Abstract
Background:
Men of African-ancestry have elevated prostate cancer (PCa) incidence and mortality compared to men of other racial groups. There is support for a genetic contribution to this disparity, with evidence of genetic heterogeneity in the underlying risk alleles between populations. Studies of PCa among African men may inform the contribution of genetic risk factors to the elevated disease burden in this population.
Methods:
We conducted an association study of >100 previously reported PCa risk alleles among 571 incidence cases and 485 controls among Uganda men. Unconditional logistic regression was used to test genetic associations and a polygenic risk score (PRS) was derived to assess the cumulative effect of the known risk alleles in association with PCa risk. In an exploratory analysis, we also tested associations of 17 125 421 genotyped and imputed markers genome-wide in association with PCa risk.
Results:
Of the 111 known risk loci with a frequency >1%, 75 (68%) had effects that were directionally consistent with the initial discovery population,14 (13%) of which were nominally significantly associated with PCa risk at P < 0.05. Compared to men with average risk (25th−75th percentile in PRS distribution), Ugandan men in the top 10% of the PRS, constructed of alleles outside of 8q24, had a 2.9-fold (95%CI: 1.75, 4.97) risk of developing PCa; risk for the top 10% increased to 4.86 (95%CI: 2.70, 8.76) with the inclusion of risk alleles at 8q24. In genome-wide association testing, the strongest associations were noted with known risk alleles located in the 8q24 region, including rs72725854 (OR = 3.37, P = 2.14 × 10−11) that is limited to populations of African ancestry (6% frequency).
Conclusions:
The ~100 known PCa risk variants were shown to effectively stratify PCa risk in Ugandan men, with 10% of men having a >4-fold increase in risk. The 8q24 risk region was also found to be a major contributor to PCa risk in Ugandan men, with the African ancestry-specific risk variant rs72725854 estimated to account for 12% of PCa in this population.
Keywords: 8q24, African men, GWAS, prostate cancer
1. |. INTRODUCTION
Prostate cancer (PCa) is the second most common cancer globally among men and is one of the leading causes of cancer mortality.1,2 Long-standing racial/ethnic differences have been noted, with men of African-ancestry having elevated incidence and mortality as well as more aggressive tumors compared to other racial/ethnic groups.3 In Africa, PCa is the most common cancer among men,4 with incidence rates in Uganda being near the highest of all African countries.5 Prostate cancer is one of the most heritable cancers,6with genome-wide association studies (GWAS) having identified more than 100 risk variants, which in total explain ~30% of the familial risk.7–13 Genetic studies in men of African-ancestry have revealed risk alleles at a number of regions, including on chromosomes 8q24, 13q34, and 22q12 that are specific for men of African descent and which may contribute, in part, to the greater PCa incidence in this population.10,13,14 To date, our understanding of genetic risk for PCa in men of African ancestry is based on studies conducted primarily among African American men.10,13,15,16 Genetic studies in African men are needed to quantify the contribution of germline variation to the higher risk observed in this population, and may assist in detecting novel African-specific loci because of minimal to no non-African admixture.
In the present study, we characterized risk associations at known PCa loci and constructed a polygenic risk model comprised of all known risk loci to assess the cumulative genetic effects of genetic risk loci in this high-risk population. In an exploratory analysis, we also conducted a GWAS of PCa in Ugandan men to search for novel PCa risk alleles.
2 |. MATERIALS AND METHODS
2.1 |. Study participants
The Uganda Prostate Cancer Study (UGPCS) is a case-control study of incident PCa among Ugandan men. Between January 1, 2010 to December 31, 2014, 571 incident PCa cases over the age of 40 were enrolled from the Urology units at seven hospitals/clinics in Kampala (Mulago Hospital, Uro Care, Mengo Hospital, Nakasero Hospital, Nsambya Hospital, Kibuli Hospital, Surgeons Plaza) and six hospitals/ clinics outside of Kampala (Kagando Hospital, Nyakibale Hospital, Surgical Clinic Mbarara, Bwindi Community Hospital, Mbarara Hospital, Mbale Hospital). All cases were histologically confirmed and Gleason score was determined for 317 (55.5%) of cases. Controls (n = 485) were recruited from non-urologic clinics (ie, surgery) at the same hospital and were men over 40 with no history of PCa or current urologic conditions. To remove potential undiagnosed disease, all controls were men with a prostate-specific antigen (PSA) level <4 ng/m. Descriptive PCa risk factor information was collected using a standardized questionnaire and a saliva spit kit (Genotek) was used to collect germline DNA. Written (or a thumb print) consents were obtained from each participant. The study protocol was reviewed and approved by the Uganda National Council of Science and Technology, the Makerere University Research and Ethics Committee, as well as the Institutional Review Board of the University of Southern California.
2.2 |. Genotype calling and quality control
We genotyped 571 cases and 485 controls in UGPCS with the Illumina OncoArray17 as part of the ELLIPSE GAME-ON Consortium.14 For quality control we combined these samples with those from the ELLIPSE GAME-ON consortium. Quality control processes for genotypes included removing SNPs with call rate <0.95, replicate concordance <99.8% based on QC replicate samples, or due to poor clustering after visual inspection. Additional removal criteria included monomorphic SNPs, SNPs with estimated MAF that deviated or had mismatched alleles in comparison to the AFR individuals in phase III 1000 Genome Project (1KGP) data, and INDELs not identified within 1KGP; 448 939 SNPs were available for imputation (described below). Samples were removed with invalid sample (n = 3), unknown duplication (n = 4) and call rate <0.95 (n = 6). Another three individuals were further removed because they were 1st degree relative pairs. After QC, the remaining sample size available for analysis was 580 cases and 460 controls.
2.3 |. Imputation
The genotypes were phased by SHAPEIT18 and imputed with Minimac3 Version 1.0.12, using the phase III 1KGP cosmopolitan reference panel. SNPs with MAF <0.01 in the UGPCS population or with imputation quality score<0.3 in the combined dataset were excluded, leaving a total of 17 125 421 variants for statistical analysis.
2.4 |. Statistical analyses
Principal component analysis (PCA) was performed using EIGENSTRAT19 together with the 1KGP populations. For each SNP, per-allele odds ratios (genotyped counts or imputed dosage) and P values were estimated using unconditional logistic regression, adjusting for age and the first 10 principal components. A quantile-quantile plot was produced to assess the influence of hidden population stratification.
We tested for association with 118 known risk variants for PCa in UGPCS, (20 at 8q24, and 98 at non-8q24 regions). The known risk alleles were selected based on previous GWAS,7,11 with African-specific risk alleles (rs75823044 at 13q34 and rs78554043 at 22q12.1) discovered in a previous GWAS meta-analysis in the African Ancestry Prostate Cancer Consortium (AAPC),14 which included subjects in UGPCS, and an expanded variant list at 8q24 as different studies report different variants likely representing the same signals. Directional consistency of effect was defined as ORs in Ugandan men that were in the same direction (ie, >1) as those reported previously in the population that discovered the variant. A nominal P-value of 0.05 was used to determine statistical significance. Risk allele frequency comparisons were conducted between UGPCS controls and African Americans in AAPC,10 as well as European ancestry samples in phase III 1KGP.
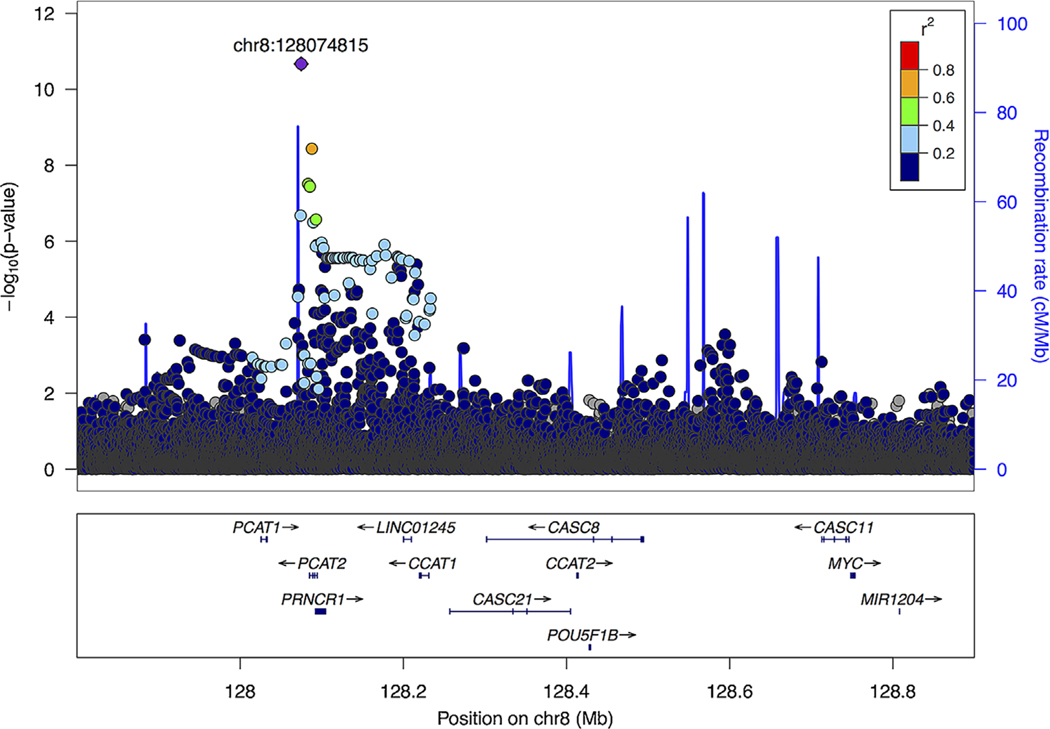
Given the importance of variation at 8q24 in PCa and the observation of multiple independent risk alleles,10,20–22 we performed a forward-selection stepwise logistic regression procedure for the region 127.8–128.8 Mb to assess the number of independent signals in UGPCS men. The correlation (r2) between the independent SNPs identified by the stepwise regression procedure (P< 0.001), and previous reported PCa risk alleles in the 8q24 region, was calculated in the phase III 1KGP African populations. An 8q24 regional association plot was generated using LocusZoom.23
We estimated the aggregate effect of known risk alleles, using a weighted polygenic risk score, , for each individual. gim is the risk allele dosage for individual i at SNP m; C defines a set of 92 non-8q24 risk SNPs with MAF >0.01 in UGPCS (6 non-8q24 known risk alleles were excluded because of MAF ≤ 0.01), together with five independent 8q24 risk alleles identified in the forward-selection stepwise procedure in UGPCS. The weights for non-8q24 risk alleles were marginal logORs from logistic regression adjusting for 10 PCs and age in UGPCS. For variants in the 8q24 region, we first obtained conditional logORs from the regression model in the forward-selection stepwise procedure, and then to correct for potential bias in effect estimation of newly discovered variants, we implemented a fully Bayesian version of a weighted correction.24 The risk score was then categorized by percentile (<10%, 10–25%, 25–75%, 75–90%, ≥90%) and the risk for each category was estimated relative to the interquartile range (25–75%) using logistic regression with covariates including 10 PCs and age. We assessed the influence of 8q24 on the PRS by comparing risk score values computed before and after the inclusion of the 5 8q24 risk alleles. Also, to evaluate the relative improvement on classification performance of PRS in distinguishing cases from controls after including 8q24 variants, we calculated the area under the receiver-operating-characteristic (ROC) curve (AUC).
We conducted case-case analysis to examine the association between PCa aggressiveness with the independent risk variants in 8q24 identified in UGPCS, the known risk alleles and PRS, using logistic models adjusted for PCs and age. We defined cases with Gleason score ≥ 8 as aggressive and <8 as non-aggressive. For variants that were nominally statistically significant in case-case testing (Phet < 0.05), stratified analyses were run in which separate logistic models were fitted using cases among each stratum and all controls (ORagg and ORnon-agg).
In the exploratory GWAS analysis, the genetic inflation factor (λ = 1.026) indicated no evidence of over-dispersion. A P-value of <5 × 10−8 was used as the threshold for genome-wide significance.
3 |. RESULTS
The mean age of the cases and controls included in the analysis were 70.9 (±9.5) and 65.1 (±8.9), respectively (Supplementary Table S1). Of the 560 cases, Gleason score was available for 309, of which, 136 (44%) had a Gleason Score ≥ 8. Supplementary Figure S1 illustrates genetic comparisons between Ugandan men in UGPCS and African ancestry samples from phase III 1KGP, and highlights close genetic relationships between Ugandan men and the Luhya in Webuye, Kenya (LWK) compared to men of African ancestry men from Western Africa, the United States and the Caribbean.
Of the 118 known risk loci, two were monomorphic (rs12621278, rs76934034) and another five had a MAF ≤ 0.01 (one at 8q24). Of the 111 variants with a MAF >0.01, directionally consistent associations were noted with 75 (67.6%) variants, of which 14 were significantly associated with PCa risk at P < 0.05 (Table 1, Supplementary Table S2). Of the three African-specific risk alleles for PCa reported outside of 8q24, variant rs7210100 at 17q2113 was not significantly associated with PCa risk (OR = 1.04, P = 0.86; RAF = 0.04), while the effect sizes for rs75823044 at 13q34 and rs78554043 at 22q12.1 were similar to those reported previously in men of African ancestry,14 with rs75823044 nominally statistically significant in UGCPS (rs75823044: OR = 2.02, P = 0.04; RAF = 0.01; rs78554043: OR = 1.53, P = 0.44; RAF = 0.01). In comparing the frequency of the known risk alleles between populations, on average, the risk allele frequency in UGPCS controls was only 0.001 smaller than that observed among African Americans in AAPC (P = 0.82, t-test). A larger non-significant difference in risk allele frequency distribution was noted between UGPCS controls and European ancestry populations (1000 Genomes), being 0.04 larger in UGPCS controls on average (P = 0.11, t-test), and with 29 (25.4%) having opposite minor alleles: 12 alleles (10.5%) had an RAF>0.5 in European ancestry populations and RAF<0.5 in UGPCS whereas 17 (14.9%) had a RAF <0.5 in European ancestry populations that was >0.5 in UGPCS. Five of the known variants were detected as nominally associated with PCa aggressiveness in case-case analysis (PHet < 0.05), including rs6763931 [PHet = 2.1 × 10−4; ORnon-agg = 3.39 (95%CI: 1.73, 6.67), ORagg = 0.78 (95%: 0.49, 1.22)], rs1218582 [PHet = 2.9 × 10−4; ORnon-agg = 0.67 (95% CI: 0.51, 0.87), ORagg = 1.31 (95%CI: 0.98, 1.76)], rs8014671 [PHet = 0.01; ORnon-agg = 1.21 (95%CI: 0.92, 1.59), ORagg = 0.72 (95% CI: 0.53, 0.99)], rs1465618 [PHet = 0.02; ORnon-agg = 1.67 (95%CI: 0.99, 2.80), ORagg = 0.80 (95%CI: 0.39, 1.64)], and rs2292884 [PHet = 0.04; ORnon-agg = 1.08 (95%CI: 0.82, 1.42), OR = 0.72 (95%CI: 0.54, 0.97)].
TABLE 1.
Known prostate cancer risk alleles that were nominally statistically significant (P < 0.05) among Ugandan men
| SNP ID | Region | Position | Risk allele | RAFa | OR (95%CI)b | P-valuec |
|---|---|---|---|---|---|---|
| rs72725854 | 8q24.21 | 128074815 | T | 0.06 | 3.37 (2.36, 4.82) | 2.14 × 10−11 |
| rs114798100 | 8q24.21 | 128085434 | G | 0.04 | 2.92 (2.00, 4.28) | 3.63 × 10−8 |
| rs72725879 | 8q24.21 | 128103969 | T | 0.36 | 1.58 (1.30, 1.93) | 4.73 × 10−6 |
| rs16901979 | 8q24.21 | 128124916 | A | 0.47 | 1.45 (1.20, 1.76) | 1.01 × 10−4 |
| rs6983561 | 8q24.21 | 128106880 | C | 0.50 | 1.39 (1.16, 1.67) | 3.91 × 10−4 |
| rs111906932 | 8q24.21 | 128086204 | A | 0.01 | 3.50 (1.60, 7.66) | 0.0017 |
| rs1512268 | 8p21.2 | 23526463 | T | 0.65 | 1.31 (1.07, 1.60) | 0.0087 |
| rs3096702 | 6p21.32 | 32192331 | A | 0.09 | 0.62 (0.43, 0.89) | 0.0090 |
| rs11568818 | 11q22.2 | 102401661 | T | 0.53 | 1.23 (1.03, 1.48) | 0.0237 |
| rs10086908 | 8q24.21 | 128011937 | T | 0.71 | 1.27 (1.03, 1.56) | 0.0258 |
| rs684232 | 17p13.3 | 618965 | C | 0.62 | 1.24 (1.02, 1.50) | 0.0277 |
| rs7463326 | 8q24.21 | 128027954 | G | 0.88 | 1.41 (1.03, 1.94) | 0.0312 |
| rs12549761 | 8q24.21 | 128540776 | C | 0.97 | 1.97 (1.03, 3.79) | 0.0412 |
| rs7153648 | 14q23.1 | 61122526 | C | 0.38 | 1.21 (1.01, 1.45) | 0.0417 |
| rs75823044 | 13q34 | 110360784 | T | 0.01 | 2.02 (1.02, 4.00) | 0.0437 |
| rs1218582 | 1q21.3 | 154834183 | G | 0.69 | 0.83 (0.69, 1.00) | 0.0497 |
Risk allele frequency in UGPCS controls.
Adjusted for age and 10 principle components.
Wald test.
At 8q24 (Figure 1), five independent signals were defined (P < 0.001; Table 2) in the stepwise selection procedure. In addition to rs72725854, the variants included rs28556804, which is highly correlated with the previous reported risk allele rs7463326 (r2 = 0.95, AFR phase III 1KGP; Supplementary Table S3), rs1456315, previously reported in Japanese25 and Chinese26 studies and moderately correlated with another previous reported risk allele rs72725879 (r2 = 0.41, AFR phase III 1KGP), and variants rs6470538 and rs73707269, located in close proximity (35–155 kb) to MYC with little correlation to known risk alleles (r2 < 0.01; Supplementary Table S3). We did not detect statistically significant differences in effect by aggressiveness for the 8q24 variants.
FIGURE 1.
Regional association plot of the 8q24 risk region (127.8–128.9 MB) in Ugandan men. Single-nucleotide polymorphisms (SNPs) are plotted by position (x-axis) and −log10 P-value (y-axis). LDs were estimated from AFR individuals in phase III 1000 Genomes Project (1KGP) data using r2 statistics. The most statistically significant associated SNP (purple diamond) is rs72725854, and the surrounding SNPs are colored to indicate pairwise correlation with the index SNP
TABLE 2.
Prostate cancer risk alleles at 8q24 in Ugandan men
| SNP ID | Chromosome position | Allelesa | Effect allele frequency (case|control) | Marginal OR (95%CI)b | Marginal P-value | Conditional OR (95%CI)c | Conditional P-value | Largest correlation (r2) with known risk variants at 8q24d |
|---|---|---|---|---|---|---|---|---|
| rs72725854 | 128074815 | T|A | 0.14|0.06 | 3.37 (2.36, 4.82) | 2.14 × 10−11 | 3.62 (2.50, 5.26) | 1.20 × 10−11 | 0.54 with rs1147981009 |
| rs6470538 | 128594189 | T|C | 0.39|0.32 | 1.44 (1.18, 1.76) | 2.87 × 10−4 | 1.47 (1.21, 1.80) | 1.18 × 10−4 | 0.01 with rs698326720 |
| rs1456315 | 128103937 | T|C | 0.62|0.53 | 1.47 (1.22, 1.77) | 6.50 × 10−5 | 1.52 (1.24, 1.87) | 6.90 × 10−5 | 0.41 with rs727258799 |
| rs28556804 | 128014315 | A|G | 0.91|0.87 | 1.46 (1.08, 1.97) | 1.28 × 10−2 | 1.80 (1.31, 2.48) | 2.94 × 10−4 | 0.95 with rs746332613 |
| rs73707269 | 128712597 | G|C | 0.95|0.92 | 2.00 (1.30, 3.07) | 1.51 × 10−3 | 2.19 (1.40, 3.44) | 6.23 × 10−4 | 0.01 with rs1009015420 |
Effect|Reference allele.
Adjusted for age and 10 principle components.
Adjusted for all the SNPs shown, age and 10 principle components.
r2 determined in AFR populations in 1000 Genomes.
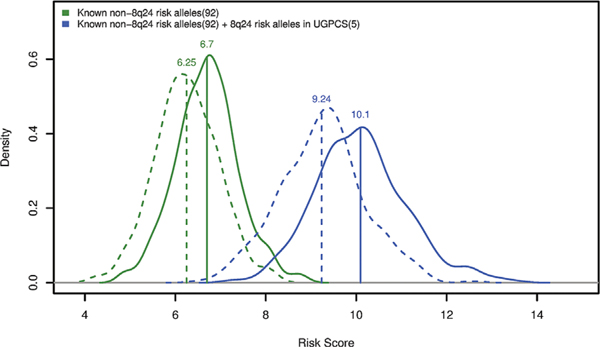
In estimating a PRS for the 92 non-8q24 risk alleles, the mean weighted score was 6.70 for PCa cases and 6.25 for controls (P < 2.2× 10−16, t-test). The average PRS increased to 10.09 in PCa cases and 9.24 in controls (P < 2.2 × 10−16, t-test) with the inclusion of the five risk alleles at 8q24 (Figure 2). Similarly, for non-8q24 risk alleles, men in the top 10% PRS had a 2.9-fold (95% CI: 1.75, 4.97) elevated risk compared to those with the average risk (PRS in 25th-75th percentiles), which increased to 4.86 (95%CI: 2.70, 8.76) with the inclusion of risk alleles at 8q24 (Table 3). Moreover, the AUC of the PRS also improved by adding the 5 8q24 variants, increasing from 0.67 (95%CI: 0.65, 0.71) to 0.73 (95%CI: 0.70, 0.76). The PRS was equally associated with aggressive and non-aggressive PCa in case-case analysis (aggressive vs non-aggressive OR = 0.92, P = 0.49).
FIGURE 2.
Density plots of the polygenic risk scores. The dotted lines are controls and the solid lines are cases. The green lines are density distributions of the polygenic risk score (PRS) derived from 92 known non-8q24 risk SNPs. The blue lines are distributions of the PRS derived from 92 known non-8q24 risk SNPs plus 5 independent risk alleles in the 8q24 region identified among UGPCS men. The labeled values are the mean PRS of the corresponding categories
TABLE 3.
A polygenic risk score for prostate cancer in Ugandan men
| UGPCS | AAPC | EUR | |||||
|---|---|---|---|---|---|---|---|
| Polygenic risk score categorya | Number of cases | Number of controls | OR (95%CI)b | P-valuec | OR (95%CI)d | P-valuec | OR (95%CI)e |
| 1–10% | 19 | 74 | 0.18 (0.10, 0.32) | 2.19 × 10−9 | 0.28 (0.17, 0.47) | 2.10 × 10−6 | 0.31 (0.28–0.35) |
| 10–25% | 55 | 101 | 0.42 (0.28, 0.63) | 2.29 × 10−5 | 0.57 (0.39, 0.84) | 4.48 × 10−3 | 0.52 (0.48–0.55) |
| 25–75% (baseline) | 282 | 238 | — | — | — | — | — |
| 75–90% | 114 | 42 | 2.25 (1.48, 3.42) | 1.37 × 10−4 | 1.72 (1.15, 2.55) | 7.61 × 10−3 | 1.78 (1.68–1.88) |
| 90–99% | 78 | 15 | 4.55 (2.48, 8.36) | 1.06 × 10−6 | 4.79 (2.64, 8.71) | 2.75 × 10−7 | 2.93 (2.75–3.12) |
PRS was calculated using 97 SNPs, including 92 known non-8q24 risk alleles (MAF>0.01) and 5 independent 8q24 risk variants identified in Ugandan men.
Adjusted for age and 10 principle components; weights were marginal log10(OR) from Ugandan men, with a Bayesian adjustment for the 5 8q24 allele for their “winner’s curse.”
Wald test P-value.
Adjusted for age and 10 principle components; weights were log10(OR) from a large African American sample.14
Estimates were from previous paper using 100 SNPs to construct PRS.7
In the genome-wide analysis, only five SNPs, all located in the risk region at 8q24.21 (Supplementary Table S4, Supplementary Figure S3) were genome-wide significant. Variant rs72725854 was found to be the most statistically significant in association with PCa risk (OR = 3.37, P = 2.14 × 10−11), with a risk allele frequency (RAF) of 0.14 in cases and 0.06 in controls. This variant is only found in men of African ancestry and is the most strongly associated risk variant for PCa found to date in men of African ancestry.14 The other four genome-wide significant variants were all located in the vicinity (within ~10 kb) and were correlated with rs72725854 (r2 = 0.52–0.61) (Supplementary Table S4).
4 |. DISCUSSION
In this study, we characterized known genetic risk factors for PCa in Ugandan men. Of the 118 established risk alleles for PCa, 111 are common in Ugandan men (MAF>0.1%) and ~70% had ORs in UGPCS that were directionally consistent with previous studies, which suggests that most of the known PCa susceptibility variants are likely to be markers of PCa risk in East African men. Just over half (56.7%) of the effects sizes observed in UGPCS were smaller than those observed in European ancestry populations (or had opposite directions), and only 14 risk alleles achieved nominal statistical significance. This observation is likely due to many factors, including variability of effect estimation due to small sample size, true differences in the effect of these susceptible variants across ethnic groups and population differences in LD between the index risk SNPs and causal alleles. Larger studies in men of African ancestry will be required to disentangle these possibilities.
The overwhelming importance of genetic variation at 8q24 in contributing to PCa risk was confirmed in UGPCS, with five independent risk alleles observed in this region. The polygenic risk score analysis further demonstrated the substantial contribution of 8q24 to PCa susceptibility, with the mean risk score increasing by ~50% with the inclusion of the five independent risk SNPs in the 8q24 region (9.70) to the PRS of the 92 non-8q24 known risk alleles (6.49). The change in the AUC of 0.06 when adding 5 8q24 alleles suggests variation in this region is important for risk classification, although overfitting likely exists in this study and external validation is needed.
A predictive PRS for PCa may be effective for identifying high-risk populations who are most likely to benefit from biopsy and subsequent treatment, and in reducing the over-diagnosis of indolent disease.27 Studies among men of European descent (using 100 risk SNPs) indicate that men in the 90–99% of the PRS have a ~3-fold increase in PCa risk compared to the population average (25th-75th percentile of the PRS distribution).7 In our study, using a comparable set of 97 risk SNPs, Ugandan men in the 90–99% of the PRS demonstrated an increased PCa risk of 4.55-fold (95%CI: 2.48,8.36) compared with the average risk. This elevated risk might be attributed to African-specific risk alleles included in our PRS, especially rs72725854, which has a greater effect size and is limited to Ugandan men, or, perhaps variability in effect estimation due to the limited sample size of our study. To address this, we also constructed the PRS using per-allele log odds ratios obtained from the large AAPC meta-analysis (>10 000 cases) in men of African Ancestry as weights,14 which includes some of the UGPCS samples. Here, we found that men in the 90–99% risk stratum had a relative risk of 4.79 (95%CI: 2.64, 8.71), which is still larger than that reported in previous studies among Whites, and is similar to that observed using Uganda-specific weights (ie, ORs) suggesting that the effects and/or frequencies of PCa risk alleles may be greater in men of African ancestry. However, the risk may be higher in our study because we removed controls with PSA levels ≥4 ng/m, which is a criterion that is not applied consistently in other studies. We found no evidence of heterogeneity in the PRS by disease aggressiveness which is consistent with other studies.28 Other studies have provided evidence of heterogeneity in PRS effects by PCa family history,28 but unfortunately, family history was not available in our study, so we were not able to assess this hypothesis here.
A major limitation in this study is the small sample size, with statistical power for testing of known risk loci and for risk allele discovery being underpowered. For known risk alleles with MAF of 20%, the power to detect ORs of 1.25 at a nominal significant level (P < 0.05) was only 55%. In the exploratory genome-wide analyses, for alleles with MAF of 20%, there was only a priori adequate power (80%) to detect alleles with large effects (ORs >1.90) at genome-wide significance (P <5× 10−8).
The most genome-wide significant association was noted with rs72725854. The risk variant rs72725854 is tri-allelic, with the risk allele T only reported in African ancestry populations, and is correlated with other published African ancestry-specific 8q24 risk alleles rs114798100 (r2 = 0.54) and rs111906932 (r2 = 0.39). This variant is located in an intergenic region near a long non-coding RNA, PCAT1 (Prostate Cancer Associated Transcript 1), and was previously discovered in the AAPC GWAS which included the majority of UGPCS subjects.14 While the RAF of this variant is similar across African ancestry populations (5–11% in AFR populations in 1KGP) the odds ratio was slightly higher in Ugandan men (OR = 3.37; 95%CI, 2.36–4.82) compared to that reported in AAPC, which included mainly African Americans (OR = 2.13; 95%CI, 1.97–2.32).14
In summary, we found that the known PCa risk variants can effectively stratify PCa risk in Ugandan men, with 10% of men having a >4-fold increase in risk. These results also emphasize the importance of germline variation at the 8q24 risk region in the etiology of PCa in Ugandan men. Larger studies in African populations will be required to identify additional PCa risk alleles that aid in risk stratification and that contribute to the higher PCa incidence in this population.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants U19CA148537 and R01CA165862.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Rebbeck TR, Devesa SS, Chang B-L, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer. 2013:560857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC—Prostate Cancer Rates by Race and Ethnicity. https://www.cdc.gov/cancer/prostate/statistics/race.htm. Accessed December 15, 2016.
- 4.Parkin DM, Nambooze S, Wabwire-Mangen F, Wabinga HR. Changing cancer incidence in Kampala, Uganda, 1991–2006. Int J Cancer. 2010;126:1187–1195. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on day/month/year. [Google Scholar]
- 6.Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Olama AA, Kote-Jarai Z, Berndt SI, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann TJ, Van Den Eeden SK, Sakoda LC, et al. A large multiethnic genome-wide association study of prostate cancer identifies novel risk variants and substantial ethnic differences. Cancer Discov. 2015;5:878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook MB, Wang Z, Yeboah ED, et al. A genome-wide association study of prostate cancer in West African men. Hum Genet. 2014;133:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Y, Rand KA, Hazelett DJ, et al. Prostate cancer susceptibility in men of african ancestry at 8q24. J Natl Cancer Inst. 2016;108:djv431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eeles RA, Al Olama AA, Benlloch S, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45:385–391, 391–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Olama AA, Dadaev T, Hazelett DJ, et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet. 2015;24:5589–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haiman CA, Chen GK, Blot WJ, et al. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet. 2011;43:570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conti DV, Wang K, Sheng X, et al. Two novel susceptibility loci for prostate cancer in men of african ancestry. JNCI J Natl Cancer Inst. 2017;109:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Y, Hazelett DJ, Wiklund F, et al. Integration of multiethnic fine-mapping and genomic annotation to prioritize candidate functional SNPs at prostate cancer susceptibility regions. Hum Mol Genet. 2015;24:5603–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haiman CA, Chen GK, Blot WJ, et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011;7:e1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amos CI, Dennis J, Wang Z, et al. The OncoArray consortium: a network for understanding the genetic architecture of common cancers. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2017;26:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaneau O, Marchini J. 1000 genomes project consortium, 1000 genomes project consortium. Integrating sequence and array data to create an improved 1000 genomes project haplotype reference panel. Nat Commun. 2014;5:3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. [DOI] [PubMed] [Google Scholar]
- 20.Cropp CD, Robbins CM, Sheng X, et al. 8q24 risk alleles and prostate cancer in african-Barbadian men: 8q24 risk alleles and prostate cancer in AB men. Prostate. 2014;74:1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haiman CA, Patterson N, Freedman ML, et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet. 2007;39:638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Olama AA, Kote-Jarai Z, Giles GG, et al. Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat Genet. 2009;41:1058–1060. [DOI] [PubMed] [Google Scholar]
- 23.Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinforma Oxf Engl. 2010;26:2336–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong H, Prentice RL. Bias-reduced estimators and confidence intervals for odds ratios in genome-wide association studies. Biostat Oxf Engl. 2008;9:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takata R, Akamatsu S, Kubo M, et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet. 2010;42:751–754. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Mo Z, Ye D, et al. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31. 2 and 19q13. 4. Nat Genet. 2012;44:1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pashayan N, Duffy SW, Neal DE, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med Off J Am Coll Med Genet. 2015;17:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Olama AA, Benlloch S, Antoniou AC, et al. Risk analysis of prostate cancer in PRACTICAL, a multinational consortium, using 25 known prostate cancer susceptibility loci. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2015;24:1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.