Nitro-fatty acids (NO2-FAs) have emerged as a new class of bioactive lipids that mediate metabolic and anti-inflammatory signaling actions. Formed endogenously by the reaction of reactive nitrogen species with unsaturated fatty acids, NO2-FAs modulate signaling actions mainly via nitro-alkylation of cysteine or histidine residues in transcriptional regulatory proteins. Initial studies identified NO2-FAs as potent activators of peroxisome proliferator-activated receptors (PPAR), mainly PPARɣ [1]. Subsequently, NO2-FAs were found to potently inhibit nuclear factor kappa B (NF-κB) signaling [2], and to induce nuclear factor erythroid 2-related factor 2 (Nrf2) signaling [3]. Thus, mediated through post translational modification of key regulatory proteins, NO2-FAs modulate glucose and lipid metabolism as well as inflammatory and anti-oxidant responses. As these pathways play major roles in the pathogenesis of cardiometabolic diseases, NO2-FAs have demonstrated protective effects in numerous relevant disease models.
Characterized by imbalanced lipid metabolism, increased oxidative stress and inflammation, atherosclerosis is the underlying cause of most cardiovascular diseases (CVD). While NO2-FAs are known to reduce vascular inflammation by inhibiting toll-like receptor 4 and NF-κB signaling [4], recent reports have improved our understanding of NO2-FA metabolism during inflammation and their anti-inflammatory properties. Conjugated linoleic acid (CLA) is a major fatty acid target of nitration reactions. Formation of nitrated CLA (NO2-CLA) was recently identified in response to acute inflammation both in vitro and in vivo. In turn, NO2-CLA inhibited NF-κB-dependent gene expression, decreased pro-inflammatory cytokine production and induced Nrf2-regulated anti-oxidant proteins [5*]. The stimulator of interferon genes (STING) has emerged as a critical signaling molecule in immunity and inflammation. NO2-CLA was found to be formed in response to virus infection and together with nitrated oleic acid (NO2-OA) was shown to inhibit STING signaling via nitro-alkylation, attenuating the release of type I INF (INF) [6**]. Indeed, the potent anti-inflammatory and anti-oxidant properties of NO2-FAs were shown by Rudolph et al. [7**] to reduce atherosclerosis in apolipoprotein E-deficient (Apoe−/−) mice fed an atherogenic diet.
The potent effects of NO2-FAs were described in the major cell types involved in the pathogenesis of atherosclerosis (Figure 1). In endothelial cells, nitrated linoleic acid (NO2-LA) and NO2-OA inhibited lipopolysaccharides (LPS)-induced expression of vascular cell adhesion molecule-1 and monocyte adhesion [2]. This was recently supported by unbiased RNA sequencing, revealing a distinct transcriptome regulated by NO2-CLA in primary human coronary artery endothelial cells. Modulation of pathways regulating inflammation, oxidative stress, fluid shear stress and atherosclerosis suggested that NO2-CLA inhibited endothelial dysfunction, a crucial step in atherosclerosis initiation [8]. Macrophage foam cell formation is a hallmark feature of early atherosclerosis and was shown to be attenuated by NO2-OA which inhibited oxidized low-density lipoprotein (oxLDL)-induced phosphorylation of signal transducer and activator of transcription-1 [7**], increased activity of endogenous anti-oxidants (glutathione and paraoxonase-2) and modulated lipid metabolism in macrophages [9]. Vascular smooth muscle cells are involved in various processes throughout the progression of atherosclerosis. Unbiased transcriptomic profiling revealed that NO2-CLA regulated critical pathways in human coronary artery smooth muscle cells, including cell proliferation, anti-oxidant response, lipid metabolism and inflammation [10].
Fig. 1. NO2-FAs for the co-treatment of atherosclerosis and NAFLD: molecular and metabolic mechanisms.
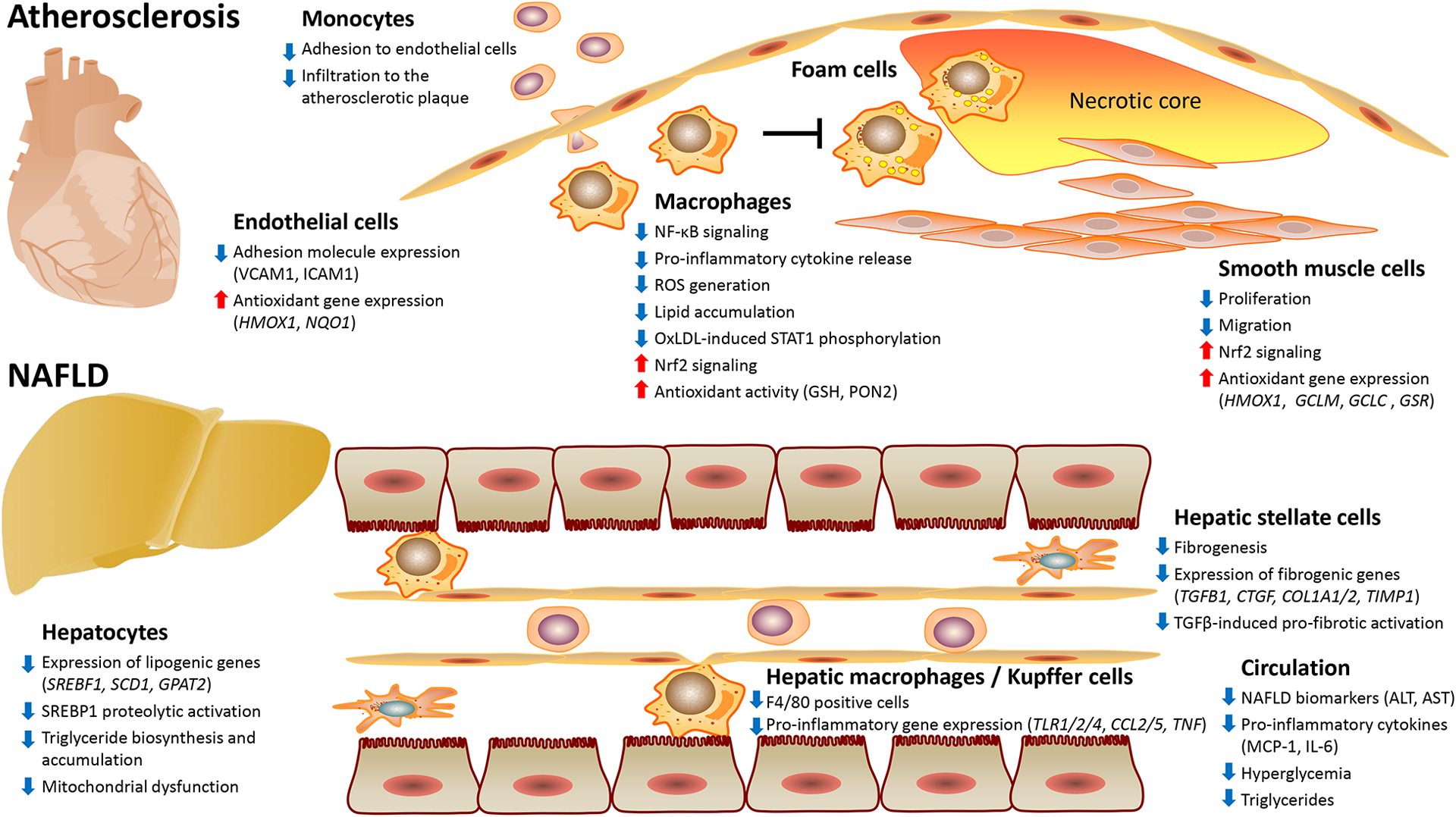
ALT, alanine aminotransferase; AST, aspartate aminotransferase; COL1A, collagen, type I, alpha; CTGF, connective tissue growth factor; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase regulatory subunit; GPAT2, glycerol-3-phosphate acyltransferase-2; GSH, glutathione; GSR, glutathione reductase; HMOX1, heme oxygenase-1; ICAM1, intercellular adhesion molecule-1; IL-6, interleukin 6; MCP-1, monocyte chemoattractant protein-1; NAFLD, non-alcoholic fatty liver disease; NF-κB, nuclear factor kappa B; NQO1, NAD(P)H quinone dehydrogenase-1; Nrf2, nuclear factor erythroid 2-related factor-2; OxLDL, oxidized low-density lipoprotein; PON2, paraoxonase-2; ROS, reactive oxygen species; SCD-1, stearoyl-CoA desaturase-1; SREBF1, sterol regulatory element-binding transcription factor-1; SREBP1, sterol regulatory element-binding protein-1; STAT-1, signal transducer and activator of transcription-1; TGFB1, transforming growth factor beta-1; TIMP1, TIMP metallopeptidase inhibitor-1; TLR, toll-like receptor; Tumor necrosis factor; VCAM1, vascular cell adhesion molecule-1.
A large body of evidence indicates that atherosclerosis is promoted by non-alcoholic fatty liver disease (NAFLD) which is reaching global epidemic proportions. CVD is the leading cause of death in NAFLD patients, particularly those with non-alcoholic steatohepatitis (NASH) [11]. Recently, we and others identified the therapeutic potential of NO2-FAs against NAFLD. In mice fed high-fat diet (HFD) for 20 weeks, Khoo et al. [12**] found that treatment with NO2-OA prevented HFD-induced triglyceride accumulation and mitochondrial dysfunction in the liver. We applied a long-term (24 weeks) murine NASH model induced by high-fructose, high-fat and high-cholesterol diet. Steatosis and fibrosis were confirmed prior to NO2-OA administration using non-invasive and histological approaches. We found a robust reduction of circulating liver damage markers, hepatic steatosis, lobular inflammation and fibrosis by NO2-OA. Unbiased transcriptomics uncovered inflammation, fibrogenesis and lipogenesis as major pathways suppressed by NO2-OA in the liver and a robust inhibition of sterol regulatory element-binding protein-1 (SREBP1) proteolytic activation was noted. Accordingly, NO2-OA inhibited triglyceride biosynthesis and accumulation in hepatocytes and suppressed fibrogenesis in hepatic stellate cells (Figure 1) [13**]. During these studies, we also tested the effects of NO2-OA in Apoe−/− mice fed a Western diet and reported reduced hepatic steatosis [13**]. Herein, we show that treatment with NO2-OA simultaneously reduced atherosclerosis in Apoe−/− mice (Figure 2).
Fig. 2. NO2-OA prevents Western diet-induced atherosclerosis in Apoe−/− mice.
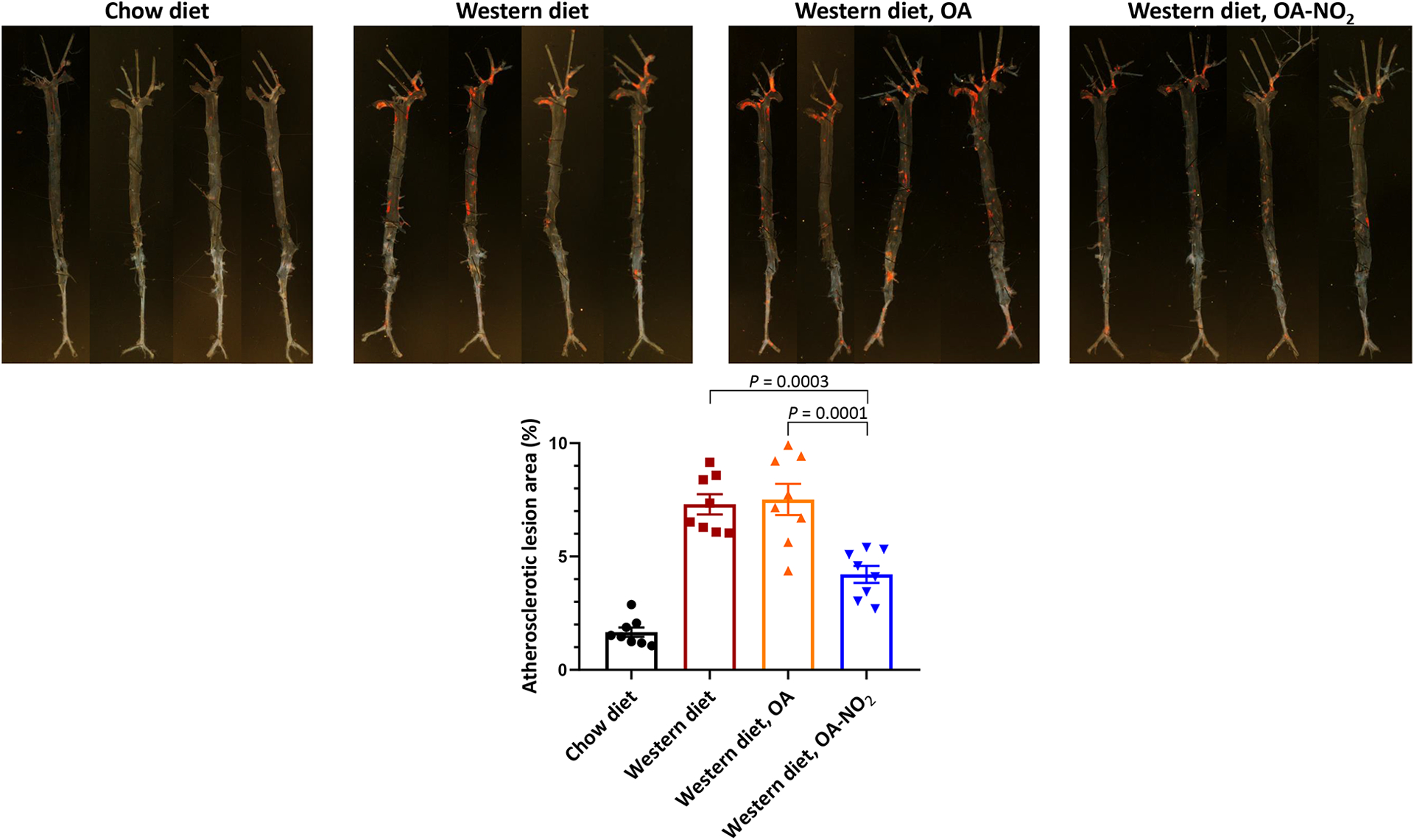
Male Apoe−/− mice were fed standard chow diet or Western diet (Envigo TD.88137, 42% fat and 0.2% cholesterol) for 8 weeks and treated with 8 mg/kg/day of NO2-OA via subcutaneously implanted osmotic mini-pumps. Mice fed chow diet and administrated vehicle (polyethylene glycol) as well as mice fed Western diet and administrated vehicle or 8 mg/kg/d of non-nitrated OA were served as controls. Oil Red O staining was used for en face analysis of atherosclerotic lesions in the aortic tree. Representative aortas from each experimental group are shown. Statistical differences were compared by one-way ANOVA followed by Bonferroni post-hoc test (n=8 mice per group).
Beyond the above studies applying various experimental models, NO2-FAs are currently evaluated in several clinical trials. Particularly, CXA-10, a specific regioisomer of NO2-OA (10-nitro-octadec-9-enoic acid) underwent pharmaceutical development as a drug candidate for the treatment of inflammatory and fibrotic diseases using oral and intravenous formulations. As phase II studies are currently ongoing in patients with focal segmental glomerulosclerosis and pulmonary arterial hypertension, a recent phase I study reported reductions in serum biomarkers of inflammation (monocyte chemoattractant protein-1, MCP1) and metabolic dysfunction (triglycerides) following oral administration of CXA-10 to obese subjects [14**]. Considering the supportive findings from preclinical and clinical studies, the lack of approved drugs for NAFLD and its global burden together with the challenges of simultaneously treating CVD and NAFLD [15], the co-treatment of atherosclerosis and NAFLD using NO2-FAs warrants further clinical evaluation.
Financial support and sponsorship:
O.R., Y.E.C. and M.A. were supported by the Michigan-Israel Partnership for Research and Education. O.R. was supported by the National Institute of Health grant K99HL150233 and by the American Heart Association Postdoctoral Fellowship 19POST34380224. L.C. was supported by the National Institute of Health grant HL122664 and Y.E.C. by grants HL068878 and HL137214.
Footnotes
Conflicts of interest: There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
- 1.Schopfer FJ, Lin Y, Baker PR, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102(7):2340–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui T, Schopfer FJ, Zhang J, et al. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 20064;281(47):35686–35698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kansanen E, Bonacci G, Schopfer FJ, et al. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem. 2011;286(16):14019–14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villacorta L, Chang L, Salvatore SR, et al. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res. 2013;98(1):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villacorta L, Minarrieta L, Salvatore SR, et al. In situ generation, metabolism and immunomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox Biol. 2018;15:522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study demonstrated formation, metabolism, anti-inflammatory and anti-oxidant properties of NO2-CLA under inflammatory conditions. Macrophages activated with LPS/IFN-γ were shown to mediate CLA nitration via inducible nitric oxide synthase (iNOS) activity. In situ NO2-CLA formation was also demonstrated using a mouse model of zymosan-A induced peritonitis. In turn, administration of exogenous NO2-CLA as well as endogenous NO2-CLA inhibited the transcription of NF-κB-dependent genes and activated Nrf2 signaling, leading to suppression of pro-inflammatory cytokine production and over-expression of cytoprotective phase 2 proteins.
- 6.Hansen AL, Buchan GJ, Rühl M, et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc Natl Acad Sci U S A. 2018;115(33):E7768–E7775. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study improved our understanding of NO2-FA formation in response to virus infection and identified a novel mechanism behind the anti-inflammatory activities of NO2-FAs. In a murine model of vaginal HSV-2 infection and in macrophages infected with HSV-2, NO2-CLA was shown to be formed in an iNOS-dependent manner. In turn, NO2-CLA and NO2-OA inhibited induction of type I IFN in response to HSV-2. Interestingly, the inhibition of type I IFN release was found to be mediated via suppression of STING, which is implicated in several chronic inflammatory conditions. NO2-OA was found to mediate the nitro-alkylation of STING in two cysteine residues (Cys88 and Cys91) and a histidine residue (His16). This inhibited STING palmitoylation, STING signaling, and subsequently the release of type I IFN.
- 7.Rudolph TK, Rudolph V, Edreira MM, et al. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30(5):938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study described the anti-atherogenic properties of NO2-FAs for the first time. In Apoe−/−mice fed an atherogenic diet, administration of NO2-OA, but not equivalent levels of non-nitrated OA, reduced atherosclerosis in the aortic root and along the whole aorta. While no significant changes were found in circulating lipid profile, NO2-OA suppressed aortic oxidant generation and inhibited adhesion molecule expression. Importantly, NO2-OA inhibited infiltration of inflammatory cells to atherosclerotic plaques, macrophage foam cell formation and oxLDL-induced phosphorylation of STAT-1. Of note, although this study applied chronic high fat feeding, the effects on NO2-OA on hepatic steatosis were not evaluated.
- 8.Lu H, Sun J, Liang W, et al. Novel gene regulatory networks identified in response to nitro-conjugated linoleic acid in human endothelial cells. Physiol Genomics. 2019;51(6):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenblat M, Rom O, Volkova N, Aviram M. Nitro-Oleic Acid Reduces J774A.1 Macrophage Oxidative Status and Triglyceride Mass: Involvement of Paraoxonase2 and Triglyceride Metabolizing Enzymes. Lipids. 2016;51(8):941–53. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Chang Z, Zhu T, et al. Transcriptomic sequencing reveals diverse adaptive gene expression responses of human vascular smooth muscle cells to nitro-conjugated linoleic acid. Physiol Genomics. 2018;50(4):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol. 2018;68(2):335–352. [DOI] [PubMed] [Google Scholar]
- 12.Khoo NKH, Fazzari M, Chartoumpekis DV, et al. Electrophilic nitro-oleic acid reverses obesity-induced hepatic steatosis. Redox Biol. 2019;22:101132. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study evaluated the effects of NO2-OA versus rosiglitazone, a potent PPARɣ agonist, in a murine model of diet-induced obesity. Mice were fed HFD for 20 weeks and were treated with rosiglitazone, NO2-OA or vehicle during the last 6 weeks. Both rosiglitazone and NO2-OA lowered hyperglycemia and improved glucose tolerance, but mice treated with NO2-OA had lower body weight gain and fat mass than mice treated with rosiglitazone. Interestingly, both rosiglitazone and NO2-OA lowered the plasma levels of alanine aminotransferase and triglycerides, but only NO2-OA reduced hepatic triglyceride accumulation and improved mitochondrial function. This study indicated potential benefits of NO2-OA for the treatment of obesity-induced hepatic steatosis.
- 13.Rom O, Xu G, Guo Y, et al. Nitro-fatty acids protect against steatosis and fibrosis during development of nonalcoholic fatty liver disease in mice. EBioMedicine. 2019;41:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study demonstrated for the first time the protective effects of NO2-OA against NAFLD and NASH in two preclinical settings: 1) Western diet-induced progressive steatosis in Apoe−/− mice. 2) Established NASH featuring advanced hepatic steatosis and fibrosis induced by long-term (24 weeks) feeding of high-fat, high-cholesterol, high-fructose diet in wild-type mice. Applying a plethora of experimental approaches including analyses of circulating NAFLD biomarkers, indirect calorimetry, non-invasive liver imaging and classical histology as well as unbiased transcriptomic profiling, NO2-OA was found to reduce clinically-relevant markers of liver damage and inflammation, protect against hepatic lipid accumulation, lobular inflammation and fibrosis and improve body composition and energy metabolism. Mechanistically, NO2-OA suppressed major pathways regulating hepatic lipogenesis, inflammation and fibrogenesis with a robust inhibition of SREBP-1 proteolytic activation in the liver. NO2-OA inhibited triglyceride biosynthesis and accumulation in hepatocytes and inhibited pro-fibrotic activation of hepatic stellate cells. Further analysis of aortas isolated from the Apoe−/− mice revealed that NO2-OA treatment can simultaneously reduce hepatic steatosis and atherosclerosis (Figure 2).
- 14.Garner RM, Mould DR, Chieffo C, Jorkasky DK. Pharmacokinetic and Pharmacodynamic Effects of Oral CXA-10, a Nitro Fatty Acid, After Single and Multiple Ascending Doses in Healthy and Obese Subjects. Clin Transl Sci. 2019;12(6):667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This report describes phase I studies in healthy and obese subjects aimed at evaluating the pharmacokinetics, safety and tolerability of oral CXA-10, a specific regioisomer of NO2-OA (10-nitro-octadec-9-enoic acid). Importantly, in obese subjects, administration of CXA-10 at 150 mg dose, but not at 25 or 450 mg doses, resulted in a consistent decrease from baseline in biomarkers of inflammation and metabolic dysfunction. After 14 days of oral CXA-10 administration, significant reductions in plasma MCP-1 and triglycerides were reported. Based on these phase I studies, phase II trials were initiated in patients with focal segmental glomerulosclerosis and pulmonaryarterial hypertension to test the efficacy and tolerability of CXA-10 at doses of 75, 150, or 300 mg/day.
- 15.Nielsen JB, Rom O, Surakka I, et al. Loss-of-function genomic variants with impact on liver-related blood traits highlight potential therapeutic targets for cardiovascular disease. bioRxive 2019. 10.1101/597377/ [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER RECOMMENDED READING
Key studies describing the beneficial properties of NO2-FAs in various cardiovascular and metabolic disease models:
- Kelley EE, Baust J, Bonacci G, et al. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res. 2014;101(3):352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Villacorta L, Chang L, et al. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res. 2010;107(4):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph V, Rudolph TK, Schopfer FJ, et al. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res. 2010;85(1):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MP, Rudolph TK, Khoo NK, et al. Nitro-fatty acid inhibition of neointima formation after endoluminal vessel injury. Circ Res. 2009;105(10):965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacorta L, Zhang J, Garcia-Barrio MT, et al. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293(1):H770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]; In 2018, the ‘nitro-lipids series’ was published in the journal Nitric Oxide, summarizing the body of knowledge on different aspects of NO2-FAs accumulated during the last two decades. Below are key reviews relevant to NO2-FA discovery, signaling actions, roles in cardiovascular and metabolic diseases as well as potential clinical applicability:
- Freeman BA, O’Donnell VB, Schopfer FJ. The discovery of nitro-fatty acids as products of metabolic and inflammatory reactions and mediators of adaptive cell signaling. Nitric Oxide. 2018;77:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom O, Khoo NKH, Chen YE, Villacorta L. Inflammatory signaling and metabolic regulation by nitro-fatty acids. Nitric Oxide. 2018;78,140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer M, Mehrkens D, Rudolph V. Nitrated fatty acids in cardiovascular diseases. Nitric Oxide. 2018;78,146–153. [DOI] [PubMed] [Google Scholar]
- Schopfer FJ, Vitturi DA, Jorkasky DK, Freeman BA. Nitro-fatty acids: New drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide. 2018;79:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


