Abstract
Introduction
Up to 20% of patients undergoing total knee replacement (TKR) surgery report no or suboptimal pain relief after TKR. Moreover, despite chances of recovering to preoperative functional levels, patients receiving TKR have demonstrated persistent deficits in quadriceps strength and functional performance compared with healthy age-matched adults. We intend to examine if low-load blood flow restricted exercise (BFRE) is an effective preoperative method to increase functional capacity, lower limb muscle strength and self-reported outcomes after TKR. In addition, the study aims to investigate to which extent preoperative BFRE will protect against surgery-related atrophy 3 months after TKR.
Methods
In this multicentre, randomised controlled and assessor blinded trial, 84 patients scheduled for TKR will be randomised to receive usual care and 8 weeks of preoperative BFRE or to follow usual care-only. Data will be collected before randomisation, 3–4 days prior to TKR, 6 weeks, 3 months and 12 months after TKR. Primary outcome will be the change in 30 s chair stand test from baseline to 3-month follow-up. Key secondary outcomes will be timed up and go, 40 me fast-paced walk test, isometric knee extensor and flexor strength, patient-reported outcome and selected myofiber properties.
Intention-to-treat principle and per-protocol analyses will be conducted. A one-way analysis of variance model will be used to analyse between group mean changes. Preintervention-to-postintervention comparisons will be analysed using a mixed linear model. Also, paired Student’s t-test will be performed to gain insight into the potential pretraining-to-post-training differences within the respective training or control groups and regression analysis will be used for analysation of associations between selected outcomes.
Ethical approval
The trial has been accepted by the Central Denmark Region Committee on Biomedical Research Ethics (Journal No 10-72-19-19) and the Danish Data Protection Agency (Journal No 652164). All results will be published in international peer-reviewed scientific journals regardless of positive, negative or inconclusive results.
Trial registration number
Keywords: blood flow restriction exercise, knee osteoarthritis, total knee replacement surgery, preconditioning, functional capacity
Strengths and limitations of this study.
The trial is a multicentre, randomised controlled assessor blinded trial.
This is the first clinical trial to investigate the effect of low-load ischaemic-resistance training as a preconditioning method prior to elective knee replacement surgery.
Patients will not be blinded to their allocation into intervention groups (blood flow restricted vs control).
This is a protocol paper.
Introduction
Knee osteoarthritis (OA) is a degenerative joint disease associated with pain, reduced physical activity and quality of life and affects almost 40% of all individuals ≥60 years of age.1–5 Approaching end-stage knee OA, total knee replacement (TKR) is often the preferred treatment choice to reduce pain and regain functional capacity. That is, TKR is considered a highly successful treatment to improve quality of life and long-term function.6 However, despite being considered highly successful, approximately 20% of the patients undergoing TKR experience a suboptimal outcome,6 which has often been suggested to be related to incomplete restoration of physical function.7 In addition, TKR patients typically demonstrate long-lasting deficits in quadriceps strength and functional performance.2 4 This failure to return to ‘normal’ strength levels has been suggested to be associated with preoperatively lower limb muscle strength and function.2
Preconditioning exercise designed to prepare the musculoskeletal system to better tolerate stressful events such as the impact of invasive surgery has been suggested to be applicable prior to elective TKR.6 This is supported by the results of two randomised controlled trials indicating that preoperative heavy-resistance strength training (HRST) may enhance functional capacity and knee extensor muscle strength 3 months postoperatively.7 8 Joint pain resulting from the high mechanical loads associated with HRST may represent a barrier to this type of training in some patients suffering from severe knee OA.1 9 Therefore, a more tolerable, yet effective, alternative is needed for this population. Also, three recent systematic reviews investigating the topic of preoperative physiotherapy-based exercise before TKR all warrant high-quality, well-powered evidence to investigate the efficacy of preoperative physiotherapy before TKR.10–12
Resistance training with low exercise loads (~30% one repetition maximum) performed with concurrent partial blood flow restriction to the working limb (blood flow restricted exercise, BFRE) has received increasing clinical interest during the last decade.1 13–32 The application of low muscle/tendon/joint forces in BFRE has been documented to increase human skeletal muscle size and to cause substantial strength gain in healthy young and old individuals, as well as some patient populations, despite the low magnitude of mechanical stress imposed on the trained tissue.13 25 26 When applied in the clinical setting, BFRE has demonstrated positive effects on skeletal muscle hypertrophy, strength, and functional capacity in mild-degree knee OA patients1 9 33 34 although not observed in all studies.33 Importantly, BFRE appears to be feasible with a high training adherence in knee OA patients.1 33 34 The use of different restrictive pressures (absolute restrictive pressures: 160–200 mm Hg and individualised pressure of 70%; the pressure needed to provide complete arterial blood flow restriction (total limb occlusion pressure, LOP) has been applied without any adverse events in mild-degree knee OA.1 33 34 This is in line with Hughes et al13 who suggested that when BFRE is performed correctly, it has been demonstrated to be as safe as free-flow exercise methods.13
Currently, no consensus exists about the appropriate restrictive pressure to induce favourable muscle adaptation in patients suffering from knee OA. This might be due to the fact that the effective occlusion pressure seems to be dictated by the exercise load/intensity.35 Thus, the effective occlusion pressure varies between studies due to use of different exercises or differences in exercise load and intensity. Restrictive pressures ranging from 40% to 80% LOP have been suggested to be sufficient to evoke muscular adaptation in healthy adults.14 17 18 36 If the load is less than 30% 1RM, higher restrictive pressures seems required to evoke muscle hypertrophy, while lower pressures (40% LOP) requires training loads of 30% 1RM or above to be performed.36 Injury or joint pain (ie, from the knee) might limit the amount of resistance applied during strength testing, and may thus compromise the ability to rely fully on a given 30% 1RM estimation. Therefore, higher pressures than 40% LOP are suggested to be used in clinical settings.36 On the other hand, higher pressures are associated with more discomfort during exercise and in between-set rest pauses,14 which potentially can affect exercise motivation negatively in patients. Thus, an occlusion pressure sufficiently high to evoke measurable muscle adaptation despite potentially exercising at loads lower than 30% 1RM; yet tolerable to maintain a high adherence, seems a favourable choice for this particular patient population.
The adaptive mechanisms evoked by BFRE seem to involve accumulation of metabolites, ischemia (transient tissue hypoxia), which may increase recruitment of higher threshold (type II) fibres through stimulation of group III and IV afferent nerve fibres,37 38 and also activation of myogenic muscle stem cells (satellite cells, SC).13 26 31 SC are cells positioned between the sarcolemma and the myofiber basal lamina.31 39 SCs play an important role in human skeletal muscle growth due to their ability to donate new myonuclei to the muscle fibres.31 40–44 That is, the human skeletal muscle fibres are multinucleated cells with each myonucleus controlling the protein synthesis of a certain cytoplasmatic area in the muscle fibre.40–42 45 Myonuclei transcriptional activity can be fully maximised with exercise, hence requiring new myonuclei to support further muscle tissue accretion.41 42 44 It has been suggested that exercise-related addition of SC and myonuclei by means of BFRE might reduce the muscle atrophy related to bedrest and/or prolonged inactivity.31 46 Previous studies applying short-term (10 days) preoperative BFRE before an anterior cruciate ligament rupture–reconstruction found no atrophy protective effect or higher postoperative muscle strength compared with performing a low-load exercise without blood flow restriction (placebo). However, it might be questionable if the applied training frequency, intensity and training period have been sufficient to promote SCs and myonuclei addition. Thus, longer periods of intensive training might be necessary to promote the desired muscle morphological adaptations (addition of myonuclei and increased SC content).
Aim and hypothesis of the trial
The primary aim of this trial is to investigate the efficacy of 8 weeks of BFRE compared with receiving usual care prior to TKR on postoperative chair stand performance. We hypothesise that 8 weeks of preoperative BFRE will lead to increased 30 s chair stand performance (30 s chair stand test: 30 s CST) when assessed 3 months postoperatively. Secondary aims are to investigate the efficacy of preoperative BFRE on lower limb muscle strength 3 months after TKR and investigate the potential relationship to functional capacity and quality of life. Furthermore, it will be investigated to which extent 8 weeks of BFRE induce myofiber hypertrophy and gain in SC number and myonuclei content in the knee extensor musculature.
Material and methods
Design
The trial is designed as a multicentre (two sites), randomised, assessor blinded, controlled trial following the Consolidated Standards of Reporting Trials (CONSORT) guidelines.47 Primary endpoint will be 3 months after TKR. Additional and secondary endpoints will be evaluated during the week of TKR, 6 weeks after TKR (questionnaires only) and 12 months after TKR. Muscle biopsies will be obtained from all patients undergoing surgery at Horsens Regional Hospital at baseline, during surgery and 3 months after TKR.
Participants
Patients will be recruited from the Departments of Orthopedic Surgery at Horsens and Silkeborg Regional Hospitals in Denmark. Patient enrolment will start 2 September 2019 at Horsens Regional Hospital and 1 October 2019 at Silkeborg Regional Hospital. Patient recruitment is expected to be completed in June 2021. All patients are expected to have completed baseline testing in September 2021. To account for surgery and intervention, the 3-month follow-up will be concluded in April 2022. Thus, at the end of September 2022, all patients are expected to have completed 12-month follow-up testing.
Inclusion criteria
(1) Patients ≥50 years scheduled for TKR due to knee OA at Horsens or Silkeborg Regional Hospital.
Exclusion criteria
(1) Severe cardiovascular diseases (New York Heart Association class III and IV), previous stroke incident, thrombosis incident; (2) traumatic nerve injury in affected limb (3) unregulated hypertension (systolic ≥180 or diastolic ≥110 mm Hg) (4) spinal cord injury; (5) planned other lower limb surgery within 12 months; (6) cancer diagnosis and currently undergoing chemotherapy-, immunotherapy or radiotherapy; (7) inadequacy in written and spoken Danish; (8) an existing prosthesis in the index limb; (9) living more than 45 min from either Horsens Regional Hospital or Silkeborg Regional Hospital and (10) pregnancy.
All patients will be screened for eligibility by four orthopaedic chief physicians at Horsens Regional Hospital and by three orthopaedic chief physicians at Silkeborg Regional Hospital who will perform the initial inclusion of study participants and hand out written project information. All patients accepting to participate will be asked to complete a written informed consent allowing the physiotherapist (at Horsens Regional Hospital and Silkeborg Regional Hospital) to contact the patients by phone for a final eligibility and exclusion criteria-screening and book an appointment for baseline testing. If the patient agrees to participate in the trial, he/she will sign a written informed consent to participate in the project. Subsequently, the patient will be baseline tested at the hospital by a blinded (to group allocation) assessor. Patients declining to participate in the RCT will be offered the option of participating in a parallel observational cohort trial. All patients included in the project will be scheduled for a TKR. Two to three weeks before surgery, all patients will be invited to a, preoperative information meeting where nurses, surgeons and physiotherapists will provide detailed information on pain management, nutrition, the surgical procedure, physical activity, postoperative home-based rehabilitation (table 1A,1B), load management (usual care).48 On the day of surgery, patients will be hospitalised at Horsens Regional Hospital or Silkeborg Regional Hospital where an orthopaedic chief physician will perform the TKR procedure. The day after surgery all patients will receive physiotherapy-supervised training once or twice per day by a physiotherapist in order to fulfil the discharge criteria (table 2).48 Patients will generally be discharged within 1–2 days after fulfilling all the discharge criteria listed above. After discharge, all patients will receive a standard home-based rehabilitation programme focusing on improving knee joint mobility, increasing the tolerance for standing without assistive devices and lower extremity muscle strength. Variations in the selection of exercises and exercise variables exist in the standard home-based rehabilitation programmes between the respective hospitals; however, the purpose of the programmes is identical. If the patients do not fulfil the discharge criteria, they will be offered supervised knee-specific exercise therapy at a municipal rehabilitation centre or specialised hospital-based rehabilitation after discharge from the hospital.
Table 2.
Discharge criteria at Horsens regional hospital and Silkeborg regional hospital
| Outcome | Horsens Regional Hospital | Silkeborg Regional Hospital |
| Minimum knee flexion range of motion | 60° | 90° |
| Maximal knee extension deficit | 15° | 5° |
| In-and-out of bed | Independent | Independent |
| Sit-to-stand | Independent | Independent |
| Walking with/without assistive devices | Independent | Independent |
| Stair negotiation with/without assistive devices | Independent | Independent |
| Activities of daily living | Independent | Independent |
| Understanding of the home-based postoperative exercise programme | Sufficient | Sufficient |
Table 1A.
Postoperative rehabilitation programme, Horsens Regional Hospital
| Step | Exercise | Repetitions | Sets | Resistance |
| Week 0–3 | ||||
| Step 1 and 2 | Supine peristaltic pump exercise with feet above heart level | 20 min | 3–4/day | – |
| Step 1 | Supine knee extension mobilisation | 20 s | 3 sets | – |
| Step 1 | Supine unilateral knee and hip extension and flexion mobilisation with slipper under the heel | 5 repetitions | 3 sets | Slipper minimises floor friction |
| Step 2 | Seated knee extension and flexion mobilisation with slipper under the foot | 5 repetitions | 3 sets | Slipper minimises floor friction |
| Step 2 | Standing weight transfer exercise | 15 repetitions each side | 1 set | Body weight |
| Step 2 | Sit to stand from a high chair or the edge of table | 5 repetitions | 3 sets | Body weight |
| Week 3 and onwards | ||||
| Step 1 and 2 | Supine peristaltic pump exercise with feet above heart level | 20 min | 3–4/day | – |
| Step 1 | Seated knee extension mobilisation | 20 s | 4 rounds | Arms can be used to apply pressure onto the knee to help extend the knee |
| Step 1 | Step up exercise | 10–15 repetitions | 2–3 sets | Bodyweight |
| Step 1 | Standing knee isometric knee towel press | 10–15 repetitions | 2–3 sets | Ball/towel rolled together |
| Step 1 | Sit to stand from a chair | 10–15 repetitions | 2–3 sets | Body weight |
| Step 1 | One leg standing | 30 s | 1 set | Body weight |
| Step 2 | Standing hip flexion | Not informed | Not informed | Elastic band |
| Step 2 | Standing hip abduction | Not informed | Not informed | Elastic band |
| Step 2 | Partial frontal plane sliding lunge | 10 repetitions | 3 sets, 2–3/day | Body weight |
| Step 2 | Partial back sliding lunge | 10 repetitions | 3 sets, 2–3/day | Body weight |
| Optional | Cycling | 10–20 min | 1 set | Light resistance can be added when it is possible to perform a full round with the operated limb. |
Step 1 is performed in the morning and step 2 is performed in the afternoon. All exercises are performed once per day.
Table 1B.
Postoperative rehabilitation programme, Silkeborg Regional Hospital
| Step | Exercise | Repetitions | Sets | Resistance |
| Week 0–2 | ||||
| Optional | Cycling | 5–10 min | 2/day | |
| – | Supine peristaltic pump exercise | Not informed | Not informed | – |
| – | Rest with leg above heart level | 30 min | 4/day | – |
| – | Seated isometric knee extension | 3 s | 10 sets | Lower leg and the foot |
| – | Seated knee flexion mobilisation | 3 s | 10 sets | – |
| – | Seated knee extension mobilisation | 30 s | 3 sets | Apply pressure to the knee joint using the arms |
| – | Supine isometric knee extension | 3 s | 10 sets | Lower leg and the foot |
| – | Supine passive knee extension mobilisation | Gravity will extend the knee joint | ||
| Week 2 and onwards | ||||
| – | Supine knee isometric knee towel press | 3 s hold | 10 sets | Lower leg and the foot |
| – | Sit to stand | 10 repetitions | 1 set | Body weight |
| – | Standing knee flexion mobilisation | 3 s | 10 sets | Body weight |
| – | Step up exercise | 10 repetitions | 1 set | Body weight |
All exercises are performed twice per day. Cycling ergometer exercise is optional.
Randomisation
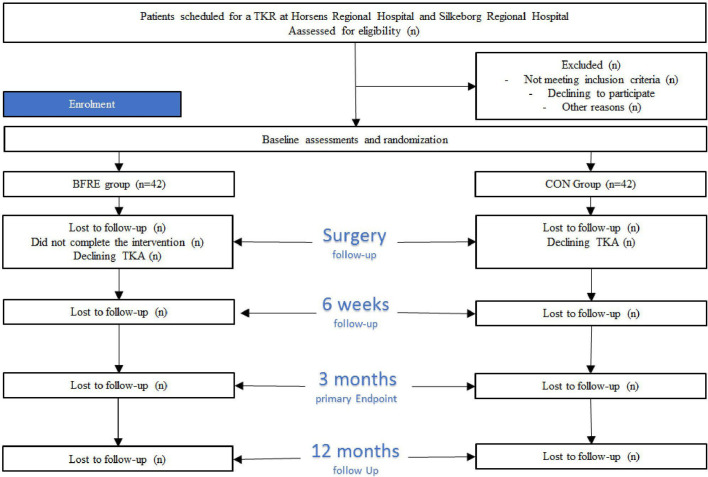
After baseline assessment, patients will be randomised (1:1) using the Research Electronic Data Capture (REDCap) randomisation system to either the training (BFRE) group or the control (CON) group. Prior to randomisation, all patients will be booked for follow-up test sessions and surgery. All randomisation procedures will be performed by the physiotherapists in charge of the BFRE training. Assessors performing the tests will be blinded to group allocation until completion of the trial. A flow chart of the patient allocation procedures is depicted in figure 1.
Figure 1.
Flow chart of the enrolment, treatment and follow-up phases. BFRE, low-load blood flow restricted exercise; CON, control; TKR, total knee replacement.
CON group
Participants in CON will receive usual care (see above) prior to TKR and be encouraged to continue their usual lifestyle up until TKR.
BFRE group
In addition to receiving usual care (cf. above), participants in the BFRE group will perform supervised BFRE sessions three times per week for 8 weeks supervised by a physiotherapist educated in administering BFRE. All BFRE training will be performed at Horsens Regional Hospital and Silkeborg Regional Hospital.
Intervention procedures
BFRE
Each BFRE session will consist of a 10 min warm up (ergometer cycling), followed by two different unilateral lower-limb-resistance training exercises: (1) leg press and (2) knee extension performed on standard strength training machines. Each exercise will be performed with the affected lower limb only and consist of four rounds interspaced by 30 s of rest (table 3). First round: 30 repetitions (reps); second round: 15 reps; third round: 15 reps; fourth round: until exhaustion (table 1A,1B). If patients can perform more than 15 repetitions in the fourth exercise set, the exercise load will be increased with the minimum extra load possible.30 Participants will be instructed to perform both the eccentric and concentric contraction phases using a steady 2 s pace duration. The fourth and final exercise set will be performed to the point of exhaustion defined as being unable to complete the final concentric contraction phase in 2 s. During the 30 s rest period, patients will rest in a standardised resting position while maintaining the initial cuff-pressure. Between each exercise, patients will have a 5 min ‘free-flow’ rest period. The 5 min rest period applied between exercises was chosen based on experiences from a previous pilot project (Jorgensen & Bohn 2019, unpublished data) and experience with applying BFRE in clinical practice. In both situations, we often experienced that patients stayed seated in the leg press machine for >2 min after the last (fatiguing) set to feel sufficiently rested and confident to walk from one exercise machine to another. The cuff will be released immediately after completion of the final exercise set.
Table 3.
Exercise variables for the blood-flow restricted exercise (BFRE) protocol
| Exercise variable | Weeks 1–8 |
| Level of LOP | 60% LOP |
| Sets | 4 |
| Load intensity | 30% 1RM |
| Repetitions 1st set | 30 |
| Repetitions 2nd and 3rd set | 15 |
| Repetitions 4th set | To volitional failure |
| Contraction modes per repetition | |
| Concentric | 2 s |
| Isometric | 0 s |
| Eccentric | 2 s |
| Rest between repetitions | 0 s |
| Time under tension per repetition | 4 s |
| Range of movement | Maximum |
| Rest between sets | 30 s |
| Rest between sessions | ≥36 hours |
| Progression | The minimal possible load (5 kg) is added when patients perform >15 repetitions in 4th set |
LOP, total limb occlusion pressure; RM, repetition maximum.
The occlusion pressure during both exercises will be set at 60% of LOP and the starting load intensity will be 30% with 1 repetition maximum (1RM) in both exercises.
Individual LOP will be determined using a pneumatic, conically shaped, 12 cm wide, rigid cuff (Occlude Aps, Denmark) attached to the patient’s most proximal area of the thigh on the affected side. While sitting on an examination table with the ankle and 1/3 of the lower limb off the table, a vascular Doppler probe (EDAN Instruments, China) will be placed posterior to the medial malleolus over the posterior tibial artery to capture the auscultatory pulse. To determine the cuff pressure (mm Hg) needed for total blood flow occlusion, the cuff will gradually be inflated in 20 mm Hg steps until reaching the pressure where the auscultatory pulse is interrupted (ie, LOP). The first time the auscultatory pulse is interrupted, the examiner releases 10–20 mm Hg pressure from the cuff until the auscultatory pulse is present again. When the auscultatory pulse reappears, the cuff is inflated with 10 mm Hg until the LOP is found again. If the second LOP is identical to the first, it will be defined as the LOP for that specific patient. Otherwise, the procedure will be repeated until determining an identical LOP two consecutive times.
Outcome variables
Outcome assessments will be performed at baseline (before randomisation), 3–4 days before surgery, 6 weeks after TKR, 3 months after TKR and 12 months after TKR. To reduce the number of postoperative visits, only questionnaires; The Knee disability and Oteoarthritis Outcome Score (KOOS), EuroQol Group 5-dimensions-Level 5 (EQ-5D-L5) and reporting of adverse event or receiving supervised physiotherapy postoperatively will be sent via email 6 weeks after surgery. Two testers (two trained physiotherapists) blinded to group allocation will perform all baseline and follow-up measurements. Bergström needle muscle biopsies49 will be taken from vastus lateralis of the quadriceps muscle in both lower limbs from patients included at Horsens Regional Hospital only at baseline, during surgery, and 3 months after TKR by doctors trained in performing the procedure. An overview of the data collection parameters is presented in table 4.
Table 4.
Outcome measures to be collected
| Outcome measures | Data collection instrument | Time points of assessment |
| Primary outcome | ||
| Sit-to-stand function | 30 s chair stand test | B, S, 3 and 12 months |
| Secondary outcomes | ||
| Ambulatory capacity | Timed up and go | B, S, 3 and 12 months |
| Gait speed | 4x10 m walk test | B, S, 3 and 12 months |
| 1RM leg press strength | Leg press machine | B, S, 3 and 12 months |
| 1RM knee extension strength | Knee extension machine | B, S, 3 and 12 months |
| Isometric knee extensor muscle strength | Handheld Dynamometer | B, S, 3 and 12 months |
| Isometric knee flexion muscle strength | Handheld Dynamometer | B, S, 3 and 12 months |
| Myofiber morphology | Muscle Biopsies | B, S, 3 months |
| Myogenic stem cell content | Muscle Biopsies | B, S, 3 months |
| Pain | KOOS | B, S, 6 weeks, 3 and 12 months |
| Symptoms | KOOS | B, S, 6 weeks, 3 and 12 months |
| Activities of daily living | KOOS | B, S, 6 weeks, 3 and 12 months |
| Sports and recreation | KOOS | B, S, 6 weeks, 3 and 12 months |
| Quality of life | KOOS | B, S, 6 weeks, 3 and 12 months |
| Socioeconomic costs | EQ-5D | B, S, 6 weeks, 3 and 12 months |
| Adverse events | Questionnaire and medical records | 3 months |
| Exercise compliance and progression | Physiotherapist records | BFRE |
| Pain during visits | NRS for pain | B, BFRE, S, 3 and 12 months |
| Declining to be operated | Questionnaire | 3 months |
| Postoperative supervised physiotherapy | Questionnaire | 6 weeks, 3 and 12 months |
| Knee joint range of motion | Goniometer | B, S, 3 and 12 months |
| Patient characteristics and related | Questionnaire | B |
| Measurements | Questionnaire | B |
| Gender | Tape measure | B |
| Age | Electronic body mass scale | B |
| Height | Questionnaire | B |
| Body mass | Questionnaire | B |
| Civil status | Questionnaire | B |
| Educational level | Questionnaire | B |
| Employment status | Questionnaire | B |
| Substance use (alcohol, smoking) | Questionnaire | B |
| Duration of knee symptoms | Questionnaire | B |
| Pain medication during the last week | Questionnaire | B |
| Comorbidities | Questionnaire | B |
B, baseline; BFRE, low-load blood flow restricted exercise; D, during surgery; EQ-5D, EuroQol Group 5-dimension; KOOS, knee disability and osteoarthritis outcome score; 12 months, 12 months after TKR; 3 months, 3 months after TKR; NRS, Numeric Rating Scale; RM, repetition maximum; S, 0–2 days before surgery.
Before starting the baseline testing, all assessors will be thoroughly trained in performing the tests according to the standardised test procedures for each test method. All assessors will be blinded to intervention allocation (presurgery BFRE training or usual care). Further, assessors will be trained in how to communicate with the participants at follow-up test sessions to avoid break of blinding due to miscommunication. Also, all cases where blinding is being broken will be registered. Also, the physiotherapist in charge of LL-BFRE will be thoroughly trained in performing the exercise on healthy subjects before applying LL-BFRE on study-patients. At the last scheduled exercise session (ie, 24th session), the physiotherapists in charge of LL-BFRE will carefully remind the participants not to reveal their group allocation to any assessors at any time point during post-testing.
The primary investigator will be in weekly contact with the physiotherapists supervising the LL-BFRE at Horsens Regional Hospital and Silkeborg Regional Hospital where day-to-day-retraining and supervision can be arranged. Furthermore, physiotherapists supervising the LL-BFRE will receive in-depth retraining every 3 months.
Outcomes
Primary outcome
The 30s-CST
The 30s-CST will be assessed using a 44 cm (seat height) chair with armrests. The 30s-CST measures the number of sit-to-stand repetitions completed within 30 s. The 30s-CST is considered a valid and sensitive measure of lower-extremity sit-to-stand function with good to excellent intraobserver and interobserver reliability.50–52
Secondary outcomes
The timed up and go test
The timed up and go test (TUG) assesses the time required for patients to stand from a 44 cm (seat height) chair walk around a tape mark 3 m away and sit into the chair at return. The patients will be instructed to walk as fast and safely as possible towards the tape mark (and touch the tape mark (with at least one foot), turn around and return to the chair and sit down. Use of armrests is allowed. The fastest of two trials will be used for further analysis. Up to 1 min of rest will be allowed between trials.53 54 Good inter-rater reliability has been demonstrated with the TUG test.52
4×10 m walk test
4×10 m walk test (40m-FWT) measures the total time it takes to walk 4×10 m excluding turns (m/s).52 Patients will be instructed to walk as quickly and as safely as possible without running to a visible mark 10 metres away, return and repeat for a total distance of 40 m.52 Prior to the test, one practice trial will be provided to check understanding. The 40m-FWT is a valid and responsive measure for assessing short distance maximum walking speed with excellent inter-rater reliability.52
1RM leg press strength
1RM leg press strength will be estimated from a 5-8RM leg press test. Patients perform three low-load warm-up sets. The first and second warm-up sets consist of 12 repetitions, and the third warm-up set consists of eight repetitions. The load of each warm-up set will be increased with 10 kilos. After warm-up, the load will be increased to determine the 5RM. If the 5RM cannot be determined within three trials, a fourth all-out trial (as many repetitions as possible) will be performed. The 1RM will be calculated as [1RM=load (kg)/1.0278–0.0278·number of repetitions)].55
1RM knee extension strength
1RM knee extension strength will be estimated from 5-8RM knee extension test as described above for the estimation of 1RM leg press test (55).
Maximal isometric voluntary contraction of the knee
Maximal isometric voluntary contraction (MVC) of the knee will be measured using a handheld dynamometer (HHD). The patients will be seated on an examination table with knees and hips positioned at 90° flexion. The patients will be instructed to remain seated in an upright position and place both hands on the shoulder to avoid compensation. The HHD will be fixed with a rigid belt to the examination table. Adjustable straps will be used to allow MVCs of the knee extensors to be performed at 90° knee flexion in all patients. The HDD will be positioned 5 cm above the medial malleolus.56 The patients will be instructed to produce as much force as possible into the HHD. Good to excellent inter-rater and intrarater reliability has previously been demonstrated on group-level in patients suffering from knee OA for maximum knee extensor muscle strength testing with HDD.56 Patients will receive four trials. For analysis, the mean maximal strength of the second, third and fourth measures will be calculated and corrected for bodyweight56
MVC of the knee flexors
MVC of the knee flexors will be measured and performed using HHD at 90° knee flexion with the patients seated identically as during MVC for the knee extensors.56 The HHD will be positioned posterior aspect of calcaneus56 and patients will be instructed to produce as much force as possible into the HHD. Good to excellent inter-rater and intrarater reliability has previously been demonstrated on group-level in patients suffering from knee OA for maximum knee flexor muscle strength testing with HDD.56 Patients will receive four trials. For analysis, the mean maximal strength of the second, third and fourth measures will be calculated and corrected for bodyweight56
Myofiber cross-sectional area, muscle fibre type composition, SC content and myonuclei number
Myofiber cross-sectional area (CSA), muscle fibre type composition, SC content and myonuclei number will be assessed by obtaining needle biopsies (100–150 mg) from all patients enrolled at Horsens Regional Hospital. The biopsies will be obtained bilaterally from the middle portion of the vastus lateralis muscle using the percutaneous needle biopsy technique of Bergström.49 57 58 Biopsies will be performed by two experienced orthopaedic surgeons (chief physicians) trained in performing the needle muscle biopsy technique at Horsens Regional Hospital. Efforts will be made to extract tissue from the same region (2–3 cm apart) and depth (~1–2 cm).49 The tissue samples will be dissected of all visible blood, adipose tissue and connective tissue and mounted in Tissue-Tec (4583, Sakura Finetek, Alphen aan den Rijn, The Netherlands), frozen in isopenate precooled with liquid nitrogen, and stored at −80°C.31 49 59 All muscle samples will be analysed as previously described by Nielsen et al31 using immunofluorescence microscopy. Transverse serial sections (8 µm) of the embedded muscle biopsy specimen will be cut at −22°C using a cryostat (HM560; Microm, Walldorf, Germany) and will be mounted on glass slides for subsequent analysis as described in detail elsewhere.31 Myogenic stem cells ((SC) will be visualised with an antibody against Pax7.31 Type I (stained) and type II (unstained) myofibers will be differentiated, and muscle fibre area will be determined31: MSC-derived nuclei will stain positive for Pax7 and be within the basal lamina; nuclei (DAPI stained) with a sublaminar placement will be considered myonuclei.31
Knee disability and osteoarthritis outcome score
KOOS is a patient-administered knee-specific questionnaire comprising five subscales: Pain; Symptoms; Activities of daily living; Sport & Recreation and Knee-Related Quality of Life. Each item is scored from 0 to 4.60 The raw score for each of the five subscales is the total sum of the associated item scores. Scores can be transformed to a 0–100 scale. The scores of the five subscales can be expressed as a composite outcome profile, higher scores indicating fewer problems.61 The KOOS questionnaire is valid and reliable in patients suffering from knee OA and patients on the waiting list for TKA for knee OA.60 62 63
EuroQol Group 5-dimension-Level 5
EQ-5D-5L is a self-completion questionnaire consisting of two parts; the first part of the EQ-5D-5L comprises five dimensions involving mobility, self-care, usual activities, pain/discomfort and anxiety/depression. All dimensions have five response categories (no problems, slight problems, moderate problems, severe problems and extreme problems) resulting in a five digit descriptive health state,64 which will be converted into a summary index ranging from −0.624 (worst) to 1.000 (best), using a Danish value set.65 The second part, EQ-VAS rates the overall current health status from 0 (worst imaginable health) to 100 (best imaginable health).64 The EQ-5D-5L is reliable and valid in patients with knee OA eligible for TKA.66 67
Adverse events
Adverse events will be defined as unpredicted or unintended events, signs or disease occurring during the period from inclusion until the 3-month follow-up (primary endpoint) resulting in contact with the healthcare system (hospital or general practitioner) independent of whether or not the event is related to the intervention or outcome assessments. Adverse events will be recorded and categorised in accordance with the definitions established by the US Food and Drug Administration. Continuous registration of adverse events will be performed and a short open-ended questionnaire will be administered at 3 months follow-up.
Other outcome measures
Blood pressure
Blood pressure will be measured by the orthopaedic chief physicians when patients are visiting the outpatient clinic. Blood pressure will be used to determine eligibility to participate in the project.
Exercise compliance and progression
Exercise compliance and progression will be obtained by the physiotherapist in charge of the training sessions and entered directly into the REDCap-system. The progression will be monitored as the total load lifted by the patient for exercise session.
Numeric rating scale for pain
Numeric Rating Sscale (NRS) for pain is a segmented unidimensional 11-item measure of pain intensity in adults68 that will be used to rate pain intensity during both testing and exercise sessions.68 The number ‘0’ represents no pain while ‘10’ represents worst pain imaginable.68
Declining to be operated
Declining to be operated will be measured at 3-month follow-up, where patients will be asked whether they decided to be operated or not. Patients who declined to be operated will be invited to participate in all prescheduled follow-up assessments.
Postoperative supervised physiotherapy
Postoperative supervised physiotherapy will be measured at 6 weeks, 3 months and 12 months follow-up by answering a questionnaire. If patients have participated in postoperative supervised physiotherapy, the patient must specify whether the treatment was related to the TKR or due to other circumstances.
Knee joint active range of motion
Knee joint active range of motion will be measured with a 360° plastic goniometer (scale 1°) with 16.5 cm moveable arms at baseline in the week of surgery, 3 months, and 12 months after surgery. Laying supine on an examination table, the knee joint flexion and knee joint extension will be measured separately.69 The tester then identifies the most prominent part of the trochanter, the lateral epicondyle of the femur, the lateral head of fibula and the lateral malleolus. When identified, the patient is asked to flex the knee as much as possible with the heel maintaining contact to the surface at all time.69 Second, the patients will be asked to extend the knee joint as much as possible. To allow the knee to extend as much as possible, a firm quadratic box (height: 5 cm, width: 8 cm, length: 15 cm) will be placed under the heel of the patient. The procedure of measuring knee extension will be similar to knee flexion, as the patients increases the degree of knee extension maximally69 The fulcrum of the goniometer will correspond visually to the transepicondylar axis of the knee joint. The moveable arms of the goniometer will be pointed towards the greater trochanter and the lateral malleolus.69
Data management
All data from the physical function tests will be entered into RedCap by the assessors using double data entry to ensure data quality. All patient-reported outcome data (KOOS, NRS Pain, EQ-5D-5L) will be entered directly into RedCap by the patients, and usage of the ‘required fields’ will ensure no missing items from the completed questionnaires. To reduce missing data, a reminder email will be sent automatically from the RedCap-system. All patient data will be anonymised by assigning study numbers to each patient (coding). Personal data about the patient will be located separately from the main dataset to protect confidentiality during all trial phases.
The raw dataset will be maintained for ten years after completion of the trial with indefinite restricted access due to sensitive data. After publication of the trial, a fully anonymised patient-level dataset and corresponding statistical description will be made publicly available if required by the scientific journal, in which the results are published.
Sample size
The power and sample size calculation is based on the expected differences between the two subject groups from baseline to 3-month follow-up.8 Due to lack of data on the primary outcome for investigations applying LL-BFRE before a surgical procedure, we decided to base our sample size calculation on Skoffer et al8 who investigated the efficacy of 4 weeks of preoperative and 4 weeks postoperative HRST (intervention group) compared with 4 weeks of postoperative HRST only (CON group) on 30 s CST 3 months in patients receiving a TKR.8 The authors found a between-group difference of 3–4 repetition difference (14.7±4.7 repetitions vs 11.0±4.4 repetitions) 3 months after TKR surgery.8
To reduce the probability of type I errors and enable detection of a between-group difference also, α-level is set at 0.05 (p<0.05) and β-level is set at 0.20 (80% power). Expecting a 3-repetition between-group difference 3 months postoperatively and assuming an SD of 4.7 in both groups, 39 patients are required in each group (yielding 78 patients in total). With an anticipated dropout rate of 10%, 84 patients will be recruited for the trial.
Statistical considerations
The primary efficacy analysis will be an assessment of the between group difference in change in the 30 s CST from baseline to 3-month follow-up (primary endpoint).
All descriptive statistics and tests will be reported in accordance with the recommendations of the ‘Enhancing the QUAlity and Transparency Of health Research’ network70 and the CONSORT statement.47 Intention-to-treat principle (ie, all patients as randomised independent of departures from allocation treatment, compliance and/or withdrawals) and per-protocol analysis will be conducted. A one-way analysis of variance model will be used to analyse between group mean changes in continuous outcome measures.31 The model includes changes from baseline to 12 month follow-up. Between-intervention comparison from baseline to 3 months after surgery will be analysed using a mixed linear model with patient ID as a random effect and time, group and hospital as fixed effects.31 71 Also, to gain insight into the potential pretraining-to-post-training differences within the respective training or CON groups, paired Student's t-test will be performed. Level of statistical significance is p<0.05.
Secondary outcome variables: Between-intervention comparison from baseline to the week of surgery, 6 weeks after surgery, three and 12 months after surgery will be analysed as described for the primary outcome. Regression analysis will be used to analyse the potential associations between preoperative strength and postoperative lower extremity function and self-reported outcome as well as between preoperative functional capacity and postoperative functional capacity. Additionally, regression analysis will be used to analyse the association between preoperative number of SCs and myonuclei on postoperative isometric knee extensor muscle strength, muscle fibre CSA, and functional capacity. All statistical analyses will be performed by the primary investigator using Stata (Stata 16.1, StataCorp LLC, Texas, USA).
Ethical aspects and dissemination
The trial has been accepted by the Central Denmark Region Committee on Biomedical Research Ethics (Journal No 10-72-19-19) and by the Danish Data Protection Agency (Journal No 652164). Before inclusion, all patients will provide their written informed consent in accordance with the Declaration of Helsinki. All data and information collected in regard to this trial will be treated confidentially (blinded and encrypted) by the researchers and staff connected to the trial.
All results from the trial will be published in international peer-reviewed scientific journals regardless of the results being considered positive, negative or inconclusive.
Patient and public involvement
Before developing this clinical trial, a pilot project was performed to determine the feasibility and efficacy of BFRE in patients suffering from lower limb injuries. The experiences with the training modality and the verbal feedback from patients on training duration, frequency and intensity resulted in useful knowledge that certainly has improved the development of the present clinical trial.
Discussion
To the best of our knowledge, this is the first trial to investigate the effect of preoperative BFRE on functional capacity, self-reported outcome, lower limb muscle strength and myofiber morphology/stem cell abundance in patients scheduled for TKR. Only few studies have investigated (short-term [10 days]) preoperative BFRE without finding an atrophy protective effect or difference in muscle strength compared with a CON group performing a placebo intervention (SHAM group).72 However, patients performing short term preoperative BFRE before ACL-R demonstrated higher muscle endurance compared with a SHAM group.73 Therefore, results of this trial are expected to provide novel information on longer periods of BFRE that will enable researchers to design effective exercise-based preconditioning protocols for elective TKR patients. The LL-BFRE protocol applied in the present project is widely used and follows the recommendations from a recent position stand by Patterson et al.74 The authors suggested that exercising 2–3 times per week at 20%–40% of 1RM in 2–4 sets (eg, 30-15-15-15 or sets to failure) using pressures between 40% and 80% of LOP has demonstrated to be effective when aiming at increasing muscle strength and promoting muscle hypertrophy.74
The trial is designed as an assessor blinded randomised controlled trial, thus representing the highest evidence level. However, the nature of the trial does not allow blinding of the participants which is an inherent limitation of the trial. The trial is conducted at two hospitals that consistently perform a high number of TKR procedures annually (225 and 460, respectively), thus securing a strong expertise in terms of surgery and infrastructure. Both hospitals have all equipment needed available for surgery, postoperative hospitalisation, training and testing. All outcome variables are considered valid and reliable measures and consist of both objective outcomes and self-reported patient outcomes.
No adverse health-related events have been reported in previous studies applying BFRE in patients’ suffering from knee OA or in healthy older adults.1 9 13 23 33 34 Further, in a recent review and meta-analysis, it was stated that exercise with concurrent blood-flow restriction is a safe exercise modality when occlusion procedures are applied correctly.13 The inherent invasive procedure of muscle biopsies may cause adverse events in rare occasions. Therefore, all muscle biopsy samples will be collected by trained medical doctors and performed following administration of local anaesthesia and in fully sterile conditions. The needle muscle biopsy protocol has been applied in a large number of previous investigations including very old frail subjects (97 years of age) without any reporting of adverse events besides occasional muscle soreness.31 49 57 75 76
There are some limitations of the project that must be taken into account. First, our primary end point is 3 months postoperatively. The (uncontrolled) period discharge to 3 months postoperatively renders the project vulnerable to external variabilities. However, from a pragmatic point of view, this uncontrolled period from discharge to 3-month follow-up reflects the reality that Danish patients face postoperatively. Thus, the results at 3-month follow-up will, indeed, reflect the impact of performing preoperative LL-BFRE on the postoperative outcome regardless of the external variable that can hamper the results. Second, the discharge criteria at Horsens Regional Hospital and Silkeborg Regional Hospital withhold slight differences. That is, the acceptable knee joint ROM at discharge differs between the sites, thus it can be speculated that more patients from Silkeborg Regional Hospital will be offered a postoperative, supervised rehabilitation programme. This might affect the number of patients receiving supervised physiotherapy after discharge between sites. However, all patients included in the present project will report whether they have received postoperative supervised physiotherapy at all follow-up assessments. Thus, we will be able to determine (and normalise) a potential between-site difference in patients receiving supervised physiotherapy after TKR. Also, site-specific differences in the postoperative rehabilitation protocols (table 1A,1B) may be considered a limitation. That is, the protocols contain both identical but also different exercises and progression steps. However, a recent review and meta-analysis found no difference in effectiveness between clinic-based or inpatient programmes compared with home-based rehabilitation programmes in the early subacute period after TKA27 and studies in other knee patient populations have also been unable to observe differences in main outcome variables when comparing home-based postoperative rehabilitation to supervised postoperative rehabilitation.28 29 We feel confident, therefore, that the apparent differences between the postoperative rehabilitation protocols are not highly likely to affect the results of the present study. Nonetheless, to verify this notion we will introduce site allocation (Horsens Hospital vs Silkeborg Hospital) as a separate independent variable in the mixed linear model used for the statistical analysis.
Supplementary Material
Footnotes
Contributors: SLJ, PA, MBB and IM were all part of designing the trial and approved the final version of the protocol. Also, SLJ, PA, MBB and IM wrote and revised the protocol.
Funding: This work trial is supported by Aase og Ejnar Danielsen’s Foundation (100,000 dkk), Nis-Hanssen’s Mindeslegat (163,883 dkk) and the Health Research Foundation of Central Denmark Region (99,658 dkk), Hede-Nielsen Foundation (8,000 dkk).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ferraz RB, Gualano B, Rodrigues R, et al. Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med Sci Sports Exerc 2018;50:897–905. 10.1249/MSS.0000000000001530 [DOI] [PubMed] [Google Scholar]
- 2.Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010;40:559–67. 10.2519/jospt.2010.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. 21, 2015. 10.1002/14651858.CD004376.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skoffer B, Dalgas U, Mechlenburg I. Progressive resistance training before and after total hip and knee arthroplasty: a systematic review. Clin Rehabil 2015;29:14–29. 10.1177/0269215514537093 [DOI] [PubMed] [Google Scholar]
- 5.Sundhedsstyrelsen Knæartrose - nationale kliniske retningslinjer og faglige visitationsretningslinjer, 2012. [Google Scholar]
- 6.Franz A, Queitsch FP, Behringer M, et al. Blood flow restriction training as a prehabilitation concept in total knee arthroplasty: a narrative review about current preoperative interventions and the potential impact of BFR. Med Hypotheses 2018;110:53–9. 10.1016/j.mehy.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 7.Calatayud J, Casaña J, Ezzatvar Y, et al. High-Intensity preoperative training improves physical and functional recovery in the early post-operative periods after total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2017;25:2864–72. 10.1007/s00167-016-3985-5 [DOI] [PubMed] [Google Scholar]
- 8.Skoffer B, Maribo T, Mechlenburg I, et al. Efficacy of preoperative progressive resistance training on postoperative outcomes in patients undergoing total knee arthroplasty. Arthritis Care Res 2016;68:1239–51. 10.1002/acr.22825 [DOI] [PubMed] [Google Scholar]
- 9.Bryk FF, dos Reis AC, Fingerhut D, et al. Exercises with partial vascular occlusion in patients with knee osteoarthritis: a randomized clinical trial. Knee Surgery, Sports Traumatology, Arthroscopy 2016;24:1580–6. 10.1007/s00167-016-4064-7 [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Lee M, Zhang Z, et al. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2016;6:e009857. 10.1136/bmjopen-2015-009857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesham RA, Shanmugam S. Does preoperative physiotherapy improve postoperative, patient-based outcomes in older adults who have undergone total knee arthroplasty? A systematic review. Physiother Theory Pract 2017;33:9–30. 10.1080/09593985.2016.1230660 [DOI] [PubMed] [Google Scholar]
- 12.Kwok IHY, Paton B, Haddad FS. Does pre-operative physiotherapy improve outcomes in primary total knee arthroplasty? A systematic review. J Arthroplasty 2015;30:1657–63. 10.1016/j.arth.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 13.Hughes L, Paton B, Rosenblatt B, et al. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med 2017;51:1003–11. 10.1136/bjsports-2016-097071 [DOI] [PubMed] [Google Scholar]
- 14.Counts BR, Dankel SJ, Barnett BE, et al. Influence of relative blood flow restriction pressure on muscle activation and muscle adaptation. Muscle Nerve 2016;53:438–45. 10.1002/mus.24756 [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Loenneke JP, Ye X, et al. Low-load resistance training with low relative pressure produces muscular changes similar to high-load resistance training. Muscle Nerve 2017;56:E126–33. 10.1002/mus.25626 [DOI] [PubMed] [Google Scholar]
- 16.Loenneke JP, Fahs CA, Rossow LM, et al. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol 2012;112:2903–12. 10.1007/s00421-011-2266-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loenneke JP, Kim D, Fahs CA, et al. The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. Clin Physiol Funct Imaging 2017;37:734–40. 10.1111/cpf.12367 [DOI] [PubMed] [Google Scholar]
- 18.Loenneke JP, Kim D, Fahs CA, et al. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve 2015;51:713–21. 10.1002/mus.24448 [DOI] [PubMed] [Google Scholar]
- 19.Loenneke JP, Thrower AD, Balapur A, et al. Blood flow-restricted walking does not result in an accumulation of metabolites. Clin Physiol Funct Imaging 2012;32:80–2. 10.1111/j.1475-097X.2011.01059.x [DOI] [PubMed] [Google Scholar]
- 20.Loenneke JP, Wilson JM, Balapur A, et al. Time under tension decreased with blood flow-restricted exercise. Clin Physiol Funct Imaging 2012;32:268–73. 10.1111/j.1475-097X.2012.01121.x [DOI] [PubMed] [Google Scholar]
- 21.Loenneke JP, Wilson JM, Wilson GJ, et al. Potential safety issues with blood flow restriction training, 2011: 510–8. [DOI] [PubMed] [Google Scholar]
- 22.Loenneke JP, Young KC, Fahs CA, et al. Blood flow restriction: rationale for improving bone. Med Hypotheses 2012;78:523–7. 10.1016/j.mehy.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 23.Ozaki H, Loenneke JP, Abe T. Blood flow-restricted walking in older women: does the acute hormonal response associate with muscle hypertrophy? Clin Physiol Funct Imaging 2017;37:379–83. 10.1111/cpf.12312 [DOI] [PubMed] [Google Scholar]
- 24.Ozaki H, Loenneke JP, Thiebaud RS, et al. Possibility of leg muscle hypertrophy by ambulation in older adults: a brief review. Clin Interv Aging 2013;8:369–75. 10.2147/CIA.S43837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott BR, Loenneke JP, Slattery KM, et al. Blood flow restricted exercise for athletes: a review of available evidence. J Sci Med Sport 2016;19:360–7. 10.1016/j.jsams.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 26.Scott BR, Loenneke JP, Slattery KM, et al. Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med 2015;45:313–25. 10.1007/s40279-014-0288-1 [DOI] [PubMed] [Google Scholar]
- 27.Takarada Y, Nakamura Y, Aruga S, et al. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol 2000;88:61–5. 10.1152/jappl.2000.88.1.61 [DOI] [PubMed] [Google Scholar]
- 28.Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 2002;86:308–14. 10.1007/s00421-001-0561-5 [DOI] [PubMed] [Google Scholar]
- 29.Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc 2000;32:2035–9. 10.1097/00005768-200012000-00011 [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen AN, Aagaard P, Nielsen JL, et al. Effects of blood-flow-restricted resistance training on muscle function in a 74-year-old male with sporadic inclusion body myositis: a case report. Clin Physiol Funct Imaging 2016;36:504–9. 10.1111/cpf.12259 [DOI] [PubMed] [Google Scholar]
- 31.Nielsen JL, Aagaard P, Bech RD, et al. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol 2012;590:4351–61. 10.1113/jphysiol.2012.237008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen JL, Aagaard P, Prokhorova TA, et al. Blood flow restricted training leads to myocellular macrophage infiltration and upregulation of heat shock proteins, but no apparent muscle damage. J Physiol 2017;595:4857–73. 10.1113/JP273907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segal N, Davis MD, Mikesky AE. Efficacy of blood flow-restricted low-load resistance training for quadriceps strengthening in men at risk of symptomatic knee osteoarthritis. Geriatr Orthop Surg Rehabil 2015;6:160–7. 10.1177/2151458515583088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segal NA, Williams GN, Davis MC, et al. Efficacy of blood flow–restricted, low‐load resistance training in women with risk factors for symptomatic knee osteoarthritis. PM&R 2015;7:376–84. 10.1016/j.pmrj.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jessee MB, Mattocks KT, Buckner SL, et al. Mechanisms of blood flow restriction: the new Testament. Techniques in Orthopaedics 2018;33:72–9. [Google Scholar]
- 36.Mattocks KT, Jessee MB, Mouser JG, et al. The application of blood flow restriction: lessons from the laboratory. Curr Sports Med Rep 2018;17:129–34. 10.1249/JSR.0000000000000473 [DOI] [PubMed] [Google Scholar]
- 37.Wernbom M, Augustsson J, Raastad T. Ischemic strength training: a low-load alternative to heavy resistance exercise? Scand J Med Sci Sports 2008;18:401–16. 10.1111/j.1600-0838.2008.00788.x [DOI] [PubMed] [Google Scholar]
- 38.Wernbom M, Aagaard P. Muscle fibre activation and fatigue with low-load blood flow restricted resistance exercise-An integrative physiology review. Acta Physiol 2020;228:e13302. 10.1111/apha.13302 [DOI] [PubMed] [Google Scholar]
- 39.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 1961;9:493–5. 10.1083/jcb.9.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadi F, Charifi N, Denis C, et al. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 2004;29:120–7. 10.1002/mus.10510 [DOI] [PubMed] [Google Scholar]
- 41.Olsen S, Aagaard P, Kadi F, et al. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol 2006;573:525–34. 10.1113/jphysiol.2006.107359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francaux M, Deldicque L. Exercise and the control of muscle mass in human. Pflugers Arch - Eur J Physiol 2019;471:397–411. 10.1007/s00424-018-2217-x [DOI] [PubMed] [Google Scholar]
- 43.Kadi F, Schjerling P, Andersen LL, et al. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 2004;558:1005–12. 10.1113/jphysiol.2004.065904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bazgir B, Fathi R, Valojerdi MR, et al. Satellite cells contribution to exercise mediated muscle hypertrophy and repair, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Covinsky KE, Lindquist K, Dunlop DD, et al. Effect of arthritis in middle age on older-age functioning. J Am Geriatr Soc 2008;56:23–8. 10.1111/j.1532-5415.2007.01511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruusgaard JC, Johansen IB, Egner IM, et al. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci U S A 2010;107:15111–6. 10.1073/pnas.0913935107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moher D, Hopewell S, Schulz KF, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28–55. 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 48.Knæalloplastik MB. fysioterapeutisk instruks e-dok, 2019. Available: https://e-dok.rm.dk/edok/admin/GUI.nsf/Desktop.html?Open&login
- 49.Suetta C, Andersen JL, Dalgas U, et al. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. J Appl Physiol 2008;105:180–6. 10.1152/japplphysiol.01354.2007 [DOI] [PubMed] [Google Scholar]
- 50.Gill S, McBurney H. Reliability of performance-based measures in people awaiting joint replacement surgery of the hip or knee. Physiother Res Int 2008;13:141–52. 10.1002/pri.411 [DOI] [PubMed] [Google Scholar]
- 51.Jones CJ, Rikli RE, Beam WC. A 30-S chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–9. 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- 52.Wright AA, Cook CE, Baxter GD, et al. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther 2011;41:319–27. 10.2519/jospt.2011.3515 [DOI] [PubMed] [Google Scholar]
- 53.Bloch ML, Jønsson LR, Kristensen MT. Introducing a third timed up & go test trial improves performances of hospitalized and community-dwelling older individuals. J Geriatr Phys Ther 2017;40:121–6. 10.1519/JPT.0000000000000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kristensen MT, Ekdahl C, Kehlet H, et al. How many trials are needed to achieve performance stability of the Timed Up & Go test in patients with hip fracture? Arch Phys Med Rehabil 2010;91:885–9. 10.1016/j.apmr.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 55.Hansen H. RM-testmanal. Danish Physiotherapy Society, 2012: 2. [Google Scholar]
- 56.Koblbauer IFH, Lambrecht Y, van der Hulst MLM, et al. Reliability of maximal isometric knee strength testing with modified hand-held dynamometry in patients awaiting total knee arthroplasty: useful in research and individual patient settings? A reliability study. BMC Musculoskelet Disord 2011;12:249. 10.1186/1471-2474-12-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekblom B. The muscle biopsy technique. historical and methodological considerations. Scand J Med Sci Sports 2017;27:458–61. 10.1111/sms.12808 [DOI] [PubMed] [Google Scholar]
- 58.Bergstrom J. Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens. Scand J Clin Laborat Invest 1962;14. [Google Scholar]
- 59.Aagaard P, Andersen JL, Dyhre-Poulsen P, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol 2001;534:613–23. 10.1111/j.1469-7793.2001.t01-1-00613.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roos EM, Lohmander LS. The knee injury and osteoarthritis outcome score (KOOS): from joint injury to osteoarthritis, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilsdotter AK, Lohmander LS, Klässbo M, et al. Hip disability and osteoarthritis outcome score (HOOS)–validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 2003;4:10. 10.1186/1471-2474-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lyman S, Lee Y-Y, McLawhorn AS, et al. What are the minimal and substantial improvements in the HOOS and KOOS and jr versions after total joint replacement? Clin Orthop Relat Res 2018;476:2432–41. 10.1097/CORR.0000000000000456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins NJ, Prinsen CAC, Christensen R, et al. Knee injury and osteoarthritis outcome score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage 2016;24:1317–29. 10.1016/j.joca.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 64.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wittrup-Jensen KU, Lauridsen J, Gudex C, et al. Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health 2009;37:459–66. 10.1177/1403494809105287 [DOI] [PubMed] [Google Scholar]
- 66.Bilbao A, García-Pérez L, Arenaza JC, et al. Psychometric properties of the EQ-5D-5L in patients with hip or knee osteoarthritis: reliability, validity and responsiveness. Qual Life Res 2018;27:2897–908. 10.1007/s11136-018-1929-x [DOI] [PubMed] [Google Scholar]
- 67.Buchholz I, Janssen MF, Kohlmann T, et al. A systematic review of studies comparing the measurement properties of the three-level and five-level versions of the EQ-5D. Pharmacoeconomics 2018;36:645–61. 10.1007/s40273-018-0642-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (vas pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CpGs), short Form-36 bodily pain scale (SF-36 BPs), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011;63:S240–52. 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 69.Jakobsen TL, Christensen M, Christensen SS, et al. Reliability of knee joint range of motion and circumference measurements after total knee arthroplasty: does tester experience matter? Physiother Res Int 2010;15:126–34. 10.1002/pri.450 [DOI] [PubMed] [Google Scholar]
- 70.Christensen R, Bliddal H, Henriksen M. Enhancing the reporting and transparency of rheumatology research: a guide to reporting guidelines. Arthritis Res Ther 2013;15:109. 10.1186/ar4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malcata RM, Hopkins WG, Pearson SN. Tracking career performance of successful triathletes. Med Sci Sports Exerc 2014;46:1227–34. 10.1249/MSS.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 72.Grapar Zargi T, Drobnic M, Jkoder J, et al. The effects of preconditioning with ischemic exercise on quadriceps femoris muscle atrophy following anterior cruciate ligament reconstruction: a quasi-randomized controlled trial. Eur J Phys Rehabil Med 2016;52:310–20. [PubMed] [Google Scholar]
- 73.Žargi T, Drobnič M, Stražar K, et al. Short–term preconditioning with blood flow restricted exercise preserves quadriceps muscle endurance in patients after anterior cruciate ligament reconstruction. Front Physiol 2018;9:1150. 10.3389/fphys.2018.01150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patterson SD, Hughes L, Warmington S, et al. Blood flow restriction exercise: considerations of methodology, application, and safety. Front Physiol 2019;10:533. 10.3389/fphys.2019.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersen JL, Aagaard P. Myosin heavy chain IIx overshoot in human skeletal muscle. Muscle Nerve 2000;23:1095–104. [DOI] [PubMed] [Google Scholar]
- 76.Malm C, Nyberg P, Engström M, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 2000;529:243–62. 10.1111/j.1469-7793.2000.00243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.