Highlights
-
•
Transthyretin amyloid cardiomyopathy (ATTR-AC) is an under-diagnosed disease.
-
•
Many grey areas are emerging in clinical management and prognostic stratification.
-
•
Candidates’ selection for novel drugs is crucial, but response criteria are lacking.
-
•
The impact of evidence-based therapies for HF in ATTR-AC should be investigated.
-
•
Multidisciplinary team is the way to deliver the best clinical management.
Keywords: Cardiac amyloidosis, Transthyretin, Grey zones, Disease-modifying therapies, Prognostic stratification
Abstract
Transthyretin amyloid cardiomyopathy (ATTR-AC) is an under-recognized and underdiagnosed disease. Although traditionally considered a rare condition, the epidemiology of the disease is rapidly changing due to the possibility of non-invasive diagnosis through cardiac scintigraphy with bone tracers and novel disease-modifying treatments providing survival advantages. Nevertheless, many questions and grey areas have to be addressed, such as the natural history of ATTR-AC, the role and implications of genotype–phenotype interactions, the best clinical management, prognostic stratification and the most appropriate treatments, including those already recommended for patients with heart failure. Clinicians have to cope with old beliefs and evolving concepts in ATTR-AC. A wide horizon of possibilities for physicians of many specialties is unfolding and awaits discovery.
1. Introduction
Amyloidosis represents a largely unexplored field in medicine, with many grey areas remaining. The search for the mechanism triggering protein misfolding has been an important research topic for many decades. In recent years, cardiac involvement due to extracellular infiltration by transthyretin (TTR) amyloidosis, so-called ‘TTR amyloid cardiomyopathy’ (ATTR-AC), has proven to be a frequently unrecognized condition [1]. TTR is a homotetrameric carrier protein produced mostly by the liver [2]. A higher tendency to dissociate in misfolded monomers and to fibrillate is thought to occur in the presence of favourable conditions, such as ageing with associated failure of homoeostatic mechanisms in wild-type TTR (TTRwt) [3] or destabilizing mutations in variant TTR (TTRv) [1]. Progressive interstitial infiltration and direct ‘toxic’ effects exerted by circulating TTR oligomers lead to oxidative stress and mitochondrial damage, increased cardiac wall thickness and diastolic dysfunction [1,4,5].
Although histological confirmation was traditionally required, the diagnosis of ATTR-AC can currently be made upon the presence of cardiac accumulation of bone tracers revealed by scintigraphy in patients with no evidence of monoclonal proteins [6,7]. This non-invasive strategy represents a major step forward in meeting the urgent need for early diagnosis of AC, particularly as novel treatments able to modify the natural history of disease have been discovered [2]. A major reappraisal is underway concerning ATTR-AC in the modern era of precision medicine, and many questions about accurate diagnosis, the best clinical management, prognostic stratification and the most effective therapies have to be addressed.
2. Changing perspectives on ATTR-AC
2.1. The epidemiology: moving from ‘zero’ to ‘two-digit’ prevalence
Historically, ATTR-AC has been considered a rare condition predominantly affecting elderly subjects and has mostly been recorded as an incidental finding at post-mortem examination [5]. This perception derived from low awareness of the disease, fragmented knowledge and frequent misdiagnosis due to phenotypic heterogeneity and overlap with other conditions [1].
Despite an estimated raw prevalence of 〈 1 in 100,000 individuals [8], the real epidemiology of ATTR-AC is currently unknown. Major insights into disease epidemiology have been provided by cardiac scintigraphy with bone tracers, revealing ATTRv-AC in 13% of patients 〉 60 years with left ventricular (LV) wall thickness ≥ 12 mm hospitalized for heart failure (HF) with preserved ejection fraction (EF) [9], in 5% of patients with hypertrophic cardiomyopathy and in 16% of patients with ‘paradoxical’ low-flow low-gradient (LF-LG) aortic stenosis [1,10].
These findings suggest ATTR-AC prevalence to be substantially higher than traditionally thought, leading to a paradigm shift from monoclonal light chains to ATTR-AC as the most frequent form [1].The increasing rate of diagnosed cases calls into question whether ATTR-AC should continue to be considered a rare disease or should rather be considered a common condition not adequately diagnosed.
2.2. Beyond traditional clinical phenotypes: a broadening spectrum of disease
Early recognition of ATTR-AC is crucial to address proper management and treatment [2,[11], [12], [13]]. The Transthyretin Amyloidosis Outcomes Survey (THAOS) registry has reported cardiac involvement in half of patients [11]. Significant regional differences have emerged, with the vast majority of subjects from the United States being elderly men with ATTRwt-AC and a cardiac-predominant phenotype compared to subjects in Europe (Table 1 ).
Table 1.
Characteristics of patients with amyloid cardiomyopathy due to wild type TTR and the two most diffuse variant TTR forms.
| Wild-Type | Val30Met | Val122Ile | |
|---|---|---|---|
| Median age at diagnosis | ≥ 75 years | Early-onset ~ 30 years Late-onset ≥ 50 years (variable) |
~ 70 years |
| Mode of transmission | – | Autosomal dominant | Autosomal dominant |
| Genetic abnormality | – | Single nucleotide mutation | Single nucleotide mutation |
| Geographical distribution | Worldwide | Diffused worldwide, but with endemic areas including Portugal, Japan, Sweden and Cyprus | United States, United Kingdom, Western Africa |
| Clinical phenotype [15] | Cardiac 79%, NNNeurological 2.4% Mixed 18% | Early-onset: Cardiac 3%, Neurological 80%, Mixed 17% Late-onset: Cardiac 9%, Neurological 64%, Mixed 27% |
Cardiac: predominant Neurological and Mixed: Unknown |
| History of CTS,% | 25% - 33% | 14% - 30% | ~ 30% |
| Time from CTS and AC diagnosis | 15.5 years | 13 years | Unknown |
| AF,% | 65 - 70% | 30 | ~ 50% |
| Low QRS voltages,% | 30 - 45% | Early-onset: 7.5% [20] Late-onset: 4% [20] |
~ 45% |
| Median LV Wall Thickness, mm | 18 ± 3 | 17 ± 4 | 16 +−34 mm |
| Restrictive filling pattern,% | 40% | < 20 - 25% | ~ 55% |
| LVEF,% | 51 ± 12 | Early- and late-onset: 70 ± 7 | 40 ± 14% |
| LA diameter, mm | 50 ± 10 | 43 ± 6 | 46 ± 6 mm |
| AV Valve Thickening,% | 50% | Early- and late-onset: 10% | ~40% |
| Pericardial effusion,% | ~40% | ~55% | – |
| Median Survival | ~3.5 - 6 years | 12 years (Early-onset) [20] 7 years (Late-onset) [20] |
~2.5 years (without therapy) |
Legend: AC, Amyloid Cardiomyopathy; AF, Atrial Fibrillation; AV, Atrio-Ventricular; CTS, Carpal Tunnel Syndrome; LA, Left Atrium; LVEF, Left Ventricular Ejection Fraction; TTR, Transthyretin. Epidemiologic data, including the prevalence of clinical and echocardiographic characteristics, derive from those available in literature [11,15,16,19,20,24,41].
In recent years, specific clinical presentations of ATTR-AC have been identified [14], [15], [16]. In particular, elderly patients with HF and preserved EF admitted to cardiology or internal medicine departments may harbour amyloid in their hearts. This may also be the case for patients with valvular heart disease due to aortic valve stenosis referred because of syncope or transcatheter aortic valve implantation (TAVI), particularly in relation to ‘paradoxical’ LF-LG disease. When encountering these peculiar profiles, some of which are common in everyday practice, experienced clinicians should maintain a high index of suspicion [17] and look for specific signs of cardiac infiltration (Fig. 1, Fig. 2 ). Therefore, a major shift in mental attitude is essential to recognize suggestive findings and prompt further testing to confirm or rule out the disease.
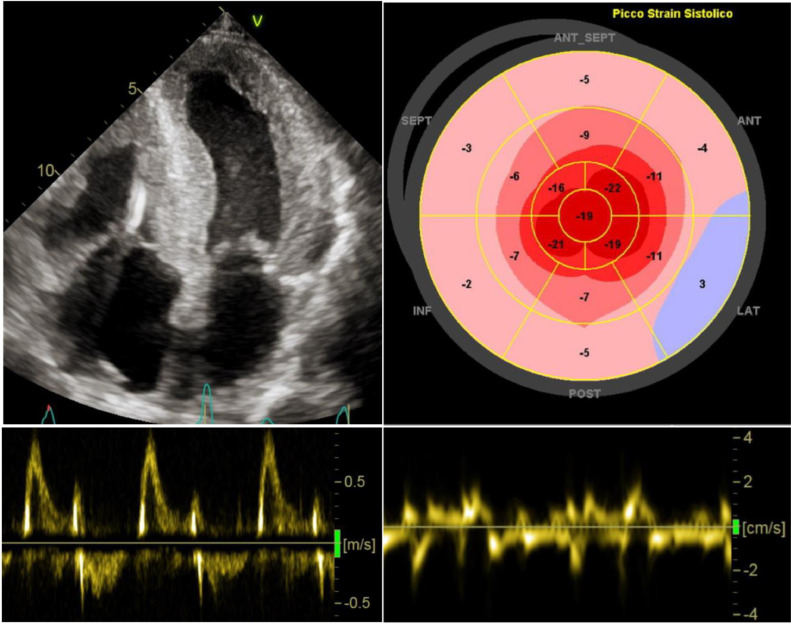
Fig. 1.
Echocardiographic findings indicating high suspicion of cardiac amyloidosis. Diffuse, concentric cardiac hypertrophy of both ventricles with bi-atrial enlargement and thickened IAS. Power Doppler showing restrictive LV filling pattern. Severely impaired LV global longitudinal strain with apical sparing pattern upon speckle-tracking analysis. Global severe reduction of regional myocardial deformation of the basal inferior septum < 5 cm/s, suggesting advanced cardiac infiltration. Legend: LV, Left Ventricle; IAS, Interatrial Septum.
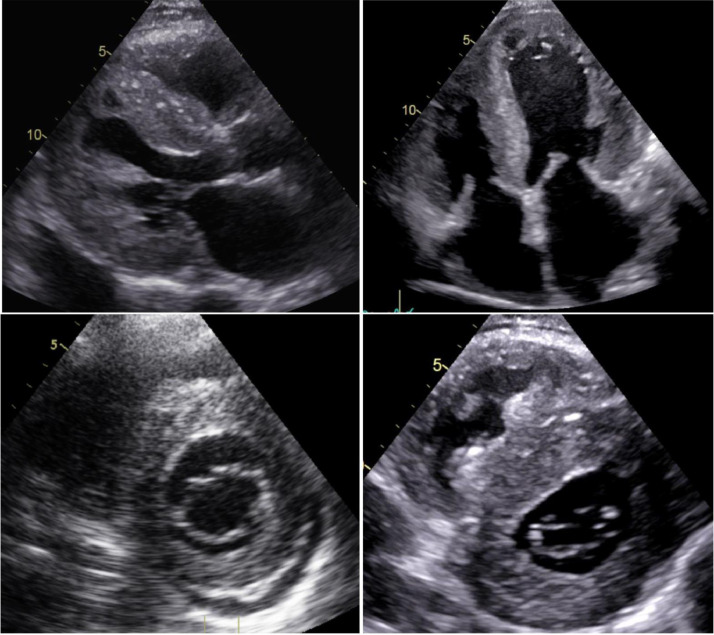
Fig. 2.
A 66-year-old man diagnosed with supposed hypertensive heart disease. Severe, concentric biventricular hypertrophy with granular sparkling appearance of the myocardium, thickened papillary muscles, bi-atrial enlargement and pericardial effusion. The regular size of the aortic root and the ascending aorta should have called into question the initial diagnosis of hypertensive heart disease. Thickened AV valves and IAS suggest long-standing infiltration. Legend: AV, atrioventricular; IAS, Interatrial Septum.
The ‘red flag’ diagnostic approach [4,18] has proven to be effective in spotting core clinical clues that point clinicians towards the diagnosis of ATTR amyloidosis, even before overt LV hypertrophy (Table 2 ). Of these clues, specific osteo-articular disorders—such as atraumatic rupture of biceps tendons, lumbar spine stenosis and hip disease—might prompt further testing for AC [1,8]. Patients with these disorders frequently undergo scintigraphy with bone tracers, and incidental cardiac retention might sometimes occur (Fig. 3 ). This finding is consistent with cardiac amyloid infiltration and warrants a comprehensive cardiac evaluation focused on AC (Table 2).
Table 2.
The comprehensive ‘red flag’ approach to TTR-AC.
| Cardiological Evaluation | Findings of high suspicion for amyloid disease |
|---|---|
| HCM diagnosed after the sixth decade of life Supposed ‘hypertensive cardiomyopathy’ in elderly patients with normal BP values and no valvular disease Recurrent syncope Need for down-titration or discontinuation of antihypertensive therapy due to poor tolerability Intolerance of ẞ-blockade in newly diagnosed HF Bowel dysfunction (constipation, diarrhoea) |
|
| Clinical Signs | HF with systemic venous congestion Aortic valve stenosis in the elderly Periorbital purpura Macroglossia Bilateral CTS, atraumatic rupture of biceps tendon and LS stenosis Unexplained neuropathic pain, mostly in non-diabetic patients Orthostatic hypotension and erectile dysfunction due to autonomic neuropathy Vitreous deposits |
| Laboratory |
Persistent elevation in serum troponin values Increased NT-proBNP values (> 5000 pg/mL), disproportional to the clinical severity of HF Monoclonal gammopathy of undetermined significance (frequently coexisting) |
| ECG | Low QRS voltages (peripheral and/or precordial leads) Discrepancies between severity of LV wall thickness and QRS voltages AV delay and/or blocks Pseudonecrosis |
| Echocardiography | Diffuse LV cardiac hypertrophy with non-dilated LV Coexisting RV hypertrophy Restrictive diastolic filling pattern Paradoxical low-flow low-gradient aortic valve stenosis Pericardial effusion Increased thickness of the interatrial septum and AV valves Granular sparkling appearance of the myocardium Reduced LV longitudinal strain with ‘apical sparing’ pattern |
Bold identifies the most specific red flags of disease according to [18]. Legend: AC, Amyloid Cardiomyopathy; AF, Atrial Fibrillation; AV, Atrio-Ventricular; BP, Blood Pressure; CA, Cardiac Amyloidosis; CAD, Coronary Artery Disease; CTS, Carpal Tunnel Syndrome; CV, Cardioversion; ECG, Electrocardiogram; EF, Ejection Fraction; HCM, Hypertrophic Cardiomyopathy; HF, Heart Failure; LS, Lumbar Spine; LV, Left Ventricle; NT-proBNP, N- terminal pro Brain Natriuretic Peptide; RV, Right Ventricular; TTR, Transthyretin.
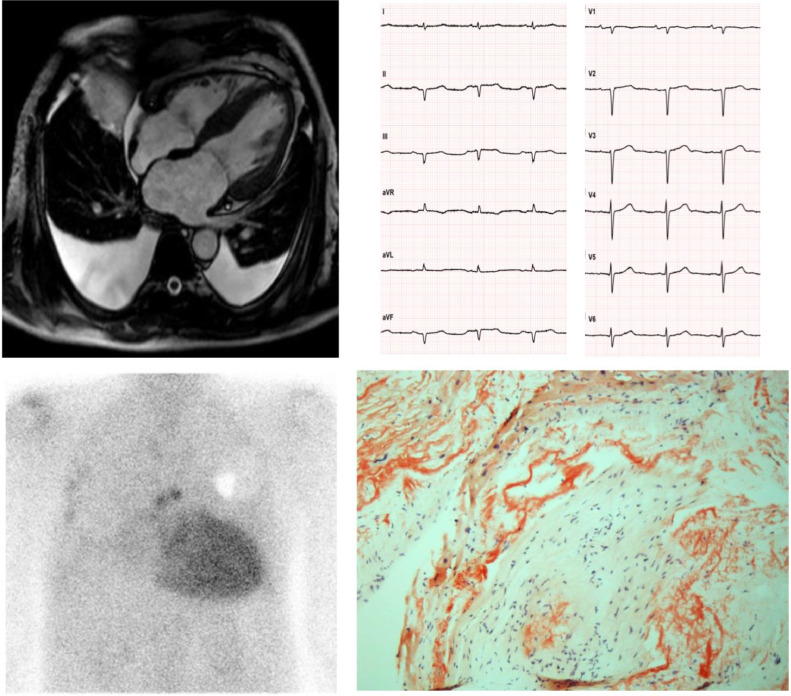
Fig. 3.
A 62-year-old man diagnosed with hypertrophic cardiomyopathy. The patient underwent a cardiological evaluation due to palpitations and a family history of sudden death (father died at 78). An ECG revealed low-voltage QRS in the peripheral leads, Q waves in the inferior leads and poor R wave progression in the precordial leads (‘pseudonecrosis’). A Holter ECG revealed paroxysmal AF and 3 consecutive ectopic ventricular beats >120 bpm. The echocardiogram and CMR showed LV hypertrophy (interventricular septum > 15 mm), left atrial dilatation and pericardial and bilateral pleural effusion. A diagnosis of hypertrophic cardiomyopathy was made, and the patient was implanted with an ICD for primary prevention of SCD. After 2 years, he was referred to our Cardiovascular Department for unexplained syncopal episodes. No arrhythmias were detected by the ICD. The patient complained of a one-year history of symptomatic bilateral carpal tunnel syndrome and discontinued anti-hypertensive drugs due to poor tolerability. An endomyocardial biopsy revealed diffuse amyloid fibrils upon Congo red staining (courtesy of Professor Rossana Bussani, MD, Institute of Pathological Anatomy and Histology, University of Trieste, Italy). Scintigraphy revealed high-grade (Perugini 3) cardiac accumulation of bone tracers, and after excluding a monoclonal component a diagnosis of ATTR-CA was made. Genetic testing revealed wild type transthyretin, and the patient was started on Tafamidis. Legend: AF, Atrial Fibrillation; CMR, Cardiac Magnetic Resonance; ECG, Electrocardiogram; ICD, Implantable Cardioverter Defibrillator; SCD, Sudden Cardiac Death.
Interestingly, carpal tunnel syndrome, particularly if bilateral at onset, can precede the diagnosis of ATTR-AC by many years (from 5 to 9 years) [19,20].These patients might exhibit normal wall thickness upon echocardiography; however, they have recently been reported to be at higher cardiovascular risk [21] and could benefit from regular cardiological evaluations.
The definite diagnosis of ATTR-AC does not rely upon specific biomarkers, but different parameters are currently under evaluation to aid in the identification of the disease and to monitor response to new disease-modifying drugs, including plasmatic concentration of TTR, retinol-binding protein-4 (RBP4), measurements of TTR kinetic stability and non-antibody peptide probes [22], [23], [24].
TTR serum levels tend to be lower than normal in subjects with destabilizing mutations and patients with ATTRwt amyloidosis. Notably, treatment with the new disease-modifying drugs has opposite effects on circulating TTR (decreased by gene silencers and increased by stabilizers), that can be used to monitor the response to therapy. RBP4 has been recently demonstrated a useful tool to detect V122I ATTR amyloidosis in elderly African Americans with HF, especially when integrated in a multi-parametric prediction model [24].
Novel markers of TTR stability (still under evaluation) include peptide probes that selectively bind to misfolded pathogenic oligomers in the serum and measurements of the rate of subunit exchange with the TTR tetramer [23].
2.3. The contribution of genetics
Many experts consider ATTRv-AC a model of genotype–phenotype correlation. Exclusively or mainly ‘cardiogenic’ or ‘neurologic’ mutations in the TTR gene have been described [4]. Val30Met substitution is associated with isolated progressive polyneuropathy (early-onset phenotype), cardiomyopathy and mixed phenotypes. Val122Ile is the most common cause of late-onset ATTRv-AC in the United States, while severe AC most commonly results from Val122Ile and Thr60Ala (early-onset phenotypes) or Ile68Leu (late-onset phenotype) in Italy and from Leu111Met (early-onset phenotype) in Denmark [1,4].
Understanding the phenotypic heterogeneity of ATTR amyloidosis is challenging because many other non-genetic determinants are often involved, such as gender, geographical distribution and endemic/non-endemic aggregation [11]. The highly variable penetrance of mutations in the TTR gene and ageing are other elements of heterogeneity [1]. Therefore, it is strongly advocated that future research investigate the role of genetic mutation in driving disease phenotype and natural history.
2.4. Prognostic stratification
Amyloidosis is considered an ominous disease carrying a high mortality burden, but recent diagnostic advances and novel treatments will change the natural history of disease [25]. Prognostic stratification remains challenging, as the diagnosis is made at different stages of the disease and the risk conferred by ATTR-AC is dynamic over time due to progressive cardiac infiltration. Patients are frequently diagnosed many years after disease onset and have commonly been managed as having hypertrophic cardiomyopathy or other phenocopies.
Some TTR mutations have been associated with more favourable or more adverse outcomes [1]. However, the presence and extent of cardiac involvement is by far the main prognostic factor, regardless of the specific amyloid precursor [11]. Therefore, dedicated staging systems have been developed to predict the outcome of ATTR-AC patients. The Mayo Clinic model validated the use of NT-proBNP and troponin T in ATTRwt-AC (cut off values of > 3000 ng/L and > 0.05 ng/mL, respectively). The estimated median overall survival when both values are above the cut-offs is 20 months [13]. More recently, Gillmore et al. have proposed another staging system based on NT-proBNP and the estimated glomerular filtration rate (eGFR) (cut off values of > 3000 ng/L and < 45 ml/min/m2, respectively), validated for both ATTRwt-AC and ATTRv-AC. The estimated median overall survival when both criteria are satisfied is 24 months [12]. The above-mentioned staging system includes only biomarkers of HF, cardiac damage and renal function. More parameters are relevant in the natural history of ATTR-CA, and a combination of blood tests and imaging parameters from echocardiography and cardiac magnetic resonance might potentially improve outcome estimation. In addition, most patients are elderly with multiple comorbidities (i.e. neoplasms) and significant competing risks of events.
Knowing the real prevalence of ATTR-AC and the natural history of disease from the initial stages of cardiac infiltration is critical to accurately predict long-term prognosis. Furthermore, the staging systems appear to perform adequately when the cardiac phenotype is apparent, but their predictive power at the initial stages of disease is currently unknown.
2.5. Disease-modifying therapies
No evidence-based treatment was available until recently, as ATTR-AC was largely underdiagnosed and frequently served as exclusion criteria in clinical trials testing HF medications. However, advances in knowledge about the ‘amyloidogenic cascade’ led to novel strategies targeting TTR synthesis and circulating TTR tetramers [2]. After years of unsuccessful pharmacological research, a stabilizer of circulating TTR called Tafamidis reduced all-cause mortality and cardiovascular hospitalization in patients with HF due to ATTR-AC [26]. In the wake of this discovery, additional drugs, such as Patisiran and Inotersen, have been tested. These are able to directly interfere with TTR synthesis via the liver, resulting in a considerable drop in circulating levels of TTR protein (approaching 80%) [2]. These drugs are promising disease-modifying approaches that have been demonstrated to promote clinical amelioration of and beneficial changes in infiltrated cardiac muscle [27,28]. The choice amongst different therapeutic approaches does not rely only on the assessment of drug safety and efficacy in different patient populations, such as those with cardiac and/or neuropathic involvement, but also on approval by regulatory authorities and the possibility of reimbursement. When therapy with Tafamidis, Patisiran or Inotersen is started, there are no defined criteria for early identification of responders and non-responders. Therefore, the decision to continue a therapy, to switch from one therapy to another or to combine two therapies is empirical. The identification of early criteria for response to treatment is an important goal of future research, particularly considering that these drugs greatly exceed conventional cost-effectiveness thresholds, as formally demonstrated for Tafamidis [29].
Currently, Tafamidis is the only approved treatment for ATTR-AC in the United States, while Patisiran and Inotersen are approved for ATTRv amyloidosis with neuropathy [2] (Table 3 ). Other drugs are being evaluated in clinical or pre-clinical studies, including:
-
•
AG-10, an oral drug that effectively stabilizes TTR tetramers (NCT03860935, NCT04418024);
-
•
AKCEA-TTR-LRx, an antisense oligonucleotide administered subcutaneously every 4 weeks that targets hepatocytes much more selectively than Inotersen, thus being more effective in silencing TTR gene expression and having less systemic adverse effects. A dedicated trial is evaluating its safety and efficacy in patients with peripheral neuropathy (NCT04136184);
-
•
Vutrisiran, a small-interfering RNA (similar to Patisiran) that can be administered subcutaneously every 3 months (NCT04153149);
-
•
Specific antibodies inducing the removal of tissue amyloid deposits. The monoclonal antibody PRX004 is able to specifically bind tissue TTR deposits, but the phase 1 trial (NCT03336580) has been prematurely terminated because of COVID 19 pandemic.
Table 3.
Focus on available treatments in TTR-AC and novel drugs under investigation.
| Drugs | Mechanism | Design | Dose | Population | Results in TTR-AC | Approved indications (FDA/EMA; June 2020) |
|---|---|---|---|---|---|---|
| Tafamidis (ATTR-ACT, 2018) [26] | TTR stabilizer | Multicentre, double-blind, placebo controlled 2:1:2 randomization |
80 mg vs. 20 mg vs. placebo for 30 months | TTR cardiomyopathy (TTRv and TTRwt) (n = 441) | Reduced all-cause mortality (HR 0.70) and CV hospitalization (RR 0.68); Less decline in 6MWT and KCCQ-OS score (both p <0.001) |
FDA: not approved EMA: Stage I TTRv-related polyneuropathy |
| AG-10 (2019) [44] | TTR stabilizer | Multicentre, double-blind, placebo controlled 1:1:1 randomization |
1600 mg vs. 800 mg vs. placebo for 28 days | TTR cardiomyopathy (TTRv and TTRwt) (n = 49) | Near-complete stabilization of TTR with restoration of normal serum levels. More effective at higher doses (average increase of 36±21% and 51±38% at 400 and 800 mg (both p<0.001) | FDA: not approved. EMA: not approved. |
| Patisiran (APOLLO, 2018) [45] | SiRNA | Multicentre, double-blind, placebo controlled 2:1 randomization |
0.3 mg/kg iv vs. placebo once every 3 weeks for 18 months | TTRv-related polyneuropathy Subgroup with cardiomyopathy (n = 126, 56%) |
Slower LV functional deterioration and promotion of favourable remodelling: decreased LV volumes, wall thickness, RWT, mass and NT-proBNP. | FDA: TTRv-related polyneuropathy EMA: Stages I–II TTRv-related polyneuropathy |
| Inotersen (2018) [46] | ASO | Multicentre, double-blind, placebo controlled 2:1 randomization |
300 mg sc vs. placebo for 64 weeks | TTRv-related polyneuropathy – Stages I–II Subgroup with cardiomyopathy (n = 108, 63%) |
Slower progression in neuropathy, no effect on echocardiographic parameters (including GLS) | FDA: TTRv-related polyneuropathy EMA: Stages I–II TTRv-related polyneuropathy |
| PRX004 (NCT03336580) | mAb | Phase I, single centre, open-label, dose escalation | 0.1 mg/kg iv once every 28 days | TTRv amyloidosis (estimated n = 36) | Aim: determine the safety, tolerability, PK, PD and MTD | Prematurely terminated because of COVID 19 pandemic |
| Ab-A (2020) [47] | mAb | Single centre, open label, murine model | 10 mg/kg vs. 5 mg/kg vs. 0.1 mg/kg vs. sham | TTRwt amyloidosis | Promoting clearance and degradation of aggregated TTR by cardiac macrophages Protecting cardiomyocytes from aggregated TTR- mediated toxicity |
– |
Legend: 6MWT, Six Minute Walking Test; AC, Amyloid Cardiomyopathy; ASO, Antisense Oligonucleotide; CV, Cardiovascular; EMA, European Medicine Agency; FDA, Food and Drug Administration; GLS, Global Longitudinal, Strain; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire Overall Summary; LV, Left Ventricular; mAb, Monoclonal Antibody; MTD, Maximum Tolerated Dose; NT-proBNP, N- terminal Brain Natriuretic Peptide; PD, Pharmacodynamics; PK, Pharmacokinetics; RWT, Relative Wall Thickness, siRNA, small interfering RNA; TTR, Transthyretin; TTRv, mutated Transthyretin; TTRwt, wild-type Transthyretin.
3. To treat or not to treat … and when?
The increasing availability of potential therapies for ATTR-AC highlights the fundamental issue of the accurate selection of candidates. In exploring uncharted horizons, relevant unsolved issues and challenging scenarios are emerging (Table 4 ) [2,30].
-
a)
TTR amyloid in the heart: disease or incidental finding of amyloid deposits? The prolongation of the average lifespan in the recent decades has been paralleled by the rise of degenerative disorders [3]. TTR amyloidosis is part of this phenomenon as a result of the failure of protein homoeostasis associated with ageing [3]. However, the mere presence of amyloid deposits in the myocardium does not directly involve a definite pathogenic role. TTR amyloidosis has been reported in the atria of > 91% of subjects over 65 years and in the aorta of ≥ 95% of those aged ≥ 80 years [31,32]. At present, cardiac retention of bone tracers on scintigraphy can reveal TTR amyloid deposits even before a detectable increase in ventricular wall thickness on echocardiography [1,8]. This approach can considerably reduce diagnostic delay, allowing the identification of subjects at an asymptomatic stage [6]. However, the clinical relevance of such deposits is undetermined, being potentially the consequence of age-related physiological disruptions. Therefore, such information should be evaluated in combination with the clinical picture and cardiologic findings to avoid misdiagnosis and the initiation of unnecessary therapies. Although severe and widespread amyloid accumulation is reasonably detrimental, the amount of amyloid burden to be considered ‘disease-related’ and prompting treatment initiation remains to be established. Furthermore, amyloid localization should be considered, as even small amounts in vulnerable areas (i.e. the atrio-ventricular node) can have major consequences.
Table 4.
Grey zones and knowledge gaps in the current management of TTR-AC.
| Reasonably indicated treatments and grey zones | |
|---|---|
| Anti-neurohormonal therapy | No survival benefit is demonstrated in TTR-AC. Beta-blockers and ACE-i/ARB can be poorly tolerated, especially in advanced stages due to vasodilatation and reduced CO. |
| Atrial fibrillation and anticoagulation | Anticoagulation with VKAs and DOACs should be administered based on the usual indications and contraindications. Patients with TTR-AC in sinus rhythm can harbour thrombi in the left atrial appendage, especially when atrial function is severely impaired. Therefore, TEE should be routinely performed before a planned cardioversion. |
| Atrial fibrillation, rate or rhythm control and catheter ablation | There is no specific recommended strategy for the management of arrhythmias. Digoxin should be avoided or used at low dosages due to concerns about potential enhanced toxicity caused by binding to amyloid fibrils. Drugs with a negative inotropic effect should be avoided (i.e. verapamil and diltiazem). The efficacy of catheter ablation seems limited, but future studies are required. |
| Transcatheter aortic valve implantation | Percutaneous or surgical aortic valve replacement should not be denied only because of coexistent AC. Definitive PM implantation may be required due to a higher risk of persistent AV blocks. |
| Ventricular arrhythmias and implantable cardioverter defibrillator | Sudden death is not infrequent in patients with advanced HF, but it is generally due to electromechanical dissociation. The role of ICD for the purpose of: - primary prevention is currently limited; - secondary prevention can be considered in select non-advanced cases with sustained VT/VF. |
| Cardiac resynchronization therapy | Very limited data are available on this topic. CRT should be considered in patients eligible for PM implantation with an estimated time of RV pacing >40%. |
| Heart transplantation | Highly selected HF patients with TTRwt or TTRv and predominant cardiac phenotype without extra-cardiac organ damage could be eligible. Combined heart and liver transplantation could be considered in very selected cases with mixed phenotype and limited neurological involvement. |
Legend: AC, Amyloid Cardiomyopathy; ACE-i, Angiotensin Converting Enzyme Inhibitors, ARBs, Angiotensin Receptor Blockers; AV, Atrio-Ventricular; CO, Cardiac Output; DOACs, Direct Oral Anticoagulants; HF, Heart Failure; LBBB, Left Bundle Branch Block; LVEF, Left Ventricular Ejection Fraction; PM, Pacemaker; RV, Right Ventricular; SCD, Sudden Cardiac Death; TEE, Transoesophageal Echocardiography; TTR-AC, Transthyretin Amyloid Cardiomyopathy; TTRv, variant Transthyretin; TTRwt, wild-type Transthyretin, VF, Ventricular Fibrillation; VKAs, Vitamin K Antagonists; VT, Ventricular Tachycardia.
Whether and when asymptomatic patients with incidental cardiac retention of bone tracers on scintigraphy, no signs of HF, normal biomarker levels, no evidence of cardiac hypertrophy or neurological manifestations should be started on disease-modifying medications is unclear. A tailored strategy—also considering the presence of TTR mutations—seems reasonable in light of the variable natural history of disease. Some patients might never progress to subsequent stages of disease or might experience only neurological manifestation without cardiac involvement [11]. Accordingly, a cardiologic screening looking for red flags and suggestive echocardiographic features would allow prompt recognition of initial cardiac involvement and treatment initiation.
-
a)
Advanced stages of the disease and comorbities. Considerable grey areas remain to be clarified in the clinical management and prognosis of ATTR-AC with advanced infiltration revealed by severe biventricular hypertrophy, a restrictive diastolic pattern and systolic dysfunction.
If present at all, the survival advantage provided by TTR stabilizers in advanced ATTR-AC seems minimal. In the “Tafamidis in Patients With Transthyretin Cardiomyopathy” trial [26], HF patients with New York Heart Association (NYHA) class IV or eGFR <25 ml/min/m2 were excluded, and Tafamidis provided greater benefits in those with early-stage ATTR-AC (NYHA I–II). Furthermore, although functional capacity and quality of life were significantly ameliorated at 6 months, the consistent mortality benefit was evident only after 18–20 months of therapy [26]. These findings raise the question of whether subjects with a lower estimated life expectancy (<6–12 months) should be treated, as they might derive no survival advantage while potentially experiencing side effects.
Prolonging the life expectancy of these patients will result in dealing with challenging scenarios not previously faced (Table 1). For instance, these patients could be eligible for implantable cardioverter defibrillator (ICD) therapy to prevent sudden arrhythmic death, cardiac resynchronization therapy to act on dyssynchrony due to left bundle branch block, percutaneous treatment of severe mitral regurgitation or aortic valve stenosis [33] or coronary artery revascularization. However, the prognostic benefit of these treatments is unknown, as are the effects of anti-neurohormonal therapies with proven impact in HF due to non-amyloidotic aetiologies [34], even in combination with novel specific treatments.
The prevention of sudden cardiac death and percutaneous aortic valve implantation are crucial issues and deserves further discussion. Although secondary prevention is a strong indication for ICD implantation also in patients with AC [35] the benefits of this therapy for the purpose of primary prevention remain uncertain. Sudden cardiac death in AC, in fact, frequently results from pulseless electric activity or elettro-mechanical dissociation (non-defibrillable rhythms) rather than tachy-arrhythmias [36]. In a single-centre retrospective experience, appropriate ICD shocks accounted for only 32% of cases at one-year follow up [37]. Furthermore, ICD therapy did not provide any overall survival benefit. Both ATTRv- and ATTRwt-AC patients experience lower rates of appropriate ICD shocks compared to AL-AC, suggesting that the risk of life-threatening arrhythmias may change in different forms of AC and highlighting the need for accurate patients selection.
Recent studies have shown that ATTR-AC frequently coexists with severe degenerative aortic stenosis (AS) (prevalence ranging from 8 to 16%) due to the shared epidemiologic substrate and the possible effect of increased myocardial strain of aortic stenosis acting on an underlying amyloidogenetic substrate [38]. The first anecdotic reports have questioned the usefulness of aortic valve replacement, even when performed by TAVI. More recent prospective studies in relatively large cohorts of patients with severe AS in the elderly with or without ATTR-AC undergoing TAVI have shown similar short and medium term results regarding overall survival (with a higher risk of hospitalization for HF amongst patients with ATTR-AC) [33,38]. Waiting for further randomized studies, these results suggest that TAVI should not be denied only on the basis of the coexistence of ATTR-AC.
-
a)
Genotype-positive phenotype-negative relatives. The clinical management of relatives carrying disease-related TTR gene mutations without evidence of cardiac and neurological diseases represents a major gap in knowledge. These patients might potentially be at higher risk of developing amyloid deposition over time and could benefit from dedicated screening programs. No official recommendations are available in this field. Asymptomatic carriers have been proposed to start regular monitoring up to 10 years before the predicted mutation-specific age of onset of symptomatic disease, most commonly between 40 and 55 years [39]. Early recognition of ATTRv amyloidosis is crucial to initiate orientated clinical evaluation and tailored treatments, genetic counselling and familial screening [2,8]. Cardiac scintigraphy with bone tracers and deformation imaging might play a future role in revealing initial cardiac infiltration in asymptomatic carriers [1,8].
Finally, several TTR variants exist. For example, non-pathogenic genetic variants have been found, the so-called TTR stabilizing mutations (i.e. Thr119Met and Arg104His). These variants slow the dissociation of the TTR tetramer acting as a trans-suppressor of amyloid fibril formation and result in a more stable TTR protein [40]. Therefore, the determinants of the clinical profile and the prognostic implications of specific TTR mutations are only partially known (also for TTRwt-AC), and further research is required.
3.1. Future directions and conclusions
Recent years have witnessed significant advances in the epidemiology, pathophysiology and diagnosis of amyloidosis [41]. ATTR-AC is increasingly recognized due to heightened awareness of the disease, more accurate aetiological characterization, the ‘red flag’ integrated approach and cardiac scintigraphy with bone tracers. Nevertheless, important issues in clinical research still need to be addressed, including:
-
•
Understanding the real prevalence of ATTR amyloidosis, particularly with the cardiac phenotype, in the various clinical scenarios and differentiating indolent cardiac accumulation of the ‘senile’ form from the authentic infiltrative disease;
-
•
The use of diphosphonate single photon emission computed tomography to better characterize patients with Perugini score 1 (by detecting localized but intense amyloid deposition, thus allowing an early diagnosis [42]) and possibly to quantify the amyloid burden;
-
•
Investigating the potential role of PET imaging with amyloid tracers (alone or in combination with bone tracer scintigraphy) for the differential diagnosis between AL and ATTR amyloidosis [43];
-
•
Defining the minimal disease threshold to justify the initiation of novel treatments with high costs and potential side effects;
-
•
Defining the degree of cardiac involvement to be considered so advanced that no significant benefit is expected from the administration of disease-modifying drugs;
-
•
Identifying the most appropriate tool to quantify the global amyloid burden and to monitor its changes under specific treatment;
-
•
Defining baseline and early on treatment criteria to identify ‘responders’ and ‘non-responders’ to disease-modifying therapies in order to guide the decision to discontinue, change or possibly associate drugs;
-
•
Defining potential parameters at baseline to predict response to treatment and consequently identifying criteria to discontinue current drugs in favour of other medications or to start on combination therapy.
All this information will be crucial to address proper clinical management and more accurate prognostic stratification through a multiparametric approach integrating clinical evaluation with imaging-derived parameters and specific genetic information. A wide horizon of possibilities is unfolding and awaits discovery.
Funding and disclosures
None
Declaration of Competing Interest
The authors declare they have no conflict of interest.
Acknowledgement
We would like to thank Fondazione CariGO and Fondazione per la Cardiologia e le Scienze Multidisciplinari Livia e Vittorio Tonolli and all the healthcare professionals for the continuous support to the clinical management of patients affected by cardiomyopathies, followed in Heart Failure Outpatient Clinic of Trieste, and their families.
References
- 1.Maurer M.S., Elliott P., Comenzo R., Semigran M., Rapezzi C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation. 2017;135:1357–1377. doi: 10.1161/CIRCULATIONAHA.116.024438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emdin M., Aimo A., Rapezzi C., Fontana M., Perfetto F., Seferović P.M. Treatment of cardiac transthyretin amyloidosis: an update. Eur Heart J. 2019;40:3699–3706. doi: 10.1093/eurheartj/ehz298. [DOI] [PubMed] [Google Scholar]
- 3.Stakos D.A., Stamatelopoulos K., Bampatsias D., Sachse M., Zormpas E., Vlachogiannis N.I. The Alzheimer's Disease Amyloid-Beta Hypothesis in Cardiovascular Aging and Disease. J Am Coll Cardiol. 2020;75:952–967. doi: 10.1016/j.jacc.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapezzi C., Lorenzini M., Longhi S., Milandri A., Gagliardi C., Bartolomei I. Cardiac amyloidosis: the great pretender. Heart Fail Rev. 2015;20:117–124. doi: 10.1007/s10741-015-9480-0. [DOI] [PubMed] [Google Scholar]
- 5.Palladini G., Merlini G. Systemic amyloidoses: what an internist should know. Eur J Intern Med. 2013;24:729–739. doi: 10.1016/j.ejim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Gillmore J.D., Maurer M.S., Falk R.H., Merlini G., Damy T., Dispenzieri A. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 7.González-Calle V., Mateos M.V. Monoclonal gammopathies of unknown significance and smoldering myeloma: assessment and management of the elderly patients. Eur J Intern Med. 2018;58:57–63. doi: 10.1016/j.ejim.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Maurer M.S., Bokhari S., Damy T., Dorbala S., Drachman B.M., Fontana M. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ Hear Fail. 2019:12. doi: 10.1161/CIRCHEARTFAILURE.119.006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González-López E., Gallego-Delgado M., Guzzo-Merello G., De Haro-Del Moral F.J., Cobo-Marcos M., Robles C. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 10.Ternacle J., Krapf L., Mohty D., Magne J., Nguyen A., Galat A. Aortic Stenosis and Cardiac Amyloidosis. J Am Coll Cardiol. 2019;74:2638–2651. doi: 10.1016/j.jacc.2019.09.056. [DOI] [PubMed] [Google Scholar]
- 11.Maurer M.S., Hanna M., Grogan M., Dispenzieri A., Witteles R., Drachman B. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey) J Am Coll Cardiol. 2016;68:161–172. doi: 10.1016/j.jacc.2016.03.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillmore J.D., Damy T., Fontana M., Hutchinson M., Lachmann H.J., Martinez-Naharro A. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 13.Grogan M., Scott C.G., Kyle R.A., Zeldenrust S.R., Gertz M.A., Lin G. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol. 2016 doi: 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Manolis A.S., Manolis A.A., Manolis T.A., Melita H. Cardiac amyloidosis: an underdiagnosed/underappreciated disease. Eur J Intern Med. 2019;67:1–13. doi: 10.1016/j.ejim.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Damy T., Kristen A.V., Suhr O.B., Maurer M.S., Planté-Bordeneuve V., Yu C.-.R. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS) Eur Heart J. 2019 doi: 10.1093/eurheartj/ehz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapezzi C., Quarta C.C., Obici L., Perfetto F., Longhi S., Salvi F. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34:520–528. doi: 10.1093/eurheartj/ehs123. [DOI] [PubMed] [Google Scholar]
- 17.Witteles R.M., Bokhari S., Damy T., Elliott P.M., Falk R.H., Fine N.M. Screening for Transthyretin Amyloid Cardiomyopathy in Everyday Practice. JACC Hear Fail. 2019;7:709–716. doi: 10.1016/j.jchf.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Vergaro G., Aimo A., Barison A., Genovesi D., Buda G., Passino C., et al. Keys to early diagnosis of cardiac amyloidosis: red flags from clinical, laboratory and imaging findings. Eur J Prev Cardiol 2019:204748731987770. 10.1177/2047487319877708. [DOI] [PubMed]
- 19.Milandri A., Farioli A., Gagliardi C., Longhi S., Salvi F., Curti S. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail. 2020;22:507–515. doi: 10.1002/ejhf.1742. [DOI] [PubMed] [Google Scholar]
- 20.Adams D., Koike H., Slama M., Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019 doi: 10.1038/s41582-019-0210-4. [DOI] [PubMed] [Google Scholar]
- 21.Fosbøl E.L., Rørth R., Leicht B.P., Schou M., Maurer M.S., Kristensen S.L. Association of Carpal Tunnel Syndrome With Amyloidosis, Heart Failure, and Adverse Cardiovascular Outcomes. J Am Coll Cardiol. 2019;74:15–23. doi: 10.1016/j.jacc.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 22.Pregenzer-Wenzler A., Abraham J., Barrell K., Kovacsovics T., Nativi-Nicolau J. Utility of Biomarkers in Cardiac Amyloidosis. JACC Hear Fail. 2020;8:701–711. doi: 10.1016/j.jchf.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Hendren N.S., Roth L.R., Grodin J.L. Disease-Specific Biomarkers in Transthyretin Cardiac Amyloidosis. Curr Heart Fail Rep. 2020;17:77–83. doi: 10.1007/s11897-020-00457-z. [DOI] [PubMed] [Google Scholar]
- 24.Arvanitis M., Koch C.M., Chan G.G., Torres-Arancivia C., LaValley M.P., Jacobson D.R. Identification of Transthyretin Cardiac Amyloidosis Using Serum Retinol-Binding Protein 4 and a Clinical Prediction Model. JAMA Cardiol. 2017;2:305. doi: 10.1001/jamacardio.2016.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porcari A., Falco L., Lio V., Merlo M., Fabris E., Bussani R. Cardiac amyloidosis: do not forget to look for it. Eur Hear J Suppl. 2020;22:E142–E147. doi: 10.1093/eurheartj/suaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer M.S., Schwartz J.H., Gundapaneni B., Elliott P.M., Merlini G., Waddington-Cruz M. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 27.Solomon S.D., Adams D., Kristen A., Grogan M., González-Duarte A., Maurer M.S. Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients With Hereditary Transthyretin-Mediated Amyloidosis. Circulation. 2019;139:431–443. doi: 10.1161/CIRCULATIONAHA.118.035831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dasgupta N.R., Rissing S.M., Smith J., Jung J., Benson M.D. Inotersen therapy of transthyretin amyloid cardiomyopathy. Amyloid. 2020;27:52–58. doi: 10.1080/13506129.2019.1685487. [DOI] [PubMed] [Google Scholar]
- 29.Kazi D.S., Bellows B.K., Baron S.J., Shen C., Cohen D.J., Spertus J.A. Cost-Effectiveness of Tafamidis Therapy for Transthyretin Amyloid Cardiomyopathy. Circulation. 2020;141:1214–1224. doi: 10.1161/CIRCULATIONAHA.119.045093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aimo A., Rapezzi C., Vergaro G., Giannoni A., Spini V., Passino C. Management of complications of cardiac amyloidosis: 10 questions and answers. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320920756. [DOI] [PubMed] [Google Scholar]
- 31.Kawamura S., Takahashi M., Ishihara T., Uchino F. Incidence and distribution of isolated atrial amyloid: histologic and immunohistochemical studies of 100 aging hearts. Pathol Int. 1995;45:335–342. doi: 10.1111/j.1440-1827.1995.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 32.Iwata T., Kamei T., Uchino F., Mimaya H., Yanagaki T., Etoh H. Pathological study on amyloidosis -relationship of amyloid deposits in the aorta to aging. Pathol Int. 1978;28:193–203. doi: 10.1111/j.1440-1827.1978.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 33.Scully P.R., Patel K.P., Treibel T.A., Thornton G.D., Hughes R.K., Chadalavada S. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur Heart J. 2020;41:2759–2767. doi: 10.1093/eurheartj/ehaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aimo A., Vergaro G., Castiglione V., Rapezzi C., Emdin M. Safety and Tolerability of Neurohormonal Antagonism in Cardiac Amyloidosis. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 36.Kristen A V., Dengler T.J., Hegenbart U., Schonland S.O., Goldschmidt H., Sack F.-.U. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Hear Rhythm. 2008;5:235–240. doi: 10.1016/j.hrthm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Lin G., Dispenzieri A., Kyle R., Grogan M., Brady P.A. Implantable Cardioverter Defibrillators in Patients with Cardiac Amyloidosis. J Cardiovasc Electrophysiol. 2013;24:793–798. doi: 10.1111/jce.12123. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblum H., Masri A., Narotsky D.L., Goldsmith J., Hamid N., Hahn R.T. Unveiling Outcomes in Coexisting Severe Aortic Stenosis and Transthyretin Cardiac Amyloidosis. Eur J Heart Fail. 2020 doi: 10.1002/ejhf.1974. ejhf.1974. [DOI] [PubMed] [Google Scholar]
- 39.Conceição I., Damy T., Romero M., Galán L., Attarian S., Luigetti M. Early diagnosis of ATTR amyloidosis through targeted follow-up of identified carriers of TTR gene mutations*. Amyloid. 2019;26:3–9. doi: 10.1080/13506129.2018.1556156. [DOI] [PubMed] [Google Scholar]
- 40.Hammarstrom P., Jiang X., Hurshman A.R., Powers E.T., Kelly J.W. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci. 2002;99:16427–16432. doi: 10.1073/pnas.202495199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruberg F.L., Grogan M., Hanna M., Kelly J.W., Maurer M.S. Transthyretin Amyloid Cardiomyopathy. J Am Coll Cardiol. 2019;73:2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grigoratos C., Aimo A., Rapezzi C., Genovesi D., Barison A., Aquaro G.D. Diphosphonate single-photon emission computed tomography in cardiac transthyretin amyloidosis. Int J Cardiol. 2020 doi: 10.1016/j.ijcard.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Genovesi D., Vergaro G., Giorgetti A., Marzullo P., Scipioni M., Santarelli M.F. [18F]-Florbetaben PET/CT for Differential Diagnosis Among Cardiac Immunoglobulin Light Chain, Transthyretin Amyloidosis, and Mimicking Conditions. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Judge D.P., Heitner S.B., Falk R.H., Maurer M.S., Shah S.J., Witteles R.M. Transthyretin Stabilization by AG10 in Symptomatic Transthyretin Amyloid Cardiomyopathy. J Am Coll Cardiol. 2019;74:285–295. doi: 10.1016/j.jacc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Adams D. Gonzalez-Duarte A, O'Riordan WD, Yang C-C, Ueda M, Kristen A V., et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 46.Benson M.D., Waddington-Cruz M., Berk J.L., Polydefkis M., Dyck P.J., Wang A.K. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.George J., Rappaport M., Shimoni S., Goland S., Voldarsky I., Fabricant Y. A novel monoclonal antibody targeting aggregated transthyretin facilitates its removal and functional recovery in an experimental model. Eur Heart J. 2020;41:1260–1270. doi: 10.1093/eurheartj/ehz695. [DOI] [PubMed] [Google Scholar]