Abstract
Purpose
This study aimed to investigate the associations between the preoperative prognostic nutritional index (PNI), systemic immune-inflammation index (SII) and overall survival (OS) and cancer-specific survival (CSS) in high-risk non-muscle-invasive bladder cancer (NMIBC) patients who received intravesical instillation of Bacillus Calmette-Guerin (BCG) after transurethral resection of bladder tumour (TURBT).
Patients and Methods
We retrospectively collected data from 387 high-risk NMIBC patients between January 2004 and December 2014. PNI was calculated as total lymphocyte count (109/L)×5+albumin concentration (g/L). SII was calculated as neutrophil count (109/L)×platelet count (109/L)/lymphocyte count (109/L). The cutoff values of PNI and SII were determined through receiver operating characteristic (ROC) analysis. OS and CSS were estimated by Kaplan–Meier analysis. The Log rank test was used to compare differences between the groups. Univariate and multivariate Cox regression analyses were performed to assess the predictive values of PNI and SII for OS and CSS. Additionally, highest-risk NMIBC patients were also divided into low or high groups according to PNI and SII. The OS and CSS of highest-risk NMIBC patients were estimated using Kaplan-Meier analysis with the Log rank test.
Results
The patients were divided into two groups according to the cutoff values of PNI (<50.17 vs ≥50.17) and SII (<467.76 vs ≥467.76). Kaplan–Meier analysis revealed that low PNI and high SII were associated with poorer OS and CSS in high-risk NMIBC patients. Univariate and multivariate Cox regression analyses revealed that PNI and SII were independent predictive factors for OS and CSS. Kaplan–Meier analysis also revealed that low PNI and high SII were related to poorer OS and CSS in highest-risk NMIBC patients.
Conclusion
These results suggest that preoperative PNI and SII, based on standard laboratory measurements, may be useful noninvasive, inexpensive and simple tools for predicting the long-term survival of high-risk NMIBC patients who received intravesical instillation of BCG after TURBT.
Keywords: prognostic nutritional index, systemic immune-inflammation index, non-muscle-invasive bladder cancer, Bacillus Calmette-Guerin, overall survival, cancer-specific survival
Introduction
Bladder cancer (BC) ranks as the 9th most commonly diagnosed cancer and is the 13th most frequent cause of cancer death worldwide.1 In the United States, it is the 5th most common cancer.2 BC is a complex disease and can be classified into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) subtypes according to the distinct clinical progression and prognosis. Of these, 75% of patients are diagnosed with NMIBC with mucosal submucosal invasion.3 NMIBC includes Ta (noninvasive papillary carcinoma), T1 (tumour invading subepithelial connective tissue) and carcinoma in situ (CIS; “flat tumour”).4 Transurethral resection of bladder tumour (TURBT) is a typical first-line treatment for NMIBC patients. However, 70% of patients may suffer from recurrence, with a high 5-year recurrence rate ranging from 30% to 80% after treatment with TURBT.5 Because of the heterogeneity of NMIBC, it is difficult to determine the standard guidelines for treatment. Thus, the stratification of risk groups based on recurrence and progression is a strategy that attempts to individualize and standardize BC patients.6 The European Association of Urology (EAU) guidelines (2019 edition) defined the high-risk NMIBC group as follows: 1) T1 tumours; 2) G3 (high-grade [HG]) tumours; 3) CIS; and 4) multiple, recurrent and large (>3 cm) TaG1G2/low-grade tumours (all features must be present).4 In addition, as an extremely hazardous subgroup of high-risk NMIBC, the highest-risk NMIBC subgroup includes T1G3/HG tumours associated with concurrent bladder CIS; multiple and/or large T1G3/HG and/or recurrent T1G3/HG tumours; T1G3/HG tumours with CIS in the prostatic urethra; some forms of variant histology of urothelial carcinoma (UC); and lymphovascular invasion.4 Patients with HG, T1 or CIS are at high risk of recurrence and progression. The prognosis is worse in high-risk NMIBC patients; for instance, the 5-year cancer-specific mortality is as high as 11.3% in T1G3 high-risk NMIBC patients.7 The EAU recommends that all high-risk NMIBC patients receive intravesical instillations of Bacillus Calmette-Guerin (BCG). Intravesical instillations of BCG can reduce the long-term risk of recurrence and progression in high-risk NMIBC patients.8
Increasing evidence shows that nutritional deficiencies and the systemic inflammatory response (SIR) might play critical roles in tumorigenesis, proliferation, progression and metastasis.9–12 Preoperative nutrition status is also associated with postoperative complications and survival in cancer patients.13 The prognostic nutritional index (PNI), which is calculated based on the peripheral blood lymphocyte count and serum albumin concentration, has been used to assess the hosts’ immune and nutritional status.14 It has also been confirmed as a powerful prognostic marker in various malignancies, including hepatocellular carcinoma and colorectal cancer.15,16 SIR markers, such as lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR), have been used to evaluate patients’ antitumour immune responses and to predict cancer prognosis in many different kinds of malignancies, including urological cancer.17–19 However, these previous inflammation-based markers integrated only two kinds of immune cells. As a novel indicator based on lymphocyte, neutrophil and platelet counts, the systemic immune-inflammation index (SII) has been proven to be a powerful predictor of recurrence and survival in non-small cell lung cancer (NSCLC), gastric cancer and pancreatic cancer.20–22 To our knowledge, the values of PNI and SII in predicting the overall survival (OS) and cancer-specific survival (CSS) of high-risk NMIBC patients have not yet been investigated. The main purpose of this study was to evaluate the values of preoperative nutrition- and inflammation-based indicators, including PNI and SII, in predicting the long-term survival of high-risk NMIBC patients who received intravesical instillation of BCG after TURBT.
Patients and Methods
Patient Selection
We retrospectively collected and examined medical data from 387 patients who met our defined high-risk NMIBC group criteria and received intravesical instillation of BCG after TURBT at the Department of Urology, Xuanwu Hospital Capital Medical University from January 2004 to December 2014. Patients who met the following criteria were included in this study: 1) complete clinical and pathological information available; 2) accordance with the stratification of high-risk NMIBC; 3) receipt of intravesical instillation of BCG after TURBT; 4) exclusion of autoimmune diseases and cancers in other systems; 5) absence of preoperative neoadjuvant chemotherapy and radiotherapy; 6) exclusion of suspected infectious diseases accompanied by neutrocytosis and/or leukocytosis; 7) exclusion of distant metastasis before surgery; and 8) full follow-up data available. This was a retrospective cohort study that was approved by the Ethics Committee of Xuanwu Hospital Capital Medical University. All patients provided written informed consent for the information to be used in our hospital database. The study protocol and all related items followed the Declaration of Helsinki guidelines.
Data Collection and Definitions
The clinicopathological data, including sex, age, body mass index (BMI), smoking status, tumour number, recurrence, tumour size, tumour grade, T stage, concomitant CIS, lymphovascular invasion (LVI) and preoperative routine laboratory data, were obtained from the patients’ medical records. The neutrophil, platelet and lymphocyte counts were collected by using a routine blood test, and the albumin concentration was obtained using the hepatic function test before breakfast within 1–2 weeks before surgery. PNI and SII were calculated as follows: PNI=total lymphocyte count (109/L)×5+albumin concentration (g/L); SII=neutrophil count (109/L)×platelet count (109/L)/lymphocyte count (109/L).
Follow-Up
All patients were regularly followed up with physical examination, blood tests, routine urine tests, biochemical tests and cystoscopy every 3 months for the first 2 years, every 6 months in the following 3 years and annually thereafter. A computed tomography scan was performed every year to assess bladder and upper tract urothelial recurrences. Intravesical BCG treatment was performed after TURBT. The BCG schedule was as follows: BCG induction therapy (weekly BCG instillation for the first 6 weeks) and maintenance therapy for up to 3 years (3 weekly maintenance instillations for 3, 6, 12, 18, 24 and 36 months).
Statistical Analysis
Statistical analysis was conducted using SPSS statistical software package version 25.0 (IBM, Armonk, USA) and SigmaPlot version 12.5 for Windows (Systat Software, Inc., San Jose, CA, USA). Continuous data are shown as the mean ± standard deviation (SD) or median with range. Categorical variables are presented as frequencies and percentages and were compared using Pearson’s chi-square test. Receiver operating characteristic (ROC) curve analysis was applied to identify the optical cut-off values of PNI and SII with the highest Youden’s index for predicting 5-year OS. The primary end point was OS, and the secondary end point was CSS. Survival analysis was performed using the Kaplan-Meier method, and survival curves were compared by the Log rank test. A Cox proportional hazards regression model was used to analyse various independent predictors of postoperative OS and CSS. Univariate analysis was used to assess various factors related to patient and tumour characteristics. Multivariate analysis was performed only for variables with p<0.1 in univariate analysis. A two-tailed p<0.05 was considered statistically significant.
Results
Clinicopathological Characteristics of the Patients
A total of 387 high-risk NMIBC patients who were diagnosed after TURBT, received intravesical instillation of BCG after TURBT and met the inclusion criteria were enrolled in the study. The median (range) follow-up duration was 108 (5–191) months. Of the 387 patients, 277 (71.58%) were male, and 158 (40.83%) were current or ex-smokers. The mean (±SD) age of the 387 patients was 69.49 (±10.84) years. The mean (±SD) BMI of the 387 patients was 24.07 (±3.35) kg/m2. A total of 214 (55.30%) patients had multifocal tumours. Over half (53.49%) of these patients experienced recurrence. The tumours of 84 (21.71%) patients were ≥ 3 cm. Most patients (n=263, 67.96%) had a high pathological grade, and 260 (67.18%) patients had stage T1 disease. Among the patients, 24 (6.20%) had concomitant CIS, and 31 (8.01%) had LVI. Eighty-two (21.19%) patients experienced tumour progression. Forty-two (10.85%) of all patients underwent radical cystectomy. The 5-year cancer-specific mortality and 5-year overall mortality for the overall patients were 11.78% and 16.80%, respectively. Of the entire population, 90 (23.26%) patients were included in the highest-risk NMIBC group. The 5-year cancer-specific mortality and 5-year overall mortality for the highest-risk NMIBC patients were 42.17% and 46.67%, respectively. The mean (SD) values of the nutrition- and inflammation-based indicators were as follows: PNI, 51.64 (SD: 11.67); SII, 447.46 (SD: 139.39) (Table 1).
Table 1.
Clinicopathological Characteristics of 387 High-Risk NMIBC Patients Who Received Intravesical Instillation of BCG After TURBT
| Characteristics | Patients, n (%) |
|---|---|
| Sex | |
| Male | 277 (71.58) |
| Female | 110 (28.42) |
| Age | |
| Mean±SD | 69.49±10.84 |
| Median (range) | 71 (34–89) |
| BMI | |
| Mean±SD | 24.07±3.35 |
| Median (range) | 23.58 (17.36–36.86) |
| Smoking status | |
| Never smoker | 229 (59.17) |
| Current or ex-smoker | 158 (40.83) |
| Tumour number | |
| 1 | 173 (44.70) |
| 2~7 | 143 (36.95) |
| ≥8 | 71 (18.35) |
| Recurrence | |
| None | 180 (46.51) |
| Yes | 207 (53.49) |
| Tumour size | |
| <3cm | 303 (78.29) |
| ≥3cm | 84 (21.71) |
| Tumour grade | |
| LG | 124 (32.04) |
| HG | 263 (67.96) |
| T stage | |
| Ta | 107 (27.65) |
| Tis | 20 (5.17) |
| T1 | 260 (67.18) |
| Concomitant CIS | |
| None | 363 (93.80) |
| Yes | 24 (6.20) |
| LVI | |
| None | 356 (91.99) |
| Yes | 31 (8.01) |
| Blood cell counts, mean (SD) | |
| NEUT (×109/L) | 3.89 (1.46) |
| PLT (×109/L) | 214.18 (46.81) |
| LYMP (×109/L) | 1.94 (0.61) |
| ALB (g/L) | 41.93 (9.59) |
| Nutrition-inflammation-based parameters, mean (SD) | |
| PNI | 51.64 (11.67) |
| SII | 447.46 (139.39) |
| Oncological outcomes | |
| Radical cystectomy | 42(10.85) |
| Upper urinary tract recurrence | 13(3.36) |
| Bladder recurrence | 194(50.12) |
| Progression | 82(21.19) |
| 5-year cancer specific mortality | 43/365 (11.78) |
| 5-year overall mortality | 65/387 (16.80) |
| 5-year cancer specific mortality of highest-risk NMIBC | 35/83 (42.17) |
| 5-year overall mortality of highest-risk NMIBC | 42/90 (46.67) |
| Follow-up duration, months | |
| Median (range) | 108 (5–191) |
Abbreviations: NMIBC, non-muscle-invasive bladder cancer; BCG, Bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumour; BMI, body mass Index; LG, low grade; HG, high grade; CIS, carcinoma in situ; LVI, lymphovascular invasion; NEUT, neutrophil count; PLT, platelet count; LYMP, lymphocyte count; ALB, albumin; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; SD, standard deviation.
The Optimal Cut-off Values of PNI, SII and Other Indexes for Estimating Prognosis
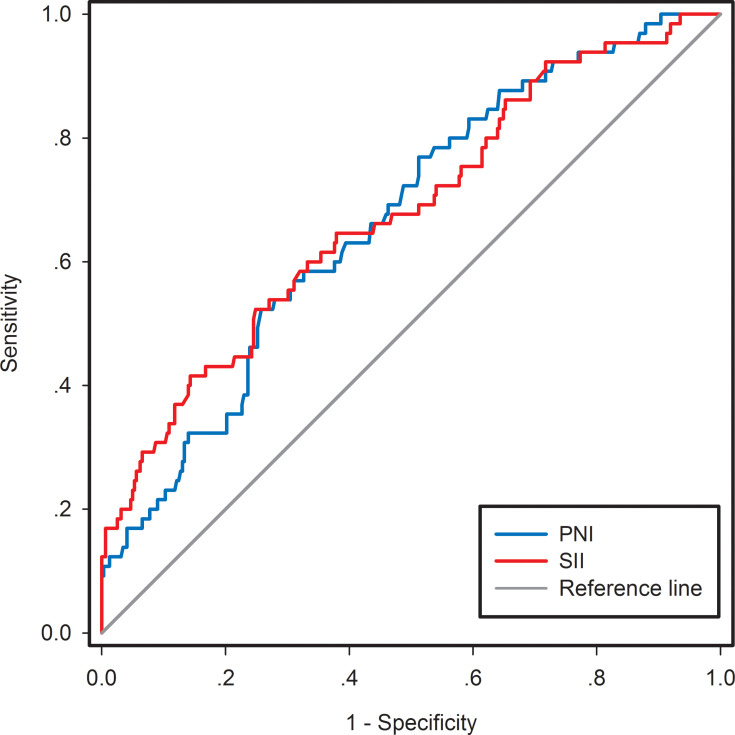
The mean values of PNI and SII for the overall study population were 51.64 and 447.46, respectively. Therefore, we attempted to determine the optimal cut-off values of the nutrition- and inflammation-based indicators, including PNI and SII, for predicting 5-year OS with the ROC analysis method. As shown in Figure 1, the areas under the curve (AUCs) for 5-year OS were 0.668 (95% CI: 0.599–0.738) and 0.671 (95% CI: 0.604–0.753) for PNI and SII, respectively. The corresponding optimal cut-off values were identified as 50.17 for PNI and 467.76 for SII according to the maximum Youden index. Similarly, 63.5 years, 22.31 kg/m2, 4.36×109/L, 235×109/L, 1.78×109/L, and 42.19 g/L were the chosen cut-offs for age, BMI, neutrophil counts, platelet counts, lymphocyte counts, and albumin levels, respectively. Based on the optical cut-off values, the patients were divided into the following groups: low PNI group (<50.17, n=116) or high PNI group (≥50.17, n=271) and low SII group (<467.76, n=242) or high SII (≥467.76, n=145) group.
Figure 1.
ROC curve analyses for the optimal cut-off values of PNI and SII. Areas under the curve (AUCs) for 5-year OS were 0.668 (95% CI, 0.599-0.738, p<0.001) and 0.671 (95% CI, 0.604-0.753, p<0.001) for PNI and SII, respectively.
Abbreviations: ROC, receiver operating characteristic; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; AUC, area under the curve; OS, overall survival.
Correlations Between PNI, SII and Clinicopathological Characteristics
The relationships between the preoperative PNI and SII and the patient clinicopathological parameters of this cohort are shown in Table 2. We found that patients with low PNI (<50.17) were more likely to have older age (p=0.009), lower BMI (p=0.006), more tumours (p=0.048), more frequent recurrence (p=0.002), larger tumour sizes (p<0.001), higher tumour grades (p=0.001), more advanced T stages (p<0.001), concomitant CIS (p=0.002) and LVI (p=0.006) than those with high PNI (≥50.17). Patients with high SII (≥467.76) were associated with lower BMI (p=0.007), more tumours (p=0.007), more frequent recurrence (p=0.005), larger tumour sizes (p<0.001), higher tumour grades (p=0.033), more advanced T stages (p=0.005), concomitant CIS (p=0.009) and LVI (p=0.004). Nevertheless, no significant correlations were observed in terms of sex (p>0.05) or smoking status (p>0.05). Unexpectedly, no association between SII and age (p>0.05) was observed.
Table 2.
Correlations Between the PNI, SII and the Clinicopathological Characteristics in High-Risk NMIBC Patients Who Received Intravesical Instillation of BCG After TURBT
| Characteristics | PNI<50.17 | PNI ≥50.17 | p-value | SII <467.76 | SII ≥467.76 | p-value |
|---|---|---|---|---|---|---|
| n=116 | n=271 | n=242 | n=145 | |||
| Sex, n (%) | 0.618 | 0.454 | ||||
| Male | 81(69.8) | 196(72.3) | 170(70.2) | 107(73.8) | ||
| Female | 35(30.2) | 75(27.7) | 72(29.8) | 38(26.2) | ||
| Age (years), n (%) | 0.009 | 0.353 | ||||
| <63.5 | 29(25.0) | 105(38.8) | 88(36.4) | 46(31.7) | ||
| ≥63.5 | 87(75.0) | 166(61.2) | 154(63.5) | 99(68.3) | ||
| BMI, n (%) | 0.006 | 0.007 | ||||
| <22.31 | 62(53.4) | 104(38.4) | 91(37.6) | 75(51.7) | ||
| ≥22.31 | 54(46.6) | 167(61.6) | 151(62.4) | 70(49.6) | ||
| Smoking status, n (%) | 0.935 | 0.060 | ||||
| Never smoker | 69(59.5) | 160(59.0) | 152(62.8) | 77(53.1) | ||
| Current or ex-smoker | 47(40.5) | 111(41.0) | 90(37.2) | 68(46.9) | ||
| Tumour number, n (%) | 0.048 | 0.007 | ||||
| Unifocal | 43(37.1) | 130(48.0) | 121(50.0) | 52(35.9) | ||
| Multifocal | 73(62.9) | 141(52.0) | 121(50.0) | 93(64.1) | ||
| Recurrence, n (%) | 0.002 | 0.005 | ||||
| None | 40(34.5) | 140(51.7) | 126(52.1) | 54(37.2) | ||
| Yes | 76(65.5) | 131(48.3) | 116(47.9) | 91(62.8) | ||
| Tumour size, n (%) | <0.001 | <0.001 | ||||
| <3cm | 75(64.7) | 228(84.1) | 208(86.0) | 95(65.5) | ||
| ≥3cm | 41(35.3) | 43(15.9) | 34(14.0) | 50(34.5) | ||
| Tumour grade, n (%) | 0.001 | 0.033 | ||||
| LG | 23(19.8) | 101(37.3) | 87(36.0) | 37(25.5) | ||
| HG | 93(80.2) | 170(62.7) | 155(64.0) | 108(74.5) | ||
| T stage, n (%) | <0.001 | 0.005 | ||||
| T1 | 93(80.2) | 167(61.6) | 150(62.0) | 110(75.9) | ||
| ≤Tis | 23(19.8) | 104(38.4) | 92(38.0) | 35(24.1) | ||
| Concomitant CIS, n (%) | 0.002 | 0.009 | ||||
| None | 102(87.9) | 261(96.3) | 233(96.3) | 130(89.7) | ||
| Yes | 14(12.1) | 10(3.7) | 9(3.7) | 15(10.3) | ||
| LVI, n (%) | 0.006 | 0.004 | ||||
| None | 100(86.2) | 256(94.5) | 230(95.0) | 126(86.9) | ||
| Yes | 16(13.8) | 15(5.5) | 12(5.0) | 19(13.1) | ||
| NEUT, n (%) | <0.001 | <0.001 | ||||
| <4.36×109/L | 82(70.7) | 252(93.0) | 237(98.0) | 97(66.9) | ||
| ≥4.36×109/L | 34(29.3) | 19(7.0) | 5(2.0) | 48(33.1) | ||
| PLT, n (%) | <0.001 | <0.001 | ||||
| <235×109/L | 97(83.6) | 259(95.6) | 233(96.3) | 121(83.4) | ||
| ≥235×109/L | 19(16.4) | 12(4.4) | 7(3.7) | 24(16.6) | ||
| LYMP, n (%) | <0.001 | <0.001 | ||||
| <1.78×109/L | 86(74.1) | 41(15.1) | 26(10.7) | 101(69.7) | ||
| ≥1.78×109/L | 30(25.9) | 230(84.9) | 216(89.3) | 44(30.3) | ||
| ALB, n (%) | <0.001 | <0.001 | ||||
| <42.19 g/L | 105(90.5) | 120(44.3) | 119(49.2) | 106(73.1) | ||
| ≥42.19 g/L | 11(9.5) | 151(55.7) | 123(50.8) | 39(26.9) |
Note: Bold values indicate statistical significance, p<0.05.
Abbreviations: NMIBC, non-muscle-invasive bladder cancer; BCG, Bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumour; BMI, body mass Index; LG, low grade; HG, high grade; CIS, carcinoma in situ; LVI, lymphovascular invasion; NEUT, neutrophil count; PLT, platelet count; LYMP, lymphocyte count; ALB, albumin; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; SD, standard deviation.
Associations of PNI and SII with OS and CSS in the Overall Population
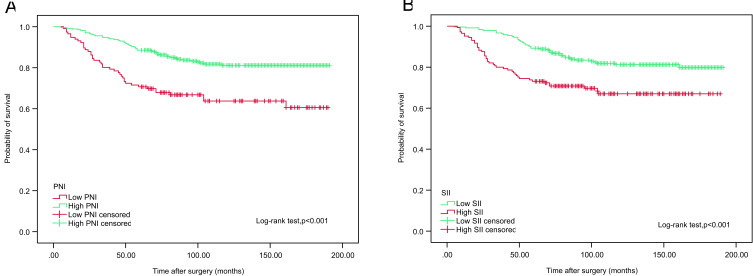
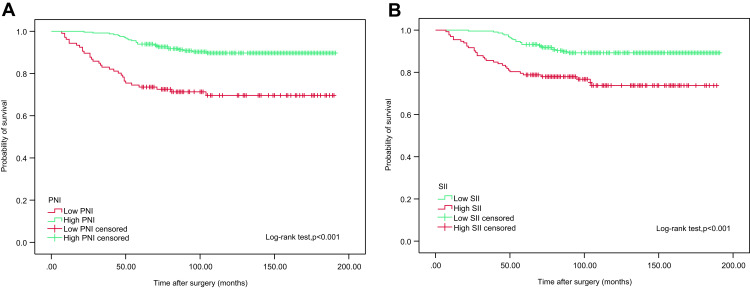
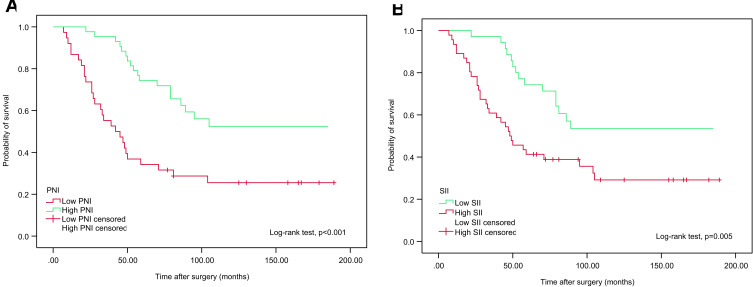
We examined whether PNI and SII were associated with CSS and OS using Kaplan-Meier survival analysis. The patients were divided into two groups based on preoperative PNI (<50.17 vs ≥50.17) and SII (<467.76 vs ≥467.76). Notably, OS and CSS times were longer in the low SII (<467.76) group. In contrast, reduced PNI (P<50.17) was associated with poorer OS and CCS, as shown in Figures 2 and 3.
Figure 2.
Kaplan–Meier analyses for OS in high-risk NMIBC patients who received intravesical instillation of BCG after TURBT according to preoperative PNI (A) and SII (B).
Abbreviations: OS, overall survival; NMIBC, non-muscle-invasive bladder cancer; BCG, Bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumour; PNI, prognostic nutritional index; SII, systemic immune-inflammation index.
Figure 3.
Kaplan–Meier analyses for CSS in high-risk NMIBC patients who received intravesical instillation of BCG after TURBT according to preoperative PNI (A) and SII (B).
Abbreviations: CSS, cancer specific survival; NMIBC, non-muscle-invasive bladder cancer; BCG, Bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumour; PNI, prognostic nutritional index; SII, systemic immune-inflammation index.
Significant Predictors of OS and CSS by Cox Univariate and Multivariate Regression Analyses
The results of univariate and multivariate Cox regression analyses of clinicopathological factors associated with OS and CSS are presented in Tables 3 and 4. Multivariate Cox regression analysis revealed that PNI (p=0.017), SII (p=0.007), age (p=0.007), BMI (p=0.021), tumour number (p<0.001), recurrence (p=0.007), tumour grade (p<0.001), T stage (p=0.001) and concomitant CIS (p=0.032) were independent predictive factors for OS after adjusting for other confounding factors. PNI (p=0.009), SII (p=0.014), age (p=0.033), tumour number (p<0.001), recurrence (p<0.001), tumour grade (p=0.003), T stage (p<0.001), concomitant CIS (p=0.007) and LVI (p=0.015) were identified as independent predictors of CSS.
Table 3.
Univariate and Multivariate Cox Regression Analyses for Overall Survival (OS) in High-Risk NMIBC Patients Who Received Intravesical Instillation of BCG After TURBT
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Sex | 0.966 | |||
| Male | 1(reference) | |||
| Female | 1.009(0.652–1.563) | |||
| Age (years) | <0.001 | 0.007 | ||
| <63.5 | 1(reference) | 1(reference) | ||
| ≥63.5 | 2.887(1.657–5.031) | 2.221(1.246–3.959) | ||
| BMI | 0.001 | 0.021 | ||
| <22.31 | 1(reference) | 1(reference) | ||
| ≥22.31 | 0.541(0.407–0.813) | 0.632(0.381–1.047) | ||
| Smoking status | 0.764 | |||
| Never smoker | 1(reference) | |||
| Current or ex-smoker | 0.937(0.612–1.434) | |||
| Tumour number | <0.001 | <0.001 | ||
| 1 | 1(reference) | 1(reference) | ||
| 2~7 | 2.148(1.151–4.006) | 1.752(0.933–3.289) | ||
| ≥8 | 10.959(6.201–19.368) | 7.518(4.108–13.761) | ||
| Recurrence | <0.001 | 0.007 | ||
| None | 1(reference) | 1(reference) | ||
| Yes | 2.911(1.810–4.681) | 2.007(1.213–3.321) | ||
| Tumour size | <0.001 | 0.718 | ||
| ≤3cm | 1(reference) | 1(reference) | ||
| >3cm | 2.620(1.704–4.031) | 1.104(0.645–1.890) | ||
| Tumour grade | <0.001 | <0.001 | ||
| LG | 1(reference) | 1(reference) | ||
| HG | 3.527(1.919–6.481) | 3.430(1.847–6.71) | ||
| T stage | 0.009 | 0.001 | ||
| Ta | 1(reference) | 1(reference) | ||
| Tis | 3.547(1.567–8.030) | 5.774(2.379–14.016) | ||
| T1 | 1.778(1.028–3.075) | 1.957(1.081–3.544) | ||
| Concomitant CIS | <0.001 | 0.032 | ||
| None | 1(reference) | 1(reference) | ||
| Yes | 3.827(2.343–6.250) | 1.843(1.055–3.218) | ||
| LVI | 0.001 | 0.170 | ||
| None | 1(reference) | 1(reference) | ||
| Yes | 3.843(1.770–8.343) | 1.159(0.766–3.311) | ||
| PNI | <0.001 | 0.017 | ||
| <50.17 | 1(reference) | 1(reference) | ||
| ≥50.17 | 0.412(0.272–0.626) | 0.602(0.433–0.894) | ||
| SII | <0.001 | 0.007 | ||
| <467.76 | 1(reference) | 1(reference) | ||
| ≥467.76 | 2.111(1.391–3.202) | 2.104(1.231–3.597) | ||
Note: Bold values indicate statistical significance, p<0.05.
Abbreviations: NMIBC, non-muscle-invasive bladder cancer; BCG, Bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumour; BMI, body mass Index; LG, low grade; HG, high grade; CIS, carcinoma in situ; LVI, lymphovascular invasion; PNI, prognostic nutritional index; SII, systemic immune-inflammation index.
Table 4.
Univariate and Multivariate Cox Regression Analyses for Cancer-Specific Survival (CSS) in High-Risk NMIBC Patients Who Received Intravesical Instillation of BCG After TURBT
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Sex | 0.873 | |||
| Male | 1(reference) | |||
| Female | 1.048(0.586–1.876) | |||
| Age | 0.002 | 0.033 | ||
| <63.5 | 1(reference) | 1(reference) | ||
| ≥63.5 | 3.088(1.511–6.309) | 2.292(1.067–4.921) | ||
| BMI | 0.009 | 0.077 | ||
| <22.31 | 1(reference) | 1(reference) | ||
| ≥22.31 | 0.561(0.291–0.969) | 0.644(0.374–1.179) | ||
| Smoking status | 0.743 | |||
| Never smoker | 1(reference) | |||
| Current or ex-smoker | 1.093(0.641–1.863) | |||
| Tumour number | <0.001 | <0.001 | ||
| 1 | 1(reference) | 1(reference) | ||
| 2~7 | 2.490(1.110–5.586) | 2.135(0.930–4.903) | ||
| ≥8 | 14.145(6.689–29.913) | 7.963(3.585–17.685) | ||
| Recurrence | <0.001 | <0.001 | ||
| None | 1(reference) | 1(reference) | ||
| Yes | 6.790(3.071–15.012) | 4.695(1.986–11.103) | ||
| Tumour size | <0.001 | 0.628 | ||
| ≤3cm | 1(reference) | 1(reference) | ||
| >3cm | 3.485(2.045–5.941) | 1.199(0.575–2.498) | ||
| Tumour grade | <0.001 | 0.003 | ||
| Low | 1(reference) | 1(reference) | ||
| High | 5.548(2.212–13.915) | 4.259(1.617–11.214) | ||
| T stage | 0.002 | <0.001 | ||
| Ta | 1(reference) | 1(reference) | ||
| Tis | 6.387(2.239–18.218) | 8.972(2.688–29.076) | ||
| T1 | 2.625(1.178–5.853) | 2.491(1.021–6.078) | ||
| Concomitant CIS | <0.001 | 0.007 | ||
| None | 1(reference) | 1(reference) | ||
| Yes | 5.355(2.988–9.596) | 2.612(1.297–5.261) | ||
| LVI | <0.001 | 0.015 | ||
| None | 1(reference) | 1(reference) | ||
| Yes | 6.099(2.748–13.534) | 3.352(1.266–8.875) | ||
| PNI | <0.001 | 0.009 | ||
| <50.17 | 1(reference) | 1(reference) | ||
| ≥50.17 | 0.269(0.158–0.459) | 0.413(0.196–0.870) | ||
| SII | <0.001 | 0.014 | ||
| <467.76 | 1(reference) | 1(reference) | ||
| ≥467.76 | 2.759(1.613–4.719) | 1.716(1.339–3.512) | ||
Note: Bold values indicate statistical significance, p<0.05.
Abbreviations: NMIBC, non-muscle-invasive bladder cancer; BCG, Bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumour; BMI, body mass Index; LG, low grade; HG, high grade; CIS, carcinoma in situ; LVI, lymphovascular invasion; PNI, prognostic nutritional index; SII, systemic immune-inflammation index.
Associations of PNI and SII with OS and CSS in Highest-Risk NMIBC Patients
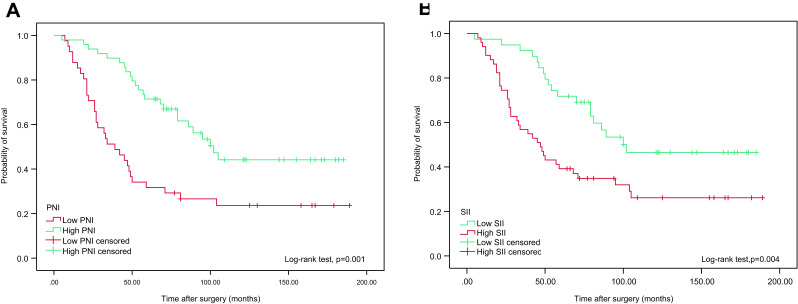
We further examined whether PNI and SII were associated with OS and CSS in highest-risk NMIBC patients who received intravesical instillation of BCG after TURBT using Kaplan-Meier survival analysis. The patients were divided into low or high groups based on their preoperative PNI (<50.17 vs ≥50.17) and SII (<467.76 vs ≥467.76). Notably, OS and CSS times were longer in the high PNI (≥50.17) group. Conversely, elevated SII (≥467.76) was associated with poorer OS and CCS, as shown in Figures 4 and 5.
Figure 4.
Kaplan–Meier analyses for OS in highest-risk NMIBC patients who received intravesical instillation of BCG after TURBT according to preoperative PNI (A) and SII (B).
Abbreviations: OS, overall survival; NMIBC, non-muscle-invasive bladder cancer; BCG, Bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumour; PNI, prognostic nutritional index; SII, systemic immune-inflammation index.
Figure 5.
Kaplan–Meier analyses for CSS in highest-risk NMIBC patients who received intravesical instillation of BCG after TURBT according to preoperative PNI (A) and SII (B).
Abbreviations: CSS, cancer specific survival; NMIBC, non-muscle-invasive bladder cancer; BCG, Bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumour; PNI, prognostic nutritional index; SII, systemic immune-inflammation index.
Discussion
Tumour-related factors, such as pathological tissue type, grade and stage, have been used to predict progression and prognosis in cancer patients. In addition, patient-related elements, including SIR markers, can predict progression and prognosis.23 Malnutrition, reflected by hypoalbuminemia, is associated with increased morbidity and mortality.24 Several studies have shown that malnutrition also interferes with the patient’s response to cancer treatment,25 and malnutrition is related to immunosuppression, which provides a favourable microenvironment for tumour recurrence.26,27 The association between inflammation and tumours was first noticed by Virchow in 1863.28 Inflammation not only plays important roles in malignant conversion and metastasis but also constitutes the local environment of the tumour.29 In this study, we investigated the preoperative nutrition- and inflammation-based factors, PNI and SII, in high-risk NMIBC patients who received intravesical instillation of BCG after TURBT. To the best of our knowledge, this is the first study to investigate the predictive values of PNI and SII for long-term survival and the associations of these two nutritional and inflammatory indicators, sex, age, BMI, smoking status, and pathological factors with the OS and CSS outcomes of a cohort of high-risk NMIBC patients who received intravesical instillation of BCG after TURBT.
PNI, which is calculated with the serum albumin concentration and lymphocyte count, was originally proposed as a marker for predicting the nutritional and immunological status of cancer patients before surgery.30,31 It is well known that the serum albumin level is one of most widely used markers in evaluating patients’ nutritional status. As another component in PNI, lymphocytes play a very important role in the cell-mediated immune response in the recurrence and progression of tumours. The vital role of lymphocytes has been clarified in several published studies that showed that lymphocytopenia is related to high cancer mortality.32,33 Worse nutritional status also leads to tumour progression via the suppression of tumour immunity.34 This immunosuppressed status in patients could result in poor oncological outcomes. Therefore, PNI was reported as a useful predictive indicator in various types of human cancers.15,35 Patients’ systemic inflammation is reported to be associated with tumour progression, recurrence, metastasis and oncological outcomes. The prognostic significance of inflammation-based markers, including SII, has been shown in many solid tumours, most notably in prostate cancer, colorectal cancer and NSCLC.36–38
The optical cut-off values of PNI and SII for predicting OS and CSS outcomes remain unclear. Jeon et al reported a PNI of 51 for renal carcinoma.35 Moric et al reported a PNI of 50 for NSCLC in their study, and the optical cut-off value was calculated by ROC curve analysis.39 Our results demonstrated that the cut-off values of PNI and SII from ROC curve analysis were 50.17 and 467.76, respectively. The PNI value was similar to that in patients with colorectal cancer.40 However, the SII value was slightly lower than those in previous studies.41 Such discrepancies might be due to differences in risk group stratification for BC, heterogeneous patient status, bias related to the number of patients, and different statistical methods.
Preoperative nutrition- and inflammation-based indicators are reported to be related to tumour progression and metastasis and affect the clinical outcomes of cancer patients. In our study, low PNI and elevated SII were associated with lower BMI, more tumours, more frequent recurrence, larger tumour sizes, higher tumour grades, more advanced T stages, concomitant CIS and LVI, indicating a more aggressive tumour phenotype. Our results were consistent with those of previously published reports.41–43 However, there were no associations between PNI and sex or smoking status. No relationships between SII and sex, age, or smoking status were found. These findings may be due to the limited number of patients and a bias resulting from patient selection.
Several noteworthy findings were identified in our study. There were significant correlations between PNI and SII and OS and CSS in all populations. We found that elevated PNI and lower SII were independently associated with better OS and CSS, while lower PNI and higher SII were related to worse OS and CSS. In addition, univariate analysis demonstrated that PNI, SII, age, BMI, tumour number, recurrence, tumour size, tumour grade, T stage, concomitant CIS and LVI were significantly associated with OS and CSS. Unlike that in previous studies, no relationship between sex and OS was found in univariate analysis. In multivariate analysis, PNI, SII, age, BMI, tumour number, recurrence, tumour grade, T stage and concomitant CIS were significantly associated with OS. Moreover, multivariate analysis also showed that PNI, SII, age, tumour number, recurrence, tumour grade, T stage, concomitant CIS and LVI were important predictors of CSS. We noticed that tumour size, which was included in the European Organisation for Research and Treatment of Cancer (EORTC) Genito-Urinary Cancer Group risk scoring system, was not an independent predictor of OS or CSS.4 From a mechanistic view, LVI is a high risk factor for NMIBC progression, and a lower BMI may be related to the hosts’ low nutritional status and increased immune response. However, in this study, LVI and BMI were not predictors of OS and CSS, respectively. This result may be due to a bias related to the limited patient pool.
Based on prognostic factors, it is necessary to substratify high-risk NMIBC patients and identify those who are at the highest risk of cancer progression. An analysis of the subgroup of highest-risk NMIBC patients who received intravesical instillation of BCG after TURBT also showed that low PNI and elevated SII were significantly associated with poor OS and CSS. These results are consistent with the results of the whole high-risk NMIBC patient group in our study. The number of patients in the highest-risk NMIBC subgroup was insufficient to analyse the relative predictive factors by using univariate and multivariate Cox regression analyses in the study.
The mechanism by which low nutritional status and increased SIR change the biological features of tumours is not sufficiently understood. The following are possible explanations for the relationships between low PNI, high SII and poor prognosis in high-risk NMIBC patients. Patients with low PNI and elevated SII often have hypoalbuminemia, neutrophilia, thrombocytosis, and lymphopenia. Previous studies have shown that malnutrition, reflected by hypoalbuminemia, provides a favourable microenvironment for tumour recurrence.25,28 A worse nutritional status leads to tumour progression through the suppression of tumour immunity.34 Inflammatory blood cells, such as neutrophilia, play a fundamental role in generating high levels of reactive oxygen species (ROS), tumour necrosis factor-α (TNF-α) and macrophage migration inhibitory factor.44 Further, neutrophilia can cause the secretion of large amounts of arginase, ROS, and nitric oxide (NO), which results in the disorder of T cell activation45 and production of vascular endothelial growth factor (VEGF), leading to tumour neovascularization.46 As reported in a variety of cancer patients, elevated platelet counts could stimulate tumour angiogenesis and protect tumour cells from cytolysis, which contributes to tumour recurrence and progression. Conversely, lymphocytes play an important antitumour role by inhibiting tumour cell proliferation and metastasis and intensifying the patient’s immune response to cancer. Cancer may reflect a reduced number of CD4+ T helper lymphocytes, which is related to cell growth, progression and migration. Another potential reason for tumour-induced lymphocytopenia is the result of both impaired lymphocyte homeostasis and elevated lymphocyte apoptosis. Additionally, proapoptotic molecules, such as Fas ligand, can result in the increased destruction of lymphocytes through the activation of the extrinsic pathway of apoptosis.47 Thus, in this study, PNI was passively associated with long-term oncological outcomes, while SII was positively related to CSS and OS in high-risk NMIBC patients.
Several limitations of this study should be acknowledged. First, this is a single-institute study with a small sample size. Second, this study has a retrospective cohort design, which may have led to selection bias during patient selection and data collection. Third, the assessment of some reported inflammation- and nutrition-based indicators, such as Glasgow prognostic score (GPS), albumin/globulin (A/G), and C-reactive protein/albumin (CRP/ALB), were not included in this study. Fourth, the AUC was relatively low. Fifth, as useful predictors in cancer, PNI and SII might also play critical roles in other inflammation- and nutrition-related diseases. The specificities of PNI and SII may not be high. Therefore, a large, multicentre prospective cohort study should be performed in the future to confirm the preliminary results of this study.
Conclusion
In this retrospective cohort study of high-risk NMIBC patients who received intravesical instillation of BCG after TURBT, we found that PNI and SII might serve as valuable independent predictors of OS and CSS. Preoperative PNI and SII, based on standard laboratory measurements, may be useful noninvasive, inexpensive and simple tools for evaluating the long-term oncological prognosis of high-risk NMIBC patients who received intravesical instillation of BCG after TURBT.
Funding Statement
The financial support of this study was provided by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (grant number: ZYLX201801). All authors made a significant contribution to the work reported, whether it was in the conception, study design, execution, data acquisition, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors thank the staff of the medical recording office of Xuanwu Hospital Capital Medical University who provided all the patients’ medical records for this study.
Disclosure
The authors report grants from Beijing Municipal People’s Government, during the conduct of the study; and grants from Beijing Municipal People’s Government, outside the submitted work. The authors declare that there are no other conflicts of interest in this study.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 2.Smith AB, Jaeger B, Pinheiro LC, et al. Impact of bladder cancer on health-related quality of life. BJU Int. 2018;121:549–557. doi: 10.1111/bju.14047 [DOI] [PubMed] [Google Scholar]
- 3.Garg T, Connors JN, Ladd IG, et al. Defining priorities to improve patient experience in non-muscle invasive bladder cancer. Bladder Cancer. 2018;4:121–128. doi: 10.3233/BLC-170138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babjuk M, Burger M, Compérat EM, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ)-2019 update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 5.Kang M, Jeong CW, Kwak C, et al. Preoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget. 2017;8:12891–12901. doi: 10.18632/oncotarget.14179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demirtaş A, Sabur V, Akınsal EC, et al. Can neutrophil-lymphocyte ratio and lymph node density be used as prognostic factors in patients undergoing radical cystectomy? Scientific World J. 2013;2013:703579. doi: 10.1155/2013/703579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cambier S, Sylvester RJ, Collette L, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus calmette-guérin. Eur Urol. 2016;69:60–69. doi: 10.1016/j.eururo.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 8.Thiel T, Ryk C, Renström-Koskela L, et al. Intravesical BCG treatment causes a long-lasting reduction of recurrence and progression in patients with high-risk non-muscle-invasive bladder cancer. World J Urol. 2019;37:155–163. doi: 10.1007/s00345-018-2375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho U, Park HS, Im SY, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One. 2018;13:e0200936. doi: 10.1371/journal.pone.0200936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imrie CW. Host systemic inflammatory response influences outcome in pancreatic cancer. Pancreatology. 2015;15:327–330. doi: 10.1016/j.pan.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 11.Dupré A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. 2018;44:566–570. doi: 10.1016/j.ejso.2018.02.209 [DOI] [PubMed] [Google Scholar]
- 12.Baracos VE. Cancer-associated malnutrition. Eur J Clin Nutr. 2018;72:1255–1259. doi: 10.1038/s41430-018-0245-4 [DOI] [PubMed] [Google Scholar]
- 13.Caccialanza R, Pedrazzoli P, Cereda E, et al. Nutritional support in cancer patients: a position paper from the italian society of medical oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J Cancer. 2016;7:131–135. doi: 10.7150/jca.13818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa MD, Vieira de Melo CY, Amorim AC, et al. Association between nutritional status, inflammatory condition, and prognostic indexes with postoperative complications and clinical outcome of patients with gastrointestinal neoplasia. Nutr Cancer. 2016;68:1108–1114. doi: 10.1080/01635581.2016.1206578 [DOI] [PubMed] [Google Scholar]
- 15.Okamura Y, Sugiura T, Ito T, et al. The optimal cut-off value of the preoperative prognostic nutritional index for the survival differs according to the TNM stage in hepatocellular carcinoma. Surg Today. 2017;47:986–993. doi: 10.1007/s00595-017-1491-0 [DOI] [PubMed] [Google Scholar]
- 16.Sagawa M, Yoshimatsu K, Yokomizo H, et al. Worse preoperative status based on inflammation and host immunity is a risk factor for surgical site infections in colorectal cancer surgery. J Nippon Med Sch. 2017;84:224–230. doi: 10.1272/jnms.84.224 [DOI] [PubMed] [Google Scholar]
- 17.Prabawa IPY, Bhargah A, Liwang F, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as a predictive value of hematological markers in cervical cancer. Asian Pac J Cancer Prev. 2019;20:863–868. doi: 10.31557/APJCP.2019.20.3.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu D, Jin J, Zhang L, et al. The neutrophil to lymphocyte ratio may predict benefit from chemotherapy in lung cancer. Cell Physiol Biochem. 2018;46:1595–1605. doi: 10.1159/000489207 [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Yao X, Xie X, et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol. 2017;35:261–270. doi: 10.1007/s00345-016-1864-9 [DOI] [PubMed] [Google Scholar]
- 20.Tomita M, Ayabe T, Maeda R, et al. Systemic immune-inflammation index predicts survival of patients after curative resection for non-small cell lung cancer. In Vivo. 2018;32:663–667. doi: 10.21873/invivo.11291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol. 2019;10:965–978. doi: 10.21037/jgo.2019.05.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, Hua YQ, Wang D, et al. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J Transl Med. 2019;17:30. doi: 10.1186/s12967-019-1782-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493503. doi: 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 24.Lach K, Peterson SJ. Nutrition support for critically ill patients with cancer. Nutr Clin Pract. 2017;32:578–586. doi: 10.1177/0884533617712488 [DOI] [PubMed] [Google Scholar]
- 25.Castillo-Martínez L, Castro-Eguiluz D, Copca-Mendoza ET, et al. Nutritional assessment tools for the identification of malnutrition and nutritional risk associated with cancer treatment. Rev Invest Clin. 2018;70:121–125. doi: 10.24875/RIC.18002524 [DOI] [PubMed] [Google Scholar]
- 26.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- 27.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 30.Ji F, Liang Y, Fu S, et al. Prognostic value of combined preoperative prognostic nutritional index and body mass index in HCC after hepatectomy. HPB. 2017;19(8):695–705. doi: 10.1016/j.hpb.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 31.Sun KY, Xu JB, Chen SL, et al. Novel immunological and nutritional-based prognostic index for gastric cancer. World J Gastroenterol. 2015;21:5961–5971. doi: 10.3748/wjg.v21.i19.5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2. doi: 10.1186/s13058-016-0794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6:74. doi: 10.1186/s40425-018-0383-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, Zhou S, Jiang W, et al. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32:201–215. doi: 10.1081/IMM-120022979 [DOI] [PubMed] [Google Scholar]
- 35.Jeon HG, Choi DK, Sung HH, et al. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol. 2016;23:321–327. doi: 10.1245/s10434-015-4614-0 [DOI] [PubMed] [Google Scholar]
- 36.Lolli C, Caffo O, Scarpi E, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front Pharmacol. 2016;7:376. doi: 10.3389/fphar.2016.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Guo X, Wu T, et al. Prognostic significance of inflammation-based indexes in patients with stage III/IV colorectal cancer after adjuvant chemoradiotherapy. Medicine. 2019;98:e14420. doi: 10.1097/MD.0000000000014420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomita M, Ayabe T, Maeda R, et al. Comparison of inflammation-based prognostic scores in patients undergoing curative resection for non-small cell lung cancer. World J Oncol. 2018;9:85–90. doi: 10.14740/wjon1097w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mori S, Usami N, Fukumoto K, et al. The significance of the prognostic nutritional index in patients with completely resected non-small cell lung cancer. PLoS One. 2015;10:e0136897. doi: 10.1371/journal.pone.0136897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noh GT, Han J, Cho MS, et al. Impact of the prognostic nutritional index on the recovery and long-term oncologic outcome of patients with colorectal cancer. J Cancer Res Clin Oncol. 2017;143:1235–1242. doi: 10.1007/s00432-017-2366-x [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Wang R, Ma W, et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Transl Med. 2019;7:431. doi: 10.21037/atm.2019.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui J, Chen S, Bo Q, et al. Preoperative prognostic nutritional index and nomogram predicting recurrence-free survival in patients with primary non-muscle-invasive bladder cancer without carcinoma in situ. Onco Targets Ther. 2017;10:5541–5550. doi: 10.2147/OTT.S146990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuk HD, Jeong CW, Kwak C, et al. Should intravesical Bacillus Calmette-Guerin (BCG) treatment be administered to patients with T0 after repeat transurethral resection of bladder tumor in patients with high-risk non-muscle invasive bladder cancer? PLoS One. 2018;13:e0208267. doi: 10.1371/journal.pone.0208267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261 [DOI] [PubMed] [Google Scholar]
- 45.Müller I, Munder M, Kropf P, et al. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522–530. doi: 10.1016/j.it.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 46.Shamamian P, Schwartz JD, Pocock BJ, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197–206. doi: 10.1002/jcp.10014 [DOI] [PubMed] [Google Scholar]
- 47.Pfeffer CM, Singh ATK. Apoptosis: a target for anticancer therapy. Int J Mol Sci. 2018;19:448. [DOI] [PMC free article] [PubMed] [Google Scholar]