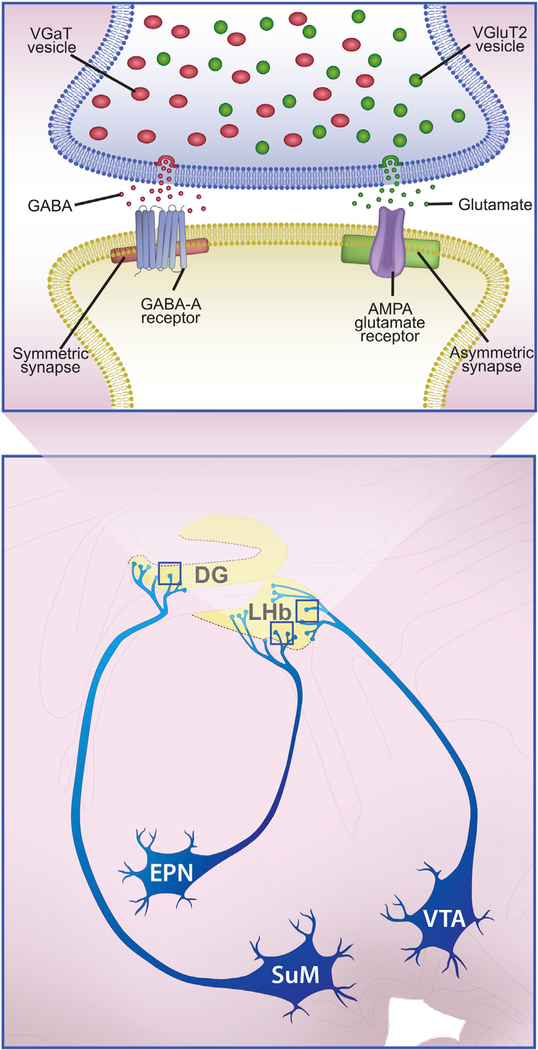
SUMMARY
For decades, it has been thought that glutamate and GABA are released by distinct neurons. However, some mouse neurons innervating the lateral habenula (LHb) co-release glutamate and GABA. Here, we mapped the distribution of neurons throughout the rat brain that co-express vesicular transporters for the accumulation of glutamate (VGluT2) or GABA (VGaT) and for GABA synthesis (GAD). We found concentrated groups of neurons that co-express VGluT2, VGaT, and GAD mRNAs within subdivisions of the ventral tegmental area (VTA), entopeduncular (EPN), and supramammillary (SUM) nuclei. Single axon terminals established by VTA, EPN, or SUM neurons form a common synaptic architecture involving asymmetric (putative excitatory) and symmetric (putative inhibitory) synapses. Within the LHb, which receives co-transmitted glutamate and GABA from VTA and EPN, VGluT2 and VGaT are distributed on separate synaptic vesicles. We conclude that single axon terminals from VGluT2 and VGaT co-expressing neurons co-transmit glutamate and GABA from distinct synaptic vesicles at independent synapses.
In Brief
Root et al. identify concentrated populations of glutamate and GABA co-transmitting neurons in VTA, SUM, and EPN. Single axon terminals from these neurons form a common synaptic architecture that co-transmits glutamate and GABA from distinct synaptic vesicles at independent asymmetric or symmetric synapses.
Graphical Abstract

INTRODUCTION
Mammalian nervous systems are primarily controlled by a delicate balance of glutamate excitatory neurotransmission and GABA inhibitory neurotransmission (Foster and Kemp, 2006). Imbalances of excitatory and inhibitory neurotransmission are associated with several disorders, including epilepsy, autism, Tourette’s syndrome, and schizophrenia (Cline, 2005). For several decades, it has been thought that glutamate and GABA are released from independent populations of neurons. However, recent studies have shown that axon terminals in the rodent lateral habenula (LHb) (Meye et al., 2016; Root et al., 2014b; Shabel et al., 2014), dentate gyrus (Boulland et al., 2009; Munster-Wandowski et al., 2013; Pedersen et al., 2017; Soussi et al., 2010), lateral superior olive (Gillespie et al., 2005; Noh et al., 2010), and cortex (Fattorini et al., 2015) have the capability to co-release both glutamate and GABA. Thus, these data have challenged our understanding of the mechanisms underlying neurotransmission.
We have recently identified a group of neurons within the ventral tegmental area (VTA) that synaptically releases both glutamate and GABA in the adult mouse (Root et al., 2014b). These neurons express vesicular glutamate transporter 2 (VGluT2; for the vesicular transport of glutamate), glutamic acid decarboxylase (GAD; for the synthesis of GABA), and vesicular GABA transporter (VGaT; for the vesicular transport of GABA) and are intermingled with dopamine neurons (Root et al., 2014b). Axon terminals from these VTA VGluT2+ VGaT+ neurons establish independent glutamate- and GABA-releasing synapses on LHb neurons (Root et al., 2014b). In addition, quantitative ultrastructural findings have shown that, within the rat LHb, more than half of the total population of LHb axon terminals co-express VGluT2 and VGaT (Root et al., 2014b), indicating a major role of GABA and glutamate co-release in LHb excitability. Studies in the rat and mouse indicate that many of these LHb dual VGluT2+ VGaT+ terminals are derived from the VTA (Root et al., 2014b) or entopeduncular nucleus (EPN) (Shabel et al., 2014; Wallace et al., 2017).
Here, we first determined the extent to which dual VGluT2+ VGaT+ neurons are present in different rat brain areas by mapping (by in situ hybridization) the brain expression of GAD and VGaT within the population of VGluT2 neurons. We next determined the ultrastructural and molecular characteristics of the synapses established by VGluT2+ VGaT+ neurons from different brain structures (by a combination of viral tract tracing, immune-detection, and electron microscopy). Lastly, to gain insight into the possible cellular mechanisms of glutamate and GABA corelease, we determined by co-immunoprecipitation and co-immunoelectron microscopy the extent to which axon terminals in the LHb have the capability to co-release glutamate and GABA from the same synaptic vesicle or from segregated synaptic vesicles.
RESULTS
To determine the extent to which neurons with the capability to co-release glutamate and GABA are present in different brain structures, we initially surveyed throughout the adult rat brain for the presence of neurons co-expressing VGluT2 and GAD (VGluT2+ GAD+ neurons). By dual in situ hybridization for the detection of both VGluT2 and GAD mRNAs, we detected VGluT2+ GAD+ neurons in the VTA (Figure S1; Table S1), EPN (Figure S2; Table S2), supramammillary nucleus (SUM) (Figure S3; Table S2), LHb (Figure S4; Table S2), the posterodorsal tegmental nucleus (PDTg) (Figure S5; Table S2), and caudal pontine reticular nucleus (PNC) (Figure S6; Table S2).
To determine whether the identified VGluT2+ GAD+ neurons are capable of accumulating GABA (synthesized by GAD) into synaptic vesicles, we looked for the presence of neurons co-expressing VGluT2 and VGaT (VGluT2+ VGAT+ neurons) within the VTA, EPN, SUM, LHb, PDTg, and PNC. By dual in situ hybridization for the detection of both VGluT2 and VGaT mRNAs, we found that while VGluT2+ neurons in the VTA, EPN and SUM frequently co-expressed VGaT (Figures 1, 2, 3, S1, S2, and S3; Tables S3 and S4), the VGluT2+ neurons detected in LHb, PDTg, and PNC rarely co-expressed VGaT (Figures S4, S5, and S6; Table S4). Further, by triple fluorescent in situ hybridization for simultaneous detection of VGluT2, VGaT, and GAD mRNAs, we confirmed that the VGluT2+ GAD+ co-expressing neurons of the VTA (Figures 1F and 1G), EPN (Figures 2F and 2G), and SUM (Figures 3G and 3H) co-expresed VGaT. In contrast, the VGluT2+ GAD+ co-expressing neurons of the LHb, PDTg, and PNC lacked VGaT (Figure S7). We also detected sporadic VGluT2+ GAD+ neurons, but not VGluT2+ VGaT+ neurons, in the lateral hypothalamus surrounding the fornix.
Figure 1. VTA VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ Neurons.

(A) Brain coronal section at the level of the VTA. Bregma: −5.40 mm.
(B–D) Higher magnification of rostral linear nucleus (boxed area in A) showing cellular expression of VGluT2 mRNA (B), VGaT mRNA (C), and both VGluT2 and VGaT mRNAs (D) in three neurons (yellow arrows). Green arrow in each panel points to a VGluT2+ VGaT− neuron.
(E) Frequency of VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ neurons across the entire VTA (mean ± SEM).
(F) Cellular expression of VGluT2 mRNA (red), VGaT mRNA (green), and GAD mRNA (white) in the VTA. Yellow arrows indicate three neurons co-expressing VGluT2, VGaT, and GAD.
(G) Frequency of VTA VGluT2+ VGaT+ GAD+ co-expressing neurons (yellow), VGluT2+ VGaT+ GAD co-expressing neurons (yellow cross-hatch), and VGluT2+ VGaT− GAD+ co-expressing neurons lacking VGaT (gray) (mean ± SEM).
(H–K) Frequency of VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ neurons across the RLi (H), IF (I), PN (J), and PBP (K) subdivisions of the VTA (mean ± SEM).
(L) Summary of VTA distribution of VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ neurons. Number of dots represents average number of neurons at bregma −5.40 mm.
fr, fasciculus retroflexus; IF, interfascicular nucleus; mp, mammillary peduncle; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; RLi, rostral linear nucleus of the raphe. Scale bars represent 1 mm in (A) and (L) and 25 μm in (B) and (F).
Figure 2. EPN VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ Neurons.

(A) Brain coronal section at the level of the central EPN. Bregma: −2.28 mm.
(B–D) Higher magnification of central EPN (boxed area shown in A) showing cellular expression of VGluT2 mRNA(B), VGaT mRNA(C), and bothVGluT2 and VGaT mRNAs (D) in three neurons (yellow arrows).
(E) Frequency of VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ neurons across the entire EPN (mean ± SEM).
(F) Cellular expression of VGluT2 mRNA (red), VGaT mRNA (green), and GAD mRNA (white) in the EPN. Yellow arrows indicates two neurons co-expressing VGluT2, VGaT, and GAD.
(G) Frequency of EPN VGluT2+ VGaT+ GAD+ co-expressing neurons (yellow), VGluT2+ VGaT+ co-expressing neurons lacking GAD (yellow cross-hatch), and VGluT2+ GAD+ co-expressing neurons lacking VGaT (gray) (mean ± SEM).
(H) Summary of EPN distribution of VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ neurons. Number of dots represents average number of neurons at bregma −1.92 mm (anterior EPN), −2.28 mm (central EPN), and −2.76 (posterior EPN).
(I) Frequency of VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ neurons across subdivisions of the EPN (mean ± SEM). Scale bars represent 1 mm in (A) and (H) and 25 μm in (B) and (F).
Figure 3. SUM VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ Neurons.

(A) Brain coronal section at the level of the SUM, showing medial (SUMM) and lateral (SUML) subdivisions. Bregma: −4.20 mm.
(B–D) Higher magnification of SUML (boxed area in A) showing cellular expression of VGluT2 mRNA(B), VGaT mRNA(C), and both VGluT2 and VGaT mRNAs (D) in four neurons (yellow arrows). Green arrows point to VGluT2+ VGaT− neurons.
(E) Frequency of VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ neurons across the entire SUML and SUMM (mean ± SEM).
(F) Summary of SUM distribution of VGluT2+ VGaT−, VGluT2− VGaT+, and VGluT2+ VGaT+ neurons. Number of dots represents average number of neurons at bregma −4.20 mm.
(G) Cellular expression of VGluT2 mRNA (red), VGaT mRNA (green), and GAD mRNA (white) in the SUML. Yellow arrows indicate one VGluT2+ VGaT+ GAD+ neuron. Yellow arrowheads indicate three VGluT2+ VGaT+ GAD− neurons.
(H) Frequency of SUML VGluT2+ VGaT+ GAD+ co-expressing neurons (yellow), VGluT2+ VGaT+ GAD− co-expressing neurons (yellow cross-hatch), and VGluT2+ VGaT− GAD+ co-expressing neurons (gray) (mean ± SEM).
Scale bars represent 1 mm in (A) and (F) and 25 μm in (B) and (G).
Taken together, these results indicate that, throughout the rat brain, (1) the vast majority of neurons release either glutamate or GABA, not both; (2) some glutamatergic neurons within the LHb, PDTg, and PNC have the capability to synthesize GABA but lack the capability to accumulate vesicular GABA via VGaT; and (3) glutamatergic neurons with the capability to synthesize and co-release GABA are concentrated within the VTA, EPN, and SUM. After establishing the localized distribution of VGluT2+ VGaT+ GAD+ neurons in the VTA, EPN, and SUM, we next determined within these brain areas the detailed topographical distribution and frequency of VGluT2+ VGaT+ GAD+ neurons.
Topography and Frequency of VGluT2+ VGaT+ GAD+ Neurons within the VTA, EPN, and SUM
We found that, across the entire VTA, the subpopulations of VGluT2+ GAD+ or VGluT2+ VGaT+ neurons represented approximately one fourth of all labeled VTA neurons and had a lateromedial increasing concentration (Figures 1A–1E, S1, S8, and S9). By triple fluorescent in situ hybridization for the simultaneous detection of transcripts encoding VGluT2, VGaT, or GAD, we found that the vast majority of VTA neurons that co-expressed VGluT2 and GAD also co-expressed VGaT (Figures 1F and 1G). The highest concentration of VGluT2+ GAD+ and VGluT2+ VGaT+ neurons was found within the rostral linear nucleus (RLi) and the interfascicular nucleus (IF) (Figures 1H, 1I, S8, and S9; Tables S1 and S3). Within the RLi, the concentration of VGluT2+ GAD+ and VGluT2+ VGaT+ neurons increased along the anteroposterior axis (Figures S8J and S9J). We confirmed the presence of single-labeled neurons within the RLi (VGluT2+ GAD− neurons, VGluT2+ VGaT− neurons, VGluT2− GAD+ neurons, or VGluT2− VGaT+ neurons), in which the VGluT2+ GAD− or VGluT2+ VGaT− neurons were abundant and the VGluT2− GAD+ and VGluT2− VGaT+ neurons were infrequent (Figures 1H, S8A, and S9A). Among the IF neurons, roughly one third were VGluT2+ GAD+ neurons, and nearly half were VGluT2+ VGaT+ neurons (Figures 1l, S8B, and S9B). The VGluT2+ GAD+ and VGluT2+ VGaT+, neurons were most prevalent in the anterior aspects of the IF, but concentrations of VGluT2− GAD+ and VGluT2− VGaT+ neurons increased across the anteroposterior axis (Figures S8H–S8J and S9H–S9J). While we detected VGluT2+ GAD+ and VGluT2+ VGaT+ neurons in the anteromedial portions of the paranigral nuclei (PN) and parabrachial pigmented nuclei (PBP) (Figure S1), these nuclei contained mostly single-labeled neurons (Figures 1J, 1K, S8C, S8D, S9C, and S9D; Tables S1 and S3). We found that, within the anterior levels of both PN and PBP, the VGluT2+ GAD− or VGluT2+ VGaT− neurons were the main type of neurons, and both VGluT2− GAD+ and VGluT2− VGaT+ neurons were the main type of neurons at caudal PN and PBP levels (Figures S8H–S8J and S9H–S9J). We also detected a small population of VGluT2+ GAD+ and VGluT2+ VGaT+ neurons within the caudal linear nucleus (CLi), concentrated in its medial aspects and intermingled with mostly VGluT2− GAD+ or VGluT2− VGaT+ neurons (Figures S1, S8E, and S9E; Tables S1 and S3). We detected the lowest concentrations of VGluT2+ GAD+ or VGluT2+ VGaT+ neurons within the parainterfascicular (PIF) and rostral ventral tegmental area (VTAR) nuclei (Figures S8F and S9F). The PIF contained mostly VGluT2- GAD+ or VGluT2− VGaT+ neurons. The VTAR, a neighboring structure to the VTA, contained mostly VGluT2+ GAD− or VGluT2+ VGaT− neurons (Figures S8G and S9G; Tables S1 and S3). Thus, VGluT2+ VGaT+ neurons populate every subdivision of the VTA (Figure 1L).
We found that the EPN is the major brain structure that contains large populations of VGluT2+ GAD+ and VGluT2+ VGaT+ neurons (Figures 2A–2D; Tables S2 and S4). The VGluT2+ GAD+ and VGluT2+ VGaT+ neurons represent nearly half of the population of all labeled EPN neurons (Figure 2E). By triple fluorescent in situ hybridization for the simultaneous detection of transcripts encoding VGluT2, VGaT, or GAD, we found that the vast majority of EPN neurons that co-expressed VGluT2 and GAD also co-expressed VGaT (Figures 2F and 2G). Within the anterior EPN, the different types of neurons had similar prevalence (Figures 2H and 2I). In contrast, we found that both VGluT2+ GAD+ and VGluT2+ VGaT+ neurons were concentrated in the central EPN and that VGluT2− GAD+ and VGluT2− VGaT+ neurons were concentrated in the posterior EPN (Figures 2H and 2I). These findings demonstrate a distinct neuronal organization within the EPN in which different classes of neurons are topo-graphically distributed along the EPN anteroposterior axis (Figure S2).
We identified the SUM as a third brain structure that contains large populations of VGluT2+ GAD+ and VGluT2+ VGaT+ neurons (Figures 3A–3D; Tables S2 and S4). Both SUM VGluT2+ GAD+ and VGluT2+ VGaT+ neurons were confined to its lateral subdivision (SUML) (Figures 3E, 3F, and S3) but SUML VGluT2+ VGaT+ neurons were clearly more numerous than VGluT2+ GAD+ neurons. By triple fluorescent in situ hybridization for the simultaneous detection of transcripts encoding VGluT2, VGaT, or GAD in the SUML, we identified a subpopulation of neurons in which most neurons that co-expressed VGluT2 and VGaT lacked GAD and a second subpopulation in which neurons that co-expressed VGluT2 and VGaT co-expressed GAD (Figures 3G and 3H). These data indicate that the discrepancy between the proportions of neurons co-expressing VGluT2 and GAD and those co-expressing VGluT2 and VGaT (using riboprobes) is due to a larger proportion of SUML VGluT2+ VGaT+ GAD- neurons than VGluT2+ VGaT+ GAD+ neurons. In addition, we detected within the SUML and the medial SUM (SUMM) high concentrations of VGluT2+ GAD− or VGluT2+ VGaT− neurons and a higher frequency of VGluT2− GAD+ and VGluT2− VGaT+ neurons in the SUMM than in the SUML (Figures 3E and 3F).
A Common Synaptic Architecture across Axon Terminals from VGluT2+ VGaT+ GAD+ Neurons
To characterize the synapses established by axon terminals from rat VGluT2+ VGaT+ GAD+ neurons, we selectively tagged axon terminals from rat VTA, EPN, or SUM neurons by injecting in each of these brain areas, in different rats, a viral vector encoding channelrhodopsin2 (ChR2)-mCherry under the control of the CaMKIIα promoter. By immunolabeling and confocal microscopy, we looked for the presence of mCherry-labeled fibers within the LHb and the hippocampus, as previous studies have reported VGluT2+ VGaT+ varicosities in the mouse LHb derived from the VTA (Root et al., 2014b) or EPN (Shabel et al., 2014) and in the hippocampus derived from SUM (Boulland et al., 2009; Soussi et al., 2010). By quadruple fluorescent immunolabeling and three-dimensional reconstruction, we found mCherry varicosities containing VGluT2, VGaT, and GAD within the LHb of animals injected in the VTA (VTA → LHb pathway; Figures 4A–4D) or in the EPN (EPN → LHb pathway; Figures 4E–4H) and in the hippocampus of animals injected in the SUM (SUM → dentate gyrus pathway; Figures 4I–4L). We found that the vast majority of LHb varicosities from the VTA (Figures 4A–4D) or from the EPN (Figures 4E–4H) co-expressed VGluT2, VGaT, and GAD (Figures 4D and 4H). In contrast, while some dentate gyrus varicosities from the SUM co-expressed VGluT2, VGaT, and GAD, others co-expressed VGluT2 and VGaT without GAD (Figure 4L).
Figure 4. Co-existence of VGluT2, VGaT, and GAD Proteins in Axon Terminals from VTA, EPN, or SUM Neurons.

To tag neurons and projections, the AAV5-CaMKIIα-ChR2-mCherry vector was delivered into the VTA (A), EPN (E), or SUM (I). The phenotype of the immunolabeled mCherry-tagged axon terminals (red) were determined by the immunofluorescence detection of VGluT2 (green), VGaT (white), and GAD (blue).
(A–C) VTA → LHb fibers were labeled with mCherry (A). (B) Low magnification of VTA → LHb fibers and VGluT2, VGaT, and GAD immunofluorescence. (C) High magnification of mCherry axons co-expressing VGluT2, VGaT, and GAD (yellow arrowheads) or only expressing VGluT2 (magenta arrow).
(D) Frequency of VTA → LHb axon terminal phenotypes (mean ± SEM). 2,273 mCherry axon terminals were analyzed by three-dimensional reconstruction (9 LHb samples; 3 rats).
(E–G) EPN → LHb fibers were labeled with mCherry (E). (F) Low magnification of EPN → LHb fibers and VGluT2, VGaT, and GAD immunofluorescence. (G) High magnification of mCherry axons co-expressing VGluT2, VGaT, and GAD (yellow arrowheads) or only expressing VGluT2 (magenta arrow).
(H) Frequency of EPN → LHb axon terminal phenotypes (mean ± SEM). 1,940 mCherry axon terminals were analyzed by three-dimensional reconstruction (10 LHb samples; 3 rats).
(I–K) SUM → dentate gyrus (DG) fibers were labeled with mCherry (I). (J) Low magnification of SUM → DG fibers and VGluT2, VGaT, and GAD immunofluorescence. (K) High magnification of mCherry boutons co-expressing VGluT2, VGaT, and GAD (yellow arrowheads) or boutons co-expressing VGluT2 and VGaT without GAD (orange arrow).
(L) Frequency of SUM → DG axon terminal phenotypes (mean ± SEM). 1,135 mCherry axon terminals were analyzed by three-dimensional reconstruction (6 DG samples; 2 rats).
Scale bars represent 100 mm in (B), (F), and (J), and 2 mm in (C), (G), and (K). MHb, medial habenula; LHb, lateral habenula.
By electron microscopy, we confirmed that mCherry-labeled varicosities corresponded to axon terminals. By double immunoelectron microscopy, we found mCherry axon terminals containing VGluT2 and VGaT in the LHb from the VTA (Figures 5A–5D) or EPN neurons (Figures 5E–5H), and in the dentate gyrus terminals from SUM neurons (Figures 5I–5L). Moreover, we found that individual axon terminals from any of these pathways simultaneously established spatially segregated asymmetric (putative excitatory) synapses and symmetric (putative inhibitory) synapses on postsynaptic neurons (Figure 5).
Figure 5. Single Axon Terminals from VGluT2+ VGaT+ Neurons Establish Both Asymmetric and Symmetric Synapses.
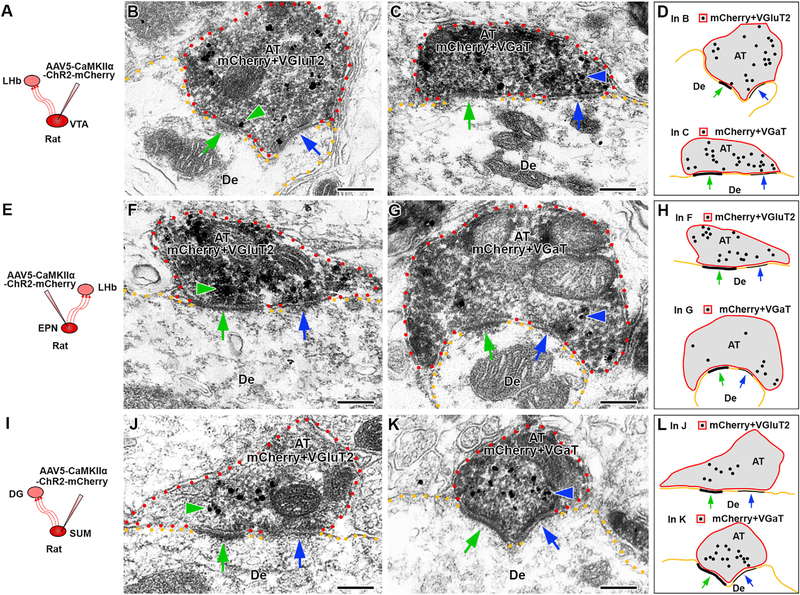
(A, E, and I) To identify axon terminals (AT) from VGluT2+ VGaT+ neurons, the AAV5-CaMKIIα-ChR2-mCherry vector was delivered into the VTA (A), EPN (E), or SUM (I). The phenotype of the mCherry-immunolabeled (scattered dark material) AT was established by immunodetection of VGluT2 (gold particles; green arrowheads in B, F, and J) and VGaT (gold particles; blue arrowheads in C, G, and K).
(A–D) VTA → LHb axon terminals.
(B) Single mCherry AT co-expressing VGluT2 and establishing asymmetric (green arrows) and symmetric (blue arrows) synapses onto a common dendrite (De).
(C) Single mCherry AT co-expressing VGaT and establishing asymmetric (green arrows) and symmetric (blue arrows) synapses onto a common De.
(D) Summary representation of single VTA → LHb axon terminals establishing individual asymmetric and symmetric synapses with VGluT2 or VGaT.
(E–H) EPN → LHb axon terminals.
(F) Single mCherry AT co-expressing VGluT2 and establishing asymmetric (green arrows) and symmetric (blue arrows) synapses onto a common dendrite (De).
(G) Single mCherry AT co-expressing VGaT and establishing asymmetric (green arrows) and symmetric (blue arrows) synapses onto a common De.
(H) Summary representation of single EPN → LHb axon terminals establishing individual asymmetric and symmetric synapses with VGluT2 or VGaT.
(I–L) SUM → DG axon terminals.
(J) Single mCherry AT co-expressing VGluT2 and establishing asymmetric (green arrows) and symmetric (blue arrows) synapses onto a common De.
(K) Single mCherry AT co-expressong VGaT and establishing asymmetric (green arrows) and symmetric (blue arrows) synapses onto a common De.
(L) Summary representation of single SUM → DG axon terminals establishing individual asymmetric and symmetric synapses with VGluT2 or VGaT. Scale bars represent 200 nm.
Distinct Pools of Glutamate and GABA Synaptic Vesicles within the LHb
To better characterize the properties of the vesicles within the VGluT2+ VGaT+ axon terminals, we focused our analysis on terminals within the LHb, because (1) more than half of the total population of LHb axon terminals co-expresses VGluT2 and VGaT (Root et al., 2014b), and (2) the synapses established within the LHb by individual VGluT2+ VGaT+ axon terminals from VTA or EPN shared similar ultrastructural features (as detailed earlier).
To begin examining the co-release of GABA and glutamate in the rat LHb from VTA or EPN terminals, we expressed ChR2-eYFP (enhanced yellow fluorescent protein) in VTA or EPN GAD+ and recorded from LHb neurons receiving ChR2-eYFP inputs from either the VTA or the EPN (Figure 6). By whole-cell recordings, we recorded LHb neurons at two different holding potentials: −60 mV, to favor detection of glutamatergic transmission produced by vesicular glutamate release; or 0 mV, to favor detection of GABAergic transmission produced by vesicular GABA release. LHb photostimulation (5 ms) of ChR2-eYFP fibers from VTA evoked outward currents at 0 mV and inward currents at −60 mV (Figures 6A–6C). Both 0-mV outward currents and −60-mV inward currents were blocked by tetrodotoxin (TTX) and rescued by the subsequent addition of 4-aminopyridine (4-AP), demonstrating the monosynaptic nature of both currents. The AMPA-receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) blocked the −60-mV inward currents, with minimal effect on the 0-mV outward currents. In some experiments, after the addition of CNQX, an outward current recorded at −60 mV was unmasked. Subsequent addition of the GABAA-receptor antagonist bicuculline blocked the 0-mV outward currents (Figures 6B and 6C). Likewise, LHb photostimulation of ChR2-eYFP fibers from EPN evoked fast inward currents at −60 mV that were blocked by CNQX and fast outward currents at 0 mV that were blocked by bicuculline (Figures 6D–6F). Taken together, these results indicate that rat VTA and EPN GAD+ axons targeting the LHb are capable of vesicular glutamate and vesicular GABA co-release onto LHb neurons.
Figure 6. LHb Glutamate and GABA Are Accumulated into Distinct Subpopulations of Synaptic Vesicles.
(A) A Cre-inducible AAV5-DIO-ChR2-eYFP vector was injected into the VTA of GAD::Cre rats. Photostimulation of ChR2-eYFP fibers from VTA and LHb cell recordings.
(B) Traces of excitatory postsynaptic currents (EPSCs) recorded at −60 mV and inhibitory postsynaptic currents (IPSCs) recorded at 0 mV after photostimulation (blue line) of VTA ChR2-eYFP expressing fibers in the LHb with the subsequent addition of TTX, 4-AP, Bicuculline and CNQX. (C)Photostimulation (blue line) of ChR2-eYFP fibers evoked excitatory postsynaptic currents (EPSCs) at −60 mV or inhibitory postsynaptic currents (IPSCs) at 0 mV, which were highly diminished by TTX(1 μM) (−75.72 ± 16.33 pA baseline at −60 mV; −2.69 ± 0.15 after TTX at −60 mV; 244.03 ± 63.38 pA baseline at 0 mV; 8.42 ± 3.78 pA after TTX at 0 mV), and restored by 4-AP (200 μM) (−75.72 ± 16.33 pA baseline at −60 mV; −77.79 ± 36.57 pA after 4-AP at −60 mV; 244.02 ± 63.38 pA baseline at 0 mV; 295.39 ± 59.64 after 4-AP at 0 mV). EPSCs recorded at −60 mV were blocked by AMPA-receptor antagonist CNQX (10 μM) (41.14 ± 8.77 pA at −60 mV) but not IPSCs recorded at 0 mV (291.79 ± 60.70 at 0 mV), which were blocked by the GABAA-receptor antagonist bicuculline (10 μM) (bicuculline, 0 mV = 10.04 ± 7.13) (−60-mV currents: F(4, 24) = 8.30, p = 0.0008, repeated-measures ANOVA, Dunnett post hoc test, n = 5 cells of 3 rats; 0-mV currents: F(4, 24) = 17.98, p < 0.0001, repeated-measures ANOVA, Dunnett post hoc test, n = 5 cells of 3 rats). *p < 0.05; **p < 0.01; ***p < 0.001. Data are mean ± SEM.
(D) A Cre-inducible AAV5-DIO-ChR2-eYFP vector injected into the EPN of GAD::Cre rats. Photostimulation of ChR2-eYFP fibers from EPN and LHb cell recordings.
(E) Traces of excitatory postsynaptic currents (EPSCs) recorded at −60 mV and inhibitory postsynaptic currents (IPSCs) recorded at 0 mV after photostimulation (blue line) of EPN ChR2-eYFP expressing fibers in the LHb with the subsequent addition of TTX, 4-AP, Bicuculline and CNQX.
(F) Photostimulation (blue line) of ChR2-eYFP fibers from EPN evoked EPSCs at −60 mV or IPSCs at 0 mV, which were highly diminished by TTX (1 μM) (−152.10 ± 56.64 pA baseline at −60 mV; −2.83 ± 0.21 pA after TTX at −60 mV; 373.69 ± 125.25 pA baseline at 0 mV; 6.53 ± 2.90 pA after TTX at 0 mV) and restored by 4-AP (200 μM) (−152.10 ± 56.64 pA baseline at −60 mV; −100.46 ± 44.54 pA after 4-AP at −60 mV; 373.69 ± 125.25 pA baseline at 0 mV; 330.92 ± 86.34 pA after 4-AP at 0 mV). EPSCs recorded at −60 mV were blocked by AMPA-receptor antagonist CNQX (10 μM) (47.31 ± 15.14 pA at −60 mV) but not IPSCs recorded at 0 mV (306.96 ± 91.97 pA), which were blocked by the GABAA-receptor antagonist bicuculline (10 μM) (6.23 ± 2.91 pA) (−60-mV currents: F(4, 24) = 6.776, p = 0.002, repeated-measures ANOVA, Dunnett post hoc test, n = 5 cells of 3 rats; 0-mV currents: F(4, 24) = 9.755, p = 0.0003, repeated- measures ANOVA, Dunnett post hoc test, n = 5 cells of 3 rats). *p < 0.05; **p < 0.01; ***p < 0.001. Data are mean ± SEM.
(G) Post-embedding detection ofVGluT2 (18-nm gold particles, black arrows) and VGaT (12-nm gold particles, black arrowheads), both VGluT2 and VGaT signals are segregated within the same AT. Note the VGluT2 vesicles proximal to asymmetric synapses (green arrows).
(H–M) Electron micrograph showing the purity and integrity of LHb-isolated synaptic vesicles (H) used for either simultaneous detection of VGluT2 and VGaT (I and K) or co-immunoprecipitation (M). (I) Dual detection of VGluT2 (arrows, 18-nm gold particles) or VGaT (arrowheads, 12-nm gold particles) are associated with different synaptic vesicles. (J) Closer view of vesicles detected wit VGluT2 (arrows, 18-nm gold particles) or (K) VGaT (arrowheads, 12-nm gold particles).
(L) Frequency (mean ± SEM) of vesicles containing VGluT2, VGaT, or both. From a total of 2,815 labeled vesicles, 59.28 ± 0.88% solely expressed VGluT2, 38.65 ± 0.56% solely expressed VGaT, and 2.07 ± 0.33% appeared to co-label forVGluT2 and VGaT; paired t test, t(2) = 14.37, p = 0.0048. Synaptic vesicles were quantified from three different preparations of isolated vesicles from rat LHb (n = 80).
(M) Western blots from total isolated vesicles before immunoprecipitation (IP, T) and after magnetic beads IP with antibodies against VGluT2 (IP: VGluT2, B). Western blots were immunolabeled (IB) with antibodies against VGluT2, VGaT, or the vesicular marker synaptophysin. The vesicular nature of each fraction was confirmed by the detection of synaptophysin. VGluT2 and VGaT were present in the total pool of vesicles (T). In contrast, onlyVGluT2, not VGaT, was detected in the sample IP with antibody to VGluT2. The western blots were repeated three times.
(N) An LHb vesicular model showing the accumulation of glutamate by VGluT2 in a vesicle different from the one that accumulates GABA by VGaT. Scale bars represent 200 nm in (G)–(I) and 50 nm in (J) and (K).
After establishing that inputs from both VTA and EPN corelease glutamate and GABA into the LHb in the rat, we determined whether glutamate and GABA are co-released from the same or different vesicles. Given that morphological differences in vesicle shape have classically been suggested to indicate excitatory (mostly spherical vesicles of uniform size) or inhibitory (oval vesicles of different sizes) synaptic vesicles (Gray, 1969), we initially examined the ultrastructural characteristics of synaptic vesicles in rat LHb ultrathin sections. Within axon terminals establishing asymmetric (putative excitatory) synapses, we detected vesicles that had a spherical shape (Figure S10A). In contrast, axon terminals establishing symmetric (putative inhibitory) synapses contained vesicles that had oval shapes of different sizes (Figure S10B). Within the same LHb ultrathin sections, we found single LHb axon terminals containing both spherical and oval-shaped synaptic vesicles (Figure S10C), suggesting that these LHb single axon terminals have pools of excitatory or inhibitory vesicles. To test this idea, we prepared rat LHb samples to optimize protein detection of VGluT2 and VGaT by dual post-embedding immunogold labeling (using secondary antibodies coupled to colloidal gold particles of different sizes). We did not use cocktails of antibodies (see Experimental Procedures for details) to avoid charge-mediated attraction between colloidal gold of different sizes, which may result in unspecific co-localization of gold particles and, thus, false interpretations of vesicular co-localization of VGluT2 immunolabeling and VGaT immunolabeling. Although we found single axon terminals containing gold particles for the detection of both VGluT2 and VGaT, these particles were segregated within the common axon terminal (Figure 6G). We observed gold particles for the detection of VGluT2, but not for VGaT, proximal to asymmetric synapses (Figure 6G; see also VGaT vesicles proximal to symmetric synapses in Figure 5).
Our findings from ultrastructural and molecular analysis of LHb ultrathin sections indicate that rat VGluT2+ VGaT+ axon terminals have segregated asymmetric and symmetric synapses and appear to have segregated pools of VGluT2 vesicles and VGaT vesicles. To further evaluate the presence of pools of VGluT2 vesicles and VGaT vesicles within the LHb, we purified vesicles from rat LHb synaptosomes. By electron microscopy analysis, we evaluated the integrity of the purified vesicles (Figure 6H) and used them for dual VGluT2 and VGaT immunolabeling using secondary antibodies coupled to colloidal gold particles of different sizes. By electron microscopy analysis, we observed two major pools of vesicles, a pool of vesicles containing VGluT2 and a pool of vesicles containing VGaT (Figures 6I–6K). From a total of 2,815 labeled vesicles, we found that 59.28 ± 0.88% were VGluT2+ VGaT−, 38.65 ± 0.56% were VGluT2− VGaT+, and a very small proportion (2.07 ± 0.33%) appeared to co-label as VGluT2+ VGaT+ (Figure 6L). The lack of coexistence of VGluT2 and VGaT at the vesicular level was next confirmed by VGluT2 co-immunoprecipitation of vesicles purified from rat LHb synaptosomes. Consistent with our ultrastructural findings, protein preparations of vesicles immunoprecipitated with anti-VGluT2 antibodies showed vesicular immunodetection of both VGluT2 and the vesicular marker synaptophysin but lacked evidence of VGaT immunodetection (Figure 6M).
Therefore, we conclude that, within the rat LHb, which receives axon terminals from VTA and EPN VGluT2+ VGaT+ neurons, glutamate and GABA are packaged and released from distinct pools of vesicles (Figure 6N). Based on these findings and the observed segregated clustering of VGluT2 and VGaT- immunolabeled vesicles within single axon terminals, we suggest that LHb axon terminals co-expressing VGluT2 and VGaT contain distinct pools of vesicles for the accumulation and release of either glutamate or GABA, with each pool concentrated close to its site of release (VGluT2 vesicles close to asymmetric synapses and VGaT vesicles close to symmetric synapses).
DISCUSSION
It is well accepted that, within the mammalian nervous system, independent groups of neurons release the excitatory neurotransmitter glutamate or the inhibitory neurotransmitter GABA. However, accumulating evidence indicates that subsets of axon terminals co-express VGluT2 and VGaT and co-release glutamate and GABA within the LHb and the hippocampus (Meye et al., 2016; Ntamati and Luscher, 2016; Root et al., 2014b; Shabel et al., 2014; Wallace et al., 2017; Yoo et al., 2016). We show now that these VGluT2+ VGaT+ axon terminals are likely to derive mostly from VTA, EPN, or SUM, as these are the only brain structures that, throughout the brain, have concentrated groups of neurons with the capability to synthesize GABA by GAD, accumulate GABA into synaptic vesicles by VGaT, and accumulate glutamate into synaptic vesicles by VGluT2. The VGluT2+ VGaT+ neurons are not homogeneously distributed within the VTA, EPN, and SUM but concentrated in specific subdivisions. Based on our molecular and ultrastructural characterizations of VGluT2+ VGaT+ axon terminals from VTA, EPN, or SUM, we demonstrate that a single VGluT2+ VGaT+ axon terminal (from VTA, EPN, or SUM) establishes juxtaposing asymmetric and symmetric synapses on a common dendrite. Based on our characterization of synaptic vesicles within axon terminals and within purified synaptic vesicles from LHb, we conclude that glutamate and GABA are mostly (if not only) accumulated into independent pools of synaptic vesicles. The segregated pools of glutamate and GABA vesicles within single axon terminals suggest independent mechanisms by which VGluT2+ VGaT+ terminals may regulate the accumulation, release, and recycling of each neurotransmitter. These results may provide new avenues to explore possible alterations in brain disorders at the level of the molecular and cellular mechanisms that regulate the vesicular accumulation of glutamate or GABA into independent vesicles within a shared axon terminal or at the level of the molecular mechanisms that regulate the sorting and recycling of glutamate vesicles and GABA vesicles within a shared axon terminal.
While we have shown that VGluT2+ VGaT+ neurons represent a major type of glutamate-GABA co-releasing neuron, other neuron types may also co-release fast-acting neurotransmitters. In addition to VGluT2, glutamate may be loaded into synaptic vesicles by VGluTI or VGluT3 (Bellocchio et al., 2000; Fremeau et al., 2001,2002; Gras et al., 2002; Herzog et al., 2001; Takamori et al., 2000). Furthermore, VGaT is capable of accumulating both GABA and glycine into synaptic vesicles (Sagné et al., 1997). VGluT3+ VGaT+ neurons that co-release glutamate and glycine onto different cell types within the rodent retina have recently been identified (Lee et al., 2016; Tien et al., 2016), as well as glutamate, GABA, and glycine co-transmission in the developing lateral superior olive (Gillespie et al., 2005; Noh et al., 2010). The segregated signaling of glutamate and glycine by retinal VGluT3+ VGaT+ neurons is similar to that of dopamine and glutamate signaling by VTA VGluT2+ dopamine+ neurons (Zhang et al., 2015) but differs from the signaling of the VGluT2+ VGaT+ neurons that co-release glutamate and GABA onto the same post-synaptic element (Root et al., 2014b; present study). VGluT3-expressing interneurons within the hippocampus co-express VGaT (Somogyi et al., 2004), and recent evidence suggests that these neurons co-release glutamate and GABA (Fasano et al., 2017). In addition, select axon terminals and synaptic vesicles within rodent cortex co-express VGluT1 and VGaT (Fattorini et al., 2009, 2015), indicating that VGluT1+ VGaT+ neurons are present in the brain and suggesting the release of glutamate together with GABA or glycine, which remains to be demonstrated.
Selective Compartmentalization of VGluT2+ VGaT+ Neurons within the VTA, EPN, and SUM
We had recently documented that the majority of axon terminals within the rat and mouse LHb contain transporters for the vesicular accumulation of glutamate and for the vesicular accumulation of GABA (Root et al., 2014b), suggesting that co-release of glutamate and GABA is a major mechanism of neurotransmission in the rodent LHb. In addition, we had previously established that some of these VGluT2+ VGaT+ axon terminals originated from VTA neurons (Root et al., 2014b). Here, we confirmed and expanded these findings by showing that VGluT2+ VGaT+ neurons are a major subset of VTA neurons located in all VTA subdivisions but concentrated in the RLi and IF. The VTA VGluT2+ VGaT+ neurons are intermingled with neurons that are purely glutamatergic in the anteromedial VTA and with neurons that are purely GABAergic in the caudolateral VTA. Previous anatomical and behavioral studies have shown that the VTA VGluT2-expressing neurons establish local (Wang et al., 2015a) and long-range connections (Qi et al., 2016; Root et al., 2014b; Yamaguchi et al., 2011), and they may mediate reward (Wang et al., 2015a), aversion (Qi et al., 2016; Root et al., 2014a), perseveration (Kabanova et al., 2015), or autism-related behaviors (Krishnan et al., 2017). However, the possible contribution of VTA VGluT2+ VGaT+ neurons to the local circuitry remains to be determined, as well as their possible role on behaviors currently ascribed to VTA VGluT2-expressing neurons.
We identified the EPN as the brain structure containing the highest percentage of VGluT2+ VGaT+ neurons throughout the brain. For almost 40 years, the EPN has been regarded as a GABA-releasing structure due to its high concentration of GAD-expressing neurons (Nagy et al., 1978; Penney and Young, 1981). However, recent studies have demonstrated that the EPN has different subpopulations of neurons, including glutamate-GABA neurons (Wallace et al., 2017). Consistent with cellular heterogeneity within the EPN, we found, within the anterior compartment of the EPN, neurons capable of co-releasing glutamate and GABA, purely releasing GABA, or purely releasing glutamate. In contrast, the central compartment of the EPN has mostly VGluT2+ VGaT+ neurons, and the posterior compartment has mostly purely GABA-releasing neurons. It is likely that the different neuronal phenotypes represented along the anteroposterior plane of the EPN result in efferents with different signaling properties that innervate different brain areas and participate in different brain functions (Wallace et al., 2017). In support of this idea, the EPN → LHb pathway (also known as the habenula-projecting globus pallidus) (Robertson et al., 2014), likely to derive from central EPN neurons, exhibits GAD reduction in animal models of depression (Shabel et al., 2014) or VGaT reduction in animal models of cocaine addiction (Meye et al., 2016). These data suggest that the central EPN VGluT2+ VGaT+ neurons are dynamically involved in motivational disorders. In contrast, the posterior EPN, shown here to contain mostly purely GABAergic neurons, is the source of projections constituting classic pallidal GABAergic projections to the thalamus and brainstem (Takada et al., 1994). The posterior EPN appears to be involved in classic basal ganglia disorders, such as schizophrenia and movement disorders. For example, the anti-psychotic clozapine increases GAD mRNA mostly in the posterior EPN compared with the anterior EPN (Yu et al., 1999). Furthermore, torsinA, the causative protein of a severe form of dystonia, is selectively reduced in the posterior EPN from rats with levodopa-induced dyskinesia (Yamada et al., 2006). Given the heterogeneous cellular composition of the EPN, its cellular and efferent topography, and its neuroanatomical position as the output of the basal ganglia, we infer that each spatially organized EPN neuronal phenotype plays crucial roles in a wide array of behaviors and brain disorders.
We identified the SUM as another structure with a high concentration of VGluT2+ VGaT+ neurons. The detection of VGluT2+ VGaT+ neurons in the SUM is consistent with prior findings showing the co-existence of VGluT2, GAD, and VGaT proteins in axon terminals from the SUM (Boulland et al., 2009; Soussi et al., 2010). We found that the VGluT2+ VGaT+ neurons are confined to the SUML, intermingled with purely glutamatergic neurons. In contrast, neurons expressing GAD or VGaT without VGluT2 are confined to the SUMM. In both the VTA and EPN, the vast majority of neurons that co-express VGluT2 and VGaT also co-express GAD. However, in the SUML, most neurons that co-express VGluT2 and VGaT lack GAD, while a subset co-expresses VGluT2, VGaT, and GAD. Thus, VGluT2+ VGaT+ neurons have an additional unexpected diversity within their populations. This diversity may have implications for disorders involving the SUML. For instance, the SUML projection to dentate gyrus that is normally restricted to the supragranular layer exhibits aberrant innervation into the inner molecular layer in pilocarpine-treated epileptic rats, and this aberrant epileptic- induced pathway exhibits a significant increase in terminals expressing VGluT2 alone or co-expressing VGluT2 and VGaT (Soussi et al., 2015). Thus, it is of interest to determine whether VGluT2+ VGaT+ GAD+ or VGluT2+ VGaT+ GAD- SUML neurons that project to dentate gyrus are altered in epilepsy. The findings of layer-specific and neurochemical changes in the VGluT2+ VGaT+ terminals within the dentate gyrus (in animal models of epilepsy), together with those observed within the LHb (in animal models of depression or addiction), suggest a great dynamic degree of structural and functional neuroplasticity, in response to environmental challenges, within the subpopulations of VGluT2+ VGaT+ neurons that co-express VGluT2 and VGaT.
Signaling by VGluT2+ VGaT GAD+ and VGluT2+ VGaT+ GAD− Neurons
As detailed earlier, we identified within the midline aspects of the VTA, central aspects of the EPN, and lateral aspects of the SUM high concentrations of neurons with the capability to package glutamate and GABA into synaptic vesicles and likely to co-release glutamate and GABA at their target sites. In support of the co-release of glutamate and GABA by these VGluT+ VGaT+ neurons, electrophysiological findings shown here in rats and elsewhere in mice have demonstrated that the LHb receives inputs that co-release glutamate and GABA from the VTA (Root et al., 2014b) and EPN (Shabel et al., 2014) and that the dentate gyrus receives inputs from the SUM that co-release glutamate and GABA (Pedersen et al., 2017).
In contrast to the VGluT2+ VGaT+ neurons within the VTA, EPN, and SUM, we detected VGluT2+ VGaT− GAD+ neurons within the LHb, PDTg, and PNC. Due to the lack of VGaT within VGluT2-expressing neurons in these brain structures, it is unclear whether VGluT2+ VGaT− GAD+ neurons accumulate GABA into vesicles. Although it has been shown that the vesicular monoamine transporter is capable of packaging GABA into synaptic vesicles (Tritsch and Sabatini, 2012), this transporter is not expressed by neurons of the LHb, PDTg, or PNC (Hansson et al., 1998). While GABA synthesized by VGluT2+ VGaT− GAD+ neurons of the LHb, PDTg, and PNC may not be released by synaptic vesicles, it may play alternative roles in neuronal signaling. In this regard, findings from neuronal cultures indicate that endogenous GABA acts in the rough endoplasmic reticulum as a cognate chaperone ligand to promote forward trafficking of GABAa receptors and their membrane insertion (Eshaq et al., 2010; Wang et al., 2015b). In support for a similar role by GABA in the brain, GABA has been detected in the rough endo-plasmic reticulum of cortical glutamatergic neurons (Wang et al., 2015b). Future studies are necessary to determine whether GABA synthesized by VGluT2+ VGaT− GAD+ neurons of the LHb, PDTg, or PNC may play a role in regulating membrane insertion of GABAA receptors. However, it is also possible that environmental experience alters the neurochemical profile of these neurons (Zhang et al., 2018).
Single Axon Terminals Co-release Glutamate and GABA from Distinct Synapses and Distinct Synaptic Vesicles
We had previously demonstrated that single axon terminals from VTA VGluT2+ VGaT+ neurons co-express VGluT2 and VGaT and simultaneously establish asymmetric and symmetric synapses on LHb dendrites (Root et al., 2014b). We confirmed these findings and also found similar ultrastructural and biochemical properties by single axon terminals of VGluT2+ VGaT+ neurons from EPN axon terminals in the LHb and from SUM axon terminals in the dentate gyrus. These shared ultrastructural and biochemical properties suggest a common cellular mechanism among these VGluT2+ VGaT+ neurons for the synchronous or asynchronous release of glutamate and GABA (Barker et al., 2016). Moreover, the integration of multiplexed signals derived from different VGluT2+ VGaT+ neurons may be determined by specific features of their postsynaptic targets, such as nature of the postynaptic receptors, nature of the postynaptic neurons, and membrane potential of the postsynaptic neuron at the time at which glutamate and GABA are co-released (Root et al., 2014b).
It has been previously suggested that, within the LHb, single synaptic vesicles accumulate and co-release glutamate and GABA (Shabel et al., 2014). However, recent findings from single-vesicle imaging studies have shown different vesicular uptake mechanisms for glutamate- and GABA-containing vesicles, highlighting the importance of this segregation in maintaining the fidelity in synaptic neurotransmission (Farsi et al., 2016). Consistent with the concept of segregated pools of glutamate vesicles and GABA vesicles, we found that individual synaptic vesicles purified from the LHb have either VGluT2 or VGaT, suggesting that glutamate and GABA are each accumulated and released from non-overlapping subpopulations of vesicles. The segregation of glutamate and GABA to different pools of vesicles within a common axon terminal may be part of a regulatory mechanism toward avoiding an interdependent accumulation of each neurotransmitter within the same vesicle. The observed segregation of VGluT2 and VGaT to different vesicles, together with the ultra-structural results showing that single axon terminals from VGluT2+ VGaT+ neurons establish distinct asymmetric and symmetric synapses, provides strong support for the hypothesis that glutamate and GABA are co-released from separate synaptic vesicles at discrete synapses from single axon terminals. The discovery that LHb glutamate and GABA co-release occurs by segregated neurotransmitter release provides the foundation for examining specific receptors and ion channels that maintain segregated signaling of glutamate and GABA, which may lead to unique therapeutic interventions aimed at altering single neuro-transmitters released from VGluT2+ VGaT+ axon terminals.
Conclusion
We conclude that, across the brain, neurons with the capability to co-release glutamate and GABA utilizing VGluT2 and VGaT are present in the VTA, EPN, and SUM (Figure 7). Axon terminals from these VGluT2+ VGaT+ neurons share a common and unique synaptic architecture in which a single dual VGluT2+ VGaT+ axon terminal simultaneously establishes asymmetric synapses that release glutamate (from vesicles packaged by VGluT2) and symmetric synapses that release GABA (from vesicles packaged by VGaT) (Figure 7). Glutamate released from VGluT2-containing vesicles is detected by postsynaptic AMPA receptors at asymmetric synapses, and GABA released from VGaT-containing vesicles is detected by postsynaptic GABAA receptors at symmetric synapses (Root et al., 2014b). Although small sub-populations of VGluT2 neurons in the LHb, PDTg, and PNC contain GAD (and are, therefore, capable of synthesizing GABA), they are likely to synaptically release glutamate, but not GABA, due to their lack of VGaT. We found that the LHb contains a high population of individual axon terminals that co-release glutamate and GABA and accumulates each transmitter into distinct synaptic vesicles, which are likely to sustain synaptic release from highly controlled discrete sites within single VGluT2+ VGaT+ axon terminals derived from the VTA or EPN. Immunoprecipitation of hippocampal synaptic vesicles has demonstrated the presence of VGluT2- or VGaT-containing vesicles, but not dual VGluT2-VGaT vesicles (Boulland et al., 2009), indicating that the SUM → dentate gyrus pathway also contains two distinct pools of synaptic vesicles. These highly controlled synaptic microdomains that govern the co-release of glutamate and GABA may provide new avenues to explore the mechanisms of decision making and brain disorders. For example, the balance of co-transmitted glutamate and GABA in the LHb has been hypothesized to be a mechanism by which the LHb influences reward-prediction errors (Uchida, 2014). In addition, recent findings have shown that the balance of LHb glutamate and GABA co-transmission is altered in rodent models of addiction (Meye et al., 2016) or depresion (Shabel et al., 2014). Because the different types of VGluT2+ VGaT+ neurons are present in brain structures involved in aversive experience, addiction, movement disorders, epilepsy, or depression and shown to be functionally and structurally modified by these experiences (Davis et al., 1982; Meye et al., 2016; Root et al., 2014a; Shabel et al., 2014; Soussi et al., 2015; Stamatakis and Stuber, 2012), we suggest that these VGluT2+ VGaT+ neurons participate in disorders involving motivation.
Figure 7. VGluT2+ VGaT+ Neurons from Different Sources Establish a Common Synaptic Architecture for Glutamate and GABA Co-release.
The diagram shows ultrastructural properties of VTA → LHb, EPN → LHb, and SUM → DG axon terminals (top) from VGluT2+ VGaT+ neurons (bottom). Single axon terminals from VGluT2+ VGaT+ neurons contain synaptic vesicles that package GABA via VGaT or package glutamate via VGluT2. At the symmetric synapse, GABA is released, which interacts with GABAA-receptors within postsynaptic neurons. At the asymmetric synapse, glutamate is released, which interacts with AMPA receptors within postsynaptic neurons. Therefore, each VGluT2+ VGaT+ axon terminal has the capability of co-releasing glutamate and GABA from independent vesicles and independent synapses within the same axon terminal. The postsynaptic reception of the co-released glutamate and GABA may be onto the same postsynaptic dendrite.
EXPERIMENTAL PROCEDURES
All animal procedures were performed in accordance with the NIH guidelines and approved by the National Institute on Drug Abuse Animal Care and Use Committee.
Phenotypical Characterization of Glutamate and GABA Neurons
Three male Sprague-Dawley rats (10–15 weeks old) were used. Hybridization was performed by double in situ hybridization for radioactive detection of VGluT2 mRNA and nonradioactive detection of GAD (transcripts encoding the two isoforms of GAD [i.e., GAD65 and GAD67]) or VGaT mRNA. Alternatively, a triple fluorescent in situ hybridization was processed for the simultaneous detection of transcripts encoding GAD (GAD65 and GAD67), VGluT2, and VGaT using RNAscope. For details and analysis procedures, see the Supplemental Experimental Procedures.
Confocal and Electron Microscopy
Nine male Sprague-Dawley rats (5–15 weeks old) were injected with AAV5-CaMKIIa-ChR2(h134R)-mCherry in VTA, EPN, or SUM. Animals were perfused, and brains collected for microscopy and immunochemistry 8 weeks later. For details and analysis procedures, see the Supplemental Experimental Procedures.
Characterization of Glutamatergic and GABAergic Synaptic Vesicles
Twenty male Sprague-Dawley rats (14 weeks old) were used for each batch of isolation of synaptic vesicles. The isolation of synaptic vesicles was done 4 times from a total of 80 rats. For details and analysis procedures, see the Supplemental Experimental Procedures.
Electrophysiological Recordings
Six male GAD::Cre rats (5–15 weeks) were injected with AAV5-DIO- ChR2(h134R)-eYFP in VTA or EPN (350 nL, 100 nL/min). LHb neurons from 200-μm coronal slices were recorded. For details and analysis procedures, see Supplemental Experimental Procedures.
Statistical Methods
Changes in evoked current amplitude at −60 mV and 0 mV compared evoked currents at baseline and after the sequential addition of TTX, 4-AP, CNQX, and bicuculline, using a repeated-measures ANOVA followed by a Dunnett post hoc test.
Supplementary Material
Highlights.
VGluT2, VGaT, and GAD co-expressing neurons are present in VTA, SUM, and EPN
VTA, EPN, and SUM glutamate-GABA terminals establish symmetric and asymmetric synapses
VTA and EPN co-release glutamate and GABA to LHb from separate synaptic vesicles
ACKNOWLEDGMENTS
The Intramural Research Program of the National Institute on Drug Abuse supported this research. We thank the NIDA Visual Media Services, Keenan Caswell, Lynn Cournoyer, Rong Ye, and Li Jing for their excellent technical assistance.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, ten figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.05.063.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Barker DJ, Root DH, Zhang S, and Morales M. (2016). Multiplexed neuro-chemical signaling by neurons of the ventral tegmental area. J. Chem. Neuro- anat 73, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT Jr., and Edwards RH (2000). Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289, 957–960. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Jenstad M, Boekel AJ, Wouterlood FG, Edwards RH, Storm-Mathisen J, and Chaudhry FA (2009). Vesicular glutamate and GABA transporters sort to distinct sets of vesicles in a population of presynaptic terminals. Cereb. Cortex 19, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H. (2005). Synaptogenesis: a balancing act between excitation and inhibition. Curr. Biol 15, R203–R205. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, and Gendelman PM (1982). A primary acoustic startle circuit: lesion and stimulation studies. J. Neurosci 2, 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshaq RS, Stahl LD, Stone R 2nd, Smith SS, Robinson LC, and Lei-denheimer NJ (2010). GABA acts as a ligand chaperone in the early secretory pathway to promote cell surface expression of GABAA receptors. Brain Res 1346, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsi Z, Preobraschenski J, van den Bogaart G, Riedel D, Jahn R, and Woehler A. (2016). Single-vesicle imaging reveals different transport mechanismsbetween glutamatergicand GABAergicvesicles.Science 351,981–984. [DOI] [PubMed] [Google Scholar]
- Fasano C, Rocchetti J, Pietrajtis K, Zander JF, Manseau F, Sakae DY, Marcus-Sells M, Ramet L, Morel LJ, Carrel D, et al. (2017). Regulation of the hippocampal network byVGLUT3-positive CCK-GABAergic basket cells. Front. Cell. Neurosci 11, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini G, Verderio C, Melone M, Giovedì S, Benfenati F, Matteoli M, and Conti F. (2009). VGLUT1 and VGAT are sorted to the same population of synaptic vesicles in subsets of cortical axon terminals. J. Neurochem 110, 1538–1546. [DOI] [PubMed] [Google Scholar]
- Fattorini G, Antonucci F, Menna E, Matteoli M, and Conti F. (2015). Co-expression of VGLUT1 and VGAT sustains glutamate and GABA co-release and is regulated by activity in cortical neurons. J. Cell Sci 128, 1669–1673. [DOI] [PubMed] [Google Scholar]
- Foster AC, and Kemp JA (2006). Glutamate- and GABA-based CNS therapeutics. Curr. Opin. Pharmacol 6, 7–17. [DOI] [PubMed] [Google Scholar]
- Fremeau RT Jr., Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, and Edwards RH (2001). The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31, 247–260. [DOI] [PubMed] [Google Scholar]
- Fremeau RT Jr., Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, et al. (2002). The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl. Acad. Sci. USA 99, 14488–14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, and Kandler K. (2005). Inhibitory synapses in the developing auditory system are glutamatergic. Nat. Neurosci 8, 332–338. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, and El Mestikawy S. (2002). A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J. Neurosci 22, 5442–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG (1969). Electron microscopy of excitatory and inhibitory synapses: a brief review. Prog. Brain Res 31, 141–155. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Hoffman BJ, and Mezey E. (1998). Ontogeny of vesicular monoamine transporter mRNAs VMAT1 and VMAT2. I. The developing rat central nervous system. Brain Res. Dev. Brain Res 110, 135–158. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, and El Mestikawy S. (2001). The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci 21, RC181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanova A, Pabst M, Lorkowski M, Braganza O, Boehlen A, Nikbakht N, Pothmann L, Vaswani AR, Musgrove R, Di Monte DA, et al. (2015). Function and developmental origin of a mesocortical inhibitory circuit. Nat. Neurosci 18, 872–882. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Stoppel DC, Nong Y, Johnson MA, Nadler MJ, Ozkaynak E, Teng BL, Nagakura I, Mohammad F, Silva MA, et al. (2017). Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature 543, 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhang Y, Chen M, and Zhou ZJ (2016). Segregated glycine-glutamate co-transmission from vGluT3 amacrine cells to contrast-suppressed and contrast-enhanced retinal circuits. Neuron 90, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Soiza-Reilly M, Smit T, Diana MA, Schwarz MK, and Mameli M. (2016). Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat. Neurosci 19, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Munster-Wandowski A, Gomez-Lira G, and Gutierrez R. (2013). Mixed neurotransmission in the hippocampal mossy fibers. Front. Cell. Neurosci 7, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Carter DA, Lehmann J, and Fibiger HC (1978). Evidence for a GABA-containing projection from the entopeduncular nucleus to the lateral habenula in the rat. Brain Res 145, 360–364. [DOI] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, and Kandler K. (2010). Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat. Neurosci 13, 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntamati NR, and Luscher C. (2016). VTA projection neurons releasing GABA and glutamate in the dentate gyrus. eNeuro 3, ENEURO.0137–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NP, Ferrari L, Venner A, Wang JL, Abbott SBG, Vujovic N, Arrigoni E, Saper CB, and Fuller PM (2017). Supramammillary glutamate neurons are a key node of the arousal system. Nat. Commun 8, 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney JB Jr., and Young AB (1981). GABA as the pallidothalamic neuro-transmitter: implications for basal ganglia function. Brain Res 207, 195–199. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang HL, Barker DJ, Miranda-Barrientos J, and Morales M. (2016). Glutamatergic efferents from the ventral tegmental area to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci 19, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B, Kardamakis A, Capantini L, Perez-Fernandez J, Suryanar- ayana SM, Wallen P, Stephenson-Jones M, and Grillner S. (2014). The lamprey blueprint of the mammalian nervous system. Prog. Brain Res 212, 337–349. [DOI] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Qi J, and Morales M. (2014a). Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J. Neurosci 34, 13906–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang HL, Hoffman AF, Lupica CR, and Morales M. (2014b). Single rodent mesohabenular axons release glutamate and GABA. Nat. Neurosci 17, 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagné C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, and Gasnier B. (1997). Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett 417, 177–183. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Piriz J, and Malinow R. (2014). Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science 345, 1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, and Somogyi P. (2004). GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur. J. Neurosci 19, 552–569. [DOI] [PubMed] [Google Scholar]
- Soussi R, Zhang N, Tahtakran S, Houser CR, and Esclapez M. (2010). Heterogeneity of the supramammillary-hippocampal pathways: evidence for a unique GABAergic neurotransmitter phenotype and regional differences. Eur. J. Neurosci 32, 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi R, Boulland JL, Bassot E, Bras H, Coulon P, Chaudhry FA, Storm-Mathisen J, Ferhat L, and Esclapez M. (2015). Reorganization of supramammillary-hippocampal pathways in the rat pilocarpine model of temporal lobe epilepsy: evidence for axon terminal sprouting. Brain Struct. Funct 220, 2449–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, and Stuber GD (2012). Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci 75, 1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Ikai Y, and Mizuno N. (1994). Direct projections from the entopeduncular nucleus to the lower brainstem in the rat. J. Comp. Neurol 342, 409–429. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, and Jahn R. (2000). Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407, 189–194. [DOI] [PubMed] [Google Scholar]
- Tien NW, Kim T, and Kerschensteiner D. (2016). Target-specific glycinergic transmission from VGluT3-expressing amacrine cells shapes suppressive contrast responses in the retina. Cell Rep 15, 1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, and Sabatini BL (2012). Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N. (2014). Bilingual neurons release glutamate and GABA. Nat. Neurosci 17, 1432–1434. [DOI] [PubMed] [Google Scholar]
- Wallace ML, Saunders A, Huang KW, Philson AC, Goldman M, Macosko EZ, McCarroll SA, and Sabatini BL (2017). Genetically distinct parallel pathways in the entopeduncular nucleus for limbic and sensorimotor output of the basal ganglia. Neuron 94, 138–152.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Qi J, Zhang S, Wang H, and Morales M. (2015a). Rewarding effects of optical stimulation of ventral tegmental area glutamatergic neurons. J. Neurosci 35, 15948–15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Eshaq RS, Meshul CK, Moore C, Hood RL, and Leiden-heimer NJ (2015b). Neuronal gamma-aminobutyric acid (GABA) type A receptors undergo cognate ligand chaperoning in the endoplasmic reticulum by endogenous GABA. Front. Cell. Neurosci 9, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Goto S, Kaji R, and Kuratsu J. (2006). Modulation of torsinA expression in the globus pallidus internus is associated with levodopa-induced dyskinesia in hemiparkinsonian rats. Neurosci. Lett 396, 62–66. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, and Morales M. (2011). Mesocorticolimbic glutamatergic pathway. J. Neurosci 31, 8476–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, Shenasa MA, Johnson AB, Fife KH, Faget L, and Hnasko TS (2016). Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun 7, 13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Kallstrom L, Wiesel FA, and Johnson AE (1999). Neurochemical changes in the entopeduncular nucleus and increased oral behavior in rats treated subchronically with clozapine or haloperidol. Synapse 34, 192–207. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, and Morales M. (2015). Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci 18, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hernandez VS, Swinny JD, Verma AK, Giesecke T, Emery AC, Mutig K, Garcia-Segura LM, and Eiden LE (2018). A GABAergic cell type in the lateral habenula links hypothalamic homeostatic and midbrain motivation circuits with sex steroid signaling. Transl. Psychiatry 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.