Abstract
BACKGROUND
Aneurysm recurrence after coiling has been associated with aneurysm growth, (re)hemorrhage, and a greater need for follow-up. The second-generation HydroCoil Embolic System (HES; MicroVention, Inc) consists of a platinum core with integrated hydrogel and was developed to reduce recurrence through enhancing packing density and healing within the aneurysm.
OBJECTIVE
To compare recurrence between the second-generation HES and bare platinum coil (BPC) in the new-generation Hydrogel Endovascular Aneurysm Treatment Trial (HEAT).
METHODS
HEAT is a randomized, controlled trial that enrolled subjects with ruptured or unruptured 3- to 14-mm intracranial aneurysms amenable to coiling. The primary endpoint was aneurysm recurrence using the Raymond-Roy scale. Secondary endpoints included minor and major recurrence, packing density, adverse events related to the procedure and/or device, mortality, initial complete occlusion, aneurysm retreatment, hemorrhage from target aneurysm during follow-up, aneurysm occlusion stability, and clinical outcome at final follow-up.
RESULTS
A total of 600 patients were randomized (HES, n = 297 and BPC, n = 303), including 28% with ruptured aneurysms. Recurrence occurred in 11 (4.4%) subjects in the HES arm and 44 (15.4%) subjects in the BPC arm (P = .002). While the initial occlusion rate was higher with BPC, the packing density and both major and minor recurrence rates were in favor of HES. Secondary endpoints including adverse events, retreatment, hemorrhage, mortality, and clinical outcome did not differ between arms.
CONCLUSION
Coiling of small-to-medium aneurysms with second-generation HES resulted in less recurrence when compared to BPC, without increased harm. These data further support the use of the second-generation HES for the embolization of intracranial aneurysms.
Keywords: Bare platinum coil, Coil embolization, Endovascular, HydroCoil Embolic System, Intracranial aneurysm, Randomized controlled trial
ABBREVIATIONS
- BPC
bare platinum coil
- CI
confidence interval
- FDA
Food and Drug Administration
- HEAT
Hydrogel Endovascular Aneurysm Treatment
- HES
HydroCoil Embolic System
- HPB
Health Protection Branch
- MRA
magnetic resonance angiogram
- mRS
modified Rankin Scale
- OR
odds ratio
- RR
Raymond-Roy
- SAE
serious adverse event
- SD
standard deviation
While coil embolization of intracranial aneurysms has emerged as an effective minimally invasive treatment for select intracranial aneurysms, concern has lingered re-garding recurrence. Aneurysm recurrence ranges from 15% to 33% at 18 mo and has been hypothesized to be driven by unorganized and unstable thrombus formation and absence of neointima formation at the neck of coiled aneurysms.1-4 Recurrence has been associated with the need for greater imaging follow-up, additional procedures, and target aneurysm (re)hemorrhage, which potentially increases morbidity and mortality.1,5 Newer coils, including bioactive coils, have not succeeded in reducing recurrence.6-9 The HydroCoil Embolic System (HES) (MicroVention, Inc, Aliso Viejo, California) is a recent advance in coil mechanics, which includes platinum coils containing a hydrogel polymer that, once in contact with blood, expands to fill the coil lumen. A body of evidence has linked both lower packing density and poor healing within the aneurysm to higher recurrence rates, and hence the design of HES to improve both coil packing density and healing within aneurysms and at the neck.10-12 Animal studies have shown that HES achieves thicker neointima along the aneurysm wall and neck, higher endothelial deposition in the neck, higher cellular response, and better aneurysm occlusion than bare platinum coils (BPCs).13-15 Clinical trials on the first-generation HES demonstrated a trend toward reduced recurrence with HES; however, its widespread adoption was impeded by challenging handling properties.3,16,17 A second-generation HES was developed to overcome the technical difficulties associated with first-generation HES. The new-generation Hydrogel Endovascular Aneurysm Treatment Trial (HEAT) was designed to investigate whether the second-generation HES reduces recurrence without an increase in adverse outcomes after coiling of ruptured and unruptured small-to-medium intracranial aneurysms when compared with BPC.
METHODS
Trial Design
HEAT is an investigator-initiated, randomized, controlled trial that aimed to compare second-generation HES to BPC in the treatment of ruptured or unruptured, small-to-medium-sized aneurysms (3-14 mm). A total of 600 subjects were enrolled at 46 study centers in the United States and Canada. The study protocol was approved by the institutional review boards at Northwestern University, the Mayo Clinic, and all participating study sites.18 The study was funded by MicroVention, Inc (Aliso Viejo, California) through a grant that was housed at Northwestern University and Mayo Clinic but was independently managed by the senior author (B.R.B.) with his research team through Northwestern University and Mayo Clinic. The funder, MicroVention, Inc, had no role in the design or operation of the trial. However, the funder was informed about serious adverse events (SAEs) throughout the duration of the trial. The principal investigator had no relationship with MicroVention beyond the funding of the study. This study was initially conceived as the principal investigator's master's thesis as part of Northwestern University's Masters of Clinical Investigation Program. An independent imaging core lab (A.J.D. and J.A.S.) reviewed all angiographic imaging. Clinical outcomes and events were independently monitored and then reviewed by a Data Safety Monitoring Board. Clinical trial registration information for this study can be found at https://clinicaltrials.gov/ct2/show/NCT01407952 with the unique identifier “NCT01407952.”
Participants
Subjects were eligible for enrollment if they were between 18 and 75 yr of age with an untreated intracranial aneurysm (ruptured or unruptured) between 3 and 14 mm in size, which was amenable to coil embolization. Patients with ruptured aneurysms were eligible if their Hunt and Hess grade was ≤3. Exclusion criteria included the presence of concurrent intracranial pathologies or presence of serious comorbidities. Prior to randomization, informed consent was obtained from patients or a legally authorized representative following Good Clinical Practice guidelines. A detailed listing of inclusion and exclusion criteria is provided in the study protocol.18
Interventions
For subjects randomized to the BPC arm, any BPC approved by the Food and Drug Administration (FDA) or the Health Protection Branch of Health Canada (HPB) was allowed and treatment consisted exclusively of BPCs. For subjects randomized to the HES arm, at least 90% (of the total implanted coil length) of any FDA or HPB approved second-generation HES was used; BPCs were allowed for up to 10% of the total implanted coil length. The use of bioactive and other types of coils was not permitted. Assist devices including balloons and stents were permitted at the discretion of the performing physician. The use of flow diverters, however, was not permitted.
Outcomes
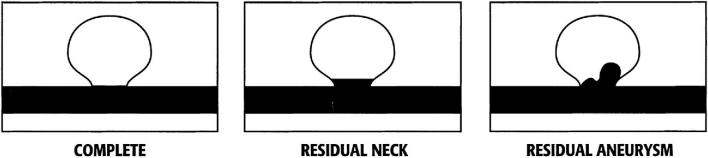
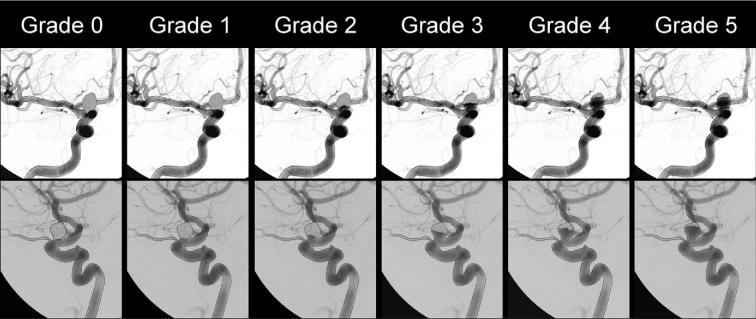
The primary endpoint of the HEAT trial was aneurysm recurrence defined as any progression on the Raymond-Roy (RR) occlusion scale (Figure 1) during final follow-up.19 The secondary endpoints of the trial included initial packing density, initial rate of complete occlusion, device- and/or procedure-related adverse events, mortality rate, clinical outcome at the 18- to 24-mo follow-up assessed by the modified Rankin Scale (mRS), retreatment rates, hemorrhage from the coiled aneurysms at any time during follow-up, aneurysm occlusion stability (assessed by any change in volumetric occlusion as evaluated by the Meyers scale presented in Figure 2), minor recurrence, and major recurrence.20 Major recurrence was defined as an increase in the RR scale from 1 to 3, 2 to 3, or a 3 with an increase in the Meyers scale. Minor recurrence was defined as an increase in the RR scale from 1 to 2. Packing density was estimated by dividing the estimated volume of the coils used by the estimated volume of the aneurysm. The volume of the coils was determined by summing the volume of all coils used. The volume of each coil was calculated by multiplying the square of the coil diameter as found in coil brochures provided by the manufacturers by the length used by π/4. The volume of the aneurysm was calculated assuming the shape of an ellipsoid, and multiplying the height × length × width × π and dividing by 6, where the aneurysm measurements were observed during an angiogram. Clinical outcome (mRS) was assessed at the 18- to 24-mo follow-up, with missing values imputed as the last observed value carried forward or as 6 if the patient died. Lastly, adverse events, including SAEs defined as life-threatening, disabling, resulting in prolonged hospitalization or death, and designated by treating physicians, were reported in alignment with FDA and HPB regulations. All adverse events and essential data points were adjudicated in alignment with FDA Risk-Based Monitoring guidance.21 Adverse events were designated as unanticipated and related to device and/or procedure at the discretion of the sites.
FIGURE 1.
Raymond-Roy aneurysm occlusion classification scale.19 Complete aneurysm occlusion; residual aneurysm neck; residual aneurysm dome.
FIGURE 2.
Aneurysm occlusion grading system as reported by Meyers et al.20 Grade 0: complete and total aneurysm occlusion. Grade 1: ≥90% volumetric aneurysm occlusion. Grade 2: 70% to 89% volumetric aneurysm occlusion. Grade 3: 50% to 69% volumetric aneurysm occlusion. Grade 4: 25% to 49% volumetric aneurysm occlusion. Grade 5: volumetric aneurysm occlusion.
Timeline
Both subject randomization and endovascular intervention were performed on the same day, which was considered day 0. Initial and follow-up clinical assessments were performed at 1 d, 3- to 28-d, 3- to 12-mo, and 18- to 24-mo follow-up intervals. Imaging parameters, angiography and/or magnetic resonance angiogram (MRA), were evaluated at the 3- to 12-mo and 18- to 24-mo visits. Whenever both MRA and catheter angiography were performed, the catheter angiographic evaluation was considered for outcome assessment. For ruptured aneurysms, the National Institutes of Health Stroke Scale and Hunt and Hess scale were recorded but not included in the trial analysis.
Sample Size
Sample size was determined based on the assumption that the use of second-generation HES to embolize intracranial aneurysms could achieve a 30% lower recurrence rate than with the use of BPC. Assuming a BPC major recurrence rate of 33%—as published in the HELPS trial—a sample size of 600 patients provided the HEAT trial with 80% power to detect an improvement in recanalization rates to 22.8% using a chi-square test at a type I error rate of 5%.
Randomization and Blinding
After informed consent was signed, subjects were randomized in a 1:1 allocation to either the HES group or the BPC group using DATATRAK (DATATRAK International, Inc, Mayfield Heights, Ohio), the study's Electronic Data Capture; randomization was stratified by participation site. Patients were given a study identification number that was used for clinical data extraction. Submitted imaging studies were evaluated by the Imaging Core Lab, which was blinded to the type of the coil used for treatment. Treating physician and subjects were not blinded to the treatment coil.
Statistical Methods
The analysis for the primary outcome was based on the intent-to-treat principle. For missing outcome data, multiple imputation methods were used, creating 5 datasets. Results were combined over the imputation analyses to present valid univariate inferences for treatment group. Due to the nature of the data, secondary analyses of the primary outcome and all secondary outcomes were based on a per-protocol analysis. Baseline demographics are presented as counts and percentages (categorical characteristics), or mean and standard deviation (SD) for age, and aneurysm dimension. Logistic regression models were fit to estimate odds ratios (OR) and 95% CI for recurrence. The secondary analysis was per protocol, with a chi-square test to determine if the recurrence rates differed by coil type, and a logistics regression model adjusted for rupture status, initial RR scale, maximal aneurysm circumference, and neck diameter. As a further sensitivity analysis, an intent-to-treat analysis was conducted, assuming patients without imaging follow-up had aneurysm recurrence. Secondary outcomes were analyzed with a chi-square test or Fisher's exact test when event rates were restrictive (minor or major recurrence, adverse events related to device or procedure, mortality, initial occlusion, retreatment, and hemorrhage), Cochran-Armitage trend test (number of adverse events related to device or procedure per person), independent t-test (packing density), Wilcoxon rank-sum test (clinical outcome), or generalized linear models with a logit link function to examine occlusion stability over time. All analyses were performed using SASv9.2 (Cary, North Carolina) and run at a 5% type I error rate.
RESULTS
Participant Flow and Recruitment
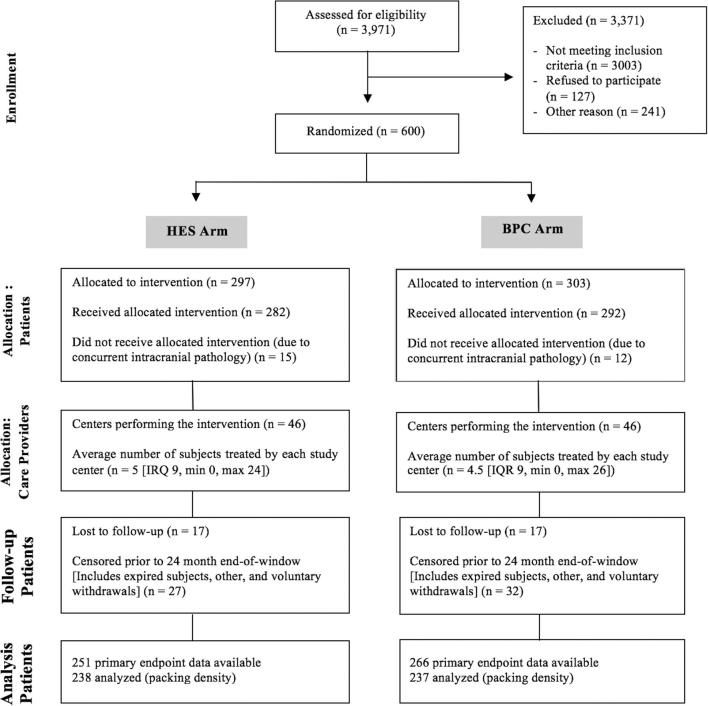
A total of 600 out of 3971 screened subjects were enrolled in the HEAT study between May 2012 and January 2016 (Figure 3). After patient randomization, 297 were assigned to the HES arm and 303 were assigned to the BPC arm. Information pertaining to the primary outcome was available from 251 (84.5%) subjects in the HES arm (222 from 18- to 24-mo imaging, 29 from 3- to 12-mo imaging) and 266 (87.8%) from the BPC arm (231 from 18- to 24-mo imaging, 35 from 3- to 12-mo imaging). In the HES arm, 17 patients were lost to follow-up, 7 patients expired, 7 subjects voluntarily withdrew from the study, and 17 subjects were not eligible for primary endpoint analysis for other reasons. In the BPC arm, 17 patients were lost to follow-up, 9 patients expired, 11 subjects voluntarily withdrew, and 10 subjects were not eligible for primary endpoint analysis for other reasons (Table, Supplemental Digital Content 1). Of note, some of the patients who exited the study were still eligible for primary endpoint analysis, as they had 3- to 12-mo imaging studies.
FIGURE 3.
HEAT participant flow CONSORT diagram.22
Baseline Characteristics
Patient demographic and clinical characteristics were similar for most categories across treatment arms (Table 1). On average, 28% of the aneurysms across both arms were ruptured (HES: 76, 25.6%, BPC: 93, 30.9%). Most patients enrolled in the trial were female (HES: 238, 80.1%, BPC: 236, 77.9%), and the mean (SD) age of patients in the HES and BPC arms was 56.65 (11.5) and 56.9 (10.3), respectively. Furthermore, aneurysm location, the size and shape of the aneurysms, dome-to-neck ratio, and the use of assist devices were similar for both arms. Of note, the number of internal carotid artery aneurysms in the HES arm (76 aneurysms, 26.6%) was lower than in the BPC arm (99 aneurysms, 34.0%).
TABLE 1.
Baseline Characteristics of Subjects in the HEAT Study
| Hydrogel coil | Bare platinum | |
|---|---|---|
| 297 | 303 | |
| Sex | ||
| Female | 238 (80.1) | 236 (77.9) |
| Male | 59 (19.9) | 67 (22.1) |
| Age | ||
| (Mean ± SD) | 56.5 ± 11.5 | 56.9 ± 10.3 |
| Ruptured status | ||
| Unruptured | 216 (74.0) | 208 (69.1) |
| Ruptured | 76 (26.0) | 93 (30.9) |
| If ruptured, Hunt and Hess | ||
| 1 | 14 (18.4) | 21 (22.3) |
| 2 | 39 (51.3) | 47 (50.0) |
| 3 | 22 (28.9) | 26 (27.7) |
| 4 | 1 (1.3) | 0 (0.0) |
| Neck size | ||
| (mm) | 3.20 ± 1.40 | 3.00 ± 1.10 |
| Dome-to-neck ratio | ||
| ≤1.5 | 33 (11.6) | 21 (7.4) |
| >1.5 | 251 (88.4) | 263 (92.6) |
| Maximum diameter (mm) | 7.30 ± 2.70 | 7.50 ± 2.70 |
| Shape | ||
| Daughter sac | 54 (18.9) | 60 (20.5) |
| Irregular | 59 (20.6) | 69 (23.6) |
| Regular | 173 (60.5) | 163 (55.8) |
| Type | ||
| Bifurcation | 156 (54.5) | 141 (48.3) |
| Sidewall | 86 (30.1) | 107 (36.6) |
| Terminal | 44 (15.4) | 44 (15.1) |
| Location | ||
| Anterior cerebral artery | 10 (3.5) | 7 (2.4) |
| Anterior communicating artery | 70 (24.5) | 64 (22.0) |
| Basilar artery | 35 (12.2) | 34 (11.7) |
| Internal carotid artery | 76 (26.6) | 99 (34.0) |
| Middle cerebral artery | 39 (13.6) | 29 (10.0) |
| Posterior communicating artery | 51 (17.8) | 53 (18.2) |
| Vertebral artery | 5 (1.7) | 5 (1.7) |
| Family history of aneurysm | ||
| No | 224 (76.7) | 227 (75.2) |
| Yes | 68 (23.3) | 75 (24.8) |
| Co-morbid conditions | ||
| No | 73 (24.6) | 87 (28.7) |
| Yes | 224 (75.4) | 216 (71.3) |
| Smoking status | ||
| Current smoker | 116 (39.7) | 114 (37.7) |
| Never smoked | 71 (24.3) | 88 (29.1) |
| Past smoker | 105 (36.0) | 100 (33.1) |
Primary Endpoint
The odds of recurrence (defined as any progression on the RR scale) for BPC relative to HES was 2.32 (95% CI [1.35, 3.97]) using an intent-to-treat imputation analysis (in which 46 patients’ outcomes were imputed in the HES arm and 37 in the BPC arm). As per-protocol analysis, aneurysm recurrence was observed in 11 (4.4%) patients in the HES arm and 41 (15.4%) patients in the BPC arm (OR 3.97 95% CI [1.99, 7.92]). After adjusting for aneurysm size, rupture status, aneurysm neck size, and procedural angiographic occlusion, results still favored greater occlusion in the HES arm (OR 2.58 95% CI [1.05, 6.34]). A conservative intent-to-treat analysis assuming that all subjects with no imaging follow-up had aneurysm recurrence showed a similar trend, but failed to reach statistical significance (OR 1.46 95% CI [0.99, 2.15]). Results are presented in Tables 2 and 3.
TABLE 2.
Primary Outcome Analyses
| Model | OR and 95% CI | P value |
|---|---|---|
| Intent to treat (multiple imputation) | 2.32 (1.356, 3.97) | .002 |
| Univariate per protocol | 3.97 (1.99, 7.92) | <.001 |
| Adjusted per protocol | 2.58 (1.05, 6.34) | .039 |
| Intent to treat (missing assumed to recur) | 1.46 (0.99, 2.15) | .055 |
TABLE 3.
Primary and Secondary Outcomes
| HydroCoil Embolic System | Bare platinum coils | P value | |
|---|---|---|---|
| Recurrence: any progression on the RR scale | 4.4% | 15.4% | .002 |
| Occlusion stability: any increase on the Meyers scale | 13% | 27% | <.001 |
| Minor recurrence: progression on the RR scale from 1 to 2 | 2 (1.0%) | 14 (5.0%) | .004 |
| Major recurrence: progression on the RR scale to 3, or a 3 with an increase in the Meyers scale | 32 (12.8%) | 55 (20.7%) | .016 |
| Packing density | 32.5% ± 14.8% | 24.7% ± 10.2% | <.001 |
| Initial occlusion | 50 (17.8%) | 82 (28.3%) | .003 |
| Occlusion stability | <.001 | ||
| 3-12 mo | 64.9% | 49.0% | |
| 18-24 mo | 68.6% | 51.5% | |
| Patients with adverse eventsa | 64 (21.6%) | 75 (24.8%) | .352 |
| Adverse events per persona | |||
| None | 233 (78.5%) | 228 (75.3%) | .294 |
| One | 44 (14.8%) | 63 (20.8%) | |
| Two | 19 (6.4%) | 8 (2.6%) | |
| Three | 1 (0.3%) | 4 (1.3%) | |
| Mortality | 7 (2.4%) | 9 (3.0%) | .641 |
| Clinical outcome | .578 | ||
| No symptoms | 181 (63.1%) | 178 (61.0%) | |
| No significant disability | 60 (20.9%) | 63 (21.6%) | |
| Slight disability | 24 (8.4%) | 28 (9.6%) | |
| Moderate disability | 13 (4.5%) | 10 (3.4%) | |
| Moderately severe disability | 2 (0.7%) | 0 (0%) | |
| Severe disability | 0 (0%) | 4 (1.4%) | |
| Expired | 7 (2.4%) | 9 (3.1%) | |
| Retreatment | 13 (5.2%) | 22 (8.3%) | .162 |
| Hemorrhagea | 5 (1.7%) | 4 (1.3%) | .714 |
aEvent related to device and/or procedure.
Secondary Endpoints
A total of 33 (13%) of HES and 71 (27%) of BPC showed recurrence based on the Meyers scale (defined as any progression on the Meyer scale), P < .001. Similarly, major recurrence (defined by an increase on the RR scale from 1 to 3, 2 to 3, or a 3 that had progression on the Meyer scale) was seen in 32 (12.8%) of HES, and 55 (20.7%) in BPC, and minor recurrence (defined by an increase on the RR scale from 1 to 2) was seen in 2 (1%) of HES and 14 (5%) of BPC (P = .016 and .004, respectively). Packing density of the aneurysms was assessed in 238 HES and 237 BPC participants. HES had an average packing density of 32.5% (SD 14.8%), whereas BPC had an average packing density of 24.7% (SD 10.2%), P < .001. On average, there were 4.9 (SD = 3.1) coils used in HES procedures and 5.6 (SD = 4.0) coils in BPC. The average coil lengths were 8.5 cm (SD = 4.5) and 9.0 cm (SD = 4.9), respectively. Initial complete occlusion was seen in 50 (17.8%) subjects in the HES group, as compared to 82 (28.3%) in the BPC group (P = .003). However, it appeared that the percentage of aneurysms with complete occlusion increased in the HES arm throughout imaging follow-up at a higher rate than in the BPC arm (P < .001); estimated complete occlusion rates were 64.9% and 49.0% at 3- to 12-mo and 68.6% and 51.5% at 18- to 24-mo for HES and BPC, respectively. A total of 16 patients expired over the course of the study: 7 (2.4%) in HES and 9 (3.0%) in BPC (OR 0.80 95% CI [0.29, 2.17]). There was no difference in mRS at final follow-up between subjects in the HES and BPC arms (P = .578). Aneurysm retreatment was noted in 13 (5.2%) of HES and 22 (8.3%) of BPC (OR 0.61 95% CI [0.30, 1.23]). Analysis of adverse events related to the procedure and/or the device as well as hemorrhage from the target aneurysm during follow-up are presented in the Harms section below.
Harms
A total of 597 adverse events were recorded by the investigators, equally distributed between arms: 286 (47.9%) in the HES arm and 311 (52.1%) in the BPC arm (P = .306). Among these, 177 were classified as SAEs: 92 (52.0%) in the HES arm and 85 (48.0%) in the BPC arm (P = .196) (Table, Supplemental Digital Content 2). The serious and nonserious adverse events are listed in Tables, Supplemental Digital Content 2 and 3, respectively. The SAEs included 3 hydrocephalus events designated as device related by the site investigators in the HES arm with none in the BPC arm. All 3 cases were in the posterior circulation and occurred after 1 yr of follow-up. There were 2 events designated as unanticipated adverse events, the first being one of the aforementioned hydrocephalus cases attributed to the device and a second case of coil detachment in the HES arm.
There were 176 adverse events related to the procedure and/or the device occurring in 139 patients: 64 (21.6%) in HES and 75 (24.8%) in BPC (P = .352). In each arm, the number of adverse events per person ranged from 0 to 3, with no significant trend in number of events per arm (OR 0.84 95% CI [0.57, 1.22]). There were 16 incidents of intracranial hemorrhage in 15 patients; of these, 9 were either related to device or procedure: 5 in HES and 4 in BPC (P = .714). Seven were procedural events and 2 were post-procedural. Due to lack of further information on the source of bleeding from the post-procedural hemorrhages, we assumed that both events were hemorrhages from target aneurysms during follow-up (2 in the HES arm and 0 in the BPC arm).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
DISCUSSION
Background
Over the past 3 decades, BPC has been considered the standard of care for endovascular aneurysm occlusion.23,24 More recently, there have been significant advancements in coil design and deployment techniques that have broadened the spectrum of aneurysms amenable to coil embolization and, to an extent, have improved the outcomes of aneurysm coiling.24-26 Despite this progress, recurrence remains high and continues to be a limitation of endovascular treatment.27,28 Multiple enhanced coil designs including bioactive and surface modified coils have not succeeded in showing a convincing reduction in aneurysm recurrence.6-9,29 In the MAPS trial, recurrence (with a follow-up of 455 d) was 13.3% in aneurysms treated with the bioactive Matrix coils (Stryker, Kalamazoo, Michigan) and 14.6% for those treated with BPC (P = .76).6 In the Cerecyte coil trial (Micrus Endovascular, San Jose, California), the occlusion status of aneurysms (complete/stable/improved) at a median follow-up time of 6 mo was similar between aneurysms coiled with the bioactive Cerecyte coils and BPC (P = .17).7
On the other hand, the first-generation HES demonstrated evidence of better outcomes as compared to BPC in several clinical studies. The HEAL registry, which assessed recurrence in aneurysms treated with various percentages of first-generation HES length, showed that the use of a higher percentage of HES was associated with lower recurrence rates at 3 to 6 mo.30 Notably, patients with ≥75% of HES had no recurrence (0/18 subjects). Subsequently, HELPS, the first randomized controlled trial of HES, showed lower major recurrence (over an 18-mo follow-up) associated with the use of first-generation HES as compared to BPC (24% vs 33%, OR: 0.73, P = .049).3 The PRET trial also evaluated recurrence over an 18-mo follow-up period in aneurysms treated with HES in comparison to BPC.16,31 PRET-1 evaluated aneurysms ≥10 mm in size, and PRET-2 was focused on aneurysms that had recurred after prior coiling. For both groups, major recurrence was similar between the HES and BPC arms.
Although the first-generation HES demonstrated results trending towards better durability than BPC, handling characteristics prevented widespread adoption. In an attempt to overcome the technical difficulties associated with the first-generation HES, a second-generation HES was developed. The latter coil is constructed with a filament of expandable hydrogel within the platinum coil unlike the first-generation coil that has an exterior hydrogel coating. The second-generation HES coils are as soft as platinum coils and thus have handling properties similar to BPC. Furthermore, the second-generation HES coils do not require any additional preparation prior to introduction into the microcatheter and they allow for longer repositioning time due to slower and reduced expansion of the gel (up to 30 min).
The GREAT trial was the first randomized, controlled trial to compare the second-generation HES to BPC.32 This trial used a composite primary endpoint that included major recurrence, retreatment, morbidity, and mortality. Over a follow-up period of 18 mo, the primary endpoint of GREAT trial was lower in the HES arm (19.9% vs 28.7%); when adjusted for rupture status, the results were still in favor of the HES arm (P = .036). Notably, major recurrence rate was lower in the HES arm (12% vs 18%). The HELPS, PRET, and GREAT studies had a composite endpoint that included angiographic and clinical outcomes.3,31,32 Clinical outcomes after aneurysm treatment depend upon multiple factors besides aneurysm occlusion including patient age, comorbidities, aneurysm rupture status, etc. Aneurysm recurrence is a clinically relevant parameter to define the success of aneurysm treatment. The clinical importance of durable aneurysm occlusion is the prevention of (re)hemorrhage. However, (re)hemorrhage itself occurs at such a low incidence that it does not represent a pragmatic primary endpoint for a randomized controlled trial—ie, a randomized controlled trial predicated upon (re)hemorrhage rates would require a much larger sample size and much longer follow-up. As such, aneurysm recurrence based upon radiological follow-up represents a well-accepted, reliable, and pragmatic surrogate endpoint. Given these considerations, the HEAT trial was powered with a primary focus on recurrence.
Key Findings
The primary endpoint of the HEAT trial (any progression on the RR scale within 24 mo) was in favor of the HES arm (intent-to-treat, OR 2.32 95% CI [1.35, 3.97]). The sensitivity analysis adjusting for aneurysm size, rupture status, aneurysm neck size, and procedural angiographic occlusion was also in favor of the HES arm (OR 2.58 CI 95% [1.05, 6.34]). These analyses demonstrate that HES reduces recurrence rates in aneurysms between 3 and 14 mm in size, regardless of their rupture status, aneurysm neck size, and procedural angiographic occlusion.
Generalizability
While the HEAT trial primary endpoint was based upon progression on the RR scale, we additionally assessed major and minor recurrences as well as aneurysm occlusion stability assessed by any decrease in volumetric occlusion as defined in the Meyers scale. Major recurrence was defined as any progression on the RR scale to 3 or any 3 that had a decrease in volumetric occlusion (using the Meyers scale). Major recurrence was defined by HELPS and PRET as large recanalization requiring placement of further coils and by GREAT as any increase on the RR scale or any volumetric change in aneurysms with residual dome.3,31,32 In HEAT, major recurrence and aneurysm occlusion stability were both in favor of the HES arm. Although recurrence is directly linked to retreatment, retreatment rates with HES were not statistically different from BPC (P = .162) in HEAT. It should be noted that the need for retreatment has been reported in the literature even beyond 15 yr of follow-up.33
Interpretation
With respect to initial complete occlusion, the HES arm was associated with a significantly lower rate of complete initial procedural occlusion than the BPC arm (17.8% vs 28.3%, P = .003). However, complete occlusion was higher for the HES arm at 3- to 12-mo and 18- to 24-mo imaging (P < .001). This could be explained by a gradual hydrogel expansion that occurs 20 to 30 min post-deployment. Conversely, the GREAT trial results showed higher initial complete occlusion in the HES arm.32 One should note that they used up to 50% BPC while the HEAT trial only allowed up to 10% use of BPC. The average percentage of HES used in the HES arm of HEAT was 95.3%. The analysis indicated higher packing density in the HES arm (P < .001). Packing density was also in favor of the HES arm of PRET and GREAT; however, PRET showed no difference in recurrence between HES and BPC.31,32
Overall adverse events and clinical outcomes in HEAT were similar in both arms. The most common SAEs were stroke and vasospasm. While no a priori plans were made to compare specific SAEs between treatment arms, it is notable that the OR and 95% CI for these 2 events comparing HES and BPC were 1.41 (0.64, 3.13) and 0.65 (0.30, 1.41), respectively. With regard to the 3 cases of hydrocephalus in the HES arm, all 3 were in unruptured aneurysms with maximal diameters of 6.8, 10.5, and 13.5 mm and occurred in a delayed fashion at 70, 57, and 54 wk following endovascular coiling, respectively. All 3 aneurysms were in the posterior circulation, involving the basilar tip (n = 2) and vertebrobasilar junction (n = 1). All 3 patients required placement of a ventriculoperitoneal shunt and ultimately all had excellent clinical outcomes. The observation that all 3 hydrocephalus cases occurred in patients treated with HES might suggest relatedness to the HES device. However, the delayed occurrence (greater than 1 yr) argues against this. In the GREAT trial, 3 cases of hydrocephalus were reported—2 in in the HES arm and 1 in the platinum arm—and the events were adjudicated as unrelated to the coil type. One of the plausible mechanisms specific to basilar tip aneurysms is the possible obstruction of the cerebral aqueduct by the mass of the aneurysm, which could theoretically be exasperated by a coil mass.23,24
Limitations
Several limitations were encountered in the HEAT trial. The trial randomization was not stratified based on baseline characteristics relevant to aneurysm prognosis. This resulted in minor imbalances in some aneurysm characteristics, including aneurysm location. Packing density was based upon the assumption of an ellipsoidal shape for all aneurysms—this is not completely accurate, especially in cases of irregular aneurysms. This assumption can underestimate the volume of some aneurysms and thereby overestimate the packing densities. In addition, the packing density calculation in the HES arm assumed full volumetric expansion of the gel, which might have not occurred in vivo. Of note, the average packing density calculation in each arm was not based on the packing density of all treated aneurysms, as the information provided on some of the coils was incomplete and did not allow for the determination of coil diameter (an essential metric for packing density calculation).
While all adverse events were recorded, data on the source of hemorrhagic events were not requested with sufficient specificity in regard to whether the hemorrhages were related to the target aneurysm or not. However, postoperative hemorrhages related to device and/or procedure were captured and reported by the sites. A retrospective review of clinical documentation for both patients with such postoperative hemorrhages was performed. Only one hemorrhage—in the HES arm—was found to be a post-procedural subarachnoid hemorrhage from the treated aneurysm. The event happened 6 h following the endovascular coiling procedure. The other patient experienced a same day post-procedural subarachnoid hemorrhage with an unclear source of bleeding. This patient had a total of 5 aneurysms, and the treating physician felt that the targeted aneurysm was not convincingly the source of bleeding. With respect to the procedure, the treating physicians were not blinded to the treatment arm, but the imaging Core Lab physicians were, which strengthens the validity of the imaging results. The attrition rate was 13.8%, 15.5% for HES and 12.2% for BPC. Finally, the trial was not powered to detect the effect of HES in reducing retreatment rates and long-term clinical outcomes beyond 2 yr.
CONCLUSION
The results of the HEAT trial demonstrate statistically significant reduced recurrence rates in 3- to 14-mm-sized ruptured and unruptured intracranial aneurysms treated with second-generation HES when compared to aneurysms treated with BPC. There were no significant differences in procedural safety and clinical outcomes detected between the 2 treatment arms throughout the period of the study. Despite expected limitations, the primary findings of the HEAT trial provide evidence in favor of using second-generation HES over BPCs for the endovascular coiling of small-to-medium-sized intracranial aneurysms.
Disclosures
The HEAT trial was funded by MicroVention Inc., but sponsored and operated through Northwestern University and Mayo Clinic. The only obligation to MicroVention is the final publication of the trial outcome. Dr Dashti reports consultant relationship with Cerenovus. Dr Woo owns equity in Vascular Simulations Inc.
Supplementary Material
Acknowledgments
We would like to thank the HEAT study investigators, site coordinators, and the subjects who enrolled in the trial. The participating sites and investigators are listed in sequential order of activation though the duration of the trial as follows: Principal Investigator Sameer Ansari, MD (15 patients), Northwestern University, Chicago, Illinois; Principal Investigator Eric Deshaies, MD (1 patient), SUNY Upstate Medical University, Syracuse, New York; Principal Investigator Sean Lavine, MD (1 patient), Columbia University, New York, New York; Principal Investigator Hormozd Bozorgchami, MD (21 patients), Oregon Health & Science University, Portland, Oregon; Principal Investigator Josser Delgado, MD (30 patients), Abbott Northwestern/Consulting Radiologists, Inc, Minneapolis, Minnesota; Principal Investigator Erol Veznedaroglu, MD (1 patient), Capital Health Regional Medical Center, Trenton, New Jersey; Principal Investigator Felipe Albuquerque, MD (18 patients), St. Joseph's Hospital, Phoenix, Arizona; Principal Investigator David Fiorella, MD (37 patients), SUNY, Stony Brook, New York; Principal Investigator Alan Boulos, MD (1 patient), Albany Medical College, Albany, New York; Principal Investigator Maria Cortes, MD (14 patients), McGill University, Montreal, Canada; Principal Investigator Hilal Kanaan, MD (20 patients), East Carolina University, Greenville, North Carolina; Principal Investigator Gaurav Jindal, MD (9 patients), University of Maryland, Baltimore, Maryland; Principal Investigator Richard Klucznik, MD (1 patient), Methodist Hospital Research Institute, Houston, Texas; Principal Investigator Guilherme Dabus, MD (31 patients), Baptist Health of Miami, Miami, Florida; Principal Investigator David Kalmes, MD (17 patients), Mayo Clinic, Rochester, New York; Principal Investigator Rabih Tawk, MD (13 patients), Mayo Clinic, Jacksonville, Florida; Principal Investigator Jean Raymond, MD (43 patients), CHUM Research Centre, Montreal, Canada; Principal Investigator Charles Romero, MD (7 patients), UPMC Hamot, Erie, Pennsylvania; Principal Investigator Andrew Xavier, MD (0 patient), McLaren Flint/Wayne State University, Flint, Michigan; Principal Investigator Muhammad Hussain, MD (20 patients), Cleveland Clinic, Cleveland, Ohio; Principal Investigator Michael Kelly, MD (9 patients), University of Saskatchewan, Saskatoon, Canada; Principal Investigator Christopher Moran, MD (5 patients), Washington University, St. Louis, Missouri; Principal Investigator Imran Chaudry, MD (35 patients), Medical University of South Carolina, Charleston, South Carolina; Principal Investigator Aditya Pandey, MD (2 patients), University of Michigan, Ann Arbor, Michigan; Principal Investigator Dennis Wang, MD (2 patients), MultiCare Health System, Tacoma, Washington; Principal Investigator Brian van Adel, MD (20 patients), Hamilton Health/McMaster University, Hamilton, Canada; Principal Investigator Genevieve Milot, MD (31 patients), CHU de Quebec, Quebec, Canada; Principal Investigator Joshua Hirsch, MD (6 patients), Massachusetts General Hospital, Boston, Massachusetts; Principal Investigator Jeffrey Carpenter, MD (9 patients), West Virginia University, Morgantown, West Virginia; Principal Investigator Ciaran Powers, MD (6 patients), Ohio State University, Columbus, Ohio; Principal Investigator Pascal Jabbour, MD (4 patients), Thomas Jefferson University, Philadelphia, Pennsylvania; Principal Investigator George Luh, MD (24 patients), Mercy General Hospital, Sacramento, California; Principal Investigator Jai Shankar, MD (22 patients), Dalhousie University, Halifax, Canada; Principal Investigator Ramanchandra Tummala, MD (5 patients), University of Minnesota, Minneapolis, Minnesota; Principal Investigator Athos Patsalides, MD (0 patient), Weill Medical College of Cornell University, New York, New York; Principal Investigator Avery Evans, MD (20 patients), University of Virginia, Charlottesville, Virginia; Principal Investigator Ankur Garg, MD (16 patients), Oklahoma University, Oklahoma City, Oklahoma; Principal Investigator Shervin Dashti, MD (50 patients), Norton Healthcare, Louisville, Kentucky; Principal Investigator Sung Lee, MD (10 patients), Queens Medical Center, Honolulu, Hawaii; Principal Investigator Roberts James, MD (2 patients), University of Louisville, Louisville, Kentucky; Principal Investigator Mahesh Jayaraman, MD (1 patient), Rhode Island Hospital, Providence, Rhode Island; Principal Investigator Sudhakar Satti, MD (1 patient), Christiana Hospital, Newark, New Jersey; Principal Investigator Eric Sauvageau, MD (0 patient), Lyerly Neurosurgery, Jacksonville, Florida; Principal Investigator Jeremy Fields, MD (16 patients), Kaiser Permanente, Clackamas, Oregon; Principal Investigator Thomas Grobelny, MD (1 patient), Advocate Health, Park Ridge, Illinois; and Principal Investigator Johnathan Hartman, MD (3 patients), Kaiser Permanente, Sacramento, California. In addition, we would like to thank the Data Safety Monitoring Board, which included Dr Adnan Siddiqui, Dr Michael Cawley, Dr Franklin Marden, and Dr Mary J. Kwasny.
Notes
The HEAT trial was presented as an oral presentation at the late-breaking abstract session of the 2018 conference of the Society of Neurointerventional Surgery on July 25, 2018 (San Francisco, California), at the plenary session of the 2019 conference of the American Association of Neurological Surgeons on April 15, 2019 (San Diego, California), at the late-breaking session of the 2019 American Heart Association Stroke conference on February 7, 2019 (Honolulu, Hawaii), and at the 2019 meeting of the Neurosurgical Society of America on June 19, 2019 (Banff, Canada).
Contributor Information
HEAT Study Investigators:
Sameer Ansari, Eric Deshaies, Sean Lavine, Hormozd Bozorgchami, Josser Delgado, Erol Veznedaroglu, Felipe Albuquerque, David Fiorella, Alan Boulos, Maria Cortes, Hilal Kanaan, Gaurav Jindal, Richard Klucznik, Guilherme Dabus, David Kalmes, Rabih Tawk, Jean Raymond, Charles Romero, Andrew Xavier, Muhammad Hussain, Michael Kelly, Christopher Moran, Imran Chaudry, Aditya Pandey, Dennis Wang, Brian van Adel, Genevieve Milot, Joshua Hirsch, Jeffrey Carpenter, Ciaran Powers, Pascal Jabbour, George Luh, Jai Shankar, Ramanchandra Tummala, Athos Patsalides, Avery Evans, Ankur Garg, Shervin Dashti, Sung Lee, Roberts James, Mahesh Jayaraman, Sudhakar Satti, Eric Sauvageau, Jeremy Fields, Thomas Grobelny, and Johnathan Hartman
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
Supplemental Digital Content 1. Table. Reasons for patient early termination from the HEAT trial.
Supplemental Digital Content 2. Table. Prevalence of serious adverse events (SAE), categorized by treatment arm.
Supplemental Digital Content 3. Table. Prevalence of non-serious adverse events with a frequency of >3%, categorized by treatment arm.
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. Byrne JV, Sohn MJ, Molyneux AJ. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999;90(4):656-663. [DOI] [PubMed] [Google Scholar]
- 2. Molyneux AJ, Kerr RS, Birks J et al.. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 2009;8(5):427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White PM, Lewis SC, Gholkar A et al.. Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (HELPS): a randomised controlled trial. Lancet North Am Ed. 2011;377(9778):1655-1662. [DOI] [PubMed] [Google Scholar]
- 4. Brinjikji W, Kallmes DF, Kadirvel R. Mechanisms of healing in coiled intracranial aneurysms: a review of the literature. Am J Neuroradiol. 2015;36(7):1216-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plowman RS, Clarke A, Clarke M, Byrne JV. Sixteen-year single-surgeon experience with coil embolization for ruptured intracranial aneurysms: recurrence rates and incidence of late rebleeding. J Neurosurg. 2011;114(3):863-874. [DOI] [PubMed] [Google Scholar]
- 6. McDougall CG, Johnston SC, Gholkar A et al.. Bioactive versus bare platinum coils in the treatment of intracranial aneurysms: the MAPS (Matrix and Platinum Science) trial. Am J Neuroradiol. 2014;35(5):935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Molyneux AJ, Clarke A, Sneade M et al.. Cerecyte coil trial: angiographic outcomes of a prospective randomized trial comparing endovascular coiling of cerebral aneurysms with either cerecyte or bare platinum coils. Stroke. 2012;43(10):2544-2550. [DOI] [PubMed] [Google Scholar]
- 8. Bendszus M, Solymosi L. Cerecyte coils in the treatment of intracranial aneurysms: a preliminary clinical study. Am J Neuroradiol. 2006;27(10):2053-2057. [PMC free article] [PubMed] [Google Scholar]
- 9. Van Rooij WJ, De Gast AN, Sluzewski M. Results of 101 aneurysms treated with polyglycolic/polylactic acid microfilament nexus coils compared with historical controls treated with standard coils. AJNR Am J Neuroradiol. 2008;29(5):991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sluzewski M, van Rooij WJ, Slob MJ, Bescós JO, Slump CH, Wijnalda D. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology. 2004;231(3):653-658. [DOI] [PubMed] [Google Scholar]
- 11. Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. Am J Neuroradiol. 2002;23(9):1580-1588. [PMC free article] [PubMed] [Google Scholar]
- 12. Brinjikji W, Amar AP, Almandoz JE et al.. GEL THE NEC: a prospective registry evaluating the safety, ease of use, and efficacy of the HydroSoft coil as a finishing device. J Neurointervent Surg. 2018;10(1):83-87. [DOI] [PubMed] [Google Scholar]
- 13. Yoshino Y, Niimi Y, Song JK, Silane M, Berenstein A. Endovascular treatment of intracranial aneurysms: comparative evaluation in a terminal bifurcation aneurysm model in dogs. J Neurosurg. 2004;101(6):996-1003. [DOI] [PubMed] [Google Scholar]
- 14. Zhang C, Chaudhary N, Gemmete JJ, Thompson BG, Xi G, Pandey AS. Reactive tissue proliferation and damage of elastic lamina caused by hydrogel coated coils in experimental rat aneurysms. J Neurointervent Surg. 2014;6(6):480-486. [DOI] [PubMed] [Google Scholar]
- 15. Cruise GM, Rivera EA, Jones RM et al.. A comparison of experimental aneurysm occlusion determination by angiography, scanning electron microscopy, MICROFIL® perfusion, and histology. J Biomed Mater Res. 2009;91(2):669-678. [Google Scholar]
- 16. Raymond J, Klink R, Chagnon M et al.. Patients prone to recurrence after endovascular treatment: periprocedural results of the PRET randomized trial on large and recurrent aneurysms. Am J Neuroradiol. 2014;35(9):1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fanning NF, Berentei Z, Brennan PR, Thornton J. HydroCoil as an adjuvant to bare platinum coil treatment of 100 cerebral aneurysms. Neuroradiology. 2007;49(2):139-148. [DOI] [PubMed] [Google Scholar]
- 18. Abi-Aad KR, Aoun RJ, Rahme RJ et al.. New generation Hydrogel Endovascular Aneurysm Treatment Trial (HEAT): a study protocol for a multicenter randomized controlled trial. Neuroradiology. 2018;60(10):1075-1084. [DOI] [PubMed] [Google Scholar]
- 19. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32(9):1998-2004. [DOI] [PubMed] [Google Scholar]
- 20. Meyers PM, Schumacher HC, Higashida RT et al.. Reporting standards for endovascular repair of saccular intracranial cerebral aneurysms. Stroke. 2009;40(5):366-379. [DOI] [PubMed] [Google Scholar]
- 21. U.S Food and Drug Administration. A Risk-Based Approach to Monitoring of Clinical Investigations Questions and Answers. Guidance document. 2013https://www.fda.gov/media/121479/download. [Google Scholar]
- 22. Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 Update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med. 2017;167(1):40-47. [DOI] [PubMed] [Google Scholar]
- 23. Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86(3):475-482. [DOI] [PubMed] [Google Scholar]
- 24. Sluzewski M, van Rooij WJ, Rinkel GJ, Wijnalda D. Endovascular treatment of ruptured intracranial aneurysms with detachable coils: long-term clinical and serial angiographic results. Radiology. 2003;227(3):720-724. [DOI] [PubMed] [Google Scholar]
- 25. Wakhloo AK, Gounis MJ, Sandhu JS, Akkawi N, Schenck AE, Linfante I. Complex-shaped platinum coils for brain aneurysms: higher packing density, improved biomechanical stability, and midterm angiographic outcome. Am J Neuroradiol. 2007;28(7):1395-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piotin M, Iijima A, Wada H, Moret J. Increasing the packing of small aneurysms with complex-shaped coils: an in vitro study. Am J Neuroradiol. 2003;24(7):1446-1448. [PMC free article] [PubMed] [Google Scholar]
- 27. Bendok BR, Rahme RJ. Complex shaped detachable platinum coil system for the treatment of cerebral aneurysms: the Codman Trufill DCS and Trufill DCS orbit detachable coil system COMPLEX registry final results. J Neurointervent Surg. 2013;5(1):54-61. [DOI] [PubMed] [Google Scholar]
- 28. Hirsch JA, Bendok BR, Paulsen RD, Cognard C, Campos J, Cronqvist M. Midterm clinical experience with a complex-shaped detachable platinum coil system for the treatment of cerebral aneurysms: Trufill DCS Orbit detachable coil system registry interim results. J Vasc Interv Radiol. 2007;18(12):1487-1494. [DOI] [PubMed] [Google Scholar]
- 29. Mehra M, Hurley MC, Gounis MJ, King RM, Shaibani A, Dabus G. The impact of coil shape design on angiographic occlusion, packing density and coil mass uniformity in aneurysm embolization: an in vitro study. J Neurointervent Surg. 2011;3(2):131-136. [DOI] [PubMed] [Google Scholar]
- 30. Cloft HJ. HydroCoil for Endovascular Aneurysm Occlusion (HEAL) study: 3–6 month angiographic follow-up results. Am J Neuroradiol. 2007;28(1):152-154. [PMC free article] [PubMed] [Google Scholar]
- 31. Raymond J, Klink R, Chagnon M et al.. Hydrogel versus bare platinum coils in patients with large or recurrent aneurysms prone to recurrence after endovascular treatment: a randomized controlled trial. AJNR Am J Neuroradiol. 2017;38(3):432-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taschner CA, Chapot R, Costalat V et al.. Second-generation hydrogel coils for the endovascular treatment of intracranial aneurysms. Stroke. 2018;49(3):667-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okada T, Ishikawa T, Moroi J, Suzuki A. Timing of retreatment for patients with previously coiled or clipped intracranial aneurysms: analysis of 156 patients with multiple treatments. Surg Neurol Int. 2016;7(Suppl 2):S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.