Abstract
Denosumab is a receptor activator of nuclear factor kappa-Β ligand inhibitor, which suppresses the bone resorption process to preserve bone mass. It is usually recommended to postmenopausal women and men with high fracture risk. With the recent publication of the results from FREEDOM study and its extension, the long-term effect of denosumab in preventing fragility fractures has been put forward. This review aims at summarising the evidence of denosumab in reducing fracture risk and its safety derived from clinical studies. Most of the evidence are derived from FREEDOM trials up to 10 years of exposure. Denosumab is reported to prevent vertebral and non-vertebral fractures. It is also proven effective in Japanese women, patients with chronic kidney diseases and breast cancer patients receiving antineoplastic therapy. Denosumab discontinuation leads to high remodeling, loss of bone mineral density and increased fracture risk. These negative effects might be preventable by bisphosphonate treatment. The safety profile of denosumab is consistent with increased years of exposure. In conclusion, denosumab is a safe and effective option for reducing fracture risk among patients with osteoporosis.
Keywords: bone mineral density, bone turnover marker, menopause, osteopenia, osteoporosis
Introduction
Osteoporosis is a metabolic disease of the skeletal system with insidious onset and affects bone mineral density (BMD) adversely.1 According to World Health Organization, osteoporosis is defined as a BMD that lies 2.5 standard deviations or more below the average value for young adult (T-score ≤ −2.5).2 Fragility fractures are the ultimate consequence of osteoporosis. An estimate showed that 75% of all vertebral and non-vertebral fractures occur in individuals aged ≥65 years, and more than 75% of hip fractures affect individuals aged ≥75 years.3 Fragility fractures are traumatic events for the octogenarians and geriatric patients because their overall health and functioning status will be severely impaired.4,5 Vertebral fractures were linked with increased comorbidity and admission events, as well as prolonged duration of hospitalization.6 Elderly men have higher chances of suffering from these complications, particularly pneumonia and musculoskeletal diseases, compared to their female counterparts.7,8 Patients with fragility fracture suffer from higher morbidity, higher risk for subsequent fracture and greater mortality risk after discharge.9 Patients with refracture possess a mortality rate of 1.2 times higher than patients without refracture patients 3 years.10
Both pharmacological and non-pharmacological strategies are required in constructing better fracture prevention care for the elderly. The National Osteoporosis Foundation Guidelines for Pharmacologic Treatment of Osteoporosis recommends pharmacological treatments to postmenopausal women and men aged ≥50 years with a history of hip/vertebral fracture, presenting with BMD T-score between −1.0 and −2.5 at the femoral neck/spine or those with a T-score ≤ −2.5 at the femoral neck/spine.11 These populations have a 10-year risk of hip fracture ≥3% or 10-year risk of major osteoporosis-related fracture ≥20% by Fracture Risk Assessment Tool (FRAX) calculation.12 Anti-osteoporotic therapy has been demonstrated to reduce mortality in patients with osteoporosis who have been reported to be at risk of high mortality.13–15 Given the higher chance of refracture among the fracture population, post-fracture anti-osteoporosis treatment is important in reducing the burden of fragility fracture.
Denosumab is a human recombinant monoclonal antibody that prevents the binding of receptor activator of nuclear factor kappa-Β (RANK) ligand (RANKL) to RANK on osteoclasts, thereby suppressing bone resorption.16,17 It is one of the most commonly prescribed antiresorptive drugs in clinical practice for the management of osteoporosis in postmenopausal women.18 Denosumab is reported to increase BMD, inhibit high bone turnover and reduce fracture risk in postmenopausal women with osteoporosis.19,20 A potential rise in the risk of multiple vertebral fractures follows discontinuation of denosumab.21 Several reviews on the effects of denosumab on BMD have been published.22–24 In this article, the effects of denosumab alone or in comparison with other anti-resorptive drugs in fracture/refracture risk reduction among the elderly are reviewed. We also addressed the mortality rate and safety of denosumab use in patients with osteoporosis.
Overview of the Human Studies
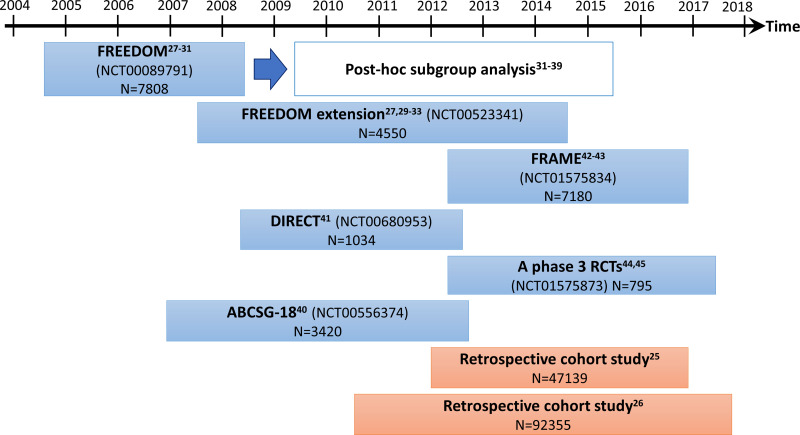
A total of 22 original research articles reporting the anti-fracture effects of denosumab from human retrospective cohort and clinical trials were included in this review. Human studies that did not report the fracture incidence or risk are not included. There are two retrospective cohort studies utilizing national data;25,26 seven articles from Fracture REduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) clinical trial and FREEDOM extension;27–33 nine articles on post hoc analysis of FREEDOM data31,39 (wherein 2 articles reporting both post hoc analysis and FREEDOM extension);32,33 one article from Austrian Breast Cancer Study Group study (ABCSG-18);40 one article from Denosumab fracture Intervention RandomizEd placebo Controlled Trial (DIRECT);41 two articles from FRActure study in postmenopausal woMen with ostEoporosis study (FRAME)42,43 and two articles from a randomized controlled trial (RCT) by Saag et al.44,45 The denosumab was administrated at 60 mg subcutaneously once in every 6 months in these clinical trials. However, the treatment period, dose and compliance of denosumab are not known for retrospective cohort studies.25,26
Two retrospective cohort studies compared the anti-fracture effects of denosumab with bisphosphonates.25,26 Behavona et al analyzed the national data (98% coverage; n=47,139) in Austria among patients aged ≥50 years, who experienced a hip fracture between January 2012 and December 2016, and then either left untreated or treated with denosumab or bisphosphonates (oral or intravenous).25 They were tracked up to 60 months for any incidence of subsequent hip fracture and all-cause mortality as study outcomes.25 Pederson et al performed a similar nationwide, population-based, historical cohort study from Danish health registries or database.26 A total of 92,355 subjects aged ≥50 years and received the first dispensing of denosumab or alendronate from May 2010 to December 2017 without any antiosteoporosis medication within 1 year were included in the retrospective analysis.26 These subjects were follow-up up to 7.5 years for any hip or other fracture as study outcomes.26 The data were analyzed by treatment condition and then further with subgroup analysis by sex, age, and prior history of hip or any fracture.26
To date, more than 6 completed clinical trials related to denosumab had been reported. FREEDOM trial is an international Phase 3 randomized, placebo-controlled trial designed to investigate the effect of denosumab on fracture risk in postmenopausal women aged 60–90 years with a BMD T-score of less than −2.5 at the lumbar spine or total hip in a 3-year follow-up period.28,46,47 It was started in August 2004 and completed in June 2008, and involved 7808 postmenopausal women as either placebo (N=3902) or denosumab groups (N=3906).28,47 Placebo or 60 mg denosumab was administered subcutaneously once every 6 months for 3 years.28,47 The new vertebral, non-vertebral or hip fracture incidence were recorded as primary outcomes.28,47 A continuation of FREEDOM trial (also known as FREEDOM extension) was conducted, wherein all subjects (both placebo and denosumab groups) who did not miss more than 1 dose of the investigational product were recruited to receive the denosumab up to an additional 7 years.27,29–33 The placebo of FREEDOM trial was noted as “cross-over group” because they received denosumab in the FREEDOM extension while denosumab group continued to receive denosumab up to 10 years and was named the “long-term group”.27,29–33
ABCSG-18 study is a prospective, double-blind, placebo-controlled, multicentre phase 3 trial on 3420 postmenopausal women with hormone receptor-positive breast cancer and underwent aromatase inhibitor as treatment.40 All subjects were randomly assigned to receive either denosumab (N=1274) or placebo (N=1188) up to 7 years with a median time of study of 38 months.40
DIRECT was a randomized, double-blind, placebo-controlled trial that evaluates the anti-fracture effects of denosumab on both Japanese postmenopausal women and men with osteoporosis.41 A total of 1034 Japanese postmenopausal women and men, aged ≥50 years, with BMD T-score <-1.7 (lumbar spine) or <-1.6 (total hip) with 1–4 prevalent vertebral fractures were randomly assigned in a 2:2:1 ratio to receive either placebo (N=416), denosumab (N=414), or 35 mg oral alendronate weekly (N=204) for 24 months.41
FRAME trial is an international, randomized, double-blind, placebo-controlled, phase 3 trial on postmenopausal women with osteoporosis.42,43 A total of 7180 postmenopausal women, aged 55–90 years and with a total hip or femoral neck BMD T-score of −3.5 to −2.5 were randomly assigned to receive either placebo or romosozumab (at a dose of 210 mg) monthly for the first 12 months; and subsequently subcutaneous administration of denosumab up to 24 months.42,43 Miyauchi et al performed a subgroup analysis on the Japanese subjects (with romosozumab (N=247) and placebo (N=245)).43
Lastly, Saag et al had conducted a phase 3, international, randomized, double-blind, double-dummy, active-controlled, non-inferiority clinical trial on patients with glucocorticoid-induced osteoporosis (N=590) with the lumbar spine, total hip or femoral neck BMD T-score ≤ −2.0 or ≤ −1.0 with fracture history or past osteoporosis-related fracture history.44,45 Subjects receiving glucocorticoid therapy for less than 3 months before screening was grouped as “glucocorticoid-initiating group” (n=253) while those with more than 3 months therapy were grouped as “glucocorticoid-continuing group” (n=438).44,45 All subjects were randomly assigned either subcutaneous denosumab and oral placebo daily for 24 months, or oral risedronate 5 mg daily with subcutaneous placebo every 6 months for 24 months.44,45
Figure 1 provides an overview of the human retrospective cohorts and clinical trials included in this review.
Figure 1.
An overview of the human retrospective cohorts and clinical trials included in this review.
The Effect of Denosumab on Vertebral Fracture Risk Reduction
In the FREEDOM trial, 36-month denosumab treatment significantly reduced the risk of new vertebral fracture, new clinical vertebral fracture, multiple new vertebral fractures, new or worsening vertebral fracture, clinical osteoporotic fractures (vertebral and non-vertebral fractures) and primary or secondary fragility fracture (new vertebral and low-trauma non-vertebral fracture).28,30,31,33–38,48 Parallelly, in ABCSG-18 trial, 36-month denosumab treatment also significantly reduced the new vertebral fracture, new or worsening vertebral fracture incidences, as well as the first clinical fracture among breast cancer patients treated with an aromatase inhibitor.40 Similar fracture risk reduction was also reported as early as 12-month denosumab treatment during FREEDOM and DIRECT study.28,30,34,35,41 Denosumab was also as effective as risedronate in reducing the fracture incidences, including any osteoporosis-related fracture and new or worsening vertebral fractures upon 24-month treatment.45 The efficacy of denosumab and risedronate were similar regardless of sex and menopause status in women.44,45
Additionally, denosumab also reduced new vertebral fracture incidence during the 2–7 years of FREEDOM extension.27,30,31 New vertebral fracture incidence was initially high in the placebo group during the FREEDOM study,30,31 but decreased sharply during the first 2 years of denosumab treatment30 and remained low throughout the 7 years of FREEDOM extension.27 The trend of fracture risk reduction in the cross-over group was similar to the FREEDOM denosumab group in the first 2 years of denosumab treatment.30 Additionally, the cumulative new vertebral fracture incidence of the long-term group was also significantly reduced in both FREEDOM study and the 7-year FREEDOM extension.27 Moreover, denosumab reduced the exposure-adjusted subsequent osteoporotic fracture rate during FREEDOM trial as well as on all subjects receiving denosumab during the 7-year FREEDOM extension.33
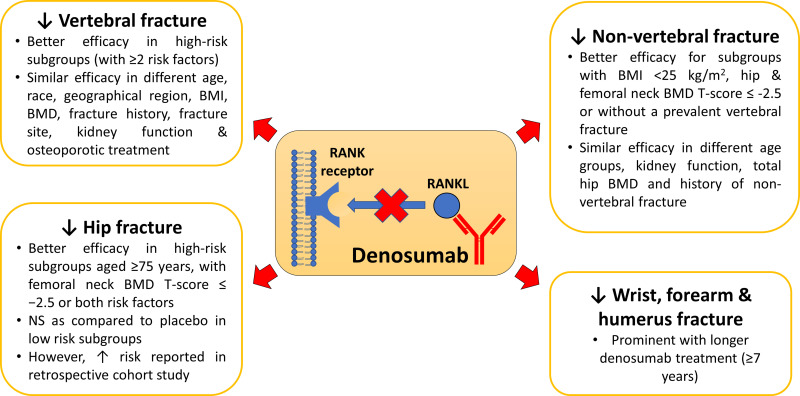
FREEDOM post hoc analyses demonstrated a greater anti-fracture effect of denosumab in certain subgroups.33–35,37,41 Denosumab significantly reduced the new vertebral fracture incidences in the high-risk subgroup with prevalent vertebral fracture and/or vertebral deformity or those with baseline femoral neck BMD T-score of ≤−2.5 or those with all above risk factors after 24 and 36 months.35 McCloskey et al also reported that the efficacy of denosumab was better on patients with moderate to high fracture risk and low body mass index.37 Additionally, 24-month denosumab reduced the risk of new or worsening vertebral fracture among Japanese postmenopausal women but not men during the DIRECT study.41 Recently, Kendler et al also reported a higher fracture risk reduction among subjects with a history of osteoporotic fracture with denosumab treatment.33
On the other hand, McClung et al reported the efficacy of denosumab in new vertebral fracture risk reduction was independent of age, race, region, body mass index, estimated creatinine clearance, femoral neck BMD, prevalent vertebral fracture, prior non-vertebral fracture and prior use of osteoporosis medications upon a post-hoc analysis on FREEDOM data.38 A similar finding by Palacios et al also reported that the anti-fracture effects of denosumab were independent of age, prior fragility fracture history, fracture site, previous osteoporotic treatment history as well as those presented with both risk factors of age and prior fragility fracture.48 Similarly, denosumab reduced the osteoporotic fracture regardless of age, femoral neck BMD value, prior fracture, parental history of hip fracture, secondary causes of osteoporosis, smoking or alcohol intake in another FREEDOM post-hoc analysis.37 Similarly, prevalent vertebral fracture, femoral neck BMD and the functionality of the kidney also did not affect the efficacy of denosumab on fracture risk reduction.35,36,40
The Effect of Denosumab on Hip Fracture Risk Reduction
Two retrospective cohort studies were conducted to evaluate the effects of denosumab in reducing bone fracture.25,26 Behavona et al reported no significant difference in subsequent hip fracture risk between denosumab and bisphosphonate groups.25 Surprisingly, patients treated with bisphosphonates and denosumab experienced a higher risk and cumulative incidence of subsequent hip fracture compared to patients without antiresorptive treatment.25 The increase in subsequent hip fracture risk was more prominent among women receiving denosumab.25 The increase of subsequent hip fracture risk with denosumab treatment might be due to a short follow-up time or confounders, such as alcohol consumption, physical activity, nutrition and/or medication adherence.25 Sample size for the denosumab group was relatively small (N=555) as compared with untreated patients (N=42,795).25 A recent retrospective cohort study by Pederson et al identified that hip fracture risk was similar between patients treated with denosumab and alendronate within 3 years of follow-up, regardless of sex, age, previous history of hip fracture or any fracture.26 Subgroup analysis revealed a marginally lower risk among women, subjects <80 years old, those with or without a history of any fracture and those without previous hip fracture compared to alendronate.26
In FREEDOM study, 36-month denosumab treatment significantly reduced the risk of hip fractures compared to placebo.28,30,35,36 Prolonged (>3 years) denosumab treatment reduced the hip fracture incidence during the first 3 years of FREEDOM trial, then further reduced and maintained it throughout the 7 years of FREEDOM extension.29–31 A similar trend was detected in the cross-over group, where the hip fracture incidence was significantly reduced, reaching a level comparable to the long-term group at the end of FREEDOM extension.27,29-31 Boonen et al reported that denosumab could significantly reduce the risk of hip fractures in the entire FREEDOM population as well as high-risk subgroups, for example, subjects aged ≥75 years; baseline femoral neck BMD T-score of ≤ −2.5 or those with both risk factors.35 There was no significant difference in hip fracture risk reduction between denosumab and placebo for low-risk subgroups due to the low baseline hip fracture risk.35 Parallelly, in the FREEDOM post-hoc subgroup analysis, denosumab did not reduce hip fractures after adjusting for age and major osteoporotic fracture probability calculated by FRAX.37
The Effect of Denosumab on Non-Vertebral Fracture Risk Reduction
In FREEDOM study, 36-month denosumab treatment significantly reduced the risk of non-vertebral fractures compared to placebo.28,30,35,36,38 The anti-fracture effects of denosumab in the non-vertebral or other minor fractures are heterogeneous, especially with shorter treatment time. Saag et al reported that the efficacy of denosumab in reducing low-trauma non-vertebral fractures was similar to risedronate during a 24-month treatment frame.44,45 However, marginal non-vertebral fracture risk reduction was reported after 12- and 24-month denosumab treatment.34,41 Additionally, Nakamura et al also reported that 24-month denosumab increased the risk of non-major non-vertebral fracture to 2.5% as compared to 0.4% among placebo.41 Thus, the efficacy of denosumab in reducing non-vertebral fracture risk needs to be investigated further.
Prolonged (>3 years) denosumab treatment resulted in a similar or even better fracture risk reduction compared with shorter treatment. Denosumab reduced the non-vertebral fracture incidence during the first 3 years of FREEDOM trial, then further reduced and maintained it throughout the 7 years of FREEDOM extension.27,29–31 A similar trend was detected in the cross-over group, where the non-vertebral fracture incidence was significantly reduced, reaching a comparable level as the long-term group at the end of FREEDOM extension.27,29–31 The non-vertebral fracture incidence was significantly higher among FREEDOM placebo and the twin-estimated placebo in FREEDOM extension.30 It can be concluded that the anti-fracture effect of denosumab is only significant after ≥3 years of treatment.
In the FREEDOM post-hoc subgroup analysis, denosumab significantly reduced the clinical osteoporotic fractures after adjusting for age and FRAX major osteoporotic fracture probability.37 Denosumab only significantly reduced the non-vertebral fracture risk in those subgroups with BMI <25 kg/m2, femoral neck BMD T-score ≤-2.5 or without a prevalent vertebral fracture.38 Additionally, the efficacy of denosumab on non-vertebral fracture was independent of age groups, kidney function, total hip BMD, and prior history of non-vertebral fracture.34,36,38 Subgroup analysis revealed that denosumab-induced non-vertebral fracture risk reduction was more prominent among subjects with the lower hip (T-score between −1.0 and −2.5) or femoral neck BMD (T-score ≤-2.5) during FREEDOM extension.29 In other words, the risk reduction effects of denosumab in a specific subgroup may be marginal and only become prominent upon a longer treatment time. Additionally, the selection of inclusion and exclusion criteria and subgrouping definition may also affect the outcome in subgroup analysis.
The Effect of Denosumab on Wrist and Other Fracture Risk Reduction
The effects of denosumab on wrist and other fractures, including forearm and humerus fractures, were also reported. However, the effects might not be as significant as major fractures.32,39 Denosumab significantly reduced the wrist fracture incidence for FREEDOM subgroup with a femoral neck T-score ≤ −2.5, but not for the entire FREEDOM population.39 Bilezikian et al also reported the beneficial effects of denosumab on upper limb fractures during the 7-year FREEDOM extension.32 Denosumab significantly reduced the humerus but not wrist and forearm fracture rate among the extension group during the 1st–3rd year of FREEDOM extension.32 The overall fracture rate for upper limbs, including wrist, forearm and humerus fractures, was significantly lower in the extension group during the 4th–7th year of FREEDOM extension.32 Parallel with vertebral and non-vertebral fracture, this observation also suggested that a longer denosumab treatment is necessary to optimize its anti-fracture effects for the upper limbs.
Figure 2 summarizes the effects of denosumab on vertebral, non-vertebral, hip, wrist and other fractures risk reduction.
Figure 2.
The anti-fracture effects of denosumab in vertebral, non-vertebral, hip, wrist and other fractures.
Notes: ↓Increase; ↑Decrease.
Abbreviations: BMI, body mass index; BMD, bone mass density; NS, non-significant; RANKL, receptor activator of nuclear factor kappa-Β ligand.
Comparison of the Fracture Risk Reduction Between Denosumab and Other Anti-Osteoporotic Drugs
The retrospective cohort study by Pedersen et al compared the risk of hip and any fracture between patients treated with denosumab and alendronate.26 The hazard ratios of hip fractures for both denosumab and alendronate were similar regardless of sex, age, or fracture history.26 On the other hand, denosumab was more potent than risedronate in improving BMD as evidenced in an RCT study.44,45 Denosumab, as compared to risedronate, significantly increased the percentage change of BMDs at the lumbar spine, total hip, femoral neck and 1/3 radius among those glucocorticoid-treated subjects.44,45 There was an early downregulation of serum BTM levels prior to the increase of BMDs upon denosumab treatment.44,45
On the other hand, the anti-osteoporotic effects of combined denosumab and romosozumab among postmenopausal women were studied in the FRAME trial.42,43 Subjects with 1 year of romosozumab followed by another year of denosumab showed a similar incidence of non-vertebral and clinical fracture.42 With the continuation of an extra 1 year of denosumab treatment, no significant reduction in new vertebral, clinical, non-vertebral, major non-vertebral, major osteoporotic, clinical new or worsening vertebral and hip fractures was observed compared to subjects with the first year of placebo and subsequent 2 years of denosumab.43 Denosumab did increase the BMDs in both groups. However, the pre-treatment of romosozumab significantly increased patients’ BMD from the baseline values.42,43 The anti-fracture effects of romosozumab-denosumab combination need to be further confirmed via a larger sample size and/or longer treatment time.
Teriparatide is the first approved anabolic agent that stimulates osteoblastic bone formation.49,50 The anti-osteoporotic effects of teriparatide, denosumab and other anti-osteoporotic agents have been examined in systematical review and meta-analysis recently.51 Both teriparatide (daily dose of 20–40 μg) and denosumab were effective in reducing vertebral fracture risk by 86% and 68%, respectively.51 However, the between-groups comparison was not performed by the authors. In the Denosumab and Teriparatide Administration randomized trial (DATA), 12-month denosumab was superior to teriparatide based on the increment of BMDs at femoral neck, total hip and distal 1/3 of radial shaft.52 In the DATA extension study, 24-month teriparatide was as effective as denosumab in increasing the BMDs at the lumbar spine, femoral neck and total hip.53 Denosumab, but not teriparatide, significantly increased the BMD at the 1/3 distal radius.53
Short-term teriparatide treatment is preferable given its potential carcinogenic effect, based on the osteosarcoma induction in rats upon long-term exposure (2-year).54,55 A shorter (3 to 6 months) but cyclic approach of teriparatide could exert a similar or slightly weaker anti-osteoporotic effect compared to standard daily teriparatide treatment.56 Moreover, the cyclic teriparatide treatment with three cycles of teriparatide (6 months) to denosumab (6 months) was similar to standard sequential teriparatide (18 months) to denosumab (18 months) treatment in increasing the BMDs at spine, total femur, femoral neck and 1/3 radius.57 On the other hand, in DATA-Switch study, co-treatment of teriparatide and denosumab for 24 months, followed by 24-month denosumab alone was more potent in increasing the BMDs at total hip, femoral neck and radius bone (but not lumbar spine) than sequential treatments with teriparatide (24 months) to denosumab (24 months) or vice versa.58,59 Similar findings were obtained in the DATA and DATA extension study, whereby the co-treatment of teriparatide and denosumab induced a greater increase in BMDs.52,53
A summary of the anti-fracture effects of denosumab is presented in Table 1.
Table 1.
The Anti-Fracture Effects of Denosumab in the Retrospective Cohort and Clinical Trials
| No. | Reference | Study Design | Subjects | Intervention Description | Outcomes |
|---|---|---|---|---|---|
| 1 | Cummings et al, 200928 | Randomised controlled trial (FREEDOM; ClinicalTrials.gov: NCT00089791) | A total of 7808 postmenopausal women aged 60–90 years with a T score < −2.5 at the lumbar spine or total hip. | Subjects received subcutaneous injections of either 60 mg denosumab or placebo every 6 months for 36 months. All women received daily supplements containing calcium (≥1 g) and vitamin D (≥400 IU). |
|
| 2 | Papapoulos et al, 201230 | FREEDOM and FREEDOM extension study (ClinicalTrials.gov: NCT00523341) | A total of 7808 subjects from FREEDOM trial and 4550 subjects from both placebo (N=2207) and denosumab groups (N=2343) who completed the 3-year FREEDOM trial and did not discontinue investigational product or miss >1 dose in the first 2 years of FREEDOM extension study. | Similar intervention as Cummings et al 2009. During the FREEDOM extension, all subjects (denosumab and placebo) were administrated 60 mg denosumab subcutaneously every 6 months for the first 2 years with daily calcium and vitamin D supplementation. Cross-over group: all subjects from placebo of FREEDOM study who received the denosumab in the extension. Long-term group: Subjects who continued denosumab for 2 years in addition to the 3 years initial treatment (total 5 years of treatment). |
|
| 3 | Ferrari et al, 201529 | FREEDOM and FREEDOM extension study (ClinicalTrials.gov: NCT00523341) |
A total of 4074 subjects (N=2343 denosumab; N=1731 placebo) who completed the 3-year FREEDOM trial and did not discontinue investigational product or miss >1 dose for the first 4 years in the FREEDOM extension. | Similar intervention as Papapoulos et al 2012 but up to 3 years. Cross-over group: all subjects from placebo of FREEDOM study who received the denosumab in the extension. Long-term group: Subjects who continued denosumab for 4 years in addition to the 3 years initial treatment (total 7 years of treatment). |
|
| 4 | Papapoulos et al, 201531 | FREEDOM and FREEDOM extension study (ClinicalTrials.gov: NCT00523341) |
A total of 7808 subjects from FREEDOM trial and 3004 subjects from both placebo (N=1462) and denosumab groups (N=1542) who completed the 3-year FREEDOM trial and did not discontinue investigational product or miss >1 dose in the first 5 years of FREEDOM extension. | Similar intervention as Cummings et al 2009. and Papapoulos et al 2012 (up to 5 years). Cross-over group: all subjects from placebo of FREEDOM study who received the denosumab in the extension. Long-term group: Subjects who continued denosumab for 5 years in addition to the 3 years initial treatment (total 8 years of treatment). |
|
| 5 | Bone et al, 201727 | FREEDOM and FREEDOM extension study (ClinicalTrials.gov: NCT00523341) |
A total of 7808 subjects from FREEDOM trial and 2626 subjects from both placebo (N=1283) and denosumab groups (N=1343) who completed the 3-year FREEDOM trial and did not discontinue investigational product or miss >1 dose for 7 years in the FREEDOM extension. | Similar intervention as Cummings et al 2009. and Papapoulos et al 2012 (up to 7 years). Cross-over group: all subjects from placebo of FREEDOM study who received the denosumab in the extension. Long-term group: Subjects who continued denosumab for 7 years in addition to the 3 years initial treatment (total 10 years of treatment). |
|
| 6 | Kendler et al, 201933 | Post-hoc analysis of FREEDOM and FREEDOM extension study (ClinicalTrials.gov: NCT00523341) |
A total of 710 FREEDOM subjects and 794 subjects from FREEDOM and FREEDOM extension respectively who had an osteoporotic fracture (new vertebral or non-vertebral fracture) and then continued post-fracture treatment at least ≥ 2 doses of placebo or denosumab. | Similar intervention as Cummings et al 2009. and Papapoulos et al 2012 (up to 7 years). Cross-over group: all subjects from placebo of FREEDOM study who received the denosumab in the extension. Long-term group: Subjects who continued denosumab for 7 years in addition to the 3 years initial treatment (total 10 years of treatment). Combined denosumab group: Denosumab users in long-term group and/or cross-over group. |
|
| 7 | Bilezikian et al, 201932 | Post hoc analysis of FREEDOM and FREEDOM extension study (ClinicalTrials.gov: NCT00523341) |
A total of 2207 subjects from FREEDOM extension study who had an osteoporotic fracture (new vertebral or non-vertebral fracture) and then continued post-fracture treatment at least ≥ 2 doses of placebo or denosumab. A total of 441 subjects were identified in a BMD sub-study with 209 placebo and 232 denosumab. |
Similar intervention as Cummings et al 2009. and Papapoulos et al 2012 (up to 7 years). Cross-over group: all subjects from placebo of FREEDOM study who received the denosumab in the extension. Long-term group: Subjects who continued denosumab for 7 years in addition to the 3 years initial treatment (total 10 years of treatment). Extension group: Cross-over and long-term groups. |
|
| 8 | Boonen et al, 201135 | Post hoc analysis of FREEDOM data | A total of 7808 postmenopausal women from FREEDOM trial, who were grouped as high- and low-risk subgroups based on fracture prevalent, femoral neck BMD and/or age. | Similar intervention as Cummings et al 2009. |
|
| 9 | Jamal et al, 201136 | Post-hoc analysis of FREEDOM data | A total of 7808 postmenopausal women from FREEDOM trial, who were grouped based on the modified National Kidney Foundation classification of CKD. |
Similar intervention as Cummings et al 2009. |
|
| 10 | McClung, et al 201238 | Post-hoc analysis of FREEDOM data | A total of 7808 postmenopausal women from FREEDOM trial, who were grouped into subgroups (age, body mass index, estimated creatinine clearance, region, femoral neck BMD, prevalent vertebral fracture, prior non-vertebral fracture, race and prior use of osteoporosis medications) with their new vertebral fracture, non-vertebral fracture and femoral neck BMD outcomes. | Similar intervention as Cummings et al 2009. |
|
| 11 | Austin et al, 201234 | Post-hoc analysis of FREEDOM data | A total of 7808 postmenopausal osteoporotic women from FREEDOM study with their total hip BMD and fracture outcomes. | Similar intervention as Cummings et al 2009. |
|
| 12 | McCloskey et al, 201237 | Post-hoc analysis of FREEDOM data | Calculation of FRAX based on clinical risk factors and BMD data from the 7808 postmenopausal osteoporotic women in FREEDOM study. | Similar intervention as Cummings et al 2009. |
|
| 13 | Simon et al, 201339 | Post-hoc analysis of FREEDOM data | A total of 7808 postmenopausal women from FREEDOM trial with wrist fracture incidence, radius BMD, bone mineral content and strength data. | Similar intervention as Cummings et al 2009. |
|
| 14 | Palacios et al, 201548 | Post-hoc analysis of FREEDOM data | A total of 7808 postmenopausal women from FREEDOM trial with the onset of secondary fragility fracture. | Similar intervention as Cummings et al 2009. |
|
| 15 | Cosman et al 201642 | An international, randomized, double-blind, placebo-controlled, phase 3 fracture study on Japanese postmenopausal women with osteoporosis [FRActure study in postmenopausal woMen with ostEoporosis (FRAME) study]; (ClinicalTrials.gov: NCT01575834) |
A total of 7180 postmenopausal women, age 55–90 years with osteoporosis (BMD T-score −3.5 to −2.5 at total hip or femoral neck). | Subjects were randomized 1:1 to receive subcutaneous romosozumab 210 mg (N=3589) or placebo (N=3591) once monthly for 12 months. All subjects were transitioned to open-label denosumab 60 mg subcutaneously every 6 months for the first 12 months. All subjects received a minimum daily calcium (500–1000 mg) and vitamin D (600–800 IU) supplementation throughout the study. |
|
| 16 | Miyauchi et al, 201943 | Sub-group analysis of FRAME data |
A total of 492 Japanese postmenopausal women, age 55–90 years with osteoporosis (BMD T-score −3.5 to −2.5 at total hip or femoral neck) were used in this FRAME sub-analysis with a total of 7180 subjects. | Subjects were randomized 1:1 to receive subcutaneous romosozumab 210 mg (N=190) or placebo (N=209) once monthly for 12 months. All subjects were transitioned to open-label denosumab 60 mg subcutaneously every 6 months for another 24 months. All subjects were prescribed a minimum daily calcium (500–1000 mg) and vitamin D (600–800 IU) supplementation throughout the study. |
|
| 17 | Nakamura et al, 201441 | A randomized, double-blind, placebo-controlled trial on Japanese postmenopausal women and men with osteoporosis [Denosumab fracture Intervention RandomizEd placebo Controlled Trial (DIRECT)]; (ClinicalTrials.gov: NCT00680953) |
A total of 1034 Japanese postmenopausal women and men aged 50 years or older with osteoporosis (BMD T-score <-1.7 (lumbar spine) or <-1.6 (total hip) with one to four prevalent vertebral fractures completed the study. | Subjects were randomly assigned in a 2:2:1 ratio to either receive placebo (N=416), denosumab 60 mg subcutaneous every 6 months (N=414), or open-label oral alendronate 35 mg weekly (N=204) for 24 months. At least 600 mg calcium and 400 IU vitamin D were supplemented daily throughout the study period. |
|
| 18 | Gnant et al, 201540 | A prospective, double-blind, placebo-controlled, multicentre phase 3 study on postmenopausal women with hormone receptor-positive breast cancer and aromatase inhibitor as treatment [Austrian Breast Cancer Study Group (ABCSG-18); (ClinicalTrials.gov: NCT00556374) |
A total of 3420 postmenopausal women with early-stage hormone receptor-positive breast cancer and underwent aromatase inhibitor treatment completed the study. | Subjects received either 60 mg subcutaneous denosumab (N=1274) or placebo (N=1188) every 6 months up to 7 years with a median time of study of 38 months. Daily administration of 500 mg calcium and at least 400 IU vitamin D were supplemented throughout the study period. |
|
| 19 | Saag et al, 201844 | A phase 3, international, randomized, double-blind, double-dummy, active-controlled, non-inferiority study on glucocorticoid-initiating and glucocorticoid-continuing patients (ClinicalTrials.gov: NCT01575873) | A total of 691 glucocorticoid-treated participants with osteoporosis (BMD T-score ≤ −2.0 or ≤ −1.0 with fracture history) or past osteoporosis-related fracture history. | Subjects with glucocorticoid therapy for < 3 months before screening was grouped as “glucocorticoid-initiating group” (N=253) while those with > 3 months therapy were grouped as “glucocorticoid-continuing group” (N=438). Subjects were then randomized 1:1 within each group to receive either subcutaneous denosumab 60 mg every 6 months and oral placebo daily for 12 months, or oral risedronate 5 mg daily with subcutaneous placebo every 6 months for 12 months. All patients were assigned to receive daily supplementation with at least 1000 mg calcium and 800 IU vitamin D. |
|
| 20 | Saag et al, 201945 | A phase 3, international, randomized, double-blind, double-dummy, active-controlled, parallel-group study on glucocorticoid-initiating and glucocorticoid-continuing patients (ClinicalTrials.gov: NCT01575873) | A total of 590 glucocorticoid-treated participants with osteoporosis (BMD T-score ≤ −2.0 or ≤ −1.0 with fracture history) or past osteoporosis-related fracture history. | Same as Saag et al 2018 but with 226 subjects in “glucocorticoid-initiating group” and 364 subjects in “glucocorticoid-continuing group” up to 24 months of treatment. |
|
| 21 | Behanova et al (2019)25 | A retrospective cohort study on Austria national data with propensity score matching for antiresorptive-treated and untreated patients | Previous data from a total of 47,139 patients aged ≥ 50 years old who experienced a hip fracture between January 2012 and December 2016 and follow-up with or without treatment (bisphosphonates or denosumab). | Hip fracture patients either received oral or intravenous bisphosphonates (N= 3789), denosumab (N=555) or no treatment (N=42,795), up to 60 months. However, the treatment dosage and interval are unknown. |
|
| 22 | Pedersen, Heide-Jorgensen, Sorensen, Prieto-Alhambra, and Ehrenstein, 201926 | A retrospective cohort study using a nationwide, population-based, historical cohort study from Danish health registries/database with complete follow-up | Previous data from a total of 92,355 subjects aged ≥ 50 years who received the first dispensing of denosumab or alendronate from May 2010 to December 2017 without any antiosteoporosis medication within 1 year. |
Patients either received denosumab (N=4624) or alendronate (N=87,731) and then followed-up up to 7.5 years (median= 3.3 years). However, the treatment dosage and interval are unknown. |
|
Notes: ↓Increase; ↑Decrease.
Abbreviations: aHR, adjusted hazard ratio; ARR, absolute risk reduction; BMD, bone mass density, BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; OR, odds ratio; NA, not available; RR, relative risk; RRR, relative risk reduction; SHR, subdistribution hazard ratio.
Discontinuation of Denosumab and Fracture Risk
The effect of denosumab discontinuation on fracture risk has been reviewed extensively.21,60,61 Denosumab discontinuation is closely associated with a rebound effect, indicated increased BTM levels and the parallel loss of gained BMD within 6–24 months of discontinuation, as reported in several case reports, observational study, RCTs and post-hoc analysis of FREEDOM data.62–67 Additionally, denosumab discontinuation also resulted in a higher risk of rebound-associated vertebral fracture, including multiple vertebral fractures.68–72 The simultaneous reactivation of all dormant osteoclasts at once after denosumab discontinuation, which subsequently leads to excessive bone resorption, is suggested to be the mechanism of rebound effect.73 As evidence, an early 5- to 7-fold increase of CTX and P1NP levels after the 3-month discontinuation of denosumab was observed. This effect was accompanied by an increase in RANKL level and a gradual decrease in Dickkopf-1 and sclerostin level.74 A recent histomorphological analysis on bone biopsies from fracture patients also confirmed that denosumab discontinuation caused a higher bone turnover activity and bone structures and hardness reduction, which may explain the rebound-associated fracture.75 Moreover, there is a downregulation of miR-503 and miR-222-2 (miRNAs that downregulate osteoclastogenesis) upon denosumab discontinuation, which leads to the subsequent upregulation of RANK and cathepsin K mRNAs.73 The optimum treatment after discontinuation of denosumab is not yet established. The re-initiation of denosumab was reported to increase the BMDs again, but a very recent case report from Niimi et al showed that re-initiation of denosumab did not eliminate the risk of rebound-associated vertebral fractures.70,76 A pre-treatment or follow-up course of bisphosphonates upon denosumab discontinuation was found to be protective by maintaining BMDs and CTX levels, possibly via the reduction of the dormant osteoclasts.74,77-80 Further investigation is required to elucidate the underlying mechanisms of denosumab discontinuation and establish a proper strategy to overcome fractures associated with it.
Mortality Rate and Safety of Denosumab in Patients
Subcutaneous administration of denosumab (60 mg every 6 months) is safe for human as the mortality rate is reduced or maintained at the same level as the placebo.25,27,35 A retrospective cohort study by Behanova et al reported that denosumab treatment (as well as bisphosphonate) significantly reduced the risk of mortality as compared to untreated patients.25 Subgroup analysis also identified that the mortality-reducing effect of denosumab was more prominent in men than women.25 Additionally, denosumab also significantly reduced the mortality rate among high-risk subjects based on prevalent vertebral fracture status with or without a low femoral neck BMD.35 Comparing with risedronate, there was a 1% increase in mortality rate upon denosumab treatment.44,45 However, statistical analysis was not performed.
In terms of the adverse reaction, denosumab did not significantly increase the total incidence of adverse effects including infection, cancer, hypocalcaemia, cardiovascular and peripheral vascular diseases, stroke, heart diseases. No cases of study discontinuation due to adverse events were reported.28 All and serious adverse events remained stable over time in both denosumab and placebo groups during DIRECT, FREEDOM and FREEDOM extension studies.27,30,31,41 Additionally, denosumab is also safe regardless of age groups, kidney function, fragility fracture history, as well as in patients with breast cancer treated with an aromatase inhibitor.30,35,36,40,48 No event of osteonecrosis or neutralising anti-denosumab antibodies was identified.27,28,40,81 Histological examination of 22 samples from the 10-year denosumab-treated group revealed that all lamellar bones were normally mineralized with no pathological changes and low remodelling activation.27
Denosumab, however, did slightly increase the incidence of certain adverse events including hypocalcaemia, bacterial cellulitis, infection, cardiovascular disorder, bone pain, pain in extremity, hot flush, hypertension, osteoarthritis and nervous system disorders in some subjects.40,41 Denosumab was also associated with a marginally higher incidence of eczema, flatulence and cellulitis among some FREEDOM subjects.28 These adverse events, however, became insignificant during the 10-year FREEDOM extension study.27 The adverse events and serious adverse events were found similar between denosumab and risedronate groups among glucocorticoid-treated subjects up to 24 months of treatment.44,45 Denosumab did marginally (~1%) increase the incidence of adverse events including back pain, hypertension, osteoporosis-related fractures and non-vertebral fractures compared to risedronate, but the statistical analysis was not performed.44
Conclusion
Denosumab prevents the binding of RANKL to RANK on osteoclasts, thereby preventing bone loss through resorption and lowering the fracture risk. Through the clinical trials conducted, especially FREEDOM and its extension, denosumab demonstrates efficacy in preventing vertebral and non-vertebral fractures even with 10 years of continuous treatment. The performance of denosumab is on par with other treatment agents for osteoporosis like bisphosphonates. It is also safe to be used among vulnerable patients with chronic kidney disease and breast cancer treated with an aromatase inhibitor. Its hypocalcemic effects may require the physicians to assess the calcium status of the patients before initiating treatment. Rebound bone loss and fracture risk after discontinuation is a concern, but this can be overcome by subsequent treatment with other agents like bisphosphonates. Since the efficacy of denosumab in preventing fracture is only assessed in limited studies, more comprehensive trials involving patients of diverse genetics and environmental exposure background are warranted.
Acknowledgments
The authors thank Universiti Kebangsaan Malaysia for funding them via Fundamental Research Grant (FF-2020-145). Dr. Kok-Lun Pang is a postdoctoral researcher funded by Universiti Kebangsaan Malaysia through Postdoctoral Research Scheme (RGA1).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chin KY, Kamaruddin AA, Low NY, Ima-Nirwana S. Effects of age, sex, and ethnicity on bone health status of the elderly in Kuala Lumpur, Malaysia. Clin Interventions Aging. 2016;11:767–773. doi: 10.2147/CIA.S108772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. Belgium May: Brussels; 2004:5–7. [Google Scholar]
- 3.Blain H, Masud T, Dargent-Molina P, et al. A comprehensive fracture prevention strategy in older adults: the European Union Geriatric Medicine Society (EUGMS) statement. J Nutr Health Aging. 2016;20(6):647–652. doi: 10.1007/s12603-016-0741-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rau CS, Lin TS, Wu SC, et al. Geriatric hospitalizations in fall-related injuries. Scandinavian J Trauma, Resuscitation Emergency Med. 2014;22:63. doi: 10.1186/s13049-014-0063-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matityahu A, Elson J, Morshed S, Marmor M. Survivorship and severe complications are worse for octogenarians and elderly patients with pelvis fractures as compared to adults: data from the national trauma data bank. J Osteoporos. 2012;2012:475739. doi: 10.1155/2012/475739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong T, Kantachuvesiri P, Sahota O, Gladman JRF. Characteristics and outcomes of hospitalized patients with vertebral fragility fractures: a systematic review. Age Ageing. 2018;47(1):17–25. doi: 10.1093/ageing/afx079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer K, Schwarzkopf L, Graessel E, Holle R. A claims data-based comparison of comorbidity in individuals with and without dementia. BMC Genetics. 2014;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhavan MV, Gersh BJ, Alexander KP, Granger CB, Stone GW. Coronary artery disease in patients >/=80 years of age. J Am Coll Cardiol. 2018;71(18):2015–2040. doi: 10.1016/j.jacc.2017.12.068 [DOI] [PubMed] [Google Scholar]
- 9.Nazrun AS, Tzar MN, Mokhtar SA, Mohamed IN. A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: morbidity, subsequent fractures, and mortality. Therapeutics Clin Risk Management. 2014;10:937–948. doi: 10.2147/TCRM.S72456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo J-I, Ha Y-C, Park KS, Kim R-B, Seo S-H, Koo K-H. Incidence and mortality of osteoporotic refractures in korea according to nationwide claims data. Yonsei Med J. 2019;60(10):969–975. doi: 10.3349/ymj.2019.60.10.969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unnanuntana A, Gladnick BP, Donnelly E, Lane JM. The assessment of fracture risk. J Bone Joint Surgery. American Volume. 2010;92(3):743–753. doi: 10.2106/JBJS.I.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YC, Lin WC. Poor 1st-year adherence to anti-osteoporotic therapy increases the risk of mortality in patients with magnetic resonance imaging-proven acute osteoporotic vertebral fractures. Patient Preference Adherence. 2017;11:839–843. doi: 10.2147/PPA.S131564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lems WF, Raterman HG. Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Therapeutic Adv Musculoskeletal Disease. 2017;9(12):299–316. doi: 10.1177/1759720X17732562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Bergh JP, van Geel TA, Geusens PP. Osteoporosis, frailty and fracture: implications for case finding and therapy. Nature Reviews. Rheumatology. 2012;8(3):163–172. doi: 10.1038/nrrheum.2011.217 [DOI] [PubMed] [Google Scholar]
- 16.Kostenuik PJ, Nguyen HQ, McCabe J, et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Mineral Res. 2009;24(2):182–195. doi: 10.1359/jbmr.081112 [DOI] [PubMed] [Google Scholar]
- 17.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocrine Reviews. 2008;29(2):155–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewiecki EM, Bilezikian JP. Denosumab for the treatment of osteoporosis and cancer-related conditions. Clin Pharmacology Therapeutics. 2012;91(1):123–133. doi: 10.1038/clpt.2011.268 [DOI] [PubMed] [Google Scholar]
- 19.Sanchez A, Brun LR, Salerni H, et al. Effect of denosumab on bone mineral density and markers of bone turnover among postmenopausal women with osteoporosis. J Osteoporos. 2016;2016:8738959. doi: 10.1155/2016/8738959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeks ED. Denosumab: a review in postmenopausal osteoporosis. Drugs Aging. 2018;35(2):163–173. doi: 10.1007/s40266-018-0525-7 [DOI] [PubMed] [Google Scholar]
- 21.Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 22.Yanbeiy ZA, Hansen KE. Denosumab in the treatment of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. Drug Design, Development Therapy. 2019;13:2843–2852. doi: 10.2147/DDDT.S148654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bridgeman MB, Pathak R. Denosumab for the reduction of bone loss in postmenopausal osteoporosis: a review. Clin Therapeutics. 2011;33(11):1547–1559. doi: 10.1016/j.clinthera.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 24.Wensel TM, Iranikhah MM, Wilborn TW. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. Pharmacotherapy. 2011;31(5):510–523. doi: 10.1592/phco.31.5.510 [DOI] [PubMed] [Google Scholar]
- 25.Behanova M, Reichardt B, Stamm TA, Zwerina J, Klaushofer K, Kocijan R. Treatment effects of bisphosphonates and denosumab on survival and refracture from real-world data of hip-fractured patients. Calcif Tissue Int. 2019;105(6):630–641. doi: 10.1007/s00223-019-00611-3 [DOI] [PubMed] [Google Scholar]
- 26.Pedersen AB, Heide-Jorgensen U, Sorensen HT, Prieto-Alhambra D, Ehrenstein V. Comparison of risk of osteoporotic fracture in denosumab vs alendronate treatment within 3 years of initiation. JAMA Network Open. 2019;2(4):e192416. doi: 10.1001/jamanetworkopen.2019.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomized FREEDOM trial and open-label extension. Lancet Diab Endocrinol. 2017;5(7):513–523. doi: 10.1016/S2213-8587(17)30138-9 [DOI] [PubMed] [Google Scholar]
- 28.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. New England J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493 [DOI] [PubMed] [Google Scholar]
- 29.Ferrari S, Adachi JD, Lippuner K, et al. Further reductions in non-vertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years. Osteoporosis Int. 2015;26(12):2763–2771. doi: 10.1007/s00198-015-3179-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Mineral Res. 2012;27(3):694–701. doi: 10.1002/jbmr.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papapoulos S, Lippuner K, Roux C, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporosis Int. 2015;26(12):2773–2783. doi: 10.1007/s00198-015-3234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilezikian JP, Lin CJF, Brown JP, et al. Long-term denosumab treatment restores cortical bone loss and reduces fracture risk at the forearm and humerus: analyses from the FREEDOM Extension cross-over group. Osteoporosis Int. 2019;30(9):1855–1864. doi: 10.1007/s00198-019-05020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendler DL, Chines A, Brandi ML, et al. The risk of subsequent osteoporotic fractures is decreased in subjects experiencing fracture while on denosumab: results from the FREEDOM and FREEDOM Extension studies. Osteoporosis Int. 2019;30(1):71–78. doi: 10.1007/s00198-018-4687-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austin M, Yang YC, Vittinghoff E, et al. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and non-vertebral fractures. J Bone Mineral Res. 2012;27(3):687–693. doi: 10.1002/jbmr.1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metabolism. 2011;96(6):1727–1736. doi: 10.1210/jc.2010-2784 [DOI] [PubMed] [Google Scholar]
- 36.Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Mineral Res. 2011;26(8):1829–1835. doi: 10.1002/jbmr.403 [DOI] [PubMed] [Google Scholar]
- 37.McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Mineral Res. 2012;27(7):1480–1486. doi: 10.1002/jbmr.1606 [DOI] [PubMed] [Google Scholar]
- 38.McClung MR, Boonen S, Torring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Mineral Res. 2012;27(1):211–218. doi: 10.1002/jbmr.536 [DOI] [PubMed] [Google Scholar]
- 39.Simon JA, Recknor C, Moffett AH Jr, et al. Impact of denosumab on the peripheral skeleton of postmenopausal women with osteoporosis: bone density, mass, and strength of the radius, and wrist fracture. Menopause. 2013;20(2):130–137. doi: 10.1097/GME.0b013e318267f909 [DOI] [PubMed] [Google Scholar]
- 40.Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomized, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. doi: 10.1016/S0140-6736(15)60995-3 [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Matsumoto T, Sugimoto T, et al. Clinical trials express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metabolism. 2014;99(7):2599–2607. doi: 10.1210/jc.2013-4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. New England J Med. 2016;375(16):1532–1543. doi: 10.1056/NEJMoa1607948 [DOI] [PubMed] [Google Scholar]
- 43.Miyauchi A, Dinavahi RV, Crittenden DB, et al. Increased bone mineral density for 1 year of romosozumab, vs placebo, followed by 2 years of denosumab in the Japanese subgroup of the pivotal FRAME trial and extension. Arch Osteoporos. 2019;14(1):59. doi: 10.1007/s11657-019-0608-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomized, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diab Endocrinol. 2018;6(6):445–454. doi: 10.1016/S2213-8587(18)30075-5 [DOI] [PubMed] [Google Scholar]
- 45.Saag KG, Pannacciulli N, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol. 2019;71(7):1174–1184. doi: 10.1002/art.40874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell AD, Bell BR. The FREEDOM trial is family medicine ready for biologic therapies? Canadian Family Physician • Le Médecin De Famille Canadien. 2011;57:438–441. [PMC free article] [PubMed] [Google Scholar]
- 47.ClinicalTrials.gov. A study to evaluate denosumab in the treatment of postmenopausal osteoporosis; 2020. Available from: https://clinicaltrials.gov/ct2/show/record/NCT00089791. Accessed March9, 2020.
- 48.Palacios S, Kalouche-Khalil L, Rizzoli R, et al. Treatment with denosumab reduces secondary fracture risk in women with postmenopausal osteoporosis. Climacteric. 2015;18(6):805–812. doi: 10.3109/13697137.2015.1045484 [DOI] [PubMed] [Google Scholar]
- 49.Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ. Teriparatide for osteoporosis: importance of the full course. Osteoporosis Int. 2016;27(8):2395–2410. doi: 10.1007/s00198-016-3534-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dempster DW, Zhou H, Recker RR, et al. Differential effects of teriparatide and denosumab on intact PTH and bone formation indices: AVA osteoporosis study. J Clin Endocrinol Metab. 2016;101(4):1353–1363. doi: 10.1210/jc.2015-4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng J, Silver Z, Huang E, et al. Pharmacological prevention of fractures in patients undergoing glucocorticoid therapies: a systematic review and network meta-analysis. Rheumatology. 2020. doi: 10.1093/rheumatology/keaa228 [DOI] [PubMed] [Google Scholar]
- 52.Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomized trial. Lancet. 2013;382(9886):50–56. doi: 10.1016/S0140-6736(13)60856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–1700. doi: 10.1210/jc.2013-4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH (1-34)] are dependent on duration of treatment and dose. Toxicologic Pathology. 2004;32(4):426–438. doi: 10.1080/01926230490462138 [DOI] [PubMed] [Google Scholar]
- 55.Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicologic Pathology. 2002;30(3):312–321. doi: 10.1080/01926230252929882 [DOI] [PubMed] [Google Scholar]
- 56.Cosman F, Nieves JW, Zion M, et al. Daily or cyclical teriparatide treatment in women with osteoporosis on no prior therapy and women on alendronate. J Clin Endocrinol Metab. 2015;100(7):2769–2776. doi: 10.1210/jc.2015-1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cosman F, McMahon D, Dempster D, Nieves JW. Standard versus cyclic teriparatide and denosumab treatment for osteoporosis: a randomized trial. J Bone Mineral Res. 2020;35(2):219–225. doi: 10.1002/jbmr.3850 [DOI] [PubMed] [Google Scholar]
- 58.Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomized controlled trial. Lancet. 2015;386(9999):1147–1155. doi: 10.1016/S0140-6736(15)61120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai JN, Jiang LA, Lee H, Hans D, Leder BZ. Effects of teriparatide, denosumab, or both on spine trabecular microarchitecture in DATA-switch: a randomized controlled trial. J Clin Densitometry. 2017;20(4):507–512. doi: 10.1016/j.jocd.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guanabens N, Moro-Alvarez MJ, Casado E, et al. The next step after anti-osteoporotic drug discontinuation: an up-to-date review of sequential treatment. Endocrine. 2019;64(3):441–455. doi: 10.1007/s12020-019-01919-8 [DOI] [PubMed] [Google Scholar]
- 61.Iranikhah M, Deas C, Murphy P, Freeman MK. Effects of denosumab after treatment discontinuation: A review of the literature. Consultant Pharmacist. 2018;33(3):142–151. doi: 10.4140/TCP.n.2018.142 [DOI] [PubMed] [Google Scholar]
- 62.Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–980. doi: 10.1210/jc.2010-1502 [DOI] [PubMed] [Google Scholar]
- 63.Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: A post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Mineral Res. 2017;33(2):190–198. doi: 10.1002/jbmr.3337 [DOI] [PubMed] [Google Scholar]
- 64.McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM. Observations following discontinuation of long-term denosumab therapy. Osteoporosis Int. 2017;28(5):1723–1732. doi: 10.1007/s00198-017-3919-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sosa Henriquez M, Gómez de Tejada Romero MJ, Escudero-Socorro M, et al. Hip fractures following denosumab discontinuation: three clinical cases reports. J Royal Soc Med. 2019;112(11):472–475. doi: 10.1177/0141076819861027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tripto-Shkolnik L, Fund N, Rouach V, Chodick G, Shalev V, Goldshtein I. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150. doi: 10.1016/j.bone.2019.115150 [DOI] [PubMed] [Google Scholar]
- 67.Miller PD, Wagman RB, Peacock M, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: six-year results of a Phase 2 clinical trial. J Clin Endocrinol Metabolism. 2011;96(2):394–402. doi: 10.1210/jc.2010-1805 [DOI] [PubMed] [Google Scholar]
- 68.Anastasilakis AD, Evangelatos G, Makras P, Iliopoulos A. Rebound-associated vertebral fractures may occur in sequential time points following denosumab discontinuation: need for prompt treatment re-initiation. Bone Reports. 2020;12:100267. doi: 10.1016/j.bonr.2020.100267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonzalez-Rodriguez E, Aubry-Rozier B, Stoll D, Zaman K, Lamy O. Sixty spontaneous vertebral fractures after denosumab discontinuation in 15 women with early-stage breast cancer under aromatase inhibitors. Breast Cancer Res Treatment. 2020;179(1):153–159. doi: 10.1007/s10549-019-05458-8 [DOI] [PubMed] [Google Scholar]
- 70.Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A. Second rebound-associated vertebral fractures after denosumab discontinuation. Arch Osteoporos. 2020;15(1):7. doi: 10.1007/s11657-019-0676-0 [DOI] [PubMed] [Google Scholar]
- 71.Anastasilakis AD, Makras P. Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporosis Int. 2016;27(5):1929–1930. doi: 10.1007/s00198-015-3459-5 [DOI] [PubMed] [Google Scholar]
- 72.Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Mineral Res. 2017;32(6):1291–1296. doi: 10.1002/jbmr.3110 [DOI] [PubMed] [Google Scholar]
- 73.Anastasilakis AD, Yavropoulou MP, Makras P, et al. Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. European J Endocrinol. 2017;176(6):677–683. doi: 10.1530/EJE-16-1027 [DOI] [PubMed] [Google Scholar]
- 74.Fassio A, Adami G, Benini C, et al. Changes in Dkk-1, sclerostin, and RANKL serum levels following discontinuation of long-term denosumab treatment in postmenopausal women. Bone. 2019;123:191–195. doi: 10.1016/j.bone.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 75.Jahn-Rickert K, Wolfel EM, Jobke B, et al. Elevated bone hardness under denosumab treatment, with persisting lower osteocyte viability during discontinuation. Front Endocrinol. 2020;11:250. doi: 10.3389/fendo.2020.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–229. doi: 10.1016/j.bone.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 77.Laroche M, Couture G, Ruyssen-Witrand A, Constantin A, Degboe Y. Effect of risedronate on bone loss at discontinuation of denosumab. Bone Reports. 2020;13:100290. doi: 10.1016/j.bonr.2020.100290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adami G, Giollo A, Rossini M, et al. Different fracture risk profile in patients treated with anti-osteoporotic drugs in real-life. Reumatismo. 2020;72(2):71–74. doi: 10.4081/reumatismo.2020.1267 [DOI] [PubMed] [Google Scholar]
- 79.Kendler D, Chines A, Clark P, et al. Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab. 2020;105:3. doi: 10.1210/clinem/dgz095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uebelhart B, Rizzoli R, Ferrari SL. Retrospective evaluation of serum CTX levels after denosumab discontinuation in patients with or without prior exposure to bisphosphonates. Osteoporosis Int. 2017;28(9):2701–2705. doi: 10.1007/s00198-017-4080-6 [DOI] [PubMed] [Google Scholar]
- 81.Wilcken N, Inkyung Lee C. In postmenopausal women with breast cancer treated with aromatase inhibitors, denosumab reduced fractures. Annals of Internal Medicine. 2015;163(6):Jc11. doi: 10.7326/ACPJC-2015-163-6-011 [DOI] [PubMed] [Google Scholar]