Abstract
Subsolid nodules are common on chest CT imaging and may be either benign or malignant. Their varied features and broad differential diagnoses present management challenges. Although subsolid nodules often represent lung adenocarcinomas, other possibilities are common and influence management. Practice guidelines exist for subsolid nodule management for both incidentally and screening-detected nodules, incorporating patient and nodule characteristics. This review highlights the similarities and differences among these algorithms, with the intent of providing a resource for comparison and aid in choosing management options.
Key Words: American College of Chest Physicians, British Thoracic Society, Fleischner Society, ground-glass, subsolid
Abbreviations: ACR, American College of Radiology; BTS, British Thoracic Society; CHEST, American College of Chest Physicians; GGN, ground-glass nodule; IELCAP, International Early Lung Cancer Action Project; NCCN, National Comprehensive Cancer Network; PSN, part-solid nodule; SSN, subsolid nodule
Subsolid nodules (SSNs) include both pure ground-glass nodules (GGNs) and part-solid nodules (PSNs) and are increasingly detected on chest CT scans.1, 2, 3 In addition to the spectrum of primary adenocarcinoma of the lung, potential diagnoses include a number of alternate malignancies as well as benign lesions.
A range of imaging techniques and clinical concerns need to be considered when constructing differential diagnoses and establishing management guidelines. Of particular concern is the correlation among various morphologic CT appearances, including attenuation, shape, and internal complexity, in characterization and serial assessment for potential growth. In this regard, technical factors may profoundly influence nodule detection and encourage consistency in nodule assessment and reporting.
To date, multiple management algorithms have been developed to address these challenges, in both screening and nonscreening populations. These include those of the American College of Chest Physicians (CHEST),4 , 5 the British Thoracic Society (BTS),6 the Fleischner Society,7 the American College of Radiology (ACR),8 and the National Comprehensive Cancer Network (NCCN).9
Despite considerable overlap, including prioritization of shared decision-making, no one algorithm, either for screen-detected or incidentally identified nodules, is universally accepted. The current review compares and contrasts these algorithms, providing a resource for comparison that may aid in choosing management options.
Technical Aspects of Imaging and Reporting Subsolid Nodules
Current algorithms for lung nodules share essential CT acquisition and reporting considerations, regarding slice thickness, reconstruction algorithm, display windows, and value of multiplanar reformatted images (Table 1 ).4 , 6, 7, 8, 9, 10, 11, 12, 13
Table 1.
Guidelines for SSN Reporting
| Parameter | Current Recommendations |
|---|---|
| Slice thickness | |
| Reconstruction algorithm |
|
| Display window | |
| Multiplanar reformatted images |
|
| Reporting of nodule size |
|
BTS = British Thoracic Society; NCCN = National Comprehensive Cancer Network; SSN = subsolid nodule.
Practice guidelines addressing SSN management include those for screening-detected nodules from the American College of Radiology.
Applying consistent CT parameters enables reliable comparison across serial examinations. Full inspiratory images are universally recommended for lung nodule evaluation,9 , 10 with use of the lowest possible radiation exposure.6 , 8 , 10 Contrast enhancement is unnecessary.4 , 9 The presence of contrast increases dose and measured volume, mass, and mean attenuation of SSNs.14
Contiguous thin-section images improve nodule detection and feature evaluation,6 , 7 with management algorithms recommending 1-mm slice thickness and evaluation on lung windows at thinnest collimation. Mediastinal soft tissue display windows may aid in determining the presence of solid components within an SSN.11 , 12
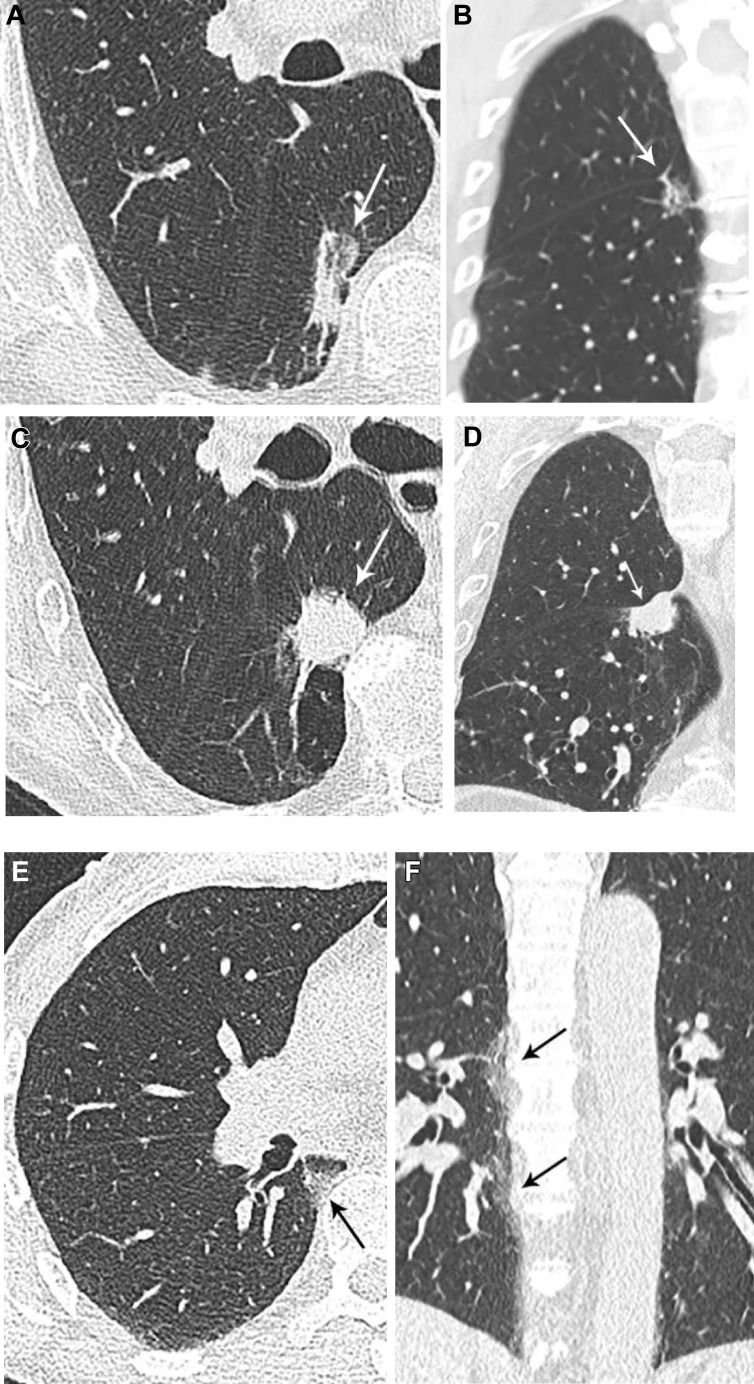
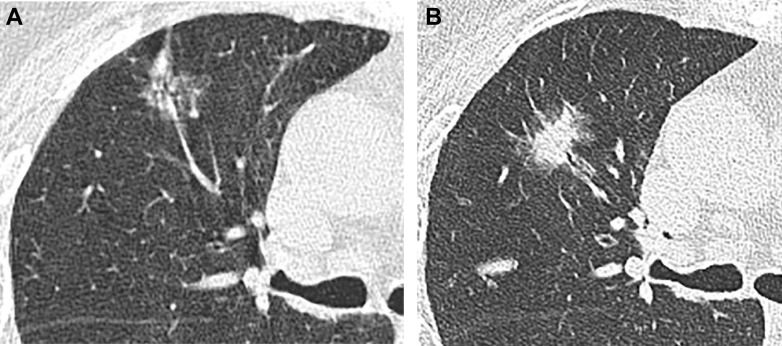
Sagittal and coronal image reconstruction aids SSN detection,6 , 7 which may be challenging in the setting of interstitial or smoking-related lung disease. Lung cancers in interstitial lung disease most often develop adjacent to or within regions of fibrosis,15 and review of non-axial reconstructions may exclude nodularity or identify convexities typical for scarring in regions of parenchymal abnormality, such as paravertebral or apical fibrosis (Fig 1 ).
Figure 1.
A-F, Value of multiplanar reformatted images for evaluating suspected lesions. A, An 83-year-old man with subsolid opacity in the paraspinal right lower lobe on axial image, believed to represent fibrosis. B, Coronal image exhibits an ovoid convex shape, atypical for scarring, and fissural tethering, which has been described with malignant lesions. On imaging 8 years later, the lesion has progressed in size and is now solid, with round shape in the axial plane (C) and increased fissural bowing in the coronal plane (D). The lesion was invasive adenocarcinoma on resection. E, Periosteophyte fibrosis may appear nodular in the axial plane. F, Coronal images show the craniocaudal distribution of fibrosis to better advantage.
Maximum and minimum intensity projection images may improve detection of solid nodules and SSNs, respectively.6 , 16 Volume rendering6 , 9 and computer-aided diagnosis9 are additional tools. Computer-aided diagnosis had a higher sensitivity for SSNs than visual detection (88.4% vs 34.2%) in 2,303 baseline screening examinations from the Multicenter Italian Lung Detection trial.17
Ultimately, nodule size is fundamental when deciding on a management approach. All nodule aspects are included in overall measurement, preferably on the high-frequency, sharp reconstruction algorithm, with reporting of size in the plane of maximal dimension.10
Approaching Management Algorithms: Patient Factors
Practice guidelines addressing SSN management include those for incidentally detected nodules from the CHEST, BTS, and Fleischner Society, and those for screening-detected nodules from the ACR (Lung-RADS) and NCCN (Table 2 ).4, 5, 6, 7, 8, 9 These algorithms specify applicable patient populations and incorporate patient and nodule-specific risk factors for lung cancer.
Table 2.
Management Algorithms for Subsolid Nodules
| Variable | Incidental SSNs |
Screen-Detected SSNs |
||||
|---|---|---|---|---|---|---|
| ACCP, 20134 | British Thoracic Society, 20156 | CHEST Clinical Practice Consensus Guidelines for Asia, 20165 | Fleischner Society, 20177 | American College of Radiology, Lung-RADS,a 20198 | National Comprehensive Cancer Network, 20209 | |
| Population for which guidelines applicable |
|
|
|
|
|
High-risk individuals:
|
| SSNs warranting imaging follow-up |
|
|
|
|
|
|
| Initial imaging intervals from baseline | Annual surveillance:
|
|
Annual surveillance
|
6-12 mo:
|
Annual surveillance (Lung-RADS 2):
|
Annual:
|
| Follow-up imaging intervals | Annual surveillance:
|
Surveillance at 1, 2, and 4 y from baseline in stable SSNs with low risk of malignancy (< 10%); malignancy risk assessed by Brock model and morphology (solid component size, pleural indentation, presence bubble lucencies), as well as factors such as smoking history and history of lung cancer | Annual:
|
Every 2 y:
|
Annual surveillance:
|
Annual:
|
| Size/density threshold for escalation | Persistent GGN:
|
|
Persistent PSN > 8 mm:
|
|
PET/CT (when solid component ≥ 8 mm) and/or tissue sampling depending on malignancy riskd and comorbidities:
|
Chest CT with contrast and/or PET:
|
| Surveillance end point for stable lesions |
|
|
|
|
|
|
CHEST = American College of Chest Physicians; GGN = pure ground-glass nodule; PSN = part-solid nodule; SSN = subsolid nodule (GGN or PSN).
Practice guidelines addressing SSN management include those for screening-detected nodules from the American College of Radiology.
Malignancy risk assessed by Brock model and morphology (eg, solid component size, pleural indentation, presence bubble lucencies), as well as factors such as smoking history and history of lung cancer.
Factors, including age, comorbidities, and treatment-associated risks, should be considered.
Endorses use of Brock calculator.
Patient Risk Profile
Risk of malignancy is a major consideration affecting nodule management guidelines and is often based on clinical judgment. The CHEST guidelines define high (> 65%), intermediate (5%-65%), and low (< 5%) malignancy risk categories incorporating clinical factors of age, smoking history, and previous cancer, and nodule features including size, margin, upper lobe location, imaging behavior (PET and serial CT imaging), and nonsurgical histopathology results.4 The CHEST risk categories are incorporated in the Fleischner Society guidelines, which recommend risk assignment based on the CHEST low-risk category and grouping of the intermediate- and high-risk categories.7 The BTS and NCCN also consider both clinical risk factors and radiologic nodule features.
Qualitative risk prediction, based on clinician judgment, or quantitative, model-based risk prediction are encouraged by the CHEST guidelines.4 There are several models, or probability calculators, synthesizing clinical and imaging features, such as the Bayesian Inference Malignancy Calculator, Brock, Herder, Mayo Clinic, Thoracic Research Evaluation and Treatment, and Department of Veterans Affairs models.18
Each of the models is derived from specific patient populations.18 Model performance is optimal when applied to populations similar to those from which the model was derived.4 , 18 For this reason, separate CHEST consensus guidelines for Asia were developed indicating that diagnostic risk calculators may not apply to Asian patients due to the higher rate of lung cancer in women, as well as the higher prevalence of TB and environmental exposures.5
The CHEST recommends the Mayo Clinic probability model in the US population, developed and validated from a patient cohort with incidentally detected nodules on chest radiography.19 The Department of Veterans Affairs model is similarly based on incidentally detected nodules, albeit in the higher risk veteran population.20 Screening data inform the Brock model, synonymous with the PanCan or Vancouver models, in reference to the screening cohorts.2
Risk assessment models can be applied at multiple points in nodule management. For example, the BTS guidelines suggest risk prediction at two separate junctures along the management algorithm, initially using the Brock calculator to determine if malignancy risk is > 10%.6 When malignancy risk is estimated to be < 10%, BTS guidelines advise CT surveillance over biopsy or resection for SSNs. For patients who undergo further evaluation with PET/CT imaging, the BTS guidelines suggest using the Herder risk assessment model, which incorporates fluorodeoxyglucose activity with Mayo-predicted probability,21 to guide subsequent management.
For screen-detected nodules, Lung-RADS categorizes findings and standardizes management, and recommends the Brock calculator for risk stratifying patients with category 4B or 4X (very suspicious) lesions.8 Brock model inputs include age, sex, family history, emphysema, nodule size, nodule spiculation, number of nodules, lobar location, and nodule attenuation (solid, partially solid, or nonsolid).2
The Brock model is the only validated model incorporating nodule attenuation and thus SSNs. Of note, the Brock model is based on a screening population (50-75 years of age) with smoking history, which may affect its performance when applied to female nonsmokers, in whom a higher incidence of SSNs/indolent lung adenocarcinomas are reported.22 Studies have evaluated the efficacy of models when applied to differing populations and roles,23 , 24 and knowledge of their performance in these scenarios is important. A future role may exist for model-based patient selection for lung cancer screening.25 Management of small nodules may also be aided by the application of models.26
Some clinical factors associated with higher lung cancer risk are not reflected in risk models. As noted in the NCCN guidelines,9 COPD and interstitial lung disease are risk factors for lung cancer but are not specifically included in the Brock model. The incidence of lung cancer in patients with interstitial lung disease and COPD is nearly threefold higher than in patients with COPD alone.27 In the National Lung Screening Trial cohort of > 25,000 participants, those with asymptomatic interstitial abnormalities (eg, baseline reticulonodular opacities, honeycombing, fibrosis, scarring) had a higher incidence of and mortality from lung cancer.28
Family history is included in both the NCCN and Brock model risk assessments. In a pooled analysis from the International Lung Cancer Consortium, having a first-degree relative with lung cancer conferred 1.5 times increased risk, after adjustment for smoking and additional risk factors.29
Young Patient Age
The risk for malignancy is low in younger patients. The incidence of all cancers in adolescents and young adults is only approximately 75.5 per 100,000, with lung cancer among neither the most common nor the most deadly cancers for patients aged < 40 years.30 However, certain clinical and radiologic features may render particular nodules more suspicious, warranting closer follow-up regardless of age. The Fleischner, BTS, and CHEST guidelines address incidentally detected nodules, with BTS guidelines applicable to adults aged ≥ 18 years and Fleischner guidelines applicable to individuals aged ≥ 35 years. In patients aged < 35 years presenting with SSNs, recommendations are therefore based on the clinical scenario.
Patients With Prior or Extrathoracic Primary Malignancy
Guidelines for incidentally detected nodules are not intended for patients with known primary neoplasms in whom metastatic disease would be a consideration.7 This rationale applies more for solid nodules; however, SSNs may uncommonly represent metastases, in which case their behavior and neoplastic potential depend on the type and grade of the primary malignancy.
Lymphomas, mucinous GI neoplasms, extrapulmonary adenocarcinomas, and tumors associated with hemorrhage creating ground-glass opacity may all present as SSNs.31 Ground-glass attenuation produced by metastases is infrequently due to the lepidic growth32 , 33 that characterizes most lung adenocarcinoma spectrum lesions. Metastatic lesions initially presenting as SSNs may exhibit aggressive rather than indolent behavior.33 , 34 Therefore, close follow-up is prudent in oncology patients with new SSNs.
Reconciling Management Algorithms: Surveilling Nodules
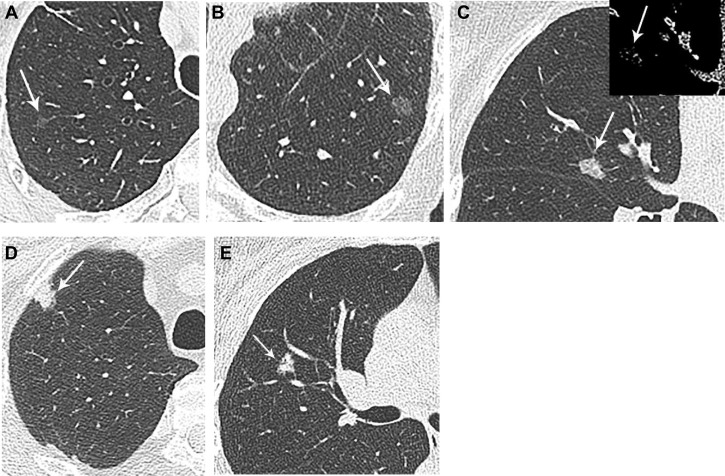
Surveillance recommendations for SSNs are guided by the main cause for persistent SSNs: lesions on the spectrum of lung adenocarcinoma. Lung adenocarcinoma spectrum lesions are currently classified pathologically by using the International Association for the Study of Lung Cancer system, which has been integrated into the World Health Organization TNM staging (Fig 2 , Table 3 13). This classification applies to small (≤3 cm) nonmucinous lung adenocarcinomas with ground-glass attenuation and lepidic growth patterns on pathology. The algorithms direct surveillance primarily based on nodule size and ground-glass or part-solid density.
Figure 2.
A-F, Lung adenocarcinoma spectrum lesions exhibit greater soft tissue density with greater invasive features. A, Atypical adenomatous hyperplasia: 76-year-old woman with multiple right upper lobe subsolid nodules, and incidental resection of 5-mm pure ground-glass nodule, atypical adenomatous hyperplasia, at time of right upper lobectomy for invasive adenocarcinoma (lesion not pictured). B, Adenocarcinoma in situ: 70-year-old woman with 11-mm pure ground-glass left lower lobe nodule, adenocarcinoma in situ on resection. C, Minimally invasive adenocarcinoma: 76-year-old woman with 11-mm subsolid right middle lobe nodule. Although there is no discrete solid component on imaging, the nodule is denser than ground-glass in attenuation and was a minimally invasive adenocarcinoma on resection. The inset shows the lesion in mediastinal windowing, confirming small solid aspects. D, Invasive adenocarcinoma: 66-year-old man with predominately solid right upper lobe nodule, found to be adenocarcinoma, with an acinar pattern predominant with lepidic, papillary, and focally solid patterns. E, Mucinous adenocarcinoma: 46-year-old woman with 9-mm subsolid right upper lobe nodule with coursing air bronchogram, found to be mucinous adenocarcinoma on percutaneous core biopsy. Invasive mucinous adenocarcinoma is a less common and distinct histopathologic subtype of lung adenocarcinoma.
Table 3.
IASLC Staging: Pathologic Criteria13
| Atypical Adenomatous Hyperplasia | Adenocarcinoma in Situ | Minimally Invasive Adenocarcinoma | Invasive Adenocarcinoma |
|---|---|---|---|
|
|
|
|
IASLC = International Association for the Study of Lung Cancer.
Determining Nodules Necessitating Follow-up: Size, Density, and Number
For incidentally detected SSNs, size threshold necessitating follow-up is typically 5 mm.4, 5, 6, 7 This reflects that nodules ≤ 5 mm correspond to atypical adenomatous hyperplasia. The CHEST consensus guidelines for Asia recommend considering surveillance for even smaller SSNs, recognizing the increased risk in this population.5 The Fleischner guidelines also suggest that CT follow-up at 2 and 4 years may be obtained in Asian populations7 for solitary nodules ≤ 5 mm, as these may represent preinvasive lesions.
PSNs are managed differently than nonsolid nodules due to their association with invasive lung adenocarcinoma. The Fleischner guidelines note a potential limitation in discerning small solid aspects of already-small nodules, thus generally considering all PSNs to be at least 6 mm in size,7 although follow-up is not precluded for smaller nodules if morphologically suspicious or the patient is high risk.7 A multiplicity of nodules, irrespective of size and/or pure ground-glass density, would be followed up in 3 to 6 months as per the Fleischner guidelines. Multiple SSNs, when persistent, most often represent synchronous or metachronous lung primaries rather than intrapulmonary metastasis.35 , 36 This pattern most often occurs in female nonsmokers, in both North American and Asian groups.37
Decisions regarding surveillance vs treatment for persisting SSNs require evaluating each nodule individually,4 such as in terms of overall and solid component size. The most suspicious nodule may not be the largest nodule.7
Establishing Nodule Persistence
Persistence of a nodule has significant implications upon differential diagnosis (Table 4 ), including malignant (Figs 2 and 3 ) and benign (Fig 4 ) causes. Establishing persistence of a subsolid lesion is recommended by the CHEST, BTS, and Fleischner guidelines, because up to 70% of SSNs may be transient.38 The CHEST Consensus Asian Guidelines further suggest that empiric antimicrobial agents may be appropriate for PSNs > 8 mm in size.5
Table 4.
Differential Considerations for Subsolid Nodules
| Primary Lung Adenocarcinoma | Non-Primary Lung Adenocarcinoma Etiologies |
|---|---|
| • Atypical adenomatous hyperplasia | • Transient infection (eg, aspergillosis, candidiasis) |
| • Adenocarcinoma in situ | • Transient inflammation |
| • Minimally invasive adenocarcinoma | • Focal interstitial fibrosis |
| • Invasive adenocarcinoma | • Organizing pneumonia |
| • Mucinous adenocarcinoma | • Eosinophilic pneumonia |
| • Alveolar sarcoid | |
| • Drug reaction | |
| • Vasculitis (granulomatosis with polyangiitis) | |
| • Endometriosis | |
| • Mucosa associated lymphoid tissue (MALT) and lymphoproliferative disorders | |
| • Metastatic lesions (including melanoma; renal carcinoma; breast, GI, and pancreatic adenocarcinomas) |
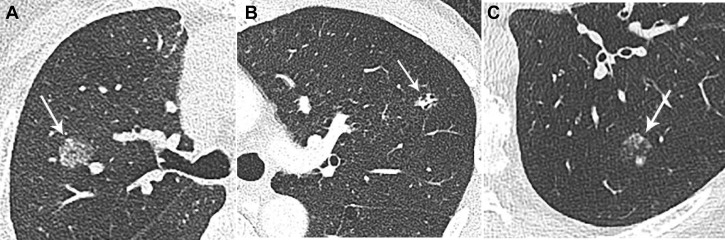
Figure 3.
A-C, Alternative neoplastic entities presenting as persistent subsolid nodules. A, Mucosa-associated lymphoid tissue: 81-year-old woman with subsolid right upper lobe nodule proven to be extranodal marginal zone lymphoma with plasmacytic differentiation on resection. Lymphomas may be uni- or multifocal and, unlike multiple synchronous primary lung adenocarcinomas, usually progress in unison. B, GI adenocarcinoma metastases: 54-year-old man with colorectal adenocarcinoma, and a left upper lobe metastasis with internal bubble lucencies. C, Melanoma: 79-year-old man with melanoma confined to the ear 4 years prior, with new mixed solid and subsolid nodule, with posterior solid component doubling in size over 3 months (baseline image not shown). On resection, the nodule was found to be metastatic melanoma.
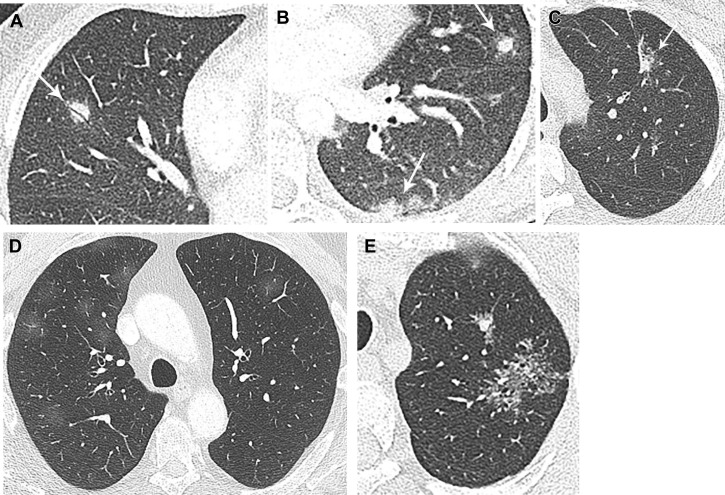
Figure 4.
A-E, Non-neoplastic etiologies for subsolid nodules. A, Organizing pneumonia: 45-year-old man with cutaneous T-cell lymphoma and multiple subsolid and solid nodules, including the imaged right middle lobe nodule with coursing air bronchogram. Wedge resections of multiple nodules, including the right middle lobe nodule, demonstrated organizing pneumonia. B, Fungal infection: 26-year-old woman with multiple predominately solid nodules with ground-glass halo and recent history of cave-diving. Percutaneous core biopsy specimen revealed non-necrotizing granulomatous inflammation with Grocott methenamine silver-positive structures suggestive of fungal organisms. C, Focal fibrosis: 46-year-old woman with history of smoking and mildly fluorodeoxyglucose-avid left upper lobe part solid nodule, with linear pleural extension. Percutaneous core biopsy revealed fibroelastic scar. D, Drug reaction: 59-year-old man on immunotherapy for renal carcinoma, with the emergence of multiple bilateral ground-glass nodules coinciding with an increase in dosage of immunotherapy. E, Alveolar sarcoid: 63-year-old man with World Trade Center Ground Zero exposure and left upper lobe subsolid lesions. The dominant mass appears to be a confluence of perilymphatic micronodules, compatible with pathologically proven alveolar sarcoid post-left upper lobectomy.
For participants of the International Early Lung Cancer Action Project (IELCAP), nearly 20% of PSNs39 and 26% of nonsolid nodules40 identified on baseline decreased in size or resolved. Comparably, in 622 PSNs and GGNs from the National Lung Screening Trial cohort, 28% resolved on follow-up imaging.3 In addition, in 264 SSNs from the Dutch Belgian Lung Cancer Screening trial (NELSON) cohort, 63% resolved on follow-up.41 SSNs identified on follow-up rounds compared with baseline are more likely to resolve: in IELCAP, 66% of new nonsolid nodules and 70% of new PSNs decreased in size or resolved.39 , 40 Given the likely transience for new SSNs on subsequent screening examinations, Lung-RADS version 1.1 suggests that new large nodules may be surveilled at a short 1-month interval rather than proceeding to further evaluation.8 Similarly, the NCCN algorithm for a newly detected SSNs on follow-up first asks whether there is suspected infection/inflammation and, if so, recommends low-dose CT imaging in 1 to 3 months.9
New SSNs on follow-up examinations in patients without malignancy are favored to be transient given the indolent nature of SSNs, with reported volume doubling times of 457 to 568 days for PSNs and 469 to 813 days for GGNs.42 , 43 Similar to IELCAP findings, data from the NELSON trial showed that 67% of newly detected SSNs (on 1-, 3-, and 5.5-year incidence screening rounds) resolved, and new SSNs after baseline occurred in < 1% of participants.44 Although three of 16 nonresolving newly detected SSNs were malignant in NELSON (adenocarcinoma in situ in two nodules, and stage 1A invasive adenocarcinoma in one nodule), favorable staging of these lesions despite protracted referral after 1 year did not support the need for more aggressive management.44
Transient nodules are also common in patients with extrapulmonary malignancies; in a retrospective study of 78 patients with extrapulmonary malignancies, new SSNs were commonly transient (36 of 78 nodules).45
Younger age,45, 46, 47 male sex, and peripheral eosinophilia are associated with resolving subsolid opacities. Nodule features such as detection on a follow-up examination,39 , 45 , 46 multiplicity,45 , 46 ill-defined margins, nonspiculated margins, and large solid component46 are more often associated with SSN transience, as are polygonal shape (as opposed to round), mixed density (rather than pure ground-glass), and larger size.48 In contrast, pleural retraction and “bubble” lucencies are more common in persisting SSNs.45
Confirming Nodule “Growth”
Overall nodule size and solid component size are associated with pathologic staging as well as outcomes for lung adenocarcinoma spectrum lesions.13 , 49 Establishing nodule growth is based on change in size ≥ 2 mm as per the Fleischner and BTS guidelines6 , 50 and > 1.5 mm as per the Lung-RADS version 1.1 of the ACR,8 as smaller changes are within measurement error. Volumetric growth is an additional parameter included in both the BTS and Lung-RADS algorithms6 , 8 and may facilitate earlier lung cancer diagnosis.51 The NELSON trial classified screen-detected nodules into growth and subsequent management categories based on volume doubling time; in SSNs, volumetric segmentation was applied to the solid portion and diameter for the overall nodule.52 This method recognizes the challenges in defining SSNs that can contribute to inaccurate segmentations.12 , 53, 54, 55
Nodule progression may also manifest as new or increasing solid component, or uniform increase in attenuation. Nodule mass, incorporating both attenuation and volume, is associated with less intraobserver and interobserver variability compared with either diameter or volume alone, and an earlier indicator of nodule growth for SSNs.56 A change in this measure would reflect an increase in nodule size and/or density. Nodule mass assessment is recommended in the BTS guidelines, although it requires volumetric segmentation, and it may be more broadly recommended in the future.6
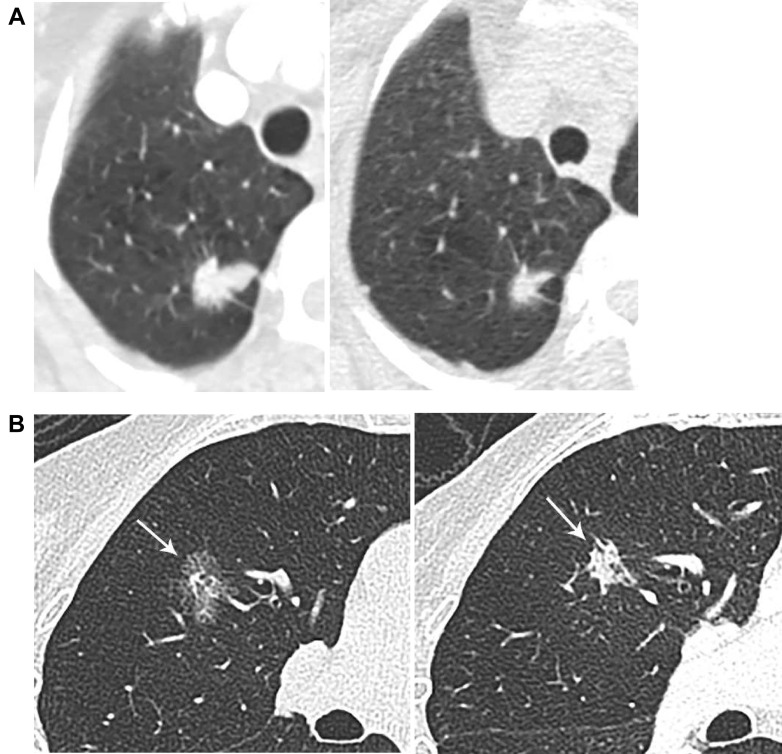
Accurate assessment of growth on CT imaging may be more difficult because of nodule attenuation, shape, location, and scan interval.6 Lesions adjacent the mediastinum or lung base may be affected by cardiac or inspiratory motion, and greater inspiratory effort inversely affects volume of solid nodules.57 Growth is more evident when comparing examinations separated by longer intervals,6 , 10 highlighting the need for comparison vs baseline studies in addition to the immediately prior imaging, which is especially useful for lesions with indolent behavior (Fig 5 ). Growth-rate precision also increases with a greater time interval between scans.51
Figure 5.
A-B, Indolent lesion growth. A, A 79-year-old woman with subsolid right upper lobe opacity, which demonstrated little interval change on successive examinations. B, Baseline image, as opposed to immediate prior exams, better demonstrated the change in lesion size, density, and morphology, as evidenced in this follow-up image 15 years later. This was a primary lung adenocarcinoma.
Contracting nodules are an uncommonly encountered pitfall, as nodules may at times decrease in size at points in their growth curve.58 Progressing nodules may contract in one or both dimensions with increasing soft tissue, related to fibrotic alveolar collapse, or increasing invasive components.59 , 60 Spurious contraction may be due to inflammatory components of cancers, which can be misinterpreted in the absence of continued follow-up imaging or investigation (Fig 6 ).
Figure 6.
A-B, Contracting nodules proven malignant. A, A 73-year-old woman with brain mass found to have a 2.4-cm right upper lobe nodule, with very mild surrounding ground-glass. Biopsy was requested prior to inpatient neurosurgery. Four days later, the lesion decreased to 1.5 cm in size. Results of percutaneous core biopsy revealed invasive adenocarcinoma. The decrease in nodule size was attributed to the high-dose steroids administered for cerebral edema. B, An 85-year-old woman with multiple lung adenocarcinomas and a right lung subsolid nodule (2.2 × 1.7 cm) that contracted over 18 months (1.3 × 1 cm), as solid density increased.
Stable Large Pure GGNs
Larger size in pure GGNs is associated with higher probability of invasive adenocarcinoma.61, 62, 63 Liu et al61 found that 35.4% (56 of 158) of pure GGNs represented invasive adenocarcinomas, significant for tumor volume ≥ 1,125 mm3. Lim et al62 reported that 39% of persistent pure GGNs > 16 mm were invasive adenocarcinomas. Nevertheless, the malignancy potential of stable or slowly growing nonsolid nodules ≥ 30 mm is classified as Lung-RADS category 2, which implies a risk of malignancy estimate of < 1%.
Recent risk-based stratification models suggest that the probability of malignancy for SSNs currently assigned to Lung-RADS categories 2 and 3 may be higher (3% and 13%, respectively) vs current risk predictions of <1% and 1% to 2%.3 Future iterations may consider additional size-based risk stratifications, given that Lung-RADS category 2 GGNs that are > 10 mm in size have greater malignancy risk than subcentimeter GGNs. Current CHEST guidelines recommend that biopsy or resection may be considered for pure GGNs > 10 mm, and the BTS guidelines recommend the same approach even for stable persistent GGNs in which malignancy risk is > 10%. The BTS guidelines also suggest that resection and nonsurgical treatment may be considered for GGNs increasing in size by ≥ 2 mm. Lung-RADS has no recommendation for tissue sampling for pure GGNs, although category 4x may suggest tissue sampling for GGNs ≥ 30 mm. These lesions may have indolent behavior, and it is unclear if aggressive management translates into improved outcomes.
Reaching Management Determinations: Escalation and End Points
The purpose of follow-up is to guide decision-making in the patient’s best interest. This includes an emphasis on shared decision-making.
Surveillance End Points and Delayed Progression
The choice between surveillance and action is influenced by an increase in nodule size, new or increasing solid component, and pace of growth as indicated by surveillance intervals. Biopsy, resection, or nonsurgical treatment can also be pursued for subsolid lesions in the setting of > 10% malignancy risk per the BTS guidelines.
The appropriate length of imaging surveillance for nonscreening patients with stable SSNs is an unanswered question. SSNs may exhibit lengthy volume doubling times, consistent with their often-indolent behavior. For example, in a retrospective cohort of 97 patients with SSNs, median volume doubling times ranged from 759 to 1,832 days, with more rapid volume doubling time for those nodules with solid components > 5 mm.64 In this cohort, the upper limits of median volume doubling time for ground-glass lesions reached over 12 years.
For stable SSNs, CHEST recommends a minimum follow-up duration of 3 years, BTS 4 years, and Fleischner 5 years. The CHEST Clinical Practice Consensus Guidelines for Asia encourage consideration of ongoing surveillance beyond 3 years.5 A reasonable end point for surveillance of stable SSNs in nonscreening populations includes patient counseling, such as whether diagnosis and treatment would be pursued for progressing nodules.65 ACR and NCCN screening guidelines similarly suggest cessation of follow-up if patients are no longer candidates for definitive treatment, have life-limiting comorbidities, or would defer eventual treatment.
PET/CT Imaging
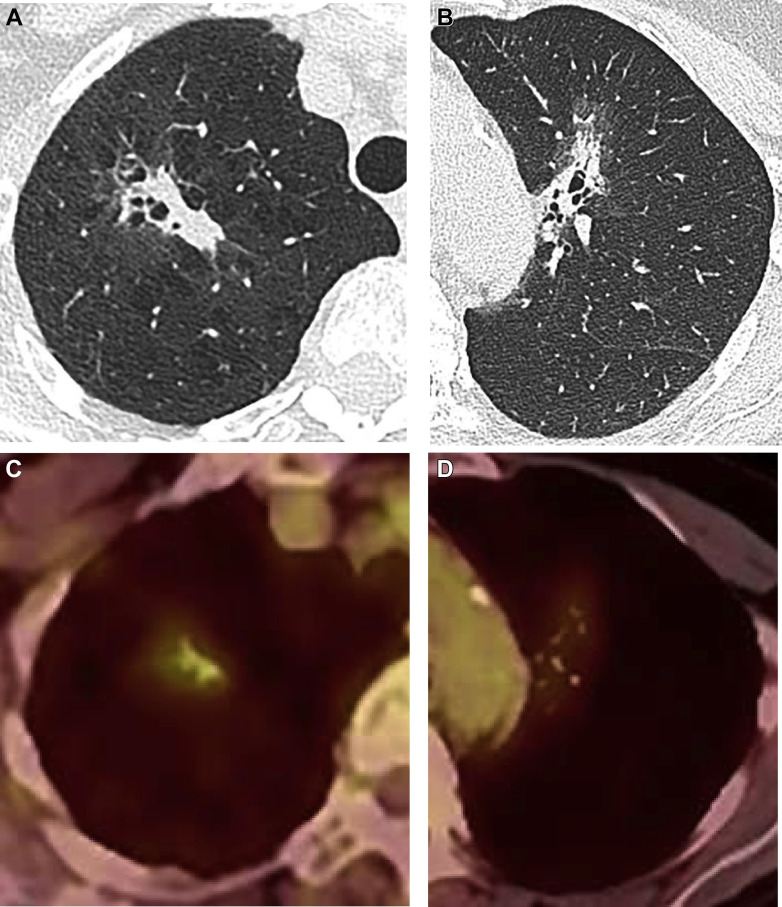
PET/CT imaging is not recommended to characterize GGNs or other SSNs with small solid components.4 Fluorodeoxyglucose avidity is nonspecific, and both false-positive and false-negative findings may occur66 , 67 (Fig 7 ). Infectious/inflammatory processes may result in false-positive outcomes for subsolid opacities.68 , 69 False-negative findings in SSNs may be due to lower metabolism of indolent lesions, lesions or solid components below the threshold for PET-CT spatial resolution, mucinous lesions, or location mis-registration.66 , 70 PET is also of limited utility for preoperative staging of T1 SSNs.71
Figure 7.
A-D, Fluorodeoxyglucose (FDG) avidity may be nonspecific. A-B, Subsolid predominately soft tissue right upper lobe mass, demonstrating FDG avidity; however, it was found to be organizing pneumonia following a right upper lobectomy. C-D, Subsolid left upper lobe mass demonstrating FDG avidity, found to be invasive lung adenocarcinoma on resection.
Tissue Sampling and Treatment Options
The rate of benign diagnoses for SSNs following biopsy ranges from 6% to 39%72, 73, 74, 75 and may be affected by differing indications, referral patterns, and patient preferences, including desire for diagnostic certitude and level of risk tolerance.4 Benign diagnoses included fibrosis, organizing pneumonia, or presumed infection/inflammation. Biopsy of subsolid lesions has been associated with lower diagnostic accuracy compared with solid lesions,76 which may be due to lower cellularity.72 However, others have shown comparable diagnostic accuracy for malignancy comparing SSNs and solid nodules77 and up to 97% diagnostic accuracy in a series of 67 patients with ground-glass lesions sampled by using percutaneous core needle biopsy.73
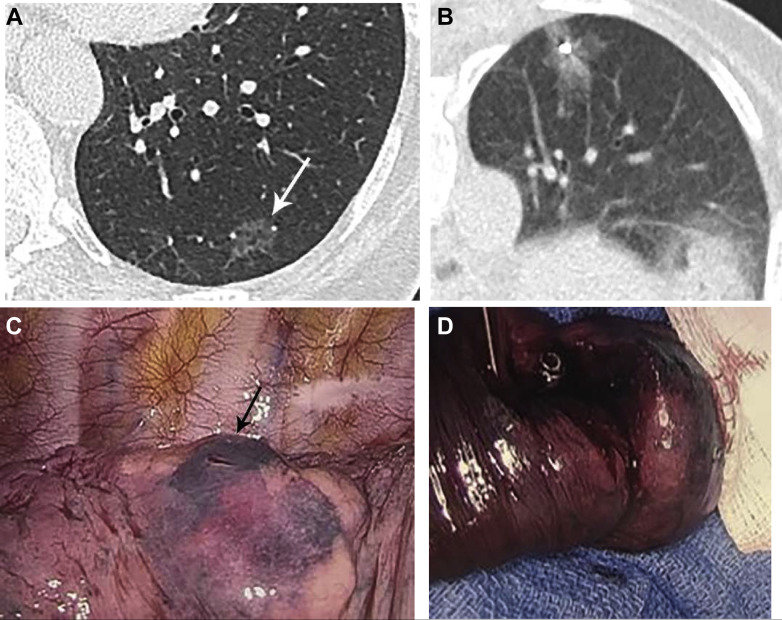
For primary invasive lung cancers in which definitive local therapy is possible, the NCCN and BTS guidelines favor parenchymal-sparing surgical resection. Radiotherapy and ablative therapies are additional local treatment options.78 For GGNs, localization may be necessary prior to surgical resection. Options for localization include CT-guided wire or marker placement; percutaneous injection of dye, radiotracer, or other material (Fig 8 ); or navigational bronchoscopic localization.79
Figure 8.
A-D, Subsolid nodule localization and surgical resection. A, Left lower lobe ground-glass nodule in a patient electing surgical resection. B, Intraprocedural prone image demonstrating coil deployment adjacent to the nodule, and methylene blue injected upon needle retraction. C, Intraoperative photo shows the methylene blue along the lung surface, and needle puncture mark. (Image courtesy of Dr Bernard Crawford.) D, Gross resection image demonstrates the metallic coil. (Image courtesy of Dr Bernard Crawford.)
Radiation and other ablative therapies are most often pursued in nonsurgical candidates6 and increasingly as a lung-sparing treatment option in surgical candidates. This approach is especially relevant for patients presenting with multiple synchronous primary lesions. Simulation modeling has suggested superior outcomes with stereotactic body radiation therapy compared with lobectomy or nontherapy for SSNs.80 However, trials comparing these treatments to establish noninferiority of ablative options have failed to accrue participants. The gold standard remains surgical resection for patients who are surgical candidates.
Conclusions
Although there are overlapping and distinct aspects to the various algorithms for incidentally and screen-detected SSNs, all algorithms highlight shared decision-making and patient counseling to reach practical management approaches. The side-by-side presentation of these guidelines may help prioritize the management options, with the understanding that ongoing research will refine recommendations.81 Lesion, patient, and even local epidemiologic factors are considerations needed to arrive at balanced management decisions. Future guidelines, such as those from CHEST, BTS, and the Fleischner Society, will continue to evolve in step with knowledge of SSN behavior and management.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. P. N. is a consultant for Google, Inc. and on the advisory boards of Biodesix, Inc., and Exact Science, Inc. None declared (L. A., J. P. K., W. H. M.).
References
- 1.Henschke C.I., Yankelevitz D.F., Mirtcheva R., McGuinness G., McCauley D., Miettinen O.S. CT Screening for Lung Cancer. Am J Roentgenol. 2002;178(5):1053–1057. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 2.McWilliams A., Tammemagi M.C., Mayo J.R. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369(10):910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammer M.M., Palazzo L.L., Kong C.Y., Hunsaker A.R. Cancer risk in subsolid nodules in the National Lung Screening Trial. Radiology. 2019;293(2):441–448. doi: 10.1148/radiol.2019190905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gould M.K., Donington J., Lynch W.R. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Chest. 2013;143(5):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai C., Choi C.M., Chu C.M. Evaluation of pulmonary nodules: clinical practice consensus guidelines for Asia. Chest. 2016;150(4):877–893. doi: 10.1016/j.chest.2016.02.650. [DOI] [PubMed] [Google Scholar]
- 6.Callister M.E.J., Baldwin D.R., Akram A.R. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70(suppl 2):ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 7.MacMahon H., Naidich D.P., Goo J.M. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 8.Lung Rads American College of Radiology. [cited 2020 Mar 9] https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads
- 9.Wood DE, Kazerooni EA, Chair ф V, et al. NCCN Guidelines Version 1.2020 Lung Cancer Screening Continue NCCN Guidelines Panel Disclosures. 2019.
- 10.Bankier A.A., MacMahon H., Goo J.M., Rubin G.D., Schaefer-Prokop C.M., Naidich D.P. Recommendations for measuring pulmonary nodules at CT: a statement from the Fleischner Society. Radiology. 2017;285(2):584–600. doi: 10.1148/radiol.2017162894. [DOI] [PubMed] [Google Scholar]
- 11.Yanagawa M., Kusumoto M., Johkoh T. Radiologic–pathologic correlation of solid portions on thin-section CT images in lung adenocarcinoma: a multicenter study. Clin Lung Cancer. 2018;19(3):e303–e312. doi: 10.1016/j.cllc.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Kakinuma R., Noguchi M., Ashizawa K. Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol. 2016;11(7):1012–1028. doi: 10.1016/j.jtho.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Travis W.D., Asamura H., Bankier A.A. The IASLC Lung Cancer Staging Project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2016;11(8):1204–1223. doi: 10.1016/j.jtho.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J.G., Goo J.M., Yoo R.E. The effect of late-phase contrast enhancement on semi-automatic software measurements of CT attenuation and volume of part-solid nodules in lung adenocarcinomas. Eur J Radiol. 2016;85(6):1174–1180. doi: 10.1016/j.ejrad.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Oh S.Y., Kim M.Y., Kim J.E. Evolving early lung cancers detected during follow-up of idiopathic interstitial pneumonia: serial CT features. Am J Roentgenol. 2015;204(6):1190–1196. doi: 10.2214/AJR.14.13587. [DOI] [PubMed] [Google Scholar]
- 16.Li W., Chu Z., Zhang Y., Li Q., Zheng Y., Lv F. Effect of Slab thickness on the detection of pulmonary nodules by use of CT maximum and minimum intensity projection. Am J Roentgenol. 2019;213(3):562–567. doi: 10.2214/AJR.19.21325. [DOI] [PubMed] [Google Scholar]
- 17.Silva M., Schaefer-Prokop C.M., Jacobs C. Detection of subsolid nodules in lung cancer screening: complementary sensitivity of visual reading and computer-aided diagnosis. Invest Radiol. 2018;53(8):441–449. doi: 10.1097/RLI.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 18.Choi H.K., Ghobrial M., Mazzone P.J. Models to estimate the probability of malignancy in patients with pulmonary nodules. Ann Am Thorac Soc. 2018;15(10):1117–1126. doi: 10.1513/AnnalsATS.201803-173CME. [DOI] [PubMed] [Google Scholar]
- 19.Swensen S.J. The probability of malignancy in solitary pulmonary nodules. Arch Intern Med. 1997;157(8):849. [PubMed] [Google Scholar]
- 20.Gould M.K., Ananth L., Barnett P.G. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131(2):383–388. doi: 10.1378/chest.06-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herder G.J., Van Tinteren H., Golding R.P. Clinical prediction model to characterize pulmonary nodules: validation and added value of18F-fluorodeoxyglucose positron emission tomography. Chest. 2005;128(4):2490–2496. doi: 10.1378/chest.128.4.2490. [DOI] [PubMed] [Google Scholar]
- 22.Arenberg D. Bronchioloalveolar lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(suppl 3):306S–313S. doi: 10.1378/chest.07-1383. [DOI] [PubMed] [Google Scholar]
- 23.Isbell J.M., Deppen S., Putnam J.B. Existing general population models inaccurately predict lung cancer risk in patients referred for surgical evaluation. Ann Thorac Surg. 2011;91(1):227–233. doi: 10.1016/j.athoracsur.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair V.S., Sundaram V., Desai M., Gould M.K. Accuracy of models to identify lung nodule cancer risk in the National Lung Screening Trial. Am J Respir Crit Care Med. 2018;197(9):1220–1223. doi: 10.1164/rccm.201708-1632LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ten Haaf K., Bastani M., Cao P. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5):466–479. doi: 10.1093/jnci/djz164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tammemägi M.C., Haaf K. Ten., Toumazis I. Development and validation of a multivariable lung cancer risk prediction model that includes low-dose computed tomography screening results: a secondary analysis of data from the National Lung Screening Trial. JAMA Netw Open. 2019;2(3) doi: 10.1001/jamanetworkopen.2019.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi W. I.l., Park S.H., Park B.J., Lee C.W. Interstitial lung disease and lung cancer development: a 5-year nationwide population-based study. Cancer Res Treat. 2018;50(2):374–381. doi: 10.4143/crt.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittaker Brown S.A., Padilla M., Mhango G. Interstitial lung abnormalities and lung cancer risk in the National Lung Screening Trial. Chest. 2019;156(6):1195–1203. doi: 10.1016/j.chest.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 29.Coté M.L., Liu M., Bonassi S. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur J Cancer. 2012;48(13):1957–1968. doi: 10.1016/j.ejca.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henley S.J., Ward E.M., Scott S. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126(10):2225–2249. doi: 10.1002/cncr.32802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo J.B., Im J.G., Goo J.M., Chung M.J., Kim M.Y. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics. 2001;21(2):403–417. doi: 10.1148/radiographics.21.2.g01mr17403. [DOI] [PubMed] [Google Scholar]
- 32.Okita R., Yamashita M., Nakata M., Teramoto N., Bessho A., Mogami H. Multiple ground-glass opacity in metastasis of malignant melanoma diagnosed by lung biopsy. Ann Thorac Surg. 2005;79(1) doi: 10.1016/j.athoracsur.2004.03.096. [DOI] [PubMed] [Google Scholar]
- 33.Kang M.J., Kim M.A., Park C.M., Lee C.H., Goo J.M., Lee H.J. Ground-glass nodules found in two patients with malignant melanomas: different growth rate and different histology. Clin Imaging. 2010;34(5):396–399. doi: 10.1016/j.clinimag.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 34.Borghesi A., Bercich L., Michelini S., Bertagna F., Scrimieri A., Maroldi R. Pulmonary metastases from malignant epithelioid schwannoma of the arm presenting as fast-growing subsolid nodules: report of an unusual case. Eur J Radiol Open. 2019;6:307–314. doi: 10.1016/j.ejro.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung J.H., Choe G., Jheon S. Epidermal growth factor receptor mutation and pathologic-radiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol. 2009;4(12):1490–1495. doi: 10.1097/JTO.0b013e3181bc9731. [DOI] [PubMed] [Google Scholar]
- 36.Girard N., Deshpande C., Lau C. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol. 2009;33(12):1752–1764. doi: 10.1097/PAS.0b013e3181b8cf03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Detterbeck F.C., Marom E.M., Arenberg D.A. The IASLC lung cancer staging project: background data and proposals for the application of TNM staging rules to lung cancer presenting as multiple nodules with ground glass or lepidic features or a pneumonic type of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11(5):666–680. doi: 10.1016/j.jtho.2015.12.113. [DOI] [PubMed] [Google Scholar]
- 38.Godoy M.C.B., Sabloff B., Naidich D.P. Subsolid pulmonary nodules. Curr Opin Pulm Med. 2012;18(4):304–312. doi: 10.1097/MCP.0b013e328354a5f2. [DOI] [PubMed] [Google Scholar]
- 39.Henschke C.I., Yip R., Smith J.P. CT screening for lung cancer: part-solid nodules in baseline and annual repeat rounds. Am J Roentgenol. 2016;207(6):1176–1184. doi: 10.2214/AJR.16.16043. [DOI] [PubMed] [Google Scholar]
- 40.Yankelevitz D.F., Yip R., Smith J.P. CT screening for lung cancer: nonsolid nodules in baseline and annual repeat rounds. Radiology. 2015;277(2):555–564. doi: 10.1148/radiol.2015142554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholten E.T., de Jong P.A., de Hoop B. Towards a close computed tomography monitoring approach for screen detected subsolid pulmonary nodules? Eur Respir J. 2015;45(3):765–773. doi: 10.1183/09031936.00005914. [DOI] [PubMed] [Google Scholar]
- 42.Hasegawa M., Sone S., Takashima S. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73(876):1252–1259. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 43.Lindell R.M., Hartman T.E., Swensen S.J. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007;242(2):555–562. doi: 10.1148/radiol.2422052090. [DOI] [PubMed] [Google Scholar]
- 44.Walter J.E., Heuvelmans M.A., Yousaf-Khan U. New subsolid pulmonary nodules in lung cancer screening: the NELSON trial. J Thorac Oncol. 2018;13(9):1410–1414. doi: 10.1016/j.jtho.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Choi W.S., Park C.M., Song Y.S., Lee S.M., Wi J.Y., Goo J.M. Transient subsolid nodules in patients with extrapulmonary malignancies: their frequency and differential features. Acta Radiol. 2015;56(4):428–437. doi: 10.1177/0284185114528325. [DOI] [PubMed] [Google Scholar]
- 46.Lee S.M., Park C.M., Goo J.M. Transient part-solid nodules detected at screening thin-section CT for lung cancer: comparison with persistent part-solid nodules. Radiology. 2010;255(1):242–251. doi: 10.1148/radiol.09090547. [DOI] [PubMed] [Google Scholar]
- 47.Oh J.Y., Kwon S.Y., Yoon H.I.L. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer. 2007;55(1):67–73. doi: 10.1016/j.lungcan.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Felix L., Serra-Tosio G., Lantuejoul S. CT characteristics of resolving ground-glass opacities in a lung cancer screening programme. Eur J Radiol. 2011;77(3):410–416. doi: 10.1016/j.ejrad.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Cho J.Y., Leem C.S., Kim Y. Solid part size is an important predictor of nodal metastasis in lung cancer with a subsolid tumor. BMC Pulm Med. 2018;18(1):151. doi: 10.1186/s12890-018-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naidich D.P., Bankier A.A., MacMahon H. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304–317. doi: 10.1148/radiol.12120628. [DOI] [PubMed] [Google Scholar]
- 51.Ko J.P., Berman E.J., Kaur M. Pulmonary nodules: growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology. 2012;262(2):662–671. doi: 10.1148/radiol.11100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu D.M., Gietema H., de Koning H. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer. 2006;54(2):177–184. doi: 10.1016/j.lungcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Scholten E.T., Jacobs C., van Ginneken B. Detection and quantification of the solid component in pulmonary subsolid nodules by semiautomatic segmentation. Eur Radiol. 2015;25(2):488–496. doi: 10.1007/s00330-014-3427-z. [DOI] [PubMed] [Google Scholar]
- 54.Cohen J.G., Goo J.M., Yoo R.E. Software performance in segmenting ground-glass and solid components of subsolid nodules in pulmonary adenocarcinomas. Eur Radiol. 2016;26(12):4465–4474. doi: 10.1007/s00330-016-4317-3. [DOI] [PubMed] [Google Scholar]
- 55.Garzelli L., Goo J.M., Ahn S.Y. Improving the prediction of lung adenocarcinoma invasive component on CT: value of a vessel removal algorithm during software segmentation of subsolid nodules. Eur J Radiol. 2018;100:58–65. doi: 10.1016/j.ejrad.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 56.de Hoop B., Gietema H., van de Vorst S., Murphy K., van Klaveren R.J., Prokop M. Pulmonary ground-glass nodules: increase in mass as an early indicator of growth. Radiology. 2010;255(1):199–206. doi: 10.1148/radiol.09090571. [DOI] [PubMed] [Google Scholar]
- 57.Gietema H.A., Schaefer-Prokop C.M., Mali W.P.T.M., Groenewegen G., Prokop M. Pulmonary nodules: interscan variability of semiautomated volume measurements with multisection CT—influence of inspiration level, nodule size, and segmentation performance. Radiology. 2007;245(3):889–894. doi: 10.1148/radiol.2452061054. [DOI] [PubMed] [Google Scholar]
- 58.Lindell R.M., Hartman T.E., Swensen S.J., Jett J.R., Midthun D.E., Mandrekar J.N. 5-Year lung cancer screening experience: growth curves of 18 lung cancers compared to histologic type, CT attenuation, stage, survival, and size. Chest. 2009;136(6):1586–1595. doi: 10.1378/chest.09-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko J.P., Azour L. Management of incidental lung nodules. Semin Ultrasound CT MRI. 2018;39(3):249–259. doi: 10.1053/j.sult.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Kakinuma R., Ohmatsu H., Kaneko M. Progression of focal pure ground-glass opacity detected by low-dose helical computed tomography screening for lung cancer. J Comput Assist Tomogr. 2004;28(1):17–23. doi: 10.1097/00004728-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y., Sun H., Zhou F. Imaging features of TSCT predict the classification of pulmonary preinvasive lesion, minimally and invasive adenocarcinoma presented as ground glass nodules. Lung Cancer. 2017;108:192–197. doi: 10.1016/j.lungcan.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Lim H., Ahn S., Lee K.S. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan. Chest. 2013;144(4):1291–1299. doi: 10.1378/chest.12-2987. [DOI] [PubMed] [Google Scholar]
- 63.Jin X., Zhao S.H., Gao J. CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol. 2015;25(9):2532–2540. doi: 10.1007/s00330-015-3637-z. [DOI] [PubMed] [Google Scholar]
- 64.Song Y.S., Park C.M., Park S.J., Lee S.M., Jeon Y.K., Goo J.M. Volume and mass doubling times of persistent pulmonary subsolid nodules detected in patients without known malignancy. Radiology. 2014;273(1):276–284. doi: 10.1148/radiol.14132324. [DOI] [PubMed] [Google Scholar]
- 65.Naidich D.P., Azour L. Managing stable subsolid lung nodules: a possible approach. Radiology. 2020;295(2):456–457. doi: 10.1148/radiol.2020200047. [DOI] [PubMed] [Google Scholar]
- 66.Chang J.M., Lee H.J., Goo J.M. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006;7(1):57–69. doi: 10.3348/kjr.2006.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nomori H., Watanabe K., Ohtsuka T., Naruke T., Suemasu K., Uno K. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer. 2004;45(1):19–27. doi: 10.1016/j.lungcan.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Tae J.K., Kyung W.L., Hyae Y.K. Simple pulmonary eosinophilia evaluated by means of FDG PET: the findings of 14 cases. Korean J Radiol. 2005;6(4):208–213. doi: 10.3348/kjr.2005.6.4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mcdermott S., Kilcoyne A., Wang Y., Scott J.A., Halpern E.F., Ackman J.B. Comparison of the 18 F-FDG avidity at PET of benign and malignant pure ground-glass opacities: a paradox? 2019 [cited 2019 Nov 30] [DOI] [PubMed]
- 70.Higashi K., Ueda Y., Seki H. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med. 1998;39(6):1016–1020. [PubMed] [Google Scholar]
- 71.Suh Y.J., Park C.M., Han K. Utility of FDG PET/CT for preoperative staging of non–small cell lung cancers manifesting as subsolid nodules with a solid portion of 3 cm or smaller. Am J Roentgenol. 2020;214(3):514–523. doi: 10.2214/AJR.19.21811. [DOI] [PubMed] [Google Scholar]
- 72.Hur J., Lee H.J., Nam J.E. Diagnostic accuracy of CT fluoroscopy–guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. Am J Roentgenol. 2009;192(3):629–634. doi: 10.2214/AJR.08.1366. [DOI] [PubMed] [Google Scholar]
- 73.Yamauchi Y., Izumi Y., Nakatsuka S. Diagnostic performance of percutaneous core needle lung biopsy under multi-CT fluoroscopic guidance for ground-glass opacity pulmonary lesions. Eur J Radiol. 2011;79(2):e85–e89. doi: 10.1016/j.ejrad.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 74.Kiranantawat N., McDermott S., Petranovic M. Determining malignancy in CT guided fine needle aspirate biopsy of subsolid lung nodules: is core biopsy necessary? Eur J Radiol Open. 2019;6:175–181. doi: 10.1016/j.ejro.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ha Y.K., Young M.S., Kyung S.L., Han J., Yi C.A., Yoon K.K. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007;245(1):267–275. doi: 10.1148/radiol.2451061682. [DOI] [PubMed] [Google Scholar]
- 76.De Filippo M., Saba L., Concari G. Fattori che predicono l’accuratezza diagnostica dell’agobiopsia transtoracica TC-guidata dei noduli polmonari solidi non calcifici, subsolidi e misti. Radiol Medica. 2013;118(7):1071–1081. [Google Scholar]
- 77.Yun S., Kang H., Park S., Kim B.S., Park J.G., Jung M.J. Diagnostic accuracy and complications of CT-guided core needle lung biopsy of solid and part-solid lesions. Br J Radiol. 2018;91(1088):20170946. doi: 10.1259/bjr.20170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ettinger DS, Wood DE, Chair V, et al. Continue NCCN Guidelines Panel Disclosures NCCN Guidelines Version 6.2020 Non-Small Cell Lung Cancer. 2020.
- 79.Chen D., Dai C., Kadeer X., Mao R., Chen Y., Chen C. New horizons in surgical treatment of ground-glass nodules of the lung: experience and controversies. Ther Clin Risk Manag. 2018;14:203–211. doi: 10.2147/TCRM.S152127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hammer M.M., Palazzo L.L., Eckel A.L., Barbosa E.M., Kong C.Y. A decision analysis of follow-up and treatment algorithms for nonsolid pulmonary nodules. Radiology. 2019;290(2):506–513. doi: 10.1148/radiol.2018180867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazzone P.J., Gould M.K., Arenberg D.A. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST Expert Panel Report. Chest. 2020;158(1):406–415. doi: 10.1016/j.chest.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]