Abstract
Patients with coronavirus disease 2019 (COVID-19) are prone to pulmonary artery hypertension (PAH) and right ventricular pressure overload due to severe bilateral infiltrates, high ventilation pressures, persistent hypoxemia, pulmonary fibrosis, and/or pulmonary embolism. In patients on extracorporeal membrane oxygenation (ECMO), this potentially leads to increased recirculation. In the current report, the authors present a case in which continuous inhaled nitric oxide (iNO)-enriched ventilation was effective in terms of PAH and recirculation reduction in a COVID-19 patient on veno-venous ECMO.
Key Words: COVID-19, acute respiratory distress syndrome, extracorporeal membrane oxygenation, inhaled nitric oxide
VENO-VENOUS extracorporeal membrane oxygenation (V-V ECMO) can be used as an invasive life-saving measure in patients with coronavirus disease 2019 (COVID-19) with severe acute respiratory distress syndrome (ARDS),1 in appropriately selected patients.2
Before considering initiation of V-V ECMO, guidelines recommend the use of salvage techniques such as prone positioning and administration of neuromuscular blocking agents.3 , 4 Additionally, these guidelines mention the use of inhaled pulmonary vasodilators as potential alternative options. In these cases, pulmonary vasodilators could be beneficial in some patients through a reduction of pulmonary pressures and an improvement in ventilation-perfusion match through their vasodilatory effect.5 The class IIb recommendation is, however, supported by a low-quality level of evidence.3
In the current report, the case of a COVID-19 patient with severe ARDS on V-V ECMO is presented, in whom iNO had a beneficial effect on pulmonary hypertension and ECMO recirculation.
Case Presentation
A 45-year-old man, with an unremarkable history, presented to a regional hospital's emergency department with progressive dyspnea for three weeks. Computed tomography at admission (Video 1) and nasal swab confirmed COVID-19 pneumonia, and absence of pulmonary emboli. Despite optimal oxygen therapy, the patient had persistent oxygenation problems, with saturation levels <90% while exhaling. The patient was intubated and mechanically ventilated three days after admission. Neuromuscular blockade (Rocoronium bromide, Esmeron, Kenilworth, NJ) and prone positioning were used as salvage maneuvers for severe persisting hypoxemia, with an initial satisfactory response of oxygenation and ventilation. However, on day six of admission, the authors’ tertiary referral ECMO team was consulted for progressive, refractory hypoxemia (Pao 2:Fio 2 ratio <80 mmHg for >6 hours with high positive end-expiratory pressure (PEEP) strategy). Fulfilling the criteria for V-V ECMO, a Vf-Vj configuration6 was initiated with cannulation of the right internal jugular vein (19Ch Biomedicus arterial cannula, Medtronic, Dublin, Ireland) and left femoral vein (25Ch HLS venous cannulae, Getinge, Stockholm, Sweden), with a blood flow of 4.5 L/min, airflow 3.5 L/min, and Fio 2 90%. Double-lumen cannulation was considered, but not performed due to off-center cannulation and rapid desaturation after reversal of prone positioning. Unfractionated heparin was used as anticoagulation therapy, with target activated partial thromboplastin time (aPTT) of 60-to -80 seconds. Transthoracic echocardiography (TTE) on V-V ECMO after transport showed good left ventricular function and normal right ventricular (RV) dimensions and contractility, with adequate cannulae position and a recirculation fraction of 21%, as measured by ultrasound dilution.7 Due to progressive renal failure, continuous renal replacement therapy with ultrafiltration was started four days after ECMO initiation.
In the days following, hypoxemia progressed (Po 2 <50 mmHg [<6.6 kPa], Spo 2 84%) in spite of maximal ECMO (blood flow 4.7 L/min, airflow 8.0 L/min, Fio 2 100%) and ventilator support (pressure-controlled, 24/min, inspiratory pressure 24 cm H2O, PEEP 10 cm H2O, Fio 2 100%, tidal volume [VT] 170 mL, VTindex 2.2 mL/kg). TTE showed a notably dilated RV due to RV pressure overload, with severe tricuspid regurgitation (TR). Right ventricular systolic pressure was >50 mmHg, with a flattened intraventricular septum and a left ventricular ejection fraction of 45% to 50% and a cardiac output of 6.0 L/min. In addition, the authors observed a recirculation fraction of 50% (reported values in literature vary between 2% and 57%8), despite optimal cannula position on TTE and chest/abdominal X-ray (Fig 1 ). The authors concluded that RV pressure overload and TR caused backflow of oxygenated ECMO blood and increased recirculation.
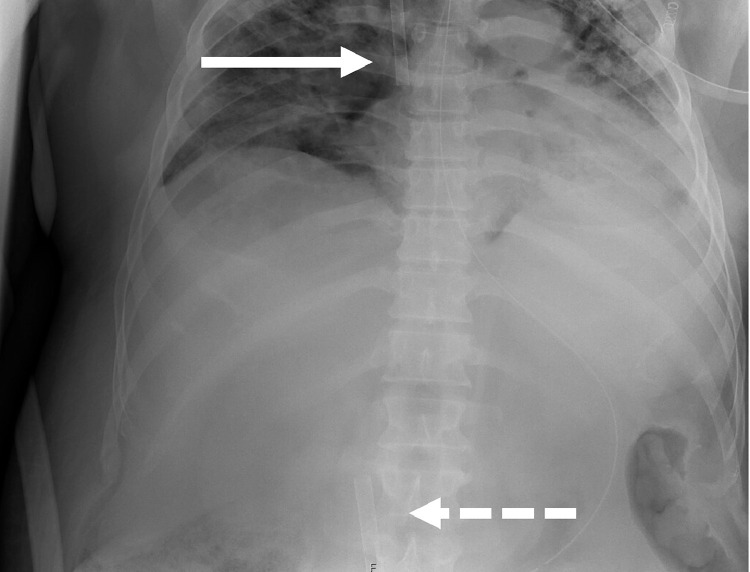
Fig 1.
Chest/abdominal x-ray demonstrating adequate Vf-Vj cannula position (interrupted arrow demonstrates the drainage cannula through the femoral vein in the inferior caval vein; uninterrupted arrow demonstrates the perfusion cannula through the jugular vein in the superior caval vein and right atrium).
To reduce pulmonary vascular resistance, first an aerosolized prostacyclin analog was administered at an initial dose of 2.5 µg and a second dose of 5.0 µg (iloprost-trometamol, 2 µg/mL, Ventavis, Bayer, Germany), with a limited effect.
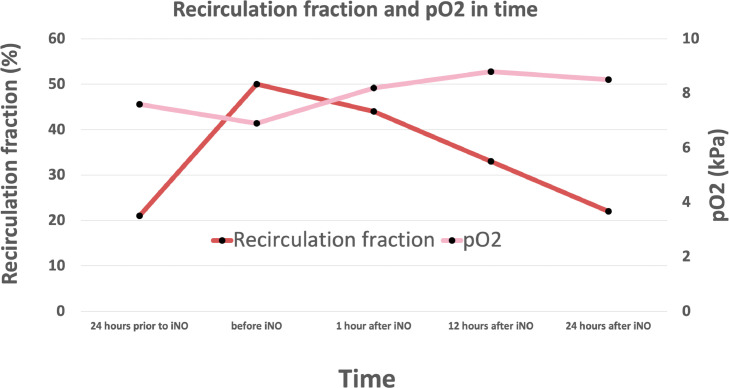
After treatment effect of iloprost ceased, continuous iNO was initiated (starting dose 20 ppm, dose increase to 30 ppm), followed by a remarkable Po 2 increase after which oxygenation remained stable (Table 1 , Fig 2 ). Direct follow-up TTE showed a significant reduction in RV dimensions with good contractility, no residual TR, and a significant reduction of blood recirculation (33%). Moreover, recirculation improved to 22% after 24 hours (Table 1). Cardiac output improved the morning after to 7.5 L/min with an increase in mean arterial blood pressure from 62 mmHg to 80 mmHg.
Table 1.
Echocardiographic Characteristics, Ventilator and ECMO Settings, and Their Effect in Relation to iNO Administration
| 24 h Prior to iNO | Start iNO | 1 h After iNO | 12 h After iNO | 24 h After iNO | |
|---|---|---|---|---|---|
| Echocardiography | |||||
| RV diameter | Dilated | Normal | |||
| TR | Severe | Absent | |||
| V-V ECMO | |||||
| Bloodflow, L/min | 4.7 | 4.6 | 4.6 | 4.9 | 4.8 |
| Airflow, L/min | 8.0 | 8.0 | 8.0 | 8.0 | 9.0 |
| Fio2, % | 100 | 100 | 100 | 100 | 100 |
| Recirculation, % | 21 | 50 | 44 | 33 | 22 |
| Ventilator Settings | |||||
| Inspiratory pressure, cm H2O | 26 | 26 | 26 | 26 | 28 |
| PEEP, cm H2O | 10 | 10 | 10 | 10 | 10 |
| Fio2, % | 100 | 100 | 100 | 100 | 100 |
| Arterial Blood Gas | |||||
| Po2, kPa/mmHg | 7.6/57 | 6.9/52 | 8.2/61 | 8.8/66 | 8.5/64 |
| pCO2, kPa/mmHg | 6.1/46 | 5.5/41 | 6.2/47 | 6.8/51 | 6.2/47 |
| Cardiac output, L/min | 6.0 | 7.5 |
Abbreviations: iNO, inhaled nitric oxide; PEEP, positive end-expiratory pressure; RV, right ventricular; TR, tricuspid regurgitation; V-V ECMO, veno-venous extracorporeal membrane oxygenation.
Fig 2.
Graph displaying recirculation fraction and Po2 improvement in function of time before and after iNO administration. Abbreviation: iNO, inhaled nitric oxide; Po2, partial pressure of oxygen.
After initial respiratory improvement, the clinical course was, however, again complicated by superinfection with refractory multi-organ failure, which proved unresponsive to maximal supportive therapy, after which treatment was ceased, without considering a conversion to alternative ECMO configurations, due to a futile prognosis.
Discussion
COVID-19 can cause severe ARDS for which V-V ECMO can be used as a lifesaving treatment modality. Currently, 44% of V-V ECMO COVID-19 patients survive until discharge,9 which is comparable to patients with severe ARDS in the EOLIA-trial.10
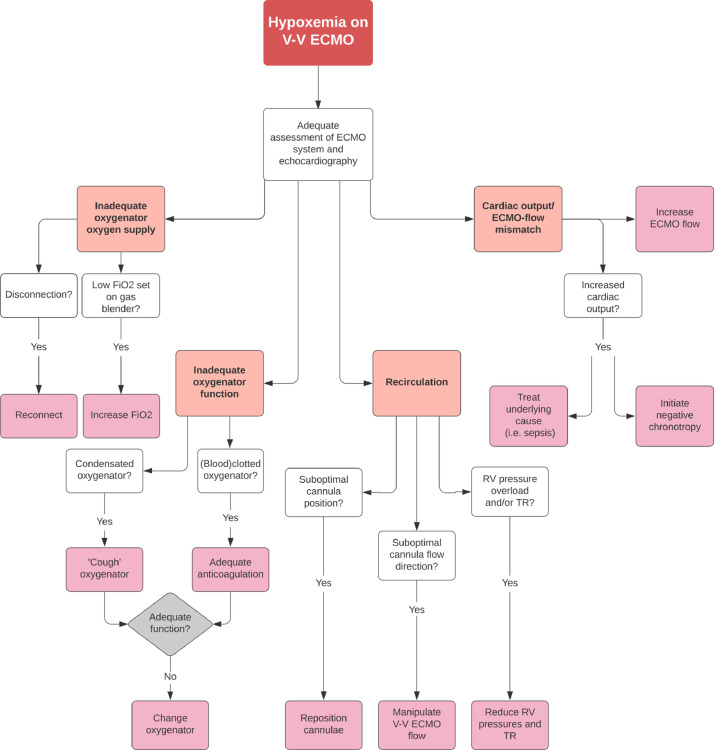
The effectiveness of ECMO treatment is dependent on a variety of patient and therapy-related factors, including ECMO blood flow, patient cardiac output, metabolic demand, oxygenator membrane performance, and the amount of recirculation within the ECMO circuit.11 The work-up of hypoxemia during V-V ECMO is therefore complicated and requires a structured approach, which is presented in the flow-chart in Figure 3 .
Fig 3.
Flow-chart demonstrating a structured approach to hypoxemia on V-V ECMO.
Abbreviations: Fio2, fraction of inspired oxygen; RV, right ventricle; TR, tricuspid regurgitation; V-V ECMO, veno-venous extracorporeal membrane oxygenation.
In the femoral-to-jugular vein configuration (Vf-Vj), a closer proximity of the inferior to the superior vena cava cannula can induce a marked recirculation. Additionally, increased RV end-diastolic filling pressures and subsequent TR can redirect infused blood flow toward the drainage cannula, increasing the blood recirculation fraction. RV failure secondary to pulmonary artery hypertension (PAH) commonly is seen in ARDS and has a multifactorial etiology in general,12 and may be precipitated by pulmonary emboli and/or fibrosis in COVID-19 patients in particular.13 , 14 In such patients, a switch to a veno-arterial configuration can be considered, although this potentially could lead to differential hypoxemia (also known as Harlequin's syndrome or North/South syndrome) when using a peripheral approach. Alternatively, a veno-arterial/venous configuration could support the RV in specific cases of refractory RV failure and avoid differential oxygenation.15 , 16
It was opted to improve the recirculation fraction by pharmacologically reducing PH and subsequent TR. In current guidelines, iloprost is prescribed 6-to-9 times per day, as it has a relatively short half-life (15-30 minutes) and short working period,17 potentially explaining the limited effect compared with continuously administered iNO in the authors’ patient. Although previous studies have demonstrated aerosolized iloprost to have similar or superior hemodynamic effects (PH reduction, RV output) compared with iNO,18 these studies were performed in patients with (pseudo)normal VTs. However, this usually is not the case in V-V ECMO patients, especially not in the presented case (<200 mL). As demonstrated in earlier studies, and perhaps illustrated by the limited response in this patient, aerosolized therapy in mechanically ventilated patients might not be as effective due to high drug losses in the ventilator components, and subsequent low lung drug delivery (varying between 1% and 10% in vitro and vivo19). Moreover, reduced VTs could even attenuate this effect and should therefore be ideally >500 mL,20 , 21 which can potentially be harmful to this patient category.
Conclusion
COVID-19 patients are prone to PAH and RV pressure overload. In patients on V-V ECMO this may lead to increased recirculation. By manipulating RV pressure overload and TR severity, recirculation can be reduced. To achieve this pharmacologically, continuous iNO ventilation appeared to be more effective than aerosolized prostacyclin-analogs in low VT situations and could therefore serve as first-line therapy in such cases.
Conflict of Interest
None declared.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.jvca.2020.09.137.
Appendix. Supplementary materials
Video 1. Axial computed tomography images demonstrating typical features of bilateral COVID-19 pneumonia and absence of pulmonary emboli.
References
- 1.Delnoij T.S., Driessen R., Sharma A.S., et al. Venovenous extracorporeal membrane oxygenation in intractable pulmonary insufficiency: Practical issues and future directions. Biomed Res Int. 2016;2016 doi: 10.1155/2016/9367464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray B.W., Haft J.W., Hirsch J.C., et al. Extracorporeal life support: Experience with 2,000 patients. ASAIO J. 2015;61:2–7. doi: 10.1097/MAT.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poston J.T., Patel B.K., Davis A.M. Management of critically ill adults with COVID-19. JAMA. 2020;323:1839–1841. doi: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett R.H., Ogino M.T., Brodie D., et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moloney E.D., Evans T.W. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003;21:720–727. doi: 10.1183/09031936.03.00120102. [DOI] [PubMed] [Google Scholar]
- 6.Conrad S.A., Broman L.M., Taccone F.S., et al. The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in Extracorporeal Life Support. A position paper of the Extracorporeal Life Support Organization. Am J Respir Crit Care Med. 2018;198:447–451. doi: 10.1164/rccm.201710-2130CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darling E.M., Crowell T., Searles B.E. Use of dilutional ultrasound monitoring to detect changes in recirculation during venovenous extracorporeal membrane oxygenation in swine. ASAIO J. 2006;52:522–524. doi: 10.1097/01.mat.0000237589.20935.a4. [DOI] [PubMed] [Google Scholar]
- 8.Broman M., Frenckner B., Bjallmark A., et al. Recirculation during veno-venous extra-corporeal membrane oxygenation—A simulation study. Int J Artif Organs. 2015;38:23–30. doi: 10.5301/ijao.5000373. [DOI] [PubMed] [Google Scholar]
- 9.Extracorporeal Life Support Organization. https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx. Accessed May 1, 2020.
- 10.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 11.Abrams D., Bacchetta M., Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J. 2015;61:115–121. doi: 10.1097/MAT.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 12.Mekontso Dessap A., Boissier F., Charron C., et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: Prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 13.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Z., Zhang Y., Wang Y., et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorusso R., Raffa G.M., Heuts S., et al. Pulmonary artery cannulation to enhance extracorporeal membrane oxygenation management in acute cardiac failure. Interact Cardiovasc Thorac Surg. 2019;30:215–222. doi: 10.1093/icvts/ivz245. [DOI] [PubMed] [Google Scholar]
- 16.Bunge J.J.H., Caliskan K., Gommers D., et al. Right ventricular dysfunction during acute respiratory distress syndrome and veno-venous extracorporeal membrane oxygenation. J Thorac Dis. 2018;10:S674–S682. doi: 10.21037/jtd.2017.10.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olschewski H. Inhaled iloprost for the treatment of pulmonary hypertension. Eur Respir Rev. 2009;18:29–34. doi: 10.1183/09059180.00011111. [DOI] [PubMed] [Google Scholar]
- 18.Hoeper M.M., Olschewski H., Ghofrani H.A., et al. A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension. German PPH study group. J Am Coll Cardiol. 2000;35:176–182. doi: 10.1016/s0735-1097(99)00494-5. [DOI] [PubMed] [Google Scholar]
- 19.Golshahi L., Longest P.W., Azimi M., et al. Intermittent aerosol delivery to the lungs during high-flow nasal cannula therapy. Respir Care. 2014;59:1476–1486. doi: 10.4187/respcare.02903. [DOI] [PubMed] [Google Scholar]
- 20.Dhand R., Tobin M.J. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med. 1997;156:3–10. doi: 10.1164/ajrccm.156.1.9610025. [DOI] [PubMed] [Google Scholar]
- 21.Rothenberg S.J., Swift D.L. Aerosol deposition in the human lung at variable tidal volumes: Calculation of fractional deposition. Aerosol Sci Technol. 1984;3(2):215–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Axial computed tomography images demonstrating typical features of bilateral COVID-19 pneumonia and absence of pulmonary emboli.