Abstract
Despite the vital role of vaccines in fighting viral pathogens, effective vaccines are still unavailable for many infectious diseases. The importance of vaccines cannot be overstated during the outbreak of a pandemic, such as the coronavirus disease 2019 (COVID-19) pandemic. The understanding of genomics, structural biology, and innate/adaptive immunity have expanded the toolkits available for current vaccine development. However, sudden outbreaks and the requirement of population-level immunization still pose great challenges in today’s vaccine designs. Well-established vaccine development protocols from previous experiences are in place to guide the pipelines of vaccine development for emerging viral diseases. Nevertheless, vaccine development may follow different paradigms during a pandemic. For example, multiple vaccine candidates must be pushed into clinical trials simultaneously and manufacturing capability must be scaled up in early stages. Factors from essential features of safety and efficacy, manufacturing, and distributions, to administration approaches, are taken into consideration based on advances in materials science and engineering technologies. In this review, we present recent advances in vaccine development by focusing on vaccine discovery, formulation, and delivery devices enabled by alternative administration approaches. We hope to shed light on developing better solutions for faster and better vaccine development strategies through the use of biomaterials, biomolecular engineering, nanotechnology, and microfabrication techniques.
Keywords: COVID-19, pandemics, infectious disease, vaccine, immunotherapy, drug discovery, drug delivery, biomedical devices
Graphical Abstract
The appearance of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) has caused unprecedented global disruption to health, economy, and social stability.1 SARS-CoV-2 causes coronavirus disease 2019 (COVID-19) in which individuals can suffer from mild to severe symptoms once infected.2 Global scientific communities are working together to fight the crisis caused by this pandemic. The initial efforts to combat this pandemic included identifying infected patients and ramping up clinical trials of repurposing existing drugs.3 However, the development of effective vaccines to prevent SARS-CoV-2 infection is believed by many to be the most effective long-term solution.4
The pathway from identifying a virus to having a vaccine is lengthy and expensive. After billions of dollars spent and multiple years of trials, researchers generally face high rates of failure in the traditional vaccine development paradigms (Figure 1a).4 Despite rapid responses from the scientific community and the pharmaceutical industry, vaccines have historically been not ready even after an epidemic becomes manageable, such as severe acute respiratory syndrome (SARS), Zika, and Ebola.5 Considering economic factors, funding for vaccine development may be reallocated after a substantial drop in infection cases is observed, as in the 2015-2016 Zika and SARS epidemics. The 2014-2016 Ebola outbreak in Africa resulted in over 28,000 cases and 11,000 deaths.6 After continuous research funding support, in Dec. 2019, the FDA granted the first approval to Ervebo (Single-dose, live attenuated virus vaccine from Merck) for Ebola virus prevention.7 Benefiting from egg- and cell-based platforms, vaccine approval for the most recent 2009 H1N1 Pandemic was relatively fast following the peak of the outbreak, and the vaccine was ultimately incorporated in the seasonal influenza vaccine.8 Since the initiation and ending of epidemics are highly unpredictable, the rapid deployment of vaccine development may still be too slow for a sudden outbreak.4,5
Figure 1.
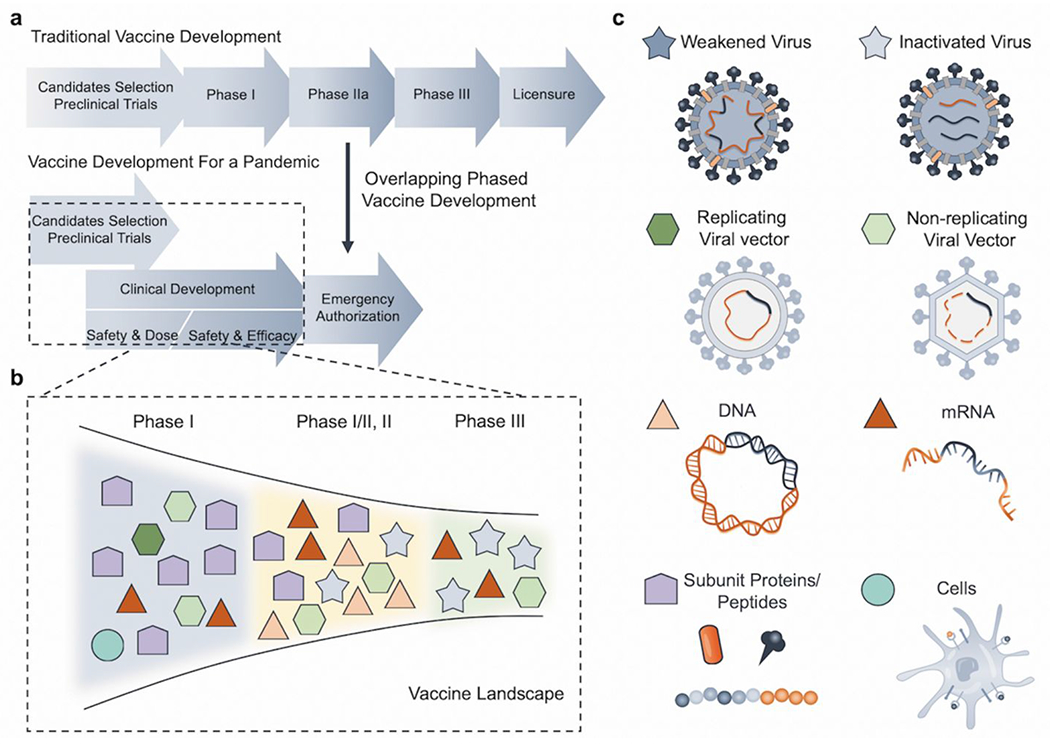
Summary of vaccine development paradigms and major types of vaccines. (a) Paradigms of vaccine development in a traditional condition vs. during a pandemic.5 (b) Snapshot of vaccine landscape for COVID-19 with the different types of vaccine candidates in various clinical stages as of Aug. 25, 2020.10 (c) Schematic of major types of vaccines, including virus-based (weakened and inactivated), viral vector-based (replicating and non-replicating), nucleic acid-based (DNA and mRNA), protein and peptide-based, and cell-based.13
To tackle a pandemic, a different vaccine development process is needed to rapidly produce safe and effective vaccines against novel viruses. This process is characterized by overlapping phases, multiple potential vaccine candidates, and early ramp-up of manufacturing capacities (Figure 1a).4 After the initial release of the genetic sequence of SARS-CoV-2, researchers worldwide started to develop vaccines for the prevention of COVID-19. The first dose was administered into humans by Moderna Inc. on Mar. 16, 2020.9 This method utilized lipid nanoparticles (LNP) with a formulated mRNA vaccine.9 This work was fast-tracked at an unprecedented pace, even though mRNA-based vaccines were never licensed before.9 A snapshot of the landscape of COVID-19 vaccines (Figure 1b) shows that around 31 vaccines have entered clinical trials as of Aug. 25, 2020.10 A high diversity of vaccine candidates is seen in these forerunners, which includes six mRNA-based, five inactivated virus-based, five non-replicating viral vector-based, one replicating viral vector-based, four DNA-based, one cell culture-based vaccines, as well as nine protein-based vaccines (Figure 1b, c). The incomplete understanding of the SARS-CoV-2 interactions with the human immune system became one of the major obstacles in vaccine development. A recent report on a second COVID-19 infection on the same patient further raises the question of protective immunity and immune memory. For instance, it is still unknown how durable the immune memory response induced by SARS-CoV-2 could be or what is the threshold of antibodies titers that can protect the patients against reinfection.11,12 In addition, protective immunity against SARS-Cov-2 should also be comprehensively studied, where the balance of cellular and humoral immunity, the ratio of effector to memory T cells, the maintenance of memory B cells, and functional features of activated T cells should be characterized.11,12 These aspects could ultimately facilitate vaccine designs and evaluations.
In this review, we aim to provide engineering insights on vaccine development by covering vaccine discovery, vaccine formulations in terms of different materials (lipid, polymer, and inorganic particles), and vaccine delivery devices. We will present how some of the existing challenges could potentially be addressed by nano/micro/macroscale engineering approaches. We will also discuss some emerging technologies that may contribute to future vaccine development pipelines.
VACCINE DISCOVERY
After a virus is identified, different categories of vaccines can be developed, which includes virus-, viral vector-, nucleic acid-, protein/peptide-, and/or cell-based vaccines (Figure 1c).13 Virus-based vaccines commonly require patient-derived viruses, while other types can utilize the genomic sequence information of viruses to accelerate vaccine development. Previously approved vaccines mostly fall into the virus-based and the recombinant protein-based categories, but not into the nucleic acid-based one (Figure 2a).14 The discovery process of each vaccine type is distinct with corresponding pros and cons (Figure 2b–d).
Figure 2.
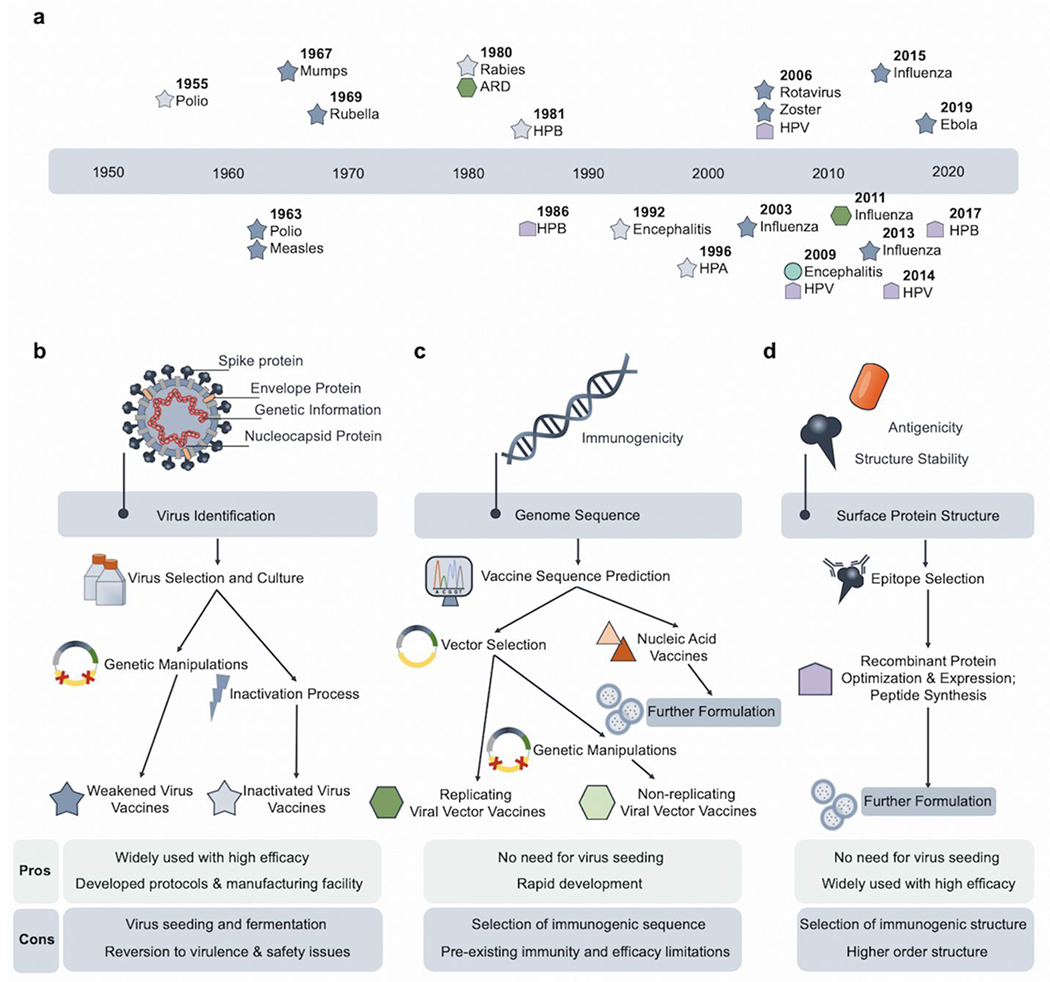
Vaccine discovery processes for different types of vaccines. (a) Timeline of previously approved antiviral vaccines.14 (b) Virus-based vaccines require the seeding and culture of specific viruses derived from patients. By manipulating the genetic sequences of the virus or inactivating the virus directly with chemicals, weakened and inactivated virus vaccines can be produced.15 (c) Viral vector-based and nucleic acid-based vaccines are independent of virus culture and rely on the genetic sequence of the virus and/or the selection of immunogenic sequences of the virus. By selecting commonly developed vectors, virus-specific sequences can be inserted.16 Nucleic acid vaccines require further formulations for optimal efficacy. (d) Structure-based understanding of native viral proteins can validate the expressed recombinant proteins and predict the peptide sequences desirable as vaccines. Protein/peptide vaccines require further formulations for optimal efficacy.17
Virus-based vaccines:
Virus-based vaccines, including the weakened and inactivated types, are still the most widely used to date. The effective prevention of the polio epidemic by vaccination in the 20th century represented a landmark success in medical history.18 This vaccination regimen included the use of the inactivated polio vaccine (IPV) and oral polio vaccine (OPV).18 The IPV was made by inactivating lab-cultured poliovirus by formalin. The OPV was weakened live poliovirus.18 Weakened viruses are highly immunogenic and are commonly developed by the deletion of viral genes. Nevertheless, mutations could cause virulence reversion as observed for vaccine-derived polioviruses that have been responsible for recent polio outbreaks.19 This represents an obstacle for polio eradication, and more importantly, a concern for the weakened virus-based vaccines in general. Further genetic manipulation strategies are therefore required to prevent this issue. For instance, modifying the 5’ untranslated region (UTR), 2C coding region, and 3D polymerase region resulted in a genetically stabilized OPV strain that was less likely to regain virulence and exhibited limited viral adaptability.20
On the contrary, inactivated virus vaccines generally do not have virulence reversion issues. They also do not rely on extensive genetic manipulations, but the inactivation process may cause the loss of antigenicity of the immunogen. This vaccine type has been quickly adapted to cope with COVID-19 where patient-derived viruses were inactivated with β-propiolactone, followed with purification by chromatography, and formulated with Al(OH)3 as adjuvant.15 The vaccine protected non-human primates from SARS-Cov-2 challenges with no notable side effects.15 Advantages of these virus-based methods include well-developed methodologies, well-established regulation policies, and existing manufacturing facilities from previous practices. Nevertheless, the requirement of virus seeding and culture along with unknown safety issues necessitates alternative practices for the development of other vaccines.
Viral vector-based vaccines:
Viral vector-based vaccines work by inserting viral antigen-encoding DNA sequences into host cells, which leads to the expression of viral antigens by the host cells.16 The rationale is based on the capability of viruses to infect cells efficiently through viral integration mechanisms.16 This method could also be problematic since the genome of the host could be altered and cause other diseases. The selection of viruses is therefore crucial in balancing the efficacy (efficient infectivity) and safety (limited pathogenicity). Another common obstacle is that the host’s pre-existing immunity to certain vectors that would mitigate the efficacy of a vaccine. The selected viral vectors could be further genetically modified to be non-replicable by disrupting genes responsible for replication. Adenoviruses (Ad) are the most extensively used vectors for vaccines because of several advantages: infectious to various cell types, efficient transgene expression, high in vitro growth, lack of genome integration, and genetic stability.21 For instance, Johnson & Johnson (J & J) deployed the Ad26 vector to deliver genes encoding for surface proteins of the SARS-CoV-2; and CanSino Biological used the Ad5 vector.22 The spike glycoprotein of SARS-CoV-2 was cloned with the tissue plasminogen activator signal peptide gene in the early region 1 (E1) and early region 3 (E3) deleted Ad5 vector. The E1 and E3 deletion render the Ad5 vector non-replicating. Even though rapid humoral and T cell responses were induced by the vaccine, pre-existing anti-Ad5 immunity partially diminished the immune responses among healthy adults.22 To address the pre-existing immunity to Ad, chimpanzee Ad (ChAdOX1) represents an alternative solution that provided broad protective immunity against Middle East Respiratory Syndrome Coronavirus (MERS-Cov).23 An investigational vaccine for SARS-CoV-2 from the University of Oxford is based on this vector.23 Their preclinical study showed the prevention of SARS-CoV-2 pneumonia in rhesus macaques with no evidence of subsequent immune-enhanced diseases.23 However, viral loads were more significantly reduced in the lower respiratory tract but not in the nose swabs. In general, because of previous vaccine programs, the manufacturing capacity and protocols for this method are already well-established; therefore, less effort may be needed to shift towards the large-scale production of vector-based vaccines. However, both the pre-existing immunity towards certain vectors and previously demonstrated safety concerns over the increased risk of human immunodeficient virus type 1 (HIV-1) acquisition should be carefully evaluated.22
Nucleic acid-based vaccines:
DNA- and mRNA-based vaccines have the greatest potential for rapid development because of their synthetic nature that circumvents the need for cell culture or fermentation of viruses. The synthetic nature of this method also results in a high safety profile and rapid manufacturing ability.24 However, this method generally requires further delivery formulations or better delivery devices to boost vaccine efficacy.24 In contrast to RNA, the increased stability of DNA decreases the need for frozen storage and low-temperature transportation.24 One of the forerunners among COVID-19 vaccines is DNA-based (Inovio).10 Similar DNA-based vaccines have had promising results for the prevention of MERS.25 In the discovery phase of DNA-based vaccines, strategies such as codon/RNA optimization and the addition of highly efficient immunoglobulin leader sequences are needed to enhance the magnitude and breadth of the immune response.26 For instance, Muthumani et al. designed a consensus DNA sequence for the spike protein by analyzing S protein sequences, where sequences from clades A and B can be both involved.26 To enhance the in vivo expression, the immunoglobulin E (IgE) leader sequence was added to the immunogen sequence. The insert was further incorporated into the pVax1 vector, which allowed high-level transient expression of proteins of interest in mammalian cells.26 The strategies of adding the IgE leader sequence and the use of the pVax1 vector could be quickly adapted to the different virus sequences as well.
Due to their high potency, rapid development, and low cost for manufacturing, mRNA vaccines have emerged as a promising alternative vaccination development strategy for various infectious diseases and cancers.27 No mRNA-based vaccines are currently FDA-approved. Several strategies have been proposed to solve the common drawbacks of mRNA-based vaccines, which include mRNA instability and high innate immunogenicity.28 The 5’ and 3’ UTR of mRNA has significant regulatory influences on both stability and translatability.28 Half-life and expression can be enhanced when these sequences come from viral genes: a 5’ cap is essential for efficient protein expression, and a poly (A) tail affects mRNA translation and stability.29 Furthermore, the replacement of rare codons with frequently used synonymous codons also enhances protein expression.30 Additional optimization strategies include increasing G:C content and introducing modified nucleosides.31,32 In spite of the innate immunostimulatory effect of exogenous or unpurified mRNA, further investigations are required to assess the advantages of their use for vaccines specifically.33 Innate immune sensing mechanisms of mRNA has resulted in inhibition of antigen expression and a decreased immune response.34 Therefore, further formulations for vaccine delivery could potentially reduce innate mRNA-associated immunogenicity and involve favorable immune activation.
Subunit protein/peptide vaccines:
Unlike nucleic acid-based vaccines, which produce viral protein after administration, the direct application of viral protein antigens may be a more straightforward method to trigger immunity. This approach currently represents the largest category of COVID-19 vaccines under preclinical investigation.4,10 Full-length proteins are advantageous because they can induce the development of antibodies against multiple epitopes. Importantly, there is a higher probability that the developed antibodies could bind to the native conformation of the viral protein. However, this also increases the chance of inducing non-specific cross-reactive antibodies.35 In addition to the high cost of recombinant protein technologies, the recombinant protein products may not fully match all the molecular features of the corresponding viral proteins, which may lead to ineffective induction of broad neutralizing antibodies (bNAb).35 Optimizing peptide sequences in the discovery phase can improve the stabilization of epitopes associated with bNAb while reducing the antigenicity of non-NAb epitopes.17
Given that only specific amino acid sequences of the full-length protein antigens are responsible for effective immune responses, minimally immunogenic peptides, mimicking B and T cell epitopes, have been proposed for vaccines.17 Early approaches used short peptide sequences (8-10 amino acids) that could potentially be presented by Major Histocompatibility Complex (MHC) I molecules. Importantly, the memory immune response of cytotoxic T cells could not be efficiently induced with such T cell epitope-mimicking peptides.36 One proposed rationale is that in vivo direct loading of short peptides occurs for all cells expressing MHC molecules, including T and B cells. Unlike DCs, they are incapable of generating effective immune response.37 These early findings benefitted the design of synthetic long peptide (SLP) vaccines where both helper T cells (TH) peptides and TLR ligand or peptides could be hybridized to enhance the immune response. For example, Jackson et al. demonstrated one single SLP vaccine that can target TLR 2 can serve as self-adjuvant and contain both MHC I- and II-specific peptides (T cell epitopes).38 SLP cannot directly bind with MHC I and have to be internalized by DCs for further presentation. Furthermore, in silico computer-based approaches can be used to benefit the discovery of peptide vaccines by enabling quicker screening and more accurate predictions of peptide sequences with high immunogenicity and binding affinities to MHC I and II molecules.39 These approaches have been used to accelerate the development pipeline of vaccines, and particularly for emerging viral diseases such as COVID-19. These computational approaches can predict the binding affinity of specific peptides sequences to either MHC I and II molecules or B cell receptors/antibodies. In this regard, theoretical B and T cell epitopes specific to SARS-CoV-2 were synthesized to generate a multi-epitope protein.39 The antigenicity, allergenicity, physiochemical parameter, secondary and tertiary structures of these multi-epitope proteins were determined in silico. However, the processing in the endosomal/lysosomal compartments of the designed multi-epitope SARS-CoV-2 antigens remains to be addressed.
Cell-based vaccines:
DCs are the most potent antigen-presenting cells (APCs) at the interface of innate and adaptive immunity to sense the “danger signals”, process the antigens, and present the non-self-antigens.40 Unlike other types of vaccines, DC vaccines are manipulated ex vivo to be antigen-specific and thus they are ready to initiate immune responses after being re-infused in vivo. The DC vaccine is quality-controllable and natively able to traffic efficiently in vivo. Different methodologies have been developed to produce modified DC vaccine ex vivo, including loading DCs with immunogenic peptides or proteins (subunit DCs), viral transduction to express immunogenic peptides,41 or using mRNA-based DCs.42 Different strategies may produce DC vaccines with different potency, for instance, genetically modified DCs may be more efficacious in immune activation than subunit DC vaccines41. In addition, supplementary immunomodulatory genes could be incorporated to enhance the potency. However, the process of producing cell-based vaccines is labor-intensive and expensive.43 Thus, this method could be the most challenging to be scaled up as a universal vaccine for a global pandemic.43
VACCINE FORMULATIONS
Action mechanisms:
Vaccines are commonly administered intramuscularly, making it less efficient in interacting with the abundant immune cells present within the skin. Alternative strategies, including oral or intranasal deliveries, are also widely studied because of their ease of use.44 The effectiveness of alternative strategies is based on mucosal immunity.44 Nonetheless, different strategies rely on the activation of the adaptive immune system, which involves the interactions between T cells and APCs (DCs and B cells).12 As shown in Figure 3a, APCs can internalize exogenous antigens and process them into antigenic peptide fragments. The peptide fragments can subsequently be loaded onto the MHC II molecules and displayed on the surface of the APCs.12,45 CD4+ helper T cells could recognize peptides presented by MHC class II through T cell receptors (TCRs), which could induce the production of cytokines, such as IL-2, that are essential to the normal function of immune cells.12,45 B cells can directly capture antigens through B-cell receptors (BCRs). The antigens can be internalized and subsequently processed into antigenic peptides for presentation by MHC class II to CD4+ helper T cells. This process is required for the clonal expansion and differentiation of B cells into IgG antibody-secreting plasma cells and memory B cells, which is classified as humoral immunity.12,45 Generally, only endogenous antigens could be degraded into peptides and loaded onto MHC class I molecules for presentation to CD8+ T cells.12 Cross-presentation is the ability of certain APCs to uptake, process, and present extracellular antigens in an MHC class I-dependent manner to CD8+ T cells. Cross-presentation can be achieved by several cell types, including DCs (the primary cell type), neutrophils, macrophages, and endothelial cells.12 The MHC class I-presented peptide fragments can activate CD8+ cytotoxic T cells through TCR recognition. Antigen-specific CD8+ cytotoxic T cells can then recognize these peptide fragments presented by target cells and kill them, which refers to cellular immunity.12,45 Lastly, DCs are also capable of detecting “danger signals” via an array of receptors called pattern recognition receptors (PRRs). PRRs are capable of recognizing pathogenic molecules as part of the innate immunity for a response to virus infection by identifying pathogen-associated molecular patterns (PAMPs) from viruses or microbes. The understanding of innate immunity could benefit the development of adjuvants to enhance the immune response further. Together, the understanding of the action mechanisms facilitates the design of vaccine formulations to boost their efficacy.46
Figure 3.
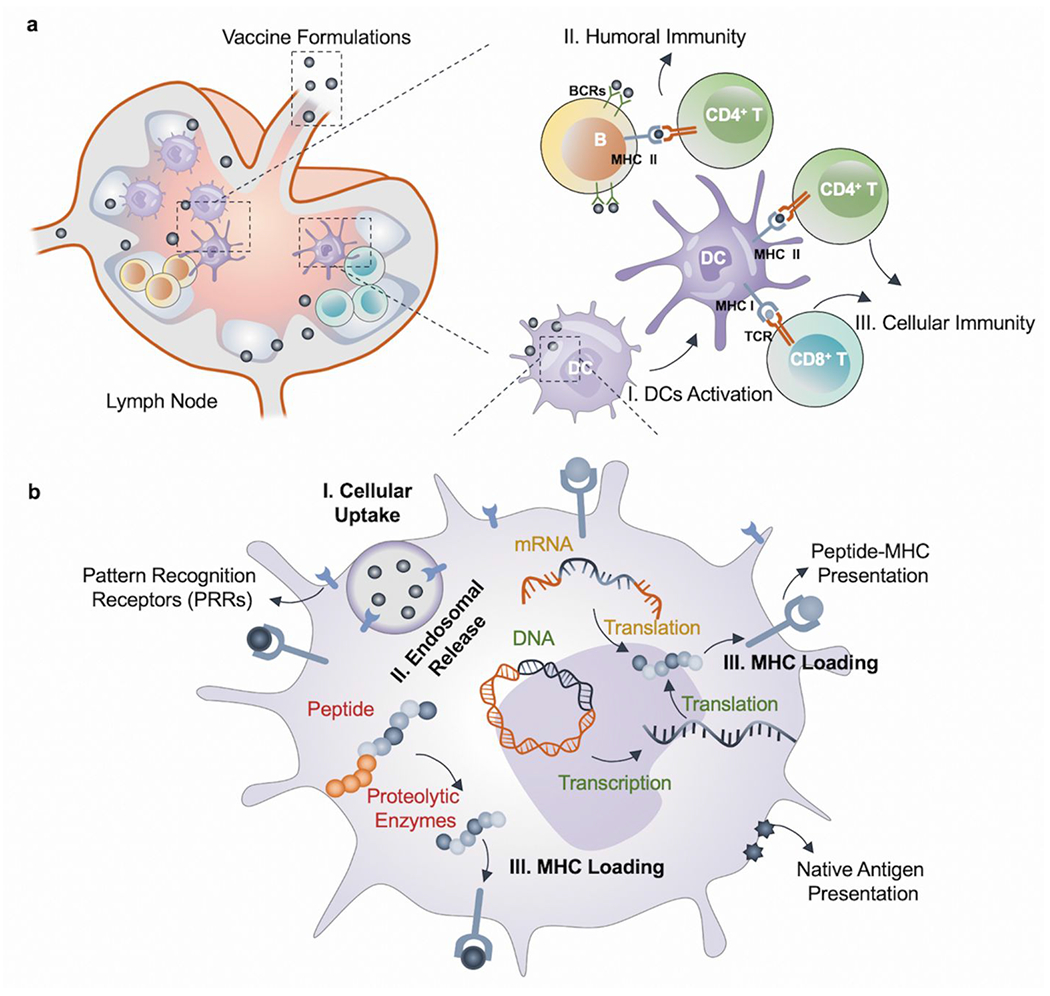
Action mechanisms of vaccine formulations. (a) Vaccines generate humoral and cellular immunity within lymph nodes (LNs): I. DCs can process the antigens and present the peptide fragments via both MHC class I and class II molecules. II. B cells can directly recognize the antigens via BCRs and present the antigenic peptide fragments by MHC class II to helper T cells (CD 4+). Stimulated B cells can subsequently initiate a humoral immune response. III. Cytotoxic T cells (CD 8+) can recognize the antigenic peptide fragments presented by MHC class I through TCRs and trigger the cellular immune response.12,45 (b) Intracellular response of DCs to antigen presentation for different types of vaccines through PRRs. I. Vaccine formulations can be effectively uptaken by cells, followed by II. Endosomal release. Before III. MHC loading of antigen peptide, peptide vaccine undergoes enzymatic processing, DNA vaccine undergoes transcription and translation, and mRNA vaccine undergoes translation.24,27,37
Requirement of formulations:
Based on the current understanding of eliciting optimal immune responses, materials-based nanoengineering approaches could improve the efficacy of vaccines. In this regard, different methodologies could be applied based on different types of vaccines, with either distinct or shared requirements. Virus and viral vector-based vaccines generally do not require further formulations and their efficacy and safety profiles are largely dependent on the purification and inactivation strategies, the optimization of culturing of viral particles, and the choice of vectors.47 They have efficient innate advantages in delivery due to their small sizes, and they are easily recognized by the immune system. Although inactivated or weakened virus-based vaccines still make up most of the effective vaccines today, they are not effective for all pathogens.47 Similarly, the potency of DC vaccines is mostly dependent on how DCs are manipulated ex vivo. Strategies in further potentiating cell-based immunotherapies, such as the decoration with nanoparticles (NPs),48 are mainly developed for cancer immunotherapies, but they may be less suitable for large scale manufacturing that is needed for the production of antiviral vaccines.
In contrast, synthetic vaccines (nucleic acid-based and protein/peptide-based ones) generally require additional formulation designs for optimal efficacy.49 These synthetic molecules all have essential secondary or higher-order structures that could be disrupted in physiological conditions.12 Various endogenous enzymes (such as nuclease or protease) can digest these synthetic molecules before reaching immunocompetent cells, such as APCs, or immunocompetent sites, such as secondary lymphoid organs.12 Furthermore, physiochemical features of these synthetic molecules may pose challenges for cells to efficiently uptake and process them. For instance, DNA and mRNA are too large to directly diffuse across the cellular membrane and their dense negative charge repels them from cell membranes, which further prevents efficient cellular uptake.27 Even after penetrating the membrane, the vaccine formulations need to reach the endosomal compartments either to be processed into peptides (protein/peptide-based vaccines), released for further transcription (DNA-based vaccines), or translation (mRNA-based vaccines). The resulting peptide fragments can be further loaded onto MHC molecules and exported to the membrane surface for antigen presentation to T cells (Figure 3b).50
We refer vaccine formulations to engineering approaches that could facilitate the in vivo delivery of vaccines and enhance immune responses.51 We categorize the vaccine formulations in terms of commonly used materials: lipids, polymeric materials, and inorganic particles (Figure 4). Commonly pursued feature goals include protection of cargo, improving loading capacity, and enhancing cellular uptake (Figure 4a).52 In addition, the ideal microenvironment for initiating and amplifying immune responses is secondary lymphoid organs where APCs, T cells, and B cells are in close proximity for cell-to-cell interactions. Therefore, targeting DC or LN can be one type of approach to pursue (Figure 4b).53 To further boost immune responses, immunostimulatory components or adjuvants are usually required, and co-formulation approaches have been widely explored for developing immunostimulatory vaccine formulations (Figure 4c).
Figure 4.
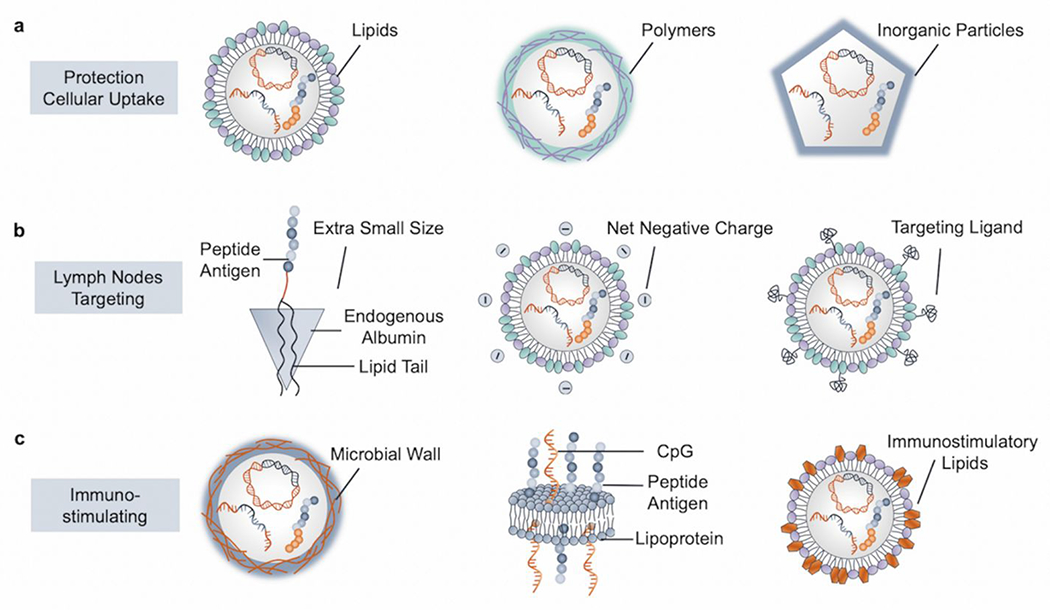
Materials-based vaccine formulations for important immunological functions. (a) Vaccine formulations that protect the active “cargo” and enhance cellular uptake may be based on lipids, polymeric materials, and/or inorganic particles. (b) Vaccine formulations that allow LN targeting by forming extra small-sized albumin-based vaccine complexes, tuning the net charge to be negative, and incorporating targeting ligands. (c) Vaccine formulations which promote immunostimulatory effects by using microbial wall derived polysaccharides as nanocarriers, co-delivering adjuvants with peptide antigens, and exploring innate immunostimulatory lipids.
Lipids:
Lipid molecules are suitable for vaccine formulations because of their high safety profile and tunable physiochemical features.55,59 These advantages also make them the leading platform in developing vaccines quickly. Currently, there are two mRNA vaccine forerunners in development for COVID-19 that are based on lipid formulations (Moderna and BioNTech).10 Lipids are a class of amphiphiles that contain hydrophilic head groups and hydrophobic tails. Interactions between those molecules can drive the formation of spherical vesicles to encapsulate vaccine formulations either with one (unilamellar) or more (multilamellar) lipids bilayers.55 Lipid molecules have also been explored to achieve distinct biological functions.59 For instance, cationic lipids are commonly used for efficient cellular uptake and increase the loading of negatively charged mRNA and DNA.56 DOTMA, DOTAP, and DOPE are common examples.56 However, cationic lipids are commonly associated with high toxicity and neutralization by anionic serum proteins, which reduces the delivery efficacy.83 Therefore, ionizable lipids that can change their charge state at different pH conditions can be utilized to maintain efficacy, to reduce toxicity, and to facilitate the endosomal release of cargos.84 Some common strategies to improve the efficacy of LNPs include enhancing cellular uptake and promoting endosomal escape by incorporating cholesterol, polyethylene glycol (PEG) lipids, or helper lipids DOPE.56 Hydrophobic cholesterol can fill the gaps between lipid tails to stabilize the vesicle. PEG lipids can shield the vesicles and protect them from clearance when systemically delivered.56 Helper lipids have unsaturated bonds to form an unstable hexagonal lamellar phase that enhance the endosomal escape.85 In addition, good biocompatibility of lipids makes it possible for expedited FDA approvals.86 Therefore, LNPs can be optimized in vaccine formulations to stabilize and enhance uptake of cargo, to improve immunostimulatory functions for antigen, and for targeted delivery. Oberli et al. utilized an ionizable lipid, a phospholipid, cholesterol, and a PEG anchored lipid as LNP formulations for mRNA vaccine delivery, which resulted in stable nanoformulations, efficient antigen presentation, and elicitation of a strong T cell response.54 However, the in vivo potency of LNP-based vaccines could be limited by their suboptimal physiological stability, which can cause the fast release of antigens before initiating any immune response. Moon et al. developed a hyperstabilized lipid-based vaccine formulation by covalently crosslinking two lipid molecule layers between their head groups using a mild chemical condition that is compatible with loaded antigens.55 The resulting vesicles are stabilized multilamellar structures that could trap high levels of protein antigens. Combined with PEGylation as a well-established stabilization strategy, the hyperstabilized NPs can sustain the release of antigens up to 30 days in the presence of serum, which significantly enhanced humoral immune response.87 By adding the lipid-like monophosphoryl lipid A (MPLA) as adjuvants, the vaccine formulation can further enhance T cell-mediated humoral responses.
In addition, LNPs formulations can be optimized for LN targeting thereby enhancing the immune response in situ. One notable study by Kranz et al. demonstrated that a designed mRNA-lipoplexes (RNA-LPX) could target DCs in vivo simply by using lipid molecules.56 They established a library of RNA lipoplexes by tuning the lipid to RNA ratios. Cationic lipids DOTMA or DOTAP and the helper lipid DOPE or cholesterol were used. The charge ratio, size, zeta potential, colloidal properties, and RNA stability also varied with the shifting of lipid to RNA ratios. Interestingly and unexpectedly, all negatively charged particles displayed selective targeting to the spleen. The negatively charged formulation (1.3:2 lipid: RNA) displayed effective targeting of DCs and formed monodisperse NPs (~300 nm) that protected encapsulated mRNA. The authors demonstrated in vivo efficacy using RNA-LPX encoding for influenza virus hemagglutinin (HA) where DCs, NK, B, and T cells were activated by a single intravenous injection of HA-LPX. They also observed an enhanced IFN-α production by vaccination, which is a typical hallmark of APCs sensing for antiviral environment development induced by RNA virus infections through TLR3 and TLR7.88
To enhance immunostimulatory effects, a common strategy is to co-deliver adjuvants to stimulate immune responses. Kuai et al. designed an antigen and adjuvant codelivery nanodisc platform based on synthetic high-density lipoprotein (sHDL).89 One of the striking features of the nanodisc is its extra-small size: ~10 nm in diameter, which contributed to the enhanced trafficking to LNs.57 Exploiting the endogenous role of HDL as a cholesterol carrier, adjuvants CPG were modified with cholesterol to efficiently insert into pre-formed nanodiscs. This strategy also allowed the co-loading of more than one adjuvant, which promoted synergistic immune activation.58 Together, these features contributed to the sustained antigen presentation by DCs. Recently, there is a growing interest in exploring innate immunostimulatory functions of lipid molecules. To expand the diversity of lipid molecules, Miao et al. developed a library of lipids through a one-step three-component reaction that included amines, isocyanides, and alkyl ketones.59 This strategy significantly increased the diversity of synthesized lipids compared to traditional two-component reactions.59 They showed that the immunostimulatory effect of lipids was dependent on the head groups. The identified lipids with cyclic amino head groups displayed immune cell activation efficacy through the intracellular stimulator of interferon genes (STING) pathway. The lipids could be further formulated with mRNA to form LNPs as mRNA vaccine formulations to efficiently deliver the oligonucleotide products. Another advantage is that the formulation could activate the mRNA-independent intracellular STING pathway rather than TLRs, which could reduce systemic toxicity. In addition, inhibition of antigen expression related to the TLRs binding by exogenous mRNA was prevented to favor optimal immune response.27
Polymers:
Aside from the lipids system, polymers have been widely explored for the delivery of different vaccine candidates. Specifically, cationic and ionizable polymers are promising in nucleic acid-based vaccine formulations. Positively charged polymers can condense nucleic acids as nanocomplexes for efficient cellular uptake and endosomal release. In addition, early studies showed that cationic materials tend to induce an inflammatory response.90 One commonly applied polymer category is polyamines, such as polyethylene imine (PEI) and its derivatives.91 Chahal et al. utilized modified dendrimer for antigen-encoding mRNA replicon complexation to form monodisperse NPs.60 This delivery system, with self-amplifying mRNA, enabled immunization in mice, which protected them from various virus challenges, including H1N1 influenza, Ebola virus, and the Toxoplasma gondii parasite.60 However, their high molecular weight (MW) and branched polymer structure (needed to allow a massive loading of vaccine therapeutics) also lead to severe cytotoxicity that limits wider applications.92 Zhao et al. developed a low MW PEI with modifications (stearic acid and 2 kDa PEI conjugates) to form self-assembled micelles for delivering mRNA encoding HIV-1 group-specific antigen (gag).62 Li et al. used 2 kDa PEI conjugated with cyclodextrin for intranasal delivery of HIV antigen-encoding mRNA that was able to stimulate HIV-specific immune response.63 Cyclodextrin modification provided mucosal permeation and mitigated cytotoxicity by lowering the charge density. Other commonly used polymers include polyester-based, such as poly (lactic-co-glycolic acid) (PLGA) and poly (lactic acid) (PLA), because of their biocompatibility and biodegradability.93 Several studies have demonstrated that the delivery of protein antigens in PLGA NPs with enhanced immune responses as compared to soluble formulations.94,95 In addition, such solid polymer particles can control the kinetics of antigens released from the polymer core based on different biodegradation profiles.64,96 However, non-modified PLGA is generally negatively charged, and as a result, it has limited cellular uptake efficiency and loading of anionic antigens.66 Therefore, various coating or modification strategies (such as PEI,65,97 chitosan,66 and poly-L-lysine (PLL) coatings) have been demonstrated to enable the delivery of vaccines and promote interactions with negatively charged cellular membranes.67 Notably, the cationic polymeric delivery carrier itself could induce T helper cell responses through TLR-4 dependent IL-12 secretion.67,98 Poly (β-amino esters) (PBAE) is also extensively used in delivering plasmids DNA or mRNA vaccines because of its non-toxic and biodegradable nature.99 Fields et al. developed NPs formulations for plasmid DNA delivery by combining PLGA and PBAE to reconcile the drawbacks from each component: the low DNA loading efficiency of PLGA and the cytotoxicity of PBAE.68 Other polymeric materials have also been explored, such as polysaccharides100 and block co-polymers.101
In terms of targeted delivery to immune cells, versatility in polymer chemistry shows great potential in developing tissue-selective formulations. For instance, a large library of PBAE-based polymers could be easily synthesized and screened for desirable target polymers.69 Kowalski et al. designed an ionizable amino polyester (APE) for mRNA delivery through ring opening of lactones with amino alcohols.69 Tissue selective delivery was achieved by different APE candidates, including APCs within the spleen.69 Oligopeptides have been used to modify PBAE to allow the tuning of the charge status of NPs.102 Initially, this was done to optimize cargo loading and mitigate cytotoxicity. Later, Fornaguera et al. demonstrated that specific oligopeptide modifications lead to APCs targeting within the spleen and exhibited efficient transfection of mRNA.70 Inspirations also come from using native LN trafficking molecules. Clinically, visualization of sentinel LNs is achieved by injecting dyes that bind to albumin because of the LN trafficking capability of albumin particles.103 Inspired by the endogenous role of albumin, Liu et al. designed a vaccine by conjugating the adjuvants or antigens to the albumin-binding lipid tail.71 The vaccines displayed notable increased accumulation in the LNs, which decreased the systemic distribution of the vaccines and prolonged the vaccine bioavailability. These features limited the systemic toxicity while increasing the potency of vaccination for OVA antigen, HIV antigen, and Human Papillomavirus (HPV) antigen.71
Some polymeric delivery carriers have been demonstrated to have immunostimulatory effects, such as polymers with cationic charge and hydrophobic domains.104,105 In addition, inspired by microbes’ immunostimulatory effect, Son et al. designed sugar capsules that were derived from microbial cell walls for vaccine NPs coating.72 The coating process was achieved by using silicate NPs (Si NPs) as a template followed by its removal after the mRNA was loaded and the sugar capsules were entirely coated. Notably, the removal of Si NPs renders relatively flexible nanostructures that are beneficial for lymphatic drainage and accumulation.72 The mRNA cargos can be efficiently loaded by PEI coating due to electrostatic interactions. The PEI coating was also expected to facilitate the endosomal release for mRNA translation and antigen presentation. The sugar capsules itself functioned as a strong DC activator by eliciting the production of a variety of pro-inflammatory factors, including IL-6, TNF-α, and IL-12 p40, without the use of exogenous adjuvants.72
Inorganic particles:
Inorganic materials have been used in vaccine formulations for a long time. Back in 1926, an aluminum compound (Alum) was discovered to be able to precipitate the toxoid to enhance the immunogenicity.106 Alum is still the most widely used adjuvant today. In spite of over 80 years of development, the underlying mechanisms for Alum as an adjuvant are still controversial.107 Popular explanations include the “depot theory” for extended antigen release106 and enhanced uptake by APCs through antigen absorption on alum.108 Various immunity signaling and activation pathways have also been identified to contribute to the function of the adjuvants.109 Recently, Moyer et al. utilized the interaction between alum and phosphoserine (pSer) to engineer a short peptide-immunogen conjugate to significantly prolong the immunogen release from pSer-immunogemalum complexes.73 The complexes could form NPs for efficient trafficking to LNs and initiated antigen processing and presentation by APCs.73 Site-specific pSer modification on the base of the HIV turner could enable the immunogen (HIV trimer) to be displayed with a specific orientation, which could prevent the induction of undesired NAbs.73 Apart from Alum, other inorganic materials have been proposed that may stimulate vaccine-specific immune responses, such as silica crystal110 and calcium phosphate.109
In addition to the traditional use as immunostimulatory adjuvants, a wide range of biocompatible inorganic particles have also been developed as vaccine formulations,111 including quantum dots (QDs),80 gold nanoparticles (Au NPs),77 carbon nanotubes (CNTs),82 and Si NPs.79 The physical properties of formulations, such as surface charge, hydrophobicity, and aspect ratios, are closely associated with the efficacy of vaccines.112 Inorganic materials have a high degree of tunability to allow them to fulfill the requirements of vaccine formulations. Easy preparation and good storage stability are additional advantages of inorganic particles.113 For example, Au NPs have been chemically functionalized to investigate the relationship between surface hydrophobicity and the associated immune activity.74 It is also expected that other functional groups may bring a pathway-dependent immune activation that is specifically desirable for antiviral defense. Xu et al. described surface-engineered Au nanorods (NRs) for plasmid DNA vaccine delivery.75 With certain surface modifications and aspect ratios, cellular toxicity of Au NRs can be lowered, internalization can be enhanced, and intrinsic adjuvant features can be utilized.76 In vivo vaccination with HIV-1 envelope glycoprotein (Env) plasmids demonstrated enhanced cellular and humoral immunity.75 Zhou et al. synthesized a series of Au NPs (15 to 80nm) for co-delivery of antigens (OVA peptides) and adjuvants (CpG) by surface conjugation.77 Particles of 60 and 80 nm outperformed other sizes (15, 30, and 40 nm) in activating DCs; this was most likely due to a higher payload of antigens. Notably, they promoted DC homing to the LNs and induced potent cellular immune response.77 High tunability is also achievable in other organic particles, such as adjuvant-based mesoporous Si NPs,78 mesoporous Si NPs-based antigen/adjuvant co-delivery systems,79 antigen density tunable QDs,80 size-controlled antiviral QDs,81 and surface oxidized antigen/adjuvant-based multiwall CNTs.82
VACCINE DELIVERY DEVICES
If the vaccine formulations are considered as utilizing nanoscale engineering, vaccine delivery devices utilize micro/macroscale engineering to improve vaccine efficiency and efficacy. Beyond the optimization of the vaccine itself and designing appropriate delivery carriers, the efficacy of a vaccine also depends on pharmacokinetics (PK) of the antigens after administration.114 When the body is exposed to an exogenous antigen, both humoral and cellular immune responses are initiated to neutralize it, which also leads to immunological memory.12 During a virus infection, the replication process typically takes one to several weeks to sustainably stimulate the immune system with a continuous supplement of antigens.115 In contrast, typical vaccines show rapid clearance and can only be detected in LNs within days.114 Although still poorly understood, the PK of a vaccine, which refers to the dynamics of antigens presence during immunization, has a profound impact on immune response.87 One study indicated that increasing the dosage of vaccines over two weeks at both priming and boost stages induced durable immune response and increased antibody production compared to traditional bolus immunization.87 Also, computational models suggested that antigens can be better captured in GCs if antigen availability is extended.116 In addition to the advantages of extended antigen availability, traditional vaccination strategies often fail to yield the desired optimized immune responses in a single dose. Furthermore, vaccine regimes are often designed to be multiple shots, thus achieving even longer immune protection by re-challenging the immune system with the same antigens in secondary “boosting” shots administered after the primary shots.117 Therefore, vaccine delivery devices have been widely investigated, to develop single-dose vaccines, minimal invasively administered vaccines, and self-administrable vaccines (Figure 5a, b). However, its potential can only be realized if various design principles are met.
Figure 5.
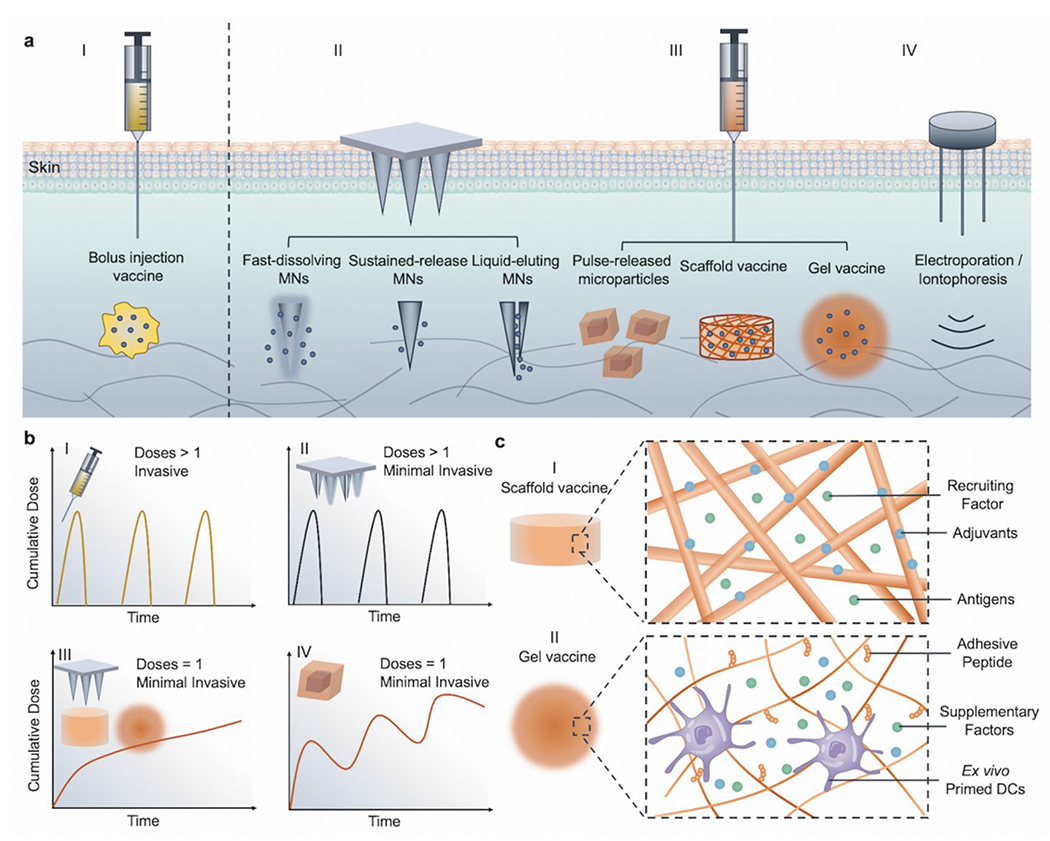
Summary and characteristics of various vaccine delivery devices. (a) Drug delivery devices developed for vaccine administration: I. Traditional vaccine-containing solutions for bolus injection. II. Minimal invasive microneedle devices (including fast-dissolving, sustained-released, and liquid-eluting hollow microneedles). III. Injectable drug delivery devices (such as microparticles, solid scaffolds, and hydrogel-based). IV. Other devices for enhancing vaccine efficacy (such as electroporation and iontophoresis). (b) Schematic for representative PKs of vaccine and administration features for different vaccine delivery devices: I. Traditional bolus injection is invasive and may require multiple doses. II. Fast dissolving or liquid eluting MNs can be self-administrable and minimally invasive but still require multiple doses.125 III. Sustained released MNs can be both minimally invasive, with only one dose necessary,122 while scaffold and gel vaccines could be injectable to avoid implantation and also only require one dose. IV. Pulsatile released microparticles can mimic a multiple doses regimen with only one injection.127,128 (c) Schematic of representative mechanisms for vaccine delivery devices. I. Scaffold vaccines: the scaffold could sustainably release recruiting factors to home DCs, and encapsulated antigens could further be processed by DCs in situ.129 II. Gel vaccines: hydrogel microenvironment can be engineered to support encapsulated cell vaccines (such as DC vaccines) by incorporating cell adhesive peptides and supplementary factors.136
Microneedles (MNs) vaccines:
Transdermal MNs patches have been proposed as a promising drug delivery device for immunotherapies144–147 based on the minimally invasive nature of MNs and the highly active immune environment of the skin. Additionally, since MNs patch-based vaccines can be easily administered without the need for significant medical training, these vaccines can be easily deployed both in areas with limited medical resources, such as developing countries and during times of significant medical crisis, as seen during an outbreak or pandemic. Early studies include the use of vaccine coated metal MNs118 and fast dissolving polymer-based MNs.119 Recently, the dissolving MNs (carboxymethyl cellulose-based) were used for adjuvants and Ad5-based vaccine co-delivery in an attempt to address COVID-19.147 One-month storage of MNs vaccine at 4 °C maintained the immunogenicity of Ad, where no significant loss in antibody responses was shown in mice studies.147 The similar dissolving MNs are also being investigated to deliver SARS-CoV-2 subunit spike protein (S1).120 Potent virus-specific antibody responses were elicited in mice as early as 2 weeks after the immunization with MNs. The dissolvable MNs for influenza vaccine has been previously evaluated in a phase I clinical trial compared to the traditional intramuscular hypodermic injection of a single-dose vaccine.121 The MN patch was well-tolerated and immunogenic after a single-dose vaccination. In addition, self-administration exhibited efficacies comparable to the administration by medical workers. The dissolving feature leaves no sharp wastes, and self-administration could significantly relieve the pressure on medical resources, which could be especially critical in coping with a pandemic. Beyond these milestones, advances in microfabrication, materials engineering, and vaccine formulation optimization have provided additional opportunities to enhance vaccine efficacies.148
Recent advances in microfabrication, such as 3D printing and stereolithography, can allow for more sophisticated MNs designs.125 Liquid-based vaccine formulations are still the most widely used.125 However, liquid-based vaccine formulations are incompatible with most solid-based MNs forms. Inspired by the capability of snake-fangs to efficiently inject venom, Bae et al. used an exposure lithography-based strategy to produce MNs patches with snake-fang architectures for efficient liquid formulations delivery.125 The design was compatible with most existing vaccine formulations and the patch can be applied with the pressure of the thumb and achieve an efficient transdermal delivery.125 Nevertheless, solid-based vaccines, including MNs-based ones, also attracted significant attention because of the decreased cost of vaccine manufacturing, transport, and storage when compared to the liquid-based formulations.149 One of the key challenges faced by these MNs patches is the loss of vaccine activity during the drying or heating process of MNs patch fabrication,150 where molecular structures of vaccines may be disrupted.151 Different strategies can be adopted, such as the use of lyoprotective polymers as MNs matrices, which include silk fibroin,122 gelatin, dextran, and PVP;152 or adding sugar molecules as stabilizing excipients, including trehalose and sucrose.147,152 In the vaccine discovery phase, the introduction of certain mutations can improve the thermal stability of vaccines as well,148 which can be further applied to specific MNs formulations. Additionally, a hyperstabilized vaccine formulation, such as the interbilayer-crosslinked multilamellar vesicles, helped protect vaccines during the drying process of MNs fabrication and have outperformed other traditional delivery strategies in inducing potent antigen-specific immune response.123
MNs also provide advantages in tuning optimal release profiles of vaccines. As mentioned earlier, the sustained availability of vaccines is a desirable feature for immunization. Vaccines targeting different infectious diseases may vary in terms of optimal release profiles based on the unique features of each virus. Initially, MNs for vaccination were mainly an upgrade to traditional bolus delivery by using a fast-dissolving MNs matrix,119 which requires multiple doses for sufficient immunity. With the exploration of the desired PK of vaccines, MNs can play a much more important role. For example, a silk matrix with crystallized structures could be used as an MNs matrix to sustainably release loaded vaccines over two weeks and to elicit potent immune responses with one single administration.122 In addition, biodegradable polymers with known degradation kinetics can be used to encapsulate the vaccines and tune antigen release profiles. These polymers include biodegradable cationic PBAE123 and PLGA.124
Injectable material-assisted vaccines:
Macroscale biomaterials strategies have recently boosted the field of immunotherapy.153 There are many advantages in controlled release and encapsulation of various cargos, ranging from small molecules to proteins and cells.153 These varied cargo elements can help to meet the need for diverse types of vaccine candidates. In terms of controlling PKs of vaccines, both sustained and pulsatile release could be possibly achieved.153 In addition, materials engineering can enable devices that do not require surgically invasive administrations.153 Together, they could minimize the requirement of medical resources without mitigating the vaccine efficacy.
PLGA has also been developed for a single-shot vaccine based on the tunability of its biodegradation profile.126 In the case of polio vaccines, two to three administrations over several months are required. Tzeng et al. demonstrated that IPV could be encapsulated within PLGA microspheres stably and released through two bursts that were one-month apart.126 They examined the effect of adding cationic excipients (Eudragit E, PLL, and branched PEI) to stabilize IPV antigens, protecting them from denaturation by acidic byproducts, and modulate degradation profiles of PLGA microspheres.126 Notably, a single shot of PLGA microsphere-based vaccines could elicit the production of NAb as potent as two bolus injections.126 Combined with the advances in microfabrication and additive manufacturing, McHugh et al. fabricated microscale (~400 μm) hollow PLGA-based microparticles that were injectable.127 the microparticles can be filled with vaccine formulations and the degradation rate of the microparticle shell (a lactic/glycolic copolymer) can be tuned to achieve controlled release spanning from a few days to a few months.127 Such design can accomplish the goals of general transdermal vaccine injection and achieved temporally controlled pulsed delivery of antigens in a single injection.127 Recently, a similar PLGA-based microparticles platform was applied to release STING agonist (cyclic guanosine monophosphate-adenosine monophosphate (cGAMP)) in a pulsatile manner over a long period (~ 16 days).128 One single injection of the pulsatile drug-releasing microparticles induced potent immunity as effective as three separate injections. These engineered platforms are versatile in terms of encapsulating different cargos required by vaccine formulations and tuning release kinetics correspondingly. However, even though the use of FDA-approved PLGA could facilitate the clinical translation to a certain degree, several design limitations need to be overcome for translating PLGA-based drug delivery devices into clinical applications. For instance, the pulsatile release was achieved with hollow microparticles that have thick PLGA shells to encapsulate the drugs in the hollow core for controlled release. This design significantly sacrificed their loading efficiency due to the limited volume of the core. Clinically, patients need high drug dosage, and this formulation needs further optimization to meet the dosage requirement. In addition, PLGA can create an acidic microenvironment during biodegradation, damaging the encapsulated drugs.126 Furthermore, the encapsulated vaccines need to survive the fabrication process, brief heating requires thermally stable vaccines.127,128 All these aspects could compromise their applications for a broader collection of drugs or vaccines.
Traditionally, ex vivo modification of antigen-specific DC vaccines is a multi-step process associated with high costs and high levels of complexity.43 It was proposed that in situ DC recruitment, activation, and further trafficking to LNs would be more desirable compared to the ex vivo modification strategy (Figure 5c).129 The early study by Ali et al. demonstrated the use of macroporous scaffold vaccines based on PLGA, which presented cytokines (GM-CSF), danger signals (CpG), and antigens in a spatiotemporally defined manner. GM-CSF can be sustainably released from the scaffolds over 20 days for effective DC recruitment.129 By co-loading the CpG through PEI-mediated electrostatic interactions, CpG was immobilized on the scaffolds for local internalization by the recruited DCs. This process mimicked an infection and was able to continuously recruit and activate DCs.129 This specific scaffold vaccine was surgically implanted; nevertheless, it encouraged further development of injectable vaccines that work similarly. Kim et al. demonstrated the use of mesoporous silica rods (MSRs) with a high-aspect-ratio that could be injected with a syringe needle.130 They could self-assemble in vivo as macroporous scaffolds to initiate potent immune responses.130 The DCs could be recruited into the scaffolds where the inflammatory signals (GM-CSF), adjuvants (CpG), and antigens (OVA) were sustainably released from the scaffolds for in situ DC activation and maturation. It was also demonstrated that locally injected scaffold vaccines based on MSRs could elicit strong systemic immune responses with the increase in levels of antigen-specific B cells, helper T cells, and cytotoxic T cells.130 A PEI coating was later added to the design to enable antigen loading through adsorption for enhanced immunogenicity.131 This specific system was recently utilized for synthetic peptide vaccines and small antigens.132 This strategy was able to bypass multiple immunizations requirements but sustained a more robust antibody response, compared to traditional bolus or alum vaccine formulations.132
Scaffold-based vaccines have displayed efficacy in inducing systemic immune response through in situ immune modulations. In vivo self-assembled scaffolds provide a good alternative to bypass surgically implantation; however, the self-assembling process is commonly undefined in terms of how long it could take for self-assembling.133 Materials engineering facilitates the fabrication of scaffolds maintaining injectability. Bencherif et al. described a strategy for fabricating injectable scaffolds with shape memory based on cryotropic gelation.134 The cryogels were based on alginate methacrylate, and they were capable of being reversibly deformed, over 90% strain, after being chemically crosslinked in a frozen condition (− 20 °C).134 The ice crystals hindered the crosslinking in frozen spots and therefore formed the macroporous pores that not only enabled the shape memory for the gel but also allowed the DCs infiltration.134
Hydrogel has been extensively explored in the field of immunotherapy.153 It has advantages in terms of controllable delivery of multiple cargos, in situ immunomodulation, and mild fabrication process for various vaccine formulations (Figure 5c). Early pioneering work includes the loading of ex vivo antigen-primed DC vaccines within Ca2+ crosslinked alginate gel by Hori. et al..135 Activated DCs have a short lifespan, and continuous immune responses may be suppressed if no sufficient host DCs and T cells could be recruited and primed in vivo.135 Therefore, cell-based vaccines delivered by gels are expected to improve cell survival and prolong the presence of DCs.136 Both host DCs and cytotoxic T cells can be recruited to the injection site by cytokines and chemokines secreted from the injected DCs. In addition, the enhanced efficacy of transplanted cells can be enhanced by supplementing the gels with other immunomodulatory molecules, such as cytokines (IL-2) or adjuvants (CpG).137 The increased persistence of transplanted cells could be achieved by designing gels mimicking ECM by the incorporation of cell-adhesive peptides, such as RGD.154
Apart from cell-based vaccines, other types of vaccines could also be incorporated into gel systems, where the release profiles can be tuned. Roy et al. utilized the in situ crosslinking mechanism of tetrafunctional polyethylene oxide amine and polyethylene oxide succinimidyl glutarate to encapsulate plasmid DNA.138 The gelation did not interfere with the supercoiled structure of plasmid DNA and the gelling ability can be tuned by adjusting the concentrations of these two components. Notably, gene expression levels were comparable to bare DNA, but the duration of expression was significantly longer. Other in situ gel systems explored for vaccination include temperature responsive gels139 and Michael addition type hydrogels.140 Singh et al. engineered dextran and PEG-based hydrogels that could be crosslinked in situ for antigen/adjuvant delivery.141,142 Umeki et al. designed an immunostimulatory CpG DNA-based hydrogel for antigen delivery.143 To achieve sustained release of antigens, they proposed to cationize the antigens by ethylenediamine conjugation where antigens could form complexes with negatively charged DNA hydrogel matrix. The innate immunostimulatory function of gel and prolonged antigen presence leads to an enhanced antigen-specific immune response with less toxicity.143 In situ gelling strategies or physically crosslinked gels from these studies generally take minutes to hours, which renders limited control on cargos release and material properties during the gelling period.143 Therefore, the development of gel systems that are both injectable and display defined cargo release/biodegradation profiles could be desirable for vaccine administration.
Other Delivery Devices:
Many more investigations are currently ongoing to engineer alternative drug delivery devices. For instance, to promote the uptake of DNA vaccine by cells, electroporation is one of the traditional and effective strategies to enhance the uptake of plasmids.155 Different parameters, such as electrical features (voltage, current, and frequency) and length of the electrode (intradermal or intramuscular targets), need to be optimized to ensure efficacy, safety, and to minimize pain. This process has recently demonstrated promising clinical phase I results for MERS.25 The same electroporation device (CELLECTRA®) is also being investigated for a DNA vaccine of SARS-CoV-2 from Inovio.156 The spike protein of SARS-CoV-2 encoding DNA vaccine demonstrated potent antigen-specific T cell responses and the production of neutralization antibodies in preclinical mice and guinea pigs studies.156 In order to incorporate the advantages of MNs, electroactive MNs devices have also been developed for the electroporation of DNA vaccine in a minimally invasive manner.157 Similar devices using iontophoresis can also enhance the transdermal delivery of vaccines.158 Recently, Tadros et al. designed millimeter-scale star-shaped particles, termed STAR particles, based on aluminum oxide for topical vaccine delivery,159 and the minimally invasive STAR particles elicited immune response comparable to intramuscular injection.159 Even though STAR particles are simple to manufacture and well-tolerated by the immune system, a much higher dosage was administered compared to intramuscular injection. In addition, potential human-to-human variability in administration and drug leftovers on the skin may result in differential efficacy and undesirable toxicity.
In terms of reducing the demands for medical resources, vaccines that could be intranasally or orally administered are desired if their efficacy is comparable to transdermal delivery.44 The main strategy for intranasal or oral vaccine development is currently limited to weakened viral vaccines mimicking natural infections. Non-living vaccines are safer but less immunogenic, and thus may benefit greatly from drug delivery formulations and devices.160 A variety of drug delivery devices have been proposed for oral vaccination delivery. For instance, the micromotor-driven microparticles for oral delivery of antigens.161,162 The micromotor vaccine can efficiently enter the intestine to enhance the antigen uptake. Aran et al. designed a needle-free oral microjet where the high-pressure liquid jet of the vaccine was produced by a self-contained gas-producing reaction.163 It was shown that buccal immunity response was enhanced and this method was able to elicit a potent humoral immune response both locally and systemically.163 Abramson et al. engineered a self-orienting system for oral delivery that could attach to the gastric wall by autonomously positioning to maximize the delivery efficiency.164 Innovations in drug delivery devices will significantly transform traditional vaccination regimes.
SUMMARY AND FUTURE DIRECTIONS
The devastating disruptions caused by the sudden outbreak of COVID-19 has urged vaccine development at an unprecedented speed.4 Within 70 days of the first release of the genetic information of SARS-CoV-2, the first dose of the vaccine was administered to human participants.9 As of Aug. 25, 2020, 31 vaccine candidates are in various stages of clinical trials, and around 142 candidates are in preclinical trials.10 Apart from the high diversity of the vaccine candidates, many candidates are based on different technological platforms, such as nanotechnology delivered mRNA vaccine, electroporation device enabled DNA vaccine, and MN-delivered vaccine. The COVID-19 pandemic has accelerated the deployment of different vaccine formulations and delivery devices. It is reasonable to expect that more efforts would be dedicated to further optimize the vaccine designs to help fight future outbreaks.
Here, we reviewed recent research efforts in the field of vaccine discovery, vaccine formulation development, and vaccine delivery device designs. Because of the deployment of various engineering technologies, it is expected that future vaccines will not only need to demonstrate safety and efficacy, but also to be relatively convenient and simple to use for better copping with population-level vaccinations or sudden outbreaks worldwide. Nanoengineered formulations could enhance the immune response by delivering the required components together and maximizing their uptake by immunocompetent cells. To expedite the future preparedness of vaccines for the occurrence of a pandemic, synthetic vaccines have demonstrated great potential for rapid clinical applications because they are molecularly defined and independent of the time-consuming culture of viruses, which can accelerate the initial vaccine development. However, synthetic vaccines are also the ones that need to be formulated the most, which constitutes the major part of their time costs. To accelerate the development of synthetic vaccines, the nanoengineered formulations need to be simple and robust to manufacture in terms of chemistry or the selection of raw materials.165,166 In terms of vaccine delivery devices, self-administrable vaccines, single-shot vaccines, and solid MN-based vaccines could further enhance the availability of vaccines to a broader population. For instance, self-administrable vaccines can be mailed to patients at home without the need of medical experts.165 Single-shot vaccines can be much more convenient compared to the traditional immunization regimens that require multiple shots. Furthermore, solid MN-based vaccines that are cold chain free could simplify the vaccine transportation and distribution process. All of these aspects could be enabled by engineering approaches to accelerate the development of vaccines at a pandemic speed.165,166 Although combining the advantages of vaccine formulations and vaccine delivery devices is promising, micro/macro-engineering still needs to develop solutions that do not require “harsh” conditions to be compatible with nanoengineered formulations.167 Expectedly, platform technologies that can be quickly adapted to address emerging viral diseases could speed up the pace of vaccine development.
However, several challenges remain before these goals can be fully achieved. First, a differently identified virus is unknown in terms of its interactions with our immune system and which type of immune responses are most desirable to trigger prolonged immunity. Improved understanding of our immune system and its interactions with viruses are the basis for designing an effective vaccine, which also guides the steps for designing corresponding vaccine administration regimens. Second, innovative formulations or delivery devices for vaccines are mostly in the preclinical stage, which have not been approved by the FDA yet. For the example of MN-based vaccines, the encouraging results from a phase I trial of dissolvable MN-based influenza vaccine indicated the promise of using it as an alternative strategy for vaccination.121 However, patient compliance with this approach and its efficacy needs to be tested in the larger population considering only 100 human subjects are currently involved. Also, the efficacy of vaccines by self-administration should be further validated in comparison to those performed by medical experts. In addition, the proposed formulations and delivery devices should be easy to manufacture with minimal batch-to-batch variability to simplify the quality control process. The raw materials used could prioritize the FDA-approved ones to simplify the regulatory process, and the required chemistry is preferred to be simple and robust. Even though advanced manufacturing technologies excel in reducing batch-to-batch variability, high-cost may be associated. The improved efficacy of a vaccine from these formulations and delivery devices should not be complicated by the fabrication process so that it can facilitate the clinical translation and regulatory approvals.165
Additional challenges for vaccine development also exist during a pandemic. Multiple vaccine candidates flush into different stages of clinical trials that could burden the regulatory process by the FDA for selecting promising candidates and deciding final ones.4 Furthermore, human trials are starting even before the most suitable animal models for safety and efficacy are defined.168 Based on previous successful preclinical and early-stage clinical data for a similar platform, animal studies are even skipped directly for human administrations.10 Other engineering approaches such as organs-on-a-chip169–171 or screening using organoids172 may serve as a promising tool in future clinical trials for efficacy and toxicity determination. Ethically controversial human challenge trials by deliberately infecting humans with a virus have been proposed and pursued to accelerate the process of vaccine development.173 Therefore, potentially we should have vaccine clinical trials specifically designed for different scenarios by incorporating engineering advances and regulatory guidelines readily outlined for unusual trials. In conclusion, human health and societal stability are constantly challenged by sudden virus outbreaks; the rapid development of effective vaccines is believed to be an ultimate solution. We expect to observe faster and more efficient vaccine development paradigms in the future by combining the advances in different engineering fields.
Table 1:
List of the representative formulations used for engineering antiviral vaccines.
| Materials | Formulations | Cargos (vaccine types) | functions | References |
|---|---|---|---|---|
| Lipids | Ionizable lipid | mRNA (glycoprotein 100 (gp100) and tyrosinase-related protein 2 (TRP 2)) and adjuvants lipopolysaccharide (LPS) | Stabilization, Enhanced Cellular uptake | 54 |
| Covalently crosslinked lipid bilayers | Protein/ Adjuvants (Monophosphoryl lipid A (MPLA)) | Stabilization, Sustained delivery, Co-delivery | 55 | |
| Net negative charged formulations by tuning cationic lipids and mRNA ratio | mRNA (influenza virus hemagglutinin (HA) and OVA) | LN targeting, Stabilization | 56 | |
| Nanodiscs via synthetic high-density lipoprotein | Peptide neoantigens/Adjuvants (CpG); Dual adjuvants (CpG and MPLA) | Co-delivery, Enhanced uptake | 57, 58 | |
| Lipids with cyclic amino head group | mRNA (OVA and E7) | Immunostimulatory function | 59 | |
| Polymers | Modified dendrimer | mRNA (H1N1 influenza, Ebola viruses, and Toxoplasma gondii parasite) | Enhanced loading, cellular uptake, and endosomal release | 60 |
| Stearic acid conjugated Low MW PEI | Peptide (TRP 2) and mRNA (HIV-1) | Lowered charge density, Higher biocompatibility | 61,62 | |
| Cyclodextrin conjugated PEI | mRNA (HIV) | Lowered charge density, Higher biocompatibility | 63 | |
| PLGA NPs | Protein (OVA) | Controlled antigen release | 64 | |
| PEI/ Chitosan/Poly-lysine coated PLGA/PLA | Protein (HBV surface antigen) | Enhanced loading and cellular uptake, Intrinsic immunostimulatory function | 65–67 | |
| PBAE and PLGA | Plasmid DNA (Luciferase) | Enhanced loading and lower cytotoxicity | 68 | |
| Ionizable amino polyester | mRNA (Luciferase) | Spleen targeting | 69 | |
| Oligopeptide modified PBAE | mRNA (Enhanced green fluorescent protein) | APC targeting | 70 | |
| Albumin and albumin binding lipids | Peptide (OVA)/Adjuvant (CpG) | LN targeting | 71 | |
| Polysaccharide from microbial cell wall and PEI | mRNA (OVA) | Immunostimulatory, Enhanced loading and endosomal release | 72 | |
| Inorganic Particles | Aluminum hydroxide | Phosphoserine conjugated protein antigen (HIV trimer) | Immunostimulatory, Sustained release | 73 |
| Chemically functionalized Au NPs with hydrophobicity | - | Intrinsic immunostimulatory function | 74 | |
| Aspect ratio optimized and surface modified Au NRs | Plasmid DNA (HIV-1) | Enhanced cellular uptake, Intrinsic immunostimulatory function | 75,76 | |
| Sized optimized Au NPs | Peptide (OVA) and adjuvant (CpG) | Enhanced DC activation | 77 | |
| Mesoporous Si NPs | Adjuvant; Protein (E2 of diarrhea virus) | Intrinsic immunostimulatory function | 78,79 | |
| Antigen density tunable/ Size controllable QDs | Peptide (self-antigen from multiple sclerosis) | LN homing and higher density of antigens leads to more effective tolerance; Intrinsic anti-viral effects | 80,81 | |
| Oxidized multiwalled CNTs | Protein (NY-ESO-1) and adjuvant (CpG) | Co-delivery, enhanced uptake | 82 |
Table 2:
Summary of typical designs, advantages, and limitations of different vaccine delivery devices.
| Delivery Devices | Designs | Advantages | Limitations | References | |
|---|---|---|---|---|---|
| MNs vaccine (minimally invasive; minimal requirement on medical professionals and medical equipment) | Fast-dissolving MNs | Fast dissolving polymers as MNs matrix | Fast and enhanced transdermal delivery; could be cold-chain free | Multiple administrations; vaccine formulations may get denatured from MNs fabrication | 118–121 |
| Sustained-release MNs | Controlled biodegradable materials as MNs matrix | Potential to be single shot; Protect the vaccine formulations in the physiological environment; Could be cold-chain free | Vaccine formulations may get denatured from MNs fabrication | 122–124 | |
| Liquid-eluting MNs | Hollow MNs with different architectures | Compatible with liquid vaccine formulation; fast and enhanced transdermal delivery | Multiple administrations; Requirement of cold-chain transport | 125 | |
| Injectable materials assisted vaccine (potential for single-shot vaccines to reduce the requirement of medical resources) | Pulse-released microparticle | Materials with different biodegradation profiles | Optimal release for a potent immune responses; Release kinetic can be optimized by materials engineering | vaccine formulations may get denatured during the fabrication | 126–128 |
| Scaffold vaccine | Solid scaffolds that could tune the release of different immunomodulatory factors | Continuously modulate the immune response in situ | Injectability need to be achieved to avoid surgical implantation | 129–134 | |
| Hydrogel Vaccine | Biomimicking gellable materials with tunable biophysical properties and high cytocompatibility | Compatible with various types of vaccines, including cell-based vaccines; Simple fabrication process and the water environment help protect the cargos | In situ gelation may be weak to achieve sustained release | 135–143 |
ACKNOWLEDGMENT
This work was supported by National Institute of Health (CA214411, GM126831, AR069564).
VOCABULARY
- Coronavirus disease 2019 (COVID-19)
a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) and has been declared as a pandemic by WHO since Mar. 11, 2020
- Antiviral vaccine
a biological preventive preparation strategy to train the immune systems to fight against viral pathogens
- Vaccine formulations
biomaterial engineering strategies aiming to boost the efficacy or reduce side effects of vaccines through co-delivering adjuvants, targeting immune cells, or protecting vaccines activities.
- Vaccine delivery devices
tools for administering the vaccine formulations and they can be used by medical workers or the patients themselves
- Pattern recognition receptors (PRRs)
proteins that can recognize pathogenic molecules as part of innate immunity in response to virus infection, including identifying pathogen-associated molecular patterns (PAMPs) from a virus.
- Adjuvants
supplementary agents that could be codelivered with vaccines to boost the immune response, minimize the dosage of antigens, and maintain longer immunity.
- Lymph nodes
round or bean-shaped clusters of cells containing abundant immune cells that can initiate the robust immune response towards viral infections.
REFERENCES
- (1).Bedford J; Enria T; Giesecke J; Heymann DL; Ihekweazu C; Kobinger G; Lane HC; Memish Z; Oh M.-d.; Sall AA; Schuchat A; Ungchusak K; Wieler LH, COVID-19: Towards Controlling of a Pandemic. Lancet 2020, 395, 1015–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Berlin DA; Gulick RM; Martinez FJ, Severe Covid-19. N. Engl. J. Med 2020, DOI: 10.1056/NEJMcp2009575 [DOI] [PubMed] [Google Scholar]
- (3).Rome BN; Avorn J, Drug Evaluation during the Covid-19 Pandemic. N. Engl. J. Med 2020, 382, 2282–2284. [DOI] [PubMed] [Google Scholar]
- (4).Lurie N; Saville M; Hatchett R; Halton J, Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med 2020, 382, 1969–1973. [DOI] [PubMed] [Google Scholar]
- (5).Fauci AS; Morens DM, Zika Virus in the Americas — Yet Another Arbovirus Threat. N. Engl. J. Med 2016, 374, 601–604. [DOI] [PubMed] [Google Scholar]
- (6).Regules JA; Beigel JH; Paolino KM; Voell J; Castellano AR; Hu Z; Munoz P; Moon JE; Ruck RC; Bennett JW; Twomey PS; Gutiérrez RL; Remich SA; Hack HR; Wisniewski ML; Josleyn MD; Kwilas SA; Van Deusen N; Mbaya OT; Zhou Y, et al. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine. N. Engl. J. Med 2015, 376, 330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Mulangu S; Dodd LE; Davey RT; Tshiani Mbaya O; Proschan M; Mukadi D; Lusakibanza Manzo M; Nzolo D; Tshomba Oloma A; Ibanda A; Ali R; Coulibaly S; Levine AC; Grais R; Diaz J; Lane HC; Muyembe-Tamfum J-J; the PWG, A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med 2019, 381, 2293–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Fineberg HV, Pandemic Preparedness and Response — Lessons from the H1N1 Influenza of 2009. N. Engl. J. Med 2014, 370, 1335–1342. [DOI] [PubMed] [Google Scholar]
- (9).Le TT; Andreadakis Z; Kumar A; Roman RG; Tollefsen S; Saville M; Mayhew S, The COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov 2020, 19, 305–306. [DOI] [PubMed] [Google Scholar]
- (10).Draft Landscape of COVID-19 Candidate Vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed 2020-08-28)
- (11).Cox RJ; Brokstad KA, Not Just Antibodies: B Cells and T Cells Mediate Immunity to COVID-19. Nat. Rev. Immunol 2020, DOI: 10.1038/s41577-020-00436-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Irvine DJ; Swartz MA; Szeto GL, Engineering Synthetic Vaccines Using Cues from Natural Immunity. Nat. Mater 2013, 12, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Callaway E, The Race for Coronavirus Vaccines: A Graphical Guide. Nature 2020, 580, 576. [DOI] [PubMed] [Google Scholar]
- (14).Nabel GJ, Designing Tomorrow’s Vaccines. N. Engl. J. Med 2013, 368, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gao Q; Bao L; Mao H; Wang L; Xu K; Yang M; Li Y; Zhu L; Wang N; Lv Z; Gao H; Ge X; Kan B; Hu Y; Liu J; Cai F; Jiang D; Yin Y; Qin C; Li J, et al. Development of an Inactivated Vaccine Candidate for SARS-CoV-2. Science 2020, 369, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ura T; Okuda K; Shimada M, Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hos BJ; Tondini E; van Kasteren SI; Ossendorp F, Approaches to Improve Chemically Defined Synthetic Peptide Vaccines. Front. Immunol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Donlan AN; Petri WA, Mucosal Immunity and the Eradication of Polio. Science 2020, 368, 362–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Macklin GR; O’Reilly KM; Grassly NC; Edmunds WJ; Mach O; Santhana Gopala Krishnan R; Voorman A; Vertefeuille JF; Abdelwahab J; Gumede N; Goel A; Sosler S; Sever J; Bandyopadhyay AS; Pallansch MA; Nandy R; Mkanda P; Diop OM; Sutter RW, Evolving Epidemiology of Poliovirus Serotype 2 following Withdrawal of the Serotype 2 Oral Poliovirus Vaccine. Science 2020, 368, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Yeh MT; Bujaki E; Dolan PT; Smith M; Wahid R; Konz J; Weiner AJ; Bandyopadhyay AS; Van Damme P; De Coster I; Revets H; Macadam A; Andino R, Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe 2020, 27, 736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Robert-Guroff M, Replicating and Non-Replicating Viral Vectors for Vaccine Development. Curr. Opin. Biotechnol 2007, 18, 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zhu F-C; Li Y-H; Guan X-H; Hou L-H; Wang W-J; Li J-X; Wu S-P;Wang B-S; Wang Z; Wang L; Jia S-Y; Jiang H-D; Wang L; Jiang T; Hu Y; Gou J-B; Xu S-B; Xu J-J; Wang X-W; Wang W, et al. Safety, Tolerability, and Immunogenicity of a Recombinant Adenovirus Type-5 Vectored COVID-19 Vaccine: A Dose-Escalation, Open-Label, Non-Randomised, First-in-Human Trial. Lancet 2020, 395, 1845–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).van Doremalen N; Haddock E; Feldmann F; Meade-White K; Bushmaker T; Fischer RJ; Okumura A; Hanley PW; Saturday G; Edwards NJ; Clark MHA; Lambe T; Gilbert SC; Munster VJ, A Single Dose of ChAdOx1 MERS Provides Protective Immunity in Rhesus Macaques. Sci. Adv 2020, 6, eaba8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gary EN; Weiner DB, DNA Vaccines: Prime Time Is Now. Curr. Opin. Immunol 2020, 65, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Modjarrad K; Roberts CC; Mills KT; Castellano AR; Paolino K; Muthumani K; Reuschel EL; Robb ML; Racine T; Oh M-D; Lamarre C; Zaidi FI; Boyer J; Kudchodkar SB; Jeong M; Darden JM; Park YK; Scott PT; Remigio C; Parikh AP, et al. Safety and Immunogenicity of an Anti-Middle East Respiratory Syndrome Coronavirus DNA Vaccine: A Phase 1, Open-Label, Single-Arm, Dose-Escalation Trial. Lancet Infect. Dis 2019, 19, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Muthumani K; Falzarano D; Reuschel EL; Tingey C; Flingai S; Villarreal DO; Wise M; Patel A; Izmirly A; Aljuaid A; Seliga AM; Soule G; Morrow M; Kraynyak KA; Khan AS; Scott DP; Feldmann F; LaCasse R; Meade-White K; Okumura A, et al. A Synthetic Consensus Anti–Spike Protein DNA Vaccine Induces Protective Immunity against Middle East Respiratory Syndrome Coronavirus in Nonhuman Primates. Sci. Transl. Med 2015, 7, 301–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pardi N; Hogan MJ; Porter FW; Weissman D, mRNA Vaccines — A New Era in Vaccinology. Nat. Rev. Drug Discov 2018, 17, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Suschak JJ; Williams JA; Schmaljohn CS, Advancements in DNA Vaccine Vectors, Non-Mechanical Delivery Methods, and Molecular Adjuvants to Increase Immunogenicity. Human Vaccines Immunother. 2017, 13, 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Gallie DR, The Cap and Poly (A) Tail Function Synergistically to Regulate mRNA Translational Efficiency. Genes Dev. 1991, 5, 2108–2116. [DOI] [PubMed] [Google Scholar]
- (30).Gustafsson C; Govindarajan S; Minshull J, Codon Bias and Heterologous Protein Expression. Trends biotechnol. 2004, 22, 346–353. [DOI] [PubMed] [Google Scholar]
- (31).Karikó K; Muramatsu H; Welsh FA; Ludwig J; Kato H; Akira S; Weissman D, Incorporation of Pseudouridine into mRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Mol. Ther 2008, 16, 1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Karikó K; Buckstein M; Ni H; Weissman D, Suppression of RNA Recognition by Toll-Like Receptors: the Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [DOI] [PubMed] [Google Scholar]
- (33).Chen N; Xia P; Li S; Zhang T; Wang TT; Zhu J, RNA Sensors of the Innate Immune System and Their Detection of Pathogens. IUBMB life 2017, 69, 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kariko K; Muramatsu H; Ludwig J; Weissman D, Generating the Optimal mRNA for Therapy: HPLC Purification Eliminates Immune Activation and Improves Translation of Nucleoside-Modified, Protein-Encoding mRNA. Nucleic Acids Res. 2011, 39, 142–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Cohen J, Designer Proteins Produce Potent HIV Defense. Science 2015, 348, 1297. [DOI] [PubMed] [Google Scholar]
- (36).Bijker MS; van den Eeden SJ; Franken KL; Melief CJ; Offringa R; van der Burg SH, CD8+ CTL Priming by Exact Peptide Epitopes in Incomplete Freund’s Adjuvant Induces a Vanishing CTL Response, Whereas Long Peptides Induce Sustained CTL Reactivity. J. Immunol 2007, 179, 5033–5040. [DOI] [PubMed] [Google Scholar]
- (37).Bijker MS; van den Eeden SJ; Franken KL; Melief CJ; van der Burg SH; Offringa R, Superior Induction of Anti-Tumor CTL Immunity by Extended Peptide Vaccines Involves Prolonged, DC-Focused Antigen Presentation. Eur. J. Immunol 2008, 38, 1033–1042. [DOI] [PubMed] [Google Scholar]
- (38).Jackson DC; Lau YF; Le T; Suhrbier A; Deliyannis G; Cheers C; Smith C; Zeng W; Brown LE, A Totally Synthetic Vaccine of Generic Structure That Targets Toll-Like Receptor 2 on Dendritic Cells and Promotes Antibody or Cytotoxic T Cell Responses. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Enayatkhani M; Hasaniazad M; Faezi S; Guklani H; Davoodian P; Ahmadi N; Einakian MA; Karmostaji A; Ahmadi K, Reverse Vaccinology Approach to Design a Novel Multi-Epitope Vaccine Candidate against COVID-19: An In Silico Study. J. Biomol. Struct. Dyn 2020, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kapsenberg ML, Dendritic-Cell Control of Pathogen-Driven T-Cell Polarization. Nat. Rev. Immunol 2003, 3, 984–993. [DOI] [PubMed] [Google Scholar]
- (41).Malowany JI; McCormick S; Santosuosso M; Zhang X; Aoki N; Ngai P; Wang J; Leitch J; Bramson J; Wan Y; Xing Z, Development of Cell-Based Tuberculosis Vaccines: Genetically Modified Dendritic Cell Vaccine Is a Much More Potent Activator of CD4 and CD8 T Cells Than Peptide- or Protein-Loaded Counterparts. Mol. Ther 2006, 13, 766–775. [DOI] [PubMed] [Google Scholar]
- (42).Benteyn D; Heirman C; Bonehill A; Thielemans K; Breckpot K, mRNA-Based Dendritic Cell Vaccines. Expert Rev. Vaccines 2015, 14, 161–176. [DOI] [PubMed] [Google Scholar]
- (43).Perez CR; De Palma M, Engineering Dendritic Cell Vaccines to Improve Cancer Immunotherapy. Nat. Commun 2019, 10, 5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Vela Ramirez JE; Sharpe LA; Peppas NA, Current State and Challenges in Developing Oral Vaccines. Adv. Drug Deliv. Rev 2017, 114, 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Melief CJM; van der Burg SH, Immunotherapy of Established (Pre)malignant Disease by Synthetic Long Peptide Vaccines. Nat. Rev. Cancer 2008, 8, 351–360. [DOI] [PubMed] [Google Scholar]
- (46).Napolitani G; Rinaldi A; Bertoni F; Sallusto F; Lanzavecchia A, Selected Toll-Like Receptor Agonist Combinations Synergistically Trigger a T Helper Type 1-Polarizing Program in Dendritic Cells. Nat. Immunol 2005, 6, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Coffman RL; Sher A; Seder RA, Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Stephan MT; Moon JJ; Um SH; Bershteyn A; Irvine DJ, Therapeutic Cell Engineering with Surface-Conjugated Synthetic Nanoparticles. Nat. Med 2010, 16, 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Yong CY; Ong HK; Yeap SK; Ho KL; Tan WS, Recent Advances in the Vaccine Development against Middle East Respiratory Syndrome-Coronavirus. Front. Microbiol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Hajj KA; Whitehead KA, Tools for Translation: Non-Viral Materials for Therapeutic mRNA Delivery. Nat. Rev. Mater 2017, 2, 17056. [Google Scholar]
- (51).Moyer TJ; Zmolek AC; Irvine DJ, Beyond Antigens and Adjuvants: Formulating Future Vaccines. J. Clin. Invest 2016, 126, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Sun W; Hu Q; Ji W; Wright G; Gu Z, Leveraging Physiology for Precision Drug Delivery. Physiol. Rev 2016, 97, 189–225. [Google Scholar]
- (53).Moon JJ; Huang B; Irvine DJ, Engineering Nano-and Microparticles to Tune Immunity. Adv. Mater 2012, 24, 3724–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Oberli MA; Reichmuth AM; Dorkin JR; Mitchell MJ; Fenton OS; Jaklenec A; Anderson DG; Langer R; Blankschtein D, Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Moon JJ; Suh H; Bershteyn A; Stephan MT; Liu H; Huang B; Sohail M; Luo S; Ho Um S; Khant H; Goodwin JT; Ramos J; Chiu W; Irvine DJ, Interbilayer-Crosslinked Multilamellar Vesicles as Synthetic Vaccines for Potent Humoral and Cellular Immune Responses. Nat. Mater 2011, 10, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Kranz LM; Diken M; Haas H; Kreiter S; Loquai C; Reuter KC; Meng M; Fritz D; Vascotto F; Hefesha H; Grunwitz C; Vormehr M; Hüsemann Y; Selmi A; Kuhn AN; Buck J; Derhovanessian E; Rae R; Attig S; Diekmann J, et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [DOI] [PubMed] [Google Scholar]
- (57).Irvine DJ; Read BJ, Shaping Humoral Immunity to Vaccines through Antigen-Displaying Nanoparticles. Carr. Opin. Immunol 2020, 65, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kuai R; Sun X; Yuan W; Ochyl LJ; Xu Y; Hassani Najafabadi A; Scheetz L; Yu M-Z; Balwani I; Schwendeman A; Moon JJ, Dual TLR Agonist Nanodiscs as a Strong Adjuvant System for Vaccines and Immunotherapy. J. Control. Release 2018, 282, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Miao L; Li L; Huang Y; Delcassian D; Chahal J; Han J; Shi Y; Sadtler K; Gao W; Lin J; Doloff JC; Langer R; Anderson DG, Delivery of mRNA Vaccines with Heterocyclic Lipids Increases Anti-Tumor Efficacy by STING-Mediated Immune Cell Activation. Nat. Biotechnol 2019, 37, 1174–1185. [DOI] [PubMed] [Google Scholar]
- (60).Chahal JS; Khan OF; Cooper CL; McPartlan JS; Tsosie JK; Tilley LD; Sidik SM; Lourido S; Langer R; Bavari S; Ploegh HL; Anderson DG, Dendrimer-RNA Nanoparticles Generate Protective Immunity against Lethal Ebola, H1N1 Influenza, and Toxoplasma Gondii Challenges with a Single Dose. Proc. Natl. Acad. Sci. U.S.A 2016, 113, E4133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Zeng Q; Jiang H; Wang T; Zhang Z; Gong T; Sun X, Cationic Micelle Delivery of Trp2 Peptide for Efficient Lymphatic Draining and Enhanced Cytotoxic T-Lymphocyte Responses. J. Control. Release 2015, 200, 1–12. [DOI] [PubMed] [Google Scholar]
- (62).Zhao M; Li M; Zhang Z; Gong T; Sun X, Induction of HIV-1 Gag Specific Immune Responses by Cationic Micelles Mediated Delivery of Gag mRNA. Drug Deliv. 2016, 23, 2596–2607. [DOI] [PubMed] [Google Scholar]
- (63).Li M; Zhao M; Fu Y; Li Y; Gong T; Zhang Z; Sun X, Enhanced Intranasal Delivery of mRNA Vaccine by Overcoming the Nasal Epithelial Barrier via Intra- and Paracellular Pathways. J. Control. Release 2016, 228, 9–19. [DOI] [PubMed] [Google Scholar]
- (64).Demento SL; Cui W; Criscione JM; Stem E; Tulipan J; Kaech SM; Fahmy TM, Role of Sustained Antigen Release from Nanoparticle Vaccines in Shaping the T Cell Memory Phenotype. Biomaterials 2012, 33, 4957–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Gu P; Wusiman A; Zhang Y; Liu Z; Bo R; Hu Y; Liu J; Wang D, Rational Design of PLGA Nanoparticle Vaccine Delivery Systems to Improve Immune Responses. Mol. Pharm 2019, 16, 5000–5012. [DOI] [PubMed] [Google Scholar]
- (66).Chen X; Liu Y; Wang L; Liu Y; Zhang W; Fan B; Ma X; Yuan Q; Ma G; Su Z, Enhanced Humoral and Cell-Mediated Immune Responses Generated by Cationic Polymer-Coated PLA Microspheres with Adsorbed HBsAg. Mol. Pharm 2014, 11, 1772–1784. [DOI] [PubMed] [Google Scholar]
- (67).Chen H; Li P; Yin Y; Cai X; Huang Z; Chen J; Dong L; Zhang J, The Promotion of Type 1 T Helper Cell Responses to Cationic Polymers in Vivo via Toll-Like Receptor-4 Mediated IL-12 Secretion. Biomaterials 2010, 31, 8172–8180. [DOI] [PubMed] [Google Scholar]
- (68).Fields RJ; Cheng CJ; Quijano E; Weller C; Kristofik N; Duong N; Hoimes C; Egan ME; Saltzman WM, Surface Modified Poly(β Amino Ester)-Containing Nanoparticles for Plasmid DNA Delivery. J. Control. Release 2012, 164, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Kowalski PS; Capasso Palmiero U; Huang Y; Rudra A; Langer R; Anderson DG, Ionizable Amino-Polyesters Synthesized via Ring Opening Polymerization of Tertiary Amino-Alcohols for Tissue Selective mRNA Delivery. Adv. Mater 2018, 30, 1801151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Fornaguera C; Guerra-Rebollo M; Ángel Lázaro M; Castells-Sala C; Meca-Cortés O; Ramos-Pérez V; Cascante A; Rubio N; Blanco J; Borrós S, mRNA Delivery System for Targeting Antigen-Presenting Cells in Vivo. Adv. Healthc. Mater 2018, 7, 1800335. [DOI] [PubMed] [Google Scholar]
- (71).Liu H; Moynihan KD; Zheng Y; Szeto GL; Li AV; Huang B; Van Egeren DS; Park C; Irvine DJ, Structure-Based Programming of Lymph-Node Targeting in Molecular Vaccines. Nature 2014, 507, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Son S; Nam J; Zenkov I; Ochyl LJ; Xu Y; Scheetz L; Shi J; Farokhzad OC; Moon JJ, Sugar-Nanocapsules Imprinted with Microbial Molecular Patterns for mRNA Vaccination. Nano Lett. 2020, 20, 1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Moyer TJ; Kato Y; Abraham W; Chang JYH; Kulp DW; Watson N; Turner HL; Menis S; Abbott RK; Bhiman JN; Melo MB; Simon HA; Herrera-De la Mata S; Liang S; Seumois G; Agarwal Y; Li N; Burton DR; Ward AB; Schief WR, et al. Engineered Immunogen Binding to Alum Adjuvant Enhances Humoral Immunity. Nat. Med 2020, 26, 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Moyano DF; Goldsmith M; Solfiell DJ; Landesman-Milo D; Miranda OR; Peer D; Rotello VM, Nanoparticle Hydrophobicity Dictates Immune Response. J. Am. Chem. Soc 2012, 134, 3965–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Xu L; Liu Y; Chen Z; Li W; Liu Y; Wang L; Liu Y; Wu X; Ji Y; Zhao Y; Ma L; Shao Y; Chen C, Surface-Engineered Gold Nanorods: Promising DNA Vaccine Adjuvant for HIV-1 Treatment. Nano Lett. 2012, 12, 2003–2012. [DOI] [PubMed] [Google Scholar]
- (76).Qiu Y; Liu Y; Wang L; Xu L; Bai R; Ji Y; Wu X; Zhao Y; Li Y; Chen C, Surface Chemistry and Aspect Ratio Mediated Cellular Uptake of Au Nanorods. Biomaterials 2010, 31, 7606–7619. [DOI] [PubMed] [Google Scholar]
- (77).Zhou Q; Zhang Y; Du J; Li Y; Zhou Y; Fu Q; Zhang J; Wang X; Zhan L, Different-Sized Gold Nanoparticle Activator/Antigen Increases Dendritic Cells Accumulation in Liver-Draining Lymph Nodes and CD8+ T Cell Responses. ACS Nano 2016, 10, 2678–2692. [DOI] [PubMed] [Google Scholar]
- (78).Mercuri LP; Carvalho LV; Lima FA; Quayle C; Fantini MCA; Tanaka GS; Cabrera WH; Furtado MFD; Tambourgi DV; Matos JDR; Jaroniec M; Sant’Anna OA, Ordered Mesoporous Silica SBA-15: A New Effective Adjuvant to Induce Antibody Response. Small 2006, 2, 254–256. [DOI] [PubMed] [Google Scholar]
- (79).Mahony D; Cavallaro AS; Mody KT; Xiong L; Mahony TJ; Qiao SZ; Mitter N, In Vivo Delivery of Bovine Viral Diahorrea Virus, E2 Protein Using Hollow Mesoporous Silica Nanoparticles. Nanoscale 2014, 6, 6617–6626. [DOI] [PubMed] [Google Scholar]
- (80).Hess KL; Oh E; Tostanoski LH; Andorko JI; Susumu K; Deschamps JR; Medintz FL; Jewell CM, Engineering Immunological Tolerance Using Quantum Dots to Tune the Density of Self-Antigen Display. Adv. Funct. Mater 2017, 27, 1700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Hu Z; Song B; Xu L; Zhong Y; Peng F; Ji X; Zhu F; Yang C; Zhou J; Su Y; Chen S; He Y; He S, Aqueous Synthesized Quantum Dots Interfere with the NF-κB Pathway and Confer Anti-Tumor, Anti-Viral and Anti-Inflammatory Effects. Biomaterials 2016, 108, 187–196. [DOI] [PubMed] [Google Scholar]
- (82).Faria PCBD; Santos LID; Coelho JP; Ribeiro HB; Pimenta MA; Ladeira LO; Gomes DA; Furtado CA; Gazzinelli RT, Oxidized Multiwalled Carbon Nanotubes as Antigen Delivery System to Promote Superior CD8+ T Cell Response and Protection against Cancer. Nano Lett. 2014, 14, 5458–5470. [DOI] [PubMed] [Google Scholar]
- (83).Zhang L; Chan JM; Gu FX; Rhee J-W; Wang AZ; Radovic-Moreno AF; Alexis F; Langer R; Farokhzad OC, Self-Assembled Lipid–Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2008, 2, 1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Walsh CL; Nguyen J; Tiffany MR; Szoka FC, Synthesis, Characterization, and Evaluation of Ionizable Lysine-Based Lipids for siRNA Delivery. Bioconjugate Chem. 2013, 24, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Jokerst JV; Lobovkina T; Zare RN; Gambhir SS, Nanoparticle PEGylation for Imaging and Therapy. Nanomedicine 2011, 6, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Beltrán-Gracia E; Lñpez-Camacho A; Higuera-Ciapara I; Velázquez-Fernández JB; Vallejo-Cardona AA, Nanomedicine Review: Clinical Developments in Liposomal Applications. Cancer Nano 2019, 10, 11. [Google Scholar]
- (87).Tam HH; Melo MB; Kang M; Pelet JM; Ruda VM; Foley MH; Hu JK; Kumari S; Crampton J; Baldeon AD; Sanders RW; Moore JP; Crotty S; Langer R; Anderson DG; Chakraborty AK; Irvine DJ, Sustained Antigen Availability during Germinal Center Initiation Enhances Antibody Responses to Vaccination. Proc. Natl. Acad. Sci. U.S.A 2016, 113, E6639–E6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Diebold SS; Kaisho T; Hemmi H; Akira S; Reis e Sousa C, Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 1529–1531. [DOI] [PubMed] [Google Scholar]
- (89).Kuai R; Ochyl LJ; Bahjat KS; Schwendeman A; Moon JJ, Designer Vaccine Nanodiscs for Personalized Cancer Immunotherapy. Nat. Mater 2017, 16, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Dobrovolskaia MA; McNeil SE, Immunological Properties of Engineered Nanomaterials. Nat. Nanotechnol 2007, 2, 469–478. [DOI] [PubMed] [Google Scholar]
- (91).Akinc A; Thomas M; Klibanov AM; Langer R, Exploring Polyethylenimine-Mediated DNA Transfection and the Proton Sponge Hypothesis. J. Gene. Med 2005, 7, 657–663. [DOI] [PubMed] [Google Scholar]
- (92).Boussif O; Lezoualc H,F; Zanta MA; Mergny MD; Scherman D; Demeneix B; Behr JP, A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in Vivo: Polyethylenimine. Proc. Natl. Acad. Sci. U.S.A 1995, 92, 7297–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Anderson JM; Shive MS, Biodegradation and Biocompatibility of PLA and PLGA Microspheres. Adv. Drug Deliv. Rev 2012, 64, 72–82. [DOI] [PubMed] [Google Scholar]
- (94).Sunshine JC; Perica K; Schneck JP; Green JJ, Particle Shape Dependence of CD8+ T Cell Activation by Artificial Antigen Presenting Cells. Biomaterials 2014, 35, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Wang Q; Tan MT; Keegan BP; Barry MA; Heffernan MJ, Time Course Study of the Antigen-Specific Immune Response to a PLGA Microparticle Vaccine Formulation. Biomaterials 2014, 35, 8385–8393. [DOI] [PubMed] [Google Scholar]
- (96).Chan JM; Zhang L; Yuet KP; Liao G; Rhee J-W; Langer R; Farokhzad OC, PLGA–Lecithin–PEG Core–Shell Nanoparticles for Controlled Drug Delivery. Biomaterials 2009, 30, 1627–1634. [DOI] [PubMed] [Google Scholar]
- (97).Sun W; Wang J; Hu Q; Zhou X; Khademhosseini A; Gu Z, CRISPR-Cas12a Delivery by DNA-Mediated Bioresponsive Editing for Cholesterol Regulation. Sci. Adv 2020, 6, eaba2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Dong L; Xia S; Chen H; Chen J; Zhang J, Anti-Arthritis Activity of Cationic Materials. J. Cell. Mol. Med 2010, 14, 2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Little SR; Lynn DM; Ge Q; Anderson DG; Puram SV; Chen J; Eisen HN; Langer R, Poly-β Amino Ester-Containing Microparticles Enhance the Activity of Nonviral Genetic Vaccines. Proc. Natl. Acad. Sci. U.S.A 2004, 101, 9534–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Liu Z; Jiao Y; Wang Y; Zhou C; Zhang Z, Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Advanced Drug Delivery Reviews 2008, 60, 1650–1662. [DOI] [PubMed] [Google Scholar]
- (101).Acharya AP; Sinha M; Ratay ML; Ding X; Balmert SC; Workman CJ; Wang Y; Vignali DAA; Little SR, Localized Multi-Component Delivery Platform Generates Local and Systemic Anti-Tumor Immunity. Adv. Funct. Mater 2017, 27, 1604366. [Google Scholar]
- (102).Dosta P; Segovia N; Cascante A; Ramos V; Borrós S, Surface Charge Tunability as a Powerful Strategy to Control Electrostatic Interaction for High Efficiency Silencing, Using Tailored Oligopeptide-Modified Poly(Beta-Amino Ester)s (PBAEs). Acta Biomater. 2015, 20, 82–93. [DOI] [PubMed] [Google Scholar]
- (103).Tsopelas C; Sutton R, Why Certain Dyes Are Useful for Localizing the Sentinel Lymph Node. J. Nucl. Med 2002, 43, 1377–1382. [PubMed] [Google Scholar]
- (104).Seong S-Y; Matzinger P, Hydrophobicity: An Ancient Damage-Associated Molecular Pattern That Initiates Innate Immune Responses. Nat. Rev. Immunol 2004, 4, 469–478. [DOI] [PubMed] [Google Scholar]
- (105).Shokouhi B; Coban C; Hasirci V; Ay din E; Dhanasingh A; Shi N; Koyama S; Akira S; Zenke M; Sechi AS, The Role of Multiple Toll-Like Receptor Signalling Cascades on Interactions between Biomedical Polymers and Dendritic Cells. Biomaterials 2010, 31, 5759–5771. [DOI] [PubMed] [Google Scholar]
- (106).Glenny A; Pope C; Waddington H; Wallace U, Immunological Notes, xvii–xxiv. The Journal of Pathology and Bacteriology 1926, 29, 31–40. [Google Scholar]
- (107).Levitz, Stuart M; Golenbock, Douglas T, Beyond Empiricism: Informing Vaccine Development through Innate Immunity Research. Cell 2012, 148, 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Mannhalter J; Neychev H; Zlabinger G; Ahmad R; Eibl M, Modulation of the Human Immune Response by the Non-Toxic and Non-Pyrogenic Adjuvant Aluminium Hydroxide: Effect on Antigen Uptake and Antigen Presentation. Clin. Exp. Immunol 1985, 61, 143–151. [PMC free article] [PubMed] [Google Scholar]
- (109).McKee AS; Munks MW; Marrack P, How Do Adjuvants Work? Important Considerations for New Generation Adjuvants. Immunity 2007, 27, 687–690. [DOI] [PubMed] [Google Scholar]
- (110).Hornung V; Bauernfeind F; Halle A; Samstad EO; Kono H; Rock KL; Fitzgerald KA; Latz E, Silica Crystals and Aluminum Salts Activate the NALP3 Inflammasome through Phagosomal Destabilization. Nat. Immunol 2008, 9, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Hess KL; Medintz IL; Jewell CM, Designing Inorganic Nanomaterials for Vaccines and Immunotherapies. Nano Today 2019, 27, 73–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Reddy ST; van der Vlies AJ; Simeoni E; Angeli V; Randolph GJ; O’Neil CP; Lee LK; Swartz MA; Hubbell JA, Exploiting Lymphatic Transport and Complement Activation in Nanoparticle Vaccines. Nat. Biotechnol 2007, 25, 1159–1164. [DOI] [PubMed] [Google Scholar]
- (113).Sokolova V; Epple M, Inorganic Nanoparticles as Carriers of Nucleic Acids into Cells. Angew. Chem.-Int. Edit 2008, 47, 1382–1395. [DOI] [PubMed] [Google Scholar]
- (114).Moon JJ; Suh H; Li AV; Ockenhouse CF; Yadava A; Irvine DJ, Enhancing Humoral Responses to a Malaria Antigen with Nanoparticle Vaccines That Expand Tfh Cells and Promote Germinal Center Induction. Proc. Natl. Acad. Sci. U.S.A 2012, 109, 1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Lin W-HW; Kouyos RD; Adams RJ; Grenfell BT; Griffin DE, Prolonged Persistence of Measles Virus RNA Is Characteristic of Primary Infection Dynamics. Proc. Natl. Acad. Sci. U.S.A 2012, 109, 14989–14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Pape KA; Catron DM; Itano AA; Jenkins MK, The Humoral Immune Response Is Initiated in Lymph Nodes by B Cells That Acquire Soluble Antigen Directly in the Follicles. Immunity 2007, 26, 491–502. [DOI] [PubMed] [Google Scholar]
- (117).McHugh KJ; Guarecuco R; Langer R; Jaklenec A, Single-Injection Vaccines: Progress, Challenges, and Opportunities. J. Control. Release 2015, 219, 596–609. [DOI] [PubMed] [Google Scholar]
- (118).Zhu Q; Zarnitsyn VG; Ye L; Wen Z; Gao Y; Pan L; Skountzou I; Gill HS; Prausnitz MR; Yang C; Compans RW, Immunization by Vaccine-Coated Microneedle Arrays Protects against Lethal Influenza Virus Challenge. Proc. Natl. Acad. Sci. U.S.A 2009, 106, 7968–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Sullivan SP; Koutsonanos DG; del Pilar Martin M; Lee JW; Zarnitsyn V; Choi S-O; Murthy N; Compans RW; Skountzou I; Prausnitz MR, Dissolving Polymer Microneedle Patches for Influenza Vaccination. Nat. Med 2010, 16, 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Kim E; Erdos G; Huang S; Kenniston TW; Balmert SC; Carey CD; Raj VS; Epperly MW; Klimstra WB; Haagmans BL; Korkmaz E; Falo LD; Gambotto A, Microneedle Array Delivered Recombinant Coronavirus Vaccines: Immunogenicity and Rapid Translational Development. EBioMedicine 2020, 55, 102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Rouphael NG; Paine M; Mosley R; Henry S; McAllister DV; Kalluri H; Pewin W; Frew PM; Yu T; Thornburg NJ; Kabbani S; Lai L; Vassilieva EV; Skountzou I; Compans RW; Mulligan MJ; Prausnitz MR; Beck A; Edupuganti S; Heeke S, et al. The Safety, Immunogenicity, and Acceptability of Inactivated Influenza Vaccine Delivered by Microneedle Patch (TIV-MNP 2015): A Randomised, Partly Blinded, Placebo-Controlled, Phase 1 Trial. The Lancet 2017, 390, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Boopathy AV; Mandal A; Kulp DW; Menis S; Bennett NR; Watkins HC; Wang W; Martin JT; Thai NT; He Y; Schief WR; Hammond PT; Irvine DJ, Enhancing Humoral Immunity via Sustained-Release Implantable Microneedle Patch Vaccination. Proc. Natl. Acad. Sci. U.S.A 2019, 116, 16473–16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).DeMuth PC; Moon JJ; Suh H; Hammond PT; Irvine DJ, Releasable Layer-by-Layer Assembly of Stabilized Lipid Nanocapsules on Microneedles for Enhanced Transcutaneous Vaccine Delivery. ACS Nano 2012, 6, 8041–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Zaric M; Lyubomska O; Touzelet O; Poux C; Al-Zahrani S; Fay F; Wallace L; Terhorst D; Malissen B; Henri S; Power UF; Scott CJ; Donnelly RF; Kissenpfennig A, Skin Dendritic Cell Targeting via Microneedle Arrays Laden with Antigen-Encapsulated Poly-d,l-lactide-co-Glycolide Nanoparticles Induces Efficient Antitumor and Antiviral Immune Responses. ACS Nano 2013, 7, 2042–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Bae W-G; Ko H; So J-Y; Yi H; Lee C-H; Lee D-H; Ahn Y; Lee S-H; Lee K; Jun J; Kim H-H; Jeon NL; Jung W; Song C-S; Kim T; Kim Y-C; Jeong HE, Snake Fang–Inspired Stamping Patch for Transdermal Delivery of Liquid Formulations. Sci. Transl. Med 2019, 11, eaaw3329. [DOI] [PubMed] [Google Scholar]
- (126).Tzeng SY; McHugh KJ; Behrens AM; Rose S; Sugarman JL; Ferber S; Langer R; Jaklenec A, Stabilized Single-Injection Inactivated Polio Vaccine Elicits a Strong Neutralizing Immune Response. Proc. Natl. Acad. Sci. U.S.A 2018, 115, E5269–E5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).McHugh KJ; Nguyen TD; Linehan AR; Yang D; Behrens AM; Rose S; Tochka ZL; Tzeng SY; Norman JJ; Anselmo AC; Xu X; Tomasic S; Taylor MA; Lu J; Guarecuco R; Langer R; Jaklenec A, Fabrication of Fillable Microparticles and Other Complex 3D Microstructures. Science 2017, 357, 1138–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Lu X; Miao L; Gao W; Chen Z; McHugh KJ; Sun Y; Tochka Z; Tomasic S; Sadtler K; Hyacinthe A; Huang Y; Graf T; Hu Q; Sarmadi M; Langer R; Anderson DG; Jaklenec A, Engineered PLGA Microparticles for Long-Term, Pulsatile Release of STING Agonist for Cancer Immunotherapy. Sci. Transl. Med 2020, 12, eaaz6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Ali OA; Huebsch N; Cao L; Dranoff G; Mooney DJ, Infection-Mimicking Materials to Program Dendritic Cells in Situ. Nat. Mater 2009, 8, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Kim J; Li WA; Choi Y; Lewin SA; Verbeke CS; Dranoff G; Mooney DJ, Injectable, Spontaneously Assembling, Inorganic Scaffolds Modulate Immune Cells in Vivo and Increase Vaccine Efficacy. Nat. Biotechnol 2015, 33, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Li AW; Sobral MC; Badrinath S; Choi Y; Graveline A; Stafford AG; Weaver JC; Dellacherie MO; Shih T-Y; Ali OA; Kim J; Wucherpfennig KW; Mooney DJ, A Facile Approach to Enhance Antigen Response for Personalized Cancer Vaccination. Nat. Mater 2018, 17, 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Dellacherie MO; Li A; Lu BY; Verbeke CS; Gu L; Stafford AG; Doherty EJ; Mooney DJ, Single-Shot Mesoporous Silica Rods Scaffold for Induction of Humoral Responses against Small Antigens. Adv. Funct. Mater 2020, DOI: 10.1002/adfm.202002448 [DOI] [Google Scholar]
- (133).Shih T-Y; Blacklow SO; Li AW; Freedman BR; Bencherif S; Koshy ST; Darnell MC; Mooney DJ, Injectable, Tough Alginate Cryogels as Cancer Vaccines. Adv. Healthc. Mater 2018, 7, 1701469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Bencherif SA; Sands RW; Bhatta D; Arany P; Verbeke CS; Edwards DA; Mooney DJ, Injectable Preformed Scaffolds with Shape-Memory Properties. Proc. Natl. Acad. Sci. U.S.A 2012, 109, 19590–19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Hori Y; Winans AM; Huang CC; Horrigan EM; Irvine DJ, Injectable Dendritic Cell-Carrying Alginate Gels for Immunization and Immunotherapy. Biomaterials 2008, 29, 3671–3682. [DOI] [PubMed] [Google Scholar]
- (136).Guo Z; Zhang M; Tang H; Cao X, Fas Signal Links Innate and Adaptive Immunity by Promoting Dendritic-Cell Secretion of CC and CXC Chemokines. Blood 2005, 106, 2033–2041. [DOI] [PubMed] [Google Scholar]
- (137).Hori Y; Winans AM; Irvine DJ, Modular Injectable Matrices Based on Alginate Solution/Microsphere Mixtures That Gel in Situ and Co-deliver Immunomodulatory factors. Acta Biomater. 2009, 5, 969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Roy K; Wang D; Hedley ML; Barman SP, Gene Delivery with In-Situ Crosslinking Polymer Networks Generates Long-Term Systemic Protein Expression. Mol. Ther 2003, 7, 401–408. [DOI] [PubMed] [Google Scholar]
- (139).Wu Y; Wei W; Zhou M; Wang Y; Wu J; Ma G; Su Z, Thermal-Sensitive Hydrogel as Adjuvant-Free Vaccine Delivery System for H5N1 Intranasal Immunization. Biomaterials 2012, 33, 2351–2360. [DOI] [PubMed] [Google Scholar]
- (140).Hiemstra C; van der Aa LJ; Zhong Z; Dijkstra PJ; Feijen J, Rapidly in Situ-Forming Degradable Hydrogels from Dextran Thiols through Michael Addition. Biomacromolecules 2007, 8, 1548–1556. [DOI] [PubMed] [Google Scholar]
- (141).Singh A; Suri S; Roy K, In-Situ Crosslinking Hydrogels for Combinatorial Delivery of Chemokines and siRNA–DNA Carrying Microparticles to Dendritic Cells. Biomaterials 2009, 30, 5187–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Singh A; Qin H; Fernandez I; Wei J; Lin J; Kwak LW; Roy K, An Injectable Synthetic Immune-Priming Center Mediates Efficient T-Cell Class Switching and T-Helper 1 Response against B Cell Lymphoma. J. Control. Release 2011, 155, 184–192. [DOI] [PubMed] [Google Scholar]
- (143).Umeki Y; Mohri K; Kawasaki Y; Watanabe H; Takahashi R; Takahashi Y; Takakura Y; Nishikawa M, Induction of Potent Antitumor Immunity by Sustained Release of Cationic Antigen from a DNA-Based Hydrogel with Adjuvant Activity. Adv. Funct. Mater 2015, 25, 5758–5767. [Google Scholar]
- (144).Prausnitz MR; Mikszta JA; Cormier M; Andrianov AK, Microneedle-Based Vaccines. Curr. Top Microbiol. Immunol 2009, 333, 369–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Chen G; Chen Z; Wen D; Wang Z; Li H; Zeng Y; Dotti G; Wirz RE; Gu Z, Transdermal Cold Atmospheric Plasma-Mediated Immune Checkpoint Blockade Therapy. Proc. Natl. Acad. Sci. U.S.A 2020, 117, 3687–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Ye Y; Wang C; Zhang X; Hu Q; Zhang Y; Liu Q; Wen D; Milligan J; Bellotti A; Huang L; Dotti G; Gu Z, A Melanin-Mediated Cancer Immunotherapy Patch. Sci. Immunol 2017, 2, eaan5692. [DOI] [PubMed] [Google Scholar]
- (147).Erdos G; Balmert SC; Carey CD; Falo GD; Patel NA; Zhang J; Gambotto A; Korkmaz E; Falo LD, Improved Cutaneous Genetic Immunization by Microneedle Array Delivery of an Adjuvanted Adenovirus Vaccine. J. Invest. Dermatol 2020, DOI: 10.1016/j.jid.2020.03.966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Lee K; Goudie MJ; Tebon P; Sun W; Luo Z; Lee J; Zhang S; Fetah K; Kim H-J; Xue Y; Darabi MA; Ahadian S; Sarikhani E; Ryu W; Gu Z; Weiss PS; Dokmeci MR; Ashammakhi N; Khademhosseini A, Non-Transdermal Microneedles for Advanced Drug Delivery. Adv. Drug Deliv. Rev 2019, DOI: 10.1016/j.addr.2019.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).van der Maaden K; Jiskoot W; Bouwstra J, Microneedle Technologies for (Trans)dermal Drug and Vaccine Delivery. J. Control. Release 2012, 161, 645–655. [DOI] [PubMed] [Google Scholar]
- (150).Kim Y-C; Quan F-S; Compans RW; Kang S-M; Prausnitz MR, Stability Kinetics of Influenza Vaccine Coated onto Microneedles during Drying and Storage. Pharm. Res 2011, 28, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Sanders RW; van Gils MJ; Derking R; Sok D; Ketas TJ; Burger JA; Ozorowski G; Cupo A; Simonich C; Goo L; Arendt H; Kim HJ; Lee JH; Pugach P; Williams M; Debnath G; Moldt B; van Breemen MJ; Isik G; Medina-Ramírez M, et al. HIV-1 Neutralizing Antibodies Induced by Native-Like Envelope Trimers. Science 2015, 349, aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Wang W, Lyophilization and Development of Solid Protein Pharmaceuticals. Int. J. Pharm 2000, 203, 1–60. [DOI] [PubMed] [Google Scholar]
- (153).Dellacherie MO; Seo BR; Mooney DJ, Macroscale Biomaterials Strategies for Local Immunomodulation. Nat. Rev. Mater 2019, 4, 379–397. [Google Scholar]
- (154).Wu Y; Jane Grande-Allen K; West JL, Adhesive Peptide Sequences Regulate Valve Interstitial Cell Adhesion, Phenotype and Extracellular Matrix Deposition. Cell. Mol. Bioeng 2016, 9, 479–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Diehl MC; Lee JC; Daniels SE; Tebas P; Khan AS; Giffear M; Sardesai NY; Bagarazzi ML, Tolerability of Intramuscular and Intradermal Delivery by CELLECTRA® Adaptive Constant Current Electroporation Device in Healthy Volunteers. Human Vaccines Immunother. 2013, 9, 2246–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Smith TRF; Patel A; Ramos S; Elwood D; Zhu X; Yan J; Gary EN; Walker SN; Schultheis K; Purwar M; Xu Z; Walters J; Bhojnagarwala P; Yang M; Chokkalingam N; Pezzoli P; Parzych E; Reuschel EL; Doan A; Tursi N, et al. Immunogenicity of a DNA Vaccine Candidate for COVID-19. Nat. Commun 2020, 11, 2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Choi S-O; Kim Y-C; Lee JW; Park J-H; Prausnitz MR; Allen MG, Intracellular Protein Delivery and Gene Transfection by Electroporation Using a Microneedle Electrode Array. Small 2012, 8, 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Banga AK; Bose S; Ghosh TK, Iontophoresis and Electroporation: Comparisons and Contrasts. Int. J. Pharm 1999, 179, 1–19. [DOI] [PubMed] [Google Scholar]
- (159).Tadros AR; Romanyuk A; Miller IC; Santiago A; Noel RK; O’Farrell L; Kwong GA; Prausnitz MR, STAR Particles for Enhanced Topical Drug and Vaccine Delivery. Nat. Med 2020, 26, 341–347. [DOI] [PubMed] [Google Scholar]
- (160).Lamson NG; Berger A; Fein KC; Whitehead KA, Anionic Nanoparticles Enable the Oral Delivery of Proteins by Enhancing Intestinal Permeability. Nat. Biomed. Eng 2020, 4, 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Wei X; Beltrán-Gastélum M; Karshalev E; Esteban-Fernández de Ávila B; Zhou J; Ran D; Angsantikul P; Fang RH; Wang J; Zhang L, Biomimetic Micromotor Enables Active Delivery of Antigens for Oral Vaccination. Nano Lett. 2019, 19, 1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Karshalev E; Esteban-Fernández de Ávila B; Beltrán-Gastélum M; Angsantikul P; Tang S; Mundaca-Uribe R; Zhang F; Zhao J; Zhang L; Wang J, Micromotor Pills as a Dynamic Oral Delivery Platform. ACS Nano 2018, 12, 8397–8405. [DOI] [PubMed] [Google Scholar]
- (163).Aran K; Chooljian M; Paredes J; Rafi M; Lee K; Kim AY; An J; Yau JF; Chum H; Conboy I; Murthy N; Liepmann D, An Oral Microjet Vaccination System Elicits Antibody Production in Rabbits. Sci. Transl. Med 2017, 9, eaaf6413. [DOI] [PubMed] [Google Scholar]
- (164).Abramson A; Caffarel-Salvador E; Khang M; Dellal D; Silverstein D; Gao Y; Frederiksen MR; Vegge A; Hubálek F; Water JJ; Friderichsen AV; Fels J; Kirk RK; Cleveland C; Collins J; Tamang S; Hayward A; Landh T; Buckley ST; Roxhed N, et al. An Ingestible Self-Orienting System for Oral Delivery of Macromolecules. Science 2019, 363, 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Shin MD; Shukla S; Chung YH; Beiss V; Chan SK; Ortega-Rivera OA; Wirth DM; Chen A; Sack M; Pokorski JK; Steinmetz NF, COVID-19 Vaccine Development and a Potential Nanomaterial Path Forward. Nat. Nanotechnol 2020, 15, 646–655. [DOI] [PubMed] [Google Scholar]
- (166).Weiss C; Carriere M; Fusco L; Capua I; Regla-Nava JA; Pasquali M; Scott JA; Vitale F; Unal MA; Mattevi C; Bedognetti D; Merkoçi A; Tasciotti E; Yilmazer A; Gogotsi Y; Stellacci F; Delogu LG, Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [DOI] [PubMed] [Google Scholar]
- (167).Fang J; Hsueh Y-Y; Soto J; Sun W; Wang J; Gu Z; Khademhosseini A; Li S, Engineering Biomaterials with Micro/Nanotechnologies for Cell Reprogramming. ACS Nano 2020, 14, 1296–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Sun J; Zhuang Z; Zheng J; Li K; Wong RL-Y; Liu D; Huang J; He J; Zhu A; Zhao J; Li X; Xi Y; Chen R; Alshukairi AN; Chen Z; Zhang Z; Chen C; Huang X; Li F; Lai X, et al. Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment. Cell 2020, 182, 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Si L; Bai H; Rodas M; Cao W; Oh CY; Jiang A; Nurani A; Zhu DY;Goyal G; Gilpin SE; Prantil-Baun R; Ingber DE, Human Organs-on-Chips as Tools for Repurposing Approved Drugs as Potential Influenza and COVID19 Therapeutics in Viral Pandemics. bioRxiv 2020, 10.1101/2020.04.13.039917v1 (accessed 2020-08-25) [DOI]
- (170).Sun W; Luo Z; Lee J; Kim H-J; Lee K; Tebon P; Feng Y; Dokmeci MR; Sengupta S; Khademhosseini A, Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv. Healthc. Mater 2019, 8, 1801363. [DOI] [PubMed] [Google Scholar]
- (171).Segaliny AI; Li G; Kong L; Ren C; Chen X; Wang JK; Baltimore D; Wu G; Zhao W, Functional TCR T Cell Screening Using Single-Cell Droplet Microfluidics. Lab Chip 2018, 18, 3733–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Monteil V; Kwon H; Prado P; Hagelkrüys A; Wimmer RA; Stahl M; Leopoldi A; Garreta E; Hurtado del Pozo C; Prosper F; Romero JP; Wirnsberger G; Zhang H; Slutsky AS; Conder R; Montserrat N; Mirazimi A; Penninger JM, Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Shah SK; Miller FG; Darton TC; Duenas D; Emerson C; Lynch HF; Jamrozik E; Jecker NS; Kamuya D; Kapulu M; Kimmelman J; MacKay D; Memoli MJ; Murphy SC; Palacios R; Richie TL; Roestenberg M; Saxena A; Saylor K; Selgelid MJ, et al. Ethics of Controlled Human Infection to Address COVID-19. Science 2020, 368, 832–834. [DOI] [PubMed] [Google Scholar]


