Abstract
More than 1,500 construction and demolition debris (CDD) landfills operate in the United States (U.S.), and U.S. federal regulations do not require containment features such as low-permeability liners and leachate collection systems for these facilities. Here we evaluate groundwater quality from samples collected in groundwater monitoring networks at 91 unlined, permitted CDD landfills in Florida, U.S. A total of 460,504 groundwater sample results were analyzed, with a median of 10 years of quarterly or semi-annual monitoring data per site including more than 400 different chemical constituents. Downgradient concentrations of total dissolved solids, sulfate, chloride, iron, ammonia-nitrogen, and aluminum were greater than upgradient concentrations (p<0.05). At downgradient wells where sulfate concentrations were greater than 150 mg/L (approximately 10% of the maximum dissolved sulfate concentration in water, which suggests the presence of leachate from the landfill), iron and arsenic were detected in 91% and 43% of samples, with median concentrations of 1,900 μg/L and 11 μg/L, respectively. These results show that although health-based standards can be exceeded at unlined CDD landfills, the magnitude of detected chemical concentrations is generally small and reflective of leached minerals from components (wood, concrete, and gypsum drywall) that comprise the bulk of discarded CDD by mass.
Keywords: landfill, C&D, construction, waste, groundwater, contamination, leachate, reductive dissolution
Introduction
An estimated 610–780 million metric tons of construction and demolition debris (CDD) were generated in 2002 in the United States (U.S.) from the construction, renovation, repair, and demolition of residential and commercial buildings and infrastructure such as roads and bridges1. As a point of comparison, approximately 214 million metric tons of municipal solid waste (MSW) were generated in the U.S. in 20022. The overall composition of CDD varies based on the activity that produces the debris, but typically consists of concrete, wood products, metal, asphalt, gypsum drywall, masonry products and trace quantities of miscellaneous construction products like paints, solvents, and adhesives. The composition of CDD disposed depends on the degree and components of CDD that are recycled; reliable estimates of US-wide CDD disposal and recycling are not available. In the U.S., rules dictating the disposal and recycling of CDD vary by state, but disposal normally occurs in dedicated landfills that only accept CDD or in municipal landfills that accept MSW, industrial wastes, and CDD.
U.S. federal regulations currently require landfills that accept hazardous waste and MSW for disposal be constructed with liners and leachate collection systems to protect underlying and adjacent water resources3; similar requirements for the disposal of coal combustion residuals are currently under consideration4. U.S. rules do not require CDD disposal facilities to be constructed with liners and leachate collection and removal systems (LCRS), but approximately half of U.S. states require such containment systems and about half of U.S. states require routine groundwater monitoring5.
In the early 1990s, the U.S. EPA initiated rulemaking to fulfill the statutory requirement to develop landfill design and operational requirements for landfills that dispose of conditionally exempt small quantity generator (CESQG) hazardous waste. As CDD landfills were considered a potential recipient of CESQG hazardous waste such as paints, adhesives, and roofing cements6, the U.S. EPA commissioned an investigation to assess the state of knowledge regarding the impact of CDD disposal facilities on the environment, specifically on groundwater resources. Since groundwater monitoring data for such facilities were very limited, available lined CDD landfill leachate concentrations were used to evaluate the likelihood of groundwater contamination at unlined sites, and seven constituents of concern were identified as having a potential to result in groundwater concentrations above appropriate regulatory standards or risk-based water quality thresholds. Four of these chemicals have a primary drinking water standard (cadmium, lead, methylene chloride, and 1,2-dichloroethane) and three have a secondary standard (total dissolved solids (TDS), iron, and manganese)7. The mean leachate concentrations for the four primary constituents were relatively close to the applicable standard and were deemed to pose minimal threat to groundwater (dilution and attenuation would be expected). The mean iron and manganese leachate concentrations were found to be 37 and 59 times their respective regulatory standard, but as secondary standards they were not viewed as presenting a high level of risk. Based on these results and other data and feedback regarding the potential for CDD landfill groundwater impacts, the U.S. EPA promulgated rules requiring groundwater monitoring at facilities that accept CESQG hazardous waste, but did not require liners and LCRS.
In the time since the U.S. EPA’s 1995 analysis, a substantial amount of additional research and data generation regarding CDD and corresponding disposal facilities has occurred. Several laboratory and field studies have been conducted to simulate the chemical quality of CDD landfill leachate8–10, similar to historical studies conducted on MSW landfill leachate11. Major observations of the recent CDD leachate work are that material components that make up the majority of discarded CDD by mass (e.g., wood, concrete, drywall) have the potential to produce a leachate that often exceeds the secondary drinking water standards for TDS, aluminum, iron, manganese, and sulfate12 and that biological activity often occurs as a result of biological sulfate reduction prompted by the large amounts of gypsum drywall often found in CDD13, 14. Some studies have focused on specific problematic waste components in CDD that may leach chemicals of concern at high concentrations, including treated wood15–18 and lead-based paint debris19. These results found that some components have the potential to be relatively mobile in the CDD landfill environment (e.g., arsenic) while other components are largely immobile (copper, lead).
Although previously-reported analyses have helped to inform decision-makers of likely leachate constituents and their potential mobility in the landfill environment, and have allowed for an indirect assessment of possible groundwater concentrations at unlined CDD facilities, actual published groundwater quality data from operating landfill sites are limited. Although leachate constituents are expected to undergo dilution and attenuation when discharged into the environment, the extent to which attenuation and dilution actually occur over the range of conditions occurring remains debated15, 20. With the growing availability of routine groundwater monitoring data at CDD landfills since the mid-1990s, the opportunity now exists to take a fresh look at the potential for unlined CDD landfills to negatively affect groundwater resources using monitoring data from operating facilities, and thus provide needed information to decision-makers (e.g., regulatory agencies) charged with regulating these resources. As a side benefit, analysis of large groundwater monitoring data sets can help to inform data and underlying assumptions in life-cycle modeling of CDD management at the end-of-life stage.
Here we conduct an analysis of a groundwater monitoring data set across a large geographic area to evaluate groundwater quality impacts at CDD landfills. Florida (USA), which had a reported 50% (by mass) CDD recycling rate in 201321, was selected as the study location because: (i) CDD landfills are not required to have bottom liners and LCRS, (ii) routine groundwater monitoring in the saturated portion of the uppermost aquifer within the zone of discharge (less than 15.2 m from the edge of the landfill) is required22, (iii) the surficial aquifer at most of the landfills lies in close proximity to the land surface23, and (iv) multiple years of historical monitoring data are available for a large number of sites24. The objectives of the analysis were to analyze quality-assured data for all of the CDD landfills in Florida over a multiyear period and compare aggregated groundwater quality at locations up-gradient and down-gradient from disposal areas to assess the frequency and magnitude of the measured chemical concentrations. The relative occurrence of signature chemicals is used to assess the degree of dilution occurring at these facilities and on the pathways of pollutant migration. The goal was not to statistically analyze the data on a site-by-site basis, but rather assess the bulk data set to meet the stated research objectives.
Methods
Semiannual groundwater monitoring is required at CDD landfills in Florida per the Florida Administrative Code (Chapter 62–701 and 62–520, Florida Administrative Code) and the results of groundwater monitoring must be submitted to the state regulatory agency, the Florida Department of Environmental Protection (FDEP). CDD landfills must have at least one groundwater monitoring well installed hydraulically up-gradient (referred to as a background well) and at least two detection wells located hydraulically down-gradient within 15.2 m from the edge of the disposal area. Most facilities have more than one detection well – the total number of detection wells depends on a site’s specific permit conditions.
The FDEP maintains an Electronic Document Management System that serves as a repository for the regulatory-required groundwater quality monitoring data collected at permitted landfills in Florida. A screening analysis was conducted to establish a population of landfills that met specific criteria to isolate those landfills that only accepted CDD. The database contained information on 382 active, inactive, and closed CDD landfills, of which 121 had groundwater data available. Permits and other identifying information for each of the 121 landfills were reviewed and sites that accepted waste materials other than CDD were eliminated from consideration. A total of 91 CDD landfills (59 active and 32 closed or inactive) remained after the screening analysis and were analyzed further. Figure 1 presents a geographical distribution of the sites whose data were examined in this study. Data for each site were downloaded from the FDEP database and compiled into a Microsoft Access database. Only sample data corresponding to each site’s permitted groundwater monitoring system were considered in this study.
Figure 1.
Geographical Distribution of Florida (U.S.) CDD Landfills with Groundwater Quality Data that were Analyzed in this Study
The data fields in the files downloaded from FDEP included sampling date and time, location information about the CDD landfill site, unique identification numbers for each groundwater well, well type (e.g., background, detection), sample type (e.g., field measurement, organic compound, inorganic compound), sample result and units, laboratory qualifier (e.g., detected, not detected), and sampling method (e.g., peristaltic pump).
Four distinct analyses were conducted in this study. First, the distribution of exceedances of Florida’s water quality standards were analyzed. In Florida, these water quality standards are referred to as groundwater cleanup target levels (GCTLs) and are published in Chapter 62–777, Florida Administrative Code. The standards comprise numerical criteria based on risk thresholds or federal drinking water standards that serve as a basis for evaluating whether or not groundwater is negatively impacted. In this study, an exceedance is considered a measurement that is greater than the GCTL.
The second step of the analysis identified the chemical parameters that most frequently exceeded the GCTLs. The database was organized and up-gradient and down-gradient measurements were aggregated and each result was compared to the corresponding GCTL. Up-gradient wells were included in the analysis since the exceedance of a GCTL in an up-gradient well may suggest naturally-occurring groundwater concentrations that are elevated or could be an indication of variable groundwater flow direction.
Next, up-gradient and down-gradient sample results were plotted to develop box-and-whisker diagrams comparing the 10th, 25th, 75th, and 90th percentile concentrations of the chemicals that most frequently exceeded the GCTL. A two-sample t-test was conducted (Minitab, State College, PA) on the up-gradient and down-gradient data on seven data sets (up-gradient and down-gradient detected concentrations of TDS, sulfate, sodium, chloride, iron, nitrate, and aluminum) that appeared to have differing concentrations based on the aggregated up-gradient and down-gradient data in the box plots. Although groundwater monitoring samples are collected from fixed points at routine intervals, the U.S. EPA25 suggests that the criterion of sample independence required for the t-test is satisfied when quarterly or semiannual groundwater sampling is conducted, and the results of a t-test are valid since such large data sets are used 26.
Following the aggregation of the data, the fourth component of the analysis considered elevated sulfate concentrations as an indicator of impacts from CDD leachate. A sulfate concentration of 150 mg/L (down-gradient concentration) was used as a benchmark – all wells with a measured sulfate concentration greater than 150 mg/L were further analyzed with a focus on the presence and magnitude of other chemicals at these wells. The 150 mg/L threshold was selected as it represents a dilution attenuation factor (DAF) of 10 (this assumption is discussed in greater detail in the next section). In the ICF analysis in 19957, a DAF of 100 was cited as likely for a landfill based on previous rulemaking, and a DAF of 10 was frequently described in the context of a minimum DAF.
Results and Discussion
The database included 460,504 individual measurements of more than 400 chemical constituents at 91 CDD landfills, including field-collected parameters, organic compounds, and inorganic compounds. Data for each site represented monitoring events ranging from as early as May 1994 and as late as June 2012. The number of sampling data points and the number of years of data at each site varied based on site age and availability of data in the FDEP database. The number of groundwater sampling data points at a site ranged from 144 to 34,484, with a median number of measurements of 3,543; the median number of years of data analyzed for each landfill was 10.
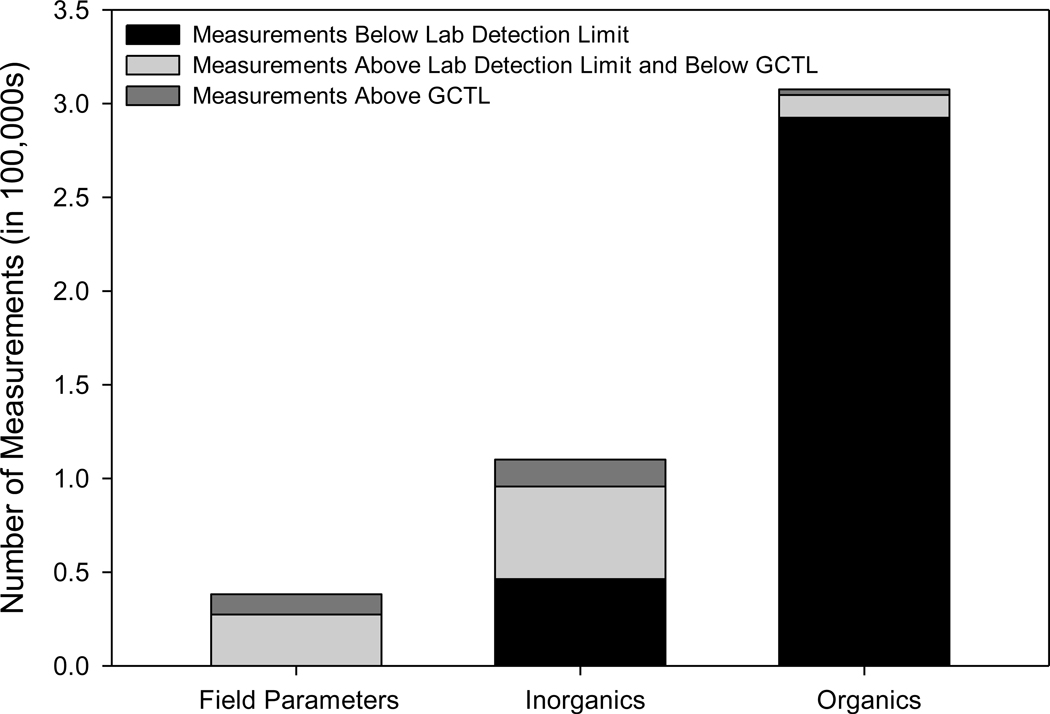
Figure 2 presents the number of all measurements below the laboratory detection limit (DL), greater than the DL but below the GCTL, and greater than the GCTL. Approximately 800 of these exceedances had laboratory-reported qualifiers such as “analyte was detected in blanks,” “the sample was improperly preserved” or that “the sample was held beyond the required holding time,” suggesting a data quality issue – these samples were eliminated from further analysis. Approximately 25,800 data points exceeded the respective GCTL. Approximately 13% of field measurements (only reflective of pH, which has a drinking water standard range of 6.5 to 8.5), 13% of inorganic compound measurements, and 1% of organic compound measurements had reported concentrations that exceeded the GCTL, as shown in Figure 2. Many chemicals (n = 153) included in the data set do not have a GCTL listed in the FDEP regulations, and are thus not included in Figure 2.
Figure 2.
Summary of Total Groundwater Sample Measurements, Measurements Greater than the Laboratory Detection Limit, and Results that Exceeded the Florida GCTL for Field-Measured Parameters (pH Outside of the Range of 6.5 – 8.5), Inorganics, and Organics
More than 95% of organic compound measurements were reported at concentrations less than the laboratory DL. Organic compounds that were measured at more than 10 sites and that exceeded the respective GCTL in more than 1% of measurements included acrylonitrile, aldrin, benzene, bis (2-chloroisopropyl) ether, bis (2-ethyhexyl) phthalate, bromodichloromethane, cyanide, dibromochloromethane, methylene chloride, phenols, 1,1,2,2-tetrachloroethane, and vinyl chloride. Phenols, benzene, vinyl chloride, bromodichloromethane, dibromochloromethane, and 1,1,2,2-tetrachloroethane constituted 32%, 16%, 11%, 8%, 7%, and 7%, respectively of the total GCTL exceedances for organic compounds. Phenols, benzene, vinyl chloride and bromodichloromethane exceeded the GCTL in 19%, 8.6%, 5.3% and 3.6% of samples, respectively. Methylene chloride was one of the two organic chemicals identified in the ICF (1995) analysis.
Forty-three of the 79 inorganic or conventional parameters measured exhibited at least one exceedance of the corresponding GCTL. Iron, aluminum, TDS, ammonia, arsenic, and sulfate constituted 28%, 21%, 16%, 11%, 6%, and 5%, of the total GCTL exceedances, respectively. TDS and iron were both among the parameters identified in the ICF analysis7; manganese was also identified in the ICF analysis, but it is not included in this analysis because it is not a regulatory-required monitoring parameter in Florida. Overall, these parameters constituted more than 85 percent of the total GCTL exceedances for all inorganic parameters measured.
The 20 chemical parameters (organic and inorganic) with the largest number of sites with down-gradient-only exceedances of the GCTL are provided in Figure 3. The data show that for 15 of the 20 most commonly-detected chemicals detected at concentrations greater than the GCTL, more sites exhibited exceedances in down-gradient wells only compared to sites that exhibited exceedances of GCTLs in both up-gradient and down-gradient wells. This observation suggests that the impacts observed at most sites reflects an impact from the landfill itself for many chemicals, although the potential mechanism underlying this impact warrants further discussion.
Figure 3.
Comparison of the Number of Sites with Exceedances of the GCTL for the 20 Most Commonly Detected Chemicals, Including a Sites with Downgradient Only Exceedances Compared to Downgradient and Upgradient Exceedances in Groundwater Monitoring Wells
The observed elevated concentrations of some chemical constituents can likely be attributed to the leaching of specific components common to CDD (e.g., sulfate from gypsum drywall). Detected concentrations of other chemicals, however, could be the result of a combination of leached chemicals from the waste and from naturally-occurring chemicals in the environment. Interestingly, the chemicals that were most often observed in both the down-gradient and up-gradient wells (compared to down-gradient wells alone) included constituents often associated with naturally-occurring minerals (e.g., iron, aluminum) as well as anthropogenic ones (e.g., 1,1,2,2-tetrachloroethane, dibromochloromethane). While some naturally-occurring chemicals may occur at elevated concentrations in groundwater regardless of whether a landfill is present, others (e.g., iron) are known to become mobilized as a result of reducing conditions caused by the presence of the landfill itself as opposed to a leached chemical from the waste8, 10, 27, 28.
Figure 4 presents a comparison of the distribution of the median of individual parameter measurements for up-gradient and down-gradient wells for the 20 parameters included in Figure 3 for all 91 sites. The median down-gradient concentrations of TDS, ammonia, sulfate, sodium, chloride and iron are approximately 2 to 5 times greater than that measured at up-gradient wells. The median concentration of several chemicals was greater in down-gradient wells than up-gradient wells: ammonia (58 of 91 sites), chloride (61 of 91), iron (57 of 91), sodium (59 of 91), sulfate (62 of 91), and TDS (68 of 91). Similarly, the median concentration at down-gradient wells was greater than the up-gradient wells for aluminum (32 of 91 sites), arsenic (23 of 91), chromium (24 of 91), and nitrate (32 of 91).
Figure 4.
Chemical Concentration Distribution of Selected Parameters Measured in Upgradient (U) and Downgradient (D) Groundwater Monitoring Wells at 91 CDD Landfills in Florida (Median Years of Semi-Annually Collected Data = 10); Measurements Below the Detection Limit Treated as Equal to Detection Limit
The box-and-whisker plots for the parameters most commonly exceeding a GCTL were evaluated further to compare measured up-gradient and down-gradient concentrations. Seven constituents (TDS, sulfate, sodium, chloride, iron, ammonia-nitrogen, and aluminum) were evaluated and the results showed that the down-gradient concentrations of all of these parameters were significantly greater than up-gradient concentrations (p < 0.05). TDS and iron were also among the chemicals previously identified as potentially problematic CDD leachate constituents7.
Iron is one of the parameters found to be most troublesome in Florida from a regulatory perspective. Iron concentrations in groundwater from the sites analyzed here ranged from below the DL to 170 mg/L. In this analysis, approximately 17 and 46 % of detected up-gradient and down-gradient measurements, respectively, exhibited an iron concentration greater than a Florida-specific risk-based iron concentration limit of 4,200 μg/L29. As previously stated, iron is well documented to be released from natural soils as a result of reductive dissolution10, 28, but it is also a constituent of CDD leachate reported at concentrations up to several hundred mg/L30. However, when comparing the ratio of iron to chloride (which is considered to be a conservative tracer) in CDD leachate and groundwater at CDD landfills, the results support natural soils as the primary contributor of iron. The release of naturally-occurring iron has been observed when simulated CDD leachate was passed through columns of iron-rich soil (concentrations were an order of magnitude larger than the CDD leachate itself)31. As suggested by the iron exceedances in the up-gradient wells (and the control columns presented in the previously described study31), iron can be mobilized even without leachate contact since other factors can promote reducing conditions, but the presence of leachate greatly promotes the reductive dissolution process for iron.
Although salt water intrusion represents a possible source of sodium, chloride, sulfate, and TDS based on a landfill’s proximity to a coastal zone32, the landfilled waste was likely the major contributor at the landfills examined here, as most of the sites were well inland as shown in Figure 1. In addition, sulfate concentrations are typically elevated in CDD leachate, a reflection of the large amount of gypsum drywall typically found in discarded CDD (7% to 15% on a weight basis33–35). Laboratory and field-scale leaching evaluations of CDD leachate have reported sulfate concentrations as high as 1,700 mg/L with a typical range of 600 to 1,000 mg/L8, 9, 30, 36. The solubility of gypsum in water is approximately 2,600 mg/L at 25 °C 37, which is equivalent to a maximum dissolved sulfate concentration of approximately 1,500 mg/L at 25 °C (although this could change in the presence of other dissolved ions38.)
As sulfate is an anticipated signature constituent of CDD leachate, and because of its limited solubility, an examination of sulfate concentrations in the Florida CDD landfill groundwater data set provided a unique opportunity to assess the degree of dilution and attenuation occurring at the Florida sites in this study. The monitoring well data were aggregated into those data with a sulfate concentration > 150 mg/L and those < 150 mg/L (this was performed for both up-gradient and down-gradient wells). Given an approximate sulfate solubility from gypsum of 1,500 mg/L, an observation of sulfate at 150 mg/L or more would suggest a dilution attenuation factor (DAF) of 10 or less. Since leached sulfate may be converted to hydrogen sulfide via sulfate reduction13, 14, 39 and then to low solubility sulfide mineral precipitates, this approach is conservative in that a 150 mg/L sulfate concentration would indicate a DAF of 10 or less (since the original sulfate concentration may have been higher).
In the database, sulfate was measured in 5,532 samples collected from down-gradient wells and the sulfate concentration was greater than 150 mg/L in 992 (17.9%) of these samples. This compares to 7% of those wells that were greater than 150 mg/L in the up-gradient wells. Following the assumption that samples with a sulfate concentration >150 mg/L were affected by CDD leachate, the presence and magnitude of other chemicals measured in these samples were evaluated to see whether they were more likely to occur where leachate impact was suspected. Table 1 presents the results of this analysis for arsenic, iron, mercury, and lead, and shows that down-gradient arsenic and iron concentrations were significantly greater (p < 0.05) in samples that exhibited sulfate concentrations >150 mg/L (mercury and lead were not statistically different). The results of the sulfate DAF analysis further support the previously-described leachate-induced reductive dissolution process as a key contributor to elevated iron concentrations. Given that the dissolution of iron is often coupled with a release of arsenic31, 40–42, it is also possible that naturally-occurring arsenic was a source of the elevated arsenic in the groundwater. However, given that laboratory studies support that arsenic should be released from CDD landfills as a result of arsenical-treated wood17, 18, the specific cause of the elevated arsenic in groundwater cannot be determined.
Table 1.
Comparison of Selected Measured Chemical Concentrations in Downgradient Wells Where the Sulfate Concentration was ≥150 mg/L (suggesting likely impacts from leachate) and <150 mg/L (suggesting limited or no impacts from leachate)
| Parameter | Downgradient Wells With Sulfate >=150 mg/L | Downgradient Wells With Sulfate <150 mg/L | High Sulfate Wells Statistically Significantly Greater than Low Sulfate Wells for the Selected Constituent (p < 0.05)? | ||||
|---|---|---|---|---|---|---|---|
| Number of Measurements | % Detected | Median Detected Value (μg/L) | Number of Measurements | % Detected | Median Detected Value (μg/L) | ||
| Arsenic | 1610 | 43% | 11.1 | 3247 | 21% | 8 | Yes |
| Iron | 1917 | 91% | 1900 | 3626 | 80% | 1100 | Yes |
| Mercury | 1648 | 8% | 0.23 | 3304 | 11% | 0.29 | No |
| Lead | 1654 | 7% | 4.6 | 3300 | 9% | 5 | No |
The relative occurrence and magnitude of several volatile organic compounds VOCs identified in Figures 3 and 4 were also compared among samples with sulfate concentrations greater than and less than 150 mg/L. Most VOCs would be expected to result from anthropogenic sources such as chemical products disposed as part of the CDD, and thus one might expect that VOC concentrations would be more frequently encountered in samples that were more strongly impacted by leachate. The results of this assessment, however, found that VOCs were mostly found in similar frequencies and magnitudes when comparing the >150 mg/L and <150 mg/L data sets for sulfate. This observation, coupled with the results shown in Figure 4 where VOCs are observed in similar concentration ranges in up-gradient and down-gradient wells, suggests that groundwater at CDD landfills may on occasion be impacted by VOCs, but that leachate might not be the dominant pathway. VOCs are known to migrate to groundwater as a result of landfill gas at MSW landfills43, and the results here indicate a similar phenomenon may be occurring at CDD landfills since limited differences in up-gradient and down-gradient concentrations of VOCs were observed.
Implications
The Florida data set provides an opportunity to reevaluate earlier opinions regarding whether unlined CDD landfills have the potential to impact underlying groundwater resources. Because of Florida’s climate (e.g., annual precipitation at the sites analyzed ranging from 1,270 – 1,788 mm) and the proximity of the groundwater table to the land surface in many locations (approximately 1–10 m23), one may expect that groundwater impacts from CDD leachate might be more noticeable in comparison to other locations in the U.S. In support of U.S. federal rulemaking in the 1990s7, the median concentration of CDD landfill leachate constituents was compared to regulatory benchmarks and the conclusion was that although multiple constituents exceeded the benchmark, only two exceeded the benchmark by more than factor of 10, and that the chemicals had secondary water quality standards which are aesthetics-based. Frequently cited in this analysis was that a dilution factor of 100 might be expected based on previous regulatory investigations.
The results here demonstrate that groundwater samples in Florida routinely exceed regulatory thresholds at multiple sites, although the vast majority of measurements are well below any health-based limits. Common to the earlier work, secondary water quality constituents such as TDS, sulfate, sodium, chloride, iron, and aluminum represent those chemicals most likely to be elevated in down-gradient wells compared to up-gradient wells. The presence of these chemicals, in addition to ammonia, is to be expected in groundwater at unlined sites as they are inherent to the materials that comprise the majority of discarded CDD by mass (wood, concrete, drywall). The mobilization of naturally-occurring iron through reductive dissolution is likely enhanced by leachate. Trace elements with health-based thresholds (e.g., primary drinking water standard chemicals) are observed above regulatory thresholds on occasion, but their occurrence is limited. Arsenic is the inorganic trace chemical with a health-based threshold that appears to be of possible concern, although two plausible sources were identified (mobilization after iron reductive dissolution and arsenic-containing leachate) but the magnitude of these sources could not be distinguished. Trace organic chemicals, primarily VOCs, also occur on occasion; the data here suggest that landfill gas may play a role in the transport of these chemicals.
When the types of chemical constituents identified here are compared with those highlighted in the U.S. EPA’s previous evaluation, common themes of secondary drinking water quality parameters and sporadic trace chemicals emerge. A primary difference between these evaluations relates to dilution and attenuation. The earlier analysis suggested that DAF should be relatively large between the landfill and the groundwater; the ICF evaluation in 1995 cited a DAF of 100 as likely7, and based this on the 100-fold factor developed for the Toxicity Characteristic Leaching Procedure. The results of this analysis, particularly based on an examination of sulfate concentrations, suggest, at least for Florida, DAF of much less than 100 is likely, and that in many cases the DAF is less than 10. Based on the analysis presented herein, 18% or more of the down-gradient samples exhibited a DAF of less than 10.
When deciding upon the necessity of liners and LCRS for CDD landfills, decision-makers should expect that groundwater quality will be altered at unlined CDD disposal facilities. A bulk of the chemicals will not represent a direct risk to human health and the environment, but may render the water quality in vicinity of the landfill non-potable. Depending on site-specific geologic and hydrologic conditions, naturally-occurring elements may become mobilized, and this might be manifested in up-gradient as well as down-gradient monitoring wells. Frequent occurrence of trace chemicals that exceed health-based risk thresholds would be unlikely. Decision-makers can use the Florida results as an indication of the relative frequency at which constituent exceedances might occur, but should consider regional-specific differences in dilution and attenuation.
Furthermore, data and observations in this study can be used in life-cycle models for CDD landfills, with the requisite due care, as part of examining impacts from chemical release to the groundwater. Specifically, the suite of specific chemical releases to groundwater, the phenomenon of reductive dissolution, and the DAF results (which suggest DAF less than 10 can occur), will help to more accurately model life-cycle analysis scenarios that include CDD disposal.
Acknowledgments
This research was funded by the U.S. EPA’s Office of Research and Development. The manuscript has not been subjected to the Agency’s internal review, therefore, the opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the U.S. EPA. Any mention of products or trade names does not constitute recommendation for use by the U.S. EPA. The authors acknowledge the contributions of Ali Bigger and Lizmarie Maldonado in reviewing aspects of the data and the report, and Matthew Shupler and Luis Ruiz for data processing.
Contributor Information
Jon T. Powell, Innovative Waste Consulting Services, LLC, 6628 NW 9th Blvd. Suite 3, Gainesville, FL 32605, USA..
Pradeep Jain, Innovative Waste Consulting Services, LLC, 6628 NW 9th Blvd. Suite 3, Gainesville, FL 32605, USA..
Justin Smith, Innovative Waste Consulting Services, LLC, 6628 NW 9th Blvd. Suite 3, Gainesville, FL 32605, USA..
Timothy G. Townsend, Department of Environmental Engineering Sciences, University of Florida, P.O. BOX 116450, Gainesville, FL 32611-6450, USA.
Thabet M. Tolaymat, Land and Nanotechnology Research, National Risk Management Research Laboratory, US Environmental Protection Agency, 26 W. Martin Luther King St., Cincinnati, OH 45268, USA.
References
- 1.Cochran KM; Townsend TG, Estimating construction and demolition debris generation using a materials flow analysis approach. Waste Manage. 2010, 30, (11), 2247–54. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Environmental Protection Agency, Municipal Solid Waste Generation, Recycling, and Disposal in the United States: Facts and Figures for 2003. In 2005. [Google Scholar]
- 3.Code of Federal Regulations, Criteria for Municipal Solid Waste Landfills. In 2012; Vol. 40 CFR; 258. [Google Scholar]
- 4.Federal Register, Disposal of Coal Combustion Residuals from Electric Utilities. In Federal Register,: 2010; p 138. [Google Scholar]
- 5.Clark C; Jambeck J; Townsend T, A Review of Construction and Demolition Debris Regulations in the United States. Crit. Rev. Env. Sci. Technol. 2006, 36, (2), 141–186. [Google Scholar]
- 6.U.S. Environmental Protection Agency, Background Document for the CESQG Rule, EPA/530-R-95–021. In 1995. [Google Scholar]
- 7.ICF Construction and Demolition Waste Landfills; May 18, 1995. [Google Scholar]
- 8.Townsend T; Yang YC; Thurn L, Simulation of Construction and Demolition Waste Leachate. J. Environ. Eng. 1999, 125, (11), 1071–1081. [Google Scholar]
- 9.Weber WJJ, Y. C; Townsend TG; Laux SJ, Leachate from Land Disposed Residential Construction Waste. J. Environ. Eng. 2002, 128, (3), 237–245. [Google Scholar]
- 10.Roussat N; Mehu J; Abdelghafour M; Brula P, Leaching behaviour of hazardous demolition waste. Waste Manage. 2008, 28, (11), 2032–40. [DOI] [PubMed] [Google Scholar]
- 11.Pohland FG, Leachate Recycle as a Landfill Management Option. J. Environ. Eng. Div. 1980, 106, (6), 13. [Google Scholar]
- 12.Jang YC; Townsend TG, Effect of Waste Depth on Leachate Quality from Laboratory Construction and Demolition Debris Landfills. Environ. Eng. Sci. 2003, 20, (3), 183–196. [Google Scholar]
- 13.Fairweather R; Barlaz M, Hydrogen Sulfide Production during Decomposition of Landfill Inputs. J. Environ. Eng. 1998, 124, (4), 353–361. [Google Scholar]
- 14.Yang K; Xu Q; Townsend TG; Chadik P; Bitton G; Booth M, Hydrogen sulfide generation in simulated construction and demolition debris landfills: impact of waste composition. J. Air Waste Manage. Assoc. 2006, 56, (8), 1130–8. [DOI] [PubMed] [Google Scholar]
- 15.Saxe JK; Wannamaker EJ; Conklin SW; Shupe TF; Beck BD, Evaluating landfill disposal of chromated copper arsenate (CCA) treated wood and potential effects on groundwater: evidence from Florida. Chemosphere 2007, 66, (3), 496–504. [DOI] [PubMed] [Google Scholar]
- 16.Moghaddam AH; Mulligan CN, Leaching of heavy metals from chromated copper arsenate (CCA) treated wood after disposal. Waste Manage. 2008, 28, (3), 628–37. [DOI] [PubMed] [Google Scholar]
- 17.Jambeck JR; Townsend TG; Solo-Gabriele HM, Landfill disposal of CCA-treated wood with construction and demolition (C&D) debris: arsenic, chromium, and copper concentrations in leachate. Environ. Sci. Technol. 2008, 42, (15), 5740–5. [DOI] [PubMed] [Google Scholar]
- 18.Dubey B; Spalvins E; Townsend T; Solo-Gabriele H, Comparison of Metals Leaching from CCA- and ACQ-Treated Wood in Simulated Construction and Demolition Debris Landfills. J. Environ. Eng. 2009, 135, (10), 910–917. [Google Scholar]
- 19.Wadanambi L; Dubey B; Townsend T, The leaching of lead from lead-based paint in landfill environments. J. Hazard. Mater. 2008, 157, (1), 194–200. [DOI] [PubMed] [Google Scholar]
- 20.Solo-Gabriele HM; Townsend TG; Khan BI; Dubey B; Jambeck J; Cai Y, Comment on “Evaluating landfill disposal of chromated copper arsenate (CCA) treated wood and potential effects on groundwater: Evidence from Florida” by Jennifer K. Saxe, Eric J. Wannamaker, Scott W. Conklin, Todd F. Shupe and Barbara D. Beck [Chemosphere 66 (3) (2007) 496–504]. Chemosphere 2008, 70, (10), 1930–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FDEP, Florida Municipal Solid Waste Collected and Recycled (2013); 2015. [Google Scholar]
- 22.FDEP, Solid Waste Management Facilities. Chapter 62–701, Florida Administrative Code. [Google Scholar]
- 23.FDEP, Depth to Water Table in the Surficial Aquifer System. 2015. [Google Scholar]
- 24.FDEP, Water Assurance Compliance System. http://appprod.dep.state.fl.us/www_wacs/Reports/SW_Facility_Count.asp. 2013.
- 25.U.S. Environmental Protection Agency, Statistical Analysis of Groundwater Monitoring Data at RCRA Facilities, Unified Guidance. 2009. [Google Scholar]
- 26.Ott RL, Longnecker M, An Introduction to Statistical Methods and Data Analysis. 5 ed.; Duxbury Thompson Learning: Pacific Grove, CA, 2001. [Google Scholar]
- 27.Townsend T; Tolaymat T; Leo K; Jambeck J, Heavy metals in recovered fines from construction and demolition debris recycling facilities in Florida. Sci. Total Environ. 2004, 332, (1–3), 1–11. [DOI] [PubMed] [Google Scholar]
- 28.Rhue DT, T.; Wang Y; Dubey B Soils Underneath Florida Landfills and Their Role in the Occurrence and Fate of Iron and Arsenic in Groundwater; Hinkley Center for Solid and Hazardous Waste Management: July 1, 2008, 2008; p 100. [Google Scholar]
- 29.Center for Environmental & Human Toxicoloty Technical Report: Development of Cleanup Target Levels (CTLs) for Chapter 62–777, F.A.C.; University of Florida: February 2005, 2005; p 310. [Google Scholar]
- 30.Melendez BA A Study of Leachate Generated from Construction and Demolition Waste Landfills Master’s Project, University of Florida, Gainesville, FL, 1996. [Google Scholar]
- 31.Wang Y; Sikora S; Kim H; Dubey B; Townsend T, Mobilization of iron and arsenic from soil by construction and demolition debris landfill leachate. Waste Manage. 2012, 32, (5), 925–932. [DOI] [PubMed] [Google Scholar]
- 32.Maddox GLL, J. M; Scott TM; Upchurch SB; and Copeland R, Florida’s Ground-Water Quality Monitoring Program, Background Hydrogeochemistry. In 34 ed.; Florida Geological Survey: 1992; p 342. [Google Scholar]
- 33.MSW Consultants Construction and Demolition Debris Composition Study, Mecklenburg County, North Carolina; 2008. [Google Scholar]
- 34.CDM Smith City of Chicago Department of Environment Waste Characterization Study; 2010. [Google Scholar]
- 35.ODEQ 2002 Oregon Solid Waste Characterization and Composition; 2004. [Google Scholar]
- 36.Jang Y-C; Townsend T, Sulfate leaching from recovered construction and demolition debris fines. Adv. Environ. Res. 2001, 5, (3), 203–217. [Google Scholar]
- 37.Speight JG, Lange’s Handbook of Chemistry. McGraw-Hill: New York, NY, 2005. [Google Scholar]
- 38.Shternina EB, Solubility of gypsum in aqueous solutions of salts. Int. Geol. Rev. 1960, 2, (7), 605–616. [Google Scholar]
- 39.Xu Q; Powell J; Jain P; Townsend T, Modeling of H2S migration through landfill cover materials. J. Hazard. Mater. 2014, 264, (0), 254–260. [DOI] [PubMed] [Google Scholar]
- 40.deLemos JL; Bostick BC; Renshaw CE; Sturup S; Feng X, Landfill-stimulated iron reduction and arsenic release at the Coakley Superfund Site (NH). Environ. Sci. Technol. 2006, 40, (1), 67–73. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh A; Mukiibi M; Saez AE; Ela WP, Leaching of Arsenic from Granular Ferric Hydroxide Residuals Under Mature Landfill Conditions. Environ. Sci. Technol. 2006, 40, (19), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian H; Shi Q; Jing C, Arsenic Biotransformation in Solid Waste Residue: Comparison of Contributions from Bacteria with Arsenate and Iron Reducing Pathways. Environmental Science & Technology 2015, 49, (4), 2140–2146. [DOI] [PubMed] [Google Scholar]
- 43.Kerfoot HB, Landfill Gas Effects on Groundwater Samples at a Municipal Solid Waste Facility. J. Air Waste Manage. Assoc. 1994, 44, (11), 6. [DOI] [PubMed] [Google Scholar]