= 52) | Any STI+(n= 65) | No infection (n= 300) | TV+(n= 24) | No infection (n= 41) | |
| Any symptom | 5 (31%) | 19 (37%) | 22 (34%) | 110 (37%) | 6 (25%) | 14 (34%) |
| Breakdown | ||||||
| Abnormal vaginal discharge* | 2 (13%) | 8 (15%) | 10 (15%) | 41 (14%) | 2 (8.3%) | 7 (17%) |
| Genital lesions** | 1 (6.3%) | 3 (5.8%) | 3 (4.6%) | 15 (5.0%) | 1 (4.2%) | 2 (4.9%) |
| Genital warts | 0 | 0 | 0 | 10 (3.3%) | 0 | 0 |
| Genital itching/ discomfort | 1 (6.3%) | 8 (15%) | 8 (12%) | 15 (5.0%) | 2 (8.3%) | 6 (15%) |
| Dyspareunia | 2 (13%) | 5 (9.6%) | 7 (11%) | 46 (15%) | 2 (8.3%) | 4 (9.8%) |
Symptoms are not mutually exclusive. Thus, symptom breakdowns by column may not add to the total symptom count. Abbreviations: NAAT, nucleic acid amplification test; CT/NG, Chlamydia trachomatis or Neisseria gonorrhoeae; TV, Trichomonas vaginalis.
Abnormal in the sense of color, consistency, or odor
Blisters, sores, or ulcers
Discussion
We determined the acceptability of a prenatal STI screening program in the Democratic Republic of Congo, a resource-limited country where syndromic diagnoses are the standard for STI case detection.25, 26 Despite the stigma associated with STI testing, we found that clinician-collected cervical swabs were nearly universally accepted among a group of pregnant women attending antenatal care clinics in Kisantu across all trimesters. Women testing positive were also overwhelmingly amenable to taking a course of antibiotics for their infections (97%), and demonstrated moderate success at delivering partner therapy. A vast majority of STI-positive women returned to their healthcare setting for at least one follow-up test of clearance, demonstrating a high willingness to participate in health seeking behaviors during the antenatal period. The high social acceptance for prenatal point-of-care STI testing and treatment among Congolese women described here also stands in accordance with that seen in screening programs for pregnant women across other countries in Africa, the Caribbean, and Asia.19–21, 27, 28
Our findings reinforce the potential value of screening programs for pregnant women in the developing world. Screening programs provide laboratory detection for infections which are frequently asymptomatic or susceptible to mismanagement by syndromic approaches alone.29 Misdiagnosis of STIs during pregnancy can result in undertreatment of mothers resulting in serious reproductive health consequences, including pelvic inflammatory disease, pregnancy complications, and transplacental neonatal infections.30, 31 In our cohort, only one-third of women screening positive for an STI described any urogenital symptoms during their questionnaires, aligning with other similar reports.27, 32 Conversely, erroneous syndromic diagnoses may lead to overtreatment of uninfected women. Not only does this represent a waste of medical resources, but it poses serious threats to perpetuating antimicrobial resistance, which has already been described widely for NG throughout Africa.33 The challenges with syndromic management may be even greater during pregnancy when urogenital side effects of gestation frequently manifest similarly to those of STIs, further reducing the specificity of syndromic methods.34, 35
Point-of-care testing systems like the GeneXpert® used here offer a several advantages to traditional laboratory testing methods. First, health care workers can learn to operate the equipment with little training, removing burdensome costs associated with establishing on-site laboratory infrastructure or sample shipment for offsite processing. Second, point-of-care testing allows clinicians to deliver rapid results and treatment to their patients, improving operational efficiency and patient outcomes, while reducing attrition related to follow-up.36 Our findings align well with other reports suggesting point-of-care testing for urogenital infections are a practical means of improving case detection and infection management in low-resource settings.27, 35 Despite this, several barriers to operation of the GeneXpert® and other tools like it remain, including a high initial investment cost in the machinery, which may be out of reach for underfunded health centers. Machine maintenance and operational electricity requirements may also pose challenges to routine use of automated NAAT hardware, particularly in rural environments. Other assays such as bacterial culture, wet mount microscopy, antigen detection, and DNA hybridization may offer alternative strategies for STI testing at a lower principal unit cost, but come with their own set of limitations including longer test result wait times, lower sensitivity and/or specificity for infection detection, and in some cases greater laboratory infrastructure demands, including reagent stockage and trained personnel requirements.37–39 Specific logistic considerations for each country and individual health center should be considered in determining appropriate testing strategies for pregnant women as diagnostic screening takes the place of syndromic STI management.
The modest clearance of TV after an initial round of antibiotic treatment stands in contrast to the 100% clearance of CT and NG among study participants. The reason for this difference is not entirely clear, although our results suggest that a lower feasibility of partner treatment may have hindered the success of personal treatment plans by putting women at risk of reinfection. Alternatively, this phenomenon may be due to under-treatment with a single dose of metronidazole, or less likely due to metronidazole-resistant infection or an oversensitive diagnostic test that detects TV DNA despite infection clearance.39, 40
Our study has several limitations worth considering. Given the small sample size, it is difficult to generalize our findings out beyond the study area, and future work will be required to strengthen evidence about STI prevalence and screening success in the Democratic Republic of Congo. That being said, several other studies have shown the utility of STI screening in low- and middle-income countries, including evaluations of nucleic acid amplification tests compared with symptom-based diagnosis in pregnant and non-pregnant women.32, 34, 35 Additionally, we were limited in our ability to directly treat sex partners of our participants, increasing the likelihood that women become re-infected. Partner treatment is challenging for a number of reasons; fear of disclosure due to embarrassment, taboo, or fear of retribution, and reluctance of partners to accept treatment may influence the lower success of partner treatment. Future research should be undertaken to better understand barriers to partner treatment in the DRC specifically. Finally, our questionnaire did not inquire about antenatal clinic visits or comprehensive health seeking behaviors prior to study enrollment, which may have influenced STI rates in this cohort. Women with prior antenatal care visits may have been previously exposed to symptom-based treatment for reproductive tract infections, downwardly biasing our snapshot of STI prevalence in this population. Furthermore, a growing body of literature suggests that intermittent preventive treatment in pregnancy (IPTp) alone and in combination with azithromycin may offer promising benefits to mothers, in the form of reduced burden of malarial and reproductive tract infections, and their offspring, in the form of decreased incidence of adverse birth outcomes such as preterm delivery.24,41 Specifically, sulfadoxine-pyrimethamine, an IPTp drug utilized nationally in DRC, appears to protect against sexually transmitted and reproductive tract infections, as well as unspecified causes of adverse birth outcomes in addition to its primary function as an antimalarial.41 Thus, it is unclear if exposure to IPTp with sulfadoxine pyrimethamine prior to and during the study may have influenced the prevalence of STIs among this cohort at baseline or increased the efficacy of STI treatment at each follow-up period, especially among women receiving azithromycin to treat chlamydia or gonorrhea.
To conclude, we found that prenatal STI screening and treatment had high acceptability and feasibility among pregnant women in the DRC, with moderate success for partner treatment. CT, NG, and TV infections were prevalent among the study population, with a particularly high prevalence of TV. Prenatal screening and treatment present an opportunity to reduce adverse birth outcomes in the DRC by detecting cases that would not have been found through syndromic management. Furthermore, antenatal screening could prevent the development of additional antibiotic resistance in the DRC, where antibiotics may currently be overprescribed when administered on the basis of urogenital symptoms alone. There is a need for continued research to evaluate the feasibility and cost-effectiveness of antenatal STI screening programs to prevent adverse reproductive outcomes in the Democratic Republic of Congo. Continued evaluation of the efficacy and acceptability of point-of-care testing should also be undertaken in low-resource settings as the basis of clinical decision-making shifts from syndromic to rapid diagnostic approaches.
Footnotes
Prenatal azithromycin administration, in addition to exhibiting preventive and curative effects against N. gonorrhoeae and C. trachomatis, also appears to increase the birth weight, mid-upper arm circumference, and head circumference of offspring born in settings with a high burden of pathogen exposure.23,24
References
- 1.Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates. Bulletin of the World Health Organization, 2019; 97(8): 548–562P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joseph Davey DL, Shull HI, Billings JD, et al. Prevalence of curable sexually transmitted infections in pregnant women in low-and middle-income countries from 2010 to 2015: a systematic review. Sex Transm Dis 2016; 43(7): 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotch MF, Pastorek JG, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis, 1997; 24(6): 353–60. [DOI] [PubMed] [Google Scholar]
- 4.de Attayde MJPM, Florêncio GLD, Gabiatti JRE, et al. Perinatal morbidity and mortality associated with chlamydial infection: a meta-analysis study. Braz J Infect Dis, 2011; 15(6): 533–9. [DOI] [PubMed] [Google Scholar]
- 5.Gencay M, Koskiniemi MA, Pirkko S, et al. Chlamydia trachomatis seropositivity is associated both with stillbirth and preterm delivery. APMIS, 2000; 108(9): 584–588. [DOI] [PubMed] [Google Scholar]
- 6.Schachter J, Grossman M, Sweet R, et al. Prospective study of perinatal transmission of Chlamydia trachomatis. JAMA, 1986; 255(24): 3374–3377. [PubMed] [Google Scholar]
- 7.Heggie AD, Lumicao GG, Stuart LA, Gyves MT. Chlamydia trachomatis infection in mothers and infants: A prospective study. Am J Dis Child, 1981; 135(6): 507–511. [DOI] [PubMed] [Google Scholar]
- 8.Laga M, Nzanze H, Brunham R, et al. Epidemiology of ophthalmia neonatorum in Kenya. Lancet, 1986; 328(8516): 1145–1149. [DOI] [PubMed] [Google Scholar]
- 9.Holmes KK. Sexually transmitted diseases. 4th ed NewYork: McGraw‐Hill Medical; 2008. [Google Scholar]
- 10.Adachi K, Klausner JD, Bristow CC, et al. Chlamydia and gonorrhea in HIV-infected pregnant women and infant HIV transmission. Sex Transm Dis, 2015; 42(10): 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol, 2004; 2(1): 33–42. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Guidelines for the management of sexually transmitted infections. 2003. [PubMed] [Google Scholar]
- 13.Medline A, Joseph Davey D, Klausner JD. Lost opportunity to save newborn lives: variable national antenatal screening policies for Neisseria gonorrhoeae and Chlamydia trachomatis. Int J STD AIDS 2017; 28(7): 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst de Cortina S, Bristow CC, Joseph Davey D, Klausner JD. A systematic review of point of care testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Infect Dis Obstet Gynecol 2016; 2016: 4386127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locksmith G, Duff P. Infection, antibiotics, and preterm delivery Seminars in Perinatology. Vol. 25 No. 5. WB Saunders, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Temmerman M, Njagi E, Nagelkerke N, et al. Mass antimicrobial treatment in pregnancy. A randomized placebo-controlled trial in a population with high rates of sexually transmitted disease. J Reprod Med, 1995; 40(3): 176–180. [PubMed] [Google Scholar]
- 17.Reekie J, Roberts C, Preen D, et al. Chlamydia trachomatis and the risk of spontaneous preterm birth, babies who are born small for gestational age, and stillbirth: a population-based cohort study. Lancet Infect Dis, 2018; 18(4): 452–460. [DOI] [PubMed] [Google Scholar]
- 18.Adamson PC, and Klausner JD. Treating chlamydial infections in pregnancy and preventing adverse birth outcomes. Lancet Infectious Diseases 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bristow CC, Mathelier P, Ocheretina O, et al. Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis screening and treatment of pregnant women in Port-au-Prince, Haiti. Int J STD AIDS, 2017; 28(11): 1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wynn A, Ramogola-Masire D, Gaolebale P, et al. Acceptability and feasibility of sexually transmitted infection testing and treatment among pregnant women in Gaborone, Botswana, 2015. BioMed Res Int 2016; 2016: 1251238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mudau M, Peters RP, De Vos L, et al. High prevalence of asymptomatic sexually transmitted infections among human immunodeficiency virus-infected pregnant women in a low-income South African community. Int J STD AIDS, 2018; 29(4): 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabeza J, García PJ, Segura E, et al. Feasibility of Chlamydia trachomatis screening and treatment in pregnant women in Lima, Peru: a prospective study in two large urban hospitals. Sex Transm Infect 2015; 91(1): 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chico RM, Hack BB, Newport MJ, et al. On the pathway to better birth outcomes? A systematic review of azithromycin and curable sexually transmitted infections. Expert Rev Anti Infect Ther, 2013; 11(12): 1303–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallamaa L, Cheung YB, Luntamo M, et al. The impact of maternal antenatal treatment with two doses of azithromycin and monthly sulphadoxine-pyrimethamine on child weight, mid-upper arm circumference and head circumference: A randomized controlled trial. PLoS One, 2019; 14(5): e0216536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Guidelines for the management of sexually transmitted infections. 2003, World Health Organization: Geneva. [PubMed] [Google Scholar]
- 26.WHO. Global strategy for the prevention and control of sexually transmitted infections: 2006–2015. 2007, World Health Organization: Geneva, Switzerland. [Google Scholar]
- 27.Morikawa E, Mudau M, Olivier D, et al. Acceptability and Feasibility of Integrating Point-of-Care Diagnostic Testing of Sexually Transmitted Infections into a South African Antenatal Care Program for HIV-Infected Pregnant Women. Infect Dis Obstet Gynecol, 2018; 2018: 3946862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen M, et al. Acceptability and feasibility of sexually transmissible infection screening among pregnant women in Hanoi, Vietnam. Sexual health, 2019. [DOI] [PubMed] [Google Scholar]
- 29.Farley TA, Cohen DA, and Elkins W Asymptomatic sexually transmitted diseases: the case for screening. Preventive Medicine, 2003; 36(4): 502–509. [DOI] [PubMed] [Google Scholar]
- 30.Mullick S, Watson-Jones D, Beksinska M, et al. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect, 2005; 81(4): 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsevat DG, Wiesenfeld HC, Parks C, et al. Sexually transmitted diseases and infertility. Am J Obstet Gynecol, 2017; 216(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verwijs MC, Agaba SK, Sumanyi JC, et al. Targeted point-of-care testing compared with syndromic management of urogenital infections in women (WISH): a cross-sectional screening and diagnostic accuracy study. Lancet Infect Dis, 2019; 19(6): 658–669. [DOI] [PubMed] [Google Scholar]
- 33.Unemo M and Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev, 2014; 27(3): 587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romoren M, Sundby J, Velauthapillai M, et al. Chlamydia and gonorrhoea in pregnant Batswana women: time to discard the syndromic approach? BMC Infect Dis, 2007; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romoren M, Velauthapillai M, Rahman M, et al. Trichomoniasis and bacterial vaginosis in pregnancy: inadequately managed with the syndromic approach. Bull World Health Organ, 2007; 85(4): 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katoba J, Kuupiel D, and Mashamba-Thompson TP. Toward Improving Accessibility of Point-of-Care Diagnostic Services for Maternal and Child Health in Low- and Middle-Income Countries. Point Care, 2019; 18(1): 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines: Chlamydial infections. Accessed 1 October 2019: https://www.cdc.gov/std/tg2015/chlamydia.htm.
- 38.Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines: Gonococcal infections. Accessed 1 October 2019: https://www.cdc.gov/std/tg2015/gonorrhea.htm.
- 39.Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines: Trichomoniasis. Accessed 1 October 2019: https://www.cdc.gov/std/tg2015/trichomoniasis.htm.
- 40.Keizur EM and Klausner JD. The need for new treatment recommendations for trichomoniasis among women. The Lancet Infectious Diseases 1811 (2018): 1168–1169. [DOI] [PubMed] [Google Scholar]
- 41.Chico RM, Chaponda EB, Ariti C, and Chandramohan D. Sulfadoxine-Pyrimethamine Exhibits Dose-Response Protection Against Adverse Birth Outcomes Related to Malaria and Sexually Transmitted and Reproductive Tract Infections. Clin Infect Dis, 2017; 64(8): 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]