Abstract
BACKGROUND
COVID-19 poses a risk to the endoscopic skull base surgeon. Significant efforts to improving safety have been employed, including the use of personal protective equipment, preoperative COVID-19 testing, and recently the use of a modified surgical mask barrier.
OBJECTIVE
To reduce the risks of pathogen transmission during endoscopic skull base surgery.
METHODS
This study was exempt from Institutional Review Board approval. Our study utilizes a 3-dimensional (3D)-printed mask with an anterior aperture fitted with a surgical glove with ports designed to allow for surgical instrumentation and side ports to accommodate suction ventilation and an endotracheal tube. As an alternative, a modified laparoscopic surgery trocar served as a port for instruments, and, on the contralateral side, rubber tubing was used over the endoscrub endosheath to create an airtight seal. Surgical freedom and aerosolization were tested in both modalities.
RESULTS
The ventilated mask allowed for excellent surgical maneuverability and freedom. The trocar system was effective for posterior surgical procedures, allowing access to critical paramedian structures, and afforded a superior surgical seal, but was limited in terms of visualization and maneuverability during anterior approaches. Aerosolization was reduced using both the mask and nasal trocar.
CONCLUSION
The ventilated upper airway endoscopic procedure mask allows for a sealed surgical barrier during endoscopic skull base surgery and may play a critical role in advancing skull base surgery in the COVID-19 era. The nasal trocar may be a useful alternative in instances where 3D printing is not available. Additional studies are needed to validate these preliminary findings.
Keywords: Endoscopic skull base surgery, Ventilated mask, Endonasal surgery, COVID-19, Aerosolization reduction, COVID-19 infection prevention
ABBREVIATIONS
- CT
computed tomography
- DIY
Do-It-Yourself
- EES
endoscopic endonasal surgery
- FDM
fused deposition modeling
- PDMS
polydimethylsiloxane
- PPE
personal protective equipment
- PLA
polylactic acid
- SF
surgical freedom
- TPE
thermoplastic elastomer
- 3D
3-dimensional
- VPM
ventilated upper airway endoscopic procedure mask
In the swiftly evolving era of the novel coronavirus pandemic, endoscopic endonasal surgery (EES) is experiencing a seismic shift to accommodate evidence suggesting that EES puts the surgeon and the surgical team at particular risk from aerosolized virus particles.1-3
Although no definitive evidence exists, anecdotal reports have suggested that endoscopic skull base procedures can potentially increase the risk of COVID-19 transmission.1,4 This concern in addition to objective data demonstrating the presence of increased nasal viral load 5,6 and persistence of aerosolized virus7 has raised questions regarding the safety of EES and upper airway endoscopy and what measures can be taken to diminish potential risks.
Workman et al2 provided a novel workaround for skull base surgery using a modified surgical facemask. However, their mask does not prevent aerosolization during high-speed drilling, which is an essential part of the surgical armamentarium. As we move forward in the COVID-19 era, there is a necessity to reduce the risks of transmission and increase surgical safety while using all essential instrumentation in order to maintain current standards of care.
This study approaches this unique problem in a novel fashion, utilizing readily available and innovative equipment as a means of creating a well-sealed 3-dimensional (3D) printed protective mask during endoscopic skull base surgery.
METHODS
Ventilated Upper Airway Endoscopic Procedure Mask 3D Design
The design was inspired by existing open-source 3D models that have been used as “Do-It-Yourself” (DIY) respirator masks (https://www.makethemasks.com/) to address ongoing COVID-19 personal protective equipment (PPE) shortages.
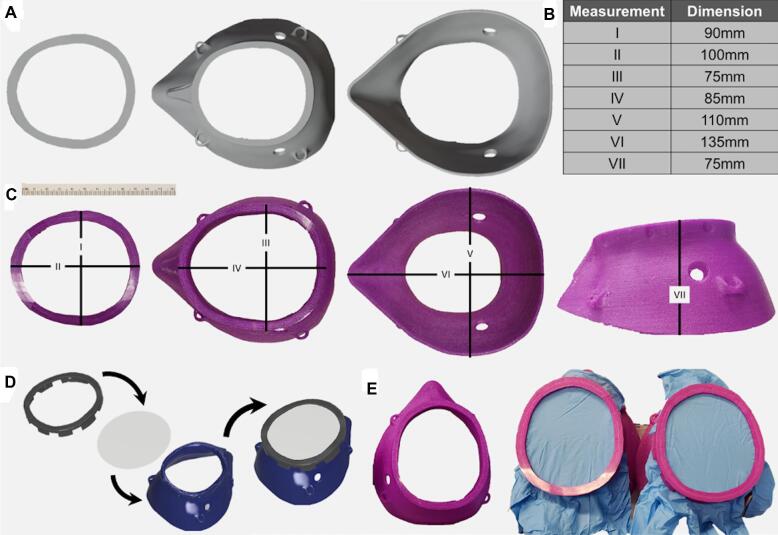
The authors then used 2 cadaveric models to provide improved facial model references to fit the 3D designs (Figure 1A). Based on these preliminary contour tests, the mask edge circumference was redesigned to enclose more of the midface and added a 9.5-mm inlet/outlet to the left and right of the mask to allow passage and retention of intubation tubing (left port) as well as vacuum suction to evacuate surgical debris during procedures (right port).
FIGURE 1.
3D design and printing of the endoscopic procedure mask. CAD design of the 2-part system A and specific dimensions for the pilots B and C that would fit most adult faces are listed. Proposed assembly of the 3D rendered system D and the pilot endoscopic procedure masks using surgical gloves E is also shown.
The anterior opening was expanded and the distance between the patient's nose and the surgical insertion sites was constrained to allow for easier surgical tool insertion and unobstructed surgical access. Full mask measurements are provided in Figure 1B and 1C.
An assembled model of the mask is presented as computer aided design (CAD; Figure 1D), and the 3D printed pilots to be used in the surgical procedure (Figure 1E) are also shown. The design presented here encompasses the majority of patient facial sizes, and is further aided by a vacuum seal between the face and mask. The 3D design modifications were performed using the Blender 3D modeling software (www.blender.org) and Autodesk Fusion 360 CAD software (https://www.autodesk.com/products/fusion-360/overview). Three-dimensional CAD files will be provided upon request to the corresponding author (Dr Helman).
Three-Dimensional Printing and Assembly of the Ventilated Upper Airway Endoscopic Procedure Mask
The ventilated upper airway endoscopic procedure mask (VPM) was 3D printed using Ultimaker 2 (www.ultimaker.com) and Pulse XE (www.matterhackers.com) fused deposition modeling (FDM) 3D printers using either thermoplastic polyurethene (TPU), or NylonX (www.matterhackers.com) filaments, using standard 3D printing profiles in Ultimaker Cura 4.5 (www.ultimaker.com) and a hardened steel 0.4-mm nozzle. Soft polylactic acid (PLA) (www.matterhackers.com) was also tested, and while it was sufficiently soft to generate a mask that could contour to the face, it did not stand up to the repeated flexing, which would be part of the procedure. Three-dimensional printing profiles that were optimized for Ultimaker 2 and Pulse XE for TPU/TPE (thermoplastic elastomer) and NylonX are available upon request. We envision that other commonly used 3D printers, such as Ender 3 Pro (Creality) and Prusa i3 (PrusaResearch), capable of printing with flexible materials can be readily used to generate such protective masks with minimal profile adjustments.
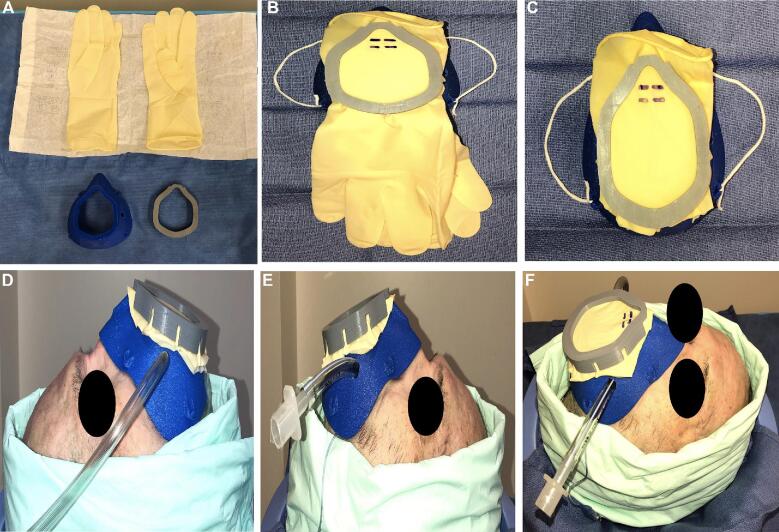
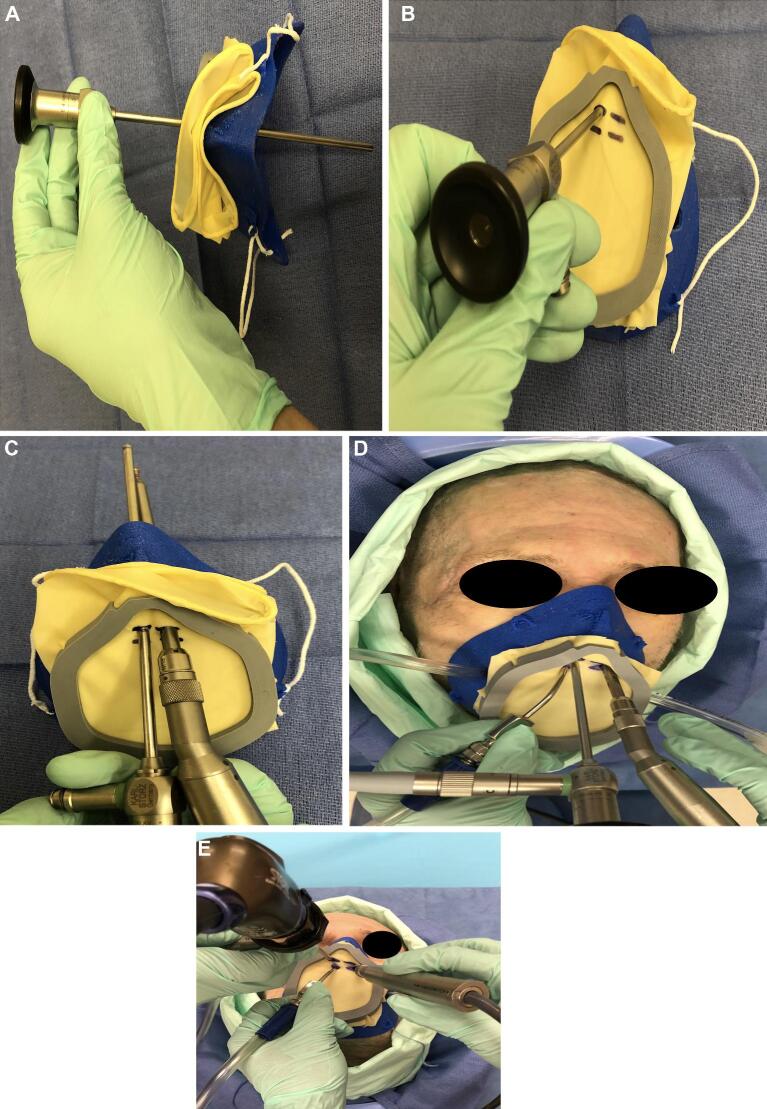
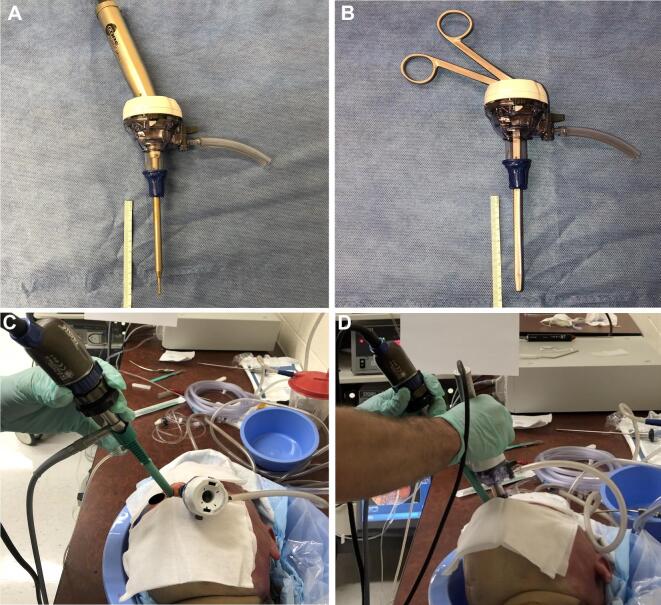
The VPM was sterilized using 90% ethanol prior to use and then 2 surgical glove material inserts (ENCORE®, Latex Ortho) were sandwiched between the mask frame and the retainer clip. An excess glove was trimmed. The cadaveric specimen was intubated with a size 7.5 endotracheal tube and inserted into the mask's endotracheal tube port. The mask was fitted to the cadaver's face and secured with elastic ties. Four superior procedural ports were fashioned using an 11 blade in the glove barrier at the level of the nasal inlet. The VPM’s right-sided suction port was connected with the female-end of a MediChoice®1/4” × 12” suction tube. Wall suction was then engaged (Figure 2A-2F). A 4-handed and a 2-handed surgical approach was performed, and the VPM seal was observed to identify a break in the surgical seal (Figure 3A-3E).
FIGURE 2.
Surgical gloves A are attached to the anterior surface of the 3D printed mask and cut to size B and C. The endotracheal tube is BROUGHT THROUGH the left-side port with the male adapter removed (F) and then replaced once the mask is secured D-F.
FIGURE 3.
A surgical GLOVE placed over the anterior aperture and then cut to size to accommodate instrumentation. Here are superior A and anterior B views of the mask with an endoscope placed through a cut port. The endoscope and surgical drill fit easily and are afforded an adequate range of motion during experimentation C-E.
Nasal Trocar
A 12-mm Universal Trocar Stability Sleeve (Johnson and Johnson, Ethicon, ENDOPATH XCEL®, New Brunswick, New Jersey) was used as the basis for the nasal trocar.
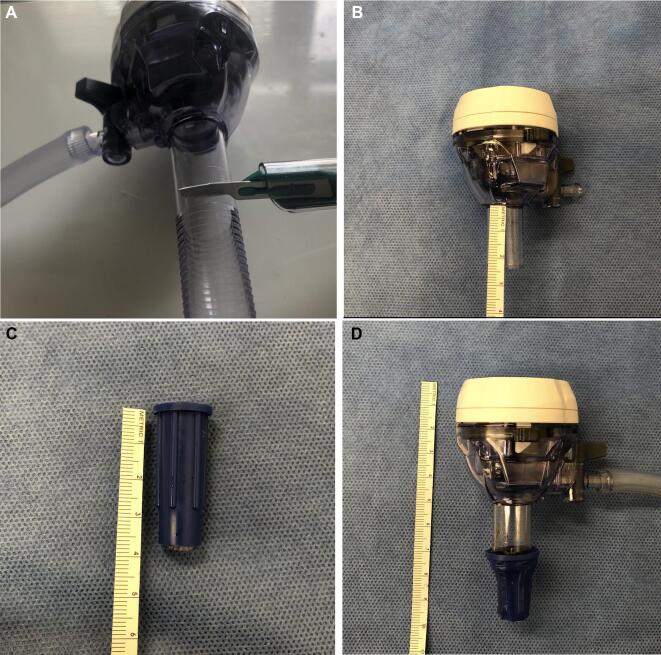
The trocar was cut at the proximal portion of the trocar shaft, leaving 2.0 cm of linear shaft tubing, and was then retrofitted with a previously fashioned adapter created by cutting and trimming the female adapter of a MediChoice®1/4” × 12” suction tube to accommodate the left-sided nasal inlet (Figure 4A-4D). To ensure an effective seal, the Endoscrub endosheath was covered lengthwise with a rubber tubing from a Lukens tube (61/4 [160 mm] polystyrene Aspiration Tube and Stopper, CardinalHealthTM) (Figure 5A-5D). A two-handed surgery approach was employed to drill the sphenoid rostrum and sellar face (Video).
FIGURE 4.
A 12-mm laparoscopic surgery trocar cut transversely 1.5 cm from its base A and B. The end of surgical suction tubing cut to adapt the trocar tip C. The end of surgical tubing modified and attached to the distal end of the trocar with the purpose of creating a seal at the nostril. The trocar has an incorporated suction port with the function of suctioning droplets and debris mainly for the passage of powered instrumentation, particularly the high-speed drill D.
FIGURE 5.
The trocar can accommodate instrumentation ideally longer than 15 cm, as shown with a high-speed drill A and an endoscopic forcep B. The trocar is placed in the left nostril for the passage of instrumentation. The right nostril has rubber tubing taken from a Lukens tube, which will be used to pass the endoscope and ensure an adequate seal C. Placement of endoscope in the right nostril and high-speed drill through the trocar in left nostril D.
Surgical Freedom
Surgical freedom (SF) was calculated.8-10 It indicates the maximum area in which a surgeon is able to move the proximal end of the instrument while the distal end is fixed on a target and is determined by measuring the maximum distance that the portion of the instrument 1 cm above the nostril can move over an axial and sagittal plane in order to determine an area. Measurements were taken with the distal end of the instrument placed over the tuberculum sellae under 3 different conditions: without any barriers, with the trocar, and with the ventilated procedure mask.
Testing of Aerosolization
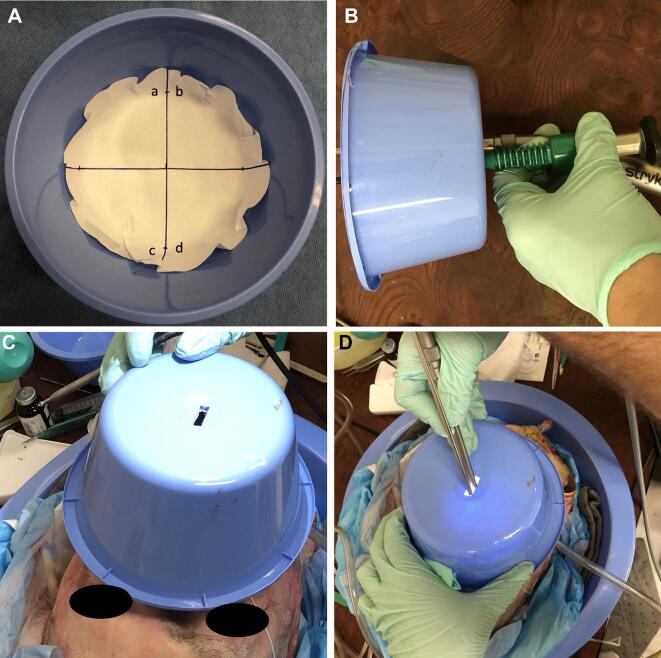
A nasal spray bottle filled with 2.5 mL of fluorescein (AK-FLOR® 10%, 100 mg/mL) was used to coat the nasal cavity for each trial. To contain particle dispersal, a blue surgical basin was lined with printer paper, which contained 4 quadrants (Figure 6A) with an operative port fashioned at the midsection to accommodate a rigid zero-degree endoscope (Karl Storz Endoscopy®) and a high-speed Stryker CORE Sumex drill with a long-angled attachment at 70 000 rpm using a Stryker 4.0-mm Round Fluted Cutting Bur 4 (Figure 6B and 6D) and was placed 6.5 cm from the cadaver's nare (Figure 6C).
FIGURE 6.
White piece of paper cut out to fit the blue basin. Quadrants were marked and labeled A. A small port was made on the basin to allow passage of instruments B. The basin was placed over the specimen C with subsequent passage of instruments D. This was done for all scenarios (without mask, with mask, and with trocar).
The first trialed condition was intranasal drilling of the rostrum, remnant posterior septum, and midnasal floor with the mask and without. The second iteration was meant to mimic anterior nasal drilling and included drilling of the midnasal and anterior nasal septa and anterior nasal floor followed by activation of the drill 0.5 cm outside of the nasal inlet for 5 s. The nasal trocar and rubber tubing system was then tested for aerosolization in a similar fashion.
Following each testing iteration, the printer paper was removed and was placed against a dark surface and then imaged using a blue light filter, as described by Singh et al (Supergel Filter #74 Night Blue, Rosco Laboratories Incorporated, Stamford, Connecticut).11 Each quadrant was imaged separately.
Image Processing
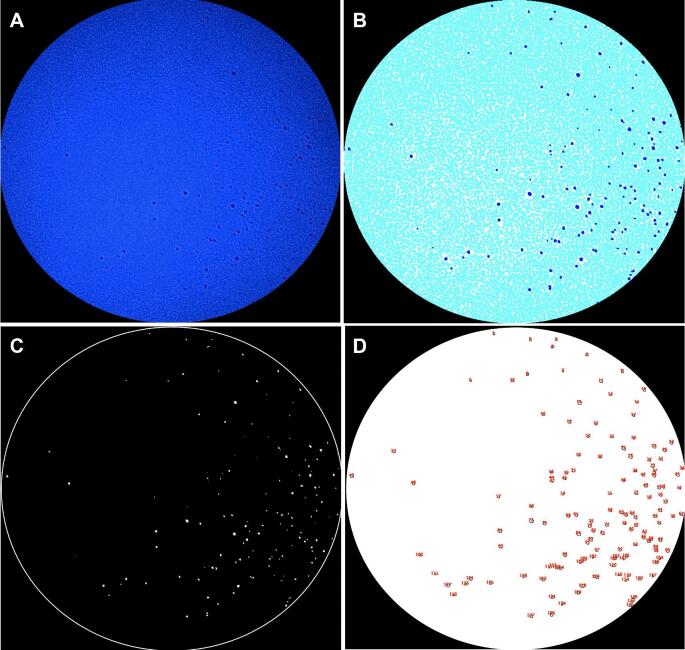
Endoscopic images of each quadrant were exported for analysis and uploaded to Image J (version 1.52v) (Figure 7A). Images underwent brightness and contrast adjustment to 0-pixel intensity and filtering with a minimum of 1 pixel (Figure 7B) and were converted into grayscale and underwent thresholding with a lower limit of 0 and an upper limit of 100 (Figure 7C). Images with droplets present underwent particle analysis to determine the number of droplets present in each image (Figure 7D).
FIGURE 7.
Representative images demonstrating the original cropped image A and image following brightness and contrast adjustment B and thresholding C with visible, accentuated droplets in both images C. D, Analysis of particles with quantification of droplets.
No living subjects were involved; therefore, this study was exempt from review by the Emory University Institutional Review Board.
RESULTS
Ventilated Upper Airway Endoscopic Procedure 3D Printed Mask
Surgical approaches were performed without difficulty and with a continuous facial seal using the 3D printed VPM. SF allowed for the traditional access needed for septoplasty, maxillary antrostomy, total ethmoidectomy, frontal sinusotomy, and sphenoidotomy. In our specimens, the posterior septectomy was widened, the sphenoid floor was drilled flush to the clivus, and the sellar face was skeletonized. Debris and smoke were ventilated with a size 8 Frazier-tip suction, and ambient gas was additionally suctioned by the negative-pressure VPM. Without any barriers an SF of 1.0 cm2 was obtained. SF with the VPM was equivalent (1.0 cm2) to the SF with no mask.
Nasal Trocar
SF was found to be reduced by 55% (SF = 0.45 cm2), and surgery anterior to the sphenoid rostrum was limited when using the trocar system. However, in the presence of a posterior septectomy, the distal end of the instrument could be easily moved between the orbits and could reach critical structures (tuberculum, sella, clivus). This technique accommodated smoke and small particle evacuation during midline skull base approaches to the clivus, sella, and tuberculum (Video, Table 1).
TABLE 1.
Results of Aerosolization Test in the Anterior Nasal Cavity. Results Reported in Number (n) of Droplets
| NO mask | Mask | Mask, spillage | |
|---|---|---|---|
| (n) | (n) | reduction (%) | |
| Quadrant A | 5 | 2 | 60% |
| Quadrant B | 137 | 11 | 92% |
| Quadrant C | 9 | 3 | 67% |
| Quadrant D | 24 | 9 | 63% |
| Total | 175 | 25 | 86% |
Particulate Capture
The presence of the mask reduced particle spillage by 86% with the most significant reduction in quadrant B. For posterior surgery, the mask reduced overall particle spillage by 71%, while the trocar system reduced spillage by 97% (Tables 1 and 2, Table, Supplemental Digital Content 1). The mask led to reductions of 92% for quadrant B and 63% for quadrant D during anterior drilling (Table 1). During posterior drilling, aerosolization was less frequent than anterior drilling, with quadrants B (20 droplets) and D (10 droplets) most frequently contaminated by aerosol. The presence of the VPM reduced aerosolization by 80% in quadrant B and 50% in quadrant D during posterior drilling. The nasal trocar was most successful in aerosol reduction with only a single aerosolized particle noted in quadrant B, representing 95% spillage reduction (Table 2).
TABLE 2.
Results of Aerosolization Test in the Posterior Nasal Cavity. Results Reported in Number (n) of Droplets
| NO mask | Mask | Trocar | Mask, spillage | Trocar, spillage | |
|---|---|---|---|---|---|
| (n) | (n) | (n) | reduction (%) | reduction (%) | |
| Quadrant A | 3 | 1 | 0 | 67% | 100% |
| Quadrant B | 20 | 4 | 1 | 80% | 95% |
| Quadrant C | 5 | 1 | 0 | 80% | 100% |
| Quadrant D | 10 | 5 | 0 | 50% | 100% |
| Total | 38 | 11 | 1 | 71% | 97% |
DISCUSSION
Our study illustrates a novel and easy-to-manufacture mask that can accommodate safer skull base surgery. Our mask can be readily printed on FDM printers (Ultimaker 2 and Pulse XE) and with nonspecialty and easy-to-acquire flexible filaments. While one-time use of the mask is advisable due to its low price (approximately 2 USD per mask) and expedient production (4 h), the thermosplastic VPM can also be cleaned by soaking in 90% ethanol or running through hydrogen peroxide vapor sterilization if repeated use is planned.
Our results indicate that the VPM markedly reduces particle dispersal, allows for an appropriate surgical range of motion, and is augmented by negative pressure to accommodate for differences in facial topography. The small degree of spillage appeared to occur during instrument removal. Future iterations will identify material that better reduces this spillage.
We did not have issues in our cadaveric specimens with the surgical seal. While the universal mask works well for most individuals, variation in facial anatomy may perturb the air-tight seal. Therefore, future design iterations will incorporate preexisting computed tomography (CT) images to better match the printed mask with patient topographic features. Additional applications for this mask include upper airway endoscopy and in-office nasal procedures, and we are actively working on these applications.
Limitations
This study has several limitations, including 3D printer availability, mask generalizability to narrow faces or particularly elongated noses, and limited sterilization options due to the masks being printed in a thermoplastic polymer at a low glass transition temperature. However, future designs can be better tailored for patient-specific anatomic concerns, especially in instances where CT scans have been performed, or there is access to a 3D scanner. We urge caution during instances where there is a breach in the dura. While we anticipate that the mask will only create a small vacuum at the level of the nasal cavity, it is unclear the extent of pressure that will be created once the dura has been breached. Sterilization of the mask also has to be considered, as the autoclave may deform the mask structure. However, sterilization via 90% ethanol or low-temperature methods is a viable option that is widely available. Our aerosolization studies provide a helpful snapshot of the degree difference in aerosolization between mask and no-mask conditions. A clear limitation of this method of assessment is that the degree of aerosolization for a full procedure was not assessed. It is certainly possible that the glove material, after repeated passes, may break down and the aperture may widen. While several materials exist that could supplant the glove for the mask's anterior aperture, gloves are cheap and ubiquitous. As such, the glove may have to be replaced during the course of surgery if visible wear and tear is noted. We intend to determine the extent of seal interruption during future studies and will further assess the ease of use of additional surgical materials such as silicone rubber membranes, polydimethylsiloxane (PDMS), and soft urethane rubber. Membrane opening can also be performed with a round needle (diameters can vary from 3 mm/3F to 35 mm/34F), which can allow for better structural integrity than the slit made by an 11 blade.
The nasal trocar system demonstrates a strong seal during posterior drilling procedures. We developed nasal trocar surgery as a workaround for institutions that do not have 3D printers. Anterior surgery, such as septoplasty, or functional endoscopic sinus surgery is likely to be not realistic with this approach due to the limited range of motion with the nasal trocars. This technique is best employed in instances where a posterior septectomy has already been performed and only drilling remained to be completed. From a technical standpoint, fashioning the trocar can be time-consuming during initial attempts.
CONCLUSION
We present a 3D printed mask with modifications that allow for appropriate surgical degrees of freedom, maneuverability, low cost, and safety during EES. The nasal trocar serves as a helpful workaround to allow drilling during EES in instances where a 3D printer is not available.
Disclosures
Dr Levy has funding from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR002378 and KL2TR002381. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental Digital Content 1. Table. Particles were collated by direct inspection by two authors (SNH, RMS) and then correlated to the Image J software. Data demonstrate impressive aerosolization not readily discernable to the naked eye.
COMMENT
The COVID-19 pandemic posed several obstacles in health care and at the same time stimulated creativity and innovation to overcome these challenges. This is an interesting pilot study on strategies to deal with aerosolization that occurs in endoscopic endonasal surgeries, particularly during drilling.
The authors proposed the use of a 3D-printed mask with an anterior aperture and a modified laparoscopic surgery trocar with the goal to reduce the risks of pathogen transmission during endoscopic endonasal skull base surgery.
Assuming that there is a direct correlation between these procedures and increased risk of COVID-19 transmission to healthcare workers, the modified facemask and trocars are ingenious tools to reduce this risk. Although the “wear and tear” effect on the devices was not evaluated, it is a factor to be considered in long procedures where the effectiveness of the seal may be compromised.
Whenever considering solutions to eliminate or reduce aerosolization in the surgical field, there is a challenging trade-off between maximal surgical maneuverability and seal. Having the instruments inside a closed space, such as the “intubation box” being used by anesthesiologists, would be ideal by providing the maximal protection and no impact in surgical instruments maneuverability. Nevertheless, the main limitation for endoscopic endonasal surgeries would be the need to have all the instruments inside the closed space to maintain seal of the surgical field.
The authors have done a great job in the qualitative assessment of their devices and should be commended for the ingenuity and innovation.
André Beer-Furlan
Chicago, Illinois
REFERENCES
- 1. Patel ZMFernandez-Miranda JHwang PHet al.. Letter: precautions for endoscopic transnasal skull base surgery during the COVID-19 pandemic. Neurosurgery. published online: April 15, 2020 (doi:10.1093/neuros/nyaa125). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Workman ADWelling DBCarter BSet al.. Endonasal instrumentation and aerosolization risk in the era of COVID-19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. published online: April 3, 2020 (doi:10.1002/alr.22577). [DOI] [PubMed] [Google Scholar]
- 3. Coronavirus Disease (COVID-2019) Situation Reports . World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Last accessed April 17, 2020. [Google Scholar]
- 4. Huang X, Zhu W, Zhao H, Jiang X. In reply: precautions for endoscopic transnasal skull base surgery during the COVID-19 pandemic. Neurosurgery. published online: April 17, 2020 (doi:10.1093/neuros/nyaa145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo YRCao QDHong ZSet al.. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou LRuan FHuang Met al.. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Doremalen NBushmaker TMorris DHet al.. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maza G, Omar AMM, Subramaniam S, Otto BA, Prevedello DM, Carrau RL. Modified endoscopic endonasal approach with a minimally invasive transoral approach—an adjunct to infrapetrous approaches. Laryngoscope. 2019;129(2):339-343. [DOI] [PubMed] [Google Scholar]
- 9. Wilson DA, Williamson RW, Preul MC, Little AS. Comparative analysis of surgical freedom and angle of attack of two minimal-access endoscopic transmaxillary approaches to the anterolateral skull base. World Neurosurg. 2014;82(3-4):e487-e493. [DOI] [PubMed] [Google Scholar]
- 10. Upadhyay SDolci RLBuohliqah Let al.. Effect of incremental endoscopic maxillectomy on surgical exposure of the pterygopalatine and infratemporal fossae. J Neurol Surg B. 2016;77(1):066-074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh NP, Roberts DN. An inexpensive blue filter for fluorescein-assisted repair of cerebrospinal fluid rhinorrhea. Laryngoscope. 2014;124(5):1103-1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.