Abstract
Objectives
To describe the clinical evolution and predictors of symptom persistence during 2 months' follow-up in adults with noncritical coronavirus disease 2019 (COVID-19).
Methods
We performed descriptive clinical follow-up (day (D) 7, D30 and D60) of 150 patients with noncritical COVID-19 confirmed by real-time reverse transcriptase PCR at Tours University Hospital from 17 March to 3 June 2020, including demographic, clinical and laboratory data collected from the electronic medical records and by phone call. Persisting symptoms were defined by the presence at D30 or D60 of at least one of the following: weight loss ≥5%, severe dyspnoea or asthenia, chest pain, palpitations, anosmia/ageusia, headache, cutaneous signs, arthralgia, myalgia, digestive disorders, fever or sick leave.
Results
At D30, 68% (103/150) of patients had at least one symptom; and at D60, 66% (86/130) had symptoms, mainly anosmia/ageusia: 59% (89/150) at symptom onset, 28% (40/150) at D30 and 23% (29/130) at D60. Dyspnoea concerned 36.7% (55/150) patients at D30 and 30% (39/130) at D60. Half of the patients (74/150) at D30 and 40% (52/130) at D60 reported asthenia. Persistent symptoms at D60 were significantly associated with age 40 to 60 years old, hospital admission and abnormal auscultation at symptom onset. At D30, severe COVID-19 and/or dyspnoea at symptom onset were additional factors associated with persistent symptoms.
Conclusions
Up to 2 months after symptom onset, two thirds of adults with noncritical COVID-19 had complaints, mainly anosmia/ageusia, dyspnoea or asthenia. A prolonged medical follow-up of patients with COVID-19 seems essential, whatever the initial clinical presentation.
Keywords: Description, Follow-up, Mild COVID-19, Moderate COVID-19, Noncritical COVID-19, Outcomes, Persisting symptoms
Introduction
The most frequent symptoms of coronavirus disease 2019 (COVID-19) at disease onset are cough, fever, asthenia, myalgia and altered smell or taste, including anosmia/ageusia. Respiratory distress can occur, mostly between 7 to 10 days after symptom onset [[1], [2], [3], [4]].
Recent studies investigating predictors of poor prognosis at an early stage identified potential risk factors for severe COVID-19 [1,5,6]. One recent study in Italy by Carfi et al. [7] described persistent symptoms after hospitalization for COVID-19. Such evidence has not been reported for mild to moderate COVID-19. Our objective was to describe the clinical evolution and predictors of symptom persistence at D30 and D60 in patients with initial noncritical COVID-19. The aim was to highlight the initial key symptoms of COVID-19 to alert practitioners and patients of the risk of longer symptom duration in individuals with noncritical COVID-19.
Materials and methods
Study design and population
This epidemiologic study, based on a prospective follow-up, was carried out in our academic university hospital from 17 March to 3 June 2020. Inclusion criteria were: every adult patients (≥18 years old) with a confirmed diagnosis of COVID-19 (positive real-time reverse transcriptase PCR (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and medical care in our hospital, either in hospitalized patients or after consultation at the hospital's outpatient clinical evaluation centre (OCEC). The OCEC has been developed to avoid consulting with a general practitioner or visiting the emergency department to reduce the risk of cross-transmission. The OCEC allowed the following: diagnosing COVID-19 by an RT-PCR test for SARS-CoV-2 with a nasopharyngeal swab, assessing the risk of critical illness/deterioration and the need for surveillance and eventually proposing hospital admission to monitor the patient. Exclusion criteria were: patients deceased or admitted to the intensive care unit (ICU) (considered as critical disease according to the World Health Organization (WHO) guidance for clinical management of COVID-19) [8], residents of retirement/nursery homes or long-term care facilities, patients transferred to another healthcare facility (i.e. other hospital, rehabilitation institution, retirement home), those unable to answer a phone questionnaire and those lost to follow-up at D30.
The infectious diseases unit was in charge of the outpatient follow-up with confirmed COVID-19 but noncritical disease. Patients with or without clinical signs of pneumonia but without a need for oxygen therapy were defined as having mild/moderate COVID-19. Patients with signs of pneumonia requiring oxygen therapy but not needing ICU admission were defined as having severe COVID-19, according to the WHO definition [8]. The infectious diseases unit assessed these patients' clinical presentation at week 1 (day (D) 7), 1 month (D30) and 2 months (D60) after symptom onset by using a specific case report form they developed. Phone interviews were conducted with a clinical decision algorithm to guide care advice messages, triage (evaluation of life-threatening conditions, especially at D7) and screening for COVID-19 symptom persistence or emergence. Baseline characteristics were retrospectively collected from patients' electronic medical records.
Outcome
Persisting symptoms at D30 or D60 were defined as the presence of at least one of the following: weight loss ≥5%, grade 2–4 dyspnoea according to the modified Medical Research Council scale [9], asthenia grade 3 or 4 according the World Health Organization (WHO) performance status classification [10], persisting chest pain, palpitations, anosmia/ageusia, headache, cutaneous signs (free description), arthralgia, myalgia, persisting digestive disorders (i.e. diarrhoea, vomiting, pain), fever (temperature >38°C) or sick leave.
Data collection
Demographic and initial clinical and laboratory data were collected from patients' electronic medical records (consultation or hospitalization). The relevant comorbidities were those considered to confer high risk for severe COVID-19 (i.e. obesity (body mass index > 30 kg/m2), chronic respiratory disease, dialysis, heart failure or previous cardiovascular event, liver cirrhosis, insulin-dependent diabetes, immunosuppression, pregnancy) [11].
The follow-up information was collected by phone at D7, D30 and D60 with use of the specific standardized case report form:
-
•
At D7 after symptom onset, the following information was collected by phone call for outpatients or from the electronic medical records for inpatients: dyspnoea, fever, weight loss, chest pain, influenzalike symptoms (headache, asthenia, myalgia), digestive disorders (i.e., diarrhoea, vomiting), anosmia and ageusia (Supplementary Material S1).
-
•
At D30 and D60 after symptom onset, COVID-19 evolution was tracked by use of a specific standardized case report form (Supplementary Material S2) by phone call: persistence or emergence of sick leave, general condition (worse, same or better than before COVID-19), dyspnoea using the modified Medical Research Council scale, chest pain and triggering factor, palpitations, anosmia and ageusia on an analogue scale (from 0, total anosmia/ageusia, to 10, normal) at the worst moment of the disease and at 1 month's follow-up, headache, asthenia (WHO), temperature >38°C, myalgia, arthralgia, digestive disorders (i.e. diarrhoea, vomiting, pain) and cutaneous signs (Supplementary Material S2). Each symptom was considered only if it had not existed before the disease.
For a few clinical variables with a high proportion of missing data, physicians manually reviewed patient charts. Missing data concerned 0 to 29% of the data according to the variables at symptom onset, mainly for dyspnoea, chest pain and abnormal auscultation. At D30 and D60, there were few missing data, except for weight evolution (57% and 33% missing data at D30 and D60 respectively).
Statistical analyses
Descriptive statistics included frequency analyses (percentages) for categorical variables and mean (standard deviation) for quantitative variables. To identify predictors of clinical symptom persistence at D30 and D60, we used comparative analyses with chi-square test or Fisher test for categorical variables or Student t test or Mann-Whitney test for quantitative variables. On bivariate analysis, odds ratios along with their 95% confidence intervals were calculated using logistic regression modelling. Analyses were performed by SAS Enterprise Guide 71 64-bit software (SAS Institute, Cary, NC, USA). p < 0.05 was considered statistically significant. All tests were two sided.
Ethics
All patients were informed of the potential reuse of their data for research purposes and could refuse to participate. This study was registered (no. 2020_049) in the teaching hospital registry of processing operations performed with personal data, as advised by the French authority Commission Nationale de l’Informatique et des Libertés (CNIL). We also received the approval of the local ethics committee in human research (no. 2020_039).
Results
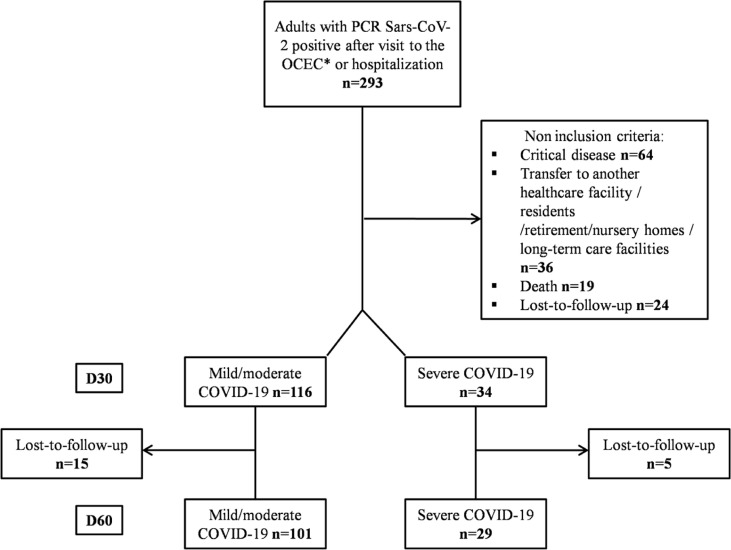
Over the first 6 weeks of the epidemic, 293 patients sought care at our hospital as inpatients or outpatients with RT-PCR–confirmed COVID-19. After excluding 64 ICU patients, 36 residents of nursing home or long-term care facilities or patients transferred to another healthcare facility, 24 lost to follow-up at D30 and 19 deaths, we finally included 150 patients with noncritical COVID-19 presentation. Between D30 and D60, 20 more patients were lost to follow-up (Fig. 1 ).
Fig. 1.
Flowchart of study design. OCEC, outpatient clinical evaluation centre.
The male/female ratio was 0.79 (female, 56%, 84/150); the mean age was 49 ± 15 years. More than half of the patients (54%, 80/150) had at least one comorbid condition, and half were healthcare professionals (75/150). Despite the 20 patients lost to follow-up, demographic characteristics at D30 and D60 were similar (Supplementary Material S3).
The most common symptoms at disease onset were flulike symptoms (87%, 129/150), anosmia/ageusia (59%, 89/150) and fever (51%, 76/150) (Table 1 ).
Table 1.
Patient symptoms at COVID-19 onset and at day 30 (D30) and 60 (D60)
| Characteristic | Onset (n = 150) | D30 (n = 150) | D60 (n = 130) |
|---|---|---|---|
| Fever (temperature >38°C) | 76 (51.4) | 5 (3.6) | 0 |
| Dyspnoea/shortness of breatha | 49 (42.2) | 16 (10.7) | 10 (7.7) |
| Chest pain | 15 (14.0) | 27 (18.0) | 17 (13.1) |
| Abnormal auscultation | 46 (39.3) | — | — |
| Flulike symptomsb | 129 (87.2) | 54 (36.0) | 28 (21.5) |
| Digestive disordersc | 48 (33.1) | 26 (17.3) | 15 (11.5) |
| Including diarrhoead | 44 (91.7) | 13 (50.0) | 5 (33.3) |
| Weight, mean ± standard deviation | 78.0 ± 19.4 | 77.2 ± 20.2 | 75.6 ± 18.0 |
| Weight loss ≥5% | — | 13 (15.9) | 15 (17.2) |
| Anosmia/ageusia | 89 (59.3) | 40 (27.8) | 29 (22.7) |
| Palpitations | — | 9 (6.5) | 14 (10.9) |
| Arthralgia | — | 13 (9.8) | 21 (16.3) |
| Cutaneous signs | — | 21 (15.4) | 15 (11.5) |
| Initial hospitalization | 53 (35.3) | — | — |
| Initial clinical presentation | |||
| Mild/moderate COVID-19 | 116 (77.3) | — | — |
| Severe COVID-19 | 34 (22.7) | — | — |
| Sick leave | — | 26 (19.7) | 14 (11.2) |
Data are presented as n (%) unless otherwise indicated. COVID-19, coronavirus disease 2019.
Grade 2–4 dyspnoea according the modified Medical Research Council scale.
Myalgia, headache and/or asthenia.
Digestive disorders (i.e. diarrhoea, vomiting).
Denominator is digestive disorders.
For the follow-up at D30 and D60, phone calls were performed at a mean of 32.7 ± 2.5 days (range, 27–37 days) and 59.7 ± 1.7 days (range, 57–67 days) after symptom onset. At D30, 103 (68%) of 150 patients reported at least one symptom compared to 86 (66%) of 130 at D60 (Table 2 ). However, each symptom was less frequently reported at D60 than D30, except for arthralgia. At D30, 46% (73/150) of patients still felt ill or were in a worse clinical condition than at COVID-19 onset versus 37% (48/130) at D60.
Table 2.
Patient characteristics at days 30 (D30) and 60 (D60) after symptom onset for patients with one or more persisting symptom
| Characteristic | Total |
One or more persisting symptom at: |
|||
|---|---|---|---|---|---|
| D30 (n = 150) |
D60 (n = 130) |
||||
| n (%) | n (%) | p | n (%) | p | |
| Patients | 150 (100) | 103 (68) | 86 (66.1) | ||
| Female | 84 (56.0) | 59 (57.3) | 0.6 | 48 (55.8) | 0.3 |
| Age | 0.06 | 0.026 | |||
| <30 years | 16 (10.7) | 7 (6.8) | 4 (4.7) | ||
| 30–39 years | 32 (21.3) | 21 (20.4) | 19 (22.1) | ||
| 40–49 years | 27 (18.0) | 24 (23.3) | 23 (26.7) | ||
| 50–59 years | 37 (24.7) | 28 (27.2) | 21 (24.4) | ||
| 60–69 years | 19 (12.7) | 11 (10.7) | 10 (11.6) | ||
| ≥70 years | 19 (12.7) | 12 (11.7) | 9 (10.5) | ||
| Healthcare professional | 75 (50.0) | 49 (47.6) | 0.38 | 43.0 (50.0) | 0.6 |
| No. of comorbid conditionsa | 0.75 | 0.5 | |||
| 0 | 69 (46.0) | 46 (45.6) | 42 (48.8) | ||
| 1 | 52 (34.7) | 35 (34.0) | 25 (29.1) | ||
| 2 or more | 28 (18.7) | 21 (20.4) | 19 (22.1) | ||
| Symptoms at onset | |||||
| Fever | 76 (51.4) | 54 (53.5) | 0.45 | 44 (52.4) | 0.8 |
| Dyspnoea | 49 (42.2) | 38 (50.0) | 0.02 | 28 (45.2) | 0.3 |
| Chest pain | 15 (14.0) | 11 (15.9) | 0.4 | 9 (15.8) | 0.6 |
| Abnormal auscultation | 46 (39.3) | 38 (47.5) | 0.0095 | 32 (47.1) | 0.046 |
| Other respiratory signsb | 135 (91.2) | 90 (90.1) | 0.5 | 76 (90.5) | 0.6 |
| Flulike symptomsc | 129 (87.2) | 88 (87.1) | 0.99 | 73 (86.9) | 0.7 |
| Diarrhoea | 44 (30.8) | 31 (32.0) | 0.65 | 26 (32.5) | 1 |
| Anosmia/ageusia | 89 (59.3) | 63 (61.2) | 0.5 | 55 (64.0) | 0.2 |
| Initial hospitalization | 53 (35.3) | 43 (41.7) | 0.017 | 37 (43) | 0.011 |
| Initial clinical presentation | 0.02 | 0.2 | |||
| Mild/moderate COVID-19 | 116 (77.3) | 74 (71.8) | 64 (74.4) | ||
| Severe COVID-19 | 34 (22.7) | 29 (28.2) | 22 (25.6) | ||
p values are compared to patients without persisting symptom. COVID-19, coronavirus disease 2019.
Obesity (body mass index >30 kg/m2), chronic respiratory disease, dialysis, heart failure or previous cardiovascular event, liver cirrhosis, insulin-dependent diabetes, immunosuppression, pregnancy.
Cough, sneeze and/or rhinitis.
Myalgia, headache and/or asthenia.
The most frequent symptom reported at D30 and D60 was anosmia/ageusia (Table 1). On an analogue scale (from 0, total anosmia/ageusia, to 10, normal), at the worse moment of the disease, the mean anosmia and ageusia scores were 1.5 ± 2.1 (range, 0–8) and 1.9 ± 2.5 (range, 0–8) respectively; at D30, the mean scores were 7 ± 2.9 (range, 0–10) and 7.7 ± 2.3 (range, 0–10); and at D60, they were 7.1 ± 2.3 (range, 0–10) and 8.3 ± 1.6 (range, 5–10) respectively.
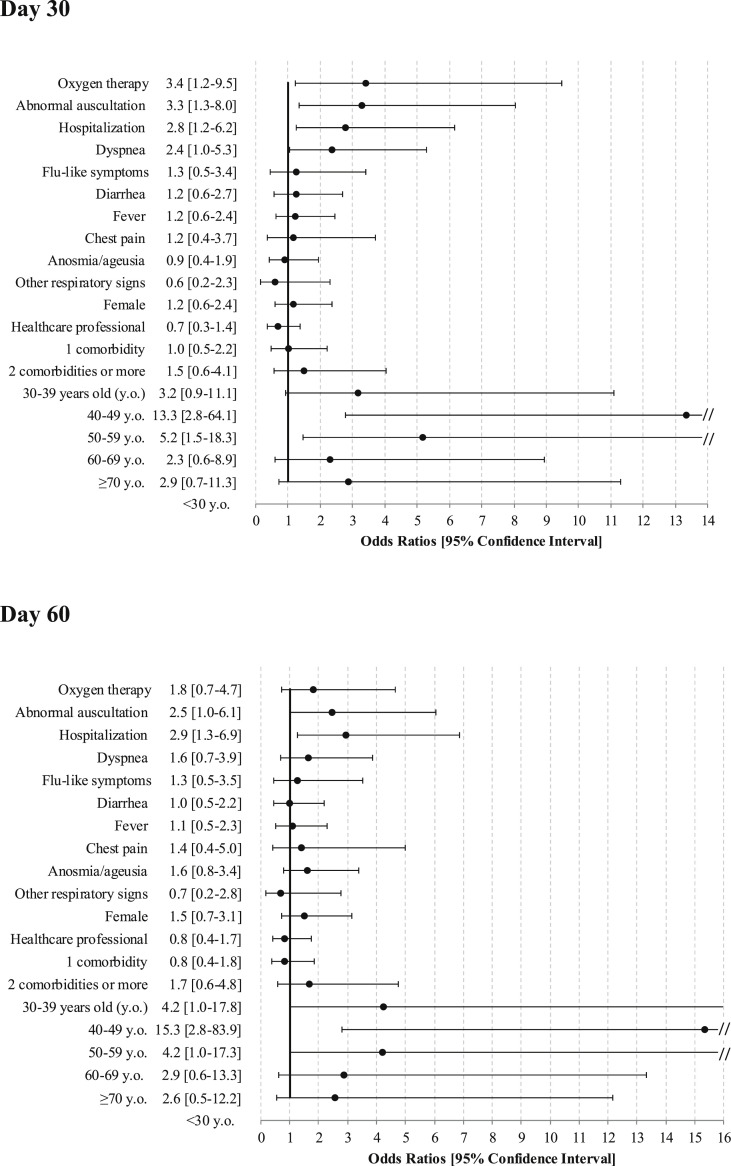
Symptoms persisting at D30 were significantly associated with hospital admission at symptom onset, initial clinical presentation, dyspnoea and abnormal auscultation (Fig. 2 ). Persisting clinical symptoms at D30 were associated with age class 40 to 60 years old but not with preexisting comorbid conditions. At D60, the associations remained for hospital admission and abnormal auscultation at symptom onset as well as the same age class 40 to 60 years old.
Fig. 2.
Predictors of persistent coronavirus disease 2019 (COVID-19) symptoms.
Discussion
This study showed that the medium-term course of 150 patients with mild or moderate COVID-19 was unfavourable: two-thirds of patients still reported symptoms at D30 and D60, and more than one third felt still ill or in a worse clinical condition at D60 than at onset of COVID-19. These prolonged symptoms were significantly associated with age 40 to 60 years old, hospital admission at symptom onset, severe COVID-19 and dyspnoea or abnormal auscultation.
The main strength of our study was a well-documented prospective follow-up of patients with a noncritical COVID-19 presentation at the early stage and at D30 and D60. Indeed, the recent international literature has provided evidence of the clinical presentation and evolution of COVID-19 patients [2,[12], [13], [14], [15]], although mainly regarding the most severe cases in ICUs and predictors of initial deterioration. The WHO has stated that median time from illness onset to recovery is about 2 weeks for mild cases and 3 to 6 weeks with severe or critical disease [16]. Small-scale studies in Wuhan, China, showed that survivors continued to have poor lung and heart function [1].
The initial clinical presentation for patients with mild or moderate COVID-19 (frequent respiratory and flulike symptoms) was similar to that of previous studies [17,18] including patients with more severe clinical presentation at symptom onset described in New York (USA), the United Kingdom or Italy. However, the patients included in our study were younger (mean age 49, vs. 62 to 73 years in severe cases) [[13], [14], [15]], and health professionals were overrepresented because of the national recommendations at this date and their prioritizing access to diagnostic tests [19].
For patients with mild or moderate COVID-19, different symptoms such as anosmia/ageusia, myalgia or headache persisted. In a recent study, Lechien et al. [17] reported persistent olfactory dysfunction in 37.5% of patients at least 7 days after the end of mild to moderate COVID-19. The precise mechanism of this symptom is unknown. Patients should be informed of this anomaly and referred to a specialist.
In our study, at D30, half of the patients still felt ill or in a worse clinical condition than before symptom onset, and 7% reported severe asthenia (3.1% at D60). One third of the patients had dyspnoea and approximately one sixth had chest pain. This situation is particularly frightening for patients. Rigorous studies with chest explorations seem necessary. Indeed, the evaluation at D30 and D60 was declarative over a phone call, without available physical, biological or imaging assessment. We controlled for this potential reporting bias by using standardized questionnaires administrated by trained investigators (Supplementary Data S1 and S2). However, subjective complaints are worth the attention and focus of the medical community and need to be taken into account in medical care. Moreover, several infectious diseases such as primary cytomegalovirus or Epstein-Barr virus infection are known to be associated with persistent symptoms, without necessarily any obvious anomaly at physical examination [[20], [21], [22]].
Marked inflammatory response associated with symptomatic COVID-19 could promote such prolonged convalescence and persisting symptoms. Some authors also suggest the possibility of posttraumatic stress disorder after COVID-19, which could contribute to a more prolonged experience of symptoms such asthenia or poor well-being [23,24]. These complex tardive psychological disorders have already been shown after acute respiratory distress syndrome [25,26]. This hypothesis could not be detailed in our study because of a lack of a reproducible psychological assessment but should probably be explored.
We found prolonged symptoms to be significantly associated in bivariate analysis with age 40 to 60 years, hospital admission at symptom onset, severe COVID-19 and dyspnoea or abnormal auscultation. Because patients' baseline characteristics were partially retrospectively collected, data for potentially contributive factors were missing, thereby preventing multivariate modelling, as the main contributive factors in bivariate analysis (dyspnoea, abnormal auscultation) had up to 29% of data missing. However, the findings of the bivariate analysis were clinically relevant. Patients' smoking status was not available; it would have been interesting to look for an association with duration of symptoms (especially anosmia/ageusia or chronic dyspnoea).
With this observational study allowing the prospective follow-up of 150 patients with noncritical COVID-19, we were able to assess the evolution of the disease and demonstrate that even the mildest presentation was associated with medium-term symptoms requiring follow-up. Thus, the COVID-19 pandemic will involve a care burden long after its end.
Transparency declaration
All authors report no conflicts of interest relevant to this article.
Acknowledgements
We thank all clinical and nursing staff who recruited and cared for the patients at Tours University Hospital and staff (especially Léonard Bachellier, Côme Schmitt, Marie Schneider, Anne-Sophie Lavedrine, Marie Leidlinger, Clotilde Laffitte, Gabrielle Valente, Amélie Gomez, Claire Corbillé, Camille Langbour, Marie Caroline Gabriel, Marion de Quillacq, Rémi Gervais, Sami El Meziani, Marie Fortin, Gaëlle Ragot, Julien Broustaille, Dorian Gagnadoux, Eloise Bonnin, Léa Octrovée, Louise Huertas, Romuald Boivin, Bérenger Le Roux, Nathalie Sayamath, Hortense Glérant) for logistical follow-up. We thank Laura Smales for English-language editorial work. None of these individuals received compensation for their role in the study.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.09.052.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020 doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cecconi M., Piovani D., Brunetta E., Aghemo A., Greco M., Ciccarelli M. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for Covid-19 infection in Lombardy, Italy. J Clin Med. 2020;9:1548. doi: 10.3390/jcm9051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Clinical management of COVID-19. https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19 Available at:
- 9.Fletcher C.M., Elmes P.C., Fairbairn A.S., Wood C.H. Significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oken M.M., Creech R.H., Tormey D.C., Horton J., Davis T.E., McFadden E.T. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 11.Haut Conseil de la Santé Publique (HCSP) HCSP; Paris: 2020. Provisional statement: recommendations on prevention and management of Covid-19 in patients at risk of severe forms.https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=799 Available at: [Google Scholar]
- 12.Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Report of the World health organization (WHO)-China joint mission on coronavirus disease 2019 (COVID-19) https://www.who.int/publications-detail-redirect/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) Available at:
- 17.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haut Conseil de la Santé Publique (HCSP) HCSP; Paris: 2020. Provisional statement: patients at risk of severe forms of Covid-19 and prioritising access to diagnostic tests.https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=798 Available at: [Google Scholar]
- 20.Balfour H.H., Holman C.J., Hokanson K.M., Lelonek M.M., Giesbrecht J.E., White D.R. A prospective clinical study of Epstein-Barr virus and host interactions during acute infectious mononucleosis. J Infect Dis. 2005;192:1505–1512. doi: 10.1086/491740. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz C.A., Henle W., Henle G., Snover D., Rudnick H., Balfour H.H. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore) 1986;65:124–134. doi: 10.1097/00005792-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Wreghitt T.G., Teare E.L., Sule O., Devi R., Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603–1606. doi: 10.1086/379711. [DOI] [PubMed] [Google Scholar]
- 23.Mahase E. Covid-19: what do we know about ‘long Covid’? BMJ. 2020;370:m2815. doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- 24.Xiao S., Luo D., Xiao Y. Survivors of COVID-19 are at high risk of posttraumatic stress disorder. Glob Health Res Policy. 2020;5:29. doi: 10.1186/s41256-020-00155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutheil F., Mondillon L., Navel V. PTSD as the second tsunami of the SARS-CoV-2 pandemic. Psychol Med. 2020 doi: 10.1017/S0033291720001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu N., Zhang F., Wei C., Jia Y., Shang Z., Sun L. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: gender differences matter. Psychiatry Res. 2020;287:112921. doi: 10.1016/j.psychres.2020.112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.