Abstract
The sympathetic nervous system plays a pivotal role in the long-term regulation of arterial blood pressure through the ability of the central nervous system to integrate neurohumoral signals and differentially regulate sympathetic neural input to specific end organs. Part 1 of this review will discuss neural mechanisms of salt-sensitive hypertension, obesity-induced hypertension, and the ability of prior experiences to sensitize autonomic networks. Part 2 of this review focuses on new therapeutic advances to treat resistant hypertension including renal denervation and carotid baroactivation. Both advances lower arterial blood pressure by reducing sympathetic outflow. We discuss potential mechanisms and areas of future investigation to target the sympathetic nervous system.
RÉSUMÉ
Le système nerveux sympathique joue un rôle essentiel dans la régulation à long terme de la pression artérielle grâce à la capacité du système nerveux central à intégrer les signaux neurohumoraux et à réguler de manière différentielle la contribution neurale sympathique à des organes terminaux précis. La première partie de cette revue de littérature portera sur les mécanismes neuronaux de l’hypertension sensible à l’apport en sel, de l’hypertension induite par l’obésité et sur la capacité des expériences passées à sensibiliser les réseaux autonomes. La deuxième partie de cette revue de littérature se concentre sur les nouvelles avancées thérapeutiques pour traiter l’hypertension résistante, y compris la dénervation rénale et la stimulation des barorécepteurs de la carotide. Ces deux avancées permettent de réduire la pression artérielle en diminuant la réponse sympathique. Nous discutons des mécanismes potentiels et des domaines de recherche future ciblant le système nerveux sympathique.
The sympathetic nervous system regulates arterial blood pressure (ABP) by functionally influencing the vasculature, kidney, and heart. Indeed, altered sympathetic function is firmly established in the development, maintenance, and pathophysiology of numerous cardiovascular diseases including hypertension.1,2 Sympathetic hyperactivity in human hypertension has been revealed through elevated norepinephrine spillover, increased muscle sympathetic nerve activity via microneurography, surgical sympathectomy, and greater depressor responses to acute ganglionic blockade (see the paper by Grassi et al.2 for review). Notably, some antihypertensive pharmacotherapies lower ABP by targeting the sympathetic nervous system.2,3 Recent advances regarding the role of the sympathetic nervous system in hypertension have focused on brain pathways or associated signalling mechanisms that increase or decrease ABP. Parallel investigations have attempted to identify which sympathetic nerve(s) become hyperactive or whose modulation lowers ABP. This article discusses recent insights regarding the role of the brain and sympathetic nervous system in hypertension that specifically pertains to high salt intake and salt-sensitive hypertension, obesity-induced hypertension, and neuroplasticity driven by prior experiences. The second half of the article will discuss renal denervation and carotid baroactivation—two therapeutic advances to treat drug-resistant hypertension by targeting the sympathetic nervous system.
Salt-Sensitive Hypertension
Salt-sensitive hypertension is simply defined as an increase (or decrease) in ABP during chronic salt-loading (or salt-restriction). To date, clinical criteria or tests to identify salt-sensitive subjects have not been established, and the magnitude of the ABP changes (and change in dietary salt content) to define salt-sensitive hypertension varies from study to study. However, a neurogenic component of salt-sensitive hypertension is strongly supported by several lines of evidence that include the following: (1) salt-sensitive hypertension is associated with activation of the sympathetic nervous system,4–7 (2) blockade of sympathetic outflow or sympathetic nerve transection lowers ABP,8,9 (3) interruption of neurotransmission in several sympathetic-regulatory nuclei lowers and even normalizes sympathetic nerve activity (SNA) and/or ABP,10–12 and (4) lesion of the anteroventral third ventricular region of the hypothalamus prevents/attenuates the development or severity of multiple experimental models of salt-sensitive hypertension.13–15
One signal postulated to initiate and support sympathoexcitation in salt-sensitive hypertension is elevated plasma or cerebrospinal fluid NaCl concentration.16 A high-salt diet elevates plasma or cerebrospinal fluid [NaCl] by 2–6 mM in multiple experimental models including Dahl salt-sensitive, spontaneously hypertensive rat, Grollman renal wrap, and deoxycorticosterone-salt.7,17–25 Similar observations have been reported in human subjects.7,18,26,27 However, plasma electrolyte measurements from many human studies should be interpreted with caution as the majority of samples are collected in fasted subjects that may not reflect daytime electrolyte values. Acutely, an infusion of hypertonic NaCl in experimental animals produces a complex sympathetic response characterized by increased lumbar (or muscle) and adrenal SNA,28,29 no change in cardiac or splanchnic SNA,28–30 and decreased renal SNA.28–30 In humans, acute elevation in plasma NaCl increases muscle SNA.31 Both acute and chronic intracerebroventricular infusion of hypertonic NaCl to produce physiological changes in cerebrospinal fluid [NaCl] increase ABP.29,32–34 The relative importance of changes in plasma vs cerebrospinal fluid [NaCl] is not yet defined. Furthermore, the specific sympathetic nerve(s) or end organ(s) that contribute to salt-sensitive hypertension are not yet defined. However, celiac ganglionectomy reduces ABP in angiotensin II (AngII)-salt and Dahl-salt–sensitive hypertension,9,35 whereas renal denervation reduces ABP in deoxycorticosterone-salt and late-stage Dahl-salt–sensitive models.35,36
Changes in extracellular NaCl concentration are detected by specialized neurons located in circumventricular nuclei of the rostral hypothalamus—the subfornical organ and organum vasculosum of the lamina terminalis (OVLT)37,38 (Fig. 1). These structures are juxtaposed to the third ventricle and lack a complete blood-brain barrier, thereby sensing and responding to changes in electrolyte concentrations and neurohumoral factors in the circulation and cerebrospinal fluid.22,37,39 These structures project mono- or polysynaptically to hypothalamic, thalamic, and cortical structures to impact autonomic function.37–40 OVLT neurons are intrinsically sensitive to extracellular [NaCl] within physiological ranges (2–10 mM).28 Local injection of hypertonic NaCl into the OVLT increased lumbar SNA, adrenal SNA, and ABP. Inhibition of these neurons attenuated sympathoexcitatory responses to central infusion of hypertonic NaCl.28 Finally, the lesion of the OVLT region attenuates both AngII-salt and deoxycorticosterone-salt hypertension.41,42 The mechanism by which circumventricular organ neurons sense changes in [NaCl] remains unknown, but candidates include an N-terminal variant of the transient receptor potential vanilloid-1, Nax, ouabain, and the epithelial sodium channel (see the papers by Stocker et al.,16 Kinsman et al.,38 and Blaustein et al.43 for reviews) (Fig. 1).
Figure 1.
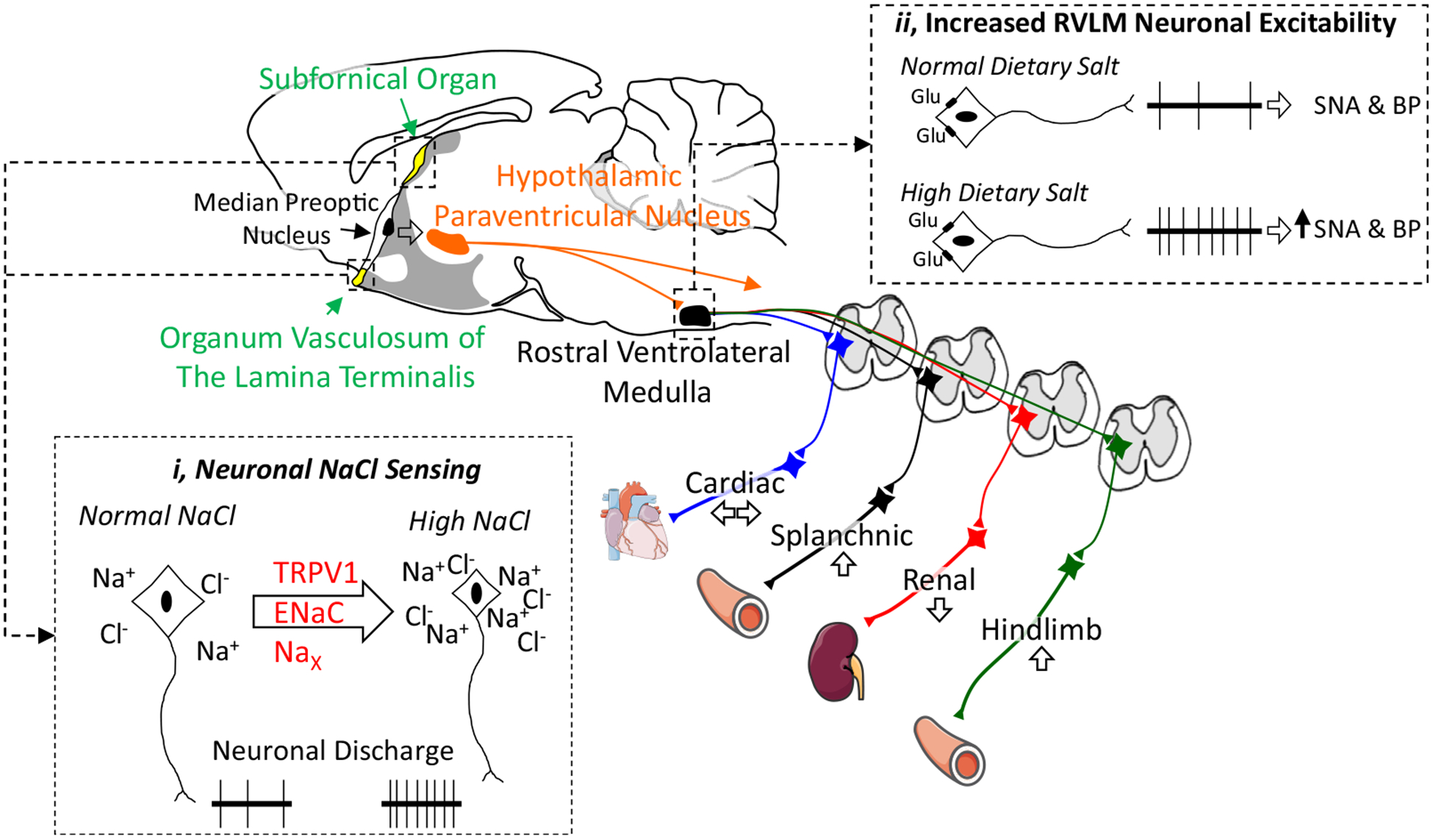
Dietary salt alters autonomic function by excitation of NaCl-sensing neurons in the lamina terminalis or increasing gain/excitability of bulbospinal neurons in the rostral ventrolateral medulla. Salt-sensitive hypertension is associated with increased sympathetic outflow to the splanchnic or hindlimb vasculature. A midsagittal section of the rodent brain illustrates key autonomic centres involved in salt-sensitive hypertension. (i) Neurons in the organum vasculosum of the lamina terminalis and subfornical organ sense changes in extracellular NaCl to increase sympathetic nerve activity. Potential NaCl-sensing mechanisms include an N-terminal variant of the transient receptor potential cation channel subfamily V member 1 (TRPV1), the epithelial sodium channel (ENaC), and the Nax channel. (ii) Dietary salt also increases the excitability or gain of bulbospinal sympathetic neurons of the rostral ventrolateral medulla. Thus, glutamatergic (or GABAergic) input onto RVLM neurons results in an exaggerated discharge and change in sympathetic nerve activity. BP, blood pressure; RVLM, rostral ventrolateral medulla; SNA, sympathetic nerve activity. Adapted from Servier Medical Art by Servier licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
The downstream pathways by which NaCl-sensitive pathways regulate SNA likely involve the hypothalamic paraventricular nucleus and rostral ventrolateral medulla (Fig. 1). Blockade of excitatory amino acid, AngII type 1, or vasopressin receptors in the hypothalamic paraventricular nucleus blunt acute pressor responses to hypertonic NaCl.44,45 Neurons of the hypothalamic paraventricular nucleus project to sympathetic preganglionic neurons of the spinal cord or bulbospinal neurons of the rostral ventrolateral medulla. The sympathoexcitatory and pressor responses to acute, central infusion of hypertonic NaCl are mediated by the latter.29 This circuitry is clinically significant as blockade of excitatory amino receptors or angiotensin type I receptors in the rostral ventrolateral medulla reduced ABP in Dahl-salt–sensitive rats fed a high-salt diet.10,11 Whether signalling mechanisms within these critical nuclei or circumventricular organs located outside the blood-brain barrier can be exploited for future therapeutic targets remains an area of future investigation.
Dietary salt intake may also sensitize autonomic circuits and predispose animals or humans to the development of hypertension. The majority of laboratory animals are “salt-resistant,” but excess dietary salt intake exaggerates or amplifies sympathetic and ABP responses of these animals to activation of sciatic afferents,46,47 exercise,48 stimulation of the aortic depressor nerve or vagal afferents,47,49 volume expansion,47 air-jet stress,33 insulin,50 and central NaCl.33,47 These effects occur independent of changes in baseline mean ABP or basal level of SNA.33,47 These amplified responses are attributed to a direct change in the excitability of neurons in the rostral ventrolateral medulla17,46,49,51,52 (Fig. 1). Exaggerated sympathetic reflexes due to chronic high salt intake were also associated with increased blood pressure variability.47 This observation has significant clinical ramifications as increased blood pressure variability predisposes individuals to end-organ damage and development of cardiovascular disease.53,54 In fact, increased blood pressure variability is a well-established predictor of future adverse cardiovascular events and disease.55–57 The mechanism(s) by which a high-salt diet “sensitizes” central autonomic networks without changes in baseline SNA or ABP has not been identified.
Obesity-Induced Hypertension
Obesity is a major risk factor for hypertension and may account for approximately two-thirds of essential hypertension.58,59 Sympathoexcitation in obesity-induced hypertension is supported by: (1) increased renal, but not cardiac, norepinephrine spillover in obese vs lean subjects;60,61 (2) muscle SNA is elevated in obese vs lean humans;62,63 (3) chronic sympathetic nerve recordings in rats or rabbits fed high-fat diets indicate that obesity has elevated lumbar or renal SNA;64–66 (4) pharmacologic blockade of adrenergic receptors67 or ganglionic blockade68 reduces ABP more in obesity vs lean subjects; and (5) bilateral renal denervation prevents the development of hypertension in dogs fed high-fat diet.69
The sympathoexcitation in obesity may be driven by several factors, most notably the peptide hormone insulin and the adipokine leptin (Fig. 2).58,59 Acute administration of leptin or insulin increases SNA in rodents and/or humans. Importantly, intracerebroventricular infusion of leptin or insulin receptor antagonists lowers ABP in high-fat–fed rabbits; however, receptor blockade for leptin but not insulin reduces renal SNA.70
Figure 2.
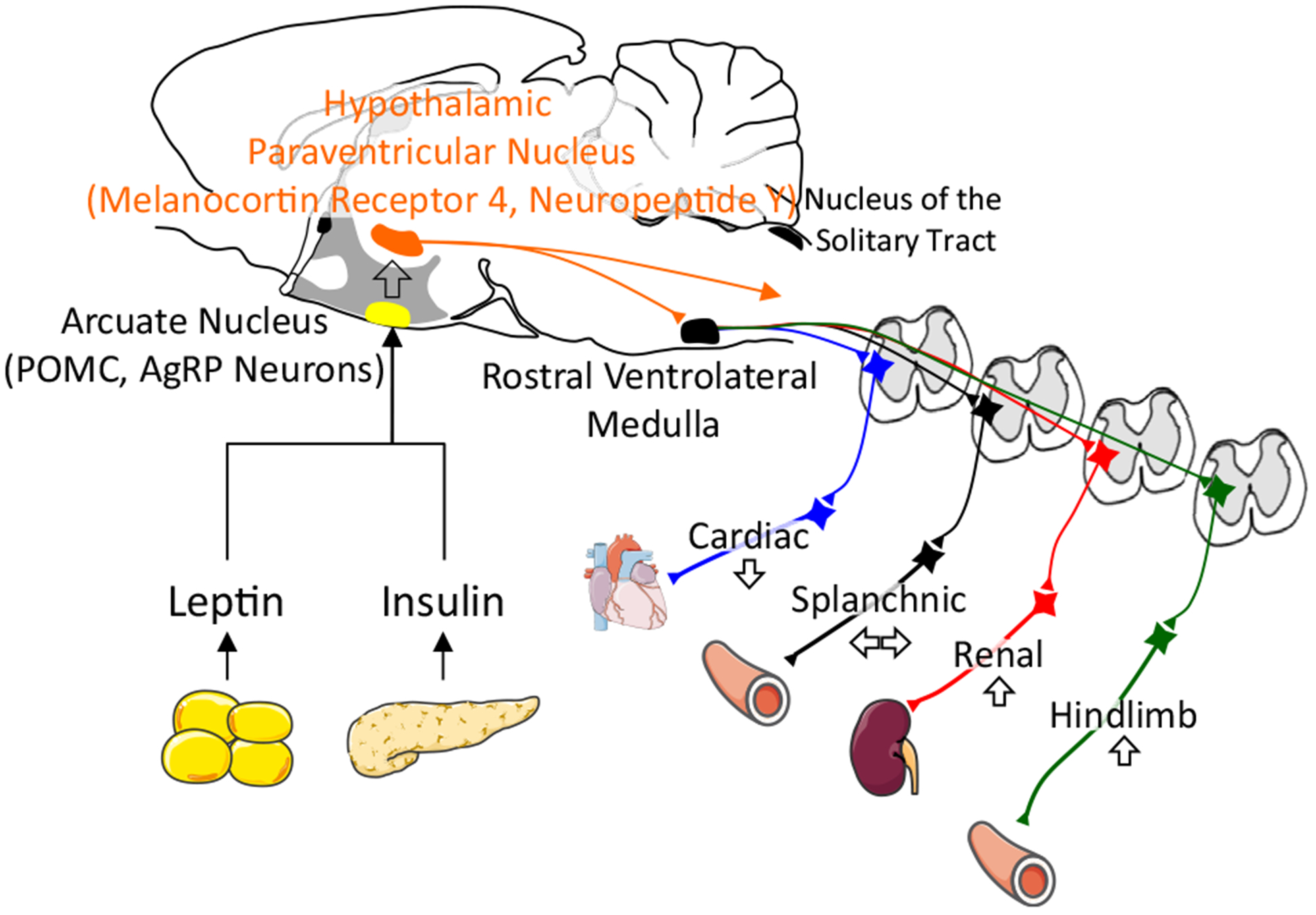
Model of sympathoexcitation in obesity-induced hypertension. Adipokines such as leptin and insulin act through proopiomelanocortin (POMC) or agouti related peptide/neuropeptide Y (AgRP) neurons in the arcuate nucleus to activate downstream pathways through the hypothalamic paraventricular nucleus and rostral ventrolateral medulla to increase sympathetic nerve activity to the kidney and hindlimb vasculature. Adapted from Servier Medical Art by Servier licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
The sympathoexcitatory actions of leptin and insulin are mediated by two distinct neuronal populations in the hypothalamic arcuate nucleus (Fig. 2). First, injection of leptin or insulin into the arcuate nucleus (location of proopiomelanocortin and agouti related peptide / neuropeptide Y neurons) increases SNA and/or ABP.71,72 Second, deletion of leptin receptors73 or neutralization of insulin via anti-insulin affibody71 within the arcuate nucleus attenuates the sympathoexcitatory responses. Third, deletion of leptin receptors in the arcuate nucleus lowers ABP in diet-induced obese mice.73 Downstream pathways likely involve the melanocortin system as pharmacologic blockade of central melanocortin receptors or deletion of melanocortin-4 receptors attenuates acute and chronic sympathoexcitatory effects.66,74–76 Selective deletion of leptin receptors on proopiomelanocortin neurons lowers ABP and prevents leptin-induced hypertension.77 The latter effects are replicated by interruption of leptin-associated signalling in arcuate and/or proopiomelanocortin neurons such as Src homology-2 tyrosine phosphatase, signal transducer and activator of transcription 3, insulin receptor substrate-2, and mammalian target of rapamycin (see the papers by Lim et al.58 and do Carmo et al.59 for review). Leptin and insulin may also act in multiple hypothalamic nuclei including the ventromedial or dorsomedial nuclei, the subfornical organ, hypothalamic paraventricular nucleus, and rostral ventrolateral medulla to increase SNA (see the papers by Lim et al.58 and do Carmo et al.59 for review). A parallel neuropeptide Y pathway may also contribute to leptin and insulin-induced sympathoexcitation.78,79
Sensitization of Autonomic Pathways to Produce Hypertension
Hypertension likely results from the combination of predisposing prior experiences, environmental factors, and genetic backgrounds. Recent evidence suggests that autonomic circuits can be sensitized by prior exposures to stimuli that subsequently alter future cardiovascular responses.80 For example, a 1-week subpressor AngII infusion followed by a 1-week washout produced exaggerated hypertensive responses to a second infusion of AngII.81 Central administration of AngII produced a similar sensitization effect that was prevented by brain AngII-receptor blockade.81 In addition, aldosterone,82 high-fat diet,83 leptin,84 and tumour necrosis-factor-alpha83 can also sensitize the hypertensive effect of a subsequent AngII infusion. Importantly, these effects are not specific to AngII-induced hypertension as prior exposures can exaggerate hypertensive effects of 2% NaCl loading.85 Within the brain, the mechanisms for autonomic circuit sensitization may be mediated by the brain renin-angiotensin-aldosterone system, N-methyl-d-aspartate receptor function, changes in cellular excitability via growth factors (ie, brain-derived neurotropic growth factor), and transcription factors.80 Notably, the prior discussion suggested that dietary salt intake “sensitizes” autonomic networks to a variety of inputs. A key difference between the two paradigms is the timing of the manipulations. As described above, various sympathetic reflexes were assessed during chronic salt loading. Here, the two stimuli are separated by time. Either situation produces exaggerated neurogenic cardiovascular responses to the second stimulus. Although the molecular mechanism(s) still need to be defined, the relevance of this paradigm is evident in considering life experiences that may impact the development of hypertension.
Therapeutic Advances to Treat Hypertension That Target the Sympathetic Nervous System
Hypertension increases the risk for adverse cardiovascular events.86 Although traditional lifestyle modifications and pharmacotherapies effectively reduce ABP, a significant proportion of hypertensive individuals remain uncontrolled due to treatment nonadherence or resistant hypertension.87,88 This population is associated with elevated or inappropriate levels of SNA.2,87,88 For this reason, novel adjunctive therapies have been developed. Here, we briefly discuss two advancements to reduce ABP through modulation of the sympathetic nervous system.
Renal nerves and renal denervation
Several, but not all, models of experimental hypertension are associated with elevated renal SNA, and renal denervation has been repeatedly reported to lower ABP in these particular models (see the papers by Lohmeier and Hall,89 Kiuchi et al.,90 and Osborn and Foss91 for review). Previous and ongoing clinical trials use various technology-based platforms (radio-frequency ablation, ultrasound energy deliver, vascular brachytherapy, and chemical ablation) to denervate renal nerves and lower ABP in human subject populations (reviewed elsewhere89,90). The majority of clinical trials report a reduction in systolic of approximately 6–9 mm Hg for 24-hour ABP and approximately 11–16 mm Hg for office ABP measurements after sham group and baseline adjustments.89,90
Renal nerves are composed of efferent (sympathetic) and afferent (sensory) fibres that coordinate renal function, central haemodynamics, and ABP (Fig. 3). Renal efferent nerves increase renin secretion, promote tubular sodium reabsorption, and regulate renal blood flow.92 These three functions are proposed to underlie the antihypertensive effects of renal nerve denervation.91,93,94 Alternatively, stimulation of renal afferent fibres in animals increases ABP,95–97 and renal afferent nerve activity is elevated in deoxycorticosterone-salt rats36 and renovascular hypertensive mice.97 In these models, dorsal rhizotomy98,99 or selective chemical ablation of renal afferent fibres reduces ABP to the same extent as total renal denervation.97 Importantly, renal afferent denervation does not always lower ABP in a model where total renal denervation is effective.89,100 In human studies, renal denervation has been reported to lower muscle SNA101–103 and plasma glucose,104,105 and reduce cardiac arrhythmias,106 thereby supporting a potential role for a neurohumoral signal originating from the kidney. Renal afferent fibres densely innervate the pelvic wall, renal vasculature, and tubules,107,108 and respond to both mechano- and chemosensitive stimuli (Fig. 3). These neurons contain calcitonin gene-related peptide and substance P,109 but the neurochemical profiles have not been linked to sensory modalities and haemodynamic responses. Whether neurochemically distinct populations of renal efferent or afferent neurons, brain pathways, or signalling mechanisms are useful to manipulate systemic haemodynamics in hypertension warrants future investigation.
Figure 3.
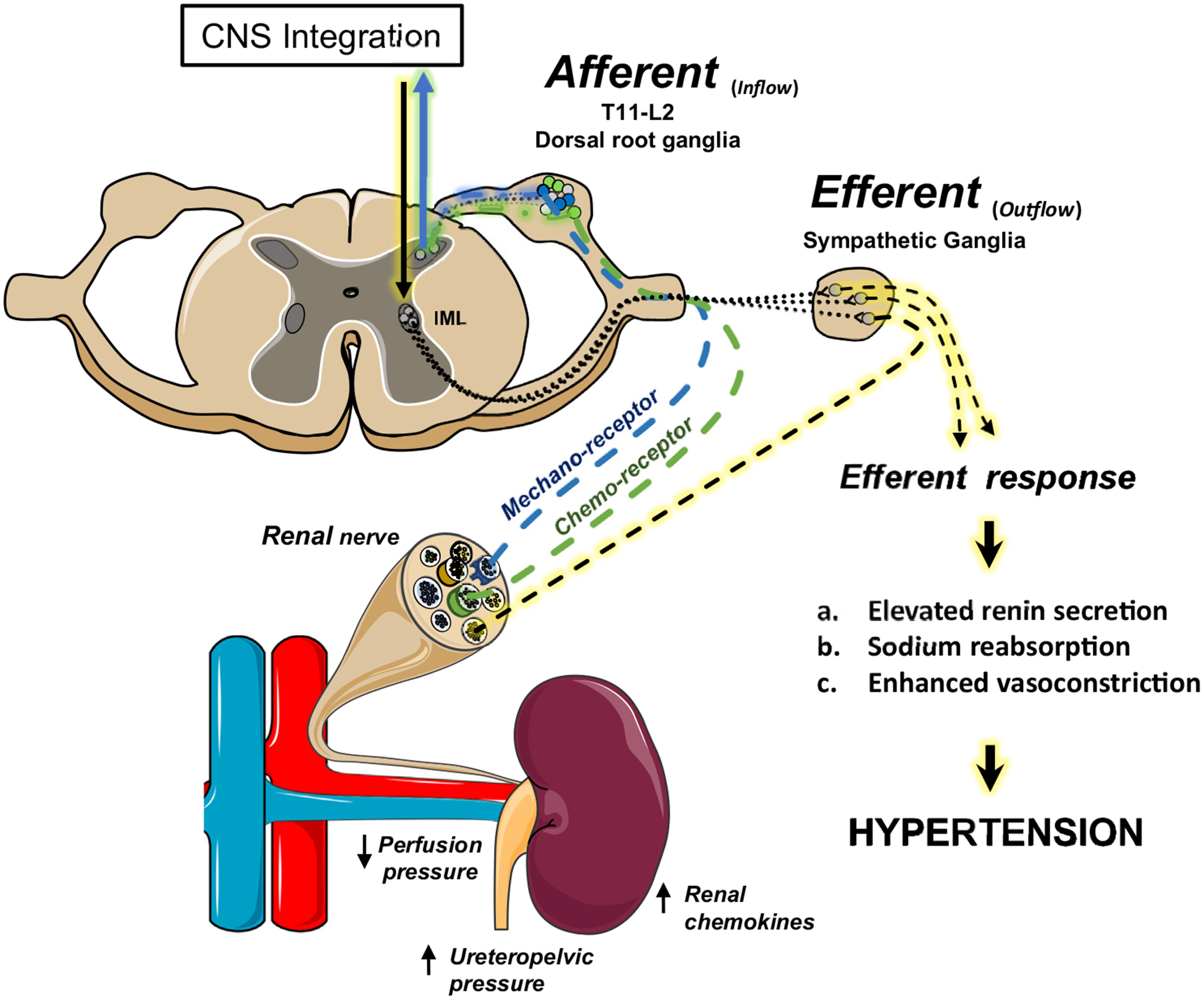
Renal efferent and/or afferent fibres contribute to hypertension. Sympathetic efferent nerves increase renin secretion, promote tubular sodium reabsorption, and regulate renal blood flow. Renal sensory nerves are activated by altered renal perfusion pressure or accumulation of parenchymal chemokines and project through the dorsal root ganglion to the central nervous system (CNS). Adjunctive renal nerve ablation interrupts both afferent sensory responses and efferent signals crucial for regulating the renin-angiotensin-aldosterone system, fluid-electrolyte balance, and peripheral haemodynamics. IML, intermediolateral cell column. Adapted from Servier Medical Art by Servier licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
Major obstacles regarding the clinical applicability of renal denervation are assessment of the degree of denervation and identification of patient cohorts who effectively respond to denervation therapy. First, there is currently no intraoperative validation measurement to test the degree of denervation after adjunctive ablation. Outcome measurements largely rely on follow-up ABP measurements. Transvascular pacing of the aorticorenal ganglion,110 renal norepinephrine spillover measurements,111 and guided intraoperative renal nerve stimulation112,113 have emerged as potential solutions to: (1) test the degree of renal denervation after ablation and (2) improve denervation efficacy by anatomically mapping renal arteries for neurally innervated regions. Second, a subset of experimental hypertension models have elevated or inappropriate levels of renal SNA. Currently, there is no routine or reliable technique to assess renal SNA in clinical practice. Thus, the failure of renal denervation to lower ABP in experimental or human hypertension should not be interpreted as a lack of sympathetic hyperactivity, but rather a function of hypertension etiology. In fact, celiac ganglionectomy has recently been shown to reduce ABP in experimental models associated with normal or reduced renal SNA.9,35,114,115
Baroreceptors and chronic baroactivation
Arterial baroreceptors provide a continuous ABP signal to the brain through mechanosensitive sensory endings embedded mainly in the aortic arch or carotid sinus116–118 (Fig. 4). These afferent fibres (composed of A and C types with distinct properties) travel through the carotid sinus and aortic depressor nerve, project to the brainstem via IXth and Xth cranial nerves, and synapse on second-order sensory neurons in the nuclei of the solitary tracts. Increased afferent activity due to an elevated ABP causes a reflexive decrease in ABP that reduces SNA directed to the vasculature and heart (thereby decreasing total peripheral resistance and cardiac output) and increases parasympathetic drive to the heart (thereby decreasing cardiac output). There is still uncertainty regarding the mechanosensitive transduction mechanisms116 and how such mechanisms respond to chronic disease states. Although baroreceptors likely adapt to chronic changes, referred to as baroreceptor resetting, how much baroreflex resetting occurs or can be attributed to peripheral vs central processing remains unclear.116–118
Figure 4.
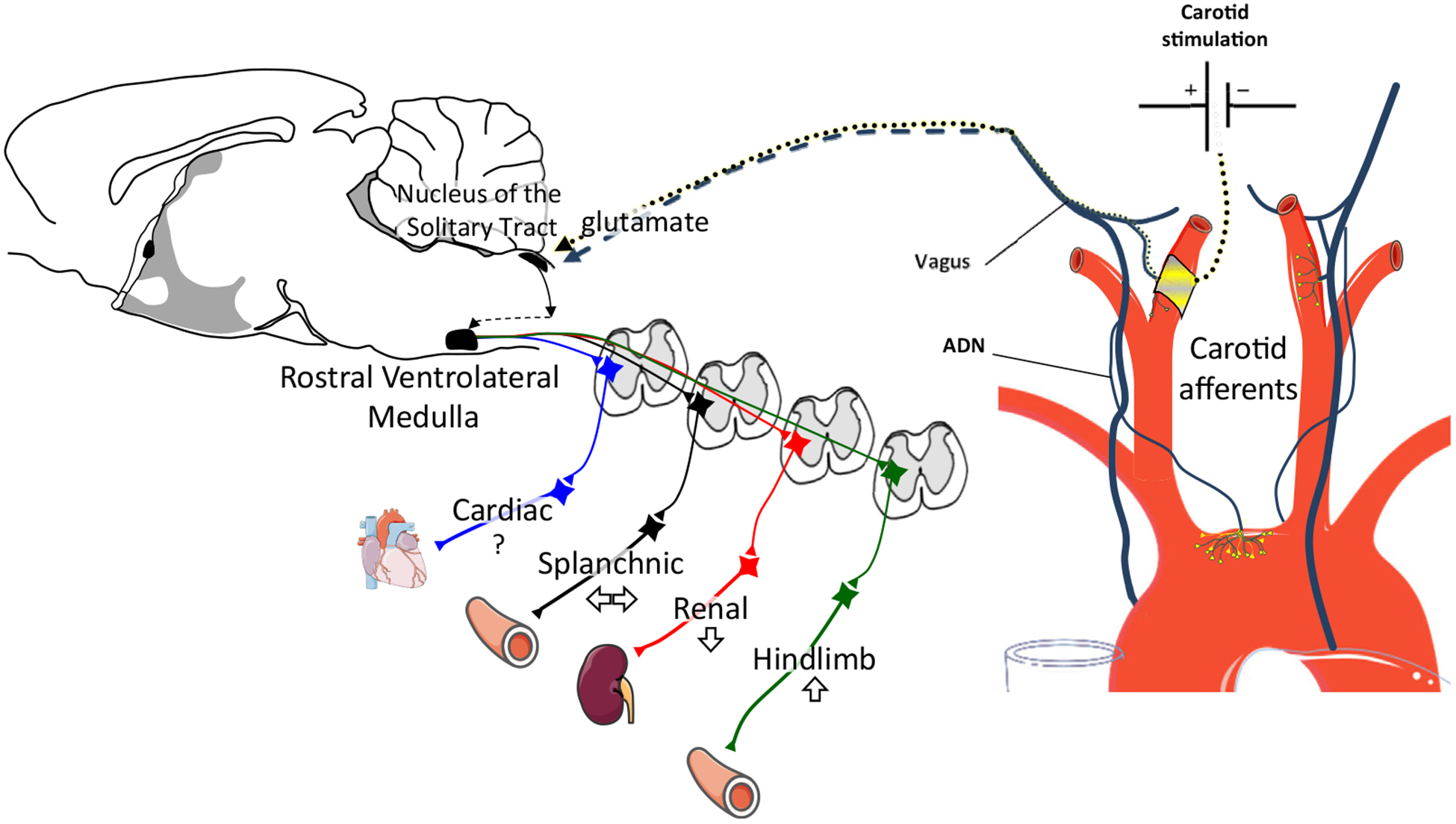
Carotid baroactivation lowers ABP. Chronic electrical stimulation of carotid afferent fibres produces a renal sympathoinhibition and reduces hypertension in some but not all experimental models of hypertension. The chronic activation of carotid afferent fibres likely activates a multisynaptic pathway in the hindbrain to increase GABAergic-mediated inhibition of bulbospinal neurons in the rostral ventrolateral medulla. How the elevated activity of carotid afferents due to the electrical stimulation is integrated with aortic baroreceptor afferents (which are not electrically stimulated) to produce a sustained decrease in sympathetic nerve activity and ABP remains unknown. ABP, arterial blood pressure; ADN, aortic depressor nerve. Adapted from Servier Medical Art by Servier licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
Multiple clinical trials have reported that chronic electrical stimulation of carotid baroreceptor afferent nerves lowers ABP (see the paper by Lohmeier and Hall89 for review) and is supported by experimental work that showed sustained reductions in SNA to reduce ABP.89 Moreover, chronic bilateral stimulation of baroreceptor afferent nerves in normotensive dogs decreased ABP by 20 mm Hg over 3 weeks and reduced plasma norepinephrine levels and norepinephrine spillover.119,120 Sustained reductions in ABP were similarly obtained in a high-fat model of obesity-induced hypertension, but not AngII-induced hypertension.121,122 Why is there a differential effect of carotid baroactivation between models, and could this identify patient populations that respond to such treatments? Lohmeier et al. suggest that the lack of a sustained response in AngII hypertension is consistent with a lower level of renal SNA.89,121 Although this may explain reduced baroactivation responses, it does not fully explain why ABP reductions are not sustained during AngII-hypertension compared with obesity-induced hypertension and chronic baroactivation, and may reflect how SNA is differentially regulated. Nonetheless, these observations are important as the therapeutic efficacy may be variable and dependent on the etiology of the hypertension.
An interesting observation from both clinical and experimental studies is the rapid increase in ABP when stimulators are turned off. Clearly, chronic activation of carotid afferents suppresses ABP. This raises interesting questions regarding the synaptic integration and processing of baroreceptor inputs. For example, how are inputs from the aortic depressor nerve and the electrically stimulated carotid sinus afferents integrated within the brainstem? How are the synaptic dynamics of A- vs C-fibres impacted? Although questions remain, the clinical and experimental work of carotid stimulation to reduce ABP again highlights the sympathetic nervous system as a potential therapeutic target for the treatment of hypertension.
Funding Sources
The authors would like to acknowledge research support by National Heart, Lung, and Blood Institute Grants R01 HL-145875 (S.D.S.), R01 HL-128388 (S.D.S.), and National Institute of Diabetes and Digestive and Kidney Diseases F32 DK123994 (L.J.D.).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Esler M, Straznicky N, Eikelis N, et al. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 2006;48: 787–96. [DOI] [PubMed] [Google Scholar]
- 2.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 2015;116:976–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grassi G. Sympathomodulatory effects of antihypertensive drug treatment. Am J Hypertens 2016;29:665–75. [DOI] [PubMed] [Google Scholar]
- 4.King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 2008;294:R1262–7. [DOI] [PubMed] [Google Scholar]
- 5.Leenen FH, Ruzicka M, Huang BS. The brain and salt-sensitive hypertension. Curr Hypertens Rep 2002;4:129–35. [DOI] [PubMed] [Google Scholar]
- 6.Osborn JW, Fink GD. Region specific changes in sympathetic nerve activity in AngII-salt hypertension. Exp Physiol 2010;95:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidlin O, Forman A, Sebastian A, Morris RC Jr. Sodium-selective salt sensitivity: its occurrence in blacks. Hypertension 2007;50: 1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 2005;289: H1519–29. [DOI] [PubMed] [Google Scholar]
- 9.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 2007;50:547–56. [DOI] [PubMed] [Google Scholar]
- 10.Ito S, Hiratsuka M, Komatsu K, et al. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension 2003;41:744–50. [DOI] [PubMed] [Google Scholar]
- 11.Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension 2001;37:687–91. [PubMed] [Google Scholar]
- 12.Nakata T, Takeda K, Itho H, et al. Paraventricular nucleus lesions attenuate the development of hypertension in DOCA/salt-treated rats. Am J Hypertens 1989;2:625–30. [DOI] [PubMed] [Google Scholar]
- 13.Berecek KH, Barron KW, Webb RL, Brody MJ. Vasopressin-central nervous system interactions in the development of DOCA hypertension. Hypertension 1982;4:131–7. [PubMed] [Google Scholar]
- 14.Goto A, Ganguli M, Tobian L, Johnson MA, Iwai J. Effect of an anteroventral third ventricle lesion on NaCl hypertension in Dahl salt-sensitive rats. Am J Physiol 1982;243:H614–8. [DOI] [PubMed] [Google Scholar]
- 15.Sanders BJ, Johnson AK. Lesions of the anteroventral third ventricle prevent salt-induced hypertension in the borderline hypertensive rat. Hypertension 1989;14:619–22. [DOI] [PubMed] [Google Scholar]
- 16.Stocker SD, Monahan KD, Browning KN. Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr Hypertens Rep 2013;15:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension 2009;54: 308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension 2005;45: 98–102. [DOI] [PubMed] [Google Scholar]
- 19.O’Donaughy TL, Brooks VL. Deoxycorticosterone acetate-salt rats: hypertension and sympathoexcitation driven by increased NaCl levels. Hypertension 2006;47:680–5. [DOI] [PubMed] [Google Scholar]
- 20.O’Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension 2006;48:658–63. [DOI] [PubMed] [Google Scholar]
- 21.Stocker SD, Osborn JL, Carmichael SP. Forebrain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol 2008;35: 695–700. [DOI] [PubMed] [Google Scholar]
- 22.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 2010;588: 3375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suckling RJ, He FJ, Markandu ND, Macgregor GA. Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int 2012;81:407–11. [DOI] [PubMed] [Google Scholar]
- 24.Komiya I, Yamada T, Takasu N, et al. An abnormal sodium metabolism in Japanese patients with essential hypertension, judged by serum sodium distribution, renal function and the renin-aldosterone system. J Hypertens 1997;15:65–72. [DOI] [PubMed] [Google Scholar]
- 25.Wannamethee G, Whincup PH, Shaper AG, Lever AF. Serum sodium concentration and risk of stroke in middle-aged males. J Hypertens 1994;12:971–9. [PubMed] [Google Scholar]
- 26.Kawano Y, Yoshida K, Kawamura M, et al. Sodium and noradrenaline in cerebrospinal fluid and blood in salt-sensitive and non-salt-sensitive essential hypertension. Clin Exp Pharmacol Physiol 1992;19:235–41. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med 1978;64: 193–8. [DOI] [PubMed] [Google Scholar]
- 28.Kinsman BJ, Simmonds SS, Browning KN, Stocker SD. Organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension 2017;69:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stocker SD, Lang SM, Simmonds SS, Wenner MM, Farquhar WB. Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension 2015;66:1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frithiof R, Xing T, McKinley MJ, May CN, Ramchandra R. Intracarotid hypertonic sodium chloride differentially modulates sympathetic nerve activity to the heart and kidney. Am J Physiol Regul Integr Comp Physiol 2014;306:R567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farquhar WB, Wenner MM, Delaney EP, Prettyman AV, Stillabower ME. Sympathetic neural responses to increased osmolality in humans. Am J Physiol Heart Circ Physiol 2006;291:H2181–6. [DOI] [PubMed] [Google Scholar]
- 32.Huang BS, Ahmad M, Deng AY, Leenen FH. Neuronal responsiveness to central Na+ in 2 congenic strains of Dahl salt-sensitive rats. Hypertension 2007;49:1315–20. [DOI] [PubMed] [Google Scholar]
- 33.Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol 2001;281:H1881–9. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura M, Ohtsuka K, Nanbu A, Takahashi H, Yoshimura M. Benzamil blockade of brain Na+ channels averts Na(+)-induced hypertension in rats. Am J Physiol 1998;274:R635–44. [DOI] [PubMed] [Google Scholar]
- 35.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 2013;61:806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banek CT, Knuepfer MM, Foss JD, et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 2016;68:1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 2008;9:519–31. [DOI] [PubMed] [Google Scholar]
- 38.Kinsman BJ, Nation HN, Stocker SD. Hypothalamic signaling in body fluid homeostasis and hypertension. Curr Hypertens Rep 2017;19:50. [DOI] [PubMed] [Google Scholar]
- 39.McKinley MJ, McAllen RM, Davern P, et al. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 2003;172(III-XII):1–122. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman CA, Leib DE, Knight ZA. Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci 2017;18:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collister JP, Nahey DB, Hartson R, et al. Lesion of the OVLT markedly attenuates chronic deoxycorticosterone acetate (DOCA) salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 2018;315:R568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collister JP, Olson MK, Nahey DB, Vieira AA, Osborn JW. OVLT lesion decreases basal arterial pressure and the chronic hypertensive response to AngII in rats on a high-salt diet. Physiol Rep 2013;1: e00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaustein MP, Leenen FH, Chen L, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol 2012;302:H1031–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 2001;281:R1844–53. [DOI] [PubMed] [Google Scholar]
- 45.Son SJ, Filosa JA, Potapenko ES, et al. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron 2013;78:1036–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol 1999;276:R1600–7. [DOI] [PubMed] [Google Scholar]
- 47.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 2014;64:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamauchi K, Tsuchimochi H, Stone AJ, Stocker SD, Kaufman MP. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol 2014;306:H450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pawloski-Dahm CM, Gordon FJ. Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension 1993;22:929–33. [DOI] [PubMed] [Google Scholar]
- 50.Muntzel MS, Crespo R, Joseph T, Onwumere O. Dietary salt loading exacerbates the increase in sympathetic nerve activity caused by intravenous insulin infusion in rats. Metabolism 2007;56:373–9. [DOI] [PubMed] [Google Scholar]
- 51.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension 2007;50:354–9. [DOI] [PubMed] [Google Scholar]
- 52.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension 2008;52:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 1987;5:93–8. [DOI] [PubMed] [Google Scholar]
- 54.Tatasciore A, Renda G, Zimarino M, et al. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 2007;50:325–32. [DOI] [PubMed] [Google Scholar]
- 55.Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension 2012;59:212–8. [DOI] [PubMed] [Google Scholar]
- 56.Muntner P, Shimbo D, Tonelli M, et al. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011;57:160–6. [DOI] [PubMed] [Google Scholar]
- 57.Shimbo D, Newman JD, Aragaki AK, et al. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women’s Health Initiative. Hypertension 2012;60:625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim K, Jackson KL, Sata Y, Head GA. Factors responsible for obesity-related hypertension. Curr Hypertens Rep 2017;19:53. [DOI] [PubMed] [Google Scholar]
- 59.do Carmo JM, da Silva AA, Wang Z, et al. Obesity-induced hypertension: brain signaling pathways. Curr Hypertens Rep 2016;18:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rumantir MS, Vaz M, Jennings GL, et al. Neural mechanisms in human obesity-related hypertension. J Hypertens 1999;17:1125–33. [DOI] [PubMed] [Google Scholar]
- 61.Vaz M, Jennings G, Turner A, et al. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 1997;96:3423–9. [DOI] [PubMed] [Google Scholar]
- 62.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 2002;106:2533–6. [DOI] [PubMed] [Google Scholar]
- 63.Grassi G, Seravalle G, Cattaneo BM, et al. Sympathetic activation in obese normotensive subjects. Hypertension 1995;25:560–3. [DOI] [PubMed] [Google Scholar]
- 64.Muntzel MS, Al-Naimi OA, Barclay A, Ajasin D. Cafeteria diet increases fat mass and chronically elevates lumbar sympathetic nerve activity in rats. Hypertension 2012;60:1498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armitage JA, Burke SL, Prior LJ, et al. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension 2012;60: 163–71. [DOI] [PubMed] [Google Scholar]
- 66.Lim K, Barzel B, Burke SL, Armitage JA, Head GA. Origin of aberrant blood pressure and sympathetic regulation in diet-induced obesity. Hypertension 2016;68:491–500. [DOI] [PubMed] [Google Scholar]
- 67.Wofford MR, Anderson DC Jr, Brown CA, et al. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens 2001;14:694–8. [DOI] [PubMed] [Google Scholar]
- 68.Shibao C, Gamboa A, Diedrich A, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension 2007;49: 27–33. [DOI] [PubMed] [Google Scholar]
- 69.Kassab S, Kato T, Wilkins FC, et al. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 1995;25:893–7. [DOI] [PubMed] [Google Scholar]
- 70.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension 2013;61: 628–34. [DOI] [PubMed] [Google Scholar]
- 71.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol 2013;304:H1538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi Z, Li B, Brooks VL. Role of the paraventricular nucleus of the hypothalamus in the sympathoexcitatory effects of leptin. Hypertension 2015;66:1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harlan SM, Morgan DA, Agassandian K, et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res 2011;108:808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 2003;23:5998–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 2006;48:58–64. [DOI] [PubMed] [Google Scholar]
- 76.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 2011;57:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.do Carmo JM, da Silva AA, Cai Z, et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension 2011;57:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cassaglia PA, Shi Z, Li B, et al. Neuropeptide Y acts in the paraventricular nucleus to suppress sympathetic nerve activity and its baroreflex regulation. J Physiol 2014;592:1655–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cassaglia PA, Shi Z, Brooks VL. Insulin increases sympathetic nerve activity in part by suppression of tonic inhibitory neuropeptide Y inputs into the paraventricular nucleus in female rats. Am J Physiol Regul Integr Comp Physiol 2016;311:R97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson AK, Zhang Z, Clayton SC, et al. The roles of sensitization and neuroplasticity in the long-term regulation of blood pressure and hypertension. Am J Physiol Regul Integr Comp Physiol 2015;309: R1309–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertension 2012;59:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension 2012;60:1023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xue B, Thunhorst RL, Yu Y, et al. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II-elicited hypertension. Hypertension 2016;67:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xue B, Yu Y, Zhang Z, et al. Leptin mediates high-fat diet sensitization of angiotensin II-elicited hypertension by upregulating the brain renin-angiotensin system and inflammation. Hypertension 2016;67:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clayton SC, Zhang Z, Beltz T, Xue B, Johnson AK. CNS neuroplasticity and salt-sensitive hypertension induced by prior treatment with subpressor doses of ANG II or aldosterone. Am J Physiol Regul Integr Comp Physiol 2014;306:R908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360: 1903–13. [DOI] [PubMed] [Google Scholar]
- 87.Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment-resistant hypertension in the United States: comparison of the 2008 and 2018 American Heart Association Scientific Statements on Resistant Hypertension. Hypertension 2019;73: 424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai A, Calhoun DA. Resistant hypertension: an update of experimental and clinical findings. Hypertension 2017;70:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lohmeier TE, Hall JE. Device-based neuromodulation for resistant hypertension therapy. Circ Res 2019;124:1071–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kiuchi MG, Esler MD, Fink GD, et al. Renal denervation update from the International Sympathetic Nervous System Summit: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:3006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Osborn JW, Foss JD. Renal nerves and long-term control of arterial pressure. Compr Physiol 2017;7:263–320. [DOI] [PubMed] [Google Scholar]
- 92.Dibona GF, Kopp UC. Neural control of renal function. Physiol Rev 1997;77:75–197. [DOI] [PubMed] [Google Scholar]
- 93.Fisher JP, Paton JFR. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens 2012;26:463–75. [DOI] [PubMed] [Google Scholar]
- 94.Foss JD, Fiege J, Shimizu Y, et al. Role of afferent and efferent renal nerves in the development of AngII-salt hypertension in rats. Physiol Rep 2018;6:e13602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst 1983;8:273–85. [DOI] [PubMed] [Google Scholar]
- 96.Calaresu F, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst 1981;3:311–20. [DOI] [PubMed] [Google Scholar]
- 97.Ong J, Kinsman B, Sved A, et al. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol 2019;122:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oparil S, Sripairojthikoon W, Wyss JM. The renal afferent nerves in the pathogenesis of hypertension. Can J Physiol Pharmacol 1987;65: 1548–58. [DOI] [PubMed] [Google Scholar]
- 99.Wyss J, Aboukarsh N, Oparil S. Sensory denervation of the kidney attenuates renovascular hypertension in the rat. Am J Physiol 1986;250: H82–6. [DOI] [PubMed] [Google Scholar]
- 100.Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol 2016;310: R262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grassi G, Seravalle G, Brambilla G, et al. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension 2015;65:1209–16. [DOI] [PubMed] [Google Scholar]
- 102.Hering D, Marusic P, Duval J, et al. Effect of renal denervation on kidney function in patients with chronic kidney disease. Int J Cardiol 2017;232:93–7. [DOI] [PubMed] [Google Scholar]
- 103.Hering D, Marusic P, Walton AS, et al. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 2014;64:118–24. [DOI] [PubMed] [Google Scholar]
- 104.Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011;123:1940–6. [DOI] [PubMed] [Google Scholar]
- 105.Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011;58:559–65. [DOI] [PubMed] [Google Scholar]
- 106.Ukena C, Mahfoud F, Ewen S, et al. Renal denervation for treatment of ventricular arrhythmias: data from an International Multicenter Registry. Clin Res Cardiol 2016;105:873–9. [DOI] [PubMed] [Google Scholar]
- 107.Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol 1991;311:389–404. [DOI] [PubMed] [Google Scholar]
- 108.Ferguson M, Bell C. Ultrastructural localization and characterization of sensory nerves in the rat kidney. J Comp Neurol 1988;274:9–16. [DOI] [PubMed] [Google Scholar]
- 109.Mulder J, Hökfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 2013;304:R675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qian PC, Barry MA, Lu J, et al. Transvascular pacing of aorticorenal ganglia provides a testable procedural endpoint for renal artery denervation. JACC Cardiovasc Interv 2019;12:1109–20. [DOI] [PubMed] [Google Scholar]
- 111.Tzafriri AR, Mahfoud F, Keating JH, et al. Procedural and anatomical determinants of multielectrode renal denervation efficacy: insights from preclinical models. Hypertension 2019;74:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tan K, Lai Y, Chen W, et al. Selective renal denervation guided by renal nerve stimulation: mapping renal nerves for unmet clinical needs. J Hum Hypertens 2019;33:716–24. [DOI] [PubMed] [Google Scholar]
- 113.Liu H, Chen W, Lai Y, et al. Selective renal denervation guided by renal nerve stimulation in canine: a method for identification of optimal ablation target. Hypertension 2019;74:536–45. [DOI] [PubMed] [Google Scholar]
- 114.Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol 2011;301:H1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci 2010;154:66–73. [DOI] [PubMed] [Google Scholar]
- 116.Stocker SD, Sved AF, Andresen MC. Missing pieces of the Piezo1/Piezo2 baroreceptor hypothesis: an autonomic perspective. J Neurophysiol 2019;122:1207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 1994;56:93–116. [DOI] [PubMed] [Google Scholar]
- 118.Kumada M, Terui N, Kuwaki T. Arterial baroreceptor reflex: its central and peripheral neural mechanisms. Prog Neurobiol 1990;35:331–61. [DOI] [PubMed] [Google Scholar]
- 119.Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension 2004;43:306–11. [DOI] [PubMed] [Google Scholar]
- 120.Lohmeier TE, Iliescu R, Dwyer TM, et al. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol 2010;299: H402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lohmeier TE, Dwyer TM, Hildebrandt DA, et al. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension 2005;46:1194–200. [DOI] [PubMed] [Google Scholar]
- 122.Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension 2007;49:1307–14. [DOI] [PubMed] [Google Scholar]


