Abstract
Small mammals are often parasitized by the immature stages of hard-bodied ticks (family Ixodidae) and may serve as reservoir hosts of tick-borne pathogens. Amblyomma maculatum, the Gulf Coast tick, is the primary vector of Rickettsia parkeri, the causative agent of R. parkeri rickettsiosis. This hard-bodied tick species is expanding its historical range from the Gulf Coast of the U.S. up the Mid-Atlantic coast. In Mid-Atlantic states, such as Virginia, R. parkeri prevalence is higher in these ticks than those found in its historical range. This high prevalence may be explained in part by small mammal populations. In this study, small mammals were trapped and checked for the presence of immature A. maculatum. The ticks as well as tissue samples from these mammals were tested for the presence of R. parkeri. This study found six rodent species acting as hosts to immature A. maculatum and three species that may play a role in the enzootic cycle of R. parkeri in Virginia.
Keywords: Amblyomma maculatum, Rickettsia parkeri, hosts, rodents, immature, enzootic cycle
Introduction
The Amblyomma maculatum species complex encompasses a group of hard-bodied tick species that have medical and veterinary importance in the Gulf and Atlantic regions of the United States (Sumner et al., 2007) and in over 10 countries in Central and South America (Santos Dias, 1993; Guglielmone et al., 2006). This species complex was originally comprised of 7 major species: A. maculatum, A. triste, A. tigrinum, A. neumanni, A. parvitarsum, A. ovale, and A. aureolatum (Camicas et al., 1998; Santos Dias, 1963; Santos Dias, 1993), all of which are known to bite humans and domestic livestock. Subsequently this species complex was redefined to encompass only 3 lineages: A. maculatum, A. triste, and A. tigrinum (Estrada-Pena et al., 2005); more recently it was suggested that A. maculatum and A. triste be synonymized Lado et al., 2018). Amblyomma maculatum, A. triste, and A. tigrinum are known vectors of Rickettsia parkeri sensu stricto, the causative agent of Rickettsia parkeri rickettsiosis (Paddock et al., 2004; Nieri-Bastos et al., 2018). The other Amblyomma species (except A. neumanni) can harbor different strains of R. parkeri that may or may not be pathogens capable of causing a human rickettsiosis (Nieri-Bastos et al., 2018). Other important veterinary pathogens associated with these Amblyomma species include Hepatozoon americanum (Ewing and Panciera, 2003) and Ehrlichia ruminantium (Mahan et al., 2000).
In the U.S., A. maculatum has recently expanded geographically from its historical range in the southern states of the Gulf Coast into North Carolina and other Mid-Atlantic states, including Virginia, Maryland, and Delaware (Sumner et al., 2007; Wright et al., 2011; Fornadel et al., 2011; Varela-Stokes et al., 2011; Jiang et al., 2012; Florin et al., 2014). This range expansion has not only resulted in intermittent populations of A. maculatum in these states, but also the introduction of R. parkeri to these areas (Nadolny and Gaff, 2018).
For humans and large domestic animals, R. parkeri infection usually follows the bite of an infected adult tick (Teel et al., 2010). Infected populations of adult A. maculatum in their historical range show a prevalence of R. parkeri ranging from ~20%-40% (Sumner et al., 2007, Nadolny et al., 2014; Mays et al., 2016); however, in southeastern Virginia, R. parkeri prevalence can reach upward of 60% at individual sites (Wright et al., 2011; Varela-Stokes et al., 2011; Fornadel et al., 2011; Nadolny et al., 2014; Wright, 2015). Because of this high pathogen prevalence, A. maculatum is of particular medical importance in the Mid-Atlantic region.
Although first reported in Virginia as early as 1898 (Cooley and Kohls, 1944), established populations of A. maculatum have only been studied since 2010 (Fornadel et al., 2011; Nadolny and Gaff, 2018). These populations could be considered transitory or fragmented because the introduction, establishment, and die off usually occurs in less than five years (Nadolny and Gaff, 2018). Habitat and host community structure could play a role in this phenomenon. Small mammals are abundant in early secondary successional habitat, but once the habitat transitions to later stages of succession with more woody plant species, small mammal abundance and diversity decreases (Rose et al., 2018). One possible reason for the decline in A. maculatum populations may be the necessity for specific rodent hosts, which disappear with the loss of herbaceous vegetation (Nadolny and Gaff, 2018). In many natural systems involving rickettsial pathogens, small mammals living in early successional habitat are often the primary hosts for immature arthropods, including ticks (Azad and Beard, 1998); such small mammals could be the primary hosts for immature A. maculatum in Virginia.
Collection of immature A. maculatum from vegetation or secondary successional environments is extremely difficult (Portugal and Goddard, 2015; Nadolny and Gaff, 2018). The most reliable methods for collecting A. maculatum species complex ticks are host-targeted techniques such as small mammal trapping (Barker et al., 2004; Portugal and Goddard, 2015; Colombo et al., 2018) or avian sampling (Teel et al., 1998; Gonzalez-Acuña et al., 2004; Moraru et al., 2012; Colombo et al., 2018).
The recorded small mammal hosts for immature A. maculatum in South Carolina (Clark et al., 1998; Clark et al., 2001), northwestern Florida (Durden et al., 2000), Mississippi (Moraru et al., 2013), and western Tennessee (Mays et al., 2016) are from two subfamilies: Sigmodontinae (includes hispid cotton rats and marsh rice rats) and Neotominae (includes woodrats, white-footed mice, and cotton mice). Overall, the studies have resulted in the collection of very few immature A. maculatum from small mammals, the exception being the South Carolina study where 179 A. maculatum larvae and 29 A. maculatum nymphs were collected over a one-year period; however, 116 of these larvae came from one rodent (Clark et al., 1998).
The goal of our study was to identify small mammal hosts of immature A. maculatum in southeastern Virginia, and to identify hosts that could be reservoirs or amplifying hosts of R. parkeri and to provide information for future studies focusing on the interactions between the pathogen, the tick, and these small mammal species.
Materials and methods
Small Mammal Live Trapping
Modified Fitch live traps (Rose, 1994) were set along pre-marked transects at 13 trap sites (Figure 1) across southeastern Virginia from 2011-2018. The traps were baited with a mixture of birdseed and sunflower seeds and supplemented with mealworms when insectivore populations were high. Polyester fiberfill was added to traps when nighttime temperatures had the potential to go below freezing. Traps were set in the evenings and checked every morning for a 2-4 day period per trapping session. Trapping sessions were conducted monthly or seasonally depending on the sampling year. Species, sex, weight, and reproductive condition of trapped mammals were recorded, and each small mammal was given an individually numbered metal ear tag. All small mammal handling was conducted in accordance with ODU IACUC permit #11-012, 16-003, 17-006 using guidelines set forth by the American Society of Mammalogists (Sikes et al., 2016). Additionally, a number of the small mammals from 2017 in our study were donated by local pest control companies operating in southeastern Virginia.
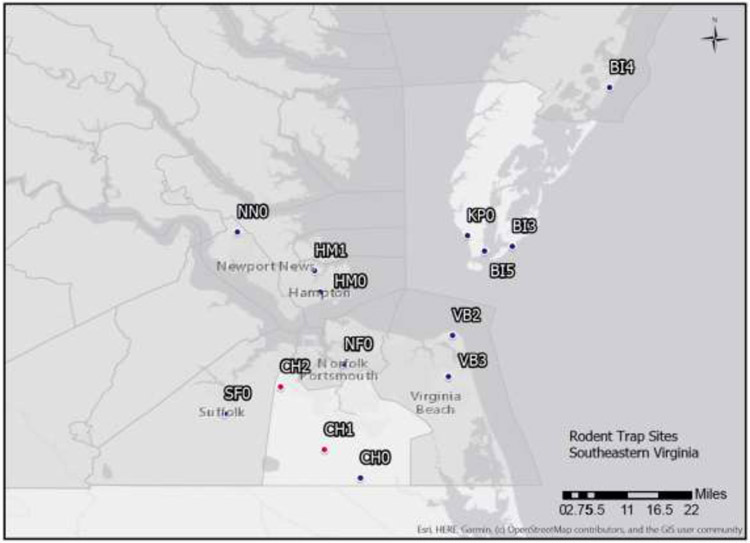
Figure 1.
Map of small mammal live-trapping sites in southeastern Virginia. Long-term monthly surveillance was done at CH1 and CH2, which area located within the City of Chesapeake. Trapping was also done at HM1 in the City of Hampton and SF0 in the City of Suffolk. Additional samples were donated from other contracted work from locations in the City of Newport News (NN0), the City of Hampton (HM0), the City of Virginia Beach (VB2 and VB3), the City of Chesapeake (CH0), in Northampton County (KP0, BI3, BI5), and in Accomack County (BI4).
Tick and Tissue Sampling
Trapped, small mammals were ear-tagged and given a full-body examination for ticks, with specific attention given to the face and ears. Ticks were removed and placed in a vial with a label corresponding to the mammal’s ear tag number. A 2 mm ear punch taken from the other ear and placed in the same vial as the ticks, if ticks were found attached to the host, or in its own vial if no ticks were present. The forceps and ear punch tool were cleaned with an alcohol swab after each use. All vials were transported back to the lab at ambient temperature then stored at −20°C for future processing.
Tick and Tissue Extraction
DNA from ticks and associated mammal tissues was extracted using the GeneJet Genomic DNA Purification Kit (ThermoFisher Scientific, Pittsburgh, PA) following the manufacturer’s instructions. Immature ticks were extracted whole following an initial pulverization using approximately the same volume (as the tick) of 1mm glass beads and one 5mm glass bead in a Mini Beadbeater (BioSpec, Inc. Bartlesville, OK, USA) to break apart the hard tick cuticle. After pulverization, there was an initial digestion in 180 μL of digestion buffer and 20 μL of Proteinase K at 57 °C overnight. DNA was eluted from columns in 100 μL of elution buffer. A subset of 183 ticks, collected prior to 2015 and used in a previous study identifying ticks on small mammals, were extracted using the DNeasy Blood and Tissue Extraction Kit (Qiagen, Valencia, CA) and DNA was eluted in 200 μl of elution buffer.
Tick Identification
All ticks collected from rodents were immature life stages (larvae or nymphs), and thus could not be easily identified morphologically because of engorgement or distortion that occurred during removal. Immature A. maculatum were identified by real-time PCR that amplified a variable region in the ITS2 gene of the tick genome (Table 1). Amblyomma maculatum were additionally confirmed using a real-time PCR assay based on the A. maculatum actin gene developed in this study (Table 1).
Table 1.
Primer pairs and probes used for tick and pathogen identification.
| Primer/Probe | Sequence (5’-3’) | Gene target |
Amplico n length (bp) |
Referenc e |
|---|---|---|---|---|
| AmacITS2F | TTGTGCGGGAAACGACCGGGTGT | ITS2 | 193 | Zemtsova et al., 2014 |
| AmacITS2R | AACGCTCGTAACGAGATACGCG | Zemtsova et al., 2014 | ||
| AmacITS2Pr | /56-FAM/ACAATGCTTGAGCAGA+G+AGAC/3IABkFQ/ | Zemtsova et al., 2014 | ||
| Amac_actin_F | GCCCTGGACTTCGAGCAG | Actin | 74 | This study |
| Amac_actin_R 2 |
CCCGTCAGGAAGTTCGTAGG | This study |
||
| Amac_actin_P r2 |
/5HEX/ACCGCCGCCT/ZEN/CGTCCTCCTC/3IABkFQ/ | This study |
||
| 16S−1 | GTCTGAACTCAGATCAAGT | 16S rDN A |
454 | de la Fuente et al., 2001 |
| 16S+1 | CTGCTCAATGATTTTTTAAATTGCTGT | Nadolny et al., 2011 | ||
| Rpa129F | CAAATGTTGCAGTTCCTCTAAATG |
omp B |
96 | Jiang et al., 2012 |
| Rpa224R | AAAACAAACCGTTAAAACTACCG | Jiang et al., 2012 | ||
| Rpa188Probe | /56- FAM/CGCGAAATTAATACCCTTATGAGCAGCAGTCGCG /BHQ-1/ |
Jiang et al., 2012 | ||
| RR190.70 (primary) |
ATGGCGAATATTTCTCCAAAA |
omp A |
590 | Blair et al., 2004 |
| RR190.701 | GTTCCGTTAATGGCAGCATCT | Blair et al., 2004 | ||
| RR190.70 (nested) | ATGGCGAATATTTCTCCAAAA | 540 | Regnery et al., 1991 | |
| RR190.622n | AGTGCAGCATTCGCTCCCCCT | Regnery et al., 1991 |
A 74 bp fragment was amplified in a 20 μL reaction composed of 10 μL 2X EconoTaq PLUS (Lugien Corp., Middleton, WI), 1 μL of each A. maculatum actin primer (10 μM), 0.5 μL of A. maculatum actin probe (10 μM), and 5 μL of extracted DNA. Thermocycler conditions for the actin assay consisted of 95°C for 3 min, followed by 40 cycles of of 95°C for 10 s and 60°C for 45 s. The A. maculatum actin assay primers and probe were created and modified based on the Ixodes actin assay developed by Graham et al. (2016). Other common tick species from our area, including I. scapularis, A. americanum, and D. variabilis controls, were used to determine accuracy of the assay for detecting only A. maculatum1 DNA. Twelve samples, representing ticks from different years, were confirmed by sequencing the tick mitochondrial 16S rRNA gene using the 16S+1 and 16S−1 primers (Table 1).
Pathogen Testing
All immature A. maculatum (collected 2011-2018) and mammal tissues (collected 2015-2018) were tested for R. parkeri using a real-time PCR assay that amplifies a fragment of the ompB gene using the Rpa129F and Rpa224R primers (Table 1). All R. parkeri-positive ticks and a total of six randomly selected R. parkeri-positive tissues taken from each year were confirmed by sequencing a portion of the ompA gene using the RR190.70, RR190.701, and RR190.622n primers (Table 1). Mammal tissue extracts were concentrated as needed by ethanol precipitation before ompA amplification when DNA concentration was low as determined by a C(q) value between 34 and 38 with a good peak from the real-time assay. The ethanol precipitation was performed by adding 0.10 times the sample volume (25 μL of DNA) of 3M sodium acetate and 2.5 volumes of 100% ethanol, followed by centrifugation at 13.2 x g for 20 min, and resuspend in 10 μL of water. Sequences were initially aligned and analyzed in Geneious (Biomatters, NZ, https://www.geneious.com/) with sequence identification determined using NCBI BLAST (http://blast.ncbi.nlm.nih.gov).
Results
During our study, we collected 1,486 ticks (490 of which were Ixodes spp.) from 833 rodents and 217 shrews (Blarina spp.); shrews were only parasitized by Ixodes ticks. The 833 rodents included: 226 hispid cotton rats (Sigmodon hispidus), 16 golden mice (Ochrotomys nuttalli), 79 house mice (Mus musculus), 76 eastern harvest mice (Reithrodontomys humulis), 164 marsh rice rats (Oryzomys palustris), 182 meadow voles (Microtus pennsylvanicus), 68 white-footed mice (Peromyscus leucopus), 9 pine voles (Pitymys pinetorum), 9 black rats (Rattus rattus), 1 Eastern flying squirrel (Glaucomys sabrinus), and 3 small mammals that were not identified due to age or body condition.. Of these small mammals, 37% (n=315) had ticks with 7% (n=22) parasitized by immature A. maculatum. The majority of immature ticks collected in this study were Dermacentor variabilis, the American dog tick (n=965 ticks), but 31 immature A. maculatum (16 larvae and 15 nymphs) were collected No adult A. maculatum were found on small mammals in this study, and no A. maculatum of any life stage were found on shrews.
Six rodent species were parasitized by immature A. maculatum including 12 hispid cotton rats, 1 golden mouse, 3 house mice, 1 eastern harvest mouse, 1 marsh rice rat, and 4 meadow voles (Table 2). No immature A. maculatum were found on any white-footed mouse, pine vole, black rat, or Eastern flying squirrel. The prevalence of immature A. maculatum for each small mammal species by tick life stage was determined by dividing the number of ticks per life stage by the number of hosts captured per species (Table 3). The golden mouse, eastern harvest mouse, and meadow vole were parasitized by only A. maculatum nymphs, whereas the house mouse and marsh rice rats only by A. maculatum larvae. Hispid cotton rats were parasitized by both immature A. maculatum life stages; most had only nymphs or larvae, but one rat had both.
Table 2.
Small mammal live trapping results by rodent species including numbers of rodents captured with R. parkeri-infected and un-infected immature A. maculatum in southeastern Virginia collected from 2011 to 2018.
| Rodent species | Scientific name | Total rodents caught |
Total rodents
with Dermacentor and Amblyomma ticks |
Total rodents with A. maculatum |
Total rodents with
R. parkeri-infected A. maculatum |
|---|---|---|---|---|---|
| Hispid cotton rat | Sigmodon hispidus | 226 | 97 | 12 | 5 |
| Golden mouse | Ochrotomys nuttalli | 16 | 8 | 1 | 0 |
| House mouse | Mus musculus | 79 | 13 | 3 | 0 |
| Eastern harvest mouse | Reithrodontomys humulis | 76 | 16 | 1 | 0 |
| Marsh rice rat | Oryzomys palustris | 164 | 59 | 1 | 1 |
| Meadow vole | Microtus pennsylvanicus | 182 | 81 | 4 | 2 |
| White-footed mouse | Peromyscus leucopus | 68 | 29 | 0 | 0 |
| Pine vole | Microtus pinetorum | 9 | 8 | 0 | 0 |
| Black rat | Rattus rattus | 9 | 1 | 0 | 0 |
| Eastern flying squirrel | Glaucomys sabrinus | 1 | 0 | 0 | 0 |
Table 3.
Immature A. maculatum collected from rodents sampled 2011-2018. The number of ticks collected from all rodents is shown in the second column and is listed by tick life stage. The percent of the rodents that represents is shown in the third column along with the total number of rodents of that species that were examined. All small mammal species not listed did not have any A. maculatum.
| Rodent
species Common name |
Life
stage (Number of ticks) |
Percentage parasitized (Number of rodents examined) |
|---|---|---|
| Hispid cotton rat | Larva (8) | 3.5% (226) |
| Nymph (7) | 3.1% (226) | |
| Golden mouse | Larva (0) | 0 (16) |
| Nymph (1) | 6% (16) | |
| House mouse | Larva (6) | 8% (79) |
| Nymph (0) | 0 (79) | |
| Eastern harvest mouse | Larva (0) | 0 (76) |
| Nymph (3) | 4% (76) | |
| Marsh rice rat | Larva (2) | 1.2% (164) |
| Nymph (0) | 0 (164) | |
| Meadow vole | Larva (0) | 0 (179) |
| Nymph (4) | 2.2% (179) |
Of the 31 immature A. maculatum collected from small mammals, 8 (25.8%) were positive for R. parkeri. These were from three small mammal species: 5 hispid cotton rats, 1 marsh rice rat, and 2 meadow voles. The majority of infected immatures were nymphs (6 of the 8 ticks). All eight infected ticks were confirmed, by sequencing, to be R. parkeri; these were 99.6-100% identical over 448-546 bp to R. parkeri str. Portsmouth.
Rodent ear punches collected (108 samples) from 2015 to 2018 were tested for R. parkeri, regardless of whether immature A. maculatum were present. Ear punches from 17 animals were positive for R. parkeri by real-time PCR. Marsh rice rats were the dominant species found with R. parkeri-infected tissues with 12 positive rats out of 108 tested (11%). Rickettsia parkeri was also detected in the tissue in 1 of 34 white-footed mice, 2 of 17 house mice, 1 of 82 meadow voles, and 1 of 9 black rats. A subsample of each of these species was sequence-confirmed for the presence of R. parkeri. Six R. parkeri-positive tissue punches were amplified using a portion of the ompA gene and were 99.8-100% identical over 437-546 bp to R. parkeri str. Portsmouth. None of the 42 cotton rats nor the one pine vole tested were positive for R parkeri.
Most Rickettsia parkeri-positive ticks came from sites with known infected adult A. maculatum populations (CHS1, CHS2, BI3). The other site, where immature A. maculatum were detected on small mammals (VB3), was not flagged so no information is available regarding infected adult A. maculatum populations. Interestingly, two sites (HM1, NN0) that did not yield immature A. maculatum did have small mammals with R. parkeri-infected tissues (Table 4).
Table 4.
Number of immature A. maculatum, rodent tissues, and number infected with R. parkeri from trapping sites in southeastern Virginia
| Trapping site* |
Year | Number of A. maculatum
removed from rodents |
Life stage** |
Number of R.
parkeri infected A. maculatum |
Number of R.
parkeri infected rodent tissues |
|---|---|---|---|---|---|
| CH1 | 2011 | 4 | 1L, 3N | 1 | Not tested |
| CH1 | 2012 | 14 | 5L, 9N | 6 | Not tested |
| CH2 | 0 | N/A | 0 | Not tested | |
| VB2 | 0 | N/A | 0 | Not tested | |
| CH1 | 2013 | 0 | N/A | 0 | Not tested |
| CH2 | 7 | 4L, 3N | 1 | Not tested | |
| SF0 | 0 | N/A | 0 | Not tested | |
| KP0 | 0 | N/A | 0 | Not tested | |
| BI3 | 0 | N/A | 0 | Not tested | |
| CH1 | 2014 | 0 | N/A | 0 | Not tested |
| KP0 | 0 | N/A | 0 | Not tested | |
| HM1 | 0 | N/A | 0 | Not tested | |
| HM1 | 2015 | 0 | N/A | 0 | 0 |
| BI3 | 2 | 2L | 0 | 13 | |
| BI4 | 0 | N/A | 0 | 0 | |
| BI5 | 0 | N/A | 0 | 0 | |
| HM1 | 2016 | 0 | N/A | 0 | 0 |
| HM1 | 2017 | 0 | N/A | 0 | 1 |
| HM0 | 0 | N/A | 0 | 0 | |
| NN0 | 0 | N/A | 0 | 1 | |
| HM1 | 2018 | 0 | N/A | 0 | 0 |
| CH0 | 0 | N/A | 0 | 0 | |
| VB3 | 4 | 4L | 0 | 2 |
The acronyms for the trapping sites correspond to those given in Figure 1
L = larva and N = nymph.
Discussion
In most enzootic cycles involving rickettsial pathogens, small mammals play a critical role as either reservoirs or amplifying hosts (Azad and Beard, 1998). To understand vector-pathogen dynamics, it is important to identify the key mammalian hosts for immature A. maculatum, because this tick and its associated pathogen, R. parkeri, are expanding into the mid-Atlantic states. Our study has identified two species of rodents (the hispid cotton rat and marsh rice rat) as potential reservoirs or amplifying hosts for R. parkeri in the geographical expansion zone of A. maculatum. Further studies into the pathogen’s ecology should focus on an assessment of reservoir competency of these two species in controlled laboratory experiments.
In Central and South America, Amblyomma spp. are widespread, and are of particular interest because they transmit numerous rickettsial pathogens endemic to the area, including R. parkeri (Guglielmone et al., 2006). Studies of small mammal populations in Central and South America show that Amblyomma spp., specifically A. triste, have an affinity for feeding on Sigmodontine rodents (Guglielmone et al., 2011). This same preference is seen with A. maculatum collected from rodents in the U.S. (Clark et al., 1998; Clark et al., 2001; Durden et al., 2000; Moraru et al., 2013; Mays et al., 2016) where rodent species hosting immature A. maculatum were from two rodent subfamilies: Sigmodontinae (includes hispid cotton rats and marsh rice rats) and Neotominae (includes woodrats, white-footed mice, and cotton mice). Muroid rodents (including house mice and black rats), however were not found to act as hosts for immature A. maculatum.
The rodent sub-family harboring the majority of immature A. maculatum in our study was Sigmodontinae. Rickettsia parkeri-infected A. maculatum and R. parkeri-infected rodent tissues were collected from hispid cotton rats and marsh rice rats, suggesting that these rodents may play an important role as reservoirs or amplifying hosts of R. parkeri. Alternatively, these rodents may only be acting as hosts to immature infected A. maculatum ticks but not be systemically infected with R. parkeri. In addition, R. parkeri-infected A. maculatum were collected from two meadow voles (subfamily Arvicolinae). One meadow vole tissue sample tested positive for R. parkeri, which may be indicative of meadow voles as another important species in the ecological maintenance of R. parkeri. Additional studies are required to explore the relationship between immature A. maculatum and rodents from these subfamilies.
The anthropophilic house mouse yielded the highest infestation rate with immature A. maculatum (Table 3), suggesting that there may be potential hosts thriving in habitats close to human settlements where other successional (and native) species of rodents may be absent. We also found R. parkeri-infected tissues from a white-footed mouse and a black rat, even though neither of these species, in our study, harbored immature A. maculatum. It is possible that immature A. maculatum ticks had previously parasitized these animals and dropped off; the vector-pathogen dynamics of R. parkeri in these species warrants additional investigation.
Positive rodent tissues were collected at sites without a known established population of adult A. maculatum, suggesting A. maculatum is potentially present but not detected or the R. parkeri is coming from some other source. It is possible that other tick species, such as D. variabilis and/or A. americanum, could be playing cryptic roles in the enzootic cycle of R. parkeri. Although these tick species are not currently known to transmit R. parkeri to humans, they have been found to be naturally infected with R. parkeri in field studies (Cohen et al., 2009; Fornadel et al., 2011; Henning et al., 2014; Wright et al., 2015). Furthermore, there is laboratory evidence of their ability to acquire R. parkeri transovarially, maintain R. parkeri transtadially, and infect animals (Harris et al., 2017; Wright et al., 2015).
It is important to note the rarity of finding immature A. maculatum: only 31 immature A. maculatum were found on 833 rodents in 8 years of study. Continued studies at our sites in southeastern Virginia, as well as at other sites where A. maculatum populations become established, will provide insight into the hosts, life history and phenology of the immature stages of A. maculatum and a better understanding of the introduction and establishment of this tick species as it continues to expand its current range.
Acknowledgements
We acknowledge Amy Johnson, Alexis White, Marc Cahill, Jana Eggleston, and the many field assistants for their help with small mammal live trapping set-up and implementation. Special thanks to David Gauthier for creating primers and probe for the A. maculatum actin real time PCR assay developed in this study. Thanks to Kasey Parker, Zach Bement, and Anna Phan for their help in extracting rodent ticks and tissues. We also thank the Nature Conservancy and city of Suffolk for permission to use their land.
Funding
This work was funded in part by NIH grant 1R01AI136035 as part of the joint NIH-NSF-USDA Ecology and Evolution of Infectious Diseases program and in part by grant no. K25AI067791 (to H.D.G.) from the National Institute of Allergy and Infectious Diseases. RMN was supported by the Science, Mathematics and Research for Transformation (SMART) scholarship from the Department of Defense and the American Society for Engineering Education. This work also was supported in part by funding from NSF grants DEB-0621014 and DEB-1237733 to the University of Virginia.
Footnotes
Animal
This study was conducted under Old Dominion University IACUC Protocols 11-012, 16-003, and 17-006. Permits: VADGIF #041740, 047118, 053333.
Declaration of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azad AF, Beard CB, 1998. Rickettsial pathogens and their arthropod vectors. Emerg Infect Dis. 4, 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RW, Kocan AA, Ewing SA, Wettemann RP, Payton ME, 2004. Occurrence of the Gulf Coast tick (Acari: Ixodidae) on wild and domestic mammals in north-central Oklahoma. J Med Entomol. 41, 170–178. [DOI] [PubMed] [Google Scholar]
- Blair PJ, Jiang J, Schoeler GB, Moron C, Anaya E, Cespedes M, Cruz C, Felices V, Guevara C, Mendoza L, Villaseca P, Sumner JW, Richards AL, Olson JG, 2004. Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J Clin Microbiol. 42, 4961–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camicas JL, Hervy JP, Adam F and Morel PC, 1998. Les tiques du monde Nomenclature, stades décrits, hôtes, répartition (Acarida, Ixodida). Paris: Orstrom, 233. [Google Scholar]
- Clark KL, Oliver JH, McKechnie DB, Williams DC, 1998. Distribution, abundance, and seasonal activities of ticks collected from rodents and vegetation in South Carolina. J Vector Ecol. 23, 89–105. [PubMed] [Google Scholar]
- Clark KL, Oliver JH, Grego JM, James AM, Durden LA, Banks CW, 2001. Host associations of ticks parasitizing rodents at Borrelia burgdorferi enzootic sites in South Carolina. J Parasitol. 87, 1379–1386. [DOI] [PubMed] [Google Scholar]
- Colombo VC, Fasano AA, Beldomenico PM, Nava S, 2018. Tick host specificity: An analysis based on host phylogeny and tick ecological features using Amblyomma triste and Amblyomma tigrinum immature stages. Ticks Tick Borne Dis. 9, 781–787. [DOI] [PubMed] [Google Scholar]
- Cooley RA, Kohls GM, 1944. The genus Amblyomma (Ixodidae) in the United States. J Parasitol. 30, 77–111. [Google Scholar]
- de la Fuente J, Van Den Bussche RA, Kocan KM, 2001. Molecular phylogeny and biogeography of North American isolates of Anaplasma marginale (Rickettsiaceae : Ehrlichieae). Vet Parasitol. 97, 65–76. [DOI] [PubMed] [Google Scholar]
- Durden LA, Hu R, Oliver JH, Cilek JE, 2000. Rodent ectoparasites from two locations in northwestern Florida. J Vector Ecol. 25, 222–228. [PubMed] [Google Scholar]
- Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA, 2005. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst Parasitol. 60, 99–112. [DOI] [PubMed] [Google Scholar]
- Ewing SA, Panciera RJ, 2003. American canine hepatozoonosis. Clin Microbiol Rev. 16, 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari FA, Goddard J, Paddock CD, Varela-Stokes AS, 2012. Rickettsia parkeri and Candidatus Rickettsia andeanae in Gulf Coast ticks, Mississippi, USA. Emerg Infect Dis. 18, 1705–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin DA, Brinkerhoff RJ, Gaff H, Jiang J, Robbins RG, Eickmeyer W, Butler J, Nielsen D, Wright C, White A, Gimpel ME, 2014. Additional US collections of the Gulf Coast tick, Amblyomma maculatum (Acari: Ixodidae), from the State of Delaware, the first reported field collections of adult specimens from the State of Maryland, and data regarding this tick from surveillance of migratory songbirds in Maryland. Syst Appl Acarol. 19, 257–262. [Google Scholar]
- Fornadel CM, Zhang X, Smith JD, Paddock CD, Arias JR, Norris DE, 2011. High rates of Rickettsia parkeri infection in Gulf Coast ticks (Amblyomma macidatum) and identification of “Candidatus Rickettsia andeanae” from Fairfax County, Virginia. Vector Borne Zoonot Dis. 11, 1535–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Acuña D, Venzal J, Skewes-Ramm O, Rubilar-Contreras L, Daugschies A, & Guglielmone AA, 2004. First record of immature stages of Amblyomma tigrinum (Acari: Ixodidae) on wild birds in Chile. Exp Appl Acarol. 33, 153–156. [DOI] [PubMed] [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ, 2016. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick Borne Dis. 7, 1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmone AA, Beati L, Barros-Battesti DM, Labruna MB, Nava S, Venzal JM, Mangold AJ, Szabó MPJ, Martins JR, González-Acuña D, Estrada-Peña A, 2006. Ticks (Ixodidae) on humans in South America. Exp Appl Acarol. 40, 83–100. [DOI] [PubMed] [Google Scholar]
- Guglielmone AA, Nava S, 2011. Rodents of the subfamily Sigmodontinae (Myomorpha: Cricetidae) as hosts for South American hard ticks (Acari: Ixodidae) with hypotheses on life history. Zootaxa. 2904, 45–65. [Google Scholar]
- Harris EK, Verhoeve VI, Banajee KH, Macaluso JA, Azad AF, Macaluso KR, 2017. Comparative vertical transmission of Rickettsia by Dermacentor variabilis and Amblyomma maculatum. Ticks Tick Borne Dis. 8, 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig TC, Orr JM, Smith JD, Arias JR, Norris DE, 2014. Spotted fever group rickettsiae in multiple hard tick species from Fairfax County, Virginia. Vector Borne Zoonotic Dis. 14, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Stromdahl EY and Richards AL, 2012. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector Borne Zoonotic Dis. 12, 175–182. [DOI] [PubMed] [Google Scholar]
- Lado P, Nava S, Mendoza-Uribe L, Caceres AG, Delgado-De La Mora J, Licona-Enriquez JD, Delgado-De La Mora D, Labruna MB, Durden LA, Allerdice ME, Paddock CD, 2018. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae) group of ticks: phenotypic plasticity or incipient speciation?. Parasites Vectors. 11, 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan SM, Peter TF, Simbi BH, Kocan K, Camus E, Barbet AF, Burridge MJ, 2000. Comparison of efficacy of American and African Amblyomma ticks as vectors of Heartwater (Cowdria ruminantium) infection by molecular analyses and transmission trials. J Parasitol. 86, 44–49. [DOI] [PubMed] [Google Scholar]
- Mays SE, Houston AE, Trout Fryxell RT, 2016. Specifying pathogen associations of Amblyomma maculatum (Acari: Ixodidae) in western Tennessee. J Med Entomol. 53, 435–440. [DOI] [PubMed] [Google Scholar]
- Moraru GM, Goddard J, Varela-Stokes AS, 2012. Observations on host preference and feeding success of immature Amblyomma maculatum (Acari: Ixodidae). J Entomol Sci. 47, 221–226. [Google Scholar]
- Moraru GM, Goddard J, Murphy A, Link D, Belant JL, Varela-Stokes A, 2013. Evidence of antibodies to spotted fever group rickettsiae in small mammals and quail from Mississippi. Vector Borne Zoonotic Dis. 13, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolny RM, Wright CL, Hynes WL, Sonenshine DE, Gaff HD, 2011. Ixodes affinis (Acari: Ixodidae) in southeastern Virginia and implications for the spread of Borrelia burgdorferi, the agent of Lyme disease. J Vector Ecol. 36, 464–467. [DOI] [PubMed] [Google Scholar]
- Nadolny RM, Wright CL, Sonenshine DE, Hynes WL, Gaff HD, 2014. Ticks and spotted fever group rickettsiae in southeastern Virginia. Ticks Tick Borne Dis. 5, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolny RM, Gaff HD, 2018. Natural history of Amblyomma maculatum in Virginia. Ticks Tick Borne Dis. 9, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieri-Bastos FA, Marcili A, De Sousa R, Paddock CD, Labruna MB, 2018. Phylogenetic evidence for the existence of multiple strains of Rickettsia parkeri in the New World. Appl Env Microbiol. 84, e02872–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SLF, Tamminga CL, Ohl CA, 2004. Rickettsia parkeri: A Newly Recognized Cause of Spotted Fever Rickettsiosis in the United States, Clin Infect Dis. 38, 805–811. [DOI] [PubMed] [Google Scholar]
- Portugal JS, Goddard J, 2015. Collections of immature Amblyomma maculatum Koch (Acari : Ixodidae) from Mississippi, U.S.A. Syst Appl Acarol. 20, 20–24. [Google Scholar]
- Regnery RL, Spruill CL, Plikaytis BD, 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 173, 1576–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RK, 1994. Instructions for building two live traps for small mammals. Va J Sci. 45, 151–158. [Google Scholar]
- Rose RK, Nadolny RM, Kiser J, Rice SE, Salamone HG, Eggleston J and Gaff HD, 2018. Compositional changes in two small mammal communities during succession in southeastern Virginia. Va J Sci. 69, 1–12. doi: 10.25778/DB6R-4R32 . [DOI] [Google Scholar]
- Santos Dias JAT, 1963. Contribuiçao para o estudo da sistemática dos ácaros da suborden Ixodoidea Banks, 1894. Mémorias e Es- tudos do Museu Zoológico da Universidade da Coimbra. 285, 34. [Google Scholar]
- Santos Dias JAT, 1993. Nova contribuiçao para o estudo da sis- temática do género Amblyomma Koch, 1844 (Acarina–Ixodidea). García de Orta, Série Zoológica. 19, 11–19. [Google Scholar]
- Sikes RS and Animal Care and Use Committee of the American Society of Mammalogists, 2016. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal. 97, 663–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD, 2007. Gulf Coast Ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 13, 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teel PD, Hopkins SW, Donahue WA, & Strey OF, 1998. Population dynamics of immature Amblyomma maculatum (Acari: Ixodidae) and other ectoparasites on meadowlarks and northern bobwhite quail resident to the coastal prairie of Texas. J Med Entomol. 35, 483–488. [DOI] [PubMed] [Google Scholar]
- Teel PD, Ketchum HR, Mock DE, Wright RE, Strey OF, 2010. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J Med Entomol. 47, 707–722. [DOI] [PubMed] [Google Scholar]
- Varela-Stokes AS, Paddock CD, Engber B, Toliver M, 2011. Rickettsia parkeri in Amblyomma maculatum ticks, North Carolina, USA, 2009–2010. Emerg Infect Dis. 17, 2350–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Nadolny RM, Jiang J, Richards AL, Sonenshine DE, Gaff HD, Hynes WL, 2011. Rickettsia parkeri in Gulf Coast ticks, southeastern Virginia, USA. Emerg Infect Dis. 17, 896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Sonenshine DE, Gaff HD, Hynes WL, 2015. Rickettsia parkeri transmission to Amblyomma americanum by cofeeding with Amblyomma maculatum (Acari: Ixodidae) and potential for spillover. J Med Entomol. 52, 1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright-Thompson CL, 2015. Determining the Prevalence and Distribution of Tick-borne pathogens in southeastern Virginia and exploring the transmission dynamics of Rickettsia parkeri in Amblyomma maculatum. Doctor of Philosophy (PhD), dissertation, Biological Sciences, Old Dominion University, DOI: 10.25777/xew9-5f34. <https://digitalcommons.odu.edu/biology_etds/1>. [DOI] [Google Scholar]
- Zemtsova GE, Watkins NE, Levin ML, 2014. Multiplex qPCR assay for identification and differentiation of Amblyomma americanum, Amblyomma cajennense, and Amblyomma maculatum (Ixodida: Ixodidae) tick species in the eastern United States. J Med Entomol. 51, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]