Abstract
During mouse embryonic development a mass of pluripotent epiblast tissue is transformed during gastrulation to generate the three definitive germ layers: endoderm, mesoderm, and ectoderm. During gastrulation, a spatiotemporally controlled sequence of events results in the generation of organ progenitors and positions them in a stereotypical fashion throughout the embryo. Key to the correct specification and differentiation of these cell fates is the establishment of an axial coordinate system along with the integration of multiple signals by individual epiblast cells to produce distinct outcomes. These signaling domains evolve as the anterior-posterior axis is established and the embryo grows in size. Gastrulation is initiated at the posteriorly positioned primitive streak, from which nascent mesoderm and endoderm progenitors ingress and begin to diversify. Advances in technology have facilitated the elaboration of landmark findings that originally described the epiblast fate map and signaling pathways required to execute those fates. Here we will discuss the current state of the field and reflect on how our understanding has shifted in recent years.
Keywords: Mouse development, axis patterning, gastrulation, cell fate specification, organogenesis
Introduction:
Normal embryonic development is the outcome of a sequence of stereotypical and coordinated cell fate decisions and morphogenetic events. While the early mouse embryo is highly plastic and resilient to perturbations, failure during key stages results in defects that are often incompatible with life. As such, the reliable and reproducible execution of key developmental steps including, (1) anterior-posterior axis patterning, (2) exit from pluripotency and specification of the definitive germ layer identities, and (3) diversification of these germ layers into organ identities are critical for successful reproduction. These three key events converge in the early mouse embryo during gastrulation, when cells of the pluripotent epiblast separate into the three germ layers: ectoderm, mesoderm, and endoderm, with cells assigned to the latter two germ layers undergoing an epithelial-to-mesenchymal transition (EMT) which facilitates their spatial relocation within the embryo.
Gastrulation in the mouse occurs mainly over three days from embryonic day (E) 6.25 to E9.5, with the anterior-posterior (AP) axis being patterned just prior to the onset of gastrulation and lineage diversification continuing thereafter. Each of these steps involves the coordination of signaling cross-talk between adjacent tissue layers, integration of multiple signals by individual cells within populations, along with cell movements to achieve a reproducible allocation of cell identities and cell positions throughout the embryo. EMT occurs throughout gastrulation at the primitive streak, a morphologically identifiable signaling hub that is positioned at the posterior epiblast (Tam et al., 1993; Williams et al., 2012). Cells that emanate from the primitive streak during gastrulation are patterned into progenitors for multiple organ systems as they relocate away from the primitive streak.
The mouse embryo comprises a radially symmetrical cup-shaped pluripotent epiblast positioned distally, and proximal extra-embryonic ectoderm (ExE, also referred to as trophoblast, which will form the fetal portion of the placenta), enveloped in a layer of extra-embryonic visceral endoderm (VE, also referred to as hypoblast, which forms extra-embryonic membranes). This architecture, which is specific to rodents, is structurally distinct from other amniotes, such as human and chick, which display a disc-shaped epiblast structure. While the geometry of embryos across species differs at the onset of gastrulation, there are many similarities and the apposition of epiblast, VE and ExE tissues appears conserved. Strikingly, the fate maps for zebrafish, chick, and mouse show a similar pattern, with, for example, cells in close proximity to the primitive streak (or its equivalent structure) before gastrulation being destined for future anterior positions (Garcia-Martinez and Schoenwolf, 1993; Keegan et al., 2004; Kinder et al., 2001a; Tam et al., 1997). Furthermore, the signaling pathways that pattern the primitive streak and its derivatives are also conserved, with knowledge gleaned in the mouse embryo having been used to develop mouse and human pluripotent stem cell (PSC) directed differentiation protocols (Murry and Keller, 2008). Recent advances have led to the development of in vitro models of gastrulation, such as micropattern differentiation, suspension aggregate gastruloids, and synthetic embryos, which may allow for deconvolution of signaling and cell-cell interactions, and higher-throughput screening of developmental mechanisms (Hadjantonakis et al., 2020).
The foundation of our knowledge regarding mouse gastrulation was established around 30 years ago, when the fate and developmental potential of the pluripotent epiblast was regionally mapped (Lawson et al., 1991; Tam and Beddington, 1987). Since then, the field has worked to dissect the molecular mechanisms involved in each step of gastrulation and has characterized a number of cardinal pathways and gene-regulatory networks involved in some detail. As available technology has advanced, we have come to understand how gastrulation and early organogenesis occurs in increasing resolution. The advent of advanced imaging, single-cell analyses, and spatial transcriptomics techniques have been a particular boon, enabling the capture of dynamics across the entire embryo in ways previously not possible.
Turning an axis on its head: establishing the anteroposterior axis.
During the window between implantation and gastrulation, at around E5.5, the embryo is comprised of three epithelial populations: a mass of pluripotent epiblast cells, the supportive extra-embryonic ectoderm (ExE), and an enveloping layer of visceral endoderm (VE) (Figure 1). At this time the pre-gastrula epiblast is radially symmetrical and the maternally oriented proximal-distal (PD) axis provides spatial information for the early embryo. The VE can be divided into embryonic VE (emVE), which overlies the distal epiblast, and extra-embryonic VE (exVE), which covers the ExE. Gastrulation will result in the formation of the first mesenchymal populations, as cells undergo epithelial-to-mesenchymal transition (EMT) at the primitive streak, and the organization of these cells into germ layers. Thus, the establishment of the AP axis is key for delimiting the location of the primitive streak, as well as subsequent allocation of cells along the anteroposterior (AP) axis as the germ layers are specified and undergo massive cell proliferation.
Figure 1: Anterior-posterior axis patterning positions the primitive streak prior to gastrulation.
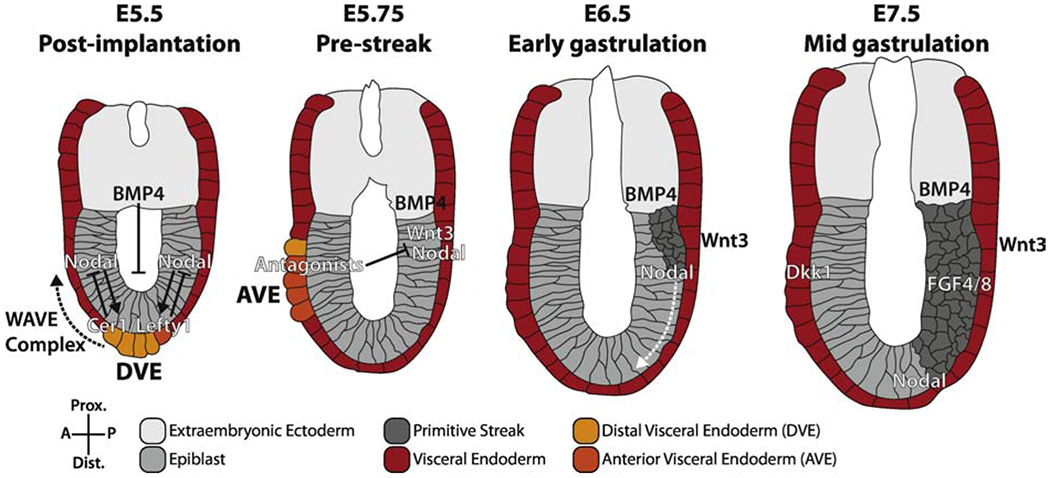
After implantation, the embryo transitions from using the proximal-distal axis to the embryonic anterior-posterior (AP) axis. The establishment of this axis depends on a sequence of events including specification of the DVE at E5.5 and formation and migration (dashed black arrow) of the AVE at E5.75. The primitive streak is restricted to the posterior pole by E6.5 and elongates distally and anteriorly (white dashed arrow) until E7.5. Solid arrows indicate activation, bar-headed lines indicate inhibition. Adapted from (Rivera-Pérez and Hadjantonakis, 2015).
Successful initiation of gastrulation depends on a stepwise process that includes the specification of a distal visceral endoderm (DVE) population within the VE at the distal pole of the conceptus, subsequent movement of the DVE to the future anterior side of the embryo, and concomitant formation of anterior visceral endoderm (AVE). Completion of these steps results in the restriction of the domain which will give rise to the primitive streak to the proximal posterior pole of the embryo. These processes involve activation and inhibition of multiple signaling pathways that organize the embryo in three-dimensional space over time, with each successive step modifying the signaling landscape of the embryo and resulting in regions with unique combinations of signals. Therefore, failure to complete any of these steps often results in severe patterning delays or a failure to gastrulate and nonviable embryo development.
DVE cells are specified from a subset of emVE based on exposure to low levels of Nodal signaling (Arnold and Robertson, 2009; Lu and Robertson, 2004; Takaoka et al., 2017). The proximal epiblast secrets pro-NODAL, which is converted to NODAL by the FURIN and PACE4 convertases in the proximally located extra-embryonic ectoderm (ExE), thereby creating a PD gradient of active NODAL (Ben-Haim et al., 2006). BMP4 secreted from the ExE acts to inhibit formation of the DVE, further restricting it to the distal pole (Yamamoto et al., 2009). The DVE subsequently expresses the Nodal inhibitors CER1 and LEFTY1 through a mechanism that depends on the downstream Nodal effector SMAD2, thereby reinforcing the Nodal signaling gradient (Yamamoto et al., 2009). The DVE population of cells migrates proximally through a Rac1-based actin cytoskeleton-dependent mechanism, where the direction of migration of this collective of cells will determine the future anterior of the embryo (Migeotte et al., 2010; Omelchenko et al., 2014; Srinivas et al., 2004). Imbalances in Lefty1 expression have been shown to be predictive of DVE migration direction, and Nodal signaling may be required to maintain DVE/AVE migration (Kumar et al., 2015; Takaoka et al., 2006; Yamamoto et al., 2009). However, much of this process remains unclear and the details surrounding DVE collective cell migration are a subject of active research.
It was previously believed that the DVE was directly converted into AVE upon migration, due in part to similarities in gene expression, morphology, and location of the two populations. It has since been shown by genetic lineage tracing with Lefty1 that the AVE is derived from a visceral endoderm population that lies just caudally to the DVE (Figure 1) (Takaoka et al., 2011). Furthermore, ablation of Eomes in the VE disrupted specification of the AVE without impacting formation of the DVE (Nowotschin et al., 2013). The formation and migration of AVE are influenced by global cell movements within the visceral endoderm that are triggered by DVE migration, as well as by high proliferation rates within the AVE (Abe et al., 2018; Antonica et al., 2019; Shioi et al., 2017). AVE maintains expression of CER1 and LEFTY1, and expresses the marker Hhex (Hoshino et al., 2015; Thomas et al., 1998). In addition, the AVE expresses the Wnt inhibitor DKK1, which restricts the signaling domain of WNT3a originating from the posterior epiblast and VE (Hoshino et al., 2015; Kimura-Yoshida et al., 2005). Thus, the AVE restricts the signaling domains of Nodal and Wnt to the proximal/posterior and posterior sides of the embryo, respectively, resulting in a two-dimensional gradient (Belo et al., 1997; Kemp et al., 2005; Perea-Gomez et al., 2002). In mutants that exhibit a failure in the specification or migration of the AVE, the primitive streak forms as a radial ring positioned at the proximal epiblast (Norris et al., 2002; Nowotschin et al., 2013). Therefore, signaling cross-talk across the AP axis is critical for proper positioning of the primitive streak to the epiblast at the posterior pole of the embryo.
While recent findings have expanded upon our understanding of this sequence of events, a number of open questions remain. Whether there are determinants of AP patterning present prior to the migration of the AVE remains elusive. Asymmetric Nodal signaling may influence the direction of DVE/AVE migration, though how a Nodal imbalance induces migration and whether additional signals act on the DVE/AVE remain to be determined (Yamamoto et al., 2004). Newly developed optogenetic approaches may enable a detailed analysis of the mechanical forces that drive DVE/AVE intra-epithelial migration and a better understanding of the cohesion of these populations (Ollech et al., 2020). Finally, it will also be of interest to learn whether and when heterogeneity within the DVE and AVE arises, something which has become feasible with new imaging and transcriptomic technologies.
Breaking free: EMT at the primitive streak.
Starting at E6.25 the primitive streak can be identified morphologically as cells begin to undergo epithelial-to-mesenchymal transition (EMT) and delaminate from the epiblast. EMT in the primitive streak is highly coordinated and is regulated by signaling pathways including Wnt, BMP, and FGF (Figure 1) (Ciruna and Rossant, 2001; Ciruna et al., 1997; Huelsken et al., 2000; Mohamed et al., 2004; Sun et al., 1999; Winnier et al., 1995). Exposure to the correct combination of signals initiates expression of a transcription factor network that triggers the switch from E- to N-Cadherin expression, adoption of a mesenchymal state and subsequent migration of cells away from the primitive streak.
Fgf4 becomes restricted to the primitive streak at the onset of gastrulation and is dependent on Fgf8 for maintained expression (Niswander and Martin, 1992; Sun et al., 1999). While gastrulation can initiate in the absence of FGF signaling, mutations in the genes encoding the FGF signaling components Fgf4, Fgf8, or Fgfr1 result in an inability to complete EMT, and cells fail to migrate away from the primitive streak (Ciruna and Rossant, 2001; Ciruna et al., 1997; Sun et al., 1999). Fgf5 is also expressed throughout the epiblast during mid gastrulation (Hebert et al., 1991). While mice null for Fgf5 do not display a gastrulation phenotype, it is unclear whether Fgf5 plays a functional role that is compensated for through redundancy of other ligands (Hébert et al., 1994). New data suggests that the Hedgehog pathway may mediate FGF signaling in the primitive streak region, potentially explaining the mesoderm defects observed in Hedgehog pathway mutants (Guzzetta et al., 2019; Zhang et al., 2001). Embryos lacking Bmp4 or the receptors Alk2, Alk3, or BmprII arrest during gastrulation, due to defects in AVE specification and primitive streak formation, and lack mesodermal lineages (Beppu et al., 2000; Gu et al., 1999; Lawson et al., 1999; Mishina et al., 1999, 1995; Winnier et al., 1995). Asymmetric canonical Wnt signaling activity is predictive of primitive streak location (Mohamed et al., 2004). Furthermore, embryos deficient for β-Catenin are unable to correctly pattern the AP axis and Wnt3 mutants fail during gastrulation (Huelsken et al., 2000; Liu et al., 1999; Tortelote et al., 2013; Yamaguchi et al., 1999). Suppression of E-Cadherin levels in Fgfr1 mutant embryos was able to restore formation of the primitive streak, suggesting a direct connection between FGF, Wnt, and E-Cadherin (Ciruna and Rossant, 2001).
The primitive streak expresses a number of transcription factors throughout gastrulation. Successful induction of EMT relies on expression of the transcription factors Eomes and Snail1, which target and repress E-cadherin (Arnold et al., 2008; Cano et al., 2000; Costello et al., 2011). Snail1 is expressed in nascent mesoderm, but not definitive endoderm, and loss of Snail1 results in accumulation of mesoderm in the primitive streak region while any cells that leave the primitive streak retain an epithelial identity (Carver et al., 2001; Pérez-González et al., 2018). Sox2 is downregulated in the primitive streak epiblast just prior to Snail1 expression, a switch that is necessary but not sufficient for mesoderm migration (Acloque et al., 2011; Bazzi et al., 2017; Ramkumar et al., 2016). Mutants that lack or misexpress Eomes do not specify definitive endoderm or cardiac mesoderm, and fail to form a morphological node (Arnold et al., 2008; Costello et al., 2011; Simon et al., 2017). The transcription factor Brachyury/T reliably marks the mesoderm-producing posterior of the streak throughout gastrulation. Tbx6 is expressed in a similar domain, and maintenance of Tbx6 expression is dependent on T (Chapman et al., 1996). The primitive streak is also marked by Mixl1, which may act to restrict the T expression domain (Hart et al., 2002). In the absence of T, posterior mesoderm is specified but fails to exit the primitive streak, resulting in an accumulation of cells at the tail bud and a loss of posterior somites (Rashbass et al., 1991; Wilson et al., 1995). Despite the mild gastrulation phenotype in T mutants, T and Eomes have been shown to increase accessibility of mesoderm and endoderm genes while repressing the pluripotency and ectoderm programs (Tosic et al., 2019). Foxa2, which marks an anterior region of the primitive streak that gives rise to definitive endoderm, is also key for patterning the primitive streak epiblast. Foxa2 mutant embryos lack an organized node and suffer from neural tube patterning defects but are capable of forming definitive endoderm (Ang and Rossant, 1994). The expression domains of T and Foxa2 are partially overlapping, and cells that co-express T and Foxa2 may give rise to a subset of cardiac mesoderm early during gastrulation and axial mesoderm during late gastrulation (Bardot et al., 2017; Burtscher and Lickert, 2009).
The first morphological event during the gastrulation EMT is the breakdown of the basement membrane that separates the epiblast and VE in the primitive streak region (Nakaya et al., 2008; Williams et al., 2012). Basement membrane removal triggers a loss of cell polarity, apical junction dissolution, and completion of EMT in a Crumbs2-dependent fashion (Ramkumar et al., 2016). Recent live imaging studies suggest that non-apical cell division within the posterior primitive streak contributes to gastrulation cell ingression through mitotic cell-rounding (Despin-guitard et al., 2020). These processes initiate at the proximal-posterior epiblast, with the primitive streak elongating anteriorly until it reaches the distal pole of the embryo. The newly specified mesoderm migrates between the epiblast and VE as morphologically identifiable wings that wrap laterally around the embryo and eventually meet at the anterior side. The definitive endoderm appears to retain E-cadherin expression, albeit isotropically, and, rapidly undergoes a mesenchymal-to-epithelial transition (MET) redistributing E-cadherin anisotropically as it intercalates into the visceral endoderm layer (Kwon et al., 2008; Viotti et al., 2014). This suggests the possibility that the definitive endoderm undergoes partial EMT, which may be executed by Eomes in regions that lack of Snail1 expression (Acloque et al., 2011; Pérez-González et al., 2018; Viotti et al., 2014). Epiblast cells exit the primitive streak continuously throughout gastrulation, proliferating to rapidly expand the embryo. As such, the remaining epiblast proliferates and is displaced into the EMT-inducing signaling environment to replace recently departed cells (Lawson and Schoenwolf, 2001; Williams et al., 2012).
The biomechanical aspects of pluripotency control and gastrulation initiation are of increasing interest to the field. There has been a recent burst of studies investigating how stem cells integrate their signaling environment, matrix stiffness, and external forces into cell state changes (recently reviewed in Kumar et al., 2017). The early embryo undergoes a series of dramatic shape and extracellular matrix (ECM) changes, including during the period surrounding gastrulation, which changes both the rigidity and signal presentation of the surrounding cells (reviewed in Sutherland, 2016). How the composition of the ECM changes during gastrulation and how that makeup influences cell behaviors remains to be fully elucidated, though recent studies have shown that local cell confinement and tension influence asymmetrical T expression in vitro (Blin et al., 2018; Muncie et al., 2020). Advances in live imaging, optogenetic perturbations and in vivo fluorescent force-sensors should enable the study of how force changes influence fate decisions during gastrulation initiation in the coming years.
From fate maps to destinations: how epiblast cells find their way.
Cells of the epiblast remain highly plastic at least until they pass through the primitive streak, as demonstrated through grafting experiments (Tam et al., 1997). Specifically, epiblast cells from multiple locations and stages could be grafted to different positions (orthotopic grafting) and would acquire fates similar to their neighbors. Conversely, cells that have already passed through the primitive streak become fate restricted and show limited potential after transplantation back into the epiblast. Thus, during normal development movement through the primitive streak acts as a determinant of cell fate acquisition, rather than the age or position of epiblast cells.
However, while cells of the epiblast are not committed to particular fates, their position relative to the primitive streak is highly predictive of their ultimate fate. These fate maps are highly reproducible and show similarity to other organisms, particularly the chick. Generally, the position of cells within the epiblast is inversely related to their location later during development and, due to the minimal neighbor exchange within the epiblast epithelium, gastrulation essentially flips their anteroposterior arrangement (Figure 2). For example, the posterior epiblast will contribute to the cranial and cardiac mesoderm, which migrates to the anterior side of the embryo, while the anterior epiblast will contribute to the ectoderm (Kinder et al., 1999; Lawson et al., 1991; Lawson and Pedersen, 1992; Quinlan et al., 1995; Tam and Beddington, 1987). Additionally, cells that meet at the midline maintain their left vs. right orientation, and new data from the chick posits a role for the ECM and programmed cell death in maintaining ipsilaterality (Maya-Ramos and Mikawa, 2020). This roadblock may serve as an important mechanism to maintain left-right asymmetry, a key axis patterning event in all bilateral organisms (reviewed in: Komatsu and Mishina, 2013; Namigai et al., 2014). Thus, fate acquisition in the epiblast depends on a logic-based allocation of cells that correlates their starting positions with their respective destinations.
Figure 2: Dynamic signaling in the gastrulating mouse embryo influences epiblast cell fate decisions.
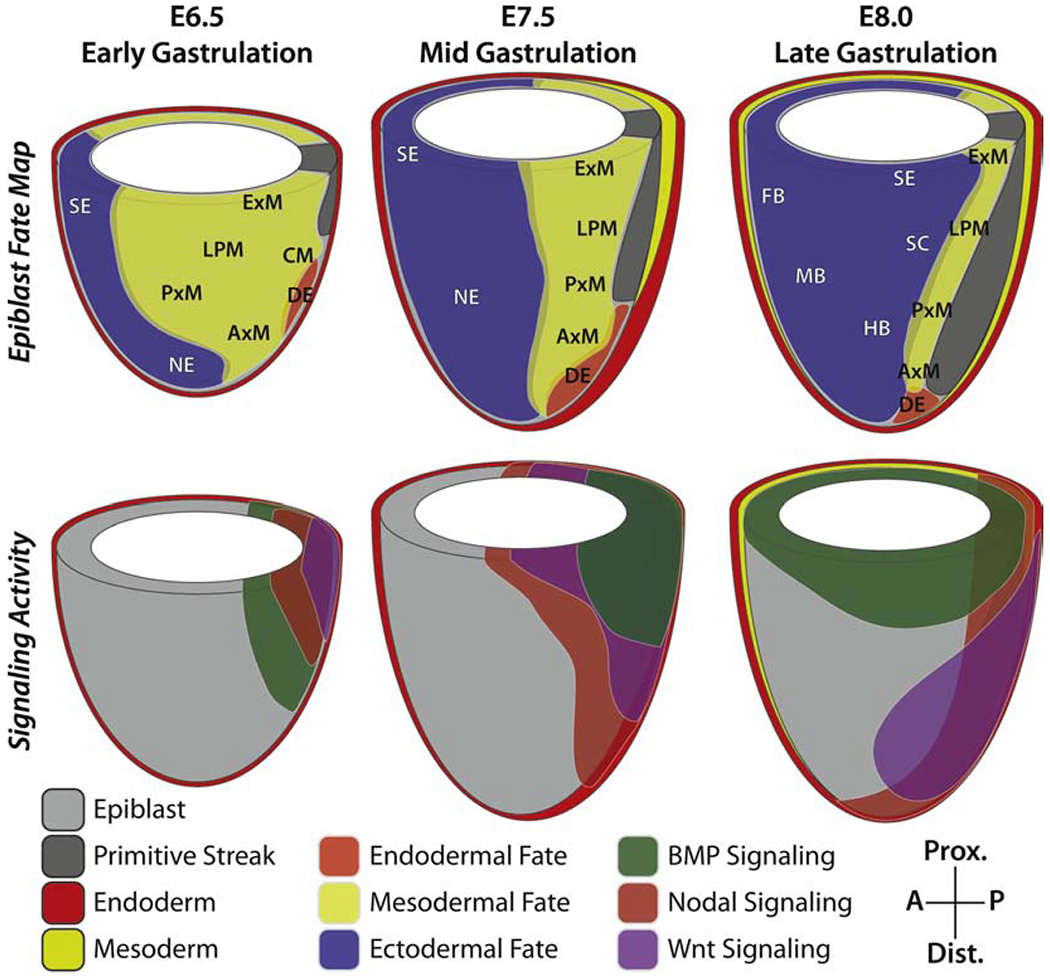
Regions of the epiblast have been shown to have reproducible lineage destinies, resulting in fate maps for multiple stages during gastrulation (top row). These fates are determined by the signaling environment cells are exposed to as they ingress through and migrate away from the primitive streak (bottom row). Thus, the combination of fate and signaling maps are instructive for how specific lineages are specified during development. DE, definitive endoderm; CM, cardiac mesoderm; ExM, extraembryonic mesoderm; LPM, lateral plate mesoderm; PxM, paraxial mesoderm, AxM, axial mesoderm; SE, surface ectoderm; SC, spinal cord; NE, neurectoderm; FB, forebrain; MB, midbrain; HB, hindbrain.
During early gastrulation (E6.5) the primitive streak is formed in a region of high BMP, Wnt, FGF, and Nodal signaling (Brennan et al., 2001; Ciruna and Rossant, 2001; Conlon et al., 1994; Huelsken et al., 2000; Liu et al., 1999; Mishina et al., 1995). Correct patterning of the anterior mesoderm relies on FGF signaling, the absence of which causes mesoderm migration and somitogenesis defects (Yamaguchi et al., 1994). Cells that ingress from the early streak, including the cranial and cardiac mesoderm, are exposed to high levels of these signals (Conlon et al., 1994; Klaus et al., 2007). The extra-embryonic mesoderm and primordial germ cells (PGCs), which represent the posterior-most cells formed, rely on high BMP signaling and must be shielded from Nodal activity (Lawson et al., 1999). BMP and Nodal are cross-inhibitory in PGCs, which migrate proximally away from the Nodal signaling source (Senft et al., 2019). Cranial and cardiac mesoderm migrate anteriorly, forming the leading edge of the mesodermal wings (Kinder et al., 1999, 2001a; McDole et al., 2018). The migratory behavior of these early mesodermal subtypes are also distinct, with embryonic mesoderm being faster and more directional than extra-embryonic mesoderm (Saykali et al., 2019).
As gastrulation progresses and the primitive streak elongates and extends distally Nodal expression remains confined to its anterior domain, eventually being expressed from the distally located node of the embryo (Conlon et al., 1994; Norris and Robertson, 1999). BMP remains highly expressed at the posterior of the embryo and the physical separation of the ExE and the node, brought about by the elongation of the streak, yields unique zones of signaling that act to pattern cell types as they are formed. During mid gastrulation (E7.5) cell fates are determined by the balance of BMP and Nodal as cells ingress through different positions along the AP axis, and these pathways act in a cross-inhibitory fashion during this window (Vincent et al., 2003; Winnier et al., 1995). Thus, the rapid proliferation within the embryo and the associated geometric changes result in a dynamic signaling environment that exposes cells to different combinations of signals throughout gastrulation (Figure 2).
The specification of lineages occurs in a posterior-to-anterior primitive streak arrangement, with lateral plate mesoderm forming from the posterior primitive streak, followed by intermediate mesoderm, anterior paraxial mesoderm, axial mesoderm, and eventually definitive endoderm from the anterior primitive streak (Lawson et al., 1991; Tam and Beddington, 1987). Whereas the lateral plate mesoderm and posterior primitive streak derivatives require high levels of BMP, definitive endoderm specification requires sustained Nodal signaling. Consistent with the anterior to posterior specification of mesoderm, the gut endoderm is formed in a similarly organized fashion (Lawson et al., 1987; Tam and Beddington, 1992). Anterior definitive endoderm is specified early and contributes to the foregut endoderm, while the mid- and hindgut are derived from later-specified definitive endoderm (Franklin et al., 2008; Lawson et al., 1986; Tam et al., 2007). The nascent gut endoderm precursors cover the surface of the embryo and will subsequently involute along the AP axis to form the gut tube.
The final stages of gastrulation are coordinated with the initiation of neurogenesis, including the specification of neuromesodermal progenitors (NMPs) and the morphogenetic resolution of the primitive streak. The anterior somites originate from paraxial mesoderm formed in the anterior primitive streak during gastrulation (Cambray and Wilson, 2007; Tam and Behringer, 1997). However, NMPs are long-perduring progenitors that contribute to the somites and spinal cord during axial elongation, and some early-specified NMPs have been shown to contribute to all somite levels (Tzouanacou et al., 2009). A population of NMPs formed near the tail bud subsequently contributes to neurectoderm of the trunk and additional paraxial mesoderm during what could be considered “secondary gastrulation” (Tzouanacou et al., 2009). The presence and contribution of this common progenitor to the spinal cord and somites is consistent with the late allocation of cells along the length of the embryo (Metzis et al., 2018; Tzouanacou et al., 2009). Recent data supports the late commitment of NMPs to either mesoderm or neurectoderm fates, showing that expansion and distribution of NMPs occurs prior to differentiation (Metzis et al., 2018).
Gastrulation concludes its initial phase at E9.5 as the primitive streak is resolved and ingression of additional cells ceases (Wilson and Beddington, 1996). The primitive streak of the late gastrula contributes to the ventral ectodermal ridge (VER), a thickened structure at the posterior of the early tailbud stage embryo. Formation of the VER occurs concomitantly with the initiation of neuropore closure, and these events mark the final stages of gastrulation. Upregulation of Noggin within the tail ventral mesoderm underlying the VER acts to inhibit BMP signaling in the late primitive streak region (Ohta et al., 2007). This inhibition acts to suppress the EMT-inducing activity of BMP, leading to restoration of E-cadherin expression in that region.
This detailed understanding of cell fate allocation is built on a series of advances made over thirty years ago. The development of techniques to culture embryos ex vivo, label specific regions of cells, and visualize their subsequent contributions provided the blueprint for further studies that determined the genetic regulators of gastrulation (Lawson et al., 1991, 1987; Tam and Beddington, 1992, 1987). The advent of genetic lineage tracing and fluorescent markers have bolstered these findings, and high resolution live imaging will continue to fill in the gaps in our knowledge of how cells get from origin to destination (Harrison and Perrimon, 1993; McDole et al., 2018; Nowotschin et al., 2019a). Furthermore, new transcriptomic and bioinformatic techniques have revealed the lineage trajectories of single cells and their gene expression changes during development (Nowotschin et al., 2019b; Peng et al., 2019; Setty et al., 2019). As these technologies continue to advance further we will only better understand how cell fate changes occur and the cell-cell interactions that mediate those decisions (Alemany et al., 2018; Chan et al., 2019; Giladi et al., 2020; McKenna et al., 2016).
Diversify and conquer: preparing for organogenesis.
Concomitant with gastrulation, newly specified cells further differentiate from the parental germ layers into progenitors for various organs and tissues. The time and position at which a given cell passes through the primitive streak dictate not only the signaling environment the cell is exposed to in the vicinity of the primitive streak, but also as it subsequently moves to its final destination. For example, cardiac mesoderm is specified from the early primitive streak where it is exposed to the BMP, Nodal, and Wnt pathways. As it migrates anteriorly, the cardiac mesoderm moves into a Wnt-inhibitory region established by the anterior endoderm (Figure 3a) (Cohen et al., 2012; Marvin et al., 2001; Schultheiss et al., 1995). This dynamic signaling environment is critical for correct differentiation of the cardiac lineage and has been recapitulated in directed differentiation of pluripotent stem cells (PSCs) to cardiomyocytes in vitro (Lian et al., 2013; Yang et al., 2008).
Figure 3: Differentiation of early organ progenitors occurs in an interdependent manner.
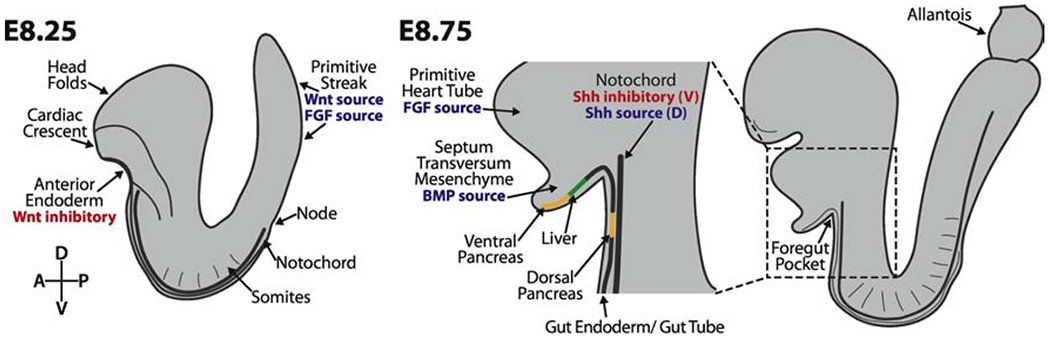
Cells fated to give rise to individual organs arrive at their destinations at different times, and are therefore exposed to different signaling environments. Late specified cells and axial progenitors are exposed to Wnt and FGF signals at the posterior of the embryo. Heart progenitors form the cardiac crescent at E8.25 adjacent to the Wnt-inhibitory anterior endoderm. As these cells differentiate and form the primitive heart tube, they express FGF ligands. These signals, in combination with BMP from the septum transversum mesenchyme, act on the foregut endoderm to induce liver fates and inhibit pancreas formation. Foregut endoderm just ventral to that domain will become the ventral pancreas. The notochord lies between the gut tube endoderm and the neural tube. The notochord secretes FGF and TGF-β to inhibit Shh expression in the gut tube endoderm, which is necessary for the formation of the dorsal pancreas, but is an important Shh source for specifying cell fates within the ventral neural tube.
As with the processes discussed to this point, lineage diversification occurs in a coordinated stepwise fashion and incorporates crosstalk between multiple cell types. Furthermore, as cells begin to differentiate, they go on to shape the signaling environment within the embryo. Newly formed cells of the endoderm and mesoderm lineages begin to inhibit Nodal at the anterior of the embryo, keeping active signaling restricted to the primitive streak as cells differentiate (Peng et al., 2019). The anterior endoderm expresses high levels of Wnt inhibitory proteins, which are critical for proper mesoderm differentiation (Kemp et al., 2005; Klaus et al., 2007; Mohammed et al., 2017). The posteriorizing Retinoic Acid (RA) pathway acts in a caudal-to-rostral fashion and patterns multiple organ systems including the heart and lungs (Hochgreb et al., 2003; Rankin et al., 2018).
This complex overlay of multiple pathways means cells are exposed to unique signaling combinations dependent on their position within the embryo, resulting in the specification of an array of cell types that subsequently organize into functional units. Recent efforts to annotate the signaling environment during gastrulation have resulted in a three-dimensional map of activating and inhibitory signals (Peng et al., 2019, 2016). In addition to providing high spatiotemporal resolution for the activity of pathways known to act during gastrulation, a potential role for Hippo signaling in early endoderm development was posited as well. Specifically, inhibition of the Hippo pathway component YAP resulted in downregulation of VE genes but did not affect expression of genes involved in later endoderm development (Peng et al., 2019). It remains to be seen what effect the loss of Hippo signaling has on cell fate decisions or gastrulation progression.
The diversification of early mesoderm has been explored in detail, with a particular focus on the cardiac and hematopoietic lineages (DeLaughter et al., 2016; Guzzetta et al., 2019; Ibarra-Soria et al., 2018; Lescroart et al., 2018; Pijuan-Sala et al., 2019; Scialdone et al., 2016). Studies of the cardiac mesoderm have added additional resolution to our understanding of the temporal specification of this lineage (Devine et al., 2014; Lescroart et al., 2018, 2014). The earliest cardiac mesoderm cells are specified at E6.5 and will contribute to the interventricular septum (IVS) and left ventricle, followed by cells that contribute to the right ventricle, atria, and finally outflow tract. This sequence corresponds to the contributions of the first and second heart fields, transient structures present at E8.25 that give rise to the IVS/left ventricle and right ventricle/outflow tract, respectively (thoroughly reviewed in Meilhac and Buckingham, 2018). While early cells are fated for first heart field derivatives, cardiac mesoderm appears to be largely homogenous until E7.25 (Lescroart et al., 2018).
Similar effort has been given to mapping the hematopoietic lineage, which is also derived from mesoderm from the early primitive streak. Generation of blood occurs in two waves: primitive hematopoiesis generates transient embryonic erythrocytes starting at E7.5, while definitive hematopoiesis occurs around E8.25 and produces erythro-myeloid progenitors that gives rise to the blood later during development (reviewed in Nandakumar et al., 2016). These two waves appear to originate from common mesodermal progenitors in the posterior primitive streak that diverge into distinct hematopoietic progenitors (Huber et al., 2004; McGrath et al., 2015; Pijuan-Sala et al., 2019; Scialdone et al., 2016). Formation of erythrocytes is dependent on the function of Tal1, a key hematopoietic transcription factor (Shivdasanl et al., 1995). Tal1 appears to function independently in primitive and definitive hematopoiesis rather than in a common progenitor, as both primitive and definitive erythrocytes are lost in Tal1−/− chimeras (Pijuan-Sala et al., 2019).
After specification and movement away from the anterior primitive streak, the definitive endoderm intercalates into the visceral endoderm on the surface of the embryo, thereby dispersing the visceral endoderm (Kwon et al., 2008; Viotti et al., 2014). The epithelial endoderm arranges at the midline of the embryo and involutes to form the embryonic gut tube by E8.75, with the most medial cells becoming the dorsal portion of the gut tube (Tam et al., 2007). The anterior definitive endoderm forms the foregut region, which gives rise to the visceral organs. More posteriorly, the midgut and hindgut regions form the small and large intestines, respectively. Notably, while most visceral endoderm ends up in the extra-embryonic region where it gives rise to the endoderm component of the yolk sac, a small proportion contributes to the posterior gut tube, ultimately resembling cells originating from neighboring definitive endoderm (Chan et al., 2019; Kwon et al., 2008; Nowotschin et al., 2019b; Pijuan-Sala et al., 2019).
The patterning of the foregut corresponds to the locations of organs that will bud off during organogenesis, with progenitors for the thymus, thyroid, lung, liver, and pancreas being arranged from anterior to posterior. While the destination of definitive endoderm along the AP axis of the gut tube can be predicted, early allocation to specific organ lineages is so far unknown. However, organ progenitors within the foregut can be delimited with high accuracy using a limited number of transcription factors, many of which are known to play key roles during the development of the respective organs (Nowotschin et al., 2019b). It remains to be seen if gut tube progenitors are prepatterned or if they constitute a multipotent pool that responds to localized signaling during organ fate commitment, though recent data supports the latter hypothesis (Han et al., 2019; Nowotschin et al., 2019b).
Data suggests that such crosstalk should play a significant role during foregut organ specification. For instance, the liver and ventral pancreas are specified from a bipotent pool of progenitors in the ventral anterior endoderm at E8.5 (Deutsch et al., 2001). Specification of liver progenitors from this pool depends on close proximity to the developing heart tube, which secretes FGFs, and the septum transversum mesenchyme, a source of BMPs, whereas the absence of these signals is required for pancreatic specification (Figure 3b) (Jung et al., 1999; Rossi et al., 2001). Similarly, specification of the dorsal pancreatic progenitors depends on proximity to the notochord, which secretes TGF-β and FGF ligands to repress Shh in the pancreatic progenitors (Figure 3b) (Hebrok et al., 1998; Kim et al., 1997). In this way, an anterior-to-posterior sequence of organ specification becomes apparent: cells that arrive at the anterior of the embryo first are specified by their surroundings and begin differentiating, which in turn influences the fate of cells that arrive thereafter. As further research into this cascade of events is performed, we will likely discover more about the importance of the order of organ specification events.
Epiblast cells that do not ingress through the primitive streak during gastrulation become the ectoderm (Lawson and Pedersen, 1992). The medially located ectoderm will thicken to form the neural plate, from which the spinal cord and central nervous system are derived. Exposure to high Wnt signaling within the posterior epiblast remodels chromatin to pattern the neurectoderm to spinal cord fates (Metzis et al., 2018). As with the ventrally located epidermal epithelium, the dorsally positioned neural plate epithelium undergoes a series of morphogenetic movements that results in the formation of an internalized neural tube. This process occurs in parallel with termination of gastrulation, and both neural plate morphogenesis and cessation of primitive streak EMT require efficient inhibition of BMP (Ohta et al., 2007; Stottmann et al., 2006; Ybot-Gonzalez et al., 2007).
Modeling gastrulation in vitro and comparisons to human development.
As discussed, gastrulation integrates timing and exposure to signaling pathways in order to specify cell types and pattern them prior to organogenesis. Knowledge gleaned from studies of gastrulation and organogenesis in vivo has been used to generate multiple cell types from pluripotent stem cells (PSCs) in vitro. These results have highlighted the replicability of these developmental pathways in vitro and have further highlighted the conservation between mouse and human developmental models. Approaches such as directed differentiation, micropattern differentiation and gastruloids, as well as synthetic embryos facilitate deconstruction of gastrulation events and enable the validation and expansion of concepts discovered in vivo and open the door to techniques that are not possible in more complex animal models.
Directed differentiation protocols have been developed for the generation of multiple cell types, including pancreatic β-cells, hepatocytes, and multiple cardiac and neural subtypes, from both mouse and human PSCs (Pagliuca et al., 2014; Peng et al., 2019; TCW et al., 2017; Touboul et al., 2010; Yang et al., 2008). These protocols harness our knowledge of early signaling pathway activities that lead to specification and differentiation of each lineage and allow for higher-throughput analysis of individual cell types. However, while the signaling environment created by neighboring cell types can be mimicked, the spatial organization of cells and the physical restraints created within the embryo are more difficult to recreate. Therefore, these methods lack contextual information that is key to better understanding in vivo development, especially when trying to study human development in the absence of in vivo experiments.
In this light, recent efforts have been directed at developing models that capture the arrangements and interactions between cell types that lead to pattern formation. Particular success has been seen by plating PSCs on micropatterned surfaces that force cells into a radial organization prior to differentiation (Morgani et al., 2018; Warmflash et al., 2014). Pluripotent stem cells differentiated on micropatterns show patterning and signal responses that are strikingly similar to those seen in vivo, suggesting that these models faithfully recapitulate aspects of normal development (Morgani et al., 2018). While this system does not recapitulate all of the cell migratory events or AP patterning associated with gastrulation, modulation of the signaling environment facilitates the formation and spatial organization of multiple germ layer cell types. Moreover, the precisely controlled nature has been leveraged to describe the activity and regulation of morphogen dynamics as the primitive streak, node, and germ layers are formed (Chhabra et al., 2019; Etoc et al., 2016; Heemskerk et al., 2019; Martyn et al., 2018).
An alternative approach has been to encourage organization of small aggregates of cells, similar to embryoid bodies, which form structures that resemble gastrulation stage embryos (Marikawa et al., 2009; ten Berge et al., 2008; Van Den Brink et al., 2014). By greatly reducing the starting number of cells relative to most embryoid body protocols, as well as limiting the time of signal induction, these gastruloids adapt asymmetrical morphologies and initiate AP patterning (Van Den Brink et al., 2014). Mouse gastruloids show an elongating tail bud structure and organize along embryonic-like axes (Beccari et al., 2018; Van Den Brink et al., 2014), and late stage gastruloids embedded in Matrigel (which is enriched in ECM components) have been shown to form neural tube and somite structures (Veenvliet et al., 2020). A major remaining question is how initial symmetry breaking is achieved in these models given the presence of a pulse of uniform Wnt induction.
Human PSCs seeded on Geltrex (which like Matrigel comprises proprietary growth factor and ECM components) and overlaid with ECM spontaneously organize into post-implantation-like asymmetric cysts that initiate a SNAI1-dependent EMT (Shao et al., 2017). The reproducibility of this behavior can be enhanced by asymmetrically delivering BMP via a microfluidic device (Zheng et al., 2019). Matrigel-embedded human gastruloids show BMP-induced symmetry breaking and initiation of EMT (Simunovic et al., 2019). Through the aggregation of PSCs with extra-embryonic-like trophoblast stem (TS) cells and primitive endoderm-like eXtra-Embryonic eNdoderm (XEN) cells, highly organized synthetic embryos could be generated that recapitulate in vivo gastrulation (Harrison et al., 2018; Kunath et al., 2005; Sozen et al., 2018; Tanaka et al., 1998). Recent reports suggest these synthetic embryos can be implanted into host mothers, and while their development arrests soon after, this further verifies their resemblance to normally developing embryos (Sozen et al., 2019; Zhang et al., 2019). Advances in the complexity of these synthetic embryos that allow for a more realistic complement of tissues may soon enable further developmental progression in utero.
This is a rapidly developing field which has recently been reviewed in greater detail (Hadjantonakis et al., 2020; Morgani and Hadjantonakis, 2020) and will likely yield additional breakthroughs in the very near future. These in vitro models offer a high degree of control and perturbation for the study of early patterning, morphogenetic and gastrulation events. They also lend themselves to combination with increasingly precise optogenetic tools, live imaging capabilities, screening platforms, and cell competition studies. While in vitro approaches have been informative for dissecting the detailed mechanisms at play during gastrulation, these findings have largely yet to be taken back to the mouse embryo for validation or further study. The coming years promise to be exciting as results from in vitro gastrulation models are explored in the embryonic context.
Concluding remarks: clarifying how we think about the germ layers.
Despite being performed 30 years ago, the pioneering studies that described how gastrulation unfolds have largely proven to hold true. In the past decade, we have added detail to these findings in ways that elaborate on the overall model. Recent identification of visceral endoderm contributing to the gut tube challenges the formerly clear trajectory of the VE to the extra-embryonic endoderm (Kwon et al., 2008; Nowotschin et al., 2019b; Pijuan-Sala et al., 2019; Viotti et al., 2014). The homogeneity of gut tube endoderm irrespective of VE vs. DE origin also raises the specter that our current understanding of lineage histories may be further modified. The combination of computational lineage trajectory mapping and lineage-recording techniques with single cell analysis approaches will blur fate maps and transcriptional trajectories, and is likely to yield more novel lineage relationships.
Within the past decade there has been new discussion over the relationships between the germ layers. Lineage trees based on earlier fate maps held that the endoderm and mesoderm were closely related, potentially even having a common mesendodermal progenitor, and that the epiblast and ectoderm were more directly related (Kinder et al., 2001b; Lawson et al., 1991; Lewis and Tam, 2006). These relationships are supported by the similar spatial origins and trajectories between lineages, with mesoderm and endoderm migrating through the primitive streak and the majority of ectoderm coming from cells that remain in the epiblast. However, recent findings have begun to reshape these lineage trees. The discovery of neuromesodermal progenitors, along with evidence that discounts the existence of mesendoderm, suggest a close relationship between the mesoderm and ectoderm at least at later stages of gastrulation, which is further supported by recent phylogenetic analysis of transcriptome data (Gouti et al., 2017; Peng et al., 2019; Tzouanacou et al., 2009; Wymeersch et al., 2019). Regardless of the existence of a bipotent mesendodermal population, the mesoderm is a diverse lineage and likely contains populations that are more similar to endoderm than ectoderm and vice versa.
The discoveries of the past 30 years have yielded a detailed map of gastrulation and early organogenesis and have been shown to be consistent across model systems both in vivo and in vitro. Going forward, emerging technologies will enable the field to revisit subtle (quantitative) phenotypes that could not be described previously, deconvolve the combinatorial action of multiple signals on a single cellular output, and explore how these diverse inputs represent the origins of congenital birth defects. Of particular interest will be to learn how the early mammalian embryo resists perturbation and compensates for errors, or how gene families act in tandem (e.g. Wnt3-Wnt3a, Eomes-Tbx6) to ensure robust development. As emerging technologies become more accessible and start to be widely applied, we may be able to better understand how genetic mutations lead to phenotypes on the cellular and organismal scale and add to our overall comprehension of the fundamental mechanisms of embryonic development; how cellular cohorts reproducibly build tissues and organs in the correct timeframe, place and size.
Highlights:
During gastrulation pluripotent epiblast generates the three definitive germ layers: endoderm, mesoderm, and ectoderm.
Gastrulation is initiated at the posteriorly positioned primitive streak, from which nascent mesoderm and endoderm progenitors ingress and begin to diversify.
A spatiotemporally controlled sequence of events results in the generation of organ progenitors positioned in a stereotypical fashion throughout the embryo.
Key to correct fate acquisition is the establishment of an axial coordinate system along with the integration of multiple signals by individual epiblast cells to produce distinct outcomes.
Signaling domains evolve as the anterior-posterior axis is established and the embryo grows in size.
Acknowledgements:
We thank Sonja Nowotschin and Claire Simon for critical reading and constructive comments on this review. Work on pluripotency and mouse gastrulation in the Hadjantonakis lab is supported by grants from the National Institutes of Health (R01-HD094868, R01-DK084391 and P30-CA00874).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Abe T, Kutsuna N, Kiyonari H, Furuta Y, Fujimori T, 2018. ROSA26 reporter mouse lines and image analyses reveal distinct region-specific cell behaviors in the visceral endoderm. Dev. 145 10.1242/dev.165852 [DOI] [PubMed] [Google Scholar]
- Acloque H., Ocaña OH, Matheu A, Rizzoti K, Wise C, Lovell-Badge R, Nieto MA, 2011. Reciprocal repression between Sox3 and Snail transcription factors defines embryonic territories at gastrulation. Dev. Cell. 10.1016/j.devcel.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany A, Florescu M, Baron CS, Peterson-Maduro J, Van Oudenaarden A, 2018. Whole-organism clone tracing using single-cell sequencing. Nature 556, 108–112. 10.1038/nature25969 [DOI] [PubMed] [Google Scholar]
- Ang SL, Rossant J, 1994. HNF-3β is essential for node and notochord formation in mouse development. Cell 78, 561–574. 10.1016/0092-8674(94)90522-3 [DOI] [PubMed] [Google Scholar]
- Antonica F, Orietti LC, Mort RL, Zernicka-Goetz M, 2019. Concerted cell divisions in embryonic visceral endoderm guide anterior visceral endoderm migration. Dev. Biol 450, 132–140. 10.1016/j.ydbio.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Flofmann UK, Bikoff EK, Robertson EJ, 2008. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development 135, 501–511. 10.1242/dev.014357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Robertson EJ, 2009. Making a commitment: Cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol 10, 91–103. 10.1038/nrm2618 [DOI] [PubMed] [Google Scholar]
- Bardot E, Calderon D, Santoriello F, Flan S, Cheung K, Jadhav B, Burtscher I, Artap S, Jain R, Epstein J, Lickert FI., Gouon-Evans V, Sharp AJ, Dubois NC, 2017. Foxa2 identifies a cardiac progenitor population with ventricular differentiation potential. Nat. Commun 8 10.1038/ncomms14428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi FI., Soroka E, Alcorn HL, Anderson KV, Flogan BLM, 2017. STRIP1, a core component of STRIPAK complexes, is essential for normal mesoderm migration in the mouse embryo. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1713535114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, Lutolf MP, Duboule D, Arias AM, 2018. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276. 10.1038/S41586-018-0578-0 [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM, 1997. Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev 68, 45–57. 10.1016/S0925-4773(97)00125-1 [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJJ, Constam DB, 2006. The Nodal Precursor Acting via Activin Receptors Induces Mesoderm by Maintaining a Source of Its Convertases and BMP4. Dev. Cell 11,313–323. 10.1016/j.devcel.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Beppu FI., Kawabata M, Flamamoto T, Chytil A, Minowa O, Noda T, Miyazono K, 2000. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev. Biol 221, 249–258. 10.1006/dbio.2000.9670 [DOI] [PubMed] [Google Scholar]
- Blin G, Wisniewski D, Picart C, Thery M, Puceat M, Lowell S, 2018. Geometrical confinement controls the asymmetric patterning of brachyury in cultures of pluripotent cells. Dev. 145 10.1242/dev.166025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RSP, Robertson EJ, 2001. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411,965–969. 10.1038/35082103 [DOI] [PubMed] [Google Scholar]
- Burtscher I, Lickert H, 2009. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development 136, 1029–1038. 10.1242/dev.028415 [DOI] [PubMed] [Google Scholar]
- Cambray N, Wilson V, 2007. Two distinct sources for a population of maturing axial progenitors. Development 134, 2829–2840. 10.1242/dev.02877 [DOI] [PubMed] [Google Scholar]
- Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, Del Barrio MG, Portillo F, Nieto MA, 2000. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol 2, 76–83. 10.1038/35000025 [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T, 2001. The Mouse Snail Gene Encodes a Key Regulator of the Epithelial-Mesenchymal Transition. Mol. Cell. Biol 21,8184–8188. 10.1128/mcb.21.23.8184-8188.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MM, Smith ZD, Grosswendt S, Kretzmer H, Norman TM, Adamson B, Jost M, Quinn JJ, Yang D, Jones MG, Khodaverdian A, Yosef N, Meissner A, Weissman JS, 2019. Molecular recording of mammalian embryogenesis. Nature 570, 77–82. 10.1038/s41586-019-1184-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE, 1996. Tbx6, a mouse T-box gene implicated in paraxial mesoderm formation at gastrulation. Dev. Biol 180, 534–542. 10.1006/dbio.1996.0326 [DOI] [PubMed] [Google Scholar]
- Chhabra S, Liu L, Goh R, Kong X, Warmflash A, 2019. Dissecting the dynamics of signaling events in the BMP, WNT, and NODAL cascade during self-organized fate patterning in human gastruloids. PLoS Biol. 17, e3000498 10.1371/journal.pbio.3000498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Rossant J, 2001. FGF Signaling Regulates Mesoderm Cell Fate Specification and Morphogenetic Movement at the Primitive Streak. Dev. Cell 1, 37–49. 10.1016/S1534-5807(01)00017-X [DOI] [PubMed] [Google Scholar]
- Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP, Rossant J, 1997. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: A role for FGFR1 in morphogenetic movement through the primitive streak. Development 124, 2829–2841. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE, 2012. Wnt5a and wnt11 are essential for second heart field progenitor development. Development 139, 1931–1940. 10.1242/dev.069377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ, 1994. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120, 1919–1928. 10.1016/0168-9525(94)90026-4 [DOI] [PubMed] [Google Scholar]
- Costello I, Pimeisl IM, Dräger S, Bikoff EK, Robertson EJ, Arnold SJ, 2011. The T-box transcription factor Eomesodermin acts upstream of Mespl to specify cardiac mesoderm during mouse gastrulation. Nat. Cell Biol 13, 1084–1092. 10.1038/ncb2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLaughter DM, Bick AG, Wakimoto H, McKean D, Gorham JM, Kathiriya IS, Hinson JT, Homsy J, Gray J, Pu W, Bruneau BG, Seidman JG, Seidman CE, 2016. Single-Cell Resolution of Temporal Gene Expression during Heart Development. Dev. Cell 39, 480–490. 10.1016/j.devcel.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despin-guitard E, Mathiah N, Stower M, Nahaboo W, Eski ES, Singh SP, Srinivas S, Migeotte I, 2020. An asymmetry in the frequency and position of mitosis in the epiblast precedes gastrulation and suggests a role for mitotic rounding in cell delamination during primitive streak epithelial-mesenchymal transition. bioRxiv. 10.1101/2020.02.21.959080 [DOI] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS, 2001. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128, 871–881. [DOI] [PubMed] [Google Scholar]
- Devine WP, Wythe JD, George M, Koshiba-Takeuchi K, Bruneau BG, 2014. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. Elife 3 10.7554/eLife.03848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etoc F, Metzger J, Ruzo A, Kirst C, Yoney A, Ozair MZ, Brivanlou AH, Siggia ED, 2016. A Balance between Secreted Inhibitors and Edge Sensing Controls Gastruloid Self-Organization. Dev. Cell 39, 302–315. 10.1016/j.devcel.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin V, Khoo PL, Bildsoe H, Wong N, Lewis S, Tam PPL, 2008. Regionalisation of the endoderm progenitors and morphogenesis of the gut portals of the mouse embryo. Mech. Dev 125, 587–600. 10.1016/j.mod.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez V, Schoenwolf GC, 1993. Primitive-Streak Origin of the Cardiovascular System in Avian Embryos. Dev. Biol 159, 706–719. [DOI] [PubMed] [Google Scholar]
- Giladi A, Cohen M, Medaglia C, Baran Y, Li B, Zada M, Bost P, Blecher-Gonen R, Salame T-M, Mayer JU, David E, Ronchese F, Tanay A, Amit I, 2020. Dissecting cellular crosstalk by sequencing physically interacting cells. Nat. Biotechnol 10.1038/s41587-020-0442-2 [DOI] [PubMed] [Google Scholar]
- Gouti M, Delile J, Stamataki D, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J, 2017. A Gene Regulatory Network Balances Neural and Mesoderm Specification during Vertebrate Trunk Development. Dev. Cell 41, 243–261.e7. 10.1016/j.devcel.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, Van Den Eijnden-Van Raaij J, Donahoe PK, Li E, 1999. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 126, 2551–2561. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Koska M, Rowton M, Kweon J, Hidalgo H, Eckhart H, Back R, Lozano S, Moon AM, Basu A, Bressan M, Pott S, Moskowitz IP, 2019. A Hedgehog-FGF signaling axis patterns anterior mesoderm during gastrulation. bioRxiv. 10.1101/736322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjantonakis A-K, Siggia ED, Simunovic M, 2020. In vitro modeling of early mammalian embryogenesis. Curr. Opin. Biomed. Eng 13, 134–143. 10.1016/j.cobme.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Koike H, Chaturvedi P, Kishimoto K, Iwasawa K, Giesbrecht K, Witcher PC, Eicher A, Nasr T, Haines L, Shannon JM, Morimoto M, Wells JM, Takebe T, Zorn AM, 2019. Single cell transcriptomics reveals a signaling roadmap coordinating endoderm and mesoderm lineage diversification during foregut organogenesis. bioRxiv 756825. 10.1101/756825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DA, Perrimon N, 1993. Simple and efficient generation of marked clones in Drosophila. Curr. Biol 10.1016/0960-9822(93)90349-S [DOI] [PubMed] [Google Scholar]
- Harrison SE, Sozen B, Zernicka-Goetz M, 2018. In vitro generation of mouse polarized embryo-like structures from embryonic and trophoblast stem cells. Nat. Protoc 13, 1586–1602. 10.1038/s41596-018-0005-x [DOI] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, Tam PPL, Elefanty AG, Robb L, 2002. Mixll is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development 129, 3597–3608. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Boyle M, Martin GR, 1991. mRNA localization studies suggest that murine FGF-5 plays a role in gastrulation. Development 112, 407–415. [DOI] [PubMed] [Google Scholar]
- Hébert JM, Rosenquist T, Götz J, Martin GR, 1994. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 78, 1017–1025. 10.1016/0092-8674(94)90276-3 [DOI] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, Melton DA, 1998. Notochord repression of endodermal sonic hedgehog permits pancreas development. Genes Dev. 12, 1705–1713. 10.1101/gad.12.11.1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk I, Burt K, Miller M, Chhabra S, Guerra MC, Liu L, Warmflash A, 2019. Rapid changes in morphogen concentration control self-organized patterning in human embryonic stem cells. Elife 8, 1–28. 10.7554/eLife.40526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CYI, Cardoso WV, Rosenthal N, Xavier-Neto J, 2003. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development 130, 5363–5374. 10.1242/dev.00750 [DOI] [PubMed] [Google Scholar]
- Hoshino H, Shioi G, Aizawa S, 2015. AVE protein expression and visceral endoderm cell behavior during anterior-posterior axis formation in mouse embryos: Asymmetry in OTX2 and DKK1 expression. Dev. Biol 402, 175–191. 10.1016/j.ydbio.2015.03.023 [DOI] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G, 2004. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432, 625–630. 10.1038/nature03122 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W, 2000. Requirement for β-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 148, 567–578. 10.1083/jcb.148.3.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Soria X, Jawaid W, Pijuan-Sala B, Ladopoulos V, Scialdone A, Jörg DJ, Tyser RCV, Calero-Nieto FJ, Mulas C, Nichols J, Vallier L, Srinivas S, Simons BD, Gottgens B, Marioni JC, 2018. Defining murine organogenesis at single-cell resolution reveals a role for the leukotriene pathway in regulating blood progenitor formation. Nat. Cell Biol 20, 127–134. 10.1038/s41556-017-0013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS, 1999. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science (80-, ). 284, 1998–2003. 10.1126/science.284.5422.1998 [DOI] [PubMed] [Google Scholar]
- Keegan BR, Meyer D, Yelon D, 2004. Organization of cardiac chamber progenitors in thezebrafish blastula. Development 131, 3081–3091. 10.1242/dev.01185 [DOI] [PubMed] [Google Scholar]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L, 2005. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev. Dyn 233, 1064–1075. 10.1002/dvdy.20408 [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Melton DA, 1997. Notochord to endoderm signaling is required for pancreas development. Development 124, 4243–4252. [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, Aizawa S, Matsui Y, Matsuo I, 2005. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev. Cell 9, 639–650. 10.1016/j.devcel.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Kinder SJ, Loebel DAF, Tam PPL, 2001a. Allocation and early differentiation of cardiovascular progenitors in the mouse embryo. Trends Cardiovasc. Med 11, 177–184. 10.1016/S1050-1738(01)00091-3 [DOI] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PPL, 1999. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126, 4691–4701. [DOI] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Wakamiya M, Sasaki H, Behringer RR, Nagy A, Tam PPL, 2001b. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development 128, 3623–3634. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W, 2007. Distinct roles of Wnt/β-catenin and Bmp signaling during early cardiogenesis. Proc. Natl. Acad. Sci. U. S. A 104, 18531–18536. 10.1073/pnas.0703113104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Mishina Y, 2013. Establishment of left-right asymmetry in vertebrate development: The node in mouse embryos. Cell. Mol. Life Sci 70, 4659–4666. 10.1007/S00018-013-1399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Lualdi M, Lyozin GT, Sharma P, Loncarek J, Fu XY, Kuehn MR, 2015. Nodal signaling from the visceral endoderm is required to maintain Nodal gene expression in the epiblast and drive DVE/AVE migration. Dev. Biol 400, 1–9. 10.1016/j.ydbio.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Placone JK, Engler AJ, 2017. Understanding the extracellular forces that determine cell fate and maintenance. Dev. 144, 4261–4270. 10.1242/dev.158469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath T, Arnaud D, Uy GD, Okamoto I, Chureau C, Yamanaka Y, Heard E, Gardner RL, Avner P, Rossant J, 2005. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development 132, 1649–1661. 10.1242/dev.01715 [DOI] [PubMed] [Google Scholar]
- Kwon GS, Viotti M, Hadjantonakis AK, 2008. The Endoderm of the Mouse Embryo Arises by Dynamic Widespread Intercalation of Embryonic and Extraembryonic Lineages. Dev. Cell 15, 509–520. 10.1016/j.devcel.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson A, Schoenwolf GC, 2001. Cell populations and morphogenetic movements underlying formation of the avian primitive streak and organizer. Genesis. 10.1002/gene.1023 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BAJ, Zeinstra LM, Davis AM, Wright CVE, Korving JPWFM, Hogan BLM, 1999. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424–436. 10.1101/gad.13.4.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA, 1991. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 113, 891–911. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA, 1986. Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev. Biol 115, 325–339. 10.1016/0012-1606(86)90253-8 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA, 1992. Clonal analysis of cell fate during gastrulation and early neurulation in the mouse. Ciba Found. Symp 10.1002/9780470514221.ch2 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA, Van De Geer S, 1987. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development 101,627–652. [DOI] [PubMed] [Google Scholar]
- Lescroart F, Chabab S, Lin X, Rulands S, Paulissen C, Rodolosse A, Auer H, Achouri Y, Dubois C, Bondue A, Simons BD, Blanpain C, 2014. Early lineage restriction in temporally distinct populations of Mespl progenitors during mammalian heart development. Nat. Cell Biol. 16, 829–840. 10.1038/ncb3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescroart F, Wang X, Lin X, Swedlund B, Gargouri S, Sànchez-Dànes A, Moignard V, Dubois C, Paulissen C, Kinston S, Göttgens B, Blanpain C, 2018. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science (80-, ). 359, 1177–1181. 10.1126/science.aao4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SL, Tam PPL, 2006. Definitive endoderm of the mouse embryo: Formation, cell fates, and morphogenetic function. Dev. Dyn 235, 2315–2329. 10.1002/dvdy.20846 [DOI] [PubMed] [Google Scholar]
- Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP, 2013. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc 8, 162–175. 10.1038/nprot.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A, 1999. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet 22, 361–365. 10.1038/11932 [DOI] [PubMed] [Google Scholar]
- Lu CC, Robertson EJ, 2004. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning. Dev. Biol 273, 149–159. 10.1016/j.ydbio.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Marikawa Y, Tamashiro DAA, Fujita TC, Alarcón VB, 2009. Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis 47, 93–106. 10.1002/dvg.20473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn I, Kanno TY, Ruzo A, Siggia ED, Brivanlou AH, 2018. Self-organization of a human organizer by combined Wnt and Nodal signaling. Nature 558, 132–135. 10.1038/S41586-018-0150-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB, 2001. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 15, 316–327. 10.1101/gad.855501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Ramos L, Mikawa T, 2020. Programmed cell death along the midline axis patterns ipsilaterality in gastrulation. Science (80-, ). 367, 197–200. 10.1126/science.aaw2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole K, Guignard L, Amat F, Berger A, Malandain G, Royer LA, Turaga SC, Branson K, Keller PJ, 2018. In Toto Imaging and Reconstruction of Post-Implantation Mouse Development at the Single-Cell Level. Cell 175, 859–876.e33. 10.1016/j.cell.2018.09.031 [DOI] [PubMed] [Google Scholar]
- McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, Kingsley PD, Koniski AD, Palis J, 2015. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell Rep. 11, 1892–1904. 10.1016/j.celrep.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Findlay GM, Gagnon JA, Horwitz MS, Schier AF, Shendure J, 2016. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science (80-. ). 353 10.1126/science.aaf7907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac SM, Buckingham ME, 2018. The deployment of cell lineages that form the mammalian heart. Nat. Rev. Cardiol 15, 705–724. 10.1038/s41569-018-0086-9 [DOI] [PubMed] [Google Scholar]
- Metzis V, Steinhauser S, Pakanavicius E, Gouti M, Stamataki D, Ivanovitch K, Watson T, Rayon T, Mousavy Gharavy SN, Lovell-Badge R, Luscombe NM, Briscoe J, 2018. Nervous System Regionalization Entails Axial Allocation before Neural Differentiation. Cell 175, 1105–1118.e17. 10.1016/j.cell.2018.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeotte I, Omelchenko T, Hall A, Anderson KV, 2010. Rac1-dependent collective cell migration is required for specification of the anterior-posterior body axis of the mouse. PLoS Biol. 8, 37–38. 10.1371/journal,pbio.1000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Crombie R, Bradley A, Behringer RR, 1999. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev. Biol 213, 314–326. 10.1006/dbio.1999.9378 [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR, 1995. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 9, 3027–3037. 10.1101/gad.9.24.3027 [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Clarke HJ, Dufort D, 2004. B-Catenin Signaling Marks the Prospective Site of Primitive Streak Formation in the Mouse Embryo. Dev. Dyn. 231,416–424. 10.1002/dvdy.20135 [DOI] [PubMed] [Google Scholar]
- Mohammed H, Hernando-Herraez I, Savino A, Scialdone A, Macaulay I, Mulas C, Chandra T, Voet T, Dean W, Nichols J, Marioni JC, Reik W, 2017. Single-Cell Landscape of Transcriptional Heterogeneity and Cell Fate Decisions during Mouse Early Gastrulation. Cell Rep. 20, 1215–1228. 10.1016/j.celrep.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgani SM, Hadjantonakis AK, 2020. Signaling regulation during gastrulation: Insights from mouse embryos and in vitro systems, 1st ed, Current Topics in Developmental Biology. Elsevier Inc. 10.1016/bs.ctdb.2019.11.011 [DOI] [PubMed] [Google Scholar]
- Morgani SM, Metzger JJ, Nichols J, Siggia ED, Hadjantonakis AK, 2018. Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning. Elife 7 10.7554/eLife.32839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncie JM, Ayad NME, Lakins JN, Weaver VM, 2020. Mechanics Regulate Human Embryonic Stem Cell Self-Organization to Specify Mesoderm. bioRxiv. 10.1101/2020.02.10.943076 [DOI] [Google Scholar]
- Murry CE, Keller G, 2008. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell 132, 661–680. 10.1016/j.cell.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Sukowati EW, Wu Y, Sheng G, 2008. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat. Cell Biol 10, 765–775. 10.1038/ncb1739 [DOI] [PubMed] [Google Scholar]
- Namigai EKO, Kenny NJ, Shimeld SM, 2014. Right across the tree of life: The evolution of left-right asymmetry in the Bilateria. Genesis 52, 458–470. 10.1002/dvg.22748 [DOI] [PubMed] [Google Scholar]
- Nandakumar SK, Ulirsch JC, Sankaran VG, 2016. Advances in understanding erythropoiesis: Evolving perspectives. Br. J. Haematol 173, 206–218. 10.1111/bjh.13938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswander L, Martin GR, 1992. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development 114, 755–768. [DOI] [PubMed] [Google Scholar]
- Norris DP, Brennan J, Bikoff EK, Robertson EJ, 2002. The Foxhl-dependent autoregulatory enhancer controls the level of Nodal signals in the mouse embryo. Development 129, 3455–3468. [DOI] [PubMed] [Google Scholar]
- Norris DP, Robertson EJ, 1999. Asymmetric and node-specific nodal expression patterns are controlled by two distinct cis-acting regulatory elements. Genes Dev. 13, 1575–1588. 10.1101/gad.13.12.1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotschin S, Costello I, Piliszek A, Kwon GS, Mao C an Klein WH, Robertson EJ, Hadjantonakis AK, 2013. The T-box transcription factor eomesodermin is essential for AVE induction in the mouse embryo. Genes Dev. 27, 997–1002. 10.1101/gad.215152.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotschin S, Garg V, Piliszek A, Hadjantonakis AK, 2019a. Ex utero culture and imaging of mouse embryos, in: Methods in Molecular Biology. 10.1007/978-1-4939-9009-2_11 [DOI] [PubMed] [Google Scholar]
- Nowotschin S, Setty M, Kuo YY, Liu V, Garg V, Sharma R, Simon CS, Saiz N, Gardner R, Boutet SC, Church DM, Hoodless PA, Hadjantonakis AK, Pe’er D, 2019b. The emergent landscape of the mouse gut endoderm at single-cell resolution. Nature 569, 361–367. 10.1038/s41586-019-1127-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S, Suzuki K, Tachibana K, Tanaka H, Yamada G, 2007. Cessation of gastrulation is mediated by suppression of epithelial-mesenchymal transition at the ventral ectodermal ridge. Development 134, 4315–4324. 10.1242/dev.008151 [DOI] [PubMed] [Google Scholar]
- Ollech D, Pflästerer T, Shellard A, Zambarda C, Spatz JP, Marcq P, Mayor R, Wombacher R, Cavalcanti-Adam EA, 2020. An optochemical tool for light-induced dissociation of adherens junctions to control mechanical coupling between cells. Nat. Commun 11 10.1038/s41467-020-14390-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko T, Rabadan MA, Hernáandez-Martínez R, Grego-Bessa J, Anderson KV, Hall A, 2014. B-Pix Directs Collective Migration of Anterior Visceral Endoderm Cells in the Early Mouse Embryo. Genes Dev. 28, 2764–2777. 10.1101/gad.251371.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA, 2014. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439. 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Suo S, Chen J, Chen W, Liu C, Yu F, Wang R, Chen S, Sun N, Cui G, Song L, Tam PPL, Han JDJ, Jing N, 2016. Spatial Transcriptome for the Molecular Annotation of Lineage Fates and Cell Identity in Mid-gastrula Mouse Embryo. Dev. Cell 36, 681–697. 10.1016/j.devcel.2016.02.020 [DOI] [PubMed] [Google Scholar]
- Peng G, Suo S, Cui G, Yu F, Wang R, Chen J, Chen S, Liu Z, Chen G, Qian Y, Tam PPL, Han JDJ, Jing N, 2019. Molecular architecture of lineage allocation and tissue organization in early mouse embryo. Nature 572, 528–532. 10.1038/S41586-019-1469-8 [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FDJ, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, Behringer RR, Ang SL, 2002. Nodal antaginists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev. Cell 3, 745–756. 10.1016/S1534-5807(02)00321-0 [DOI] [PubMed] [Google Scholar]
- Pérez-González S, Andújar C, Lantero E, Zaballos JP, 2018. On the verge of below-ground speciation: A new species complex of microendemic endogean carabid beetles, Typhlocharis Dieck, 1869 (Coleoptera: Carabidae: Anillini), from south-west Iberian Peninsula. Arthropod Syst. Phylogeny 76, 429–447. 10.13039/501100000780 [DOI] [Google Scholar]
- Pijuan-Sala B, Griffiths JA, Guibentif C, Hiscock TW, Jawaid W, Calero-Nieto FJ, Mulas C, Ibarra-Soria X, Tyser RCV, Ho DLL, Reik W, Srinivas S, Simons BD, Nichols J, Marioni JC, Gottgens B, 2019. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 566, 490–495. 10.1038/S41586-019-0933-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan GA, Williams EA, Tan SS, Tam PPL, 1995. Neuroectodermal fate of epiblast cells in the distal region of the mouse egg cylinder: Implication for body plan organization during early embryogenesis. Development 121, 87–98. [DOI] [PubMed] [Google Scholar]
- Ramkumar N, Omelchenko T, Silva-Gagliardi NF, McGlade CJ, Wijnholds J, Anderson KV, 2016. Crumbs2 promotes cell ingression during the epithelial-to-mesenchymal transition at gastrulation. Nat. Cell Biol 18, 1281–1291. 10.1038/ncb3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SA, McCracken KW, Luedeke DM, Han L, Wells JM, Shannon JM, Zorn AM, 2018. Timing is everything: Reiterative Wnt, BMP and RA signaling regulate developmental competence during endoderm organogenesis. Dev. Biol 434, 121–132. 10.1016/j.ydbio.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass P, Cooke LA, Herrmann BG, Beddington RSP, 1991. A cell autonomous function of Brachyury in T/T embryonic stem cell chimaeras. Nature 353, 348–351. 10.1038/353348a0 [DOI] [PubMed] [Google Scholar]
- Rivera-Pérez JA, Hadjantonakis AK, 2015. The dynamics of morphogenesis in the early mouse embryo. Cold Spring Harb. Perspect. Biol 7, 1–18. 10.1101/cshperspect.a015867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BLM, Zaret KS, 2001. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 15, 1998–2009. 10.1101/gad.904601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykali B, Mathiah N, Nahaboo W, Racu ML, Hammou L, Defrance M, Migeotte I, 2019. Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. Elife 8, 1–27. 10.7554/eLife.42434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss TM, Xydas S, Lassar AB, 1995. Induction of avian cardiac myogenesis by anterior endoderm. Development 121,4203–4214. [DOI] [PubMed] [Google Scholar]
- Scialdone A, Tanaka Y, Jawaid W, Moignard V, Wilson NK, Macaulay IC, Marioni JC, Gottgens B, 2016. Resolving early mesoderm diversification through single-cell expression profiling. Nature 535, 289–293. 10.1038/nature18633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft AD, Bikoff EK, Robertson EJ, Costello I, 2019. Genetic dissection of Nodal and Bmp signalling requirements during primordial germ cell development in mouse. Nat. Commun. 10, 1–11. 10.1038/s41467-019-09052-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty M, Kiseliovas V, Levine J, Gayoso A, Mazutis L, Pe’er D, 2019. Characterization of cell fate probabilities in single-cell data with Palantir. Nat. Biotechnol 10.1038/s41587-019-0068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Taniguchi K, Townshend RF, Miki T, Gumucio DL, Fu J, 2017. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun 8, 1–15. 10.1038/s41467-017-00236-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi G, Hoshino H, Abe T, Kiyonari H, Nakao K, Meng W, Furuta Y, Fujimori T, Aizawa S, 2017. Apical constriction in distal visceral endoderm cells initiates global, collective cell rearrangement in embryonic visceral endoderm to form anterior visceral endoderm. Dev. Biol 429, 20–30. 10.1016/j.ydbio.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Shivdasanl RA, Mayer EL, Orkin SH, 1995. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373, 432–434. 10.1038/373432a0 [DOI] [PubMed] [Google Scholar]
- Simon CS, Downes DJ, Gosden ME, Telenius J, Higgs DR, Hughes JR, Costello I, Bikoff EK, Robertson EJ, 2017. Functional characterisation of cis-regulatory elements governing dynamic Eomes expression in the early mouse embryo. Dev. 144, 1249–1260. 10.1242/dev.147322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic M, Metzger JJ, Etoc F, Yoney A, Ruzo A, Martyn I, Croft G, You DS, Brivanlou AH, Siggia ED, 2019. A 3D model of a human epiblast reveals BMP4-driven symmetry breaking. Nat. Cell Biol 21, 900–910. 10.1038/S41556-019-0349-7 [DOI] [PubMed] [Google Scholar]
- Sozen B, Amadei G, Cox A, Wang R, Na E, Czukiewska S, Chappell L, Voet T, Michel G, Jing N, Glover DM, Zernicka-Goetz M, 2018. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol 20, 979–989. 10.1038/s41556-018-0147-7 [DOI] [PubMed] [Google Scholar]
- Sozen B, Cox AL, De Jonghe J, Bao M, Hollfelder F, Glover DM, Zernicka-Goetz M, 2019. Self-Organization of Mouse Stem Cells into an Extended Potential Blastoid. Dev. Cell 51,698–712.e8. 10.1016/j.devcel.2019.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Rodriguez T, Clements M, Smith JC, Beddington RSP, 2004. Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development 131, 1157–1164. 10.1242/dev.01005 [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Berrong M, Matta K, Choi M, Klingensmith J, 2006. The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev. Biol 295, 647–663. 10.1016/j.ydbio.2006.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR, 1999. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13, 1834–1846. 10.1101/gad.13.14.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland AE, 2016. Tissue morphodynamics shaping the early mouse embryo. Semin. Cell Dev. Biol 55, 89–98. 10.1016/j.semcdb.2016.01.033 [DOI] [PubMed] [Google Scholar]
- Takaoka K, Nishimura H, Hamada H, 2017. Both Nodal signalling and stochasticity select for prospective distal visceral endoderm in mouse embryos. Nat. Commun 8, 1–3. 10.1038/s41467-017-01625-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Hamada H, 2011. Origin and role of distal visceral endoderm, a group of cells that determines anterior-posterior polarity of the mouse embryo. Nat. Cell Biol 13, 743–752. 10.1038/ncb2251 [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Shiratori H, Meno C, Rossant J, Saijoh Y, Hamada H, 2006. The mouse embryo autonomously acquires anterior-posterior polarity at implantation. Dev. Cell 10, 451–459. 10.1016/j.devcel.2006.02.017 [DOI] [PubMed] [Google Scholar]
- Tam PP, Beddington RS, 1992. Establishment and organization of germ layers in the gastrulating mouse embryo. Ciba Found. Symp 10.1002/9780470514221.ch3 [DOI] [PubMed] [Google Scholar]
- Tam PPL, Beddington RSP, 1987. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development 99, 109–126. [DOI] [PubMed] [Google Scholar]
- Tam PPL, Behringer RR, 1997. Mouse gastrulation: The formation of a mammalian body plan. Mech. Dev 68, 3–25. 10.1016/S0925-4773(97)00123-8 [DOI] [PubMed] [Google Scholar]
- Tam PPL, Khoo PL, Lewis SL, Bildsoe H, Wong N, Tsang TE, Gad JM, Robb L, 2007. Seqeuential allocation and global pattern of movement of the definitive endoderm in the mouse embryo during gastrulation. Development 134, 251–260. 10.1242/dev.02724 [DOI] [PubMed] [Google Scholar]
- Tam PPL, Parameswaran M, Kinder SJ, Weinberger RP, 1997. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: The role of ingression and tissue movement during gastrulation. Development 124, 1631–1642. [DOI] [PubMed] [Google Scholar]
- Tam PPL, Williams EA, Chan WY, 1993. Gastrulation in the mouse embryo: Ultrastructural and molecular aspects of germ layer morphogenesis. Microsc. Res. Tech 10.1002/jemt.1070260405 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J, 1998. Promotion to trophoblast stem cell proliferation by FGF4. Science (80-, ). 282, 2072–2075. 10.1126/science.282.5396.2072 [DOI] [PubMed] [Google Scholar]
- TCW J, Wang M, Pimenova AA, Bowles KR, Hartley BJ, Lacin E, Machlovi SI, Abdelaal R, Karch CM, Phatnani FI., Slesinger PA, Zhang B, Goate AM, Brennand KJ, 2017. An Efficient Platform for Astrocyte Differentiation from Fluman Induced Pluripotent Stem Cells. Stem Cell Reports 9, 600–614. 10.1016/j.stemcr.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R, 2008. Wnt Signaling Mediates Self-Organization and Axis Formation in Embryoid Bodies. Cell Stem Cell 3, 508–518. 10.1016/j.stem.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PQ, Brown A, Beddington RSP, 1998. Flex: A homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development 125, 85–94. [DOI] [PubMed] [Google Scholar]
- Tortelote GG, Hernandez-Hernandez JM, Quaresma AJC, Nickerson JA, Imbalzano AN, Rivera-Perez JA, 2013. Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Dev. Biol 374, 164–173. 10.1016/j.ydbio.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosic J, Kim GJ, Pavlovic M, Schroder CM, Mersiowsky SL, Barg M, Hofherr A, Probst S, Kottgen M, Hein L, Arnold SJ, 2019. Eomes and Brachyury control pluripotency exit and germ-layer segregation by changing the chromatin state. Nat. Cell Biol 21, 1518–1531. 10.1038/s41556-019-0423-1 [DOI] [PubMed] [Google Scholar]
- Touboul T, Hannan NRF, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, Santo J Di Weber A, Vallier L, 2010. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51, 1754–1765. 10.1002/hep.23506 [DOI] [PubMed] [Google Scholar]
- Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF, 2009. Redefining the Progression of Lineage Segregations during Mammalian Embryogenesis by Clonal Analysis. Dev. Cell 17, 365–376. 10.1016/j.devcel.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Van Den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, Arias AM, 2014. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Dev. 141, 4231–4242. 10.1242/dev.113001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenvliet JV, Bolondi A, Kretzmer H, Haut L, Scholze-wittler M, Schifferl D, Koch F, Heimann M, Simon P, Buschow R, Wittier L, Timmermann B, Meissner A, Herrmann BG, 2020. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. bioRxiv 1–72. 10.1101/2020.03.04.974949 [DOI] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ, 2003. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 17, 1646–1662. 10.1101/gad.1100503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti M, Nowotschin S, Hadjantonakis AK, 2014. SOX17 links gut endoderm morphogenesis and germ layer segregation. Nat. Cell Biol 16, 1146–1156. 10.1038/ncb3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH, 2014. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 11, 847–854. 10.1038/nMeth.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Burdsal C, Periasamy A, Lewandoski M, Sutherland A, 2012. Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev. Dyn 241,270–283. 10.1002/dvdy.23711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V, Beddington RSP, 1996. Cell fate and morphogenetic movement in the late mouse primitive streak. Mech. Dev 55, 79–89. 10.1016/0925-4773(95)00493-9 [DOI] [PubMed] [Google Scholar]
- Wilson V, Manson L, Skarnes WC, Beddington RSP, 1995. TheT gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development 121, 877–886. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BLM, 1995. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9, 2105–2116. 10.1101/gad.9.17.2105 [DOI] [PubMed] [Google Scholar]
- Wymeersch FJ, Skylaki S, Huang Y, Watson JA, Economou C, Marek-Johnston C, Tomlinson SR, Wilson V, 2019. Transcriptionally dynamic progenitor populations organised around a stable niche drive axial patterning. Dev. 146, 1–16. 10.1242/dev.168161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J, 1994. Fgfr-1 Is Required for Embryonic Growth and Mesodermal Patterning During Mouse Gastrulation. Genes Dev. 8, 3032–3044. 10.1101/gad.8.24.3032 [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP, 1999. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 10.1101/gad.13.24.3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Beppu H, Takaoka K, Meno C, Li E, Miyazono K, Hamada H, 2009. Antagonism between Smadl and Smad2 signaling determines the site of distal visceral endoderm formation in the mouse embryo. J. Cell Biol 184, 323–334. 10.1083/jcb.200808044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Saijoh Y, Perea-Gomez A, Shawiot W, Behringer RR, Ang SL, Hamada H, Meno C, 2004. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature 428, 387–392. 10.1038/nature02418 [DOI] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM, 2008. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528. 10.1038/nature06894 [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene NDE, Copp AJ, 2007. Neural plate morhogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development 134, 3203–3211. 10.1242/dev.008177 [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen T, Chen N, Gao D, Shi B, Kong S, West RC, Yuan Y, Zhi M, Wei Q, Xiang J, Mu H, Yue L, Lei X, Wang X, Zhong L, Liang H, Cao S, Belmonte JCI, Wang H, Han J, 2019. Implantation initiation of self-assembled embryo-like structures generated using three types of mouse blastocyst-derived stem cells. Nat. Commun 10 10.1038/s41467-019-08378-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP, 2001. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell 105, 781–792. 10.1016/S0092-8674(01)00385-3 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Xue X, Shao Y, Wang S, Esfahani SN, Li Z, Muncie JM, Lakins JN, Weaver VM, Gumucio DL, Fu J, 2019. Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421–425. 10.1038/S41586-019-1535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


