Abstract
Rationale & Objective:
The current clinical guidelines for vascular access do not have specific recommendations for older hemodialysis patients. Our study aimed to determine the association of age with arteriovenous fistula (AVF) placement, maturation, and primary and secondary patency loss among older hemodialysis recipients.
Study Design:
Retrospective cohort study.
Setting & Participants:
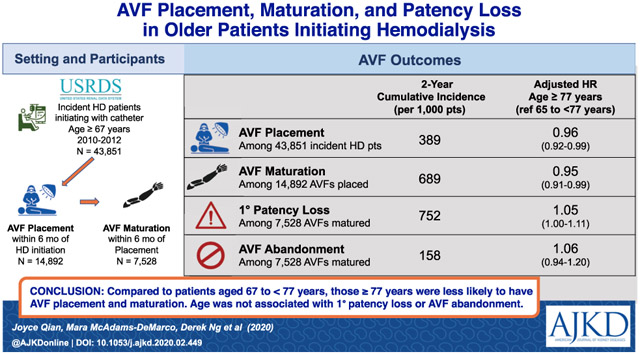
A US national cohort of incident hemodialysis patients 67 years or older (N = 43,851) assembled from the US Renal Data System.
Exposure:
Age at dialysis initiation.
Outcomes:
AVF placement, maturation, primary patency loss, and abandonment.
Analytical Approach:
Cause-specific and subdistribution proportional hazards models were used to examine the association of age and AVF outcomes, with kidney transplantation, peritoneal dialysis, and death treated as competing events. Age cutoff was identified by restricted cubic splines. We compared crude and inverse probability–weighted cumulative incidence functions using Gray’s test.
Results:
As compared with those aged 67-<77 years, patients 77 years or older had significantly lower probabilities of AVF placement (adjusted cause-specific HR [cHR], 0.96 [95% CI, 0.92-0.99]; adjusted subdistribution HR [sHR], 0.92 [95% CI, 0.89-0.95]; Gray’s test P < 0.001) and maturation (adjusted cHR, 0.95 [95% CI, 0.91-0.99]; adjusted sHR, 0.93 [95% CI, 0.90-0.97]; P < 0.001). However, age was not associated with AVF primary (adjusted cHR, 1.05 [95% CI, 1.00-1.11]; adjusted sHR, 1.04 [95% CI, 0.99-1.09]; P = 0.09) or secondary (adjusted cHR, 1.06 [95% CI, 0.94-1.20]; adjusted sHR, 1.05 [95% CI, 0.93-1.18]; P = 0.4) patency loss.
Limitations:
Reliance on administrative claims to ascertain AVF outcomes.
Conclusions:
The likelihood of AVF maturation is an important consideration for vascular access planning. Age alone should not be the basis for excluding older dialysis patients from AVF creation because maintenance of fistula patency was not reduced with older age despite a modest reduction in fistula maturation
Graphical Abstract
Older adults are the fastest growing segment of the prevalent population with end-stage renal disease (ESRD). In 2016, 47.4% of US patients undergoing hemodialysis therapy were 65 years or older1 as compared to 44.7% in 2010.2 Older dialysis recipients present a special challenge because of their heavy comorbid burdens, limited functional and cognitive ability, and short life expectancy.3,4
Current clinical guidelines recommend the arteriovenous fistula (AVF) as the optimal vascular access for hemodialysis.5 This recommendation was based on observational data that AVFs, when functional, are less prone to infection and are associated with reduced all-cause mortality as compared with arteriovenous grafts (AVGs) and central venous catheters (CVCs).6,7 In older dialysis recipients, matured AVFs achieve longer patency and require fewer interventional procedures to maintain functionality than AVGs.8 However, in the short term, AVFs are less likely to mature and need a higher frequency of interventions to achieve maturation as compared with AVGs.8 This leads to prolonged CVC use, which is related to substantially elevated rates of infection9,10 and increased all-cause mortality.11,12 Older patients, especially those who are likely to have short life expectancy and worse AVF outcomes, face the dilemma of whether they should have an AVF or AVG placed as the primary vascular access. To date, there is no clear clinical recommendation for AVF placement using age as a criterion and the clinical decision is left to the individual vascular surgeon, taking into account the patient’s preference and goals.13
In general, evidence on the association of age and AVF outcomes is scarce in the literature.14,15 Definitions of “elderly” in these studies are often inconsistent and the results of AVF outcomes are conflicting. This study aims to examine the association of age with AVF outcomes, including AVF placement, maturation, and primary and secondary patency loss in US older adults undergoing hemodialysis. Precise estimation of the association of age with AVF outcomes may provide valuable information to update clinical guidelines and assist individual vascular access planning.
Methods
Data Source and Study Population
Our primary data source was derived from the 2010 to 2015 US Renal Data System (USRDS) standard analytic files. Figure 1 shows the development of 3 study cohorts. Cohort 1, the hemodialysis cohort, included 43,851 incident hemodialysis recipients 67 years and older who initiated dialysis through a CVC between July 1, 2010, and June 30, 2012. To ensure that the CVC was the only vascular access present at the start of dialysis therapy, patients were excluded if they: (1) were using an AVF or AVG or had an AVF or AVG placed but were awaiting maturation at dialysis initiation, as reported in the Medical Evidence Form; (2) underwent AVF or AVG surgery in the 2-year period before ESRD, as assessed using Current Procedural Terminology, 4th edition (CPT-4) procedure codes 36818, 36819, 36820, 36821, and 36825 for AVF and 36800, 36810, and 36830 for AVG.
Figure 1.
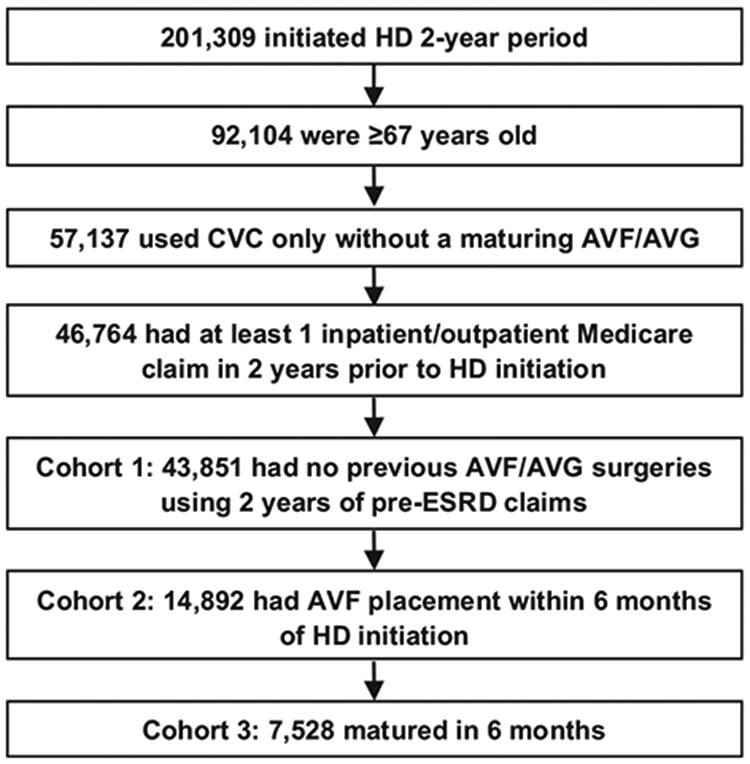
Development of 3 study cohorts. Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter; ESRD, end-stage renal disease; HD, hemodialysis.
From the hemodialysis cohort (cohort 1), we further identified 2 subcohorts. Cohort 2, the AVF-placed cohort, included 14,892 patients who underwent AVF placement within 6 months after dialysis initiation. Cohort 3, the AVF-matured cohort, included 7,528 patients whose AVFs matured within 6 months after placement.
Study Outcomes
The primary outcomes of this study were AVF placement (cohort 1), AVF maturation (cohort 2), and AVF primary and secondary patency loss (cohort 3). We identified AVF placement from the physician/supplier claims and institutional claims files, which record the exact date of AVF construction, by using the same CPT-4 codes as listed. Different from AVF placement, AVF maturation and patency loss were ascertained by month. AVF maturation was determined by using the first vascular access modifier code V7 reported from the institutional details claims file or the first AVF used with 2 needles reported from the CROWNWeb clinical file. We defined AVF primary patency loss as the first revision procedure after maturation. Codes used to identify intervention procedures are listed in Table S1. We defined AVF secondary patency loss (abandonment) as 6 consecutive months of CVC use or a new vascular access placement.
The secondary outcome of this study was AVF assisted maturation, which was defined as the performance of any percutaneous or surgical interventions before AVF maturation among patients who achieved AVF maturation within 2 years from AVF placement in cohort 2 (Table S1). Cohort 1 started at dialysis initiation and was followed up for 3 years. Cohort 2 started at AVF placement and was followed up for 2 years. Cohort 3 started at AVF maturation and was followed up for 2 years.
Competing Events
A competing event in this study was defined as kidney transplant, peritoneal dialysis transfer, or death, whichever occurred first in the follow-up period. Competing events were determined by using the transplant file, dialysis institutional claims file, and death file, respectively.
Covariates of Interest
Age at dialysis initiation was the study exposure. We extracted patient demographics, residential region, body mass index, functional status, laboratory values, primary cause of kidney failure, and predialysis nephrology care from the Medical Evidence Form. In addition, 2-year pre-ESRD claims files were used to identify major comorbid conditions. A comorbid score was calculated based on these conditions.16 ESRD Network number, facility profit status, and hospital association were ascertained from the facility file and merged to patient-level data by using facility provider identification number.
Statistical Analysis
We presented summary statistics as frequency and percentage for categorical data and mean ± standard deviation for continuous variables by patient age at dialysis initiation. In each cohort, we classified patients into 3 mutually exclusive groups according to whether they experienced the outcome of interest (AVF placement, maturation, primary patency loss, and abandonment) or a competing event: (1) patient experienced the outcome of interest, (2) patient had a competing event, and (3) patient did not experience the outcome of interest or a competing event.
To properly categorize the continuous age into groups, we tested the assumption of a linear relationship between age and log cause-specific hazard ratios (cHRs) of AVF outcomes by using restricted cubic spline function.17,18 Any breakpoint in linearity or change in the slope of cubic splines was viewed as a change in the association of age with AVF outcomes. We tested different knot numbers and locations and selected spline functions with 3 knots at the 10th, 50th, and 90th percentiles. When evidence of a nonlinear relationship was found, we identified the break points and used them to categorize age. We used the cause-specific and subdistribution proportional hazards models to explore the possible association between age group and AVF outcomes. We reported cHRs and subdistribution HRs (sHRs) for both the AVF outcomes and competing events, as recommended.19 The cHR assesses whether the rate at any point in time is higher (lower) among the older versus younger group, whereas the sHR identifies whether the cumulative risk is higher (lower) among the older versus younger group.
The proportional hazards assumption was checked by examining age and time interaction and plotting log of negative log of estimated survival function versus log of time for each AVF outcome.20 When the proportional hazards assumption is satisfied, the graph should show parallel lines and the statistical test of age and time interaction should be insignificant. When nonproportionality is present, we calculated time-varying HRs. To avoid any violation of proportional hazards assumption, we also generated the nonparametric inverse probability–weighted cumulative incidence function curves21 and implemented Gray’s test22 for equivalence of cumulative incidence function to compare weighted cumulative incidence by age groups. Inverse probability weights are the inverse of propensity scores, which are the estimated probabilities of treatment assignment conditioned on covariates. We used logistic regression to examine the association of age and 2-year assisted maturation under the assumption that patients who experienced competing events did not have assisted maturation.
Statistical analyses were performed using SAS (version 9.4; SAS Institute). All statistical tests were 2 sided, and P < 0.05 was considered statistically significant. Institutional Review Board approval for an exempt review was obtained from Bloomberg School of Public Health at Johns Hopkins University. Informed consent was not required because this is a secondary data analysis and has minimal risk to participants. We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines to improve the reporting of our observational research study.
Results
Age Categorization
Figure S1 indicated that the log HRs of AVF placement was not linear (P < 0.0001) but the log HRs of AVF maturation and primary and secondary patency loss were linear. We had tested restricted cubic splines with 3, 4, and 5 knots with locations automatically selected from the percentiles of age distribution.23 Age 76 or 77 years corresponded to a break point in the splines of log HR. HRs of AVF placement at 67-<77 years were homogeneous but decreased rapidly with age older than 77 years. Accordingly, we chose age of 77 years as a convenient cutoff value to classify patients into 2 groups.
Baseline Characteristics
Table 1 lists patient demographics, comorbid conditions, functional status, laboratory values, and care patterns by 2 categories of age at dialysis initiation. Patients 67-<77 years old were more likely to be male (53.1% vs 51.8%) and black (23.2% vs 16.7%) as compared with those 77 years or older. Not surprisingly, patients 77 years or older carried higher comorbid burdens and had worse functional status as compared with those aged 67-<77 years. Nutrition status (hemoglobin and serum albumin values) and practice patterns were similar between the 2 age groups.
Table 1.
Patient Characteristics by Age Group in Older Patients Initiating Dialysis With a Catheter
| Age at Dialysis Initiation | ||
|---|---|---|
| 67-<77 y (n = 21,722) |
≥77 y (n = 22,219) |
|
| Demographics | ||
| Male sex | 11,526 (53.1%) | 11,464 (51.8%) |
| Race | ||
| White | 15,660 (72.1%) | 17,407 (78.7%) |
| Black | 5,042 (23.2%) | 3,705 (16.7%) |
| Other/unknown | 1,020 (4.7%) | 1,01 7 (4.6%) |
| Region | ||
| Northeast | 3,425 (15.8%) | 4,356 (19.7%) |
| Midwest | 5,344 (24.7%) | 5,673 (25.6%) |
| South | 8,864 (40.8%) | 7,936 (35.9%) |
| West | 3,938 (18.1%) | 4,041 (18.3%) |
| Comorbid Conditions | ||
| Comorbid score | 8.8 ± 4.6 | 9.5 ± 4.5 |
| Primary cause of kidney failure | ||
| Hypertension/large-vessel disease | 6,191 (28.5%) | 9,263 (41.9%) |
| Diabetes | 9,917 (45.7%) | 7,107 (32.1%) |
| Glomerulonephritis | 656 (3.0%) | 695 (3.1%) |
| Other | 4,958 (22.8%) | 5,064 (22.9%) |
| Diabetes | 15,452 (71.1%) | 13,194 (59.6%) |
| Hypertension | 15,824 (72.9%) | 16,740 (75.7%) |
| Coronary artery disease | 13,259 (61.0%) | 14,580 (65.9%) |
| Myocardial infarction | 5,531 (25.5%) | 5,816 (26.3%) |
| Atherosclerosis | 12,731 (58.6%) | 14,019 (63.4%) |
| Coronary revascularization | 1,386 (6.4%) | 1,190 (5.4%) |
| Congestive heart failure | 14,476 (66.6%) | 16,143 (73.0%) |
| Peripheral vascular disease | 11,023 (50.8%) | 12,029 (54.4%) |
| Cerebrovascular disease | 6,544 (30.1%) | 7,050 (31.9%) |
| Stroke | 2,880 (13.3%) | 2,985 (13.5%) |
| Chronic obstructive pulmonary disease | 8,257 (38.1%) | 8,565 (38.7%) |
| Cancer | 4,165 (19.2%) | 4,786 (21.6%) |
| Depression | 3,984 (18.3%) | 3,649 (16.5%) |
| Dementia | 1,230 (5.7%) | 2,397 (10.8%) |
| Functional Status | ||
| Amputation | 448 (2.1%) | 225 (1.0%) |
| Inability to ambulate | 2,515 (11.6%) | 3,063 (13.8%) |
| Inability to transfer | 1,488 (6.9%) | 1,854 (8.4%) |
| Needs assistance with daily activities | 4,122 (19.0%) | 5,447 (24.6%) |
| Institutionalized | 3,191 (14.7%) | 4,612 (20.8%) |
| Laboratory Values | ||
| Body mass index, kg/m2 | 29.4 ± 7.8 | 27.0 ± 6.6 |
| Hemoglobin, g/dL | 9.6 [8.7-10.6] | 9.7 [8.8-10.6] |
| Serum albumin, g/dL | 3.0 [2.6-3.5] | 3.1 [2.6-3.5] |
| eGFR, mL/min/1.73 m2 | 13.2 ± 5.8 | 13.9 ± 5.7 |
| Care Patterns | ||
| Nephrology care | ||
| None or <6 mo | 14,463 (66.6%) | 14,661 (66.3%) |
| 6-12 mo | 3,204 (14.8%) | 3,151 (14.3%) |
| 12 mo | 4,055 (18.7%) | 4,317 (19.5%) |
| Facility type | ||
| Hospital-based | 1,956 (9.0%) | 2,089 (9.5%) |
| Freestanding | 19,716 (91.0%) | 19,985 (90.5%) |
| Profit status | ||
| For-profit | 18,143 (83.7%) | 18,287 (82.8%) |
| Nonprofit | 3,348 (15.5%) | 3,565 (16.2%) |
| Unknown | 181 (0.8%) | 222 (1.0%) |
Note: Values for categorical variables given as count (percentage); for continuous variables, as mean ± standard deviation or median [interquartile range].
Abbreviation: eGFR, estimated glomerular filtration rate.
Age and AVF Placement
Of 43,851 patients who initiated dialysis with a CVC in the hemodialysis cohort (cohort 1), the 3-year cumulative incidence was 394 per 1,000 patients for AVF placement and 408 per 1,000 patients for competing events (Table 2). Compared with patients aged 67-<77 years, those 77 years or older had a significantly lower probability to have an AVF placed (unadjusted cHR, 0.93 [95% CI, 0.90-0.96]; adjusted cHR, 0.96 [95% CI, 0.92-0.99]; unadjusted sHR, 0.86 [95% CI, 0.84-0.89]; adjusted sHR, 0.92 [95% CI, 0.89-0.95]) but higher probability of experiencing a competing event (unadjusted cHR, 1.36 [95% CI, 1.32-1.40]; adjusted cHR, 1.19 [95% CI, 1.15-1.24]; unadjusted sHR, 1.36 [95% CI, 1.32-1.40]; adjusted sHR, 1.20 [95% CI, 1.16-1.25]; Table 3). The HR was not constant over time (P = 0.01 and P < 0.001 for time and age interactions in cause-specific and subdistribution models, respectively).
Table 2.
Follow-up Time and AVF Outcomes by Age Group in Older Hemodialysis Patients
| Follow-up Time, pt-y |
N | Incidencea | Rate, per 1,000 pt-y |
Died/Tx/PD | ||||
|---|---|---|---|---|---|---|---|---|
| 1 y | 2 y | 3 y | N | 3-y Incidence | ||||
| AVF Placementb | ||||||||
| Total | 43,516 | 17,271 | 376 | 389 | 394 | 396.9 | 1 7,897 | 408 |
| 67-<77 y | 22,854 | 9,074 | 397 | 411 | 418 | 397.0 | 7,852 | 362 |
| ≥77 y | 20,662 | 8,197 | 356 | 367 | 370 | 396.7 | 10,045 | 454 |
| AVF Maturationc | ||||||||
| Total | 9,841 | 10,260 | 656 | 689 | — | 1,042.6 | 3,029 | 203 |
| 67-<77 y | 5,142 | 5,527 | 675 | 715 | — | 1,074.9 | 1,369 | 177 |
| ≥77 y | 4,699 | 4,733 | 634 | 661 | — | 1,007.2 | 1,660 | 232 |
| AVF Primary Patency Lossc | ||||||||
| Total | 4,408 | 5,664 | 695 | 752 | — | 1,284.9 | 1,045 | 139 |
| 67-<77 y | 2,399 | 2,957 | 686 | 746 | — | 1,232.6 | 530 | 134 |
| ≥77 y | 2,009 | 2,707 | 705 | 759 | — | 1,347.4 | 515 | 144 |
| AVF Abandonmentc | ||||||||
| Total | 10,510 | 1,191 | 129 | 158 | — | 113.3 | 2,600 | 345 |
| 67-<77 y | 5,691 | 613 | 125 | 155 | — | 107.7 | 1,233 | 311 |
| ≥77 y | 4,819 | 578 | 133 | 162 | — | 119.9 | 1,367 | 383 |
Abbreviations: AVF, arteriovenous fistula; PD, peritoneal dialysis; pt-y, patient-year; Tx, kidney transplant.
Cumulative incidence per 1,000 patients.
In 3 years after placement.
In 2 years after maturation.
Table 3.
Cause-Specific and Subdistribution HRs of AVF Outcomes and Competing Events by Age Group in Older Hemodialysis Patients
| AVF Outcomes |
Competing Eventsa |
|||
|---|---|---|---|---|
| Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
|
| Cause-Specific HRs | ||||
| AVF placementb,c | ||||
| 67-<77 y | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥77 y | 0.93 (0.90-0.96) | 0.96 (0.92-0.99) | 1.36 (1.32-1.40) | 1.19 (1.15–1.24) |
| AVF maturationc,d | ||||
| 67-<77 y | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥77 y | 0.95 (0.91-0.99) | 0.95 (0.91-0.99) | 1.33 (1.24-1.43) | 1.17 (1.08-1.26) |
| AVF primary patency losse | ||||
| 67-<77 y | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥77 y | 1.05 (1.00-1.11) | 1.05 (1.00-1.11) | 1.44 (1.25-1.65) | 1.12 (0.99-1.27) |
| AVF abandonmentf | ||||
| 67-<77 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥77 | 1.09 (0.97-1.22) | 1.06 (0.94-1.20) | 1.31 (1.21-1.41) | 1.19 (1.10-1.29) |
| Subdistribution HRs | ||||
| AVF placementb,c | ||||
| 67-<77 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥77 | 0.86 (0.84-0.89) | 0.92 (0.89-0.95) | 1.36 (1.32-1.40) | 1.20 (1.16-1.25) |
| AVF maturationc,d | ||||
| 67-<77 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥77 | 0.91 (0.87-0.94) | 0.93 (0.90-0.97) | 1.35 (1.25-1.45) | 1.19 (1.10-1.28) |
| AVF primary patency losse | ||||
| 67-<77 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥77 | 1.03 (0.98-1.08) | 1.04 (0.99-1.09) | 1.09 (0.97-1.23) | 1.04 (0.92-1.18) |
| AVF abandonmentf | ||||
| 67-<77 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥77 | 1.05 (0.94-1.18) | 1.05 (0.93-1.18) | 1.29 (1.19-1.39) | 1.18 (1.09-1.28) |
Abbreviations: AVF, arteriovenous fistula; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVC, central venous catheter; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Competing events included death, kidney transplant, and peritoneal dialysis transfer.
Adjusted by sex, race, diabetes, hypertension, stroke, myocardial infarction, coronary revascularization, congestive heart failure, cardiovascular disease, cancer, comorbid score, BMI, albumin level, eGFR, functional status, nephrology care, and region.
Hazards are nonproportional.
Adjusted by sex, race, diabetes, myocardial infarction, stroke, cardiovascular disease, functional status, facility type, comorbid score, BMI, eGFR, time of CVC dependency, and primary or secondary AVF.
Adjusted by sex, race, COPD, depression, region, facility type, facility profit status, comorbid score, and eGFR.
Adjusted by sex, race, hypertension, angina and atherosclerosis, congestive heart failure, peripheral vascular disease, stroke, COPD, cancer, depression, functional status, facility type, comorbid score, BMI, eGFR, and time of CVC dependency.
Time-varying HRs are presented in Table 4, which shows that cHRs and sHRs among those 77 years or older compared with patients aged 67-<77 years were initially higher but decreased quickly afterward. Results of Gray’s test for the equality of weighted cumulative incidence function also confirmed that age was statistically significantly associated with AVF placement (P < 0.001; Fig 2).
Table 4.
Time-Varying Crude and Adjusted cHRs and sHRs of AVF Placement and Maturation in Hemodialysis Patients 77 Years or Older Versus 67-<77 Years Old
| Unadjusted cHR (95% CI) |
Adjusted cHR (95% CI) |
Unadjusted sHR (95% CI) |
Adjusted sHR (95% CI) |
|
|---|---|---|---|---|
| AVF Placementa | ||||
| Baseline | 0.95 (0.79-1.16) | 1.05 (0.83-1.33) | 1.21 (1.02-1.45) | 1.34 (1.08-1.66) |
| Day 180 | 0.93 (0.89-0.96) | 0.95 (0.90-0.99) | 0.83 (0.80-0.86) | 0.88 (0.84-0.92) |
| Day 360 | 0.93 (0.88-0.98) | 0.94 (0.88-1.00) | 0.80 (0.76-0.84) | 0.85 (0.79-0.90) |
| Day 540 | 0.92 (0.87-0.98) | 0.93 (0.87-1.01) | 0.79 (0.74-0.83) | 0.83 (0.77-0.89) |
| Day 720 | 0.92 (0.86-0.99) | 0.93 (0.86-1.01) | 0.78 (0.73-0.83) | 0.82 (0.75-0.88) |
| Day 900 | 0.92 (0.86-0.99) | 0.93 (0.85-1.01) | 0.77 (0.72-0.82) | 0.81 (0.74-0.88) |
| Day 1,080 | 0.92 (0.85-1.00) | 0.93 (0.84-1.02) | 0.76 (0.71-0.82) | 0.80 (0.73-0.87) |
| AVF Maturationb | ||||
| Baseline | 2.12 (1.47-3.05) | 2.03 (1.40-2.94) | 2.78 (1.95-3.96) | 2.80 (1.95-4.01) |
| Month 6 | 0.92 (0.88-0.96) | 0.92 (0.88-0.96) | 0.86 (0.83-0.90) | 0.89 (0.85-0.93) |
| Month 12 | 0.82 (0.76-0.89) | 0.82 (0.76-0.89) | 0.74 (0.69-0.80) | 0.76 (0.71-0.82) |
| Month 18 | 0.77 (0.69-0.85) | 0.78 (0.70-0.86) | 0.68 (0.61-0.75) | 0.70 (0.63-0.77) |
| Month 24 | 0.73 (0.65-0.83) | 0.74 (0.66-0.84) | 0.63 (0.56-0.71) | 0.66 (0.58-0.74) |
Note: HRs are for the ≥77 years old group versus a reference group of 67-<77 years old. Time-varying HRs were estimated by including an age-time interaction; time was included as a continuous variable.
Abbreviations: AVF, arteriovenous fistula; BMI, body mass index; cHR, cause-specific hazard ratio; eGFR, estimated glomerular filtration rate; HR, hazard ratio; sHR, subdistribution hazard ratio.
Adjusted by sex, race, diabetes, hypertension, stroke, myocardial infarction, coronary revascularization, congestive heart failure, cardiovascular disease, cancer, comorbid score, BMI, albumin level, eGFR, functional status, nephrology care, and region.
Adjusted by sex, race, diabetes, myocardial infarction, stroke, cardiovascular disease, functional status, facility type, comorbid score, BMI, eGFR, time of central venous catheter dependency, and primary or secondary AVF.
Figure 2.
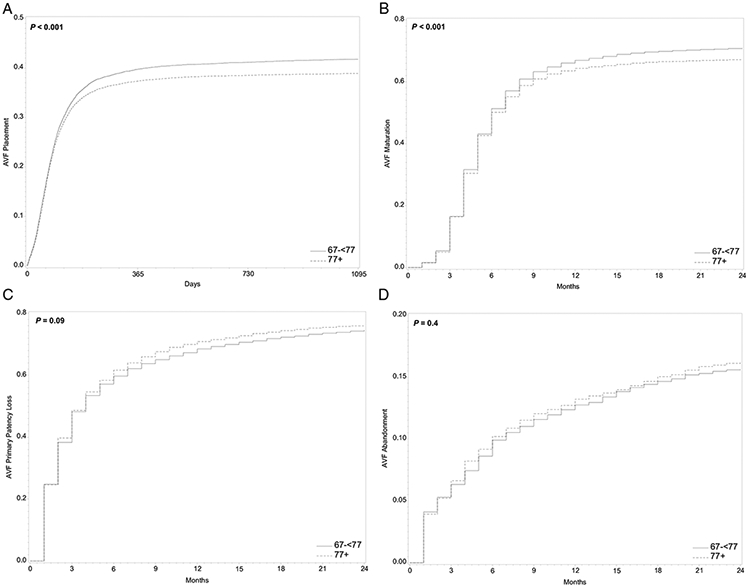
Weighted cumulative incidence functions (CIFs) of arteriovenous fistula (AVF) outcomes and competing events by age group in older hemodialysis patients. P values were obtained using Gray's test for equality of the weighted CIF. AVF outcomes included (A) AVF placement in 3 years after dialysis initiation, (B) AVF maturation in 2 years after placement, (C) AVF primary patency loss in 2 years after maturation, and (D) AVF abandonment in 2 years after maturation.
Age and AVF Maturation
In the AVF-placed cohort (cohort 2), the 2-year cumulative incidence was 689 per 1,000 patients for AVF maturation and 203 per 1,000 patients for competing events (Table 2). Proportional hazards regressions showed that AVFs placed in patients 77 years or older were less likely to mature as compared with those in patients 67-<77 years old (unadjusted cHR, 0.95 [95% CI, 0.91-0.99]; adjusted cHR, 0.95 [95% CI, 0.91-0.99]; unadjusted sHR, 0.91 [95% CI, 0.87-0.94]; adjusted sHR, 0.93 [95% CI, 0.90-0.97]; Table 3). They were more likely to have a competing event (unadjusted cHR, 1.33 [95% CI, 1.24-1.43]; adjusted cHR, 1.17 [95% CI, 1.08-1.26]; unadjusted sHR, 1.35 [95% CI, 1.25-1.45]; adjusted sHR, 1.19 [95% CI, 1.10-1.28]; Table 3).
The proportional hazards assumption was violated when comparing AVF maturation (P < 0.001 for both models). Time-varying cHRs and sHRs suggest that maturation was initially more likely to occur among patients 77 years or older as compared with those 67-<77 years old, but then less likely to mature (Table 4). Gray’s test also demonstrated that the association of age and AVF maturation was statistically significant (P < 0.001; Fig 2). Among 10,260 patients who achieved AVF maturation, 55.0% had assisted maturation. As compared with patients aged 67-<77 years, those aged 77 years or older were more likely to have an assisted maturation (unadjusted odds ratio [OR], 1.23 [95% CI, 1.15-1.31]; adjusted OR, 1.12 [95% CI, 1.05-1.21]; Table S2).
Age and AVF Primary Patency Loss
Among 7,528 patients in the AVF-matured cohort (cohort 3), the 2-year cumulative incidence was 752 per 1,000 patients for AVF primary patency loss and 139 per 1,000 patients for competing events (Table 2). The proportional hazards assumption was not rejected in both models (P = 0.2 and P = 0.9). Both cause-specific and subdistribution hazards analyses showed that age was not associated with AVF primary patency loss (unadjusted cHR, 1.05 [95% CI, 1.00-1.11]; adjusted cHR, 1.05 [95% CI, 1.00-1.11]; unadjusted sHR, 1.03 [95% CI, 0.98-1.08]; adjusted sHR, 1.04 [95% CI, 0.99-1.09]; Table 3). Gray’s test also revealed that the difference in AVF primary patency loss was not statistically significant by age groups (P = 0.09; Fig 2). The probability of experiencing a competing event was not significantly different in patients 77 years or older compared with those aged 67-<77 years (unadjusted cHR, 1.44 [95% CI, 1.25-1.65]; adjusted cHR, 1.12 [95% CI, 0.99-1.27]; unadjusted sHR, 1.09 [95% CI, 0.97-1.23]; adjusted sHR, 1.04 [95% CI, 0.92-1.18]; Table 3).
Age and AVF Abandonment
Overall, the 2-year cumulative incidence was 158 per 1,000 patients for AVF abandonment and 345 per 1,000 patients for competing events in the AVF-matured cohort (cohort 3; Table 2). The proportional hazards assumption was not rejected in both models (P = 0.7 and P = 0.8). Age was not associated with increased risk for AVF abandonment (unadjusted cHR, 1.09 [95% CI, 0.97-1.22]; adjusted cHR, 1.06 [95% CI, 0.94-1.20]; unadjusted sHR, 1.05 [95% CI, 0.94-1.18]; adjusted sHR, 1.05 [95% CI, 0.93-1.18]; P = 0.4) but patients 77 years or older had a higher likelihood of experiencing a competing event (unadjusted cHR, 1.31 [95% CI, 1.21-1.41]; adjusted cHR, 1.19 [95% CI, 1.10-1.29]; unadjusted sHR, 1.29 [95% CI, 1.19-1.39]; adjusted sHR, 1.18 [95% CI, 1.09-1.28]) as compared with those 67-<77 years old (Table 3; Fig 2).
Discussion
By using a national prospective cohort of older hemodialysis patients, we examined the association of age with AVF placement, maturation, primary patency loss, and abandonment, accounting for the competing events of death, kidney transplant, and peritoneal dialysis transfer. Among the US older adults who initiated dialysis with a CVC, patients 77 years or older had a significantly lower probability of getting an AVF placed in 3 years after dialysis initiation and matured in 2 years after placement compared with those aged 67-<77 years. However, in the subcohort of patients with a matured AVF, primary and secondary AVF patency loss was not statistically different by age group (≥77 vs 67-<77 years) in 2 years after maturation.
These results are consistent with 2 national studies of the association of age with AVF placement and maturation among older adults initiating dialysis.24,25 Lilly et al24 showed that among the US patients who initiated hemodialysis between 2005 and 2009, those 85 years and older had lower odds of AVF maturation as compared with patients aged 65-<85 years at their first dialysis session. Similarly, Harford et al25 reported that the odds of AVF maturation was significantly lower in the 80-year-and-older group compared with those aged 67-<80 years at dialysis initiation between 2005 and 2010. The association of age with AVF maturation persisted in these 2 studies after adjusting for patient demographics, comorbid conditions, insurance status, prior nephrologist care patterns, body mass index, or functional status. However, these 2 studies estimated the rate of AVF maturation in a crosssectional design without considering whether these patients had an AVF placed. Our study instead implemented a retrospective design and considered change in risk set due to competing events so that we obtained a better estimation of the impact of age on AVF placement and maturation.
Our findings of similar rates in AVF patency loss concur with the conclusions drawn from 3 retrospective single-center studies that older age did not increase the risk for AVF patency loss.26-28 In a single-center study of 335 patients, Swindlehurst et al26 reported that 25-month AVF primary and secondary patency rates were not significantly different between adult patients 65 years or older and those younger than 65 years. Another study by Weale et al27 also indicated that age group (<65, 65-79, and ≥80 years) was not associated with 1- and 2-year primary and secondary patency among 658 adult patients. Similar to our study, these 2 studies included both radiocephalic (wrist or forearm) and brachiocephalic (upper-arm) AVFs. Nonetheless, patients in these 2 studies were enrolled after ultrasound vessel screening so they were more likely to have homogeneous vein parameters regardless of age. In the third study of 444 incident AVF patients by Lok et al,28 preoperative vein mapping was not necessarily performed. Their study also demonstrated that 1- and 5-year AVF secondary patency was not significantly different between patients 65 years and older and younger than 65 years.28 Our study adds to the evidence by extending the findings from these single-center studies to a national cohort of patients.
Our study revealed that patients 77 years and older had a lower probability of AVF placement and maturation and higher probability of assisted maturation as compared with those aged 67-<77 years after controlling for patient demographics, comorbid conditions, functional status, and facility practice patterns. Notably, although a greater number of patients in the 77-year-or-older group were removed from the risk set due to competing events, the resulting cumulative incidences of AVF placement and maturation were still lower than those in the group aged 67-<77 years. This indicated that nephrologists or access surgeons were conservative with referring patients in this age group to AVF placement and the lower likelihood of AVF maturation although patients in the group 77 years or older have comparable comorbid profiles and functional status as their younger counterparts. Our study showed that these patients are more likely to experience a competing event than have an AVF placed or matured.
Our study also demonstrated that when matured, AVFs placed in those 77 years or older attained similar primary and secondary patency as compared with those aged 67-<77 years after accounting for competing events. AVF maturation could be viewed as the barrier between the initial step of AVF placement and achieving the goal of patency attainment. However, older hemodialysis patients should not be excluded for AVF placement based on their age alone. Although the cumulative incidence function was statistically lower in patients 77 years or older, the absolute difference in maturation rate per patient-year in those who received an AVF was 0.07 per patient-year difference in risk. A proper selection of patients for AVF placement using an individualized vascular access plan with consideration of the patient’s preference, likelihood of AVF maturation, frailty, clinical situation, and other factors may lead to better AVF outcomes.29-31
Our study has several strengths. Competing risk is a crucial consideration in studies of older adults undergoing dialysis because of high mortality and a frequent switch in therapy. Methods that fail to account for the presence of these competing risks, such as standard survival analysis, can overestimate the probability of AVF outcomes.32 In this study, we used the cumulative incidence estimate and competing risks regression to determine the association of age and AVF outcomes. Compared to the method using traditional survival analysis, our study provided precise estimations of the association of age with AVF outcomes and presented a full picture to better describe data and interpret results. In the presence of nonproportional hazards, we evaluated the association of AVF placement and maturation with age by using time-varying HRs. Moreover, the cutoff values of age were often arbitrarily selected in previous studies so that the association of age with AVF outcomes was forced to be homogeneous within each category. Our study instead chose the cutoff point of 77 years to categorize age based on the change in slope of the log HR function.
However, several limitations should be noted. First, we relied on AVF procedure codes to ascertain AVF outcomes. These codes were submitted by dialysis facilities for billing purposes and thus likely to be subject to misclassification and selection bias. However, they are identified by clinical experts in the field of vascular access33 and have been widely used.8,34,35 Second, USRDS data do not capture AVF location, blood vessel parameter, or surgeon technique and experience. Unmeasured confounding likely exists. Third, our analytic cohorts were not randomly selected. Those who initiate dialysis, undergo AVF placement, and achieve AVF maturation may be substantially different from those who do not based on demographics, severity of comorbid conditions, blood vessel configuration, and life expectancy.
In conclusion, our retrospective analysis of a national cohort encompassing older adults who initiated dialysis with a CVC shows that increased age is associated with reduced probabilities of AVF placement and maturation but not AVF primary or secondary patency loss. This highlights the importance of individualized vascular access planning focusing on the likelihood of AVF maturation. The chance of AVF maturation estimated based on patients’ demographics, blood vessel configuration, comorbid conditions, functional status, and life expectancy may inform the optimal vascular access for older hemodialysis patients.
Supplementary Material
Figure S1: Restricted cubic spline plots of log hazard ratios of AVF outcomes versus age with 3 knots at 10th, 50th, and 90th percentiles.
Table S1: Codes of surgical and endovascular procedures associated with AVF management.
Table S2: Odds ratios of AVF assisted maturation by age group in older hemodialysis patients.
PLAIN-LANGUAGE SUMMARY.
The role of age in making clinical recommendations for arteriovenous fistula placement is unclear. We assessed the association of age with arteriovenous fistula placement, maturation, and primary and secondary patency loss in a national retrospective cohort of older hemodialysis patients. We found that increasing age was significantly associated with a lower probability of fistula placement and maturation. However, in patients with a matured fistula, primary and secondary patency loss did not differ across age groups. The likelihood of fistula maturation is an important consideration for vascular access planning in the elderly. Older patients should not be excluded for fistula placement simply because of their age, and the decision to place a fistula should be based on an individualized approach.
Acknowledgments
Support: Dr Qian is supported by 1R01MD013818-01 from the National Institute on Minority Health and Health Disparities, 1R21DK104248-01A1 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and R03-HS-022931 from the Agency for Healthcare Research and Quality. Dr McAdams-DeMarco is supported by R01DK120518 and R01DK114074 from the NIDDK and R01AG055781 from the National Institute on Aging. These funding sources did not have a role in the study design, analysis, interpretation of data, writing the report, or the decision to submit the report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Peer Review: Received August 7, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form February 25, 2020.
Contributor Information
Joyce Z. Qian, Medical Technology and Practice Patterns Institute, Bethesda; Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD..
Mara McAdams-DeMarco, Department of Epidemiology, Bloomberg School of Public Health; Department of Surgery, School of Medicine, Johns Hopkins University, Baltimore, MD..
Derek K. Ng, Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD..
Bryan Lau, Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD..
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3)(suppl 1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2012 annual data report. Am J Kidney Dis. 2013;61(1)(suppl 1):e1–e480. [DOI] [PubMed] [Google Scholar]
- 3.Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008;73(11):1289–1295. [DOI] [PubMed] [Google Scholar]
- 4.McAdams-DeMarco MA, Tan J, Salter ML, et al. Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol. 2015;10(12):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for vascular access 2006. Am J Kidney Dis. 2006;48(suppl 1):S176–S322. [DOI] [PubMed] [Google Scholar]
- 6.Lok CE. Fistula first initiative: advantages and pitfalls. Clin J Am Soc Nephrol. 2007;2(5):1043–1053. [DOI] [PubMed] [Google Scholar]
- 7.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62:1109–1124. [DOI] [PubMed] [Google Scholar]
- 8.Lee T, Qian J, Thamer M, Allon M. Tradeoffs in vascular access selection in elderly patients initiating hemodialysis with a catheter. Am J Kidney Dis. 2018;72(4):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005;46(3):501–508. [DOI] [PubMed] [Google Scholar]
- 10.Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60(1):1–13. [DOI] [PubMed] [Google Scholar]
- 11.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis. 2006;47(3):469–47 . [DOI] [PubMed] [Google Scholar]
- 12.Raithatha A, McKane W, Kendray D, Evans C. Catheter access for hemodialysis defines higher mortality in late-presenting dialysis patients. Ren Fail. 2010;32(10):1183–1188. [DOI] [PubMed] [Google Scholar]
- 13.Viecelli AK, Howell M, Tong A, et al. Identifying critically important vascular access outcomes for trials in haemodialysis: an international survey with patients, caregivers and health professionals. Nephrol Dial Transplant. 2020;35(4):657–668. [DOI] [PubMed] [Google Scholar]
- 14.Hod T, DeSilva RN, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS. Factors predicting failure of AV “fistula first” policy in the elderly. Hemodial Int. 2014;18(2):507–515. [DOI] [PubMed] [Google Scholar]
- 15.Drew DA, Lok CE, Cohen JT, Wagner M, Tangri N, Weiner DE. Vascular access choice in incident hemodialysis patients: a decision analysis. J Am Soc Nephrol. 2015;26(1):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2009;77(2):141–151. [DOI] [PubMed] [Google Scholar]
- 17.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54(3):201–208. [DOI] [PubMed] [Google Scholar]
- 18.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057. [DOI] [PubMed] [Google Scholar]
- 19.Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66(6):648–653. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz A, Abraham AG, Matheson M, Wada N. Non-proportionality of hazards in the competing risks framework In: Lee Mei-Ling Ting Gail Mitchell, Ruth Pfeiffer, Glen Satten, Tianxi Cai, Axel Gandy, eds. Risk Assessment and Evaluation of Predictions. New York, NY: Springer; 2013:3–22. [Google Scholar]
- 21.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. [DOI] [PubMed] [Google Scholar]
- 22.Dignam JJ, Kocherginsky MN. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol. 2008;26(24):4027–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell FE. Regression Modeling Strategies, With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 24.Lilly MP, Lynch JR, Wish JB, et al. Prevalence of arteriovenous fistulas in incident hemodialysis patients: correlation with patient factors that may be associated with maturation failure. Am J Kidney Dis. 2012;59(4):541–549. [DOI] [PubMed] [Google Scholar]
- 25.Harford R, Clark MJ, Norris KC, Yan G. Relationship between age and timely placement of vascular access in incident patients on hemodialysis. Nephrol Nurs J. 2014;41(5):507–518. [PMC free article] [PubMed] [Google Scholar]
- 26.Swindlehurst N, Swindlehurst A, Lumgair H, et al. Vascular access for hemodialysis in the elderly. J Vasc Surg. 2011;53(4): 1039–1043. [DOI] [PubMed] [Google Scholar]
- 27.Weale AR, Bevis P, Neary WD, et al. Radiocephalic and brachiocephalic arteriovenous fistula outcomes in the elderly. J Vasc Surg. 2008;47(1):144–150. [DOI] [PubMed] [Google Scholar]
- 28.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV. Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int. 2005;67(6):2462–2469. [DOI] [PubMed] [Google Scholar]
- 29.Hall RK, Myers ER, Rosas SE, O’Hare AM, Colón-Emeric CS. Choice of hemodialysis access in older adults: a cost-effectiveness analysis. Clin J Am Soc Nephrol. 2017;12(6):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomonte C, Basile C, Mitra S, et al. Should a fistula first policy be revisited in elderly haemodialysis patients? Nephrol Dial Transplant. 2018;34(10):1636–1643. [DOI] [PubMed] [Google Scholar]
- 31.Misskey J, Faulds J, Sidhu R, Baxter K, Gagnon J, Hsiang Y. An age-based comparison of fistula location, patency, and maturation for elderly renal failure patients. J Vasc Surg. 2018;67(5): 1491–1500. [DOI] [PubMed] [Google Scholar]
- 32.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thamer M, Lee TC, Wasse H, et al. Medicare costs associated with arteriovenous fistulas among US hemodialysis patients. Am J Kidney Dis. 2018;72(1):10–18. [DOI] [PubMed] [Google Scholar]
- 34.Lee T, Qian J, Zhang Y, Thamer M, Allon M, et al. Long-term outcomes of arteriovenous fistulas with unassisted versus assisted maturation: a retrospective national hemodialysis cohort study. J Am Soc Nephrol. 2019;30(11):2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee T, Qian J, Thamer M, Allon M. Gender disparities in vascular access surgical outcomes in elderly hemodialysis patients. Am J Nephrol. 2019;49(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Restricted cubic spline plots of log hazard ratios of AVF outcomes versus age with 3 knots at 10th, 50th, and 90th percentiles.
Table S1: Codes of surgical and endovascular procedures associated with AVF management.
Table S2: Odds ratios of AVF assisted maturation by age group in older hemodialysis patients.


