Highlights
-
•
The IgA and IgE levels increased significantly, while C3 level decreased in non-survivors.
-
•
Decreased complement C3 level was correlated with increased odds of death.
-
•
Low level of C3 may be an alert to the attending that patients may be of additional management.
-
•
Inhibition of the complement pathway might be an effective therapeutic to COVID-19 patients.
Keywords: COVID-19, Humoral immune, Complement C3, Risk factors
Abstract
Objectives
To describe the humoral immune feature of patients with coronavirus disease 2019 (COVID-19).
Methods
The levels of total immunoglobulins (IgG, IgM, IgA, and IgE), complement (C3, C4) results were retrospectively analyzed in COVID-19 patients. Univariable and multivariable logistic regression were performed to explore the risk factors associated with the in-hospital death.
Result
A total of 236 patients were enrolled in this study, of which 169 were transferred to another institution or discharged (survival group) and 67 died in hospital (non-survival group). Compared with survivors, the levels of IgA and IgE in non-survivors increased significantly, and level of complement C3 decreased. Non-survivors also showed higher incidence of chest tightness, breath shortness and dyspnoea; higher levels of inflammatory indicators, leukocytes and neutrophils; and low levels of lymphocyte subsets. Multivariable regression showed increasing odds of in-hospital death associated with older age (HR: 1.099; 95%CI: 1.057–1.143; p < 0.0001), d-dimer greater (HR: 1.294; 95%CI: 1.138–1.473; p < 0.0001) and decreased complement C3 level (HR: 0.073; 95%CI: 0.007–0.722; p = 0.025) on admission. Finally, in survival COVID-19 patients whose humoral immunity was re-examined, C3 levels tended to increase, while in non-survivors it decreased.
Conclusion
Low level of complement C3 may be an alert to the admitted COVID-19 patients with additional management. Inhibition of the complement pathway might be an effective therapeutic to COVID-19 patients.
1. Introduction
During December 2019, several unknown pneumonia cases were reported in Wuhan, China. The numbers of infected patients in China increased rapidly and spread from Wuhan to other countries. Currently, the outbreak of coronavirus disease 2019 (COVID-19) caused by novel coronavirus (SARS-CoV-2) has attracted considerable attention worldwide.
Humoral immune response serves as a substantial role in COVID-19 development and the complement system is one of the most important components of humoral immunity [1], [2]. However, the role of the complement system in COVID-19 is considered controversial. One study, published by Delanghe et al. demonstrated that C3 polymorphism was an important determinant of COVID-19 mortality [3]. Conversely, Zhang et al. showed that complement C3 cannot predict disease progression [4]. Moreover, in another retrospective study conducted in Qin et al., they reviewed 286 severe patients and 166 mild patients, and results showed that no significant differences were found in the levels of complement C3 or C4 between the non-severe and severe groups [5].
Collectively, the discrepancies in the results of these studies reveal that complement system, complement C3 in particular, to the prognosis of COVID-19 is largely unclear. Thus, we attempt to elucidate whether the humoral immune alteration has relevance with COVID-19.
2. Methods and materials
2.1. Study design and participants
This single-center retrospective observational study was conducted at Renmin Hospital of Wuhan University, which was designated as the hospital for COVID-19 patients. This study included consecutive 236 COVID-19 patients who died or were discharged/transferred from January 31, 2020 to February 20. All patients were confirmed by detecting SARS-CoV-2 RNA in throat swab samples using a nucleic acid detection kit. The exclusion criteria were as follows: 1) age less than 18 years old; 2) individuals with autoimmune diseases, such as systemic lupus erythematosus, autoimmune liver disease, Sjogren syndrome; 3) a deficiency of clinical data. This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University. An informed consent was obtained from all patients in a written form.
2.2. Data collection
Based on available medical records, we retrospectively evaluated demographic data (age and gender), clinical data (comorbidities, signs and symptoms on admission), and laboratory results, including humoral immune function (serum IgG, IgM, IgA, IgE, C3, and C4), routine blood tests, procalcitonin (PCT), C-reactive protein (CRP), coagulation function and liver function. Criteria for discharge were the absence of fever for at least 3 consecutive days, significant improvement in both lungs on chest computed tomography (CT) scan, clinical relief of respiratory symptoms, and two consecutive negative results for nucleic acid test (interval > 24 h) [6]. The standard for transfer was that the patients whose condition became stable but had not reached the discharge criteria yet. The referrals were sent to mild-novel coronavirus pneumonia square cabin hospital designated by the government.
2.3. Humoral immune detection
The collected blood samples were sent to the laboratory of our hospital, and the plasma was separated within 2 h and tested within 24 h. The nephelometry (BN™ II System, Siemens, Germany) was used to detect the levels of immunoglobulins such as IgA, IgG, IgM and the levels of complement C3 and C4. Furthermore, C3c was the final stabilized product of C3 in the liquid phase and the antibodies against C3c was used in the laboratory of our hospital. The standardized method for complement C3 determination follows traceability. Finally, several patients re-examined the humoral immunity after one-week interval.
2.4. Statistical analysis
Categorical variables were presented as n (%) and compared by a χ2 test or Fisher’s exact test. Continuous measurements were presented as mean (with standard deviations, SD) if they were normally distributed or median and interquartile range (IQR) if they were not. For nonnormally distributed variables, we used the Mann–Whitney U test to compare differences between survivors and non-survivors. To explore the risk factors for humoral immunity-related associated with in-hospital death, univariable and multivariable logistic regression models were used.
Previous studies have shown older age [7], [8] and greater d-dimer [9], [10], [11] to be common in critically ill or fatal cases [12], [13]. In a large study in France involving 1000 participants, it was demonstrated that age and gender were as important determinants of humoral immunity [14]. Therefore, we chose age, gender, d-dimer, and also including humoral immune indicators which were meaningful in univariate analysis, as the final variables for our multivariable logistic regression model.
3. Results
3.1. Presenting characteristics
770 adult patients were hospitalized in Renmin Hospital of Wuhan University before February 20, 2020. After excluding 467 patients who were still hospitalized or suspected, 61 patients without available key humoral immune parameters, 5 patients with autoimmune diseases, and a 16-year-old adolescent patient, a total of 236 inpatients were included for further analysis.
The results showed that 67 patients died during hospitalization and 169 were discharged. The median age of the 236 patients was 57 years (IQR 44–69; range 18–98 years), and more than half of the patients were female (52%), while most of non-survivors were male (63%). Hypertension (25%) was the most common comorbidity, followed by diabetes (12%) and cardiovascular disease (11%). The most common symptoms on admission were fever (194 [82%]), cough (133 [56%]), fatigue (81 [34%]), followed by expectoration or shortness of breath (60 [25%]) and chest tightness (51 [22%]). Less common symptoms were dyspnoea, nausea or vomiting, diarrhea, dizziness or headache, and disturbance of consciousness. Compared with survivors, non-survivors were significantly older (median age, 72 years [IQR, 66–79] vs 51 years [IQR, 40–63]; p < 0.0001) with mainly chief complaints with dyspnea, chest tightness, shortness of breath, and disturbance of consciousness, and were more likely to have underlying comorbidities, including hypertension (35 [52%] vs 25 [15%], diabetes (15 [22%] vs 13 [8%]), cardiovascular disease (18 [27%] vs 9 [5%]), and respiratory system disease (10 [15%] vs 2 [1%]). Non-survivors also showed significant differences in laboratory findings, including higher white blood cell and neutrophil counts, as well as higher levels of procalcitonin, CRP, coagulation function indices (PT, APTT, and D-dimer), aspartate aminotransferase, and total bilirubin. Lymphocyte counts and eosinophil counts on admission were lower in non-survivors (median lymphocyte counts 0.6 × 109/L [IQR 0.4–0.9]; median eosinophil counts 0 × 109/L [0–0]) than survivors (median lymphocyte counts 1.2 × 109/L [1.0–1.6], p < 0.0001; eosinophil counts 0.03 mg/L [0–0.09], p < 0.0001) (Table 1 ).
Table 1.
Demographic, clinical, and laboratory findings of survival and non-survival COVID-19 patients on admission.
| Total (n = 236) | Non-survivor (n = 67) | Survivor (n = 169) | p value | |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age, years | 57 (44–69) | 72 (66–79) | 51 (40–63) | <0.0001 |
| Sex | 0.004 | |||
| Male | 113 (48%) | 42 (63%) | 71 (42%) | .. |
| Female | 123 (52%) | 25 (37%) | 98 (58%) | .. |
| Comorbidities | 93 (39%) | 48 (72%) | 45(27%) | <0.0001 |
| Hypertension | 60 (25%) | 35 (52%) | 25 (15%) | <0.0001 |
| Diabetes | 28 (12%) | 15 (22%) | 13 (8%) | 0.002 |
| Cardiovascular disease | 27 (11%) | 18 (27%) | 9 (5%) | <0.0001 |
| Cerebrovascular disease | 8 (3%) | 5 (7%) | 3 (2%) | 0.075 |
| Respiratory system disease | 12 (5%) | 10 (15%) | 2 (1%) | <0.0001 |
| Malignancy | 11 (5%) | 6 (9%) | 5 (3%) | 0.10 |
| Chronic liver disease | 5 (2%) | 1 (1%) | 4 (2%) | 1.0 |
| Signs and symptoms at admission | ||||
| Fever | 194 (82%) | 57 (85%) | 137 (81%) | 0.47 |
| Cough | 133 (56%) | 38 (57%) | 95 (56%) | 0.94 |
| Fatigue | 81 (34%) | 27 (40%) | 54 (32%) | 0.22 |
| Expectoration | 60 (25%) | 22 (33%) | 38 (22%) | 0.10 |
| Shortness of breath | 60 (25%) | 27 (40%) | 33 (20%) | 0.001 |
| Chest tightness | 51 (22%) | 22 (33%) | 29 (17%) | 0.008 |
| Dyspnoea | 35 (15%) | 19 (28%) | 16 (9%) | <0.0001 |
| Diarrhea | 31 (13%) | 9 (13%) | 22 (13%) | 0.93 |
| Dizziness or headache | 22 (9%) | 5 (7%) | 17 (10%) | 0.54 |
| Sore throat | 18 (8%) | 2 (3%) | 16 (9%) | 0.091 |
| Myalgia | 17 (7%) | 2 (3%) | 15 (9%) | 0.19 |
| Nausea or vomiting | 14 (6%) | 4(6%) | 10 (6%) | 1.0 |
| Disturbance of consciousness | 7 (3%) | 6 (9%) | 1 (0.6%) | 0.003 |
| Laboratory findings | ||||
| White blood cell count, × 109/L | 5.5 (4.2–7.7) | 8.2 (5.5–12.3) | 5.0 (3.9–6.5) | <0.0001 |
| Neutrophil count, × 109/L | 3.8 (2.5–6.1) | 7.3 (4.5–11.3) | 3.0 (2.3–4.4) | <0.0001 |
| Lymphocyte count, × 109/L | 1.1 (0.8–1.5) | 0.6 (0.4–0.9) | 1.2 (1.0–1.6) | <0.0001 |
| Monocyte count, × 109/L | 0.4 (0.3–0.6) | 0.4 (0.2–0.5) | 0.5 (0.3–0.6) | <0.0001 |
| Eosinophil count, × 109/L | 0.01 (0–0.06) | 0 (0–0) | 0.03 (0–0.09) | <0.0001 |
| Basophil count, × 109/L | 0.01 (0.01–0.03) | 0.01 (0.01–0.02) | 0.02 (0.01–0.03) | 0.021 |
| Procalcitonin, ng/mL | 0.05 (0.03–0.13) | 0.23 (0.10–0.59) | 0.04 (0.03–0.06) | <0.0001 |
| C-reactive protein, mg/L | 29 (5–73) | 92 (59–167) | 10 (5–35) | <0.0001 |
| Prothrombin time, s | 12.0 (11.4–12.7) | 12.8 (12.1–13.8) | 11.9 (11.2–12.3) | <0.0001 |
| Activated partial thromboplastin time, s | 27.8 (25.9–30.5) | 28.8 (27.0–32.5) | 27.5 (25.1–29.6) | 0.001 |
| D-dimer, mg/L | 0.73 (0.37–2.92) | 4.67 (1.15–17.16) | 0.47 (0.29–1.09) | <0.0001 |
| Alanine aminotransferase, U/L | 25 (17–43) | 25 (20–46) | 25 (16–43) | 0.256 |
| Aspartate aminotransferase, U/L | 28 (20–44) | 45 (28–63) | 24 (19–36) | <0.0001 |
| Total bilirubin, mmol/L | 10.6 (8.0–15.5) | 14.3 (9.8–20.2) | 9.8 (7.5–14.2) | <0.0001 |
3.2. Humoral immune features
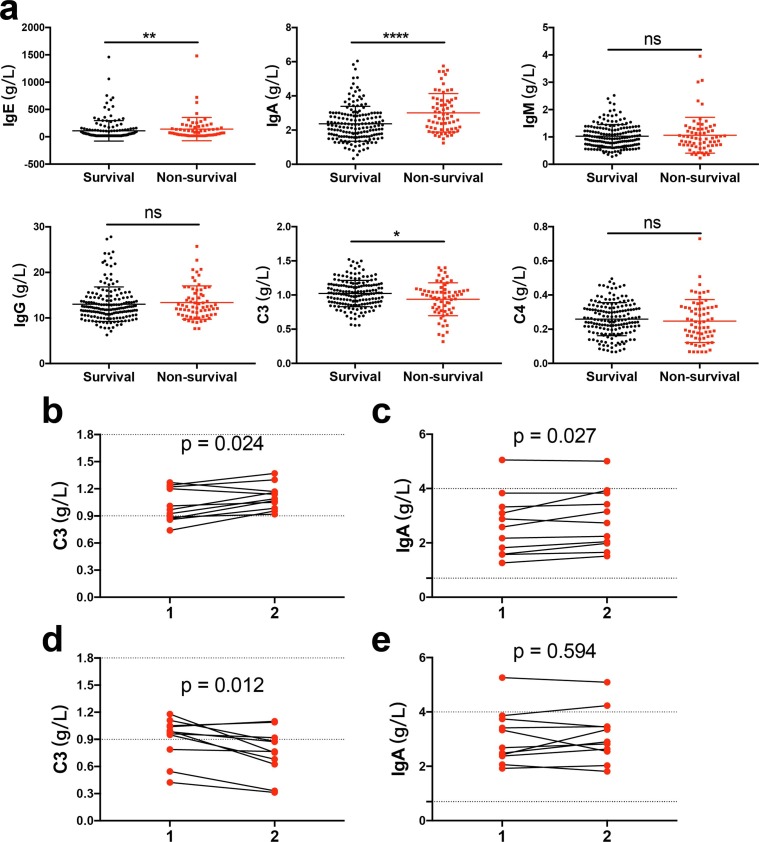
Compared to survivors, non-survivors had a significant increase of IgA (p < 0.0001) and IgE levels (p = 0.007) and a lower level of complement C3 (p = 0.027) (Fig. 1 a). In the univariable analysis, IgA (HR: 1.701; 95% CI: 1.301–2.224; p < 0.0001) level was significantly associated with higher odds of in-hospital death, while complement C3 (HR: 0.143; 95% CI: 0.035–0.581; p = 0.007) level was associated with lower odds of death. No significant difference was observed in IgE levels (HR: 1.001; 95% CI: 0.999–1.002; p = 0.279). Then, multivariable regression analysis showed that only lower complement C3 (HR: 0.073; 95% CI: 0.007–0.722; p = 0.025), older age (HR: 1.099; 95% CI: 1.057–1.143; p < 0.0001) and higher d-dimer (HR: 1.294; 95% CI: 1.138–1.473; p < 0.0001) were correlated with increased odds of death (Table 2 ).
Fig. 1.
(a) Comparison of humoral immune (immunoglobulins and complement) between COVID-19 survivors and non-survivors. (b, c) Dynamic profile of immunoglobulins A (IgA) and complement C3 levels in the COVID-19 survivors. (d, e) Dynamic profile of IgA and complement C3 levels in the COVID-19 non-survivors. The dotted line in the figure represents the normal range of complement C3 and IgA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant.
Table 2.
Univariate and multivariate analysis of risk factors associated with in-hospital death in COVID-19 patients.
| Univariable OR (95% CI) | p value | Multivariable OR (95% CI) | p value | |
|---|---|---|---|---|
| Demographics and clinical characteristics | ||||
| Age, years | 1.121 (1.085–1.158) | <0.0001 | 1.099 (1.057–1.143) | <0.0001 |
| Female sex (vs male) | 0.431 (0.241–0.772) | 0.005 | 0.529 (0.219–1.277) | 0.157 |
| Comorbidity present (vs not present) | ||||
| Hypertension | 0.159 (0.084–0.301) | <0.0001 | ||
| Diabetes | 0.289 (0.129–0.647) | 0.003 | ||
| Cardiovascular disease | 0.153 (0.065–0.363) | <0.0001 | ||
| Respiratory system disease | 0.068 (0.015–0.321) | 0.001 | ||
| Laboratory findings | ||||
| White blood cell count, × 109/L | 1.411 (1.263–1.576) | <0.0001 | ||
| Neutrophil count, × 109/L | 1.595 (1.394–1.825) | <0.0001 | ||
| Lymphocyte count, × 109/L | 0.020 (0.007–0.059) | <0.0001 | ||
| Monocyte count, × 109/L | 0.258 (0.065–1.034) | 0.056 | ||
| Prothrombin time, s | 3.285 (2.186–4.936) | <0.0001 | ||
| Activated partial thromboplastin time, s | 1.146 (1.052–1.249) | 0.002 | ||
| D-dimer, mg/L | 1.347 (1.189–1.525) | <0.0001 | 1.294 (1.138–1.473) | <0.0001 |
| Aspartate aminotransferase, U/L | 1.026 (1.014–1.039) | <0.0001 | ||
| Total bilirubin, mmol/L | 1.116 (1.061–1.173) | <0.0001 | ||
| Humoral immune parameters | ||||
| IgA, g/L | 1.701 (1.301–2.224) | <0.0001 | 1.201 (0.816–1.770) | 0.353 |
| IgE, IU/mL | 1.001 (0.999–1.002) | 0.279 | ||
| C3, g/L | 0.143 (0.035–0.581) | 0.007 | 0.073 (0.007–0.722) | 0.025 |
3.3. Dynamic changes of IgA and C3 levels during hospitalization
Furthermore, a total of 22 patients had re-examination of humoral immunity function during hospitalization, of which 11 in the non-survivors and 11 in the survival. In the survivors, the IgA (p = 0.027) and the C3 (p = 0.024) level changes tended to increase (Fig. 1b, c); and in the non-survivors, the C3 level changes tended to decrease (p = 0.012), while there was no difference in IgA levels changes during observation before they died (p = 0.594) (Fig. 1d, e).
4. Discussion
In this study, we observed that 63% of patients were older male with higher d-dimer at the time of admission in non-survivors. Our findings were consistent with other studies: the older age and higher d-dimer were potential risk factors in the context of progressive, critically ill or fatal cases [12], [13]. In addition, we reported that dysregulated humoral immune system in a cohort of 236 patients with laboratory confirmed COVID-19 in Wuhan, China. Compared to survivors, non-survivors had a significant increase of IgA and IgE levels, and a decrease of complement C3 levels, but no significant change in the IgG, IgM and complement C4. Furthermore, there was a significance tendency for the survival patients to rise in IgA and complement C3 levels, and for the non-survival patients to have a further decrease in C3 level. Collectively, these data suggested that COVID-19 damaged the humoral immune.
As with severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus (MERS-CoV), COVID-19 also led to a range of disease from asymptomatic cases to severe acute respiratory distress syndrome (ARDS) and respiratory failure [7], [15]. Moreover, complement activation formed the basis of the pathophysiology in many lung diseases, such as asthma and ARDS [16]. Complement activation occurred via three different pathways: the classical, lectin and alternate pathways, converging on C3, the central component of the complement system [17]. Stoermer et al. highlighted that they identified critical roles for the complement system in the pathogenesis of viral infection [18]. Gralinski et al. observed that SARS-CoV-infected C3-deficient mice exhibited less weight loss, respiratory dysfunction, and reduced lung pathology as well as complement deposition in the lungs of SARS-CoV infected mice. Their results suggested that complement activation resulted in immune-mediated damage to the lung [19]. Although complement activation has been proved to be related to the pathophysiology of ARDS caused by coronavirus, there are few clinical data available on the role of complement activation in the development of SARS-CoV-2-associated ARDS.
To our knowledge, it is a first COVID-19 case series that lower level of complement C3 on admission or a progressive decline during hospitalization predicted a worse prognosis. Complement activation products, C3a and C5a, interacted with their receptors C3aR and C5aR/C5L2, resulting in the recruitment of polymorphonuclear neutrophils (PMNs) and macrophages. Then, PMN and macrophages were activated and released inflammatory mediators, such as reactive oxygen species, cytokines/chemokines, myeloperoxidase and elastase. These further contributed to enhanced vascular permeability, smooth muscle contraction and tissue injury [16]. In the biopsy samples at autopsy from patients who died of COVID-19, histological examination revealed bilateral diffuse alveolar damage. And a large number of sticky secretions overflowed from the alveolar section, suggesting that COVID-19 may cause alveolar damage, including edema, proteinaceous exudate, focal reactive hyperplasia of pneumocytes with patchy inflammatory cellular infiltration, and multinucleated giant cells [20], [21], [22]. It was also reported from an autopsy examination that type II alveolar epithelial cells proliferated obviously, with some cells exfoliated. The alveolar septum was hyperemic, edema, with clear intravascular thrombosis. Focal monocytes, lymphocytes and plasma cells infiltrated the lung interstitium and secreted large amounts of pro-inflammatory factors [23]. This was manifested in the macroscopically as the secretion of sticky substances in the lung, while microscopically, it caused a “cytokine storm” in the body. Overall, the alveolar damage in patients with severe COVID-19 appeared to result from a “cytokine storm”, in which high levels of inflammatory cytokines were secreted. Similar results were also revealed in our study that increased levels of inflammatory indicators were found in non-survival COVID-19 patients, including leukocytes, neutrophils, monocytes, CRP and PCT. Therefore, the activation of complement may be involved in the injury of COVID-19 alveoli [24]. However, it still needs to be further studied whether the lower C3 level in the early stage of COVID-19, the more severe the alveolar damage of COVID-19.
Interestingly, recent autopsy also detected widespread complement activation in the lung of patients whom died from COVID-19, characterized by C3a generation and C3-debris deposition [25]. Five cases described by Cynthia et al. were severe COVID-19 patients. Their histologic findings were consistent with emerging observations, suggesting that COVID-19 patients showed extensive deposition of complement components within the lung septal microvasculature [26]. Meanwhile, they also found that alternative pathway and lectin pathway of complement activation were associated with microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection [26]. Another study further demonstrated that alternative pathway could explain many clinical manifestations of COVID-19 and addition of factor H protein could alleviate complement attack [27]. Therefore, lack of factor H or activation of alternative pathways might be the primary cause of C3 depletion in severe COVID-19 patients.
It had been shown that C3 activation was related to a series of inflammatory and pro-inflammatory pathways in SARS-CoV infection [28], and treatment with the compstatin-based C3 inhibitor AMY-101 was safe in a patient with COVID-19 [29]. Compared with C5 inhibitors function by inhibiting downstream complement effectors, such as C5a/C5aR1, C3 inhibitors could offer a broader therapeutic coverage through the control of the upstream of pro-inflammatory innate immune circuits [28], [29]. Taken together, the role of complement in the pathogenesis of COVID-19 diseases have been increasingly recognized. Complement inhibitors have broad prospects as part of a new combination therapy for the treatment of COVID-19. However, the time window for optimal intervention and the patient populations who can benefit from therapeutic complement inhibition has not yet been determined.
In addition to the older age, higher level of d-dimer, we found that a progressive decreased of complement C3 level during treatment might be a promising novel marker for the prognosis of COVID-19. Furthermore, a similar research reported by Delanghe et al. found that C3 polymorphism was a significant determinant for COVID-19 mortality [3]. The observed variation of concentration in our study might be due to variation in C3 phenotype. Although measuring the concentration of C3 rather than C3-related phenotype is widely favored in practical applications, we cannot ignore the role of C3 polymorphism in COVID-19 patients.
Moreover, our data indicated that IgA level was significantly increased in the non-survival COVID-19 patients. Compared to non-severe COVID-19 patients, Yu et al. recently found that the relative IgA level was markedly higher in severe COVID-19 patients [30]. Their subgroup analysis further indicated a significant positive association of SARS-CoV-2 specific IgA level and the APACHE-II score in critically ill patients with COVID-19 [30]. As previously reported, serum IgA regulated both anti‐inflammatory and pro‐inflammatory activities by crosslinking of the Fc alpha receptor (FcαRI) of pathogen, such as activating signals leading to phagocytosis, respiratory burst, increased antigen presentation, degranulation, and cytokine release [31], [32]. A latest study further found that SARS-CoV-2 specific CD4 T cell level was significant correlated with specific IgA level [33]. Thus, the over-activated IgA response might cause damaging effects in severe or non-survival COVID-19 patients and involved in the COVID severity. Additionally, although the IgA/C3 ratio was considered to be an effective predictor of IgA nephropathy [34], the relationship between elevated IgA levels and C3 depletion in COVID-19 patients remains unclear.
There were several limitations in our study. First, this is a retrospective study with a limited sample size. Second, we cannot offer evidences on the optimal time window for complement inhibitor intervention. Finally, more experiments and large-scale clinical trials on complement C3 activation are needed in the future.
In summary, low level of complement C3 may be an alert to the attending that patients may be of additional management. We anticipated the low C3 was secondary to excessive activation of the complement pathway and this resulted in the negative sequences in the lung and was reflected by the declining C3 level. Inhibition of the complement pathway might be an effective therapeutic to COVID-19 patients.
CRediT authorship contribution statement
Shilin Fang: Conceptualization, Writing - original draft. Haizhou Wang: Methodology, Writing - original draft, Writing - review & editing. Li Lu: Methodology, Validation. Yifan Jia: Supervision, Validation. Zhongyuan Xia: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107070.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Paces J., Strizova Z., Smrz D., Cerny J. COVID-19 and the immune system. Physiol. Res. 2020;69(3):379–388. doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forneris F., Wu J., Xue X., Ricklin D., Lin Z., Sfyroera G., Tzekou A., Volokhina E., Granneman J.C., Hauhart R., Bertram P., Liszewski M.K., Atkinson J.P., Lambris J.D., Gros P. Regulators of complement activity mediate inhibitory mechanisms through a common C3b-binding mode. EMBO J. 2016;35(10) doi: 10.15252/embj.201593673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delanghe J.R., De Buyzere M.L., Speeckaert M.M. C3 and ACE1 polymorphisms are more important confounders in the spread and outcome of COVID-19 in comparison with ABO polymorphism. Eur. J. Prevent. Cardiol. 2020;27(12):1331–1332. doi: 10.1177/2047487320931305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z., Li X., Zhang W., Shi Z.L., Zheng Z., Wang T. Clinical features and treatment of 2019-nCov pneumonia patients in Wuhan: Report of a couple cases. Virol. Sin. 2020 doi: 10.1007/s12250-020-00203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.C. Qin, L. Zhou, Z. Hu, S. Zhang, S. Yang, Y. Tao, C. Xie, K. Ma, K. Shang, W. Wang, D.S. Tian, Dysregulation of immune response in patients with COVID-19 in Wuhan, China, Clin. Infect. Dis. Off. Publ. Infectious Dis Soc. Am., 2020. [DOI] [PMC free article] [PubMed]
- 6.National Health Commission of the People’s Republic of China, Chinese management guideline for COVID-19 (version 6.0), http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf (accessed Feb 19, 2020; in Chinese) (2020).
- 7.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA internal Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.H. Han, L. Yang, R. Liu, F. Liu, K.L. Wu, J. Li, X.H. Liu, C.L. Zhu, Prominent changes in blood coagulation of patients with SARS-CoV-2 infection, Clin. Chem. Lab. Med. (2020). [DOI] [PubMed]
- 11.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of thrombosis and haemostasis: JTH. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scepanovic P., Alanio C., Hammer C., Hodel F., Bergstedt J., Patin E., Thorball C.W., Chaturvedi N., Charbit B., Abel L., Quintana-Murci L., Duffy D., Albert M.L., Fellay J. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 2018;10(1):59. doi: 10.1186/s13073-018-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarma V.J., Huber-Lang M., Ward P.A. Complement in lung disease. Autoimmunity. 2006;39(5) doi: 10.1080/08916930600739456. [DOI] [PubMed] [Google Scholar]
- 17.Varela J.C., Tomlinson S. Complement: an overview for the clinician. Hematology/oncology Clin. North America. 2015;29(3) doi: 10.1016/j.hoc.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoermer K.A., Morrison T.E. Complement and viral pathogenesis. Virology. 2011;411(2) doi: 10.1016/j.virol.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5) doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q., Wang R.S., Qu G.Q., Wang Y.Y., Liu P., Zhu Y.Z., Fei G., Ren L., Zhou Y.W., Liu L. Gross examination report of a COVID-19 death autopsy. Fa yi xue za zhi. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J. Thoracic Oncol.: Off. Publ. the Int. Assoc. Study Lung Cancer. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J. Transl. Med. 2020;18(1):164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maglakelidze N., Manto K.M., Craig T.J., Review A. Does complement or the contact system have a role in protection or pathogenesis of COVID-19? Pulmonary Therapy. 2020:1–8. doi: 10.1007/s41030-020-00118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ting Gao, Mingdong Hu, Xiaopeng Zhang, Hongzhen Li, Lin Zhu, Hainan Liu, Qincai Dong, Zhang Zhang, Zhongyi Wang, Yong Hu, Yangbo Fu, Yanwen Jin, Kaitong Li, Songtao Zhao, Yongjiu Xiao, Shuping Luo, Lufeng Li, Lingfang Zhao, Junli Liu, Huailong Zhao, Yue Liu, Weihong Yang, Jing Peng, Xiaoyu Chen, Ping Li, Yaoning Liu, Yonghong Xie, Jibo Song, Lu Zhang, Qingjun Ma, Xiuwu Bian, Wei Chen, Xuan Liu, Qing Mao, C. Cao, Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation, medRxiv (2020).
- 26.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational Res.: J. Lab. Clin. Med. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020 doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C., Huber-Lang M., Piemontese S., Assanelli A., Garlanda C., Lambris J.D., Ciceri F. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. (Orlando Fla.) 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H.Q., Sun B.Q., Fang Z.F., Zhao J.C., Liu X.Y., Li Y.M., Sun X.Z., Liang H.F., Zhong B., Huang Z.F., Zheng P.Y., Tian L.F., Qu H.Q., Liu D.C., Wang E.Y., Xiao X.J., Li S.Y., Ye F., Guan L., Hu D.S., Hakonarson H., Liu Z.G., Zhong N.S. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respiratory J. 2020;56(2) doi: 10.1183/13993003.01526-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olas K., Butterweck H., Teschner W., Schwarz H.P., Reipert B. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin. Exp. Immunol. 2005;140(3) doi: 10.1111/j.1365-2249.2005.02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leong K.W., Ding J.L. The unexplored roles of human serum IgA. DNA Cell Biol. 2014;33(12):823–829. doi: 10.1089/dna.2014.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schub D., Klemis V., Schneitler S., Mihm J., Lepper P.M., Wilkens H., Bals R., Eichler H., Gärtner B.C., Becker S.L., Sester U., Sester M., Schmidt T. High levels of SARS-CoV-2 specific T-cells with restricted functionality in severe course of COVID-19. JCI insight. 2020 doi: 10.1172/jci.insight.142167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong W.Y., Liu M., Luo D., Liu F.N., Yin L.H., Li Y.Q., Zhang J., Peng H. High serum IgA/C3 ratio better predicts a diagnosis of IgA nephropathy among primary glomerular nephropathy patients with proteinuria ≤ 1 g/d: an observational cross-sectional study. BMC Nephrol. 2019;20(1):150. doi: 10.1186/s12882-019-1331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.